Abstract
Recent advances in functional genomics have facilitated the identification of multiple genes and isoforms associated with the genetic risk of schizophrenia, yet the causal variations remain largely unclear. A previous study reported that the schizophrenia risk single-nucleotide polymorphism (SNP) rs7085104 at 10q24.32 was in high linkage disequilibrium (LD) with a human-specific variable number of tandem repeat (VNTR), and both were significantly associated with the brain mRNA expression of a human-unique AS3MTd2d3 isoform in Europeans and African Americans. In this study, we have shown the direct regulation of the AS3MTd2d3 mRNA expression by this VNTR through an in vitro minigene splicing assay, suggesting that it is likely a causative functional variation. Intriguingly, we have further confirmed that the VNTR and rs7085104 are significantly associated with AS3MTd2d3 mRNA expression in brains of Han Chinese donors, and rs7085104 is also associated with risk of schizophrenia in East Asians. Finally, the overexpression of AS3MTd2d3 in cultured primary hippocampal neurons results in significantly reduced densities of mushroom dendritic spines, implicating its potential functional impact. Considering the crucial roles of dendritic spines in neuroplasticity, these results reveal the potential regulatory impact of the schizophrenia risk VNTR on AS3MTd2d3 and provide insights into the underlying biological mechanisms.
Keywords: schizophrenia, VNTR, AS3MTd2d3, alternative splicing, dendritic spine
Introduction
Schizophrenia is a substantially heritable severe psychiatric disorder.1,2 Recent genome-wide association studies (GWAS) have identified hundreds of genomic loci associated with the risk of schizophrenia in world populations,3–5 but the underlying biological mechanisms remain largely undetermined probably because majority of these variations locate in noncoding genomic regions. Nevertheless, recent data suggest that the schizophrenia risk variations are enriched for regulatory elements affecting transcription and splicing of targeted genes,6–8 and efforts are, therefore, made to characterize the impact of such variations on gene transcription and posttranscriptional processing.9 For this purpose, several consortia (eg, CommonMind, BrainSeq, and PsychENCODE) have performed high-throughput RNA sequencing of human brain tissues from schizophrenia patients and healthy subjects,7,10,11 leading to the identification of multiple genes or spliced isoforms whose expression levels are associated with genetic risk and clinical diagnosis (eg, C4A and ZNF804A).12–14
A recent study identified a human-unique isoform AS3MTd2d3 significantly associated with schizophrenia genetic risk (eg, rs7085104) in the prominent 10q24.32 GWAS locus.15AS3MTd2d3 isoform lacks 2 exons (exons 2 and 3) compared with the wild-type full-length AS3MTfull. This truncated isoform is preferably expressed in the brain, and its expression is significantly higher in brains of schizophrenia patients relative to controls.15 A human-specific variable number of tandem repeat (VNTR) in high linkage disequilibrium (LD) with rs7085104 (ie, r2 = .94 in Europeans) showed the strongest expression quantitative trait loci (eQTL) association with brain AS3MTd2d3 expression among all the tested sequence variations in the 10q24.32–33 region in Europeans and African Americans.15 While these results strongly suggest the involvement of the VNTR and AS3MTd2d3 in schizophrenia, a few questions remain to be answered: (1) does the VNTR regulate the alternative splicing of AS3MTd2d3 isoform?, (2) The previous study primarily analyzed samples from Europeans and African Americans, is the AS3MTd2d3 isoform also a risk factor for schizophrenia in East Asians?, and (3) What is the functional relevance of this human-unique isoform in schizophrenia pathogenesis?
Using a minigene assay, we herein demonstrate that the VNTR directly affects the alternative splicing of AS3MTd2d3 in vitro, which gains further support from the robust eQTL associations between this VNTR and AS3MTd2d3 mRNA expression in Han Chinese brain samples. The single-nucleotide polymorphism (SNP) rs7085104 is also significantly associated with the risk of schizophrenia in East Asians based on public GWAS datasets.5 We then reveal that the overexpression of AS3MTd2d3 in hippocampal neurons results in a significant reduction of mushroom dendritic spine densities, providing hints for the biological basis underlying its association with schizophrenia.
Methods and Materials
AS3MT VNTR Minigene Constructs
Minigenes containing exons 1, 2, 3, and 4 of the AS3MT gene (including surrounding introns and 5′ flanking regions) were constructed into the Exontrap vector pET01 (MoBiTec GmbH, Göttingen, Germany). The targeted regions were amplified from the genomic DNA of an individual carrying 3-repeat at the AS3MT VNTR site with the following primers: forward: 5′-CAAGGGGGATGCTGATACCG-3′ and reverse: 5′-TCGCCCAGAAGAACCCCTAA-3′. The target sequence was then cloned into the pMD19T vector. Site-directed mutagenesis was performed to generate a new plasmid carrying the 2-repeat VNTR, while the sequence at all other sites was the same compared with the plasmid containing the 3-repeat VNTR. These minigenes containing different repeats of the AS3MT VNTR were then subcloned into the pET01 vector with BamHI and NotI restriction sites. The pET01 contains a SV40 promoter and plasmid-specific 5′ and 3′ exons, which are separated by a sequence of an intron. This vector has intrinsic splicing capability that permits the cloning of selected exon sequences in an inserted large DNA fragment. Sanger sequencing was conducted to ensure that the 2 recombinant constructs differed only at the VNTR site.
