Abstract
Background
Emotion dysregulation is crucial to both poor social functioning and psychotic symptom formation in patients with schizophrenia. The efficient use of emotion regulation strategies, such as cognitive reappraisal, has been less frequently observed in the early phases of psychotic disorder. It is unknown whether neurophysiological responses related to emotion regulation by cognitive reappraisal are altered in early psychosis.
Methods
Fifty-four patients with first-episode psychosis (FEP), 34 subjects at clinical high risk (CHR) for psychosis, and 30 healthy controls (HCs) participated in event-related potential recordings during a validated emotion regulation paradigm to measure the effect of cognitive reappraisal on emotion regulation. Late positive potentials (LPPs), which reflect emotional arousal, were compared across the groups and the 3 conditions (negative, cognitive reappraisal, and neutral). The relationship among LPP modulation by cognitive reappraisal and social/role functioning and severity of psychotic symptoms was investigated in the early psychosis group.
Results
The FEP and CHR participants showed comparably larger LPP amplitudes in the negative and cognitive reappraisal conditions than in the neutral condition, whereas the HCs presented larger LPPs in the negative condition than in the cognitive reappraisal and neutral conditions. LPP modulation by cognitive reappraisal was negatively correlated with positive symptom severity in the FEP patients and with disorganization severity in the CHR subjects.
Conclusions
Inefficient use of cognitive reappraisal may be related to the impaired emotion regulation and psychotic symptoms from the very beginning of psychotic disorder. This study provides the first neurophysiological evidence regarding current concepts of emotion regulation in early psychosis.
Keywords: clinical high risk, emotion regulation, first-episode psychosis, cognitive reappraisal, late positive potential, psychotic symptoms
Introduction
Patients with schizophrenia suffer from poor social functioning in addition to various psychotic symptoms. Impaired social functioning is prevalent starting at the prodromal stage of psychosis in those at clinical high risk (CHR) for psychosis and is a predictor of the onset of psychotic symptoms.1–3 Early intervention strategies for first-episode psychosis (FEP) and CHR often target social functioning; however, these strategies have not always been successful in terms of both functional and symptomatic recovery.4–7 These previous studies suggest the need for further investigation of factors influencing social functioning and psychotic symptoms in early psychosis.
Emotion plays a crucial role in social functioning by providing information about the significance of social situations and helping people respond appropriately to given social situations.8 Emotion regulation is the process that affects emotional responses to a certain social situation and can be categorized into antecedent- and response-focused strategies.9 Antecedent-focused strategies, including cognitive reappraisal, aim to modulate emotional processes before the full emotional response occurs. On the other hand, response-focused strategies, such as affective suppression, target the full emotional response itself and thus are less effective and more burdensome than antecedent-focused strategies.10,11 Studies using self-report questionnaires in schizophrenia patients and CHR subjects found that lower use of cognitive reappraisal strategies in emotion regulation was related to poor social functioning.12–15
In addition, negative affect resulting from impaired emotion regulation has been suggested to be related to psychotic symptoms of schizophrenia. Models of positive symptom formation suggest that negative affect, along with cognitive biases, contributes to emphasizing the threatening value of ambiguous experiences and thus increase the likelihood of delusional or paranoid interpretations.16,17 Klippel et al18 showed that affective disturbances mediated the pathways of the effects of stress on psychotic symptoms in FEP and CHR groups. Similarly, the negative affect produced by impaired emotion regulation comprises a part of the model explaining why social exclusion triggers paranoid ideation in CHR individuals.19 A recent meta-analysis of self-report questionnaires about emotion regulation in patients with schizophrenia supports those models in that lower use of adaptive emotion regulation strategies, such as cognitive reappraisal, was correlated with more severe positive symptoms in patients with schizophrenia.20
These previous studies provide important concepts related to impaired emotion regulation and its association with poor social functioning and psychotic symptoms in patients with schizophrenia and those experiencing early psychosis. Regarding a neurophysiological correlate of emotion regulation that supports those concepts, 2 seminal studies have investigated late positive potential (LPP), which reflects emotional arousal, during a validated emotion regulation event-related potential (ERP) paradigm developed by Foti and Hajcak21 as a marker for emotion regulation by cognitive reappraisal in chronic schizophrenia patients.22,23 Both studies showed that cognitive reappraisal failed to downregulate negative emotions in patients with schizophrenia, as reflected by an enlarged LPP in response to cognitively reappraised negative stimuli that were comparable to that of attended-to negative stimuli in an early latency LPP window. In particular, Strauss et al23 found that less modulation of LPPs by cognitive reappraisal was associated with higher scores on self-reported state and trait emotional experiences, suggesting the usefulness of LPP modulation by cognitive reappraisal as a neurophysiological marker for ineffective use of cognitive strategies in emotion regulation. However, no meaningful correlations were reported between the modulation of LPP by cognitive reappraisal and relevant clinical scale scores, and study participants were limited to relatively chronic schizophrenia patients. Therefore, further neurophysiological evidence is needed to support the concept of impaired emotion regulation and its relationship with clinical status in early psychosis.
