Abstract
Patients with schizophrenia have a lower than average life span, largely due to the increased prevalence of cardiometabolic comorbidities. There is an unmet public health need to identify individuals with psychotic disorders who have a high risk of rapid weight gain and who are at risk of developing metabolic complications. Here, we applied mass spectrometry-based lipidomics in a prospective study comprising 48 healthy controls (CTR), 44 first-episode psychosis (FEP) patients, and 22 individuals at clinical high risk (CHR) for psychosis, from 2 study centers (Turku, Finland and London, UK). Baseline serum samples were analyzed using lipidomics, and body mass index (BMI) was assessed at baseline and after 12 months. We found that baseline triacylglycerols (TGs) with low double-bond counts and carbon numbers were positively associated with the change in BMI at follow-up. In addition, a molecular signature comprised of 2 TGs (TG[48:0] and TG[45:0]) was predictive of weight gain in individuals with a psychotic disorder, with an area under the receiver operating characteristic curve (AUROC) of 0.74 (95% CI: 0.60–0.85). When independently tested in the CHR group, this molecular signature predicted said weight change with AUROC = 0.73 (95% CI: 0.61–0.83). We conclude that molecular lipids may serve as a predictor of weight gain in psychotic disorders in at-risk individuals and may thus provide a useful marker for identifying individuals who are most prone to developing cardiometabolic comorbidities.
Keywords: lipidomics, metabolomics, first-episode psychosis, metabolic co-morbidities, weight gain
Introduction
Psychotic disorders are associated with a life expectancy reduction of 15–20 years,1,2 mostly due to the high prevalence of cardiovascular disease, type 2 diabetes (T2DM), and metabolic syndrome.3–5 Metabolic comorbidities, including impaired glucose tolerance, weight gain, and obesity, often co-occur in first-episode psychosis (FEP) patients,6–8 and this increases the risk of cardiovascular disease in these individuals.9,10 Un-medicated FEP patients report a high intake of saturated fat and low levels of high-intensity exercise.11 Although unhealthy lifestyles and antipsychotic medication are associated with the development of metabolic comorbidities in psychosis patients, the underlying mechanisms remain poorly understood.3,12 Drug-induced metabolic dysregulation appears heterogeneously,13,14 while metabolic comorbidities can also occur in drug-naïve FEP patients.6,15
Metabolomics, that is, a global study of small molecules (<1500 Da) and their associated biochemical processes, is a powerful emerging tool in psychiatric research, enabling the investigation of disease etiology and treatment response from metabolic perspectives.8,16 Lipidomics is a subfield of metabolomics, which focuses on the study of lipids. By applying a lipidomics approach, we previously found that FEP patients who rapidly gain weight at follow-up have increased serum lipids at baseline; lipids which are also known to be associated with nonalcoholic fatty liver disease (NAFLD) and increased risk of T2DM.8,17 However, it is currently unclear if these lipids could be used to predict weight gain and the associated metabolic comorbidities in FEP patients. Here, we report a lipidomics study in a prospective series of plasma samples from healthy controls (CTR), FEP patients, and individuals at clinical high risk (CHR) for psychosis. The aim of the study was to investigate whether lipid profiles can identify FEP patients or CHR individuals, who are at the highest risk of rapid weight gain and occurrence of metabolic comorbidities.
Methods
Study Design and Participants
We collected plasma samples from 2 cohorts of patients receiving psychiatric early intervention services in Turku, Finland or London, UK. Ethical approval was obtained from the respective study sites in Finland (ETMK 98/180/2013) and United Kingdom (14/LO/1289). Capacity for consent was assessed and informed written consent was obtained from all volunteers. In total, 114 non-fasting blood samples were collected for this study. Plasma was separated immediately after the blood collection by centrifugation at 3000g for 10 minutes at room temperature. The plasma samples were shipped and stored at −80°C until analyzed. This case-control study included 48 healthy CTR (CTR group), 44 FEP patients (FEP group), and 22 individuals who were at CHR for psychosis (CHR group). The demographic characteristics of the study subjects are shown in table 1.
Table 1.
Clinical Characteristics of Study Population at the Baselinea
| CTR | FEP | CHR | |
|---|---|---|---|
| N (total) | 48 | 44 | 22 |
| N (Turku, Finland) | 31 | 30 | 22 |
| N (London, UK) | 17 | 14 | N/A |
| Sex (male, female) | 31, 17 | 26, 18 | 11, 11 |
| BMI (± SD) | 24.5 (3.8) | 24.3 (4.2) | 25.5 (5.7) |
| Age (± SD) (n) | 27.5 (5.9) (48) | 26.9 (6.3) (42) | 26.2 (5.0) (9) |
| GAF score (± SD) (n) | 92.0 (3.8) (36) | 46.6 (16.0) (39) | 53.0 (1) (10) |
| PANSS TOT (± SD) (n) | 30.4 (0.9) (30) | 70.9 (24.6) (40) | 55.8 (15.1) (10) |
| Antipsychotic CPZE (± SD) | |||
| Turku, Finland (n = 23 FEP, n = 9 CHR) | N/A | 221.8 (± 115.0) | 207.91(± 102.2) |
| London, UK (n = 2 FEP) | N/A | 60 (N/A) | N/A |
Note: CTR, healthy controls; FEP, first-episode psychosis group; CHR, clinical high-risk for psychosis group; SD, standard deviation; CPZE, chlorpromazine equivalence.
aStudy populations are from Turku, Finland and London, UK.