In Vitro Functional Splicing Assay
The human embryonic kidney 293T (HEK293T) cells and mouse fibroblast (NIH/3T3) cells were originally obtained from the Kunming Cell Bank, Kunming Institute of Zoology. The mouse neuroblast (Neuro-2a) cells were originally purchased from ATCC. Polymerase chain reaction (PCR) analyses were regularly performed during cell culture to ensure no mycoplasma contamination. All cells were cultured in a humidified incubator with 5% CO2 at 37°C. Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Biological Industries), 1 mM sodium pyruvate (Gibco), 1× MEM nonessential amino acids (Gibco), and 1% penicillin-streptomycin (Life Technologies) was used.
Cells were seeded into 24-well plates 24 hours before transfection. The pET01 recombinant plasmids were then quantified and transiently transfected (with consistent concentrations across each well) into cells for 24 hours using Lipofectamine 3000 (Invitrogen). All conditions were tested in triplicates per experiment, and 3 independent experiments were performed. Total RNA of cells was then purified using the TRIzol reagent (Ambion). An aliquot of 2 µg RNA was used to synthesize complementary DNA (cDNA) with a RevertAid First Strand cDNA Synthesis Kit (Thermo) in a 20-µl reaction mixture according to the standard protocol, and 180 μl of nuclease-free water was then added to the products. For real-time quantitative PCR (RT-qPCR), the reaction contained 10.0 μl 2× SYBR master mix (Roche, USA), 2.0 μl primer solution (10 μM), 2.0 μl cDNA, and 6.0 μl nuclease-free water. A 7900HT Fast Real-Time PCR System (Applied Biosystems) was used to perform the reaction with the program that firstly 95°C for 10 minutes, followed by 40 repeated cycles of 95°C for 15 seconds and 60°C for 30 seconds.
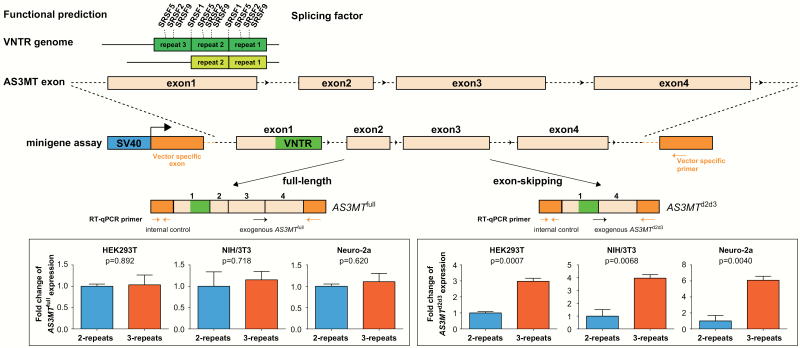
Primers were specifically designed to examine the exogenous alternative splicing in the pET01 recombinant plasmids (figure 1). A common reverse primer aligned to the pET01-specific 3′ exon (5′-TCCACCCAGCTCCAGTTG-3′) was designed for detecting both AS3MTd2d3 and AS3MTfull. Different forward primers specific for AS3MTd2d3 (5′-GCCGAGGAGACAATATTATGGCT-3′) and AS3MTfull (5′-ACACGAAGCCGTAGCCCTAAGA-3′) were designed. Internal control primers amplifying the pET01-specific 5′ exon (forward: 5′-GGATGGGGGTGTCTACGGTG-3′ and reverse: 5′-ACCACAAAGGTGCTGTTTGAC-3′) were used to normalize the transfection efficiency between different wells (figure 1). All 3 pairs of primers showed high amplification efficiencies (>90%). The relative mRNA expression levels were presented as the means of 2^–ΔΔCt, and statistical tests against different groups were conducted using 2-tailed student’s t-test. The PCR products were also separated on 2% agarose gels and processed for Sanger sequencing to ensure satisfactory yield and accuracy of the reaction products.
Fig. 1.
Experimental design and results of in vitro minigene experiments. RT-qPCR results indicate that the different repeats of variable number of tandem repeat (VNTR) exert a significant impact on the expression of AS3MTd2d3 rather than AS3MTfull. Error bars represent standard error of mean (SEM) of 3 biological replicates.
Expression Quantitative Trait Loci Analysis in Chinese Brain Sample
The study protocol has been approved by the Institutional Review Board of the Kunming Institute of Zoology, Chinese Academy of Sciences. Amygdala tissues of 60 nonpsychiatric donors were obtained from the Chinese Brain Bank Center through the human body donation program organized and implemented by the Wuhan Red Cross Society,16–18 and permissions were obtained from the donors themselves or from their relatives for brain autopsy and use of the brain tissues in research. Further detailed information of human brain tissues and relevant ethical approval regarding the sample collection have been described in a previous study.19 Genomic DNA was extracted and genotyped using the Illumina Infinium Global Screening Array (GSA) Chip (Beijing Guoke Biotechnology Co., LTD; www.bioguoke.com). Genotype data underwent a series of quality control procedures (including sex check, heterozygosity rate calculation, and identity by descent identification)20,21 and principal component analysis (PCA) using the EIGENSTRAT software.22 After quality control, low-quality genotype subjects and rare variations were removed. Genotype imputation was then performed using the prephasing imputation stepwise approach implemented in SHAPEIT and IMPUTE2,23,24 and the imputation reference was obtained from the 1000 Genomes Project Phase 3.25 SNPs with INFO > 0.8, minor allele frequency > 1%, call rate > 95%, and Hardy-Weinberg equilibrium P > 1 × 10–5 were proceeded for further analyses. The VNTR locus was genotyped through PCR, and amplicons were analyzed with both an agarose gel and Sanger sequencing to determine different alleles. The primers for genotyping of the VNTR were: forward, 5′-CCTGGTTGGAAAGCCTGTAGAGCA-3′ and reverse, 5′-GCGGGAAAGTTAGTTGAAAGGA-3′. Finally, a total of 3726 genetic variations in the 10q24.32–33 region (chr10:103900991-105504954, hg19) were included in the eQTL analysis.
