Abstract
Background
The aim of this study was to develop a standardized method to reconstruct persons’ individual viral load (VL) courses to determine viral suppression and duration of viremia for the HIV care continuum in Germany using longitudinal cohort data.
Methods
We analyzed data from two large, multi-center German cohort studies under the direction of the Robert Koch Institute. We included data from 1999 to 2018 of all diagnosed people and of people who initiated antiretroviral treatment (ART). We developed a model generating virtual VL values and an individual VL course corresponding to real VL measurements with a maximum distance of 180 days, considering ART status and VL dynamics. If the distance between VL measurements was > 180 days, the time between was defined as gap time. Additionally, we considered blips, which we defined as a single detectable VL < 1000 copies/ml within 180 days.
Results
A total of 22,120 people (164,691 person-years, PY) after ART initiation were included in the analyses. The proportion of people with viral suppression (VL < 50 copies/ml) increased from 34% in 1999 to 93% in 2018. The proportion of people with VL < 200 copies/ml increased from 47% in 1999 to 96% in 2018. The proportion of people with viremia > 1000 copies/ml decreased from 37% in 1999 to 3% in 2018. The proportion of people with gap time fluctuated and ranged between 18 and 28%. An analysis of the first VL after gap time showed that 90% showed viral suppression, 5% VL between 50- < 1000 copies/ml and 5% VL > 1000 copies/ml.
Conclusion
We provide a method for estimating viral suppression and duration of viremia using longitudinal VL data. We observed a continuous and remarkable increase of viral suppression. Furthermore, a notable proportion of those with viremia showed low-level viremia and were therefore unlikely to transmit HIV. Individual health risks and HIV drug resistance among those with low-level viremia are problematic, and viral suppression remains the goal. In 2018, 93 and 96% of people after ART initiation showed VL < 50 copies/ml and VL < 200 copies/ml, respectively. Therefore, using the threshold of VL < 200 copies/ml, Germany reached the UNAIDS 95 target of viral suppression since 2017.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-020-10088-7.
Keywords: Viral suppression, HIV care continuum, Treatment success, HIV cascade
Background
The Joint United Nations Programme on HIV/AIDS (UNAIDS) targets to accelerate the fight against HIV and to end the AIDS epidemic by 2030 aim to increase the proportion of people living with HIV (PLHIV) knowing their diagnosis, of people with diagnosed HIV infection receiving antiretroviral treatment (ART) and of people receiving ART being virally suppressed to 90% by 2020 and to 95% by 2030 [1–3].
It is estimated that, in 2018, 37.9 million people were living with HIV worldwide, and 23.3 million people were accessing ART. Globally, in 2018, 79% of PLHIV knew their status. Among people who knew their HIV status, 78% were accessing treatment. Among those people, 86% were virally suppressed. This statistic is a considerable increase in recent years compared to 2010, when only 24% of all people living with HIV were accessing treatment. New HIV infections have declined by approximately 16% since 2010 to 1.7 million new infections in 2018. Since 2010, the number of people who have died from AIDS-related illnesses worldwide has decreased by 33% to 770,000 in 2018 [4]. However, there are large differences across regions and countries regarding the HIV care continuum, with less than 50% of all people living with HIV accessing ART in Eastern Europe, central Asia, the Middle East and North Africa, and new HIV infections and AIDS-related deaths are rising in these regions [4, 5]. Additionally, for the first time since 2000, less funding was available for AIDS response in low- and middle-income countries -- almost US$ 1 billion less than in 2017 [4]. In addition, challenges with data quality, appropriate data sources and the absence of standardized definitions could hamper comparisons across countries [6].
In Germany, an increasing number of PLHIV are receiving ART [7, 8], and it is estimated that, at the end of 2018, of all PLHIV in Germany, 88% were diagnosed, and 93% of diagnosed were under ART [8]. The Robert Koch Institute (RKI) reports numbers for the German HIV care continuum to national and international stakeholders using the different available data sources. However, there is no national database containing follow-up clinical or treatment data on PLHIV. HIV surveillance, in addition to reports on diagnosed HIV/AIDS cases, requires additional surveillance tools, which are implemented with longitudinal clinical cohort studies at the RKI [9, 10]. In a former study, the RKI working group developed a method to determine the number of PLHIV receiving ART in Germany using ART prescription data and national clinical cohort data from the Clinical Surveillance of HIV Disease (ClinSurv HIV) [7]. This method, which was selected for a compendium of good HIV practices in the WHO European Region [11], has been continuously used for the second stage of the German HIV care continuum in the annual national HIV estimates of the RKI [8]. However, consistent methods for all stages of a standardized HIV continuum of care for Germany, especially for the numbers and proportions of people and their person-time with viral suppression, have not yet been published.
The main goal of ART is sustained viral suppression, which subsequently leads to several benefits. These benefits include immune recovery and decreased immune activation [12], prevention of HIV-related morbidity and mortality [13–15], reduction in non-AIDS diseases, such as cancer or cardiovascular disease [16], prevention of HIV transmission [17, 18] and avoiding the development of HIV drug resistance [19]. In Germany, effective ART evidenced by viral suppression is required by reimbursement regulations for health insurance. Hence, it is usually monitored every three months by viral load (VL) testing. Longer periods without VL controls are critical because, in cases of viral failure, immediate action would be required to avoid evolution of viral resistance or clinical progression of HIV disease.
Viral suppression is commonly defined as a VL test result below the detection limit or a certain threshold at the most recent VL test in one year [20–22]. However, such an approach does not address the dynamics of VL progression over time and could lead to biased results when the last VL is not representative of the respective year. We therefore aimed to present an alternative approach using longitudinal data, including all available VL measurements and persons’ individual ART histories.
With this study, we aimed to:
develop a model to determine the durations and proportions of viral suppression and viremia among PLHIV to be used for the HIV care continuum;
determine the numbers and proportions of PLHIV and of person-time with viral suppression and viremia between 1999 and 2018 using national clinical cohort data;
compare the results of the conventional method with those of our longitudinal model; and
evaluate the UNAIDS target of viral suppression for PLHIV in Germany.
Methods
HIV surveillance in Germany
National HIV/AIDS surveillance in Germany is regulated by the national Protection Against Infection Act and is based on mandatory reports of newly diagnosed cases of HIV infection and voluntary reporting of AIDS cases to the RKI, which is the federal institute of public health under the umbrella of the German Ministry of Health [23]. In addition, continuous monitoring of the course of HIV infection, including HIV treatment, is performed in HIV cohort studies at the RKI.
Study population and data
We analyzed data from two large German cohort studies, the Clinical Surveillance of HIV Disease (ClinSurv HIV) and the HIV-1 Seroconverter cohort; both studies are under the direction of the RKI. For this analysis, cohort data between 1999 and 2018 of people with at least two VL measurements were included.
The ClinSurv HIV cohort is the base for a nationwide, prospective, multi-center, open, long-term observational cohort study for the clinical surveillance of HIV in Germany. Data on demographics, detailed information on the initiation, composition and discontinuation of individuals’ daily ART, laboratory parameters and clinical events are collected biannually in a standardized format. ClinSurv HIV is the largest available nationwide source of PLHIV in Germany. The study design is described in detail elsewhere [9].
The HIV-1 Seroconverter cohort is the basis for a nationwide, multi-center, open, long-term observational cohort study of HIV-1-positive people with a known or reliably estimated date of HIV-1 seroconversion. Sociodemographic and clinical data from each participant were collected at the time of enrollment and at yearly follow-ups. Detailed descriptions of the study methods can be found elsewhere [10, 24, 25].
Developing a model and method to determine viral suppression and viremia
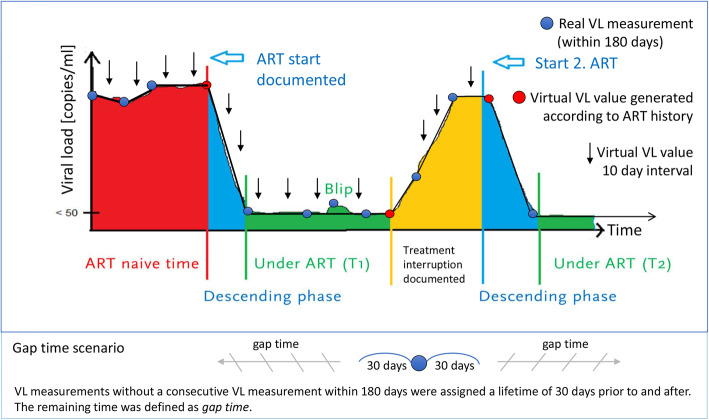
We developed a model to reconstruct the individual VL course of people to estimate the duration and proportion of viral suppression and viremia using longitudinal data, including all available VL measurements, taking into account ART status and VL dynamics. In this model, we looked for real VL measurements with a maximum distance of 180 days. We then connected the measurements linearly and generated virtual VL values for every 10-day interval along the line. The 10-day interval was chosen because it offers sufficient accuracy with manageable data volumes. Additionally, we took into account the ART status of the people when we connected the real VL measurements. For example, if a person was coming from an ART naïve time into therapy, we did not connect the VL measurements linearly, assuming that the VL decrease started with ART initiation; therefore, a virtual VL value was generated according to the ART status, and a horizontal line was drawn from the higher VL measurement to the ART start, and then the line decreased to the lower VL measurement. Similarly, if a person’s viral load increased and an interruption were documented in the ART history, we assumed that the increase did not necessarily occur from the previous VL measurement but rather stemmed from the ART interruption (see Fig. 1). VL measurements without a consecutive VL measurement within 180 days were assigned a lifetime of 30 days prior to and after the VL measurement. The remaining time not covered by our model was defined as a longer period without VL control or so-called gap time. Additionally, we considered blips, which we defined as a single detectable VL < 1000 copies/ml within 180 days with a subsequent undetectable VL.
