Abstract
Negative symptoms are characteristic of schizophrenia and closely linked to numerous outcomes. A body of work has sought to identify homogenous negative symptom subgroups—a strategy that can promote mechanistic understanding and precision medicine. However, our knowledge of negative symptom subgroups among individuals at clinical high-risk (CHR) for psychosis is limited. Here, we investigated distinct negative symptom profiles in a large CHR sample (N = 244) using a cluster analysis approach. Subgroups were compared on external validators that are (1) commonly observed in the schizophrenia literature and/or (2) may be particularly relevant for CHR individuals, informing early prevention and prediction. We observed 4 distinct negative symptom subgroups, including individuals with (1) lower symptom severity, (2) deficits in emotion, (3) impairments in volition, and (4) global elevations. Analyses of external validators suggested a pattern in which individuals with global impairments and volitional deficits exhibited more clinical pathology. Furthermore, the Volition group endorsed more disorganized, anxious, and depressive symptoms and impairments in functioning compared to the Emotion group. These data suggest there are unique negative symptom profiles in CHR individuals, converging with studies in schizophrenia indicating motivational deficits may be central to this symptom dimension. Furthermore, observed differences in CHR relevant external validators may help to inform early identification and treatment efforts.
Keywords: clinical high risk, at-risk mental state, blunted affect, alogia, anhedonia, avolition, asociality
Introduction
Negative symptoms (ie, reductions in motivation, emotion, and communicative behavior) are hallmark features of schizophrenia.1–7 These impairments have been found to be distinct from positive and disorganized symptoms.4,6,8 Notably, they are closely tied to core-pathophysiology,9,10 as well as functional outcomes and reduced rates of recovery.11–13 Despite the importance of this domain, challenges remain in identifying effective treatment options for these symptoms,14,15 potentially due to clinical heterogeneity.16 This is particularly relevant as there is increasing evidence for distinct negative symptom subgroups in schizophrenia.17–19 However, it is unknown what the nature of negative symptom profiles are among individuals at clinical high-risk (CHR) for developing a psychotic disorder. Given that negative symptoms are highly prevalent in this group, one of the earliest symptoms to emerge in the prodromal phase, and a strong predictor of conversion,20 characterizing the nature of negative symptoms within this population may be critical for early identification and prevention.
Exploratory factor analytic findings using schizophrenia samples have identified 2 negative symptom dimensions. Work in this area reveals factors including (1) diminished expression of emotion and (2) avolition-apathy.18,21–23 Related studies have sought to expand these works and assess specific negative symptom profiles on an individual level.18 Stemming from studies on deficit syndrome schizophrenia24,25 and CHR,26 this approach aims to determine if patterns of symptoms can characterize distinct patient subgroups.27 Current findings have pointed towards 2 negative symptoms subgroups using data-driven (eg, cluster analysis) approaches that reflect diminished expression and avolition-apathy in schizophrenia.18,28
In addition to research identifying unique negative symptom profiles,18 there is increasing work suggesting that the 2 dimensions of negative symptoms observed in schizophrenia samples may show differences on external validators such as clinical, functional, and cognitive measures.18,29,30 Strauss and colleagues (2013) used a data-driven approach and found evidence of symptom subgroups in a sample of schizophrenia patients, including individuals with diminished expression and avolition-apathy symptoms. When looking at external validators, this group found those with avolition-apathy symptoms had more severe pathology compared to the group with diminished expression, including more disorganized symptoms and impaired functioning. This is in line with evidence indicating that avolition is central among the negative symptom domains and may drive treatment response in schizophrenia.31–33 Furthermore, there was evidence that individuals with avolition-apathy showed deficits in social cognition.18 More generally, cognitive impairment has been commonly linked to negative symptoms particularly processing speed and social cognition.34–36
In CHR groups, factor analyses reveal 2 dimensions as well.37,38 In a study conducted by Azis and colleagues (2019), we identified 2 factors from the Structured Interview for Psychosis-Risk Syndromes (SIPS)39 interview reflecting (1) impairments in emotion (expression of emotion, experience of emotion, social anhedonia) and (2) volitional deficits (occupational functioning and avolition). While this study provided information regarding the factor structure of the SIPS negative symptom dimension, there has yet to be a study to investigate individual subgroups of these symptoms using a data-driven approach and differences on external validators using a CHR sample.
While some studies have applied data-driven approaches to assess symptoms in CHR samples such as ratings from all SIPS items,40 it is unknown what types of subgroups may emerge honing in on negative symptoms specifically. Negative symptoms have promise for yielding clinically relevant subgroups as a compelling body of work has highlighted at least 1 clinically and mechanistically distinct subgroup in schizophrenia over the past few decades.25 There is a growing body of evidence to suggest that negative symptomatology may be tied to distinct neurotransmitter abnormalities in psychosis,10 and our group has reported evidence to suggest that distinct circuits may contribute to negative vs positive symptoms in CHR individuals.41 It is also important to consider that the nature of primary vs secondary negative symptoms may be different in CHR individuals when compared with schizophrenia, with the high-risk groups evidencing significantly greater depression and anxiety.42–45 Given these lines of evidence to support meaningful heterogeneity, and increasing research indicating negative symptoms are more severe and persistent in CHR individuals that later go on to develop psychotic disorders,20,46 there are implications for understanding which negative symptom subgroups may be at risk. As a result of clinical heterogeneity, clinical course, and variability in medication use in this group, it is possible that clusters identified in samples of CHR may contrast what is observed in schizophrenia studies, although there may be some overlap, particularly when investigating conversion status.