Inclusion and Exclusion Criteria
FEP patients met the following inclusion criteria: (1) DSM-IV diagnosis of a psychotic disorder, determined by the Structured Clinical Interview for DSM Disorders (SCID)-I/P and (2) illness duration of less than 5 years. In the Turku, Finland study, FEP volunteers were taking antipsychotic medication and had diagnoses of affective or non-affective psychosis. In the London, UK cohort, FEP arm of the study, volunteers were medication-free from all pharmacological treatments for at least 6 months and had diagnoses of schizophrenia or schizoaffective disorder. In the London, UK cohort, FEP volunteers were recruited from early intervention teams in South and West London. Healthy volunteers had no current/lifetime history of an Axis-I disorder as determined by the SCID-I/P and were matched by age (age +/− 3 years).
CHR patients were identified from the clinical population of psychiatric services using structured interviews to ensure that they met criteria for an at-risk mental state and to exclude current or past psychotic disorder.18,19 Patients with brief, intermittent psychotic symptom syndrome, attenuated positive symptom syndrome or genetic risk, or deterioration syndrome were classified as CHR for psychosis patients, consistent with the standardized criteria.18
The study setting for the Finnish Institute for Health and Welfare (THL), Finland dataset, which was used as an additional dataset to build the statistical model, was described in detail previously.20
Measures
Age, sex, weight, and height were recorded. BMI was calculated as weight in kilograms divided by height in meters squared, as described previously.11 Diagnoses were recorded using the SCID-I/P. Current and previous medication histories were recorded. Chlorpromazine equivalent (CPZE) doses were calculated for current antipsychotic exposure using previously defined methods.21 Symptom severity was assessed using the Positive and Negative Syndrome Scale.22 Social and occupational functioning were assessed using the Global Assessment of Functioning scale.23
Analysis of Molecular Lipids
A total of 114 plasma samples were randomized and extracted using a modified version of the Folch procedure.24 Promptly before extraction, 10 µl of 0.9% NaCl and 120 µl of CHCl3: MeOH (2:1, v/v) containing 2.5 µg ml−1 internal standard solution (for quality control [QC] and normalization purposes) were added to 10 µl of each plasma sample. The standard solution contained the following compounds: 1,2-diheptadecanoyl-sn-glycero-3-phosphoethanolamine (PE [17:0/17:0]), N-heptadecanoyl-d-erythrosphingosylphosphorylcholine (sphingomyelins, SM [d18:1/17:0]), N-heptadecanoyl-d-erythro-sphingosine (ceramides, Cer [d18:1/17:0]), 1,2-diheptadecanoyl-sn-glycero-3-phosphocholine (phosphatidylcholines, PC [17:0/17:0]), 1-heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine (lysophosphatidylcholines, LPC [17:0]), and 1-palmitoyl-d31-2-oleoyl-sn-glycero-3-phosphocholine (PC [16:0/d31/18:1]) that were purchased from Avanti Polar Lipids, Inc. (Alabaster), as well as 3β-hydroxy-5-cholestene 3-heptadecanoate (cholesterol esters, CE [17:0]) and tripalmitin-triheptadecanoylglycerol (TG [17:0/17:0/17:0]) (Larodan AB). The samples were vortexed and incubated on ice for 30 minutes after which they were centrifuged (9400g, 3 min, 4°C); 60 µl from the lower layer of each sample was then transferred to a glass vial with an insert, and 60 µl of CHCl3: MeOH (2:1, v/v) was added to each sample. The samples were re-randomized and stored at −80°C until analysis. Calibration curves using 1-hexadecyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine (PC [16:0/18:1(9Z)]), 1-(1Z-octadecenyl)-2-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine (PC [16:0/16:0]), 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine (PC [18:0/18:0], 1-octadecanoyl-sn-glycero-3-phosphocholine (LPC [18:0]), 1-(11Z-octadecadienoyl)-sn-glycero-3-phosphocholine (LPC [18:1]), 1-(9Z-octadecenoyl)-2-hexadecanoyl-sn-glycero-3-phosphoethanolamine (PE [16:0/18:1]), (2-aminoethoxy)[(2R)-3-hydroxy-2-[(11Z)-octadec-11-enoyloxy]propoxy]phosphinic acid (LysoPE [18:1]), N-(9Z-octadecenoyl)-sphinganine (Cer [d18:0/18:1(9Z)]), 1-hexadecyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphoethanolamine (PE [16:0/18:1]) (Avanti Polar Lipids, Inc.), 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphatidylcholine (LPC [16:0]) and 1,2,3-trihexadecanoalglycerol (TG [16:0/16:0/16:0]), 1,2,3-trioctadecanoylglycerol (TG [18:0/18:0/18:0]) and ChoE (18:0), and 3β-hydroxy-5-cholestene 3-linoleate (ChoE [18:2]) (Larodan AB) were prepared at the following concentrations: 100, 500, 1000, 1500, 2000, and 2500 ng ml−1 (in CHCl3:MeOH, 2:1, v/v) including 1250 ng ml−1 of each internal standard. The samples were analyzed using an established ultra-high-performance liquid chromatography quadrupole time-of-flight mass spectrometry method (UHPLC-QTOFMS). The UHPLC system used in this work was a 1290 Infinity system from Agilent Technologies. The system was equipped with a multi sampler (maintained at 10°C), a quaternary solvent manager, and a column thermostat (maintained 7 at 50°C). Separations were performed on an ACQUITY UPLC BEH C18 column (2.1 × 100 mm, particle size 1.7 µm) by Waters. The mass spectrometer coupled to the UHPLC was a 6545 quadrupole time of flight (QTOF) from Agilent Technologies interfaced with a dual jet stream electrospray ion (dual ESI) source. All analyses were performed in positive ion mode and MassHunter B.06.01 (Agilent Technologies) was used for all data acquisition. QC was performed throughout the sample run by including blanks, pure standard samples, extracted standard samples, and control plasma samples. An aliquot of each sample was collected and pooled and used as a QC sample, together with NIST SRM 1950 reference plasma sample,25 an in-house pooled serum sample. Relative standard deviations (% RSDs) for lipid internal standards representing each lipid class in the samples (raw variation) were below 11%. The lipid concentrations in the pooled control samples were, on average, 16.4% (King's College London) and 11.4% (University of Turku). This shows that the method is reliable and reproducible throughout the sample set.