Fig. 1.
Model to determine viral suppression and viremia using longitudinal data on VL and ART history
VL was a priori stratified into the following groups: VL < 50 copies/ml, VL 50- < 200 copies/ml, VL 200- < 500 copies/ml, VL 500- < 1000 copies/ml, VL 1000- < 10,000 copies/ml, VL 10,000- < 100,000 copies/ml, VL 100,000- < 1000,000 copies/ml, and VL ≥1000,000 copies/ml.
We analyzed the proportion of person-time with viral suppression and viremia over time in the total study population, indicating that, at different points in time, different people can have the same proportion of person-time and viral suppression. We also report the number and proportion of people with viral suppression and viremia, the interquartile range (IQR) and median person-time with viral suppression and viremia, and the IQR and median proportion of viral suppression and viremia to the observation time on an individual level.
Furthermore, we analyzed the time with viral suppression and viremia on an individual level, such as the proportion of people with continuous viral suppression over a period of time.
Viral suppression and viremia from 1999 to 2018
People observed between 1999 and 2018 with at least two VLs were included. We determined viral suppression and viremia over time: (i) among all PLHIV in the cohort studies regardless of their ART status, including ART-naïve and treated person-time; and (ii) among PLHIV after ART initiation regardless of whether they were continuously under ART, including person-time with documented interruptions or gaps in treatment. Viral suppression was defined as VL < 50 copies/ml according to the German-Austrian ART guidelines [26]. When evaluating the UNAIDS target of viral suppression for PLHIV, we also report the proportion of person-time with VL < 200 copies/ml for comparability. This threshold of < 200 copies/ml for population-level monitoring is consistent with the guidelines and recommendations of several institutions, such as the US Centers for Disease Control and Prevention and the European Centre for Disease Prevention and Control [20, 27].
We determined viral suppression and viremia over time among PLHIV after ART initiation with the conventional method, using the most recent VL in each year, and we compared the results with those of our longitudinal model.
To further investigate potential misclassification using the conventional method with a single VL during one year, we determined the number and proportion of PLHIV with continuous viral suppression over a yearlong observation period on an individual level and compared it with the results of the conventional method using the most recent VL in one year.
The results on the proportion of people and person-time with viral suppression and viremia in the respective year are reported excluding gap time. The proportion of people with gap time is reported separately. Furthermore, we performed separate sensitivity analyses in the group of people with gap time to assess their VL status.
Analysis of people with longer periods without VL control (gap time)
We report the proportion of person-time with gap time on the total observation time in the study population, the number and proportion of people with gap time, the IQR and median gap time, and the IQR and median proportion of gap time to the observation time on an individual level.
In people with gap time, the last VL measurement before and the first VL measurement after having gap time were analyzed to approximate the VL status of the people during gap time. This approach is in accordance with methods used in other studies [28, 29]. The last and first VL before and after gap time were analyzed for the recent study period from 2015 to 2018. We determined the overall proportion of people with viral suppression at the last and first VL before and after gap time. Furthermore, the congruence of the last and first VL on an individual level was determined. For the analysis of the congruence between the last and first VL before and after gap time, the proportions of people with viral suppression at both VL measurements, both VL measurements 50- < 1000 copies/ml, both VL measurements > 1000 copies/ml, the proportion of people with a VL increase (last VL < first VL) and the proportion of people with a VL decrease (last VL > first VL) were determined. Among those with detectable VL, we also report the IQR and median VL in each group.
To approximate the impact of gap time on the overall viral suppression in people who initiated ART, we calculated the resulting proportion of viral suppression after considering for viremic gap time. Therefore, we determined the proportion of viremia at the first VL measurement after gap time, determined the proportion of gap time among all people who initiated ART, then determined the resulting proportion of viremic gap time and viral suppression among all people who initiated ART.
Analysis of antiretroviral treatment regimens over time
The ART regimens were separated into mainly used regimens according to the German-Austrian ART guidelines [26], consisting of nucleoside reverse transcriptase inhibitors (NRTIs) with either a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI), or an integrase strand transfer inhibitor (INSTI). Further ART was classified into triple-class regimens, NRTI only, NRTI-sparing regimens, attachment inhibitor (AI) containing, salvage regimens (3 drug classes and AI or fusion inhibitor (FI) or 4 drug classes), study medication, ART interruption, not fully active ART and ART gap.
We created our analytic sample in SQL Server Management Studio software, version 17.4 (Microsoft Corporation Redmond, WA, USA), and conducted the statistical analysis in Stata software, version 15.1 (StataCorp., College Station, TX, USA). Figures were created using Microsoft Excel and Microsoft Power Point 2019.
Results
Study population
A total of 24,569 people with at least two documented VLs from the ClinSurv HIV and the HIV-1 Seroconverter cohorts were enrolled and followed for a median of 5.9 years (IQR 2.4–11), totaling 171,990 person-years (PY). The total number of real VL measurements was 570,753, the median number of VL measurements per person was 18 (IQR 7–35), and VL monitoring occurred at a median frequency of every 91 days (IQR 64–112). With the model, 4,541,141 virtual VL values were generated. The real VL measurement and the virtual VL value occurred on the same date in 69,297 cases.
The majority of people, 88.4% (N = 21,716), were enrolled in the ClinSurv HIV cohort, 9.2% (N = 2264) were enrolled in the HIV-1 Seroconverter cohort, and 2.4% (N = 589) were enrolled in both cohort studies. Of the 24,569 people, 22,120 initiated ART, and 2449 were ART naïve at the end of observation. The characteristics of the study population are summarized in Table 1.
Table 1.
Characteristics of the study population (1999-2018)
| Patients | Study population (all diagnosed PLHIV) |
People who initiated ART | |||
|---|---|---|---|---|---|
| 24,569 | (100%) | 22,120 | (100%) | ||
| Observation time | Total PY | 171,990 | 164,691 | ||
| Sex | Male | 19,794 | (81%) | 17,794 | (80%) |
| Female | 4775 | (19%) | 4326 | (20%) | |
| HIV transmission risk | Men who have sex with men (MSM) | 13,006 | (53%) | 11,676 | (53%) |
| Heterosexual contacts | 3227 | (13%) | 2954 | (13%) | |
| High prevalence country | 3200 | (13%) | 2958 | (13%) | |
| People with injecting drug use | 1612 | (7%) | 1411 | (6%) | |
| Other | 212 | (1%) | 203 | (1%) | |
| Unknown | 3312 | (13%) | 2918 | (13%) | |
| Region of origin | Germany | 16,683 | (68%) | 15,065 | (68%) |
| Eastern Europe | 682 | (3%) | 592 | (3%) | |
| Central Europe | 1129 | (5%) | 1021 | (5%) | |
|
Western Europe (excl. Germany) |
949 | (4%) | 834 | (4%) | |
| Africa | 2998 | (12%) | 2749 | (12%) | |
| Asia | 686 | (3%) | 649 | (3%) | |
| America | 546 | (2%) | 491 | (2%) | |
| Caribbean/Ozeania | 107 | (0%) | 96 | (0%) | |
| Unknown | 789 | (3%) | 623 | (3%) | |
| Age at Enrolment (years) | Median (IQR) | 37 | (30-45) | 37 | (31-45) |
| Enrollment | 1999–2001 | 3422 | (14%) | 3098 | (14%) |
| 2002–2005 | 5454 | (22%) | 4834 | (22%) | |
| 2006–2009 | 5529 | (23%) | 4925 | (22%) | |
| 2010–2013 | 5343 | (22%) | 4819 | (22%) | |
| 2014–2018 | 4821 | (20%) | 4444 | (20%) | |
| Observation time (years) | Median (IQR) | 5.9 | (2.4-11) | 6.5 | (2.8-11.5) |
| Number of viral loads | Median (IQR) | 18 | (7-35) | 17 | (8-32) |
| Distance between viral loads (days) | Median (IQR) | 91 | (64–112) | 91 | (70–112) |
|
Viral load baseline (copies/ml) |
Median (IQR) | 49,973 | (9350–198,000) | 55,544 | (10,899–211,000) |
|
CD4 cell count baseline (cells/μl) |
Median (IQR) | 349 | (174–537) | 328 | (157–513) |
| Initiated ART | N (%) | 22,120 | (90%) | ||
| ART start period | 1999–2001 | 2416 | (11%) | ||
| 2002–2005 | 3948 | (18%) | |||
| 2006–2009 | 4833 | (22%) | |||
| 2010–2013 | 5636 | (25%) | |||
| 2014–2018 | 5287 | (24%) | |||
| not started ART | 2449 | (11%) | |||
| Age at ART start (years) | Median (IQR) | 39 | (32-46) | ||
| Time between enrollment and ART (days) | Mean (IQR) | 321 | (0–237) | ||
|
Viral load ART start (copies/ml) |
Median (IQR) | 62,000 | (12,300-212,604) | ||
|
CD4 cell count ART start (cells/μl) |
Median (IQR) | 271 | (133–429) | ||
| ART duration (years) | Median (IQR) | 5.5 | (2.3-9.9) | ||
On an individual level, a total of 88% (21,584/24,569) achieved viral suppression at any time, and 12% (2985/24,569) never achieved viral suppression. Of all subjects, 89% (21,967/24,569) showed viremia at any time, and 82% (20,249/24,569) showed viremia with VL > 1000 copies/ml. The total median observation time was 2180 days (interquartile range (IQR) 860–4020). The median person-time with viral suppression among all people was 930 days (IQR 190–2140). The resulting individual proportion of person-time with viral suppression to the observation time had a median of 52% (IQR: 18–77). Excluding gap time, the proportion of person-time with viral suppression to the observation time had a median of 75% (IQR: 37–92). The median person-time with viremia with VL > 1000 copies/ml was 120 days (IQR 40–420). The individual proportion of person-time with viremia with VL ≥1000 copies/ml to the observation time had a median of 8% (IQR: 1.8–24). Excluding gap time, the proportion of viremia with VL ≥1000 copies/ml to the observation time had a median of 12% (IQR: 2.3–40).