Total RNAs from the 60 amygdala samples were isolated using the TRIzol reagent (Ambion). The average RNA integrity number (RIN) of these RNA samples was 5.285 ± 0.157 (mean ± standard error of mean [SEM]). The cDNA was synthesized for each sample using a RevertAid First Strand cDNA Synthesis Kit (Thermo). The primers used for amplification of RPS13 (housekeeping gene) were 5′-CCCCACTTGGTTGAAGTTGA-3′ (forward) and 5′-CTTGTGCAACACCATGTGAA-3′ (reverse), and primers for AS3MTd2d3 were 5′-GCCGAGGAGACAATATTATGGCT-3′ (forward) and 5′-TGGTCATGTCTATTCCAGTCACGT-3′ (reverse). Both primers showed similar and high amplification efficiencies (>91%). The relative mRNA expression levels were presented as the means of –ΔCt through a statistical test against genotypic groups, and the P-values were calculated using linear regression covaring for sex, age, RIN, and the top 3 genomic principal components (PCs).
Summary Statistics in Published Schizophrenia GWAS
We retrieved the summary statistics of rs7085104 from a previous schizophrenia GWAS in East Asians and Europeans (accessed at https://www.med.unc.edu/pgc/)5; 22 778 cases and 35 362 controls who were Asians, and 33 640 cases and 43 456 controls who were Europeans, were included. The association was analyzed using a logistic regression model with top PCs as covariates to adjust for potential population stratification in each cohort. Detailed information of the sample recruitment, quality control, and statistical analysis can be found in the original report.5
AS3MT d2d3 Overexpression Plasmid Construction
The coding sequence of AS3MTd2d3 with a C-terminus Flag-tag was constructed into pCAG-GFP vector (www.addgene.org/11150/) for target protein overexpression. The integrity of the construct was verified through Sanger sequencing. The following antibodies were used in immunofluorescence: Chicken polyclonal GFP antibody (Abcam, #ab13970) and Rabbit monoclonal Flag antibody (Cell Signaling Technology, #14793S).
Animals
Wild-type Sprague Dawley rats were purchased from Chengdu Dossy Experimental Animals Co., Ltd (http://m.cd-dossy.cn/) and bred in house on a 12 h light/dark cycle (lights on at 08:00 am lights off at 08:00 pm) in a temperature-controlled room, 50%–60% relative humidity, and free access to food and water. All experiments were performed according to the guidelines (developed by the National Advisory Committee For Laboratory Animal Research) for ethical conduct in the care and use of animals, and all protocols were approved by the Animal Ethics Committee of Kunming Institute of Zoology before the study.
Hippocampal Neuronal Cultures
All the neuronal experiments were conducted in 3 independent times with consistent conduct and acquisition parameters. The pregnant Sprague Dawley rats (E18/19) were euthanized using a CO2 chamber. Hippocampal tissues were dissected from the embryos, treated with Papain (Worthington) and DNase I (Sigma-Aldrich), and gently triturated to generate single-cell suspensions of neurons. These neurons were seeded at a density of 1 × 106 viable cells/well in 6 well culture-plates pre-coated with poly-d-lysine (10 μg ml−1) and laminin (1.05 μg ml−1) for at least 12 h at 37°C. Cultures were maintained at 37°C with 5% CO2 in Neurobasal medium supplemented with 2% B27 (Gibco), 1× GlutaMAXTM-I (Gibco), and 2.5% FBS. During each experiment, more than 10 embryos from one pregnant Sprague Dawley rats were dissected to obtain sufficient hippocampal neurons, which were then cultured in at least 6 separate wells for transfection.
Plasmid Transfection and Image Acquisition of Dendritic Spines
Analyses of density and morphology of dendritic spines were carried out as previously described.26,27 In brief, rat neurons were cultured for 14–15 days, and then transfected with the recombinant pCAG constructs for AS3MTd2d3 or control vectors (pCAG-GFP) together with Venus using Lipofectamine 3000 (Invitrogen). Three days after the transfection, the neurons were fixed in 4% paraformaldehyde with 4% sucrose at room temperature. Immunostaining with antibody to GFP was performed to circumvent potential unevenness of GFP diffusion in spines. Images of randomly selected neurons clearly expressing both GFP and Flag were captured using an LSM 880 Basic Operation (Carl Zeiss) under consistent acquisition parameters.
Image Analyses
NeuronStudio was used to analyze spines on secondary and tertiary dendrites (total length of 60–100 μm per neuron),28 and data obtained on 2~3 dendrites from each neuron were averaged as the result for one neuron. As stated above, the neuronal culture and image acquisition were conducted for 3 independent times, and each time, at least 19 neurons from each experimental group (control or overexpression of AS3MTd2d3) were acquired for imaging analyses. We focused on thin, mushroom, and stubby spines as previously described.27 Two-tailed student’s t-tests were performed to calculate the differences of total dendritic spines between experimental groups. For experiments with 2 independent variables, 2-way ANOVA followed by Bonferroni post hoc were performed for multiple comparisons. Results are presented as mean ± SEM. (control, n = 20; overexpression of AS3MTd2d3, n = 19), and P < .05 after corrections was defined as significant. Graphs and statistics were performed using GraphPad Prism (Version 6.0).