In the current study, we sought to extend cluster analysis findings in schizophrenia18 and identify negative symptom profiles using a cluster analysis approach among CHR individuals (N = 244). In line with a recent factor analysis study,38 we predicted 2 subgroups would emerge reflecting Emotion (social anhedonia, expression of emotion, experience of emotion and self) and Volition (avolition, occupational functioning) impairments based off of ratings from the SIPS interview39 because high factor loadings can sometimes reflect the existence of distinct subgroups of participants. Furthermore, given evidence suggesting differences between negative symptom clusters on symptoms, functioning, and cognition in schizophrenia,18 we sought to investigate the distinct nature of the subgroups by comparing them on relevant external validators (eg, medications, symptoms, functioning, sources of secondary negative symptoms) and factors relevant to transitioning to a psychotic disorder.
Methods
Participants
Participants include 244 individuals, aged 12–31 (M = 20.35, SD = 3.70), identified as CHR using the SIPS39 recruited from the time frame of 2014 and 2019. This sample is a combination of participants from 3 programs in the United States. See supplementary material for further details and site-specific demographics. It is important to note the current study includes participant data used from Azis and colleagues (2019) with the addition of several more subjects enrolled throughout the year.
Measures
Demographic details (age, biological sex, parental education, race, antipsychotic medication use, selective serotonin reuptake inhibitor; SSRI use), sum scores from the SIPS39 (positive, disorganized, general), Beck Depression Inventory47 and Beck Anxiety Inventory,48 Diagnostic and Statistical Manual (DSM) of Mental Disorders49 comorbid diagnoses (anxiety, depression, alcohol use, cannabis use), functioning (Global Assessment in Functioning; GAF),50 outcomes (first-degree relative with psychosis, conversion within a 2-y period), and cognition were obtained. We used speed of processing and social cognition scores from the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS)51 Consensus Cognitive Battery. Exploratory analyses assessing differences in other MATRICS variables were also employed. See supplementary material for additional details regarding measures.
Data Analysis
Levine’s test of homogeneity of variance indicated that SIPS positive symptoms did not differ across sites. As a result, we combined data from all 3 sites. A series of analytic techniques were performed using the R statistical programming language and SPSS. First, we obtained residualized factor scores for specific Emotion and Volition factors calculated through exploratory factor analysis by Azis and colleagues (2019) with all 6 negative symptom items. The residualized scores reflect the specific Emotion (consisting of social anhedonia, expression of emotion, experience of emotions and self) and Volition (avolition and occupational functioning) factors reported in this previous study.34 This approach combines prior theory of negative symptom structure with novel data-driven empirical findings. We applied a k-means cluster analysis to the orthogonal Emotion and Volition factors to investigate whether there were distinct negative symptom subgroups. After subgroups were identified, we conducted a discriminant function analysis to validate the subgroups by confirming their stability and separation from one another. In order to determine relationships between cluster subgroups and external validators, 1-way analysis of variance (ANOVA) and chi-square tests were employed in continuous and categorical variables, respectively. For significant initial ANOVA and chi-square tests, post hoc least significant difference (LSD) contrasts (mean difference [MD]; 95% confidence intervals [95% CI] are reported) and additional chi-square tests were applied, with a Bonferroni correction employed to chi-square post hoc tests (a note is made whether analyses survive corrections for multiple comparisons). See supplementary material for information, including a zero-order correlation matrix of relationships between SIPS factors and variables of interest.
Results
Distinct Negative Symptom Cluster Groups
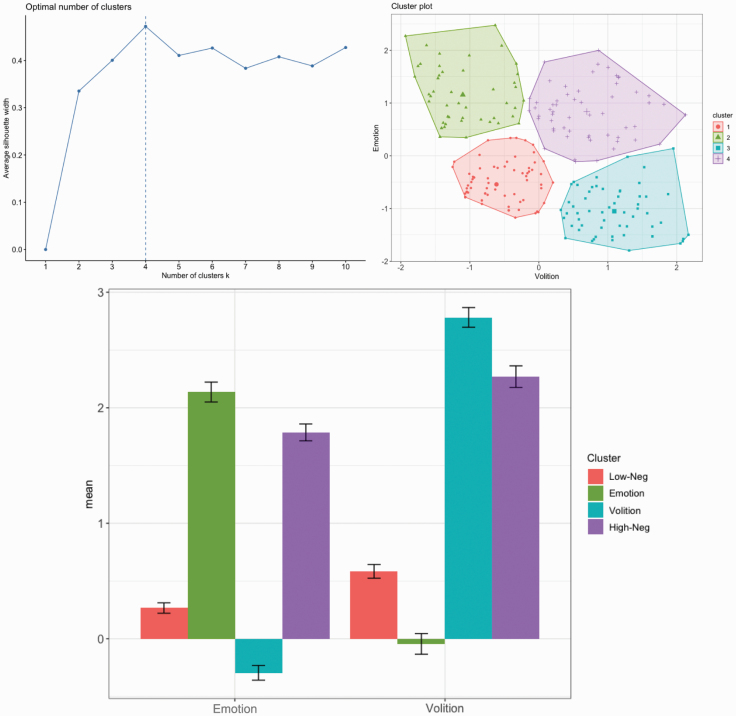
Visual inspection of silhouette plots, gap statistics, and dendrograms indicated a 4-cluster solution was optimal. Discriminant function analysis of the 4-cluster solution revealed 98% of cluster memberships were correctly classified, indicating minimal overlap in the negative symptom scores among the various clusters (ie, they were adequately separated). As depicted in figure 1, the 4 clusters reflected: (1) low negative symptom severity scores (termed, Low-Neg from this point, N = 80), (2) high emotion severity scores, but intact avolition (Emotion, N = 49), (3) high volition severity scores, but intact emotion (Volition, N = 58), and (4) high negative symptom severity scores (High-Neg, N = 57). The distribution of cluster membership did not differ between sites, χ 2(1) = 11.82, P = .07.