Viral suppression and viremia from 1999 to 2018
Among all diagnosed PLHIV
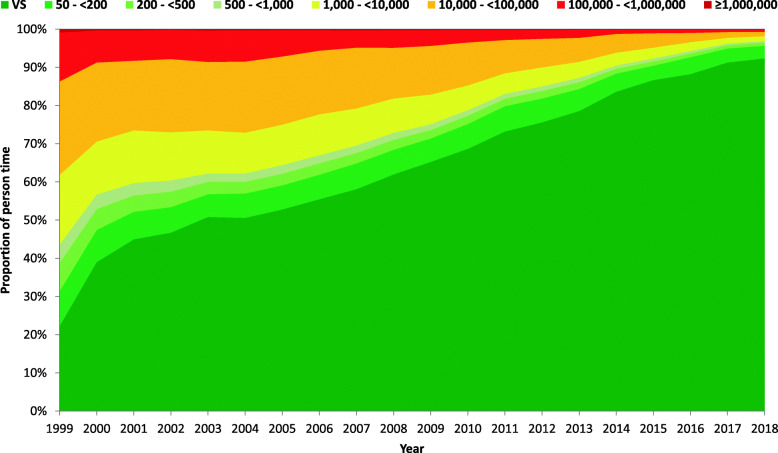
Based on the longitudinal model, the proportion of person-time with viral suppression (VL < 50 copies/ml) of the 24,569 people increased over time from 22.2% in 1999 to 92.3% in 2018. The proportion of person-time with VL < 200 copies/ml increased from 31.3% in 1999 to 95.6% in 2018. VLs of 50- < 200 copies/ml, 200- < 500 copies/ml and 500- < 1000 copies/ml were observed in 9.1, 7.4 and 4.8% of the people in 1999, respectively, and in 3.3, 0.8 and 0.4% of the people in 2018, respectively. The proportion of people with viremia > 1000 copies/ml therefore decreased from 56.4% in 1999 to 3.1% in 2018 (see Fig. 2 and Table 2 for detailed results).
Fig. 2.
Viral load levels of all diagnosed PLHIV in the study population from 1999 to 2018
Table 2.
Development of person-time with viral suppression and viremia among all diagnosed PLHIV in the cohorts, including ART-naïve and treated person-time between 1999 and 2018 based on our longitudinal model
| All diagnosed people living with HIV | ||||||||
|---|---|---|---|---|---|---|---|---|
| Year | Viral suppression (< 50) | 50 - < 200 | 200 - < 500 | 500 - < 1000 | 1000 - < 10,000 | 10,000 - < 100,000 | 100,000 - < 1.000000 | > 1000,000 |
| 1999 | 22.2 | 9.1 | 7.4 | 4.8 | 18.2 | 24.5 | 12.8 | 0.8 |
| 2000 | 39.0 | 8.4 | 5.5 | 3.8 | 13.8 | 20.7 | 8.4 | 0.3 |
| 2001 | 45.0 | 7.2 | 4.3 | 3.2 | 13.8 | 18.2 | 7.9 | 0.3 |
| 2002 | 46.7 | 6.7 | 4.1 | 2.9 | 12.6 | 19.2 | 7.6 | 0.2 |
| 2003 | 50.8 | 6.0 | 3.2 | 2.2 | 11.3 | 17.9 | 8.2 | 0.4 |
| 2004 | 50.6 | 6.4 | 3.1 | 2.2 | 10.7 | 18.7 | 8.1 | 0.3 |
| 2005 | 52.8 | 6.3 | 3.0 | 2.3 | 10.6 | 17.9 | 6.9 | 0.3 |
| 2006 | 55.5 | 6.4 | 3.0 | 2.1 | 10.7 | 16.7 | 5.3 | 0.3 |
| 2007 | 58.1 | 6.7 | 2.7 | 2.0 | 9.7 | 15.9 | 4.5 | 0.3 |
| 2008 | 62.0 | 6.5 | 2.6 | 1.8 | 9.0 | 13.3 | 4.6 | 0.3 |
| 2009 | 65.3 | 6.2 | 2.2 | 1.6 | 7.7 | 12.8 | 4.1 | 0.3 |
| 2010 | 68.7 | 6.5 | 2.2 | 1.5 | 6.5 | 11.2 | 3.2 | 0.2 |
| 2011 | 73.2 | 6.6 | 2.0 | 1.3 | 5.4 | 8.7 | 2.6 | 0.2 |
| 2012 | 75.6 | 6.2 | 2.0 | 1.2 | 5.0 | 7.5 | 2.3 | 0.2 |
| 2013 | 78.6 | 5.8 | 1.8 | 1.1 | 4.2 | 6.3 | 2.1 | 0.2 |
| 2014 | 83.6 | 4.8 | 1.3 | 0.9 | 3.3 | 4.9 | 1.1 | 0.1 |
| 2015 a | 86.7 a | 3.8 a | 1.1 | 0.8 | 2.8 | 3.8 | 0.9 | 0.1 |
| 2016 | 88.3 | 4.4 | 1.0 | 0.7 | 2.3 | 2.4 | 0.9 | 0.1 |
| 2017 b | 91.3 b | 3.7 | 0.8 | 0.5 | 1.5 | 1.5 | 0.6 | 0.1 |
| 2018 | 92.3 | 3.3 | 0.8 | 0.4 | 1.2 | 1.2 | 0.6 | 0.1 |
a The UNAIDS target of viral suppression with VL < 200 copies/ml has been met for all diagnosed PLHIV in the study population in Germany since 2015
b The UNAIDS target of viral suppression with VL < 50 copies/ml has been met for all diagnosed PLHIV in the study population in Germany since 2017
People who initiated ART
A total of 22,120 people were included in the analysis with a total follow-up time of 164,691 PY, a median observation time of 6.5 years (IQR 2.8–11.5) and a median time under ART of 5.5 years (IQR 2.3–9.9). The total number of real VL measurements was 490,352, the median number of VL measurements per person was 17 (IQR 8–32), and VL monitoring occurred at a median frequency of every 91 days (IQR 70–112). With the model, 3,974,309 virtual VL values were generated. The real VL measurements and the virtual VL values occurred on the same date in 52,205 cases.
At 88.9% (N = 19,663), the majority were enrolled in ClinSurv HIV, 8.7% (N = 1936) were enrolled in the HIV-1 Seroconverter cohort, and 2.4% (N = 521) were enrolled in both cohort studies. The characteristics of the study population who ever initiated ART are summarized in Table 1.
On an individual level, a total of 94% (20,849/22,120) achieved viral suppression after ART initiation, and 6% (1271/22,120) never achieved viral suppression. Of all, 86% (19,076/22,120) showed viremia at any time, and 77% (17,085/22,120) showed viremia with VL > 1000 copies/ml. The total median observation time was 2010 days (IQR 850–3620). The median person-time with viral suppression among all people was 1100 days (IQR 330–2310). The resulting individual proportion of person-time with viral suppression to the observation time had a median of 66% (IQR: 36–86). Excluding gap time, the proportion of person-time with viral suppression to the observation time had a median of 88% (IQR: 63–97). The median person-time with viremia with VL > 1000 copies/ml was 40 days (IQR 10–110). The individual proportion of person-time with viremia with VL ≥1000 copies/ml to the observation time had a median of 2.3% (IQR: 0.3–9). Excluding gap time, the proportion of viremia with VL ≥1000 copies/ml to the observation time had a median of 2.9% (IQR: 0.4–13).
Viral suppression and viremia from 1999 to 2018
Among PLHIV after ART initiation
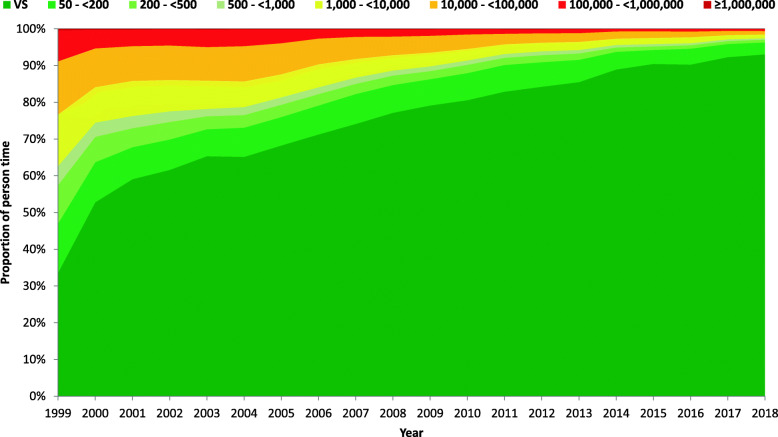
Based on the longitudinal model, the proportion of person-time with viral suppression (VL < 50 copies/ml) of the 22,120 people who ever initiated any type of ART increased over time from 33.6% in 1999 to 93.0% in 2018. The proportion of person-time with VL < 200 copies/ml increased from 47.0% in 1999 to 96.3% in 2018. VLs of 50- < 200 copies/ml, 200- < 500 copies/ml and 500- < 1000 copies/ml were observed in 13.4, 10.5 and 5.2% of the people in 1999, respectively, and in 3.3, 0.8 and 0.3% of the people in 2018, respectively. The proportion of people with viremia > 1000 copies/ml therefore decreased from 37.3% in 1999 to 2.6% in 2018 (see Fig. 3 and Table 3a for detailed results).
Fig. 3.
Viral load levels of people who ever initiated any ART in the study population from 1999 to 2018
Table 3.