Results
The VNTR Regulates the Alternative Splicing of the AS3MTd2d3 Isoform
We conducted functional prediction of the VNTR sequences using the web-based Splice-Aid tool29 and identified several splicing regulators (eg, SFRS1, SFRS2, SFRS5, and SFRS9) potentially bound to the VNTR sequences. It was predicted that the 3-repeat VNTR sequence could bind more splicing regulators than the 2-repeat VNTR sequence (figure 1). We then transfected the AS3MT VNTR minigene constructs (with different number of VNTR repeats) into HEK293T, NIH/3T3, and Neuro-2a cells, respectively, and measured the relative exogenous mRNA expression of AS3MTd2d3 and AS3MTfull isoforms. We found that mRNA levels of the exogenous AS3MTd2d3 isoform were positively and significantly associated with number of the VNTR repeats. Specifically, cells carrying 3-repeat of the VNTR had greater abundance of AS3MTd2d3 compared with those carrying the 2-repeat sequence (F(2, 2) = 5.590, df = 4, t = 9.544, P = .0007 in HEK293T; F(2, 2) = 3.502, df = 4, t = 5.133, P = .0068 in NIH/3T3; F(2, 2) = 1.939, df = 4, t = 5.949, P = .0040 in Neuro-2a; figure 1). By contrast, the number of the VNTR repeats did not predict expression variation of exogenous AS3MTfull (F(2, 2) = 19.53, df = 4, t = 0.145, P = .892 in HEK293T; F(2, 2) = 2.938, df = 4, t = 0.387, P = .718 in NIH/3T3; F(2, 2) = 13.71, df = 4, t = 0.537, P = .620 in Neuro-2a; figure 1). These results suggest that this VNTR is likely the causative variation regulating AS3MTd2d3.
The VNTR is the Leading Variation Associated With AS3MTd2d3 Expression
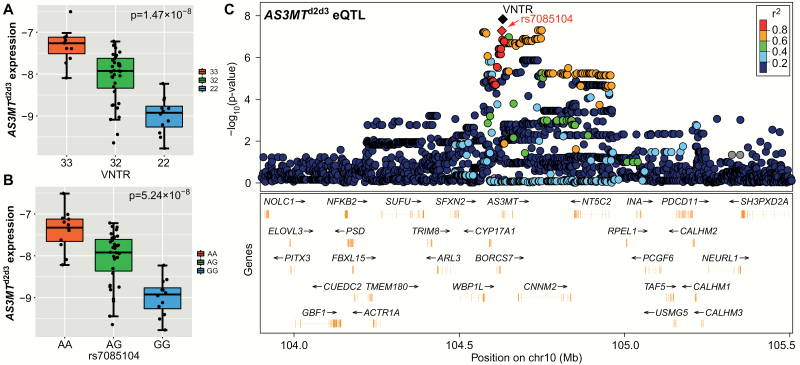
As the previous study found that the VNTR and its LD SNP rs7085104 were associated with AS3MTd2d3 expression in Europeans and African Americans,15 it is of great interest to examine whether the VNTR (and other sequence variations in the 10q24.32-33 region) was also associated with AS3MTd2d3 expression in Han Chinese. In amygdala tissues from 60 nonpsychiatric Han Chinese donors, we replicated the significant eQTL association between the VNTR and AS3MTd2d3 expression (beta = .863, standard error = .13, P = 1.47 × 10–8, figure 2), and the VNTR is again the leading eQTL variation for AS3MTd2d3 in the 10q24.32-33 region. We have also confirmed the strong LD between the VNTR and rs7085104 (r2 = .86) in our Chinese samples, and the A-allele at rs7085104 is primarily linked with 3-repeat at the VNTR. Consistently, significant eQTL association was observed between rs7085104 and AS3MTd2d3 expression (beta = .819, standard error = .13, P = 5.24 × 10–8, figure 2), with the A-allele at rs7085104 predicting higher mRNA levels of this isoform.
Fig. 2.
Association of genetic variations at 10q24.32-33 with AS3MTd2d3 expression in Chinese human brain. Associations between AS3MTd2d3 expression and (A) the VNTR, (B) rs7085104, and (C) 3726 genetic markers at 10q24.32-33 in the 60 amygdala samples from Han Chinese adult controls evaluated through RT-qPCR assays. The variable number of tandem repeat (VNTR) is marked with a black diamond and rs7085104 is marked with a red diamond in (C). P-values were calculated by linear regression on the basis of −ΔCt. Gene expression was shown using a box plot with median and interquartile range.
The VNTR High LD Index SNP rs7085104 Is Significantly Associated With Schizophrenia
In the schizophrenia GWAS in European populations, the chromosomal 10q24.32 region is one of the leading loci showing genome-wide associations,3,4 but whether the VNTR is associated with the illness in world populations remains undetermined. Since this tandem repeat is not covered by the current GWAS platforms, we used its LD SNP rs7085104 as a proxy. Based on a recent schizophrenia GWAS,5 rs7085104 is genome-wide significantly associated with the illness in 33 640 cases and 43 456 controls of European origin (P = 1.37 × 10−17, OR = 1.102 for A-allele). Intriguingly, in East Asians (22 778 cases and 35 362 controls), rs7085104 also shows strong association with schizophrenia despite not reaching genome-wide level of statistical significance (P = 5.81 × 10−5, OR = 1.058 for A-allele). In the meta-analysis combining all available GWAS results in Europeans and East Asians (56 418 cases and 78 818 controls), the magnitude of statistical significance of the association between rs7085104 and schizophrenia continuously increases (P = 3.65 × 10−20, OR = 1.084 for A-allele), suggesting that rs7085104 (and its high LD human-specific VNTR) is likely a risk factor of schizophrenia in world populations.