Fig. 1.
Negative symptom cluster membership in a clinical high-risk sample. The optimal number of clusters from a silhouette plot and cluster membership from k-means clustering are depicted. Axes represent mean residualized factor scores. The four cluster groups include participants with low negative symptom severity (Low-Neg), impaired emotion but intact volition (Emotion), higher severity scores in volition but intact emotion (Volition), and high negative symptom severity (High-Neg). Error bars represent standard error.
Differences Between Negative Symptom Subgroups on External Validators
Demographics
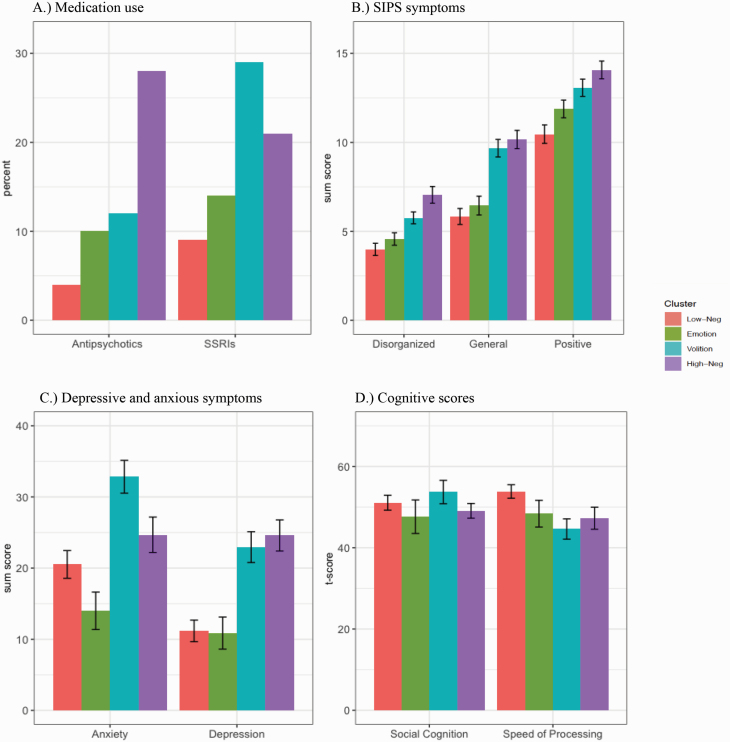
There were no significant differences between clusters in age and parental education (P > .05) (table 1). However, the High-Neg, χ 2(3) = 9.08, P = .003, and Volition, χ 2(1) = 12.96, P < .001, groups significantly differed in the number of individuals that identified as African-American when compared to the Low-Neg group, χ 2(3) = 14.38, P = .002. Additionally, the cluster groups differed in the number of participants on antipsychotics, χ 2(3) = 18.73, P < .001, and SSRIs, χ 2(3) = 10.15, P = .02, indicating a pattern in which the High-Neg group and Volition group are reporting more medication use. Specifically, post hoc tests revealed the High-Neg group had more reports of antipsychotic medication use compared to the Low-Neg, χ 2(1) = 16.89, P < .001, Emotion, χ 2(1) = 5.51, P = .019 (did not survive correction), and Volition, χ 2(1) = 4.82, P = .028 (did not survive correction), groups. Similarly, the High-Neg, χ 2(1) = 4.41, P = .036 (did not survive correction), and the Volition, χ 2(1) = 9.89, P = .002, groups had higher counts of SSRI use compared to Low-Neg (figure 2). There were no other differences in demographics between subgroups (P > .05).
Table 1.
Means, SDs, and Test Statistics in Demographics, Symptoms, Functioning, Cognition, and Predictive Variables Across 4 Negative Symptom Subgroups in a Clinical High-Risk Sample
| 1 | 2 | 3 | 4 | Group Differences | |
|---|---|---|---|---|---|
| Low-Neg | Emotion | Volition | High-Neg | ||
| Demographics | |||||
| Total | 80 | 49 | 58 | 57 | ns |
| Age | 20.13 (2.93) | 20.13 (2.93) | 21.41 (4.04) | 19.74 (4.16) | ns |
| Females | 49% | 49% | 59% | 49% | ns |
| Parent education | 15.55 (2.58) | 15.02 (2.92) | 15.04 (2.47) | 15.03 (2.65) | ns |
| Race | |||||
| Asian | 4% | 8% | 7% | 2% | ns |
| African American | 8% | 16% | 31% | 26% | 4 > 1; 3 > 1 |
| Caucasian | 69% | 49% | 52% | 54% | ns |
| Central/South American | 8% | 10% | 3% | 12% | ns |
| First Nations | 4% | 0% | 2% | 0% | ns |
| Medications | |||||
| Antipsychotics | 4% | 10% | 12% | 28% | 4 > 1, 2a, 3a |
| SSRIs | 9% | 16% | 29% | 21% | 4 > 1a; 3 > 1 |
| Symptoms | |||||
| Positive | 10.46 (4.64) | 11.88 (3.49) | 13.07 (3.69) | 14.07 (3.77) | 4 > 1, 2; 3 > 1 |
| Negativeb | 4.24 (3.33) | 9.04 (4.27) | 11.62 (3.86) | 17.51(4.21) | 4 > 1, 2, 3; 3 > 1, 2; 2 > 1 |
| Disorganized | 3.99 (3.05) | 4.57 (2.45) | 5.76 (2.53) | 7.05 (3.53) | 4 > 1, 2, 3; 3 > 1, 2 |
| General | 5.84 (4.01) | 6.45 (3.70) | 9.67 (3.75) | 10.16 (3.88) | 4 > 1, 2; 3 > 1, 2 |
| Anxiety | 20.51 (11.67) | 14.00 (10.18) | 32.83 (10.05) | 24.68 (12.46) | 4 > 2; 3 > 1, 2, 4 |
| Depression | 11.18 (9.09) | 10.87 (8.73) | 22.95 (9.44) | 24.58 (10.95) | 4 > 1, 2; 3 > 1, 2 |
| Diagnoses | |||||
| Anxiety | 27% | 23% | 35% | 54% | 4 > 2a, 1a |
| Depression | 16% | 8% | 41% | 57% | 4 > 2, 1 ; 3 > 2, 1 |
| Alcohol use | 5% | 0% | 0% | 3% | ns |
| Cannabis use | 13% | 12% | 3% | 17% | ns |
| Functioning | |||||
| GAF | 65.95 (13.33) | 60.82 (15.40) | 52.60 (10.60) | 48.04 (9.98) | 4 < 1, 2; 3 < 1, 2; 2 < 1 |
| Cognition | |||||
| Speed of processing | 53.83 (10.75) | 48.38 (13.17) | 44.60 (13.69) | 47.26 (14.10) | 4 < 1; 3 < 1 |
| Social cognition | 51.07 (11.93) | 47.63 (16.49) | 53.70 (15.72) | 49.07 (13.21) | ns |
| Predictive variables | |||||
| 1st degree psychosis | 15% | 10% | 12% | 7% | ns |
| Conversionc | 1% | 2% | 9% | 16% | 4 > 1, 2a; 3 > 1a |
| SSRIs | 0% | 0% | 5% | 17% | ns |
| No SSRIs | 1% | 2% | 10% | 16% | ns |