Development of person-time and PLHIV with viral suppression and viremia for PLHIV after ART initiation between 1999 and 2018, based on our longitudinal model (3a) and using the most recent VL in each year (3b)
| People who ever initiated ART | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Longitudinal model | Conventional method | |||||||||||||||||
| Year | Viral suppression (< 50) | 50 - < 200 | 200 - < 500 | 500 - < 1000 | 1000 - < 10,000 | 10,000 - < 100,000 | 100,000 - < 1,000,000 | > 1,000,000 | Year | Viral suppression (< 50) | 50 - < 200 | 200 - < 500 | 500 - < 1000 | 1000 - < 10,000 | 10,000 - < 100,000 | 100,000 - < 1,000,000 | > 1,000,000 | Difference in viral suppression between the methods |
| 1999 | 33.6 | 13.4 | 10.5 | 5.2 | 14.0 | 14.5 | 8.4 | 0.4 | 1999 | 51.7 | 9.4 | 7.4 | 3.6 | 12.2 | 9.9 | 5.3 | 0.5 | 18.1 |
| 2000 | 52.8 | 10.9 | 6.9 | 3.9 | 9.7 | 10.5 | 5.1 | 0.2 | 2000 | 62.2 | 7.6 | 3.4 | 2.7 | 10.7 | 8.5 | 4.4 | 0.5 | 9.4 |
| 2001 | 59.1 | 8.7 | 5.2 | 3.3 | 9.5 | 9.5 | 4.4 | 0.3 | 2001 | 64.2 | 7.5 | 3.9 | 3.1 | 8.7 | 8.5 | 3.9 | 0.2 | 5.2 |
| 2002 | 61.6 | 8.3 | 4.8 | 2.9 | 8.6 | 9.4 | 4.4 | 0.2 | 2002 | 64.2 | 7.3 | 3.5 | 2.8 | 8.7 | 8.6 | 4.5 | 0.4 | 2.6 |
| 2003 | 65.3 | 7.3 | 3.5 | 2.0 | 7.7 | 9.1 | 4.7 | 0.2 | 2003 | 68.6 | 5.6 | 3.2 | 2.1 | 7.1 | 8.7 | 4.6 | 0.2 | 3.3 |
| 2004 | 65.2 | 7.9 | 3.5 | 2.2 | 7.0 | 9.6 | 4.5 | 0.2 | 2004 | 67.1 | 7.1 | 2.9 | 2.0 | 7.6 | 8.6 | 4.3 | 0.3 | 1.9 |
| 2005 | 68.2 | 7.7 | 3.4 | 2.0 | 6.3 | 8.4 | 3.8 | 0.1 | 2005 | 69.1 | 7.2 | 3.4 | 2.3 | 6.5 | 7.8 | 3.6 | 0.1 | 0.8 |
| 2006 | 71.3 | 7.9 | 3.1 | 1.8 | 6.2 | 7.0 | 2.5 | 0.2 | 2006 | 74.6 | 6.3 | 3.2 | 1.8 | 5.4 | 6.4 | 2.1 | 0.1 | 3.3 |
| 2007 | 74.1 | 8.1 | 2.8 | 1.7 | 5.1 | 6.1 | 2.0 | 0.1 | 2007 | 75.5 | 7.4 | 3.1 | 1.9 | 4.9 | 5.2 | 1.8 | 0.2 | 1.4 |
| 2008 | 77.2 | 7.6 | 2.6 | 1.4 | 4.2 | 5.0 | 2.0 | 0.1 | 2008 | 78.9 | 6.1 | 2.9 | 1.6 | 4.3 | 4.1 | 2.0 | 0.2 | 1.8 |
| 2009 | 79.1 | 7.2 | 2.2 | 1.3 | 3.7 | 4.6 | 1.7 | 0.1 | 2009 | 80.2 | 6.2 | 2.5 | 1.6 | 3.5 | 4.0 | 1.8 | 0.2 | 1.1 |
| 2010 | 80.6 | 7.4 | 2.2 | 1.2 | 3.1 | 4.0 | 1.4 | 0.1 | 2010 | 83.2 | 5.7 | 2.4 | 1.3 | 3.1 | 3.0 | 1.2 | 0.1 | 2.7 |
| 2011 a | 82.9 | 7.3 | 1.9 | 1.1 | 2.6 | 2.8 | 1.2 | 0.1 | 2011 | 85.1 | 6.0 | 1.7 | 1.1 | 2.4 | 2.3 | 1.3 | 0.1 | 2.2 |
| 2012 | 84.3 | 6.7 | 1.9 | 1.0 | 2.4 | 2.6 | 1.0 | 0.1 | 2012 | 85.8 | 5.3 | 2.0 | 1.2 | 2.2 | 2.3 | 1.0 | 0.1 | 1.5 |
| 2013 | 85.5 | 6.1 | 1.7 | 1.0 | 2.2 | 2.4 | 1.0 | 0.1 | 2013 | 87.7 | 5.1 | 1.8 | 0.9 | 1.8 | 1.8 | 1.0 | 0.1 | 2.2 |
| 2014 | 88.9 | 4.8 | 1.2 | 0.7 | 1.7 | 2.0 | 0.6 | 0.1 | 2014 | 90.7 | 3.7 | 1.2 | 0.6 | 1.4 | 1.7 | 0.6 | 0.1 | 1.8 |
| 2015 b | 90.4 | 3.8 | 1.0 | 0.6 | 1.7 | 1.8 | 0.6 | 0.1 | 2015 | 91.9 | 3.2 | 0.9 | 0.6 | 1.3 | 1.4 | 0.6 | 0.1 | 1.4 |
| 2016 | 90.3 | 4.3 | 0.9 | 0.6 | 1.6 | 1.6 | 0.6 | 0.1 | 2016 | 91.6 | 3.6 | 0.9 | 0.4 | 1.2 | 1.5 | 0.5 | 0.1 | 1.3 |
| 2017 | 92.3 | 3.6 | 0.8 | 0.5 | 1.2 | 1.1 | 0.5 | 0.1 | 2017 | 93.0 | 3.2 | 0.8 | 0.4 | 1.0 | 0.9 | 0.5 | 0.1 | 0.7 |
| 2018 | 93.0 | 3.3 | 0.8 | 0.3 | 1.1 | 0.9 | 0.5 | 0.1 | 2018 | 93.3 | 3.2 | 0.9 | 0.3 | 0.9 | 0.9 | 0.4 | 0.1 | 0.3 |
a The UNAIDS target of viral suppression using the longitudinal model with VL < 200 copies/ml has been met for PLHIV after ART initiation in the study population in Germany since 2011
b The UNAIDS target of viral suppression using the longitudinal model with VL < 50 copies/ml has been met for PLHIV after ART initiation in the study population in Germany since 2015
Viral suppression and viremia from 1999 to 2018 using the most recent VL in each year
Among PLHIV after ART initiation
According to a conventional definition, viral suppression, as the last step of the HIV continuum of care, is defined as the number and percentage of people receiving medical care whose most recent HIV VL is suppressed. Following this definition and considering the last VL measurement in each year, the proportion of people with viral suppression among the 22,120 people who ever initiated ART and had a documented VL value increased over time from 51.7% in 1999 to 93.3% in 2018. The proportion of people with VL < 200 copies/ml increased from 61.1% in 1999 to 96.5% in 2018. VLs of 50- < 200 copies/ml, 200- < 500 copies/ml and 500- < 1000 copies/ml were observed in 9.4, 7.4 and 3.6% of the people in 1999, respectively, and in 3.2, 0.9 and 0.3% of the people in 2018, respectively. The proportion of people with viremia > 1000 copies/ml therefore decreased from 27.9% in 1999 to 2.3% in 2018 (see Table 3b for detailed results).
Continuous viral suppression over one year
A total of 11,837 people with 35,995 VL measurements were eligible for the analysis of continuous viral suppression over a one-year observation period on an individual level. In total, at the individual level, 88% (10,474/11,837) had no viral failure and showed continuous viral suppression with all VLs in 2018. The median number of VLs was 3 (IQR: 2–4), and 91% (10,792/11,837) had more than one VL. Categorizing those with 1 VL or more than 1 VL measurement, 81% (848/1045) and 89% (9626/10,792) showed continuous viral suppression, respectively. In comparison, using the last VL, 93% (11,044/11,837) showed viral suppression, which is 5% higher than the proportion with continuous viral suppression on the individual level. Using all of the available VL measurements, 93% (33,619/35,995) of the VL showed viral suppression.
Analysis of people with gap time (VL measurements > 180 days apart)
On an individual level of all 22,120 people who had ever initiated ART, 8023 (36%) had no gap time, and 14,097 (64%) had any gap time. The cumulative median gap time was 560 days (IQR: 260–1150), and the individual proportion of gap time to the observation time had a median of 27% (IQR: 12–47). The median number of gaps was 2 (IQR: 1–4), and the median gap time per gap was 223 days (IQR: 192–302).
A total of 8173 people with 15,892 VL measurements were eligible for the analysis of the last VL before and the first VL after gap time in the recent period from 2015 to 2018. Of all VL measurements, 90% (14,274/15,892) and 90% (14,293/15,892) showed viral suppression at last VL before and first VL after gap time, respectively. Furthermore, 4% (599/15,892) and 3% (531/15,892) had VL > 50- < 200 copies/ml, 1% (221/15,892) and 2% (227/15,892) had VL 200- < 1000 copies/ml, and 5% (798/15,892) and 5% (841/15,892) had VL ≥1000 copies/ml at the last VL before and the first VL after gap-time, respectively. Overall, among those with viremia, the median VL was 910 copies/ml (IQR: 104–25,700) and 1368 copies/ml (IQR: 118–31,853) for the last VL before and the first VL after gap-time, respectively.
On an individual level, of all last VLs before and first VLs after gap time, 86% were congruent with each other, with 84% showing viral suppression, 0.8% having VL 50- < 1000 copies/ml and 1.1% having VL ≥1000 copies/ml. A total of 14% were not congruent with another, with 7% having a VL increase and 7% with a VL decrease (Table 4).