In the current study, we aimed to investigate whether this neurophysiological correlate of emotion regulation by cognitive reappraisal was impaired in early psychosis, as it is in chronic schizophrenia,11,22–24 and whether the effect of cognitive reappraisal on emotion regulation was associated with social functioning or psychotic symptom severity. We hypothesized that both FEP patients and CHR individuals would show failure in emotion regulation by cognitive reappraisal, as indicated by a similarly large LPP amplitude in both the cognitive reappraisal condition and the negative condition compared with the neutral condition. On the other hand, successful emotion regulation by cognitive reappraisal, as indicated by a reduced LPP amplitude in the cognitive reappraisal condition compared with the negative condition, was expected in healthy controls (HCs). In addition, we hypothesized that these early psychosis groups would show a positive correlation between LPP modulation by cognitive reappraisal and social functioning. Furthermore, negative correlations of LPP modulation by cognitive reappraisal with psychotic symptoms in FEP patients and with prodromal psychotic symptoms in CHR individuals were expected.
Methods
Participants
A total of 54 patients with FEP, 30 subjects at CHR for psychosis, and 34 HCs underwent electroencephalographic (EEG) recording during the emotion regulation task.21 The FEP patients and CHR subjects were recruited through the Seoul Youth Clinic (SYC; www.youthclinic.org), a center for early detection of and intervention for psychosis, at the Seoul National University Hospital (SNUH).25 FEP was defined in individuals aged 16 to 40 years who satisfied the diagnosis of schizophreniform, schizophrenia, or schizoaffective disorder when assessed with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), Axis I Disorders (SCID-I), and whose duration of psychotic illness was less than 2 years. Psychotic symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS), and the duration of illness was determined by interviewing the participants and their family members. The CHR status of the participants was confirmed when they met at least 1 of the 3 criteria of the Structured Interview for Prodromal Symptoms (SIPS)26: attenuated positive symptoms (APS), brief intermittent psychotic symptoms (BIPS), and genetic risk with deterioration (GRD). Among the 34 CHR individuals, 32 met the APS criteria, 2 met the BIPS criteria, and none met the GRD criteria. Comorbid axis I diagnosis in the CHR participants was assessed using the SCID-I, and details are provided in the supplementary material. The validated Korean version of the Scale of Prodromal Symptoms (SOPS)27,28 was used to assess prodromal symptoms. In both the FEP and CHR groups, social functioning was assessed using the Global Functioning Scale: Social (GFS:S).29,30 The HCs were recruited via an internet advertisement and were screened using the SCID-I Non-patient Edition (SCID-NP). Potential HC participants were excluded if they had any first- to third-degree biological relatives with a psychotic disorder. The common exclusion criteria included substance abuse or dependence (except nicotine), neurological disease or significant head trauma, and medical illness that could be accompanied by psychiatric symptoms, sensory impairments, and intellectual disability (intelligence quotient [IQ] < 70).
All participants provided written informed consent after receiving a thorough explanation of the study procedure. For minors, informed consent was obtained from both the participants themselves and their parents. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of SNUH.
Emotion Regulation Task
We used the emotion regulation task developed by Foti and Hajcak,21 and the sequence within a trial is provided in supplementary figure S1. In brief, each trial consisted of a sequence of fixation on a gray cross on a black background (1 s), an audio description of the upcoming picture in a male’s voice (3~6 s), fixation (1 s), passive viewing of a picture stimulus (3 s) from the International Affective Picture System (IAPS),31 and the participant’s rating for negative feelings (time unlimited). Each trial was assigned to 1 of 3 conditions: a cognitive reappraisal condition to examine emotion regulation and 2 control conditions, which were the negative and neutral conditions. In the cognitive reappraisal condition, a neutral audio description that neutralized the upcoming unpleasant picture was provided to promote the use of a cognitive reappraisal strategy for emotion regulation. In the negative condition, a negative audio description that focused on the negative aspects of the upcoming unpleasant picture was given; this description prompted the participants to attend to negative feelings. A neutral audio description followed by a neutral picture was presented in the neutral condition. The task consisted of 6 practice trials (2 negative condition and 4 neutral condition trials) and 2 blocks of 75 experimental trials (25 trials for each condition in an experimental block).
EEG Recording and Preprocessing
Continuous EEG recordings were acquired during the emotion regulation task using a Neuroscan 128 Channel SynAmps system equipped with a 128-channel Quick-Cap based on the modified 10–20 international system (Neuroscan). The left and right mastoids were used as the reference. The EEG signals were digitized at a sampling rate of 1 kHz, and an online lowpass filter of 100 Hz was applied. Electrodes placed below and on the outer canthus of the left eye were used to obtain horizontal and vertical electrooculograms (EOGs). The impedance of all electrodes was less than 5 kΩ.
Curry 7 software (Compumedics) was used to analyze continuous EEG data. Up to 7% of bad channels per participant were reconstructed via the linear interpolation of the adjacent channels. The artifact reduction algorithm developed by Semlitsch et al32 was used to reduce ocular artifacts. Then, EEG recordings were rereferenced to the common average reference data and bandpass filtered between 0.1 and 30 Hz. Continuous EEG data were epoched to a 200-ms prestimulus interval and a 3000-ms poststimulus interval. Baseline correction was performed using the averaged prestimulus interval voltage. Epochs containing EEG amplitudes that exceeded ±100 μV were automatically discarded. The number of remaining epochs in each condition was not different across the groups (supplementary table S1). ERP waves were obtained by separately averaging epochs from the 3 conditions. LPP was calculated as the mean amplitude between 350 and 750 ms after stimulus onset at the Pz electrode site to focus on the time window and electrode site where the largest LPP was observed in previous studies.21–23,33 Emotion regulation scores were calculated by subtracting the mean LPP amplitudes in the cognitive reappraisal condition from those in the negative condition.