Overexpression of AS3MTd2d3 Leads to Loss of Mushroom Dendritic Spines
While elucidating the molecular mechanisms underlying most of the GWAS-identified schizophrenia genetic risk is difficult due to the limited knowledge of schizophrenia pathobiology, analyzing their correlation with the disease-related endophenotypes is believed to provide insights. To date, dendritic spine pathology and synaptic dysfunction have been repeatedly reported in the brains of schizophrenia patients,30–34 and altered dendritic spine morphogenesis and plasticity are, therefore, considered important endophenotypes for this disease.35–37 Intriguingly, accumulating studies have shown significant impacts of schizophrenia risk genes (such as ZNF804AE3E4, ANK3, TAOK2, and DISC1) on dendritic spines, suggesting that they might participate in the illness by modulating dendritic spine structure and potentially function.38–43 We, therefore, hypothesized that AS3MTd2d3 might affect the density and morphology of dendritic spines, just like multiple recently characterized schizophrenia risk genes.
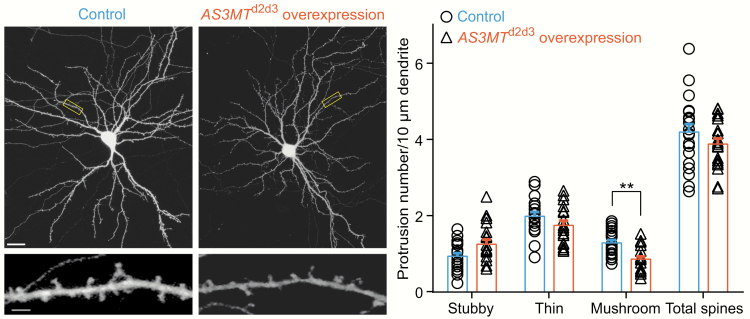
The overexpression constructs of the AS3MTd2d3 isoform or control vector were respectively transfected into rat primary hippocampal neurons. Although no obvious changes were seen in the total spine density between control neurons and those overexpressing AS3MTd2d3 (control, 4.192 ± 0.935 spines per 10 μm; overexpression of AS3MTd2d3, 3.877 ± 0.645 spines per 10 μm, F(19, 18) = 2.098, df = 37, t = 1.219, P = .231, 2-tailed student’s t-tests; figure 3), a significant interaction between experimental conditions and different spine types (interaction F(2, 111) = 7.5, P = .0009, 2-way ANOVA) was observed. We, therefore, further conducted post hoc multiple comparisons. Specifically, when AS3MTd2d3 was overexpressed, we observed a significantly decreased density of mushroom dendritic spines (control, 1.278 ± 0.355 spines per 10 μm; overexpression of AS3MTd2d3, 0.852 ± 0.326 spines per 10 μm, df = 111, t = 3.032, P = .0091, multiple comparisons using Bonferroni correction in 2-way ANOVA; figure 3), which are the most mature type of spines. However, the densities of thin (control, 1.981 ± 0.492 spines per 10 μm; overexpression of AS3MTd2d3, 1.743 ± 0.515 spines per 10 μm, df = 111, t = 1.695, P = .279, multiple comparisons using Bonferroni correction in 2-way ANOVA; figure 3) or stubby (control, 0.933 ± 0.361 spines per 10 μm; overexpression of AS3MTd2d3, 1.247 ± 0.535 spines per 10 μm, df = 111, t = 2.237, P = .0819, multiple comparisons using Bonferroni correction in 2-way ANOVA; figure 3) spines were not significantly altered after AS3MTd2d3 overexpression.
Fig. 3.
Overexpression of AS3MTd2d3 in rat primary hippocampal neurons and their impact on dendritic spine densities. Confocal images of whole neurons transfected with control (pCAG-GFP empty) and overexpression of AS3MTd2d3 (pCAG-AS3MTd2d3) vectors, and scale bars represent 20 μm. Dendritic branches were captured from each corresponding neuron, respectively, and scale bars represent 2 μm. Neuronal morphologies were visualized by staining for Enhanced Green Fluorescent Protein (EGFP). For analysis of total dendritic spine density, 2-tailed t-tests were carried out (F(19, 18) = 2.098, df = 37, t = 1.219, P = .231); for analysis of densities of each dendritic spine subtype, multiple comparisons using Bonferroni correction in 2-way ANOVA (interaction F(2, 111) = 7.5, P = .0009) were applied. Stubby (df = 111, t = 2.237, P = .0819); Thin (df = 111, t = 1.695, P = .279); Mushroom (df = 111, t = 3.032, P = .0091). In either neurons overexpressing AS3MTd2d3 or control neurons, more than 1100 spines in 39 to 40 dendrites on 19–20 neurons (control, n = 20; overexpression of AS3MTd2d3, n = 19) from more than 3 separate cultures were counted for statistical analyses. Error bars indicate standard error of mean (SEM). **P < .01. All neuronal experiments were conducted in 3 independent times with consistent conduct and acquisition parameters.
