Abstract
Many robust studies have investigated prepulse inhibition (PPI) in patients with schizophrenia. Recent evidence indicates that PPI may help identify individuals who are at clinical high risk for psychosis (CHR). Selective attention to prepulse stimulus can specifically enhance PPI in healthy subjects; however, this enhancement effect is not observed in patients with schizophrenia. Modified PPI measurement with selective attentional modulation using perceived spatial separation (PSS) condition may be a more robust and sensitive index of PPI impairment in CHR individuals. The current study investigated an improved PSSPPI condition in CHR individuals compared with patients with first-episode schizophrenia (FES) and healthy controls (HC) and evaluated the accuracy of PPI in predicting CHR from HC. We included 53 FESs, 55 CHR individuals, and 53 HCs. CHRs were rated on the Structured Interview for Prodromal Syndromes. The measures of perceived spatial co-location PPI (PSCPPI) and PSSPPI conditions were applied using 60- and 120-ms lead intervals. Compared with HC, the CHR group had lower PSSPPI level (Inter-stimulus interval [ISI] = 60 ms, P < .001; ISI = 120 ms, P < .001). PSSPPI showed an effect size (ES) between CHR and HC (ISI = 60 ms, Cohen’s d = 0.91; ISI = 120 ms, Cohen’s d = 0.98); on PSSPPI using 60-ms lead interval, ES grade increased from CHR to FES. The area under the receiver operating characteristic curve for PSSPPI was greater than that for PSCPPI. CHR individuals showed a PSSPPI deficit similar to FES, with greater ES and sensitivity. PSSPPI appears a promising objective approach for preliminary identification of CHR individuals.
Keywords: clinical high-risk individuals, schizophrenia, acoustic startle response, prepulse inhibition
Prepulse inhibition (PPI) refers to the inhibition of acoustic startle reflex by a weaker nonstartling prepulse stimulus 30–500 ms preceding the intense, startling stimulus.1 PPI can be used as an operational measure for sensorimotor gating. A “protection-of-processing” theory has been proposed by Graham for justifying the function of PPI: a weak prepulse stimulus followed by an intense stimulus can generate not only the information processing for the prepulse signal but also a gating mechanism dampening the information of the intense disruptive inputs.1 Therefore, PPI protects the early processing of the prepulse signal from interference by extraneous stimuli. Two main mechanisms are involved in the extraction of behavior-related target information from a large amount of background information: one is the brainstem-level gating mechanism,2,3 and the other the forebrain-level attention mechanism.4–6 Moreover, alternative explanations have been provided for the potential biological relevance of PPI (interruption hypothesis and protection hypothesis): prepulse may also inhibit the startle response of attenuated startle stimulus and protect the higher processing of this interference.7 PPI has been shown to be a mature neurophysiological measure that meets all of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS)/Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia criteria and are suitable for clinical studies.8
Previous studies have shown impaired PPI in several neuropsychiatric disorders, such as schizophrenia, bipolar disorder, obsessive-compulsive disorder, Huntington, Tourette, several forms of dementia, panic disorder, and autism spectrum disorders.9–12 It has been found that patients diagnosed with schizophrenia (including those with either first-episode schizophrenia [FES] or chronic schizophrenia, as well as in the acute and stable phases) had reduced PPI compared to healthy controls (HC).13–18 PPI deficits have, in part, been shown to represent state and heritable traits because they are also present in unaffected, first-degree relatives19,20; moreover, there is greater PPI deficit with higher severity of clinical symptoms measured on the Scale for the Assessment of Positive Symptoms and the Assessment of Negative Symptoms, especially hallucination and delusion.21 A plethora of studies from different laboratories, continents, and ethnic backgrounds have provided consistent evidence of PPI deficits in schizophrenia14,16,22; however, the effect size (ES) in these studies varied and there were some negative reports.
Several recent studies have demonstrated PPI disruption in individuals at clinical high risk (CHR) for psychosis. Tracing the findings as far back as 2008, we found a hypothesis that first investigated whether PPI deficits that precede the onset of schizophrenia in a prodromal state are likely to proceed to schizophrenia.23 The concept of CHR has been expounded24 to describe potential prepsychotic subsyndromal symptoms with cognitive, behavioral, and social deficits in the putative prodromal stage of schizophrenia.25 PPI impairment in CHR individuals has been replicated in other studies.26–28 These results have also been demonstrated in adolescents.29 Nonetheless, PPI deficit was not observed in another CHR sample; however, smokers with early psychosis showed evidence of PPI deficit.30 In another study, PPI impairment was particularly observed in CHR individuals who were cannabis users.31 These inconsistent results are partly attributable to small sample size, different smoking behaviors, and cannabis use.
In the large, complex, multisite genetic studies by the Consortium on the Genetics of Schizophrenia, the PPI ES ranged from 0.24 to 0.57.22 This indicated a strong overlap in PPI levels between patients and healthy people. A modified PPI paradigm is needed to increase PPI detection. The central regulatory circuit of PPI has been shown to be located in the brainstem and is considered an automated process.32 It is also regulated from the top-down by advanced cognitive activities, such as attention.33 Selective attention to prepulse stimulus can specifically enhance PPI in healthy subjects; however, this enhancement effect is not observed in patients with schizophrenia due to attention deficit.34 The modified PPI measurement uses perceived spatial separation (PSS) condition to study attentional modulation. Modified PPI uses human instinct’s binaural priority effect to create a sense of perceptual spatial separation between the target sound and the background noise; this helps improve the perception of prepulse stimulus and increases the PPI. This validity and reliability of this modified PSSPPI condition have been demonstrated.35 Therefore, we hypothesized that, under the PSSPPI condition, the enhanced selective attention would be absent in individuals with either FES or CHR, thereby being a more robust and sensitive index of PPI impairment.