Statistical Analysis
For the group comparisons of the demographic and clinical data, analysis of variance (ANOVA) was used for continuous variables, and χ 2 tests or Fisher’s exact tests were used to analyze the categorical data. Repeated measures ANOVA with condition (negative, cognitive reappraisal, and neutral) as the within-subject factor and group (FEP, CHR, and HC) as the between-subjects factor was conducted to reveal the effect of cognitive reappraisal on the mean LPP amplitude and rating scores for negative feelings across the groups. Emotion regulation scores were compared across the groups using ANOVA. Sex was considered a covariate in the group comparison analysis of LPPs. Post hoc Fisher’s least significant difference analysis was performed to find specific LPP differences across the groups and conditions. When a significant group-by-condition interaction was found, a paired samples t-test was used to reveal the specific LPP difference across the conditions within each group. The relationship between emotion regulation scores and the scores on the PANSS in the FEP patients, the scores on the SOPS in the CHR subjects, and the scores on the GFS:S in both early psychosis groups were investigated using Pearson’s correlation analysis. SPSS software ver. 23.0 (IBM Corp.) was used for the statistical analyses. Significance levels were set at P < .05.
Results
Participant Characteristics
The demographic and clinical characteristics of the participants are provided in table 1. The patients with FEP, subjects at CHR for psychosis, and HCs were not different in handedness, age, IQ, and education years. However, there were more females in the FEP group than in the CHR and HC groups (χ 2 = 16.696, P < .001). Scores on the GFS:S (t = −0.794, P = .429) were not different between the FEP and CHR groups.
Table 1.
Demographic and Clinical Characteristics of Patients With First-Episode Psychosis (FEP), Subjects at Clinical High Risk (CHR) for Psychosis, and Healthy Controls (HCs)
| FEP | CHR | HC | Statistical Analysisa | ||
|---|---|---|---|---|---|
| (N = 54) | (N = 34) | (N = 30) | F or T or χ2 | P | |
| Sex (male/female) | 19/35 | 21/13 | 24/6 | 16.696 | <.001** |
| Handedness (right/left) | 51/3 | 33/1 | 28/2 | 0.504 | .777 |
| Age (y) | 21.9 ± 3.8 | 21.3 ± 4.7 | 20.1 ± 1.8 | 2.231 | .112 |
| IQ | 101.3 ± 16.1 | 99.2 ± 12.9 | 106.1 ± 11.7 | 1.991 | .141 |
| Education (y) | 13.5 ± 2.3 | 13.0 ± 1.9 | 13.0 ± 1.4 | 0.916 | .403 |
| DOI (mo) | 7.3 ± 5.8 | — | — | — | — |
| PANSS | |||||
| Positive symptoms | 15.2 ± 5.8 | — | — | — | — |
| Negative symptoms | 14.9 ± 6.4 | — | — | — | — |
| General symptoms | 30.6 ± 10.3 | — | — | — | — |
| SOPS | |||||
| Positive symptoms | — | 10.7 ± 4.6 | — | — | — |
| Negative symptoms | — | 12.4 ± 7.2 | — | — | — |
| Disorganization | — | 3.6 ± 3.3 | — | — | — |
| General symptoms | — | 7.2 ± 4.4 | — | — | — |
| GFS:S | 5.4 ± 1.5 | 5.7 ± 1.2 | — | −0.794 | .429 |
| Prescribed medicationb | |||||
| Antipsychotics | 52 (96.3) | 4 (11.8) | — | 60.869 | <.001** |
| Antidepressants | 2 (3.7) | 2 (5.9) | — | 0.228 | .633 |
| Mood stabilizers | 10 (18.5) | 6 (17.6) | — | 0.011 | 918 |
| Anxiolytics | 32 (59.3) | 9 (26.5) | — | 0.914 | .003** |
| Antipsychotic dosec | 14.3 ± 9.7 | 0.5 ± 1.6 | — | 8.168 | <.001** |
| Anxiolytic dosed | 0.4 ± 0.5 | 0.1 ± 0.3 | — | 9.049 | .003** |
Note: IQ, intelligence quotient; DOI, duration of illness; PANSS, Positive and Negative Syndrome Scale; SOPS, Scale of Prodromal Symptoms; GFS:S, Global Functioning Scale: Social. Data are given as the mean ± standard deviation.
aAnalysis of variance, independent t-test, or Welch’s t-test if the variances were not equal; χ2 analysis or Fisher’s exact test for categorical data.
bNumber (percentage) of FEP patients and CHR subjects who were prescribed each medication at the time of late positive potential (LPP) measurement.
cOlanzapine equivalent dose of antipsychotics prescribed at the time of LPP measurement.
dLorazepam equivalent dose of anxiolytics prescribed at the time of LPP measurement.