Discussion
GWAS have successfully identified hundreds of genomic loci associated with schizophrenia. Translating these genetic risk into physiological and pathological mechanisms of the disease and eventually therapeutic targets remains an urgent task in this post-GWAS era.44–46 Growing studies have confirmed significant enrichment of schizophrenia risk loci in brain eQTL and splicing QTL (sQTL).6,7 Li et al15 previously found that schizophrenia risk variations at 10q24.32 showed significant eQTL associations with a human-unique isoform AS3MTd2d3 in brains and identified a human-specific VNTR showing robust association with AS3MTd2d3. However, whether this VNTR is the causative variation regulating AS3MTd2d3 is yet to be determined. In this study, we demonstrate that the VNTR directly regulates the splicing of the AS3MTd2d3 isoform using an in vitro assay. This VNTR also shows the strongest eQTL association with AS3MTd2d3 expression in Han Chinese brains, replicating the observation of previous eQTL analyses in European and African American populations.15 Altogether, these results support the regulatory effect of the VNTR on AS3MTd2d3. Our data also further confirm the involvement of alternative splicing in schizophrenia. Indeed, particular spliced isoforms have been identified to be relevant to schizophrenia, such as ZNF804AE3E4, NRG1 types I-IV, and KCNH2-3.1.12,47–50 Among these schizophrenia relevant spliced isoforms, AS3MTd2d3 has been discovered through high-density GWAS and high-throughput RNA-sequencing analysis in human brains.15 The association between AS3MTd2d3 and the genetic risk of schizophrenia has even surpassed the genome-wide level of statistical significance.
A recent study suggests that the schizophrenia risk allele of rs7085104 is significantly associated with striatal dopamine function in vivo, indicating a potential neural mechanism mediated by schizophrenia genetic risk.51 Besides, the AS3MT VNTR is significantly associated with the baseline brain activation during working memory in multiple regions, including the prefrontal cortex and striatum, and the 3-repeat VNTR (ie, the allele increased risk of schizophrenia) predicts lower baseline activation,52 which is consistent with the previous studies showing lowing prefrontal cortex activation in schizophrenia.53,54 The 3-repeat of the VNTR is also associated with less prefrontal cortex activation reduction induced by a month-long working memory training,52 suggesting a potential role of this variation in neuroplasticity. These findings confirm the disrupted neural plasticity in schizophrenia.55
The wild-type AS3MTfull protein is an arsenic methyltransferase.56Although arsenic toxicity is proven harmful to the central nervous system (CNS) and may increase the risk of psychiatric disorders,57,58 the AS3MTfull is not affected by schizophrenia genetic risk. On the contrary, the truncated AS3MTd2d3, which is regulated by schizophrenia risk genetic variations in previous eQTL analyses and our functional splicing assay, does not seem to have the capability to methylate arsenite due to the loss of 102 amino acids in its N-terminus.15 Therefore, an involvement of arsenic toxicity in AS3MTd2d3 relevant schizophrenia pathogenesis is not implicated, and further studies characterizing the biological function of AS3MTd2d3 are needed. Intriguingly, a recent in vivo imaging study has provided essential clues for its physiological impact.52 The authors found that individuals carrying more repeats of the VNTR had less recruitment of the prefrontal cortex and impaired prefrontal cortex plasticity during working memory tasks. In addition, Li et al15 previously found that the AS3MTd2d3 isoform was upregulated during early neuronal differentiation, and our study further suggests that elevated AS3MTd2d3 expression significantly reduces the densities of mushroom dendritic spines that are responsible for synaptic plasticity and long-term memory.59 Therefore, these convergent lines of evidence support the hypothesis that AS3MTd2d3 might participate in schizophrenia through mediating early neurodevelopment and neuroplasticity. Further investigations into the correlation between its modulatory effects on mushroom spine-related physiological processes and pathological demonstrations of schizophrenia are necessary.
Despite that our results are intriguing, several concerns are to be noted. A potential caveat is the inconsistent significance of the association between rs7085104 and schizophrenia, as it achieved genome-wide level of statistical significance in Europeans while only nominal significance in solely East Asians. Nevertheless, the sample size for the analysis in East Asians is significantly smaller than that of the Europeans, and genetic heterogeneity at this genomic locus between continental populations may also account for this inconsistency. Additionally, there are other potential functional DNA variations associated with schizophrenia in the 10q24.32 genomic region. For example, we have recently identified a schizophrenia risk-associated functional Alu polymorphism (rs71389983) in the ninth intron of the AS3MT gene.60 This Alu polymorphism (rs71389983) exerts a significant impact on the transcription of nearby genes and is in moderate LD with the VNTR in Han Chinese populations (r2 = .68 in 60 individuals). Therefore, the regulation of gene expression in this genomic region is very complicated. It should also be noted that the eQTL SNPs of AS3MTd2d3 confer genetic risk of multiple other psychiatric disorders (eg, bipolar disorder and major depressive disorder).61,62 Although the significance magnitude of its association with mood disorders is smaller than that of its association with schizophrenia, the functional impact of AS3MTd2d3 on mushroom dendritic spines likely also contributes to certain symptoms of bipolar disorder and major depressive disorder. Further in vivo analyses using transgenic mice are needed to delve deeper into its impact in the brain and behaviors.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81971259 and 81722019 to M.L.) and the Innovative Research Team of Science and Technology Department of Yunnan Province (2019HC004). M.L. was also supported by CAS Pioneer Hundred Talents Program and the 1000 Young Talents Program.
Acknowledgments
The authors thank Prof. Yongbin Chen’s lab at Kunming Institute of Zoology for providing the NIH/3T3 cells and Prof. Zhonghua Hu’s lab at Xiangya Hospital for providing the Neuro-2a cells. The authors declare no competing financial interests.