Note: Age and parental education are represented in years. Biological sex reflects percent female. Selective serotonin reuptake inhibitors (SSRIs). Positive, negative, disorganized, and general symptom scores are sum scores from the Structured Interview for Psychosis-Risk Syndromes (SIPS), with higher ratings indicating severity. Ratings from the Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI) are sum totals, with higher scores reflecting more severity in anxious and depressive symptoms, respectively. Diagnoses represent diagnoses endorsed from the Structured Clinical Interview for the Diagnostic and Statistical Manual (DSM) of Mental Disorders (SCID). Global Assessment of Functioning (GAF) variables are deduced from a 1–100 scale, with higher scores representing intact functioning. Cognitive measures are corrected t-scores from the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS)50 Consensus Cognitive Battery. The number of individuals with a first-degree relative with psychosis is described by “1st Degree Psychosis.” “SSRIs” written under “Conversion” represent the percent of individuals that converted who were taking SSRIs and “No SSRIs” are the percent that converted that are not taking SSRIs in each subgroup. Ns = nonsignificant.
aThe statistic did not survive correction for multiple comparisons.
bNegative symptom individual items were used to make cluster groups—total scores presented for each group are meant to highlight severity for descriptive purposes.
cConversion indicates the number of individuals that converted to a psychotic disorder such as schizophrenia within a 2-y period.
Fig. 2.
Differences in medication use, symptoms, and cognition between 4 negative symptom-based clusters in a clinical high-risk sample. (A) The percent of antipsychotic and selective serotonin reuptake inhibitor (SSRI) medications taken in each cluster group. (B) The total sum score of disorganized, general, and positive symptoms from the Structured Interview for Psychosis-Risk Syndromes (SIPS). (C) Total sum scores from the Beck Depression Inventory (denoted as “Depression”) and the Beck Anxiety Inventory (represented as “Anxiety”). (D) Cognitive scores are reflected as correct t-scores. Error bars represent standard error.
Symptoms
There were significant differences between the groups in positive, F(240) = 10.15, P < .001, , disorganized, F(240) = 13.43, P < .001, , general, F(239) = 20.43, P < .001, , anxious, F(89) = 8.35, P < .001, , and depressive, F(88) = 13.56, P < .001, , symptoms such that the High-Neg and Volition groups reported more severe symptoms compared to the other cluster groups (figure 2). Specifically, the High-Neg group had significantly more severe positive symptom scores compared to the Low-Neg, MD = 3.61, 95% CI (2.24–4.98), P < .001, and Emotion groups, MD = 2.19, 95% CI (0.65–3.73), P = .005. The Volition group had significantly higher positive symptom scores than the Low-Neg group, MD = 2.61, 95% CI (1.24–3.97), P < .001. In terms of disorganized symptoms, the High-Neg group had significantly more severe ratings compared to the Emotion, MD = 2.48, 95% CI (1.35–3.61), P < .001, Volition, MD = 1.29, 95% CI (0.21–2.38), P = .019, and Low-Neg group, MD = 3.07, 95% CI (2.06–4.07), P < .001. The Volition group reported significantly more severe disorganized symptoms compared to the Low-Neg, MD = 1.77, 95% CI (0.77–2.77), P = .001, and Emotion, MD = 1.19, 95% CI (0.06–2.31), P = .039, groups. Similarly, High-Neg had more severe general symptoms compared to Emotion, MD = 3.71, 95% CI (2.23–5.20), P < .001, and Low-Neg, MD = 4.32, 95% CI (3.00–5.65), P < .001. The Volition group had more general symptoms compared to the Emotion group, MD = 3.22, 95% CI (1.75–4.70), P < .001 and Low-Neg group, MD = 3.83, 95% CI (2.52–5.15), P < .001. Additionally, the High-Neg group reported more anxiety symptoms compared to the Emotion group, MD = 10.68, 95% CI (3.30–18.06), P = .005. The Volition group had significantly more severe anxious symptoms when compared to the Low-Neg, MD = 12.32, 95% CI (5.76–18.88), P < .001, Emotion, MD = 18.83, 95% CI (10.93–26.74), P < .001, and High-Neg, MD = 8.15, 95% CI (1.17–15.14), P = .023, groups. The High-Neg group and the Volition group reported higher levels of depressive symptoms when compared to the Low-Neg (MD = 13.41, 95% CI [8.31–18.51], P < .001, MD = 11.77, 95% CI [6.29–17.25], P < .001, respectively) and Emotion (MD = 13.72, 95% CI [7.42–20.01], P < .001, MD = 12.08, 95% CI [5.47–18.69], P < .001, respectively) groups. There were no other differences between subgroups in symptoms (P > .05). See supplementary material for differences between subgroups in DSM diagnoses.