The identification was carried out in a pooled serum sample, and with this information, an in-house database was created with m/z and retention time for each lipid. Identification of lipids was carried out by combining mass spectrometry (MS) and retention time, MS/MS information, and a search of the LIPID MAPS spectral database,26 and in some cases by using authentic lipid standards. MS/MS data were acquired in both negative and positive ion modes in order to maximize identification coverage. The confirmation of a lipid’s structure requires the identification of hydrocarbon chains bound to its polar moieties, and this was possible in some cases.
Data Preprocessing
MS data processing was performed using the open-source software, MZmine 2.18.27 The following steps were applied in the processing: (1) Crop filtering with a m/z range of 350 to 1200 m/z and a retention time (RT) range of 2.0 to 15.0 minutes; (2) Mass detection with a noise level of 1000; (3) Chromatogram builder with a minimum time span of 0.08 minutes, minimum height of 1200, and a m/z tolerance of 0.006 m/z or 10.0 ppm; (4) Chromatogram deconvolution using the local minimum search algorithm with a 70% chromatographic threshold, 0.05 minutes minimum RT range, 5% minimum relative height, 1200 minimum absolute height, a minimum ration of peak top/edge of 1.2, and a peak duration range of 0.08–5.0; (5) Isotopic peak grouper with a m/z tolerance of 5.0 ppm, RT tolerance of 0.05 minutes, maximum charge of 2, and with the most intense isotope set as the representative isotope; (6) Peak list row filter keeping only peaks with a minimum of 10 peaks in a row; (7) Join aligner with a m/z tolerance of 0.009 or 10.0 ppm and a weight of 2 and a RT tolerance of 0.1 minute and a weight of 1, with no requirement of charge state or ID and no comparison of isotope pattern; (8) Peak list row filter with a minimum of 53 peak in a row (10% of the samples); (9) Gap-filling using the same RT and m/z range gap filler algorithm with an m/z tolerance of 0.009 m/z or 11.0 ppm; (10) Identification of lipids using a custom database search with an m/z tolerance of 0.009 m/z or 10.0 ppm and a RT tolerance of 0.1 minutes; (11) Normalization using internal class-specific standards (PE [17:0/17:0], SM [d18:1/17:0], Cer [d18:1/17:0], LPC [17:0], TG [17:0/17:0/17:0], and PC [16:0/d30/18:1]) for identified lipids and closest internal standard for the unknown lipids, followed by the calculation of the estimated concentrations based on lipid-class calibration curves; and (12) Imputation of missing values was calculated as half of the lipid’s minimum observed value.
Data Analysis
Mann-Whitney U test was applied to compare the differences in weight gain between the study groups (eg, CTR vs FEP) and performed using GraphPad Prism v. 7.04 (GraphPad Software Inc.). In order to visualize the changes in BMI between the study groups, we created a violin plot using the ggplot2 package (version 3.2.1) in the R statistical software.28 Spearman correlation coefficients were calculated using the statistical toolbox in MATLAB 2017b (Mathworks Inc.) and P-values < .05 (2-tailed) were considered significant for these correlations. All statistical analyses involving lipid concentrations were performed on log2-transformed data. The mclust R package (version 5.4.1) was used to build lipid clusters (LCs) from the lipidomics dataset. mclust permits the modeling of data as a Gaussian finite mixture, it attempts to fit various model types, and it assesses their performance using the Bayesian Information Criterion (BIC). The highest BIC achieved by mclust form the lipidomics dataset in control subjects was used to determine both the model type and the number of clusters into which the variables should be divided.