Undertaking efforts to prevent schizophrenia before the onset of imminent psychosis may improve patient outcomes.25 Thus, there is a consensus that clinical management should prioritize the detection of CHR individuals. Dozens of studies have investigated PPI in patients with schizophrenia. The emerging findings have provided an evidence base for the CHR; however, the results have been inconsistent and demonstrated a lack of discriminative ability. In this study, we used a modified PSSPPI condition with attention modulation to investigate PPI in CHR individuals and compared the results with FES and HC; subsequently, we evaluated the accuracy of PPI in predicting CHR from HC. Furthermore, we analyzed and discussed the association of PPI with demographic, clinical characteristics, and cognitive function in the supplementary materials.
Methods
Participants
Participants were recruited at the Beijing Anding Hospital, Capital Medical University, Beijing, China, from January 2015 to January 2018. This study comprised 3 cohorts. (1) The FES group: After the Structured Clinical Interview for DSM-IV Axis I disorders—Patient Edition (SCID-I/P) screening,36 patients who initially met the diagnostic criteria for schizophrenia, those with a duration of untreated psychosis of fewer than 5 years, and those with a duration of antipsychotic treatment less than 14 days, were enrolled.37 (2) The CHR group: All subjects qualified the diagnostic criteria for 1 or more of the 3 prodromal syndromes, rated on the Structured Interview for Prodromal Syndromes (SIPS)38; these criteria included attenuated psychotic symptoms, brief limited intermittent psychotic symptoms, and genetic risk (the schizotypal personality disorder or presence of a first-degree relative with a psychotic disorder) and deterioration in function. (3) The HC group: Subjects without a family history of psychotic disorder were recruited after matching with FES group for age (but not matched with the CHR group), gender, and education level (but not matched with the FES group), and were screened by both of the above assessment tools to demonstrate the absence of psychotic disorder. The exclusion criteria were: neurological disorders, history of alcohol or drug dependence, suicidal or violent episodes, or administration of modified electroconvulsive therapy in the preceding 6 months.
Subjects or their guardians provided written informed consent for study participation. This study was approved by the Ethics Committee of the Beijing Anding Hospital, Capital Medical University.
Clinical Assessments
Clinical Scales
Symptom severity in FES patients was assessed with the Positive and Negative Syndrome Scale (PANSS).39 We used the Scale of Prodromal Syndromes (SOPS) in the SIPS interview tool to assess symptom severity in CHR individuals.
Intelligence Quotient Test
The Chinese version of the simple Wechsler adult intelligence test (WAIS-RC), which includes information, similarities, drawing completion, and block design tests, was used to assess the intelligence quotient (IQ) of the subjects.40
Cognitive Function
We chose the MATRICS Consensus Cognitive Battery (MCCB) tool to measure individual neuropsychological state.41 The MATRICS initiative was intended to develop a consensus cognitive battery for clinical studies.42 The MCCB has been recommended by the United States Food and Drug Administration (FDA) for the assessment of cognitive impairment as the primary outcome measure in registry trials of schizophrenia.41 The MCCB includes 7 cognitive domains: Speed of Processing, Attention/Vigilance, Working Memory, Verbal Learning, Visual Learning, Reasoning and Problem Solving, and Social cognition. The MCCB takes 70–90 min to be completed. In the present study, the patient’s cognitive test score was converted to a T-score according to the Chinese cognitive norm.43
PPI Measures
Participants were seated in a chair in a soundproof room, with their eyes focused on a black spot at the center of a facing screen, and attempted to reduce the number of times they blinked. Participants were not allowed to smoke for at least 30 min before the test. During the duration of the block, participants were asked to focus on the prepulse sound heard from the left ear (or right ear) and count them; finally, they were asked to report the number of sounds heard. Participants may also hear a few louder sounds, which can be ignored.
Stimulus–Response Measurements
Hearing testing was used to examine participants’ pure-tone audiometry threshold (≤40 dB HL); the threshold difference should be ≤15 dB between the right and left ears. The Xeye Human Startle Reflex System was used to record the right orbicularis oculi electromyogram of subjects to capture startle stimulation by the ag-agcl electrode filled with a conductive paste. The recording and reference electrodes were located approximately 1 cm below the pupil of the right eye and outside the lateral canthus of the right eye, respectively. Each electrode had a resistance of less than 5 kΩ. The right posterior mastid electrode was grounded to eliminate the influence of 50-Hz current.
Prior to the formal experiment, we twice played only the sound with a startle stimulus. Subjects that showed no response to the startle stimulus were excluded. Immediately thereafter, in responsive subjects, the background noise and prepulse stimulus were played. Subjects were required to be familiar with the test sound, as well as to judge the direction of the leading ear of the background noise and prepulse stimulus. Subjects with a recorded accuracy rate of 80% continued to the next experiment.
Experimental Paradigm
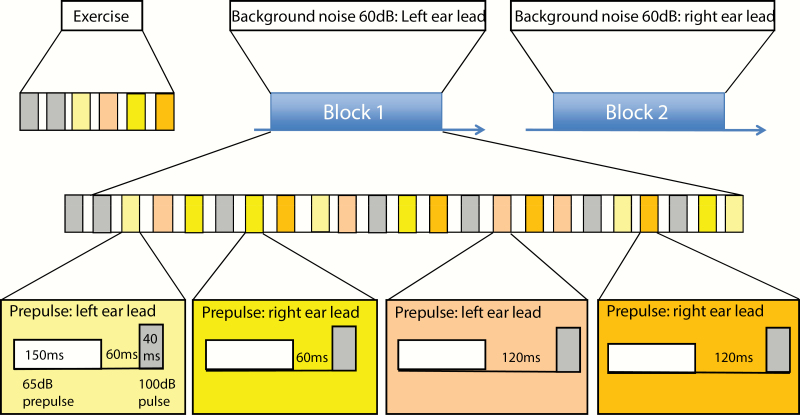
The binaural delay of background noise was set as the left or right ear leading. Participants perceive whether the background noise is emanating from the left or right direction. Based on the background noise, we randomly played pulse or prepulse + pulse. The leading ear direction of prepulse was randomly changed; 50% of it was the left ear lead, and 50% was the right ear lead. The subjects will feel that the prepulse stimulus is originating from the left or right direction. Therefore, 2 types of spatial relationships between prepulse stimulus and background noise are formed: the same direction (PSCPPI) or different directions (PSSPPI). When the binaural delay of background noise was set as the left/right ear leading, the pulse-alone trial based on background noise was abbreviated as LRA/RPA. The Prepulse + pulse trial with left or right ear leading on the basis of background noise was referred to as LNL/LNR or RNL/ RNR, respectively; with the inter-stimulus interval (ISI) of 60 and 120 ms, these were further divided into LNL/LNR60, LNL/LNR120, RNL/RNR 60, and RNL/RNR120.