Authors Contributions
H.C. and M.L. designed the study and interpreted the results. X.C. and Z.Y. performed the primary experiments. H.L. conducted SNP genotyping, imputation, and statistical analysis. X.X. and H.C. contributed to design and help the cellular and molecular experiments. X.X., H.C., and M.L. drafted the manuscript, and all authors contributed to the final version of the paper.
References
- 1. Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60(12):1187–1192. [DOI] [PubMed] [Google Scholar]
- 2. Marder SR, Cannon TD. Schizophrenia. N Engl J Med. 2019;381(18):1753–1761. [DOI] [PubMed] [Google Scholar]
- 3. Ripke S, O’Dushlaine C, Chambert K, et al. . Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45(10):1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014;511(7510):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lam M, Chen CY, Li Z, et al. . Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat Genet. 2019;51(12):1670–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takata A, Matsumoto N, Kato T. Genome-wide identification of splicing QTLs in the human brain and their enrichment among schizophrenia-associated loci. Nat Commun. 2017;8:14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jaffe AE, Straub RE, Shin JH, et al. ; BrainSeq Consortium Developmental and genetic regulation of the human cortex transcriptome illuminate schizophrenia pathogenesis. Nat Neurosci. 2018;21(8):1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu W, Li W, Cai X, et al. . Identification of a functional human-unique 351-bp Alu insertion polymorphism associated with major depressive disorder in the 1p31.1 GWAS risk loci. Neuropsychopharmacology 2020;45(7):1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huo Y, Li S, Liu J, Li X, Luo XJ. Functional genomics reveal gene regulatory mechanisms underlying schizophrenia risk. Nat Commun. 2019;10(1):670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fromer M, Roussos P, Sieberts SK, et al. . Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci. 2016;19(11):1442–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gandal MJ, Zhang P, Hadjimichael E, et al. . Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 2018;362:6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tao R, Cousijn H, Jaffe AE, et al. . Expression of ZNF804A in human brain and alterations in schizophrenia, bipolar disorder, and major depressive disorder: a novel transcript fetally regulated by the psychosis risk variant rs1344706. JAMA Psychiatry. 2014;71(10):1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang CP, Li X, Wu Y, et al. . Comprehensive integrative analyses identify GLT8D1 and CSNK2B as schizophrenia risk genes. Nat Commun. 2018;9(1):838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sekar A, Bialas AR, de Rivera H, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium Schizophrenia risk from complex variation of complement component 4. Nature 2016;530(7589):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li M, Jaffe AE, Straub RE, et al. . A human-specific AS3MT isoform and BORCS7 are molecular risk factors in the 10q24.32 schizophrenia-associated locus. Nat Med. 2016;22(6):649–656. [DOI] [PubMed] [Google Scholar]
- 16. Zhao L, Chang H, Zhou DS, et al. . Replicated associations of FADS1, MAD1L1, and a rare variant at 10q26.13 with bipolar disorder in Chinese population. Transl Psychiatry. 2018;8(1):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu W, Yan H, Zhou D, et al. . The depression GWAS risk allele predicts smaller cerebellar gray matter volume and reduced SIRT1 mRNA expression in Chinese population. Transl Psychiatry. 2019;9(1):333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li H, Chang H, Song X, et al. . Integrative analyses of major histocompatibility complex loci in the genome-wide association studies of major depressive disorder. Neuropsychopharmacology 2019;44(9):1552–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Z, Wang N, Li Z, Xiao F, Dai J. Human high intelligence is involved in spectral redshift of biophotonic activities in the brain. Proc Natl Acad Sci U S A. 2016;113(31):8753–8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, Zondervan KT. Data quality control in genetic case-control association studies. Nat Protoc. 2010;5(9):1564–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li H, Zhang C, Cai X, et al. . Genome-wide association study of creativity reveals genetic overlap with psychiatric disorders, risk tolerance, and risky behaviors. Schizophr Bull. 2020. doi:10.1093/schbul/sbaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. [DOI] [PubMed] [Google Scholar]
- 23. Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6):e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Delaneau O, Howie B, Cox AJ, Zagury JF, Marchini J. Haplotype estimation using sequencing reads. Am J Hum Genet. 2013;93(4):687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Genomes Project Consortium, Auton A, Brooks LD, et al. . A global reference for human genetic variation. Nature 2015;526(7571):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Srivastava DP, Woolfrey KM, Penzes P. Analysis of dendritic spine morphology in cultured CNS neurons. J Vis Exp. 2011. (53):e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang Z, Zhou D, Li H, et al. . The genome-wide risk alleles for psychiatric disorders at 3p21.1 show convergent effects on mRNA expression, cognitive function, and mushroom dendritic spine. Mol Psychiatry. 2020;25(1):48–66. [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One. 2008;3(4):e1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Piva F, Giulietti M, Burini AB, Principato G. SpliceAid 2: a database of human splicing factors expression data and RNA target motifs. Hum Mutat. 2012;33(1):81–85. [DOI] [PubMed] [Google Scholar]
- 30. Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57(1):65–73. [DOI] [PubMed] [Google Scholar]