Logistic ridge regression (LRR) models were developed to predict and stratify weight gain in FEP patients. The matched TGs with a regression coefficient (r ≥ .4) in Turku, Finland and London, UK cohorts, between high- vs low-weight gain subjects (ie, change in the BMIs binarized around the median), were used either singly or in combination for LR modeling. A recursive feature elimination scheme was implemented for the optimal selection of the lipids. The lipids in the LR models were incorporated or removed in an iterative manner, starting with all 9 TGs. The models were adjusted for sex and assessed by area under the receiver operating characteristic curves (AUROCs). The mean AUROC of a model was estimated by bootstrapping, ie, 1000 times re-sampling without replacement and partitioning (70% training and 30% test sets) of the lipidomic dataset using createDataPartition function coded in the caret (v. 6.0.84) R package. The model with the highest mean AUROC was considered to be the best model, which was assessed by their ROC curves using pROC 1.15.3 R package. LR and regularized LR modeling were performed using glmnet package in R. LR modeling requires a hyper-parameter “λ.” Here, the λ minimum that corresponds to the minimum cross-validation (CV) error was determined by 10-fold CV using cv.glmnet. The LR model with the highest mean AUROC was named FEP-LR model. This model was also used to predict weight gain (change in BMI from the baseline), when applied to an independent dataset (the CHR subjects).
Results
Lipidome in FEP Patients
We measured circulating molecular lipids using UHPLC-QTOFMS from the 3 study groups (figure 1), together comprising 48 healthy CTR, 44 FEP patients, and 22 CHR individuals, from 2 study centers (Turku, Finland and London, UK), at baseline as well as at 1-year follow-up (CTR, n = 21; FEP n = 13; CHR, n = 9). The demographic characteristics of the 3 study groups are shown in table 1. The lipidomics dataset included in the analysis comprised 265 identified lipids from the following lipid classes: CE, Cer, LPC, PC, PE, SM,, and TGs.
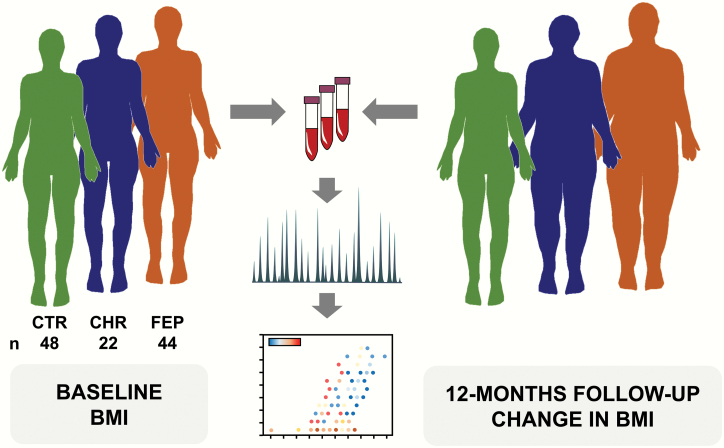
Fig. 1.
Study setting. A total of 114 plasma samples were from 48 healthy controls (CTR), 44 first-episode psychosis patients (FEP), and 22 individuals at clinical-high-risk for psychosis (CHR). Molecular lipids were analyzed using an established ultra-high-performance liquid chromatography quadrupole time-of-flight mass spectrometry method (UHPLC-QTOFMS). We analyzed lipids from baseline samples, from 2 study centers (Turku, Finland and London, UK). Body mass index (BMI) and other metabolic measures were assessed at baseline and at 12-month follow-up. Both univariate and multivariate data analyses were performed to associate the circulating lipids and future weight gain in psychosis patients and in individuals at high risk for psychosis.
In order to summarize the data, we first performed clustering using the Gaussian mixture models,29 reducing the data into 22 distinct LCs (supplementary table S1). As expected, the lipids were clustered according to the main functional lipid classes. For example, PCs and SMs predominated in LCs LC3 and LC6, while LPCs and Cers had distinct clusters (LC4 and LC5, respectively). These clusters also revealed sub-grouping according to the acyl chain carbon number and double-bond count in TGs (LC13 and LC14).
Associations Between Lipidome and Weight Gain
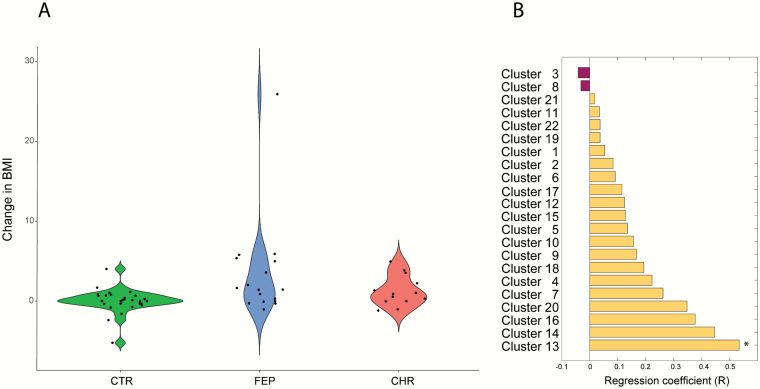
We then examined the differences in weight gain between the study groups (CTR [n = 29] vs FEP [n = 17], CTR [n = 29] vs CHR [n = 13], and CHR [n = 13] vs FEP [n = 17]). FEP patients gained weight when compared with the CTR group (figure 2A; P = .004) over 1-year period. No significant differences were observed when comparing CHR vs FEP and CTR vs CHR (P = .3851 for CHR vs FEP and P = .0561 for CHR vs CTR).