The entire test consisted of 2 blocks (figure 1). The stimulus sequence of each block was a repeating combination of a series of sound stimuli. In block 1, the background noise was always left ear leading. We randomly conducted 27 trials, which included 7 LPA, 5 LNL60, 5 LNL120, 5 LNR60, and 5 LNR120. In block 2, the background noise was always with the right ear leading; there were 27 random trials, including 7 RPA, 5 RNR60, 5 RNR120, 5 RNL60, and 5 RNL120. The intertrial interval (ITI) between the stimuli (pulses offset and prepulse onset) was pseudorandomized. The average time between each trial was 15 s (ranging from 11 to 19 s). Therefore, each block included PSCPPI60 (5 trials, RNR60 or LNL60), PSSPPI60 (5 trials, RNL60 or LNR60), PSCPPI120 (5 trials, RNR120 or LNL120), and PSSPPI120 (5 trials, RNL120 or LNR120). The detailed description of PPI is provided in the supplementary materials.
Fig. 1.
Procedure used for the prepulse inhibition paradigm.
Data Processing
On detailed examination of each trial, we excluded the myoelectric response caused by automatic blink and then determined the mean amplitude of the sampling period and peak amplitude of each trial. Valid trials were those that qualified the following criteria: maximum peak amplitude ≥ amplitude mean of the sampling period × 4 or amplitude mean of the sampling period ≥ amplitude mean of the response period. PPI = (1 – pp/p) × 100, where p represents the induced amplitude only under the condition of pulse stimulus, pp denotes the amplitude induced by prepulse + pulse stimulation. Mean startle amplitude is the average of all P in 2 blocks. Habituation = (1 − average amplitude of startle reflex in block 2/average amplitude of startle reflex in block 1) × 100. The maximum peak latency was determined according to different experimental conditions and was in the range of 50–550 ms.
Statistical Analysis
Data were analyzed using the IBM SPSS Statistics 20.0 for Windows (SPSS, Inc). The normality of the study variables was examined using the Kolmogorov–Smirnov test. One-way ANOVA was used to analyze the general demographic and clinical data of the 3 groups; the chi-squared test was used to analyze categorical variables. The t-test was used to analyze SIPS data of the CHR and HC groups (table 1). Differences between the 3 groups with respect to PPI data were analyzed using ANCOVA with gender, age, and smoking history as covariates; nonparametric tests were used for data with nonnormal distribution and heterogeneity of variance. Hochberg adjustment for multiple comparisons was used to perform post hoc pairwise comparisons of between-group differences.44 The ES (Cohen’s d) was used to differentiate levels of PPI deficits. The ranges of 0.2–0.5, 0.5–0.8, and >0.8 indicate a small, medium, and great ES, respectively.45 The effect of the interaction between the grouping (schizophrenia, CHR, and HC) and condition (PSS and PSC) on the PPI level was analyzed using the general linear models (GLM). The area under the receiver operating characteristic (ROC) curve (AUC), sensitivity, and specificity were used to assess the discriminative ability of PSCPPI and PSSPPI. The maximum value of sensitivity + specificity − 1 and its corresponding point was used as the cutoff value. AUC scores between PSCPPI and PSSPPI were analyzed by MedCalc v.16.2 software for Windows (MedCalc). Two-tailed P values <.05 were considered indicative of statistical significance.
Table 1.
Demographics and clinical feature of participants ()
| FES (n = 53) |
CHR (n = 55) |
HC (n = 53) |
Total (n = 161) |
F/t/χ2 | P | |
|---|---|---|---|---|---|---|
| Age | 24.91 ± 7.07 | 21.36 ± 5.34 | 25.02 ± 3.62 | 23.73 ± 5.75 | 18.41 | <.001 |
| Education level (years) | 12.38 ± 3.42 | 13.02 ± 3.17 | 14.06 ± 3.17 | 13.15 ± 3.30 | 2.51 | .06 |
| IQ | 99.33 ± 12.69 | 108.72 ± 11.83 | 112.40 ± 12.67 | 107.28 ± 13.43 | 21.72 | <.001 |
| Age at onset | 22.55 ± 6.50 | NA | NA | NA | NA | NA |
| Duration of illness (months) | 25.51 ± 20.70 | NA | NA | NA | NA | NA |
| Medication | ||||||
| Unmedicated (n) | 7 (13.2%) | 24 (43.6%) | NA | 31 | NA | NA |
| Antipsychotics (n)a | 43 (81.1%) | 14 (25.5%) | NA | 57 | NA | NA |
| Antidepressants (n) | 0 | 8 (14.5%) | NA | 8 | NA | NA |
| Antidepressants + antipsychotics (n) | 1 (1.9%) | 6 (10.9%) | NA | 7 | NA | NA |
| Unspecified (n) | 2 (3.8%) | 2 (3.6%) | NA | 4 | NA | NA |
| TCM (n) | 0 | 1 (1.8%) | NA | 1 | NA | NA |
| SIPS—Positive | NA | 9.42 ± 4.01 | 0.40 ± 1.26 | 4.87 ± 5.40 | −9.00 | <.001 |
| SIPS—Negative | NA | 9.42 ± 5.49 | 0.21 ± 0.81 | 4.77 ± 6.04 | −9.03 | <.001 |
| SIPS—Disorganization | NA | 4.79 ± 3.34 | 0.15 ± 0.56 | 2.45 ± 3.32 | −8.47 | <.001 |
| SIPS—General | NA | 5.40 ± 3.65 | 0.13 ± 0.48 | 2.74 ± 3.70 | −8.51 | <.001 |
| SIPS—Total | NA | 29.04 ± 12.64 | 0.89 ± 2.77 | 14.83 ± 16.80 | −9.08 | <.001 |
| PANSS—Positive | 23.21 ± 5.67 | NA | NA | NA | NA | NA |
| PANSS—Negative | 20.87 ± 7.72 | NA | NA | NA | NA | NA |
| PANSS—General | 42.12 ± 6.60 | NA | NA | NA | NA | NA |
| PANSS—Total | 84.45 ± 14.59 | NA | NA | NA | NA | NA |
| Men, n (%) | 28 (52.8) | 32 (58.2) | 29 (54.7) | 89 (55.3) | 0.32 | .85 |
| Family history, n (%) | 10 (18.86) | 17 (30.9) | 0 (0) | 27 (16.77) | 18.68 | <.001 |
| Smoking, n (%) | 5 (9.4) | 6 (10.9) | 6 (11.3) | 17 (10.6) | 1.15 | .88 |
| Married, n (%) | 8 (15.1) | 4 (7.3) | 9 (17.0) | 21 (13.0) | 2.54 | .28 |
| Unemployed, n (%) | 19 (35.8) | 6 (10.9) | 3 (5.7) | 28 (17.4) | 19.25 | <.001 |
Note: FES: first-episode schizophrenia, CHR: clinical high-risk individuals, HC: healthy controls, IQ: intelligence quotient, TCM: traditional Chinese medicine, SIPS: Structured Interview for Prodromal Syndromes, PANSS: Positive and Negative Syndrome Scale, F: 1-way ANOVA, t: 2 independent sample t-test, χ2: chi-square test. The bold values represent statistical significance.