Functioning
Findings indicated when investigating differences in GAF scores (lower scores indicate more severe functioning), F(237) = 26.92, P < .001, , the High-Neg and Volition groups endorsed overall more impaired global functioning compared to the other groups. The High-Neg group endorsed significantly lower GAF scores compared to the Low-Neg, MD = 17.91, 95% CI (13.63–22.20), P < .001, and Emotion, MD = 12.78, 95% CI (7.99–17.57), P < .001, groups. Furthermore, the Volition group had more severe ratings compared to the Low-Neg, MD = 13.35, 95% CI (9.07–17.64), P < .001, and Emotion, MD = 8.22, 95% CI (3.43–13.01), P = .001, group. Finally, the Emotion group had significantly more impaired functioning scores compared to the Low-Neg group, MD = 5.13, 95% CI (0.65–9.62), P = .025. There were no other significant differences in functioning (P > .05).
Cognitive Measures
Significant differences were observed in cognitive measures between cluster groups, F(111) = 3.40, P = .02, . The High-Neg, MD = 6.57, 95% CI (0.36–12.79), P =.038, and Volition, MD = 9.23, 95% CI [3.21–15.26], P = 003, groups endorsed significantly more severe (lower scores) speed of processing compared to the Low-Neg group. There were no significant differences in the speed of processing between any other subgroups (P > .05). Also, there were no significant differences in social cognition, F(111) = .95, P = .42. See supplementary material for differences between subgroups in other cognitive variables from the MATRICS.
Predictive Measures
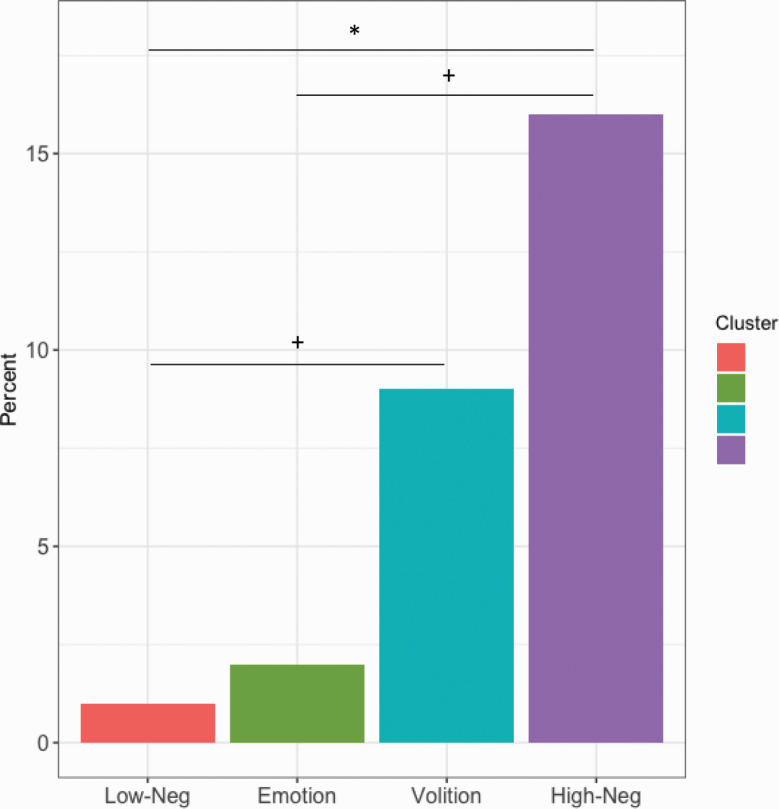
In terms of conversion to psychosis within a 2-year period (figure 3), the High-Neg group had significantly more people that converted compared to the Low-Neg group, χ 2(1) = 9.33, P = .002. Furthermore, High-Neg group had more individuals that converted to a psychotic disorder compared to the Emotion group, χ 2(1) = 5.35, P = .02 (did not survive correction). Similarly, the Volition group had more converters compared to the Low-Neg group, χ 2(1) = 4.17, P = .04 (did not survive correction). There were no significant differences in the number of converters between the other groups, including between the Emotion and Volition groups (P > .05). We also investigated group differences in conversion to psychosis between individuals taking SSRIs and those not taking these medications within each subgroup. Our findings revealed no significant differences (P > .05). Additionally, there were no significant differences in the number of first-degree relatives with a psychotic disorder, (P > .05). There were no other differences in predictive variables between subgroups (P > .05).
Fig. 3.
Conversion to psychosis based on 4 negative symptom cluster groups in a clinical high-risk sample. The percent of CHR individuals that converted to a psychotic disorder within a 2-y period. * denotes significance, P < .05. + indicates differences that did not survive corrections for multiple comparisons.
Discussion
This study sought to investigate (1) whether there are distinct negative symptom subgroups and (2) if they differ on specific external validators (a) commonly observed in the schizophrenia literature (eg, symptoms, global functioning, and cognition) and (b) characteristic of CHR individuals (anxiety, depression, rates of conversion and a first-degree relative with psychosis). This study revealed 4 distinct negative symptom subgroups indicative of individuals with lower negative symptom scores (33% of sample), globally severe negative symptom scores (23%), impairments in emotion (20%), and deficits in volition (24%). Furthermore, when examining differences in external validators, findings indicated individuals with more severe negative symptom scores and volition deficits exhibited more pathology overall.