Fig. 2.
Associations between lipidome and weight gain. (a) Difference in BMI change (12-month follow-up vs baseline) between the study groups (CTR vs FEP, CTR vs CHR, and CHR vs FEP). (b) Association between baseline lipid clusters and weight gain in the FEP group. *P < .05.
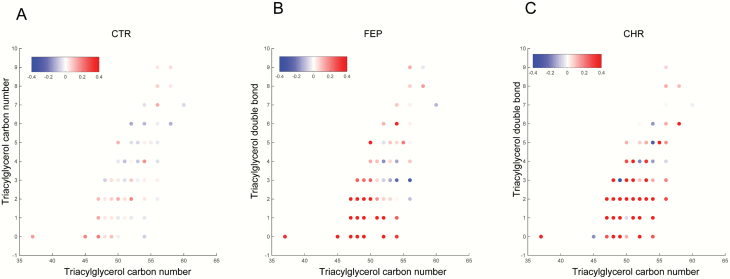
Next, we analyzed the association between the mean levels of the lipids in the baseline LCs and weight gain in CTR and FEP groups. Among the 22 LCs, the baseline level of cluster LC13 was associated with changes in BMI in the FEP group at the 12-month follow-up visit (Spearman correlation coefficient R = .53, P = .0291). The cluster LC13 contains TGs with a low double-bond count, indicating that the change of BMI in FEP patients was specifically associated with a structurally distinct subgroup of lipids. Interestingly, we observed trends of a positive association (r > .3) between weight gain and other LCs containing mainly TGs (L14, L16, and L20; figure 2B). Thus, we sought to determine the association between baseline TG composition and change of BMI (12-month follow-up vs baseline) at the molecular lipid level. The baseline levels of TGs with low carbon number and double-bond count showed positive associations with the change in BMI among the FEP patients, while the association in the CTR group remained weak (figure 3). Nine of 109 TGs at baseline, including TG (47:0), TG (47:1), TG (48:0), TG (48:0), TG (48:1), TG (48:1), TG (49:0), TG (14:0/16:0/18:1), and TG (16:0/16:0/16:0), were positively associated with the change in BMI (P < .05, supplementary table S2). Similarly, we performed correlation analysis between changes in BMI and baseline TG composition in CHR individuals; 32 out of 109 TGs remained correlated with the change in the BMI (P < .05, supplementary table S3). Baseline TGs with low carbon numbers and double-bond count showed a strong positive association with the change in BMI (figure 3C).
Fig. 3.
Correlation of individual TGs with change in BMI (12-month follow-up vs baseline). The x-axis is the acyl carbon number and the y-axis is the acyl double-bond count. (a) CTR, (b) FEP, and (c) CHR. The Spearman correlation coefficient (R) is used for the color code.
Prediction of Weight Gain in FEP Patients and CHR Individuals Using Circulating Lipids
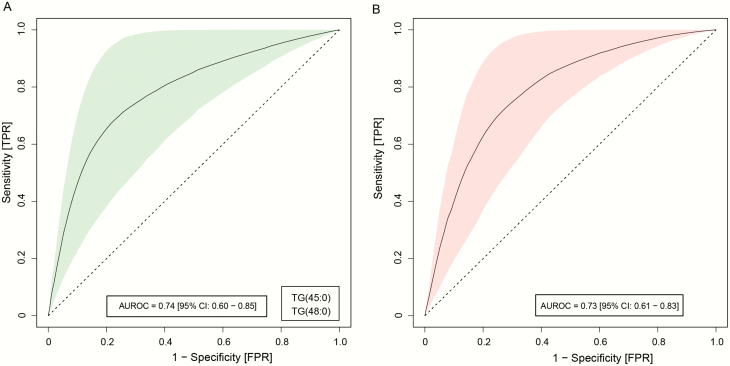
Next, we sought to determine if baseline TG concentrations predicted the risk of weight gain in FEP patients, utilizing the regularized logistic regression (LR) model. We examined the predictor model combining the data from 3 centers, including Turku, Finland, London, UK, and the matched TGs from the THL, Finland dataset. The matched TGs with regression coefficient (r ≥ .4) in Turku, Finland and London, UK cohorts were used as input to build the LR models between the high- and low-weight gain groups (binarized at their median change of BMIs from the baseline, see Methods) in FEP cases. The recursive scheme for feature selection and model reduction showed that TG (48:0) together with TG (45:0) were the best predictors for high change in BMI, with AUROC = 0.74 (figure 4A, 95% confidence interval, CI: 0.60–0.85).
Fig. 4.