- 31. Osimo EF, Beck K, Reis Marques T, Howes OD. Synaptic loss in schizophrenia: a meta-analysis and systematic review of synaptic protein and mRNA measures. Mol Psychiatry. 2019;24(4):549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berdenis van Berlekom A, Muflihah CH, Snijders GJLJ, et al. . Synapse pathology in schizophrenia: a meta-analysis of postsynaptic elements in postmortem brain studies. Schizophr Bull. 2020;46(2):374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. MacDonald ML, Alhassan J, Newman JT, et al. . Selective loss of smaller spines in schizophrenia. Am J Psychiatry. 2017;174(6):586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McKinney BC, MacDonald ML, Newman JT, et al. . Density of small dendritic spines and microtubule-associated-protein-2 immunoreactivity in the primary auditory cortex of subjects with schizophrenia. Neuropsychopharmacology 2019;44(6):1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14(3):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Forrest MP, Parnell E, Penzes P. Dendritic structural plasticity and neuropsychiatric disease. Nat Rev Neurosci. 2018;19(4):215–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Penzes P, Jones KA. Dendritic spine dynamics–a key role for Kalirin-7. Trends Neurosci. 2008;31(8):419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith KR, Kopeikina KJ, Fawcett-Patel JM, et al. . Psychiatric risk factor ANK3/ankyrin-G nanodomains regulate the structure and function of glutamatergic synapses. Neuron 2014;84(2):399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yadav S, Oses-Prieto JA, Peters CJ, et al. . TAOK2 Kinase mediates PSD95 stability and dendritic spine maturation through septin7 phosphorylation. Neuron 2017;93(2):379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hayashi-Takagi A, Takaki M, Graziane N, et al. . Disrupted-in-schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13(3):327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Russell TA, Grubisha MJ, Remmers CL, et al. . A schizophrenia-linked KALRN coding variant alters neuron morphology, protein function, and transcript stability. Biol Psychiatry. 2018;83(6):499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Forrest MP, Zhang H, Moy W, et al. . Open chromatin profiling in hiPSC-derived neurons prioritizes functional noncoding psychiatric risk variants and highlights neurodevelopmental loci. Cell Stem Cell. 2017;21(3):305–318, e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou D, Xiao X, Li M. The schizophrenia risk isoform ZNF804AE3E4 affects dendritic spine. Schizophr Res. 2020;218:324–325. [DOI] [PubMed] [Google Scholar]
- 44. Gandal MJ, Leppa V, Won H, Parikshak NN, Geschwind DH. The road to precision psychiatry: translating genetics into disease mechanisms. Nat Neurosci. 2016;19(11):1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Edwards SL, Beesley J, French JD, Dunning AM. Beyond GWASs: illuminating the dark road from association to function. Am J Hum Genet. 2013;93(5):779–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang C, Xiao X, Li T, Li M. Translational genomics and beyond in bipolar disorder. Mol Psychiatry. 2020. doi:10.1038/s41380-020-0782-9. [DOI] [PubMed] [Google Scholar]
- 47. Huffaker SJ, Chen J, Nicodemus KK, et al. . A primate-specific, brain isoform of KCNH2 affects cortical physiology, cognition, neuronal repolarization and risk of schizophrenia. Nat Med. 2009;15(5):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Law AJ, Lipska BK, Weickert CS, et al. . Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006;103(17):6747–6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ren M, Hu Z, Chen Q, et al. . KCNH2-3.1 mediates aberrant complement activation and impaired hippocampal-medial prefrontal circuitry associated with working memory deficits. Mol Psychiatry. 2020;25(1):206–229. [DOI] [PubMed] [Google Scholar]
- 50. Carr GV, Chen J, Yang F, et al. . KCNH2-3.1 expression impairs cognition and alters neuronal function in a model of molecular pathology associated with schizophrenia. Mol Psychiatry. 2016;21(11):1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. D’Ambrosio E, Dahoun T, Pardiñas AF, et al. . The effect of a genetic variant at the schizophrenia associated AS3MT/BORCS7 locus on striatal dopamine function: a PET imaging study. Psychiatry Res Neuroimaging. 2019;291:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao W, Zhang Q, Chen X, et al. . The VNTR of the AS3MT gene is associated with brain activations during a memory span task and their training-induced plasticity. Psychol Med. 2020. doi:10.1017/S0033291720000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang R, Picchioni M, Allen P, Toulopoulou T. Working memory in unaffected relatives of patients with schizophrenia: a meta-analysis of functional magnetic resonance imaging studies. Schizophr Bull. 2016;42(4):1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Forsyth JK, Lewis DA. Mapping the consequences of impaired synaptic plasticity in schizophrenia through development: an integrative model for diverse clinical features. Trends Cogn Sci. 2017;21(10):760–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lin S, Shi Q, Nix FB, et al. . A novel S-adenosyl-l-methionine:arsenic(III) methyltransferase from rat liver cytosol. J Biol Chem. 2002;277(13):10795–10803. [DOI] [PubMed] [Google Scholar]
- 57. Tyler CR, Allan AM. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep. 2014;1:132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Valeri L, Mazumdar MM, Bobb JF, et al. . The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20-40 months of age: evidence from rural Bangladesh. Environ Health Perspect. 2017;125(6):067015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Berry KP, Nedivi E. Spine dynamics: are they all the same? Neuron 2017;96(1):43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang ZH, Cai X, Qu N, et al. . Identification of a functional 339 bp Alu insertion polymorphism in the schizophrenia-associated locus at 10q24.32. Zool Res. 2020;41(1):84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li L, Chang H, Peng T, Li M, Xiao X. Evidence of AS3MTd2d3-associated variants within 10q24.32-33 in the genetic risk of major affective disorders. Mol Neuropsychiatry. 2017;2(4):213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]