These findings revealed distinct negative symptom subgroups that are in line with studies in schizophrenia.18 As noted, Strauss and colleagues (2013) found distinct clusters, and among these, diminished expression and avolition-apathy groups were detected, which mimic the current results closely, particularly in the identification of a subgroups exhibiting avolition. However, this study did not reveal separate expression and experience dimensions as observed in schizophrenia.18 The reason this may be the case is because the SIPS negative items conflate constructs and do not measure the expression dimension in the same way schizophrenia scales do. The emotion dimension encompasses both expression and experience of emotion, which likely reflects the way that the SIPS items conceptualize negative symptoms.
The results suggesting differences between cluster subgroups, particularly between the Emotion and Volition groups, on various external validators also provide additional evidence that these clusters represent distinct presentations similar to what is observed in schizophrenia.18 A common pattern was detected in that both the High-Neg and Volition group had similar deficits across domains and the Emotion and Low-Neg group resembled each other, with less overall severity and impairment. These data support the notion discussed in the schizophrenia literature suggesting volitional deficits may be central to the phenomenology of negative symptoms.31 Moreover, the Volition group endorsed more disorganized and general symptoms when compared to the Emotion group, supporting findings in schizophrenia from Strauss and colleagues (2013). Furthermore, the Volition group reported more functional impairment when compared to the Emotion group. Additionally, when looking at the speed of processing between symptom groups, the Volition group reported more deficits when compared to the Low-Neg group, also in conjunction with other studies in this area suggesting motivational impairments may be related to deficits in cognitive function.52 A future direction of this work also involves investigating differences in symptoms in regards to race and ethnicity. In the current study, there were less African-American participants in the Low-Neg group compared to the High-Neg and Volition groups. This finding is in line with Strauss and colleagues (2013), in which they also found a greater number of individuals in the avolition-apathy group that was African-American. There is evidence that African-American individuals with schizophrenia report more symptoms, including negative symptoms compared to non-African-American patients.53,54 It is possible that symptoms may be more severe in this group, given racial disparities in access to care.55,56 Additional research investigating negative symptom severity across race and ethnicity is of great importance and are warranted.
However, there are important discrepancies between findings in CHR and the broader schizophrenia literature. These findings indicating that there were no subgroup differences in social cognition offer important opportunities for future directions. In previous work using a data-driven approach in efforts to identify negative symptom subgroups, individuals with avolition-apathy were found to exhibit impairments on the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT)18 but not on other tests of social cognition. In our study, one aspect of social cognition was assessed, which was emotional intelligence. It is necessary to examine other key components of social cognition, such as emotion recognition and theory of mind in future work. This is particularly important to consider given meta-analytic evidence indicating CHR individuals show impairments in these domains of social cognition, which are also predictive of psychosis.57 Additionally, some studies point towards a complex relationship between negative symptoms and cognition.58 These findings are not well-understood in CHR samples and future work is warranted. Furthermore, findings with social cognition in this study should be interpreted with caution given that several participants did have missing data, and the resulting sample size was smaller than some of the other variables we assessed.
Investigation of subgroup and differences in external validators also provide insights that are unique to CHR individuals and relevant for prevention and intervention efforts. The Volition group reported more severe anxiety and depressive symptoms compared to the Emotion group and the difference was quite dramatic. Depression and anxiety have shown to be prevalent in CHR samples.43,59 These data highlight again the interconnected relationship between depression, anxiety, and negative symptoms. These data are in line with studies indicating both primary and secondary symptoms may be present in CHR44,45 and offer the possibility there may be differences in secondary sources such as depression depending on the negative symptom profile. Additionally, our findings revealed that the High-Neg (and Volition but this did not survive statistical correction) group had higher rates of individuals that went on to convert to a psychotic disorder within a 2-year period. The data support studies indicating that negative symptoms are predictors of transition,20 but extend these works in order to offer the possibility that specific negative symptom subgroups may be at greater risk.
While there are several strengths to the current study, including the use of a data-driven approach and a large sample size, there are also limitations that may offer potential future directions. Confirmatory factor analytic and network analysis studies indicate some measures are best conceptualized in relation to 5 rather than 2 factors60–62 and different measures might produce alternate clustering results and conclusions—additional work is needed to apply these methods across negative symptom assessment tools. Additionally, we assessed several key variables however future work may benefit from investigating other variables that may be predictive of a psychotic disorder such as other environmental risk factors like trauma, urbanicity, and migration which are gaining attention in the CHR literature.63 While our study included longitudinal data (eg, conversion status), given the limitations of using multiple sites and collecting different information and varying timepoints, there may be utility in other work to assess additional variables over time. Future large scale consortium studies (eg, CAPR, PRONET) will be invaluable for providing the statistical power necessary to evaluate these subgroups in the context of subgroup defined by other features, as well as the number of converting cases necessary to make more definitive conclusion about ultimate course.
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
This work was supported by National Institutes of Health (1F31MH121018-01A1 to T.G., RO1MH094650, 1R01MH112545, R01MH120088-01A1 to V.M., and U01 MH081988 to E.W., R01MH116039-01A1 to G.P.S., V.M., E.W.), and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to G.P.S.