aThe subjects in this study were all taking atypical antipsychotics.
Results
Demographics and Clinical Characteristics
The age range of subjects in this study was 14–40 years; all subjects were right handed. Before the experiment, all participants were subjected to the startling stimulus twice. If the startle maximum peak amplitude induced by the startle reflex in each trial was less than 4 times the amplitude mean of the sampling period, the participant was excluded. Sixteen participants who did not respond to startling stimulus were excluded after the initial screening; these included 5 cases of FES, 3 cases of CHR, and 8 cases of HC. The final data analysis included 53 FES, 55 CHR, and 53 HC. One-way ANOVA revealed significant differences in age and IQ score between the 3 groups. There was a significant difference in the degree of symptoms of SIPS between the CHR and HC groups (P < .001). Results of the chi-squared test showed significant differences in the drug use of patients with FES and CHR, when analyzed by treatment with and without medication (χ 2 = 12.37, P < .001; table 1).
Neuroelectrophysiological Data
We only assessed 120 ms ISI for modified PPI paradigm at the beginning of the study. Subsequently, we added 60-ms ISI; therefore, the sample size of the PPI experimental data of 60-ms ISI was 19 cases less than that of 120 ms. ANCOVA showed no difference in the startle reflex (F2, 158 = 0.17, P = .84), latency (F2,158 = 0.22, P = .80), and habituation (F2,158 = 0.12, P = .89) among the 3 groups. The results of the PSSPPI60 condition (F2,138 = 17.18, P < .001) and the PSSPPI120 condition (Z2,160 = 23.43, P < .001) significantly differed between the 3 groups.
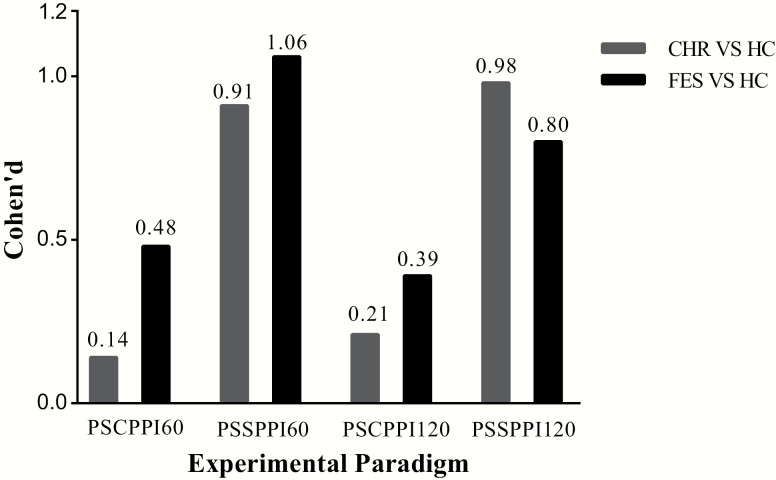
Results of pairwise comparison showed that FES (P < .001, Cohen’s d = 1.06) and CHR (P < .001, Cohen’s d = 0.91) had significantly lower PPI level in the detection of PSSPPI60. Furthermore, the PSSPPI120 condition’s ES was more than 0.8 when distinguishing between FES and HC (P < .001, Cohen’s d = 0.80) or between CHR and HC (P < .001, Cohen’s d = 0.98). All intergroup comparisons and ES values are shown in table 2 and figure 2.
Table 2.