Table 4.
Congruence of last VL before and first VL after gap time, viral suppression (VS) and median VL for each group and overall between 2015-2018
| Congruence | N | (%) | Last VL before gap time | First VL after gap time | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N no VS | (%) no VS | Median | (IQR) | N no VS | (%) no VS | Median | (IQR) | |||
| Congruent VS | 13,407 | 84.4 | – | – | – | – | – | – | ||
| Congruent 50- < 1000 | 134 | 0.8 | 134 | 100 | 88 | (66–160) | 134 | 100 | 93 | (66–149) |
| Congruent > =1000 | 170 | 1.1 | 170 | 100 | 33,180 | (7200-83,550) | 170 | 100 | 33,180 | (8900–73,827) |
|
VL decrease last VL > first VL |
1051 | 6.6 | 1051 | 100 | 1162 | (101–31,300) | 165 | 16 | 278 | (101–1820) |
|
VL increase last VL < first VL |
1130 | 7.1 | 263 | 23 | 326 | (114–2555) | 1130 | 100 | 1667 | (135–33,824) |
| Total | 15,892 | 100.0 | 1618 | 10 | 910 | (104–25,700) | 1599 | 10 | 1368 | (118–31,853) |
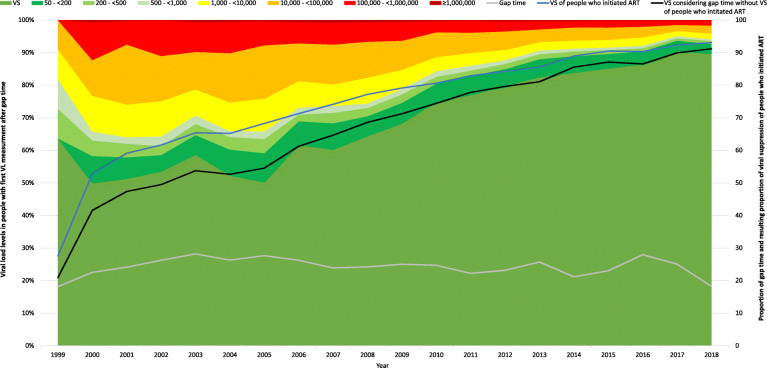
To approximate the impact of gap time on the overall viral suppression in people who initiated ART, we calculated the resulting proportion of viral suppression after considering for viremic gap time. Figure 4 shows the proportion of viral suppression and viral load levels in people with gap time at their first VL measurement after gap time between 1999 and 2018. Additionally, it shows the proportion of gap time among all people who initiated ART, the proportion of viral suppression among all people who initiated ART and the resulting proportion of viral suppression among all people who initiated ART after considering for viremic gap time. The proportion of gap time was lowest in 1999 and 2018 at 18% and highest in 2003, 2005 and 2016 at 28%, and the mean and median gap time were both 24%. The proportion of viremic gap time ranged from approximately 12% between 1999 and 2005, then decreased constantly to 2% in 2018. The resulting proportion of viral suppression among all people who initiated ART after considering for viremic gap time increased from 21% in 1999 to 90% in 2018.
Fig. 4.
Proportion of viral suppression and viral load levels in people with gap time at their first VL measurement after gap time between 1999 and 2018. Additionally, the proportion of gap time among all people who initiated ART (grey line), the proportion of viral suppression among all people who initiated ART (blue line) and the resulting proportion of viral suppression among all people who initiated ART after considering for viremic gap time (black line) is shown
Analysis of antiretroviral treatment regimens over time
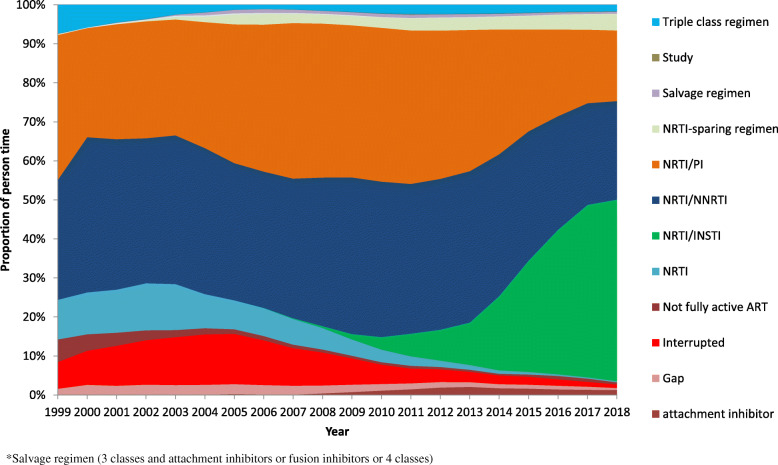
The exact composition of ART regimens in the cohort studies is shown in Fig. 5 and Table S1. Overall, NRTI/NNRTI regimens with 35% were most frequently used, followed by 32% NRTI/PI regimes and 16% NRTI/INSTI regimens. The remaining 17% were divided between less common or older regimens, and 5% had treatment interruptions. The composition of ART regimens in the cohort studies changed significantly over time. Between 1999 and 2014, NRTI/PI regimens were at approximately 35%, and this proportion decreased thereafter to 18% in 2018. NRTI/NNRTI regimens ranged from approximately 35 to 40% between 1999 and 2014 and then decreased to 25% in 2018. NRTI/INSTI regimens continuously increased after their market entry in 2006, reaching 3% in 2010 and 11% in 2013 and further increasing to 47% in 2018. In 1999, a proportion of 10% was NRTI-only regimens, and this proportion decreased from 2004 to 0.4% in 2018. NRTI sparing regimens continuously increased from 0.3% in 1999 to 4% in 2018. The proportion of not fully active ART was 6% in 1999 but continuously decreased over time to only 0.5% in 2018. Interruptions were highest in 2001 to 2006 at up to 13% and then decreased continuously from 2007 onward to 1% in 2018 (see Fig. 5 and Table S1).
Fig. 5.
Composition of ART regimens by drug classes in the cohort studies from 1999 to 2018
Discussion
Summary
We developed a model to reconstruct the individual viral load course of people to estimate the durations and proportions of viral suppression and viremia using longitudinal clinical cohort data, including all available VL measurements, additionally taking into account ART status and VL dynamics. The method provides a nationwide estimate and a useful method for calculating the number and proportion of PLHIV and of person-time with viral suppression for the HIV care continuum to evaluate the UNAIDS target of viral suppression for Germany. This model additionally allows for the determination and further analyses of people with longer periods without observation or missing VL control, defined as gap time. We determined the proportion of person-time and PLHIV with viral suppression and gap time between 1999 and 2018 using longitudinal national cohort data. We observed a continuous and remarkable increase in the proportion of person-time and of PLHIV being virally suppressed in both the whole study population and in PLHIV after ART initiation. The 90% UNAIDS target of viral suppression has been met in the whole study population of all diagnosed PLHIV since 2017 due to earlier and widespread use of ART and in PLHIV after ART initiation since 2015, respectively. Using the international comparable threshold of 200 copies/ml, the target was reached since 2015 and 2011, respectively. In 2018, 93% of PLHIV after ART initiation were virally suppressed with VL < 50 copies/ml, and 96% had VL < 200 copies/ml. Furthermore, we compared the results of the conventional method with those of our longitudinal method, showing potential misclassification of viral suppression when using only the last VL in a year. We observed a constant high proportion of gap time in these real-life cohort studies. We further analyzed people with gap time, aiming to approximate their viral load status, and we showed that, in recent years, only a slightly lower proportion of viral suppression was associated with gap time.
Longitudinal model and comparison with the conventional method
Viral suppression is conventionally determined based on the most recent HIV viral load below a certain threshold, often < 200 copies/ml for comparability across studies and different settings and because this threshold was shown to be sufficient to avoid HIV transmission [17, 18, 30]. However, such a cross-sectional approach does not address the timeliness of either reaching or the time spent at each level [6] and person-time with viral suppression and viremia. Using a single VL measurement can lead to an overestimation of durable viral suppression [6, 22]. In our approach, instead of considering only the last VL measurement in a respective year, we examined the total observation time with all available VL measurements and additionally created virtual VL values taking into account ART status and VL dynamics to reconstruct persons’ individual viral load courses. We believe that this approach provides a more accurate picture of the VL status of the study population and might be especially useful when the study population and sample size are smaller and therefore less robust. When examining a large number of people cross-sectionally, it is likely that, at each point in time, a certain more or less stable proportion of people shows viral suppression. In this study, the proportion of people with viral suppression in recent years using the conventional approach with the last VL per year was ~ 2% higher than in our longitudinal model. Although this difference is small, it might be due to the large numbers of people and measurements included, which could reflect an overestimation of durable viral suppression and could be different in smaller studies or other settings. From 1999 to 2001, when the study size was smaller, the difference was ~ 11%. Furthermore, the comparison of the conventional cross-sectional approach with the analysis of continuous viral suppression in one year on an individual level showed a notable difference of 5%. Recent studies have also demonstrated that simple, cross-sectional measures of viral suppression are prone to misclassification [31]. Viral suppression is not constant once achieved, and people often transition between suppressed and unsuppressed states, even over periods as short as one year [21]. Therefore, in agreement with the results of other studies, we believe that the dynamics of VL progression are easily overlooked with a cross-sectional assessment of the last VL measure, and longitudinal measures of VL dynamics provide more granular data with implications for HIV treatment and prevention [21, 22, 31]. Additionally, with our model, it is possible to assign, quantify and further investigate longer periods without observation or VL control. For the reasons described, we believe that our method is superior when examining trends over time in longitudinal long-term cohorts with potential observation gaps and viral load changes. In addition, it should be emphasized that this advantage can be achieved on the basis of already established standards of therapy monitoring and thus with reasonable effort.