Predictive models of weight gain (BMI change in the 12-month follow-up) in the FEP and CHR group. Logistic ridge regression (LRR) models showing triacylglycerols (TGs) as predictive markers to stratify patient groups into high and low BMI changes. (a) Receiver-operating characteristic (ROC) curves, showing the performance of the LR models with highest mean AUROCs in the FEP patients, discriminating high vs low BMI changes in the 12-month follow-up. The light green shaded area denotes the 95% confidence intervals (CI), as calculated by using bootstrapping. (b) ROC curves showing the prediction performance of the FEP-LR models with the highest mean AUROCs in the CHR individuals, discriminating high vs low BMI changes in the 12-month follow-up.
We then independently tested the potential of the FEP-LR model to predict weight gain (change in BMI) in CHR individuals. The FEP-LR model was indeed able to predict the outcome with AUROC = 0.73 (figure 4B, 95% CI: 0.61–0.83). In addition, we developed a regularized LR model to evaluate the effect of antipsychotic medication and 9 selected baseline TG concentrations on the weight gain in FEP patients. Based on the ridge coefficients, the regression model suggests that the dose of antipsychotics (converted into CPZE dose) medication is least contributing than the selected TGs toward the weight gain (median change in the BMI, supplementary figure S1). Moreover, this finding is consistent across the samples from Turku, /Finland, London, UK, and THL/Finland.
Discussion
Our study demonstrates, for the first time as far as we are aware, that circulating lipids predict the risk of future weight gain in both CHR and FEP patients. We found that plasma lipids, specifically TGs, may be a useful molecular biomarker for identifying individuals who are vulnerable to rapid weight gain. This finding is in line with and builds on our previous study, which showed that weight gain in FEP patients was positively associated with elevated TGs, containing low acyl carbon numbers and double-bond counts, independently of obesity at baseline.17,30
Glutamate and dopamine are vital neurotransmitters that are associated with the etiology of schizophrenia.31 Altered dopamine and glutamate adaptation affects the reward circuitry of the brain leading to excessive food intake, which possibly leads to weight gain.32 TGs with low double-bond count and carbon numbers, which are, in part, generated by de novo lipogenesis,33,34 are known to be elevated in NAFLD35–37 and associated with an increased risk of T2DM.38,39 Our findings thus strongly suggest that the FEP patients who go on to gain weight in the future are those who have elevated levels of liver fat but not the dietary lipids.
There is evidence that those presenting with FEP actually have a slightly lower and\or no BMI different compared with a healthy population.17 However, rapid weight gain and metabolic comorbidities are typically evident within the first year following FEP.12,40 Different antipsychotics can vary considerably in the propensity of side-effects, including weight gain.41–43 In addition, there is also considerable variability in weight gain and lipid changes among the FEP individuals with respect to antipsychotic drugs.42,44,45 However, earlier analyses suggest that the NAFLD lipid signature associates with weight gain, independent of antipsychotic medication.17 In line with this, and as a novel finding, we have here also demonstrated that the same lipid signature, predictive of weight-gain in FEP patients, is also predictive of weight gain in CHR individuals. This suggests that specific lipid disturbances in early psychosis may also contribute to the development of metabolic comorbidities, potentially independent of antipsychotic medication. Since a fraction of CHR individuals in our study received low-dose antipsychotic medication, one also cannot exclude the possibility that the metabolic consequences in some CHR individuals may have been influenced by the use of antipsychotics.46
The specific mechanisms linking psychosis, NAFLD, and increased risk of metabolic comorbidities are currently unknown. Previous work in the cohort has shown that the unmedicated FEP patients consume greater quantities of saturated fat and undertake less high-intensity exercise.11 There is a large body of literature suggesting that the endocannabinoid modulates energy intake47 and that it may be involved in the development of NAFLD.48 In line with this, Borgan et al previously showed that 2 independent cohorts of unmedicated patients49 and medicated patients50 show reductions in cannabinoid 1 receptor levels. Moreover, greater reductions in receptor levels were linked to greater total symptom severity and cognitive impairments.49 Cannabinoid receptor type 1 availability may also associate with changes in peripheral endocannabinoid levels in medicated patients.51 Furthermore, there is a large body of literature suggesting that the ECS modulates energy intake,47 and that the development of NAFLD is promoted by peripheral activation of the ECS.48 More studies are clearly needed if one is to elucidate the hypothetical role of ECS as a link between psychosis and the development of metabolic comorbidities.
This study had some limitations. First, the measurement of BMI may not correspond to the visceral fat accumulation,52 thus it may be an inaccurate assessment of the comorbidities associated with obesity. Notwithstanding this, BMI is a widely accepted measurement of the metabolic complications of obesity at the population level, and the lipidomic signature reported in our study was previously observed in patients with NAFLD. Next, the shortcoming is the limited small sample size, which did not allow us to systematically understand the metabolic outcomes of types of antipsychotics according to their propensity. However, the reported antipsychotic associations in the current study provide evidence that specific TGs signatures still remain the strong predictors of weight gain in the FEP subjects. Even though this study had a relatively small sample size, our results provide potentially clinically important findings, which need validation from larger studies.
Taken together, our study independently confirms that the lipidomic signature of NAFLD may serve as a predictor of future weight gain in FEP patients as well as in CHR individuals. This lipid signature may be used for the identification of at-risk individuals and patients who are at increased risk of developing metabolic comorbidities in psychosis. Such knowledge may be useful in targeting primary prevention of metabolic comorbidities and the identification of optimal treatment strategies for each patient.