Neuroelectrophysiological measurements among the 3 study groups (
| Variable | FES (n = 53) |
CHR (n = 55) |
HC (n = 53) |
Total (n = 161) |
F/Z | P | FES vs HC | CHR vs HC | FES vs CHR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F/Z | P | d [95% CI] | F/Z | P | d [95% CI] | F/Z | P | d [95% CI] | |||||||
| Habituation (%) | 8.80 ± 31.56 | 9.98 ± 32.98 | 11.97 ± 44.32 | 10.23 ± 36.38 | 0.12 | 0.89 | 0.53 | .47 | 0.08 [−0.30, 0.47] | 0.19 | .89 | 0.05 [−0.33, 0.43] | 0.41 | .52 | 0.04 [−0.34, 0.41] |
| Latency (ms) | 174.53 ± 65.98 | 156.83 ± 72.52 | 171.38 ± 67.01 | 167.45 ± 68.62 | 0.22 | 0.80 | 0.06 | .81 | 0.05 [−0.33, 0.43] | 0.16 | .69 | 0.21 [−0.17, 0.59] | 0.42 | .52 | 0.26 [−0.12, 0.63] |
| Startle (μV) | 67.91 ± 35.14 | 76.16 ± 36.37 | 69.92 ± 32.24 | 71.39 ± 34.61 | 0.17 | 0.84 | 0.10 | .76 | 0.06 [−0.32, 0.44] | 0.11 | .75 | 0.18 [−0.20, 0.56] | 0.25 | .62 | 0.23 [−0.15, 0.61] |
| ISI = 60 ms | n = 44 | n = 49 | n = 49 | n = 142 | |||||||||||
| PSCPPI (%) | 21.26 ± 23.52 | 29.34 ± 18.65 | 32.01 ± 20.90 | 27.75 ± 21.36 | 2.51 | 0.28 | 6.30 | .01 | 0.48 [0.07, 0.90] | 2.02 | .16 | 0.14 [−0.26, 0.53] | 2.52 | .12 | 0.38 [−0.03, 0.79] |
| PSSPPI (%) | 24.25 ± 20.20 | 27.07 ± 20.34 | 44.58 ± 18.03 | 32.27 ± 21.40 | 17.18 | <0.001 | 27.61 | <.001 | 1.06 [0.63, 1.50] | 23.52 | <.001 | 0.91 [0.49, 1.33] | 0.07 | .79 | 0.14 [−0.27, 0.55] |
| ISI = 120 ms | n = 53 | n = 55 | n = 53 | n = 161 | |||||||||||
| PSCPPI (%)a | 21.53 ± 27.20 | 26.78 ± 19.27 | 30.99 ± 20.87 | 26.43 ± 22.85 | 1.52 | 0.46 | −1.12 | .26 | 0.39 [0.01, 0.77] | −0.75 | .45 | 0.21 [−0.17, 0.59] | −0.72 | .47 | 0.23 [−0.16, 0.60] |
| PSSPPI (%)a | 22.88 ± 24.30 | 22.30 ± 17.85 | 40.10 ± 18.51 | 28.35 ± 21.89 | 23.43 | <0.001 | −3.38 | .001 | 0.80 [0.40, 1.19] | −4.71 | <.001 | 0.98 [0.57, 1.38] | −1.20 | .23 | 0.03 [−0.35, 0.41] |
Note: Habituation = (1 − average amplitude of startle reflex in block 2/average amplitude of startle reflex in block 1) × 100. The maximum peak latency was determined according to different experimental conditions and was in the range of 50–550 ms. The bold values represent statistical significance.
FES, first-episode schizophrenia; CHR, clinical high risk; HC, healthy controls; PPI, prepulse inhibition; PSCPPI, perceived spatial co-location PPI; PSSPPI, perceived spatial separation PPI; ISI, interstimulus interval; F, ANCOVA, Z, nonparametric tests; d, Cohen’s d; 95% CI, confidence interval for Cohen’s d.
aNonparametric tests.
Fig. 2.
Cohen’s d effect size of the prepulse inhibition paradigm between FES, CHR, and HC groups. FES, first-episode schizophrenia; CHR, clinical high-risk individuals; HC, healthy controls; PSCPPI60 or PSCPPI120: perceived spatial co-location prepulse inhibition, interstimulus interval 60 or 120 ms; PSSPPI60 or PSSPPI120: perceived spatial separation prepulse inhibition, interstimulus interval 60 or 120 ms.
Experimental Paradigms
GLM analysis (supplementary table S1) showed that the interaction between condition (PSSPPI and PSCPPI) and grouping (FES, CHR, and HC) had a significant influence on the PPI level (F = 3.718, P = .026). We found that the PSSPPI levels in the HC group were significantly increased, the PSSPPI levels in the FES group were mildly increased, and the PSSPPI levels in the CHR group were decreased. Due to the heterogeneity of variance of PPI data with the 120 ms ISI, we did not conduct further analysis of these data.
Sensitivity and Specificity
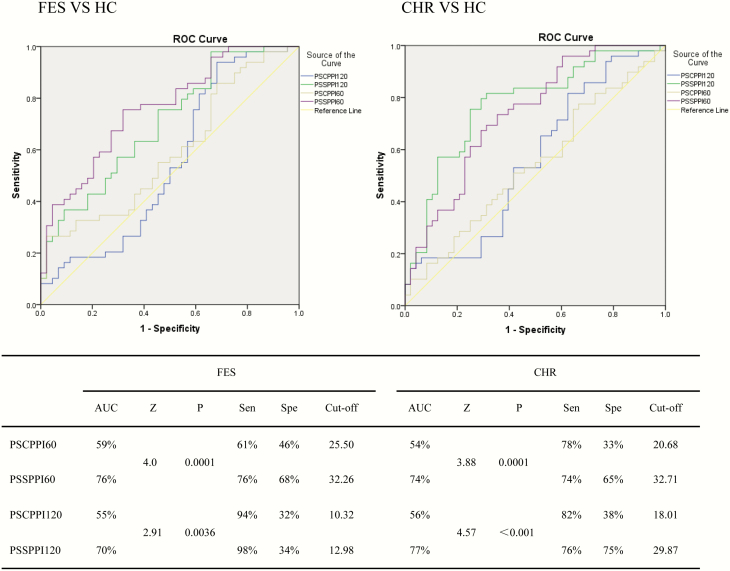
For the discrimination of FES and CHR, the area under the curve of PSSPPI and PSCPPI was greater than 50%. The ROC area under the PSSPPI curve was greater than that under the PSCPPI curve (figure 3). For the discrimination of FES, the PSSPPI60 index was better (sensitivity: 76%; specificity: 68%; optimal cutoff value: 32.26). For the discrimination of CHR, the PSSPPI120 index was better (sensitivity: 76%; specificity: 75%; cutoff value: 29.87). Statistical comparisons between AUC scores demonstrated that PSSPPI is better than PSCPPI (figure 3).
Fig. 3.