References
- 1. Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39(7):784–788. [DOI] [PubMed] [Google Scholar]
- 2. Pogue-Geile MF, Harrow M. Negative symptoms in schizophrenia: their longitudinal course and prognostic importance. Schizophr Bull. 1985;11(3):427–439. [DOI] [PubMed] [Google Scholar]
- 3. Liddle PF. The symptoms of chronic schizophrenia. Br J Psychiatry. 1987;151(2):145–151. doi: 10.1192/bjp.151.2.145 [DOI] [PubMed] [Google Scholar]
- 4. Addington J, Addington D, Maticka-Tyndale E. Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophr Res. 1991;5(2):123–134. [DOI] [PubMed] [Google Scholar]
- 5. Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5):e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214–219. doi: 10.1093/schbul/sbj053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013–1022. doi: 10.1093/schbul/sbl057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rabinowitz J, Levine SZ, Garibaldi G, Bugarski-Kirola D, Berardo CG, Kapur S. Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophr Res. 2012;137(1–3):147–150. [DOI] [PubMed] [Google Scholar]
- 9. Zec RF. Neuropsychology of schizophrenia according to Kraepelin: disorders of volition and executive functioning. Eur Arch Psychiatry Clin Neurosci. 1995;245(4–5):216–223. [DOI] [PubMed] [Google Scholar]
- 10. Millan MJ, Fone K, Steckler T, Horan WP. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 2014;24(5):645–692. [DOI] [PubMed] [Google Scholar]
- 11. Fervaha G, Foussias G, Agid O, Remington G. Impact of primary negative symptoms on functional outcomes in schizophrenia. Eur Psychiatry. 2014;29(7):449–455. [DOI] [PubMed] [Google Scholar]
- 12. Foussias G, Agid O, Fervaha G, Remington G. Negative symptoms of schizophrenia: clinical features, relevance to real world functioning and specificity versus other CNS disorders. Eur Neuropsychopharmacol. 2014;24(5):693–709. [DOI] [PubMed] [Google Scholar]
- 13. Strauss GP, Harrow M, Grossman LS, Rosen C. Periods of recovery in deficit syndrome schizophrenia: a 20-year multi-follow-up longitudinal study. Schizophr Bull. 2010;36(4):788–799. doi: 10.1093/schbul/sbn167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fusar-Poli P, Papanastasiou E, Stahl D, et al. . Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41(4):892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buckley PF, Stahl SM. Pharmacological treatment of negative symptoms of schizophrenia: therapeutic opportunity or cul-de-sac? Acta Psychiatr Scand. 2007;115(2):93–100. [DOI] [PubMed] [Google Scholar]
- 16. Carpenter W, Kirkpatrick B. The hetrogeneity of the long-term course of schizophrenia. Schizophr Bull. 1988;14(4):645–652. doi: 10.1093/schbul/14.4.645 [DOI] [PubMed] [Google Scholar]
- 17. Mueser KT, Douglas MS, Bellack AS, Morrison RL. Assessment of enduring deficit and negative symptom subtypes in schizophrenia. Schizophr Bull. 1991;17(4):565–582. [DOI] [PubMed] [Google Scholar]
- 18. Strauss GP, Horan WP, Kirkpatrick B, et al. . Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013;47(6):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmed AO, Strauss GP, Buchanan RW, Kirkpatrick B, Carpenter WT. Schizophrenia heterogeneity revisited: clinical, cognitive, and psychosocial correlates of statistically-derived negative symptoms subgroups. J Psychiatr Res. 2018;97:8–15. [DOI] [PubMed] [Google Scholar]
- 20. Piskulic D, Addington J, Cadenhead KS, et al. . Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Res. 2012;196(2–3):220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32(2):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirkpatrick B, Strauss GP, Nguyen L, et al. . The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37(2):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr Res. 2011;132(2–3):140–145. doi: 10.1016/j.schres.2011.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carpenter WT Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145(5):578–583. [DOI] [PubMed] [Google Scholar]
- 25. Kirkpatrick B, Fischer B. Subdomains within the negative symptoms of schizophrenia: commentary. Schizophr Bull. 2006;32(2):246–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Devoe DJ, Lu L, Cannon TD, et al. . Persistent negative symptoms in youth at clinical high risk for psychosis: a longitudinal study. Schizophr Res. 2020;(April). doi: 10.1016/j.schres.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kodinariya TM, Makwana PR. Review on determining number of cluster in K-means clustering. Int J Adv Res Comput Sci Manag Stud. 2013;1(6):2321–7782. [Google Scholar]
- 28. Jang SK, Choi HI, Park S, et al. . A two-factor model better explains heterogeneity in negative symptoms: evidence from the positive and negative syndrome scale. Front Psychol. 2016;7:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaiser S, Lyne J, Agartz I, Clarke M, Mørch-Johnsen L, Faerden A. Individual negative symptoms and domains - Relevance for assessment, pathomechanisms and treatment. Schizophr Res. 2017;186:39–45. [DOI] [PubMed] [Google Scholar]
- 30. Galderisi S, Bucci P, Mucci A, et al. . Categorical and dimensional approaches to negative symptoms of schizophrenia: focus on long-term stability and functional outcome. Schizophr Res. 2013;147(1):157–162. [DOI] [PubMed] [Google Scholar]
- 31. Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. 2010;36(2):359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strauss GP, Zamani Esfahlani F, Sayama H, et al. . Network analysis indicates that avolition is the most central domain for the successful treatment of negative symptoms: evidence from the roluperidone randomized clinical trial. Schizophr Bull. January 28, 2020; doi: 10.1093/schbul/sbz141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strauss GP, Esfahlani FZ, Kirkpatrick B, et al. . Network analysis reveals which negative symptom domains are most central in schizophrenia vs bipolar disorder. Schizophr Bull. 2019;45(6):1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fervaha G, Agid O, Foussias G, Siddiqui I, Takeuchi H, Remington G. Neurocognitive impairment in the deficit subtype of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2016;266(5):397–407. [DOI] [PubMed] [Google Scholar]