Gap time and retention in care
In our study, we defined longer periods without viral load control of more than 180 days between VL measurements as gap time. A notable proportion of 24% gap time was observed in these real-life cohort studies. The question of whether these people are considered successfully treated or whether having viremia is a factor of uncertainty in our analysis. However, following an approach using the last VL measure for viral suppression would not consider this proportion at all. Aiming to approximate the status of the people during gap time, we analyzed the last and first VL measurements before and after gap time. On an individual level, 84% of the people came back into observation with the same VL with which they left showing viral suppression. The overall proportion of virally suppressed before and after gap time in recent years was 90%, which is only slightly (3%) less than using our longitudinal model excluding gap time. In our opinion, it is therefore very unlikely to assume that the people had high VL only during their gap time, and we believe that the VL measurements before and after gap time are good proxies. Furthermore, the median VL of the 10% with viremia before and after gap time decreased remarkably over time. Finally, we calculated the resulting proportion of viral suppression after considering for viremic gap time and showed that this would decrease the overall viral suppression by only 2% among all people who initiated ART in 2018. One reason for viral suppression or low viremia during gap time can be that people were receiving care in non-cohort centers rather than being lost to care entirely. It is important to note again that these nationwide studies are real-life observational cohorts that reflect clinical practice. People might switch doctors or leave the country or region for a certain time and then return, or it is also possible that the gaps in observation and longer periods without VL controls are in fact gaps in documentation. These might be reasons for the constant high proportion of gap time in the studies. However, VL can be very dynamic, and after ART interruption, even in selected cases with long-term viral suppression, in the absence of plasma residual viremia and low HIV-DNA or people treated in Fiebig I acute infection, viral rebound occurred rapidly at a median time of 21 or 26 days, respectively [32, 33]. Longer periods without VL control are therefore problematic. Potentially, even the quarterly reimbursed VL testing in Germany would not be sufficient to detect every single VL even if counting them as blips, and from a researcher’s perspective, we might wish to have information about the VL status of each person for every day. However, evidence has shown that quarterly VL testing is sufficient to determine treatment success, which is reflected in guidelines [26] and reimbursement regulations. Nonetheless, at least all of the available VLs should be used to determine the proportion of virally suppressed people in one year, instead of reducing the available data to only one VL per year. In our model, we use all available VLs, additionally taking into account the ART status and VL dynamics of the people to generate virtual VL values along a line, enabling us to assign a VL status at any point in observational time. We confirmed that VL testing occurred every 91 days in our cohorts, showing again that ART in Germany is performed by highly specialized practitioners in accordance with the guidelines [26]. Conversely, we also observed a constant high proportion of 24% gap time in the cohort studies, with a slightly higher likelihood of showing viremia. Retention in care is crucial for successful treatment, and we recommend maintaining engagement and retention in care and adherence to ART, accompanied, guided and monitored by regular VL testing. We also recommend further analysis among people with gap time, which we have planned. However, the achieved improvements in HIV care and treatment by highly specialized doctors are not doubted and can be seen in the composition of ART regimens over time and not least in the remarkable increase in viral suppression over time.
Trends of viral suppression between 1999 and 2018
Between 1999 and 2018, after ART initiation, the proportions of person-time and of PLHIV with viral suppression increased from 34 to 93%. With the threshold of VL < 200 copies/ml between 1999 and 2018, the proportion of person-time and PLHIV after ART initiation increased from 47 to 96%. A remarkable increase in viral suppression has also been observed in many other studies and countries [4, 5, 34–38]. These findings are likely explained by improvements in clinical care, treatment options and ART adherence [13, 35, 38]. Although not all regimens or drugs are still being equally used, treatment options have remarkably increased since the early era after the introduction of highly active, combined ART. Not fully active ART was at 6% in 1999 but soon continuously decreased to only 0.5% in 2018. The experience of practitioners and people in using ART and the importance of adherence have improved tremendously. Resistance test-guided therapy is now the standard [26]. Much has been learned with regard to treatment interruptions, and at least since the results of the SMART study in 2006, interruptions are no longer recommended [39]. This learning can very well be seen in the proportion of interruptions in the RKI cohorts. Treatment interruptions in our study were highest at 13% in 2004 and 2005 and subsequently decreased to less than 1% in 2018. During 2015–2018 in our study population, ART interruptions still occurred in 1–2% of the people. Since ART is lifelong, we included all people who ever initiated ART even if treatment was interrupted, indicating that the proportion of viral suppression would be even higher if we restricted them to those under continuous ART. We also assessed the VL in the whole study population regardless of ART initiation, including ART-naïve people and person-time. An impressive increase was observed for all diagnosed PLHIV, which, as the results of the ClinSurv HIV and HIV-1 Seroconverter cohort showed [40], was connected with the widespread and earlier use of ART as recommended in the guidelines [26]. This achievement is a great one in terms of treatment as prevention (TasP), showing that, since early ART is common, the population of diagnosed PLHIV is not substantially contributing to HIV transmission in Germany. This fact shows that diagnosis is key to prevention. With regard to the whole HIV care continuum, we know that the potential for improvement is mostly seen at this first stage of the HIV care continuum -- the only stage for which Germany has not yet met the UNAIDS target of 90% [8]. Therefore, tailored HIV testing campaigns and enhanced access to HIV testing, including self-testing, should be further strengthened. For PLHIV after ART initiation, we recommend avoiding treatment interruptions and emphasizing adherence to ART.
Evaluation of the UNAIDS 90 target of viral suppression
The UNAIDS target of 90% viral suppression has been met among PLHIV who ever initiated ART since 2015 in these nationwide German cohort studies of PLHIV. The international comparable threshold of VL < 200 copies/ml has been met among PLHIV who ever initiated ART since 2011. In 2018, 93% of PLHIV after ART initiation were virally suppressed with VL < 50 copies/ml, and 96% had VL < 200 copies/ml. Therefore, when using the threshold of VL < 200 copies/ml, Germany reached the UNAIDS 95 target of viral suppression since 2017. On a population basis in light of the HIV transmission risk, studies have suggested that a VL up to 400 copies/ml might still be uncritical [41]. A notable proportion of our study population with viremia showed low-level viremia < 1000 copies/ml with a likely low risk of transmitting HIV [30]. However, individual health risks [42], the development of HIV drug resistance [43–45] and increased risk of viral rebound [46–48] among people with low-level viremia are problematic, and viral suppression remains the goal [49]. Therefore, further analysis of people with viral failure is essential.
Limitations
Assessing stages of the HIV continuum of care using cohorts can introduce bias since they might not be representative of all diagnosed PLHIV in a country. To estimate representativeness, we compared the demographic characteristics of our study population with all PLHIV in Germany and found them to be similar. The study population represents more than 20% of all PLHIV in Germany. ClinSurv HIV is the largest nationwide long-term cohort of HIV-positive people and the least biased source available. In a study by Gourlay et al., the authors also used country-specific cohort data to derive stages of the HIV care continuum in European Union countries [6]. Germany delivered data from ClinSurv HIV for this study and was assumed to be fairly representative of HIV people in care [6, 50, 51]. People outside of medical care, e.g., without health insurance, are not represented in ClinSurv. The HIV-1 Seroconverter cohort is assumed to be representative of men who have sex with men (MSM) in Germany [52] and therefore covers one specific group within the population of PLHIV. However, MSM is the largest group and is mainly affected by HIV; furthermore, ClinSurv HIV accounts for more than 90% of the study population in our analytic sample. In this respect, we believe that the representativeness of ClinSurv HIV applies, and following the results of Gourlay et al., our sample is fairly representative of HIV people in care in Germany. However, we cannot exclude that these studies are not representative.
As discussed, gap time is a factor of uncertainty, and although we believe that our sensitivity analysis of the last and first VL before and after gap time is a reasonable approximation, viral suppression was slightly lower, indicating that further analyses of people with gap time would be useful. Furthermore, in real-life studies, misclassification, loss to follow-up, lab-related issues and gaps in documentation can occur and influence gap time.
Conclusions
This report describes a model to estimating the number and proportion of PLHIV and person-time with viral suppression. The study provides a possible approach for estimating the number of people receiving continuously specialized HIV medical care in Germany and those with gaps in observation or VL control. With this study, we provide a nationwide estimate and a useful tool for calculating the number and proportion of PLHIV and of person-time with viral suppression and with gap time as well as trends of viral suppression and gap time between 1999 and 2018 in Germany. We observed an increase in the proportion of person-time and of diagnosed PLHIV with viral suppression. The UNAIDS 90 target of viral suppression has been met in these nationwide German cohort studies since 2015 and, when using the international comparable threshold of < 200 copies/ml, since 2011. In 2018, 93% of PLHIV after ART initiation were virally suppressed with VL < 50 copies/ml, and 96% had VL < 200 copies/ml. Germany reached the UNAIDS 95 target of viral suppression since 2017 when using the threshold of VL < 200 copies/ml. Our results suggest that the population of diagnosed PLHIV is not substantially contributing to HIV transmission in Germany. Continuous efforts toward tailored HIV testing campaigns and enhanced access to HIV testing, including self-testing, are recommended.
We also recommend regular VL testing and engagement and retention in care as well as adherence to continuous ART. Further analysis of people with viral failure is essential to understand and determine risk factors for viral failure in times of highly effective and mostly successful ART.
This approach and model to reconstruct persons’ individual viral load course can be useful for estimating the number and proportion of PLHIV with viral suppression in other countries, provided that the required resources are available. The described methodology could be used and adapted for different investigations or parameters in the future.
Supplementary Information
Acknowledgements
The authors are grateful to the patients who joined the HIV-1 Seroconverter and the ClinSurv HIV cohort and to all collaborative treatment centers. The authors would like to thank Andrea Kühne, Kerstin Dehmel, Uwe Koppe and Andrea Sailer for the management of the data collection and study management. We thank all cooperating partners.