Receiver operating characteristic (ROC) curve of PSCPPI and PSSPPI for discrimination of FES and CHR. FES, first-episode schizophrenia; CHR, clinical high-risk individuals; HC, healthy controls; PSCPPI60 or PSCPPI120: perceived spatial co-location prepulse inhibition, interstimulus interval 60 or 120 ms. PSSPPI60 or PSSPPI120: perceived spatial separation prepulse inhibition, interstimulus interval 60 or 120 ms. AUC: area under the ROC curve. Sen: sensitivity. Spe: specificity. Z and P: AUC score comparison.
Discussion
The main finding of this study was that, similar to FES, CHR had significant PSSPPI deficits in comparison with HC when the ISI was 60 and 120 ms. The expected results from PSCPPI with 60- and 120-ms ISI showed no overall difference between the CHR and HC groups. In FES and CHR groups, the PSSPPI with spatial selective attention enhancement was impaired. Moreover, the PSSPPI deficits showed greater ES in the present study. The current modified PSSPPI condition that involves attention modulation, which is an endophenotype of schizophrenia, showed more sensitivity, robustness, and stability for schizophrenia and CHR. Thus, the PSSPPI appears to be a promising objective approach for the preliminary identification of CHR for psychosis.
In the modified PSSPPI condition, spatial selective attention can enable subjects to focus on the prestimulus as soon as possible; this facilitates the process of the prepulse signal, thereby enhancing PPI.46 However, the PSCPPI condition does not incorporate this enhancement. In previous studies, the ES varied from 0.5 to 0.8 in schizophrenia.47 The modified PSSPPI condition increased the ES in schizophrenia and CHR (ES > 0.8). The ROC curve analysis confirmed the discriminative ability (sensitivity and specificity) of the PSSPPI condition. The deficits of attentional modulation of PPI in schizophrenia are closely related to symptom severity.48 In primary analysis, we observed a similar association between positive symptoms and PSSPPI120; however, the result was not significant following Bonferroni correction (supplementary materials). Deficient attention modulation of PPI may be an endophenotype of schizophrenia and CHR.
In a previous study, PPI was found related to a selective attention task in Continuous Performance Test (CPT).49 However, other studies did not observe any correlation between PPI and CPT.50 In the current study, there was no association between PPI and attention domain (CPT-Identical Pairs) from MCCB (supplementary materials). The main reason for negative results may be that the attention measurement was not applied during the PPI test; in addition, the attention domain in cognitive assessment is different from spatial selective attention used in PSSPI. There is currently no experimental paradigm that integrates attention measurement into the startle reflex system.
A follow-up study showed that attention deficits can be detected in high-risk individuals who develop asymptomatic early-stage schizophrenia pedigree disorders later in life.51 In addition, another sample of treatment-seeking CHR subjects showed significant impairment in the domain of attention.52 Attention modulation of PPI was enhanced by PSS, top-down by the medial agranular cortex, which is a subdivision of the medial prefrontal cortex (PFC).53 Similar to schizophrenia, the progression of CHR is associated with structural changes in the brain, including in the PFC.54 Studies have also shown altered PFC function in CHR.55 CHR individuals showed gray matter volume alterations in the PFC with eventual transition to overt disease.56 Absence of attention modulation of PPI may play a critical role in the etiopathogenesis of CHR.
In the current study, PSSPPI with 60-ms ISI showed the largest ES in schizophrenia (1.06) and CHR (0.91), which is greater than that in previous studies.22 Compared with HC, the ES of PSSPPI60 deficits in FES and CHR gradually changed from great to small (figure 2). The PSSPPI at the 120-ms ISI did not show such a graded effect as that of the 60-ms ISI. The ISI between prepulse and startle stimuli has a certain degree of influence on the PPI. In the PPI paradigm, the ISI was 30, 60, 120, or 240 ms. The effect of ISI on PPI appears as an inverted U-shaped, and the PPI is maximum under 60- and 120-ms ISI conditions in both HC and patients with chronic schizophrenia.57 An ISI of 60 ms appears to be the cutoff time-point for prestimulus information to be processed either automatically or consciously. Theoretically, the time-point for the conversion between intentional information processing and unintentional information processing is important for the participant to regulate the contents of consciousness and may also be an epoch of particular vulnerability in psychopathological states.58–60 Even if patients with schizophrenia have a history of atypical antipsychotic treatment or smoking, with their PPI of 60-ms ISI, we find that their social function and quality of life are impaired; this indicates that sensorimotor gating of 60-ms ISI is a particularly important pathophysiological mechanism of schizophrenia.60 Investigation of the lead interval effects on PSSPPI requires more specially designed research.
Limitations
The present study had several limitations. First, the 3 groups were not completely matched with regard to age and IQ. However, we did adjust for these variables during analysis and assessed the effects of age on PPI. Nonetheless, a complete correction for the differences is not possible. Second, menstrual data pertaining to female subjects were not collected; however, the groups were matched for the number of female subjects. Third, the relatively small sample size could limit the statistical power of the analyses. Fourth, there is no common scale for assessing the severity of symptoms in FES and CHR individuals. PANSS was used in FES, while SOPS was used in CHR individuals. Consequently, any clinical comparison in terms of symptom severity between both groups is not possible. Fifth, the procedure of 2 pretests for the startle response affects the calculation of the startle habituation data. Thus, the present habituation data are not comparable with those of previous studies. Sixth, there is a lack of evidence of the specificity of the present paradigm. PPI deficits are not diagnostic but have been shown in several neuropsychiatric disorders. It is hard to establish a correlation between PPI deficits and specific psychiatric disorders, to differentiate between psychiatric disorders, or to identify a trait of the population. Both the sensitivity and specificity of PSSPPI was <80%. Seventh, note that HC might have followed these instructions “facing screen, and attempted to reduce the number of times they blinked” better than CHR or FES. This means that HC had a more effective inhibition of blinks based on these instructions and, hence, had better PPI compared to CHR and FES. Lastly, most patients were on monotherapy or in combinations of psychotropic drugs, which cannot be easily converted to equivalent dosages. Although medication type can affect PPI, and atypical antipsychotics have a normalizing effect, exclusion of all individuals who received psychotropic drugs/antipsychotics from the analyses did not change the results of the initial comparisons between FES/CHR and HC.