**The mean difference is significant at the .005 level.
LPP and Negative Emotion Rating Results
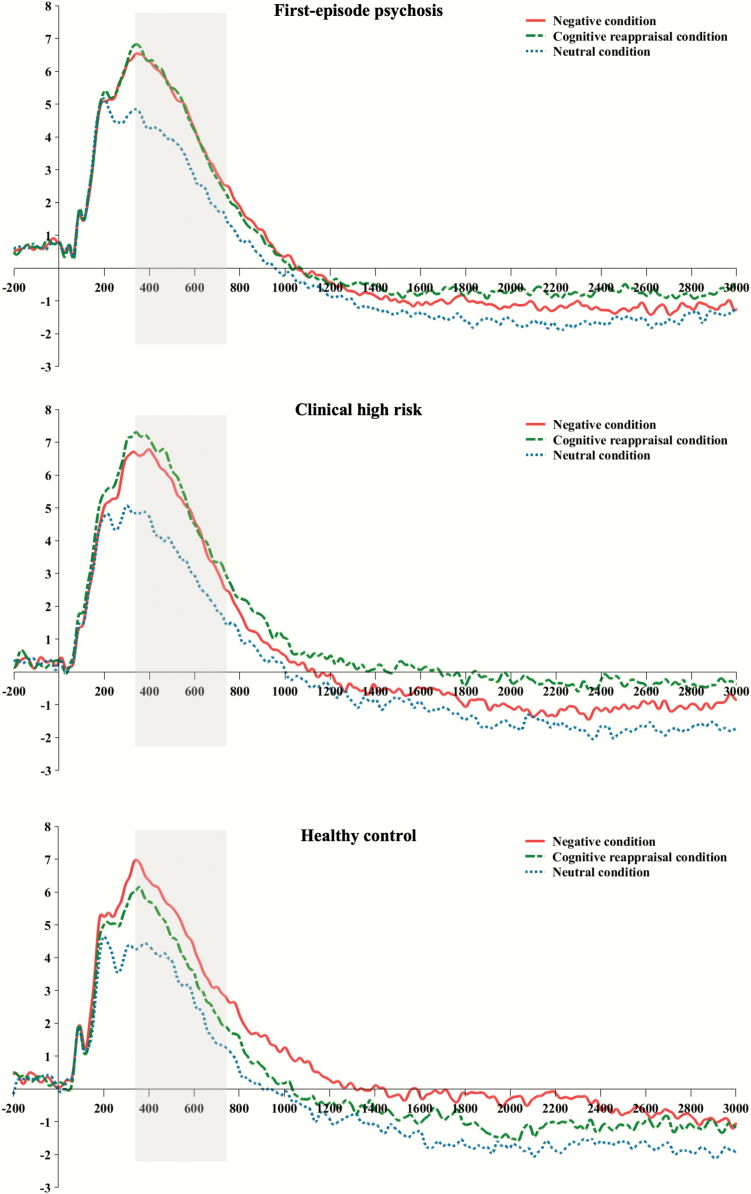
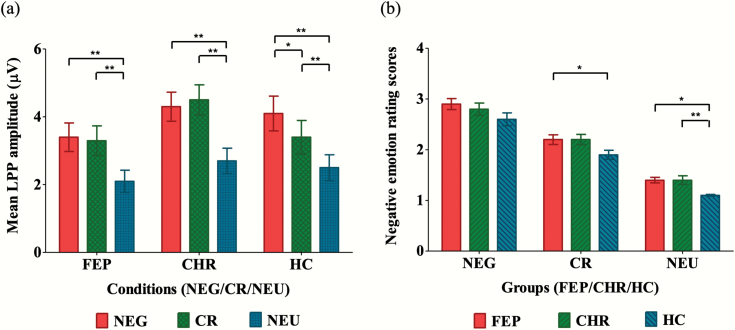
The results of the LPP and negative emotion ratings are summarized in table 2. Figure 1 displays the grand-averaged LPP waveforms across the 3 conditions in each group. Topographic maps are provided in supplementary figure S2. Repeated measures ANOVA with condition as the within-subject factor, group as the between-subjects factor, and sex as a covariate showed that there was a significant effect of condition (F = 4.201, P = .016) and group-by-condition interaction (F = 3.054, P = .018) on mean LPP amplitude at the Pz electrode site. There was no significant effect of group (F = 0.886, P = .415). Paired samples t-tests showed that mean LPP amplitudes were smaller in the cognitive reappraisal condition than in the negative condition only for the HC group (t = 2.830, P = .008; FEP: t = 0.152, P = .880; CHR: t = −1.185, P = .244), suggesting that emotion regulation by cognitive reappraisal was successful in the HCs but not in the FEP and CHR participants (figure 2a). Regarding the negative emotion rating scores, repeated measures ANOVA with condition as the within-subject factor and group as the between-subjects factor showed significant effects of group (F = 3.641, P = .029) and condition (F = 348.897, P < .001). No significant group-by-condition interaction was found (F = 0.594, P = .668). A post hoc Fisher’s least significant difference analysis showed that the FEP patients reported higher negative feelings in the cognitive reappraisal (P = .012) and neutral (P = .014) conditions than the HC subjects did. The individuals at CHR for psychosis reported higher negative feelings scores than the HCs in the neutral condition (P = .002; figure 2b).
Table 2.