The ClinSurv HIV cohort study centers
Berlin: Dirk Schürmann, Marianne Warncke (Charité, Universitätsmedizin Berlin); Bonn: Jürgen Rockstroh, Jan-Christian Wasmuth, Svetlana Hass (Universitätsklinikum Bonn); Düsseldorf: Bjoern Jensen, Cecilie Feind (Universitätsklinik Düsseldorf); Essen: Stefan Esser, Pia Schenk-Westkamp (Universitätsklinikum Essen); Frankfurt: Christoph Stephan, Annette Haberl, Peter Schott (HIV Center Frankfurt); Hamburg: Andreas Plettenberg, Thore Lorenzen, Frieder Kuhlendahl (Inst. für interdisziplinäre Medizin Hamburg); Axel Adam, Thomas Buhk, Stephan Fenske, Stefan Hansen, Christian Hoffmann, Michael Sabranski, Knud Schewe, Hans-Jürgen Stellbrink, Dennis Radzuweit, Alexander Mainka, Constantin Rickassel (ICH, Infektionsmedizinisches Centrum Hamburg); Olaf Degen, Guido Schäfer, Robin Scheiter (Universitätsklinikum Hamburg Eppendorff); Hannover: Matthias Stoll, Steve Gerschmann, Renate Beider (Medizinsche Universität Hannover); Kiel: Heinz-August Horst, Silke Trautmann (Universitätsklinikum Schleswig–Holstein); Köln: Gerd Fätkenheuer, Jörg Janne Vehreschild, Laura Hamacher, Lennart Nicksch (Universitätsklinikum Köln); München: Johannes Bogner, Barbara Sonntag, Oliver Pullen (Ludwig-Maximilians Universität München); Rostock: Carlos Fritzsche (Universitätsklinikum Rostock).
The HIV-1 Seroconverter study centers
Aachen: Heribert Knechten, Petra Panstruga (Praxenzentrum Blondelstraße Aachen); Berlin: Keikawus Arasteh, Michael Rittweger (Vivantes Auguste Viktoria Klinikum); Hans Wesselmann, Nikolai Menner (Charité - Universitätsmedizin Berlin); Ulrich Bohr (Praxiszentrum Kaiserdamm); Heiko Jessen, Arne B. Jessen (Praxis Jessen2 und Kollegen); Hubert Schulbin, Sascha Brand, (Praxis Kreuzberg); Jan Gumprecht, Beate Weninger (MVZ Infektiologie Ärzteforum Seestrasse); Heribert Hillenbrand, Heiko Karcher (Praxis City Ost); Klaus Fischer, Dietmar Schranz (Praxis Wilmersdorfer); Mathias Vallée (Praxis Dres. Vallée); Jukka Hartikainen, Stephan Grunwald (Zibp - Zentrum für Infektiologie Berlin Prenzlauer Berg - GmbH); Jörg A. Claus, Claudia Thomas (Praxis Claus und Thomas); Roland Grimm, Sarah Schoor (Schwerpunktpraxis Mitte); Christiane Cordes, Reinhold Schröder (Praxis Dr. Cordes); Tobias Glaunsinger (Praxis Prenzlauer Berg); Michael Rausch, Thomas Reineke (Praxis Schöneberg); Gordon Weinberg, Manuel Bruhy (Infektiologisches Zentrum Steglitz); Siegfried Köppe, Peter Kreckel (Praxis Köppe u. Kreckel); Andreas Berger, Sinah Lindemann (UBN/Praxis); Bochum: Norbert H. Brockmeyer, Anja Potthoff (WIR “Walk In Ruhr”); Bonn: Kathrin van Bremen, Jürgen Rockstroh (Universitätsklinikum Bonn); Dortmund: Martin Hower, Claudia Bachmann (Klinikum Dortmund gGmbH); Dresden: Petra Spornraft-Ragaller (Universitätsklinikum Carl Gustav Carus); Dieter Teichmann (Städtisches Klinikum Dresden); Düsseldorf: Bjoern Jensen, Falk Hüttig (Universitätsklinikum Düsseldorf); Essen: Stefan Esser; Pia Schenk-Westkamp (Universitätsklinikum Essen); Frankfurt: Annette Haberl, Christoph Stephan (Goethe Universität Frankfurt); Freiburg: Susanne Usadel, Matthias Müller (MVZ Clotten Freiburg, Universitätsklinikum Freiburg); Gießen: Janina Trauth, (Justus-Liebig-Universität Gießen); Hagen: Alan Chavez-Valladares, Gerd Deutschinoff (Agaplesion Allgemeines Krankenhaus Hagen Infektionsambulanz); Halle/Saale: Burkhard Kreft, Danica Lange (Martin-Luther-Universität Halle-Saale); Hamburg: Olaf Degen, Guido Schäfer (Universitätsklinik Hamburg–Eppendorf); Andreas Plettenberg, Frieder Kuhlendahl (Inst. für interdisziplinäre Medizin Hamburg); Dorothea Wiemer, Lavinia Biemann (Bundeswehrkrankenhaus Hamburg am Bernhard-Nocht Institut); Knud Schewe, Christian Hoffmann (ICH, Infektionsmedizinisches Centrum Hamburg); Hannover: Georg Behrens, Matthias Stoll, (Medizinische Hochschule Hannover); Jena: Benjamin T. Schleenvoigt, Mathias W. Pletz (Universitätsklinikum Jena); Koblenz: Ansgar Rieke (Gemeinschaftsklinikum Mittelrhein, Johannes Gutenberg-Universität Mainz); Köln: Stephan Schneeweiß, Stefan Scholten (Praxis Hohenstaufenring); Mark Oette (Krankenhaus der Augustinerinnen, Universität zu Köln); Krefeld: Peter A. Arbter, (Praxis Dr. Arbter); Leipzig: Thomas Grünewald, Jeannine Weidemann (Klinikum St. Georg gGmbH); Ines Ruck (Praxis Dr. Ruck); Ludwigshafen: Bernd Claus (Klinikum Ludwigshafen); Mainz: Martin Sprinzl, Peter R. Galle (Klinikum der Johannes-Gutenberg-Universität Mainz); Mannheim: Matthias P. Ebert, Roger Vogelmann (Medizinische Klinik Universität Heidelberg, Mannheimer Onkologie Praxis); München: Johannes Bogner, Ulrike Hellerer (Klinikum der Ludwig-Maximilians-Universität); Antoniya Todorova, Claudia Traidl-Hoffmann (Klinik und Poliklinik, TU Dermatologie); Birgit Mück (MVZ Karlsplatz); Ramona Pauli (Isarpraxis); Christoph D. Spinner, Jochen Schneider (TU München Klinikum r.d. Isar); Birgit Mück (prinzmed); Neuss: Robert Baumann (Gemeinschaftspraxis Dres. Baumann und Sels); Osnabrück: Niels Schübel, Christiane Berning (Infektiologisches Centrum, Klinikum Osnabrück); Regensburg: Franz Audebert (Praxiszentrum Alte Mälzerei); Stuttgart: Trein A., Schnaitmann E. (Infectomed GbR); Clemens Roll, Simone Marquardt (Praxis Dres Frietsch, Müller, Roll, Stuttgart); Ulm: Georg Härter, Beate Grüner (Universitätsklinikum Ulm) Wiesbaden: Cengiz Güler (Praxis Güler); Zwickau: Steve Rößler (Arztpraxis Rößler).
Abbreviations
- AI
Attachment inhibitor
- ART
Antiretroviral treatment
- ClinSurv HIV
Clinical Surveillance of HIV Disease
- FI
Fusion inhibitor
- INSTI
Integrase strand transfer inhibitor
- IQR
Interquartile range
- MSM
Men who have sex with men
- NRTI
Nucleoside reverse transcriptase inhibitor
- NNRTI
Non-nucleoside reverse transcriptase inhibitor
- PI
Protease inhibitor
- PLHIV
People living with HIV (PLHIV)
- PY
Person-years
- RKI
Robert Koch Institute
- UNAIDS
Joint United Nations Programme on HIV/AIDS
- VL
Viral load
Authors’ contributions
DS was responsible for the study design, devised the estimation approach, performed the data analysis and statistical analysis, interpreted the data and drafted the manuscript. CK was responsible for the study design, devised the estimation approach, was responsible for database management, supported the data analysis and interpretation of the data and helped to draft the manuscript. MS is site principal investigator, contributed data, supported the interpretation of the data and helped to draft the manuscript. OH was responsible for the design and implementation of the HIV-1 Seroconverter and the ClinSurv HIV cohort and supported the writing of the manuscript. BB supported the management and coordination of the study, served as the ClinSurv HIV study coordinator, contributed to improving the data quality and coverage and helped to draft the manuscript. VB supported the management and coordination of the study and helped to draft the manuscript. TK supported the data analysis and interpretation of the data and helped to draft the manuscript. All of the authors participated in the critical discussion of the results, and all of them read and approved the final manuscript.
Funding
We acknowledge the financial support of the German Federal Ministry of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to data protection and confidentiality but are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The RKI is the German national public health institute, therefore the Federal Commissioner for Data Protection is the responsible entity for studies which are conducted by the RKI. Information on HIV infection collected in ClinSurv HIV corresponds to the data reported to the RKI according to legal requirements implemented by the national Protection against Infection act (IfSG) of 2001. All patient data collected in ClinSurv HIV are generated during routine care. The German Federal Commissioner for Data Protection therefore waived the need for ethical approval for the ClinSurv HIV study.
The study protocol for the HIV-1 Seroconverter cohort was initially approved in 2005 by the Ethics Committee of the Charité, University Medicine Berlin (EA2/105/05), with approval amended and confirmed in 2013. Participants provide their written informed consent to participate in this study. The ethics committee approved this consent procedure.