Supplementary Material
Acknowledgments
The authors thank Cecilia Karlsson for assistance with lipidomics analysis and Aidan McGlinchey for editing. We would like to thank the Turku Metabolomics Centre and Biocenter Finland for their support in the metabolomics analyses. We also thank the following METSY project investigators: Raimo K. R. Salokangas, Tuula Ilonen, Päivi Jalo, Akseli Mäkelä, Tiina From, Janina Paju, Anna Toivonen, Reetta-Liina Armio, Mirka Kolkka, Maija Walta, Max Karukivi, Juha Mäkelä, Maria Tikka, Olof Solin, Merja Haaparanta-Solin, Aidan McGlinchey, Juha Pajula, Mark van Gils, Juha M. Kortelainen, Carmen Moreno, Joost Janssen, Javier Santonja, Covadonga M. Diaz-Caneja, Miriam Ayora Rodriguez, Celso Arango, Alberto Rodriguez-Quiroga, Fabian Hernández-Álvarez, Jose L. Ayuso-Mateos, Roberto Rodriguez-Jimenez, Angela Ibañez, Jaana Suvisaari, Maija Lindgren, Teemu Mäntylä, Tuula Kieseppä, Outi Mantere, Eva Rikandi, Tuukka T. Raij, Dieter Maier, Elisabeth Frank, and Markus Butz-Ostendorf. There were no conflicts of interests to declare for this study.
Funding
This project has received funding from the European Union’s Seventh Framework Programme for the project “Neuroimaging platform for characterization of metabolic comorbidities in psychotic disorders” (METSY; agreement no. 602478).
Authors’ Contributions
M.O., J.H., and O.H. initiated, designed, and supervised the study. H.L., F.B., J.S., J.H., and O.H. assisted by other METSY investigators, recruited the subjects, performed the clinical interviews, and collected the blood samples. A.M.D. and T.H. acquired serum lipidomics data. P.S., A.M.D., and S.L. analyzed the data. S.L. and M.O. wrote the first draft of the manuscript. All authors approved the final version.
References
- 1. McGrath JJ, Saha S. Thought experiments on the incidence and prevalence of schizophrenia “under the influence” of cannabis. Addiction 2007;102(4):514–515; discussion 516–518. [DOI] [PubMed] [Google Scholar]
- 2. Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry. 1997;171:502–508. [DOI] [PubMed] [Google Scholar]
- 3. Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2(5):452–464. [DOI] [PubMed] [Google Scholar]
- 4. Mukherjee S, Schnur DB, Reddy R. Family history of type 2 diabetes in schizophrenic patients. Lancet. 1989;1(8636):495. [DOI] [PubMed] [Google Scholar]
- 5. Ringen PA, Engh JA, Birkenaes AB, Dieset I, Andreassen OA. Increased mortality in schizophrenia due to cardiovascular disease – a non-systematic review of epidemiology, possible causes, and interventions. Front Psychiatry. 2014;5:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oresic M. Obesity and psychotic disorders: uncovering common mechanisms through metabolomics. Dis Model Mech. 2012;5(5):614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Orešič M, Tang J, Seppänen-Laakso T, et al. Metabolome in schizophrenia and other psychotic disorders: a general population-based study. Genome Med. 2011;3(3):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14(3):339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emul M, Kalelioglu T. Etiology of cardiovascular disease in patients with schizophrenia: current perspectives. Neuropsychiatr Dis Treat. 2015;11:2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borgan F, O’Daly O, Hoang K, et al. Neural responsivity to food cues in patients with unmedicated first-episode psychosis. JAMA Netw Open. 2019;2(1):e186893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014;9(4):e94112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Le Hellard S, Theisen FM, Haberhausen M, et al. Association between the insulin-induced gene 2 (INSIG2) and weight gain in a German sample of antipsychotic-treated schizophrenic patients: perturbation of SREBP-controlled lipogenesis in drug-related metabolic adverse effects? Mol Psychiatry. 2009;14(3):308–317. [DOI] [PubMed] [Google Scholar]
- 14. MacKenzie NE, Kowalchuk C, Agarwal SM, et al. Antipsychotics, metabolic adverse effects, and cognitive function in schizophrenia. Front Psychiatry. 2018;9:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tarricone I, Ferrari Gozzi B, Serretti A, Grieco D, Berardi D. Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychol Med. 2010;40(2):187–200. [DOI] [PubMed] [Google Scholar]
- 16. Orešič M, Seppänen-Laakso T, Sun D, et al. Phospholipids and insulin resistance in psychosis: a lipidomics study of twin pairs discordant for schizophrenia. Genome Med. 2012;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suvitaival T, Mantere O, Kieseppä T, et al. Serum metabolite profile associates with the development of metabolic co-morbidities in first-episode psychosis. Transl Psychiatry. 2016;6(11):e951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. [DOI] [PubMed] [Google Scholar]
- 19. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry. 2005;39(11-12):964–971. [DOI] [PubMed] [Google Scholar]
- 20. Keinänen J, Mantere O, Kieseppä T, et al. Early insulin resistance predicts weight gain and waist circumference increase in first-episode psychosis–a one year follow-up study. Schizophr Res. 2015;169(1-3):458–463. [DOI] [PubMed] [Google Scholar]