Results of Late Positive Potentials (LPP) at the Pz Electrode Site and Negative Emotion Ratings in the 3 Conditions
| FEP | CHR | HC | Statistical Analysisa | Post hoc Analysisb | |||||
|---|---|---|---|---|---|---|---|---|---|
| (N = 54) | (N = 34) | (N = 30) | F | P | |||||
| Mean LPP amplitudes (μV) for each condition | NEG vs CR | NEG vs NEU | CR vs NEU | ||||||
| NEG condition | 3.4 ± 3.1 | 4.3 ± 2.5 | 4.1 ± 2.8 | Group | 0.886 | .415 | |||
| CR condition | 3.3 ± 3.2 | 4.5 ± 2.6 | 3.4 ± 2.7 | Condition | 4.201 | .016* | .205 | <.001** | <.001** |
| NEU condition | 2.1 ± 2.4 | 2.7 ± 2.2 | 2.5 ± 2.1 | Group × Condition | 3.054 | .018* | .010* | .379 | .061 |
| Paired samples t-test | NEG = CR | NEG = CR | NEG > CR* | ||||||
| NEG > NEU** | NEG > NEU** | NEG > NEU** | |||||||
| CR > NEU** | CR > NEU** | CR > NEU** | |||||||
| Negative emotion rating scores (0–4) | FEP vs CHR | FEP vs HC | CHR vs HC | ||||||
| NEG condition | 2.9 ± 0.8 | 2.8 ± 0.7 | 2.6 ± 0.7 | Group | 3.641 | .029* | .708 | .112 | .262 |
| CR condition | 2.2 ± 0.7 | 2.2 ± 0.6 | 1.9 ± 0.5 | Condition | 348.897 | <.001** | .632 | .012* | .059 |
| NEU condition | 1.4 ± 0.4 | 1.4 ± 0.5 | 1.1 ± 0.1 | Group × Condition | 0.594 | .668 | .320 | .014* | .002** |
| Emotion regulation scores (μV) | FEP vs CHR | FEP vs HC | CHR vs HC | ||||||
| Mean LPP amplitude (NEG-CR) | 0.0 ± 1.3 | −0.3 ± 1.4 | 0.7 ± 1.4 | 3.443 | .019* | .316 | .024* | .004** |
Note: FEP, first-episode psychosis; CHR, clinical high risk; HC, healthy control; NEG, negative; CR, cognitive reappraisal; NEU, neutral. The NEG condition indicates a negative audio description followed by an unpleasant image. The CR condition indicates a neutral audio description followed by an unpleasant image, which promotes the use of a cognitive reappraisal strategy for emotion regulation. The NEU condition indicates a neutral audio description followed by a neutral image. Data are given as the mean ± standard deviation.
aAnalysis of variance with sex as a covariate.
bPost hoc Fisher’s least significant difference analysis.
*The mean difference is significant at the .05 level.
**The mean difference is significant at the .005 level.
Fig. 1.
Grand-averaged waveforms of the late positive potential at the Pz electrode site in negative, cognitive reappraisal, and neutral conditions across the first-episode psychosis (FEP), clinical high risk (CHR) for psychosis, and healthy control (HC) groups. Areas between 350 and 750 ms after stimulus onset are marked in gray.
Fig. 2.
(a) Comparison of mean late positive potential (LPP) amplitudes between 350 and 750 ms after stimulus onset at the Pz electrode site across the negative (NEG), cognitive reappraisal (CR), and neutral (NEU) conditions in each group. (b) Comparison of negative emotion rating scores across the first-episode psychosis (FEP), clinical high risk (CHR) for psychosis, and healthy control (HC) groups in each condition. The bars indicate the means for each condition and group, and the vertical lines indicate the standard errors. *indicates that the mean difference is significant at the .05 level; **indicates that the mean difference is significant at the .005 level.
Emotion Regulation Scores and Their Association With Clinical Status
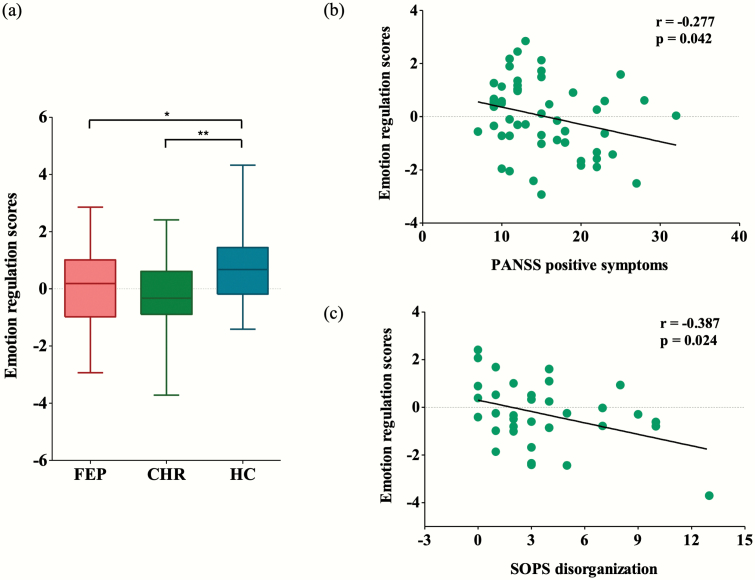
The emotion regulation scores, which were calculated by subtracting the mean LPP amplitudes in the cognitive reappraisal condition from those in the negative condition, were significantly different across the 3 groups (F = 3.433, P = .019). Post hoc Fisher’s least significant difference analysis revealed that the emotion regulation scores of the HC group were higher than those of the FEP (P = .024) and CHR (P = .004) groups (table 2; figure 3a). Pearson’s correlation analysis showed that there was a significant relationship between high emotion regulation scores and low PANSS positive symptom scores in the patients with FEP (r = −.281, P = .025; figure 3b). In the CHR group, the emotion regulation scores were negatively correlated with the SOPS disorganization scores (r = −.387, P = .024; figure 3c). Other variables, including GFS:S scores, did not show significant correlations (supplementary table S2).