Conclusions
The modified PSSPPI showed PPI impairments between CHR and HC and was more sensitive. PPI deficits appear before the onset of full-blown psychosis. This modified PSSPPI condition involved attention modulation, which is also impaired in schizophrenia and CHR, and exhibited better application. This would serve as a biomarker or endophenotype for the identification of CHR individuals. Further multisite large-scale studies are required to compare and contrast PSSPPI deficits in a prospective CHR cohort to observe the progressive course and detect the risk of transition to schizophrenia.
Funding
This study was supported by the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201807), Beijing Hospitals Authority Youth Programme (QML20171901), Beijing Municipal Natural Science Foundation (7192081), and National Natural Science Foundation of China (81901355). No investigator benefited from participating in the study.
Conflict of interest: All authors report no biomedical financial interests or potential conflict of interest.
Supplementary Material
References
- 1. Graham FK. Presidential address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12(3):238–248. [DOI] [PubMed] [Google Scholar]
- 2. Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47(2):181–188. [DOI] [PubMed] [Google Scholar]
- 3. Light GA, Braff DL. Sensory gating deficits in schizophrenia: can we parse the effects of medication, nicotine use, and changes in clinical status? Clin Neurosci Res. 2003;3(1–2):47–54. [Google Scholar]
- 4. Treisman A. Feature binding, attention and object perception. Philos Trans R Soc Lond B Biol Sci. 1998;353(1373):1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fritz JB, Elhilali M, David SV, Shamma SA. Auditory attention—focusing the searchlight on sound. Curr Opin Neurobiol. 2007;17(4):437–455. [DOI] [PubMed] [Google Scholar]
- 6. Chen J, He Y, Zhu Z, et al. Attention-dependent early cortical suppression contributes to crowding. J Neurosci. 2014;34(32):10465–10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blumenthal TD, Reynolds JZ, Spence TE. Support for the interruption and protection hypotheses of prepulse inhibition of startle: evidence from a modified Attention Network Test. Psychophysiology. 2015;52(3):397–406. [DOI] [PubMed] [Google Scholar]
- 8. Light GA, Swerdlow NR. Future clinical uses of neurophysiological biomarkers to predict and monitor treatment response for schizophrenia. Ann NY Acad Sci. 2015;1344:105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sánchez-Morla EM, Mateo J, Aparicio A, García-Jiménez MÁ, Jiménez E, Santos JL. Prepulse inhibition in euthymic bipolar disorder patients in comparison with control subjects. Acta Psychiatr Scand. 2016;134(4):350–359. [DOI] [PubMed] [Google Scholar]
- 10. Bo Q, Mao Z, Tian Q, et al. Deficits of perceived spatial separation-induced prepulse inhibition in patients with bipolar disorder compared to healthy controls. J Affect Disord. 2018;240:63–71. [DOI] [PubMed] [Google Scholar]
- 11. Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol Psychiatry. 1993;33(4):298–301. [DOI] [PubMed] [Google Scholar]
- 12. Cheng CH, Chan PS, Hsu SC, Liu CY. Meta-analysis of sensorimotor gating in patients with autism spectrum disorders. Psychiatry Res. 2018;262:413–419. [DOI] [PubMed] [Google Scholar]
- 13. Yang NB, Tian Q, Fan Y, et al. Deficits of perceived spatial separation induced prepulse inhibition in patients with schizophrenia: relationships to symptoms and neurocognition. BMC Psychiatry. 2017;17(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moriwaki M, Kishi T, Takahashi H, et al. Prepulse inhibition of the startle response with chronic schizophrenia: a replication study. Neurosci Res. 2009;65(3):259–262. [DOI] [PubMed] [Google Scholar]
- 15. Ringel TM, Heidrich A, Jacob CP, Pfuhlmann B, Stoeber G, Fallgatter AJ. Sensory gating deficit in a subtype of chronic schizophrenic patients. Psychiatry Res. 2004;125(3):237–245. [DOI] [PubMed] [Google Scholar]
- 16. Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry. 2003;54(2):121–128. [DOI] [PubMed] [Google Scholar]
- 17. Parwani A, Duncan EJ, Bartlett E, et al. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry. 2000;47(7):662–669. [DOI] [PubMed] [Google Scholar]
- 18. Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15(4):339–343. [DOI] [PubMed] [Google Scholar]
- 19. Kumari V, Das M, Zachariah E, Ettinger U, Sharma T. Reduced prepulse inhibition in unaffected siblings of schizophrenia patients. Psychophysiology. 2005;42(5):588–594. [DOI] [PubMed] [Google Scholar]
- 20. Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am J Psychiatry. 2000;157(10):1660–1668. [DOI] [PubMed] [Google Scholar]
- 21. Braff DL, Swerdlow NR, Geyer MA. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. Am J Psychiatry. 1999;156(4):596–602. [DOI] [PubMed] [Google Scholar]
- 22. Swerdlow NR, Light GA, Thomas ML, et al. Deficient prepulse inhibition in schizophrenia in a multi-site cohort: internal replication and extension. Schizophr Res. 2018;198:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quednow BB, Frommann I, Berning J, Kühn KU, Maier W, Wagner M. Impaired sensorimotor gating of the acoustic startle response in the prodrome of schizophrenia. Biol Psychiatry. 2008;64(9):766–773. [DOI] [PubMed] [Google Scholar]
- 24. Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70(1):107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Insel TR. Rethinking schizophrenia. Nature. 2010;468(7321): 187–193. [DOI] [PubMed] [Google Scholar]
- 26. Ziermans TB, Schothorst PF, Sprong M, Magnée MJ, van Engeland H, Kemner C. Reduced prepulse inhibition as an early vulnerability marker of the psychosis prodrome in adolescence. Schizophr Res. 2012;134(1):10–15. [DOI] [PubMed] [Google Scholar]
- 27. De Koning MB, Bloemen OJ, Van Duin ED, et al. Pre-pulse inhibition and striatal dopamine in subjects at an ultra-high risk for psychosis. J Psychopharmacol. 2014;28(6):553–560. [DOI] [PubMed] [Google Scholar]