- 35. İnce E, Üçok A. Relationship between persistent negative symptoms and findings of neurocognition and neuroimaging in schizophrenia. Clin EEG Neurosci. 2018;49(1):27–35. doi: 10.1177/1550059417746213 [DOI] [PubMed] [Google Scholar]
- 36. Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40(suppl. 2):107–116. doi: 10.1093/schbul/sbt197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schlosser DA, Campellone TR, Biagianti B, et al. . Modeling the role of negative symptoms in determining social functioning in individuals at clinical high risk of psychosis. Schizophr Res. 2015;169(1–3):204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Azis M, Strauss GP, Walker E, Revelle W, Zinbarg R, Mittal V. Factor analysis of negative symptom items in the structured interview for prodromal syndromes. Schizophr Bull. 2019;45(5):1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller TJ, McGlashan TH, Rosen JL, et al. . Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. [DOI] [PubMed] [Google Scholar]
- 40. Ryan AT, Addington J, Bearden CE, et al. . Latent class cluster analysis of symptom ratings identifies distinct subgroups within the clinical high risk for psychosis syndrome. Schizophr Res. 2018;197:522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dean DJ, Walther S, Bernard JA, Mittal VA. Motor clusters reveal differences in risk for psychosis, cognitive functioning, and thalamocortical connectivity: evidence for vulnerability subtypes. Clin Psychol Sci. 2018;6(5):721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McAusland L, Buchy L, Cadenhead KS, et al. . Anxiety in youth at clinical high risk for psychosis. Early Interv Psychiatry. 2017;11(6):480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Addington J, Piskulic D, Liu L, et al. . Comorbid diagnoses for youth at clinical high risk of psychosis. Schizophr Res. 2017;190:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gruber J, Strauss GP, Dombrecht L, Mittal VA. Neuroleptic-free youth at ultrahigh risk for psychosis evidence diminished emotion reactivity that is predicted by depression and anxiety. Schizophr Res. 2018;193:428–434. [DOI] [PubMed] [Google Scholar]
- 45. Vargas T, Ahmed AO, Strauss GP, et al. . The latent structure of depressive symptoms across clinical high risk and chronic phases of psychotic illness. Transl Psychiatry. 2019;9(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Velthorst E, Nieman DH, Becker HE, et al. . Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophr Res. 2009;109(1-3):60–65. [DOI] [PubMed] [Google Scholar]
- 47. Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio. 1996;78(2):490–498. [Google Scholar]
- 48. Aaron TB, Gary B, Kiyosaki RT, Lechter SL. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. [DOI] [PubMed] [Google Scholar]
- 49. First M, Gibbon M, Spitzer R. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II). Am Psychiatr Press Inc. 1997. [Google Scholar]
- 50. Miller TJ, McGlashan TH, Woods SW, et al. . Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70(4):273–287. [DOI] [PubMed] [Google Scholar]
- 51. Nuechterlein KH, Green MF, Kern RS, et al. . The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. [DOI] [PubMed] [Google Scholar]
- 52. McDowd J, Tang TC, Tsai PC, Wang SY, Su CY. The association between verbal memory, processing speed, negative symptoms and functional capacity in schizophrenia. Psychiatry Res. 2011;187(3):329–334. [DOI] [PubMed] [Google Scholar]
- 53. Mark TL, Palmer LA, Russo PA, Vasey J. Examination of treatment pattern differences by race. Ment Health Serv Res. 2003;5(4):241–250. [DOI] [PubMed] [Google Scholar]
- 54. Corcoran CM, Kimhy D, Parrilla-Escobar MA, et al. . The relationship of social function to depressive and negative symptoms in individuals at clinical high risk for psychosis. Psychol Med. 2011;41(2):251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Compton MT, Esterberg ML, Druss BG, Walker EF, Kaslow NJ. A descriptive study of pathways to care among hospitalized urban African American first-episode schizophrenia-spectrum patients. Soc Psychiatry Psychiatr Epidemiol. 2006;41(7):566–573. [DOI] [PubMed] [Google Scholar]
- 56. Anglin DM, York N, Health P, Street W, Phelan JC. Racial differences in beliefs about the effectiveness and necessity of mental health treatment. Am J Community Psychol. 2008;42:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Donkersgoed RJ, Wunderink L, Nieboer R, Aleman A, Pijnenborg GH. Social cognition in individuals at ultra-high risk for psychosis: a meta-analysis. PLoS One. 2015;10(10):e0141075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lin CH, Huang CL, Chang YC, et al. . Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophr Res. 2013;146(1–3):231–237. doi: 10.1016/j.schres.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 59. Salokangas RK, Ruhrmann S, von Reventlow HG, et al. ; EPOS group Axis I diagnoses and transition to psychosis in clinical high-risk patients EPOS project: prospective follow-up of 245 clinical high-risk outpatients in four countries. Schizophr Res. 2012;138(2–3):192–197. [DOI] [PubMed] [Google Scholar]
- 60. Strauss GP, Ahmed AO, Young JW, Kirkpatrick B. Reconsidering the latent structure of negative symptoms in schizophrenia: a review of evidence supporting the 5 consensus domains. Schizophr Bull. 2019;45(4):725–729. doi: 10.1093/schbul/sby169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ahmed AO, Kirkpatrick B, Galderisi S, et al. . Cross-cultural validation of the 5-factor structure of negative symptoms in Schizophrenia. Schizophr Bull. 2019;45(2):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Strauss GP, Esfahlani FZ, Galderisi S, et al. . Network analysis reveals the latent structure of negative symptoms in Schizophrenia. Schizophr Bull. 2018;45(5):1033–1041. doi: 10.1093/schbul/sby133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Addington J, Farris M, Stowkowy J, Santesteban-Echarri O, Metzak P, Kalathil MS. Predictors of transition to psychosis in individuals at clinical high risk. Curr Psychiatry Rep. 2019;21(6):39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.