Fig. 3.
(a) Comparison of emotion regulation scores across the first-episode psychosis (FEP), clinical high risk (CHR) for psychosis, and healthy control (HC) groups. Emotion regulation scores were calculated by subtracting the mean late positive potential amplitudes in the cognitive reappraisal condition from those in the negative condition. The horizontal lines in the group indicate the means, and the vertical lines in the group indicate the 5th to 95th percentiles. *indicates that the mean difference is significant at the .05 level; **indicates that the mean difference is significant at the .005 level. (b) The correlation between the emotion regulation scores and the scores on the Positive and Negative Syndrome Scale (PANSS) positive symptom subscale in the FEP patients. (c) The correlation between the emotion regulation scores and the scores on the Scale of Prodromal Symptoms (SOPS) disorganization subscale in the CHR individuals.
Discussion
This study is the first to investigate neurophysiological correlate of impaired emotion regulation by cognitive reappraisal in early psychosis. In both the FEP patients and the CHR individuals, LPP in the cognitive reappraisal condition was comparable to that in the negative condition. In contrast, the HCs presented larger LPP in the negative condition than in the cognitive reappraisal condition. In addition, emotion regulation scores (ie, the modulation of LPP by cognitive reappraisal) were negatively correlated with positive symptom severity in the FEP patients and with disorganization severity in the CHR subjects. Our results not only add electrophysiological evidence to the previous literature based on self-report questionnaires, which showed that impaired emotion regulation was present in the early phase of psychosis and also highlighted the association between emotion regulation difficulties, reflected by LPP modulation, and the severity of psychotic symptoms.
Compared with HCs, patients with schizophrenia have shown less use of cognitive reappraisal strategies and greater use of affective suppression in self-reported measures of emotion regulation strategy use.14,15,34 Providing biological evidence to support behavioral or self-report results is important for understanding psychopathology and related brain dysfunction. In this context, 2 previous studies reported reduced LPP modulation as a result of cognitive reappraisal in chronic schizophrenia patients, and this measure is the first neurophysiological correlate of impaired emotion regulation by cognitive strategy in patients with schizophrenia.22,23 Although self-report studies with early psychosis patients present results similar to those found for chronic schizophrenia patients,13,19,35 the neurophysiological correlate of impaired emotion regulation by cognitive reappraisal in FEP patients and CHR individuals has not yet been reported. In the current study, we found that the FEP and CHR participants presented larger LPPs (ie, greater emotional arousal) in response to cognitively appraised negative stimuli than to neutral stimuli; these LPPs were similar to those evoked by the attended-to negative stimuli, and this finding was similar to that reported for chronic schizophrenia patients.22–24 Only the HCs produced smaller LPPs in the cognitive reappraisal condition than in the negative condition, showing that cognitive reappraisal had an effect on emotion regulation in the HC group but not in the FEP and CHR groups. These findings suggest that the neurophysiological correlate of impaired emotion regulation by cognitive reappraisal presents starting in the early phases of psychotic disorder.
We found that lower emotion regulation scores (ie, less modulation of LPP by cognitive reappraisal) were associated with higher scores on the PANSS positive subscale in the FEP patients and with higher scores on the SOPS disorganization subscale in the CHR individuals. In addition, similar to chronic schizophrenia patients,23,36 both the FEP and CHR participants reported higher negative emotion rating scores in response to neutral stimuli than the HCs. It has been shown that patients with schizophrenia experience more negative affect in response to neutral stimuli due to inefficient emotion regulation, and negative affect combined with cognitive biases plays a key role in psychotic symptom formation.16,17,20 Our findings are the first to provide the neurophysiological evidence for previous study results suggesting that inefficient use of cognitive negative emotion regulation strategies was associated with psychotic symptoms starting in the early stages of psychosis, such as FEP and CHR.18,19
Unlike our initial expectation based on previous studies that reported a significant correlation between self-reported use of emotion regulation strategies and social functioning in schizophrenia patients,12,14,15 our early psychosis participants did not show significant correlations between LPP modulation by cognitive reappraisal (ie, emotion regulation) and scores on the GFS:S. It is unclear why these correlations were not significant; however, one possibility is that social functioning is affected by multiple factors, such as negative symptoms and cognitive dysfunction, rather than by emotion regulation alone. In particular, people in the early course of schizophrenia present with prominent positive symptoms rather than negative symptoms, in contrast with chronic schizophrenia patients.37 Therefore, the results of the current study, which showed a significant association between LPP modulation by cognitive reappraisal and positive symptom severity but a nonsignificant correlation between LPP modulation and social functioning, may be due to the characteristics of participants who are in the early stage of psychotic disorder and thus have rather indistinct negative symptoms.