- 21. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 23. Hall RC. Global assessment of functioning. A modified scale. Psychosomatics 1995;36(3):267–275. [DOI] [PubMed] [Google Scholar]
- 24. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 25. Simon-Manso Y, Lowenthal MS, Kilpatrick LE, et al. Metabolite profiling of a NIST Standard Reference Material for human plasma (SRM 1950): GC-MS, LC-MS, NMR, and clinical laboratory analyses, libraries, and web-based resources. Anal Chem. 2013;85(24):11725–11731. [DOI] [PubMed] [Google Scholar]
- 26. Fahy E, Sud M, Cotter D, Subramaniam S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007;35(Web Server issue):W606–W612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pluskal T, Castillo S, Villar-Briones A, Oresic M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010;11:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 29. Scrucca L, Fop M, Murphy TB, Raftery AE. mclust 5: clustering, classification and density estimation using Gaussian finite mixture models. R J. 2016;8(1):289–317. [PMC free article] [PubMed] [Google Scholar]
- 30. Davison J, O’Gorman A, Brennan L, Cotter DR. A systematic review of metabolite biomarkers of schizophrenia. Schizophr Res. 2018;195:32–50. [DOI] [PubMed] [Google Scholar]
- 31. Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol. 2015;29(2):97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fritz BM, Muñoz B, Yin F, Bauchle C, Atwood BK. A high-fat, high-sugar ‘western’ diet alters dorsal striatal glutamate, opioid, and dopamine transmission in mice. Neuroscience 2018;372:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Westerbacka J, Kotronen A, Fielding BA, et al. Splanchnic balance of free fatty acids, endocannabinoids, and lipids in subjects with nonalcoholic fatty liver disease. Gastroenterology 2010;139(6):1961–1971 e1961. [DOI] [PubMed] [Google Scholar]
- 34. Kotronen A, Seppänen-Laakso T, Westerbacka J, et al. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes 2009;58(1):203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kotronen A, Velagapudi VR, Yetukuri L, et al. Serum saturated fatty acids containing triacylglycerols are better markers of insulin resistance than total serum triacylglycerol concentrations. Diabetologia 2009;52(4):684–690. [DOI] [PubMed] [Google Scholar]
- 36. Orešič M, Hyötyläinen T, Kotronen A, et al. Prediction of non-alcoholic fatty-liver disease and liver fat content by serum molecular lipids. Diabetologia 2013;56(10):2266–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barr J, Caballería J, Martínez-Arranz I, et al. Obesity-dependent metabolic signatures associated with nonalcoholic fatty liver disease progression. J Proteome Res. 2012;11(4):2521–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suvitaival T, Bondia-Pons I, Yetukuri L, et al. Lipidome as a predictive tool in progression to type 2 diabetes in Finnish men. Metabolism. 2018;78:1–12. [DOI] [PubMed] [Google Scholar]
- 39. Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121(4):1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(Suppl 4):8–13. [PubMed] [Google Scholar]
- 41. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM. Pathways to schizophrenia: the impact of environmental factors. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S7–S13. [DOI] [PubMed] [Google Scholar]
- 44. Basile VS, Masellis M, McIntyre RS, Meltzer HY, Lieberman JA, Kennedy JL. Genetic dissection of atypical antipsychotic-induced weight gain: novel preliminary data on the pharmacogenetic puzzle. J Clin Psychiatry. 2001;62(Suppl 23):45–66. [PubMed] [Google Scholar]
- 45. Theisen FM, Gebhardt S, Haberhausen M, et al. Clozapine-induced weight gain: a study in monozygotic twins and same-sex sib pairs. Psychiatr Genet. 2005;15(4):285–289. [DOI] [PubMed] [Google Scholar]
- 46. Carr CN, Lopchuk S, Beckman ME, Baugh TB. Evaluation of the use of low-dose quetiapine and the risk of metabolic consequences: a retrospective review. Ment Health Clin. 2016;6(6):308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lau BK, Cota D, Cristino L, Borgland SL. Endocannabinoid modulation of homeostatic and non-homeostatic feeding circuits. Neuropharmacology 2017;124:38–51. [DOI] [PubMed] [Google Scholar]
- 48. Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17(4):475–490. [DOI] [PubMed] [Google Scholar]
- 49. Borgan F, O’Daly O, Veronese M, et al. The neural and molecular basis of working memory function in psychosis: a multimodal PET-fMRI study. Mol Psychiatry. 2019. doi: 10.1038/s41380-019-0619-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Borgan F, Laurikainen H, Veronese M, et al. In vivo availability of cannabinoid 1 receptor levels in patients with first-episode psychosis. JAMA Psychiatry. 2019;76(10):1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dickens AM, Borgan F, Laurikainen H, et al. Links between central CB1-receptor availability and peripheral endocannabinoids in patients with first episode psychosis. bioRxiv. 2019:664086. doi:10.1101/664086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gurunathan U, Myles PS. Limitations of body mass index as an obesity measure of perioperative risk. Br J Anaesth. 2016;116(3):319–321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.