- 28. Togay B, Çıkrıkçılı U, Bayraktaroglu Z, Uslu A, Noyan H, Üçok A. Lower prepulse inhibition in clinical high-risk groups but not in familial risk groups for psychosis compared with healthy controls. Early Interv Psychiatry. 2020;14(2):196–202. [DOI] [PubMed] [Google Scholar]
- 29. Ziermans T, Schothorst P, Magnée M, van Engeland H, Kemner C. Reduced prepulse inhibition in adolescents at risk for psychosis: a 2-year follow-up study. J Psychiatry Neurosci. 2011;36(2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cadenhead KS. Startle reactivity and prepulse inhibition in prodromal and early psychosis: effects of age, antipsychotics, tobacco and cannabis in a vulnerable population. Psychiatry Res. 2011;188(2):208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Winton-Brown T, Kumari V, Windler F, et al. Sensorimotor gating, cannabis use and the risk of psychosis. Schizophr Res. 2015;164(1–3):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yeomans JS, Li L, Scott BW, Frankland PW. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci Biobehav Rev. 2002;26(1):1–11. [DOI] [PubMed] [Google Scholar]
- 33. Peters BD, Schmitz N, Dingemans PM, et al. Preliminary evidence for reduced frontal white matter integrity in subjects at ultra-high-risk for psychosis. Schizophr Res. 2009;111(1–3):192–193. [DOI] [PubMed] [Google Scholar]
- 34. Dawson ME, Hazlett EA, Filion DL, Nuechterlein KH, Schell AM. Attention and schizophrenia: impaired modulation of the startle reflex. J Abnorm Psychol. 1993;102(4):633–641. [DOI] [PubMed] [Google Scholar]
- 35. Lei M, Luo L, Qu T, Jia H, Li L. Perceived location specificity in perceptual separation-induced but not fear conditioning-induced enhancement of prepulse inhibition in rats. Behav Brain Res. 2014;269:87–94. [DOI] [PubMed] [Google Scholar]
- 36. Spitzer RL. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version, User’s Guide. Hoboken, NJ: John Wiley & Sons, Inc.; 1996. [Google Scholar]
- 37. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995–1003. [DOI] [PubMed] [Google Scholar]
- 38. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. [DOI] [PubMed] [Google Scholar]
- 39. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 40. Pang YX, Zhang J, Yang CL, Cang Y, Wang XL. [Application of WAIS-RC short forms and adult intelligence disability scale in mental impairment assessment]. Fa Yi Xue Za Zhi. 2011;27(3):189–192. [PubMed] [Google Scholar]
- 41. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. [DOI] [PubMed] [Google Scholar]
- 42. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1–3. [DOI] [PubMed] [Google Scholar]
- 43. Shi C, Kang L, Yao S, et al. The MATRICS Consensus Cognitive Battery (MCCB): co-norming and standardization in China. Schizophr Res. 2015;169(1–3):109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–802. [Google Scholar]
- 45. Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. [DOI] [PubMed] [Google Scholar]
- 46. Li L, Du Y, Li N, Wu X, Wu Y. Top-down modulation of prepulse inhibition of the startle reflex in humans and rats. Neurosci Biobehav Rev. 2009;33(8):1157–1167. [DOI] [PubMed] [Google Scholar]
- 47. Light GA, Swerdlow NR, Rissling AJ, et al. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PLoS One. 2012;7(7):e39434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hazlett EA, Romero MJ, Haznedar MM, et al. Deficient attentional modulation of startle eyeblink is associated with symptom severity in the schizophrenia spectrum. Schizophr Res. 2007;93(1-3):288–295. [DOI] [PubMed] [Google Scholar]
- 49. Hazlett EA, Dawson ME, Schell AM, Nuechterlein KH. Attentional stages of information processing during a continuous performance test: a startle modification analysis. Psychophysiology. 2001;38(4):669–677. [PubMed] [Google Scholar]
- 50. Morales-Muñoz I, Jurado-Barba R, Fernández-Guinea S, et al. Sensory gating deficits in first-episode psychosis: evidence from neurophysiology, psychophysiology, and neuropsychology. J Nerv Ment Dis. 2016;204(12):877–884. [DOI] [PubMed] [Google Scholar]
- 51. Smith CW, Cornblatt B. Attention deficits in the development of schizophrenia: recent evidence from genetic high-risk and prodromal studies. Curr Psychosis Ther Rep. 2005;3:152–156. [Google Scholar]
- 52. Carrión RE, Goldberg TE, McLaughlin D, Auther AM, Correll CU, Cornblatt BA. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. Am J Psychiatry. 2011;168(8):806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meng Q, Ding Y, Chen L, Li L. The medial agranular cortex mediates attentional enhancement of prepulse inhibition of the startle reflex. Behav Brain Res. 2020;383:112511. [DOI] [PubMed] [Google Scholar]
- 54. de Wit S, Wierenga LM, Oranje B, et al. Brain development in adolescents at ultra-high risk for psychosis: longitudinal changes related to resilience. Neuroimage Clin. 2016;12:542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fusar-Poli P, Howes OD, Allen P, et al. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch Gen Psychiatry. 2010;67(7):683–691. [DOI] [PubMed] [Google Scholar]
- 56. Koutsouleris N, Riecher-Rössler A, Meisenzahl EM, et al. Detecting the psychosis prodrome across high-risk populations using neuroanatomical biomarkers. Schizophr Bull. 2015;41(2):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl). 2001;156(2-3):234–258. [DOI] [PubMed] [Google Scholar]
- 58. Grobstein P. Making the unconscious conscious, and vice versa: a bi-directional bridge between neuroscience/cognitive science and psychotherapy? Cortex. 2005;41(5):663–668. [DOI] [PubMed] [Google Scholar]
- 59. Kanabus M, Szelag E, Rojek E, Pöppel E. Temporal order judgement for auditory and visual stimuli. Acta Neurobiol Exp (Wars). 2002;62(4):263–270. [DOI] [PubMed] [Google Scholar]
- 60. Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63(12):1325–1335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.