This study has several limitations. First, the FEP group comprised a greater proportion of females than the CHR and HC groups did. Although one study has shown that LPP modulation by cognitive reappraisal did not differ between boys and girls,38 others have reported that females presented higher LPP amplitudes in response to negative stimuli than males did.39,40 Although sex was used as a covariate in the group comparison analysis, cautious interpretation is warranted because sex was not matched across groups in this study. Second, most of the FEP patients were taking antipsychotic and anxiolytic medication at the time of the ERP measurements, and the effect of antipsychotics and anxiolytics on emotion regulation and LPP is unknown. Although there was no significant correlation between the olanzapine equivalent dose of antipsychotics41 (r = −.043, P = .758) or the lorazepam equivalent dose of anxiolytics42 (r = −.197, P = .154) and LPP modulation by cognitive reappraisal, future studies with medication-free patients are needed to support the current study results. Third, the emotion regulation task used in the current study was designed to specifically focus on the effect of forced cognitive reappraisal on LPP modulation,21–23 in contrast with other studies that allowed participants to regulate their emotions with their own resources and preferred strategies. Therefore, interpretation of the current study’s results should be limited to the effect of specific cognitive reappraisal on LPP modulation in early psychosis patients.
The diagnosis of psychiatric disorders based on symptomatic phenotype leads to tremendously increased heterogeneity, even within a diagnosis; thus, rigorous efforts have been made to investigate biomarkers that can connect pathophysiology with behavioral observations and can be used to predict treatment response as well as prognosis.43,44 In line with these efforts, the current study results have important clinical implications in that they present the biological background underlying inefficient cognitive emotion regulation and psychotic symptoms as a target for early intervention in FEP and CHR. The modulation of LPP by cognitive reappraisal may be utilized as a potent biomarker in future longitudinal studies that aim to measure and predict responses to treatments for improving emotion regulation, psychotic symptoms, and the long-term prognosis of early psychosis patients.
Supplementary Material
Acknowledgment
The authors have no conflicts of interest to declare.
Funding
This work was supported by the Brain Research Program and the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning (grant nos. 2017M3C7A1029610 and 2019R1C1C1002457).
References
- 1. Addington J, Penn D, Woods SW, Addington D, Perkins DO. Social functioning in individuals at clinical high risk for psychosis. Schizophr Res. 2008;99(1-3):119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jang JH, Shin NY, Shim G, et al. Longitudinal patterns of social functioning and conversion to psychosis in subjects at ultra-high risk. Aust N Z J Psychiatry. 2011;45(9):763–770. [DOI] [PubMed] [Google Scholar]
- 4. Devoe DJ, Farris MS, Townes P, Addington J. Interventions and social functioning in youth at risk of psychosis: a systematic review and meta-analysis. Early Interv Psychiatry. 2019;13(2):169–180. [DOI] [PubMed] [Google Scholar]
- 5. Fowler D, Hodgekins J, French P, et al. Social recovery therapy in combination with early intervention services for enhancement of social recovery in patients with first-episode psychosis (SUPEREDEN3): a single-blind, randomised controlled trial. Lancet Psychiatry. 2018;5(1):41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fowler D, Hodgekins J, Howells L, et al. Can targeted early intervention improve functional recovery in psychosis? A historical control evaluation of the effectiveness of different models of early intervention service provision in Norfolk 1998-2007. Early Interv Psychiatry. 2009;3(4):282–288. [DOI] [PubMed] [Google Scholar]
- 7. Penn DL, Waldheter EJ, Perkins DO, Mueser KT, Lieberman JA. Psychosocial treatment for first-episode psychosis: a research update. Am J Psychiatry. 2005;162(12):2220–2232. [DOI] [PubMed] [Google Scholar]
- 8. Barrett LF, Gross J, Christensen TC, Benvenuto M. Knowing what you’re feeling and knowing what to do about it: mapping the relation between emotion differentiation and emotion regulation. Cogn Emot. 2001;15:713–724. [Google Scholar]
- 9. Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74(1):224–237. [DOI] [PubMed] [Google Scholar]
- 10. Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–291. [DOI] [PubMed] [Google Scholar]
- 11. Strauss GP, Kappenman ES, Culbreth AJ, et al. Emotion regulation abnormalities in schizophrenia: directed attention strategies fail to decrease the neurophysiological response to unpleasant stimuli. J Abnorm Psychol. 2015;124(2):288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henry JD, Rendell PG, Green MJ, McDonald S, O’Donnell M. Emotion regulation in schizophrenia: affective, social, and clinical correlates of suppression and reappraisal. J Abnorm Psychol. 2008;117(2):473–478. [DOI] [PubMed] [Google Scholar]
- 13. Kimhy D, Gill KE, Brucato G, et al. The impact of emotion awareness and regulation on social functioning in individuals at clinical high risk for psychosis. Psychol Med. 2016;46(14):2907–2918. [DOI] [PubMed] [Google Scholar]
- 14. Kimhy D, Vakhrusheva J, Jobson-Ahmed L, Tarrier N, Malaspina D, Gross JJ. Emotion awareness and regulation in individuals with schizophrenia: implications for social functioning. Psychiatry Res. 2012;200(2-3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Meer L, van′t Wout M, Aleman A. Emotion regulation strategies in patients with schizophrenia. Psychiatry Res. 2009;170(2-3):108–113. [DOI] [PubMed] [Google Scholar]
- 16. Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol Med. 2001;31(2):189–195. [DOI] [PubMed] [Google Scholar]
- 17. Preti A, Cella M. Paranoid thinking as a heuristic. Early Interv Psychiatry. 2010;4(3):263–266. [DOI] [PubMed] [Google Scholar]
- 18. Klippel A, Myin-Germeys I, Chavez-Baldini U, et al. Modeling the interplay between psychological processes and adverse, stressful contexts and experiences in pathways to psychosis: an experience sampling study. Schizophr Bull. 2017;43(2):302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lincoln TM, Sundag J, Schlier B, Karow A. The relevance of emotion regulation in explaining why social exclusion triggers paranoia in individuals at clinical high risk of psychosis. Schizophr Bull. 2018;44(4):757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ludwig L, Werner D, Lincoln TM. The relevance of cognitive emotion regulation to psychotic symptoms – a systematic review and meta-analysis. Clin Psychol Rev. 2019;72:101746. [DOI] [PubMed] [Google Scholar]
- 21. Foti D, Hajcak G. Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. J Cogn Neurosci. 2008;20(6):977–988. [DOI] [PubMed] [Google Scholar]
- 22. Horan WP, Hajcak G, Wynn JK, Green MF. Impaired emotion regulation in schizophrenia: evidence from event-related potentials. Psychol Med. 2013;43(11):2377–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strauss GP, Kappenman ES, Culbreth AJ, Catalano LT, Lee BG, Gold JM. Emotion regulation abnormalities in schizophrenia: cognitive change strategies fail to decrease the neural response to unpleasant stimuli. Schizophr Bull. 2013;39(4):872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sullivan SK, Strauss GP. Electrophysiological evidence for detrimental impact of a reappraisal emotion regulation strategy on subsequent cognitive control in schizophrenia. J Abnorm Psychol. 2017;126(5):679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwon JS, Byun MS, Lee TY, An SK. Early intervention in psychosis: insights from Korea. Asian J Psychiatr. 2012;5(1):98–105. [DOI] [PubMed] [Google Scholar]
- 26. Miller TJ, McGlashan TH, Rosen JL, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159(5):863–865. [DOI] [PubMed] [Google Scholar]
- 27. Jung MH, Jang JH, Kang DH, et al. The reliability and validity of the Korean version of the structured interview for prodromal syndrome. Psychiatry Investig. 2010;7(4):257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. [DOI] [PubMed] [Google Scholar]
- 29. Carrión RE, Auther AM, McLaughlin D, et al. The global functioning: social and role scales-further validation in a large sample of adolescents and young adults at clinical high risk for psychosis. Schizophr Bull. 2019;45(4):763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cornblatt BA, Auther AM, Niendam T, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33(3):688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lang PJ, Bradley MM, Cuthbert BN.. International Affective Picture System (IAPS): Digitized Photographs, Instruction Manual and Affective Ratings, Technical Report A-6. Gainesville, FL: University of Florida; 2001. [Google Scholar]
- 32. Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. [DOI] [PubMed] [Google Scholar]
- 33. Strauss GP, Ruiz I, Visser KH, Crespo LP, Dickinson EK. Diminished Hedonic response in neuroleptic-free youth at ultra high-risk for psychosis. Schizophr Res Cogn. 2018;12:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Visser KF, Esfahlani FZ, Sayama H, Strauss GP. An ecological momentary assessment evaluation of emotion regulation abnormalities in schizophrenia. Psychol Med. 2018;48(14):2337–2345. [DOI] [PubMed] [Google Scholar]
- 35. Liu J, Subramaniam M, Chong SA, Mahendran R. A systematic examination of cognitive emotion regulation strategies, global emotion dysregulation, and cognitive insight in relation to posttraumatic stress disorder symptoms among trauma exposed patients with early nonaffective psychosis. Psychol Trauma. 2019. [online ahead of print] doi: 10.1037/tra0000531. [DOI] [PubMed] [Google Scholar]
- 36. Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36(1):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lieberman JA, Perkins D, Belger A, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50(11):884–897. [DOI] [PubMed] [Google Scholar]
- 38. Van Cauwenberge V, Van Leeuwen K, Hoppenbrouwers K, Wiersema JR. Developmental changes in neural correlates of cognitive reappraisal: an ERP study using the late positive potential. Neuropsychologia 2017;95:94–100. [DOI] [PubMed] [Google Scholar]
- 39. Kato R, Takeda Y. Responses to affective pictures depicting humans: late positive potential reveals a sex-related effect in processing that is not present in subjective ratings. Exp Brain Res. 2017;235(1):193–204. [DOI] [PubMed] [Google Scholar]
- 40. Yang J, Zhang S, Lou Y, et al. The increased sex differences in susceptibility to emotional stimuli during adolescence: an event-related potential study. Front Hum Neurosci. 2017;11:660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686–693. [DOI] [PubMed] [Google Scholar]
- 42. Ashton CH. Benzodiazepines: How They Work and How to Withdraw. Newcastle: Newcastle University; 2006. [Google Scholar]
- 43. Clementz BA, Sweeney JA, Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173(4):373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.