Consent for publication
Not applicable.
Competing interests
DS, BGB, VB, OH and MS declare that they have no competing interests. CK is small shareholder in companies manufacturing antiretroviral drugs. TK reports outside of the submitted work to have received honoraria from Eli Lilly, Newsenselab, and Total for providing methodological advice, and from the BMJ for editorial services.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The Joint United Nations Programme on HIV/AIDS (UNAIDS) 90–90-90: An ambitious treatment target to help end the AIDS epidemic. 2014. [Google Scholar]
- 2.The Joint United Nations Programme on HIV/AIDS (UNAIDS) The Gap Report - Beginning of the end of the AIDS epidemic. Geneva: UNAIDS; 2014. p. 2014. [Google Scholar]
- 3.Assembly UG. Political declaration on HIV and AIDS: on the fast-track to accelerate the fight against HIV and to end the AIDS epidemic by 2030. New York: United Nations; 2016. [Google Scholar]
- 4.The Joint United Nations Programme on HIV/AIDS (UNAIDS) Global HIV & AIDS statistics - 2019 fact sheet. 2019. [Google Scholar]
- 5.The Joint United Nations Programme on HIV/AIDS (UNAIDS). ENDING AIDS - Progress towards the 90–90–90 targets. GLOBAL AIDS UPDATE | 2017.
- 6.Gourlay A, Noori T, Pharris A, Axelsson M, Costagliola D, Cowan S, et al. The human immunodeficiency virus continuum of care in European Union countries in 2013: data and challenges. Clin Infect Dis. 2017;64(12):1644–1656. doi: 10.1093/cid/cix212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt D, Kollan C, Stoll M, Stellbrink H-J, Plettenberg A, Fätkenheuer G, et al. From pills to patients: an evaluation of data sources to determine the number of people living with HIV who are receiving antiretroviral therapy in Germany. BMC Public Health. 2015;15(1):252. doi: 10.1186/s12889-015-1598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.an der Heiden M, Marcus U, Kollan C, Schmidt D, Gunsenheimer-Bartmeyer B, Bremer V. Schätzung der Zahl der HIV-Neuinfektionen und der Gesamtzahl von Menschen mit HIV in Deutschland, Stand Ende 2018. Epidemiologisches Bulletin. 2019;(46):483–92.
- 9.Bätzing-Feigenbaum J, Kollan C, Kühne A, Matysiak-Klose D, Gunsenheimer-Bartmeyer B, Hamouda O. Cohort profile: the German ClinSurv HIV project–a multicentre open clinical cohort study supplementing national HIV surveillance. HIV medicine. 2011;12(5):269–278. doi: 10.1111/j.1468-1293.2010.00879.x. [DOI] [PubMed] [Google Scholar]
- 10.Poggensee G, Kücherer C, Werning J, Somogyi S, Bieniek B, Dupke S, et al. Impact of transmission of drug-resistant HIV on the course of infection and the treatment success. Data from the German HIV-1 Seroconverter study. HIV medicine. 2007;8(8):511–519. doi: 10.1111/j.1468-1293.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt D, Kollan C, et al. Compendium of good practices in the health sector response to HIV in the WHO European Region Compendium of good practices in the health sector response to HIV in the WHO European region. 2018. [Google Scholar]
- 12.Al-Harthi L, Siegel J, Spritzler J, Pottage J, Agnoli M, Landay A. Maximum suppression of HIV replication leads to the restoration of HIV-specific responses in early HIV disease. Aids. 2000;14(7):761–770. doi: 10.1097/00002030-200005050-00001. [DOI] [PubMed] [Google Scholar]
- 13.Bello KJ, Mesner O, O’Bryan TA, Won SH, Lalani T, Ganesan A, et al. Factors associated with 10 years of continuous viral load suppression on HAART. BMC Infect Dis. 2016;16(1):351. doi: 10.1186/s12879-016-1677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30(Supplement_1):S5–S14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 15.Zoufaly A, Stellbrink HJ, an der Heiden M, Kollan C, Hoffmann C, van Lunzen J, et al. Cumulative HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS-related lymphoma. J Infect Dis. 2009;200(1):79–87. [DOI] [PubMed]
- 16.Freiberg MS, Chang C-CH, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, Van Lunzen J, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. Jama. 2016;316(2):171–181. doi: 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- 18.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, Degen O, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive PARTNER taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;393(10189):2428–38. [DOI] [PMC free article] [PubMed]
- 19.Clavel F, Hance AJ. HIV drug resistance. N Engl J Med. 2004;350(10):1023–1035. doi: 10.1056/NEJMra025195. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Understanding the HIV Care Continuum - fact sheet. 2019. [Google Scholar]
- 21.Kay ES, Batey DS, Mugavero MJ. The HIV treatment cascade and care continuum: updates, goals, and recommendations for the future. AIDS Res Ther. 2016;13(1):35. doi: 10.1186/s12981-016-0120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crepaz N, Tang T, Marks G, Mugavero MJ, Espinoza L, Hall HI. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012–2013. Clin Infect Dis. 2016;63(7):976–983. doi: 10.1093/cid/ciw418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamouda O. HIV/AIDS surveillance in Germany. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2003;32:S49–S54. doi: 10.1097/00126334-200302011-00008. [DOI] [PubMed] [Google Scholar]
- 24.Bartmeyer B, Kuecherer C, Houareau C, Werning J, Keeren K, Somogyi S, et al. Prevalence of transmitted drug resistance and impact of transmitted resistance on treatment success in the German HIV-1 Seroconverter cohort. PLoS One. 2010;5(10):e12718. doi: 10.1371/journal.pone.0012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.zu Knyphausen F, Scheufele R, Kücherer C, Jansen K, Somogyi S, Dupke S, et al. First line treatment response in patients with transmitted HIV drug resistance and well defined time point of HIV infection: updated results from the German HIV-1 seroconverter study. PloS one. 2014;9(5):e95956. doi: 10.1371/journal.pone.0095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deutsche Aids Gesellschaft (DAIG), Österreichische Aids Gesellschaft . Deutsch-Österreichische Leitlinien zur antietroviralen Therapie der HIV-1-Infektion. 2017. [Google Scholar]
- 27.European Centre for Disease Prevention and Control . Continuum of HIV care - Monitoring implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2017 progress report. 2017. [Google Scholar]
- 28.Xia Q, Kersanske LS, Wiewel EW, Braunstein SL, Shepard CW, Torian LV. Proportions of patients with HIV retained in care and virally suppressed in New York City and the United States: higher than we thought. JAIDS J Acquired Immune Deficiency Syndromes. 2015;68(3):351–358. doi: 10.1097/QAI.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 29.Xia Q, Wiewel EW, Braunstein SL, Kersanske LS, Torian LV. Comparison of indicators measuring the proportion of human immunodeficiency virus–infected persons with a suppressed viral load. Ann Epidemiol. 2015;25(4):226–230. doi: 10.1016/j.annepidem.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830–839. doi: 10.1056/NEJMoa1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marks G, Patel U, Stirratt MJ, Mugavero MJ, Mathews WC, Giordano TP, et al. Single viral load measurements overestimate stable viral suppression among HIV patients in care: clinical and public health implications. J Acquired immune deficiency syndromes (1999) 2016;73(2):205. doi: 10.1097/QAI.0000000000001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castagna A, Muccini C, Galli L, Bigoloni A, Poli A, Spagnuolo V, et al. Analytical treatment interruption in chronic HIV-1 infection: time and magnitude of viral rebound in adults with 10 years of undetectable viral load and low HIV-DNA (APACHE study) J Antimicrob Chemother. 2019;74(7):2039–2046. doi: 10.1093/jac/dkz138. [DOI] [PubMed] [Google Scholar]
- 33.Colby DJ, Trautmann L, Pinyakorn S, Leyre L, Pagliuzza A, Kroon E, et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med. 2018;24(7):923. doi: 10.1038/s41591-018-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helleberg M, Häggblom A, Sönnerborg A, Obel N. HIV care in the Swedish-Danish HIV cohort 1995-2010, closing the gaps. PLoS One. 2013;8(8):e72257. doi: 10.1371/journal.pone.0072257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nance RM, Delaney JC, Simoni JM, Wilson IB, Mayer KH, Whitney BM, et al. HIV viral suppression trends over time among HIV-infected patients receiving care in the United States, 1997 to 2015: a cohort study. Ann Intern Med. 2018;169(6):376–384. doi: 10.7326/M17-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin DA, Luz PM, Lake JE, Clark JL, Veloso VG, Moreira RI, et al. Improved virologic outcomes over time for HIV-infected patients on antiretroviral therapy in a cohort from Rio de Janeiro, 1997–2011. BMC Infect Dis. 2014;14(1):322. doi: 10.1186/1471-2334-14-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ledergerber B, Cavassini M, Battegay M, Bernasconi E, Vernazza P, Hirschel B, et al. Trends over time of virological and immunological characteristics in the Swiss HIV cohort study. HIV medicine. 2011;12(5):279–288. doi: 10.1111/j.1468-1293.2010.00880.x. [DOI] [PubMed] [Google Scholar]
- 38.Delaugerre C, Ghosn J, Lacombe J-M, Pialoux G, Cuzin L, Launay O, et al. Significant reduction in HIV virologic failure during a 15-year period in a setting with free healthcare access. Clin Infect Dis. 2014;60(3):463–472. doi: 10.1093/cid/ciu834. [DOI] [PubMed] [Google Scholar]
- 39.Strategies for Management of Antiretroviral Therapy Study Group CD4+ count–guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt D, Kollan C, Hamouda O, Bremer V, Gunsenheimer-Bartmeyer B. Serokonverter cohort & ClinSurv HIV cohort. A longitudinal view along the German HIV care cascade - Trends of late diagnoses and time to achieve treatment success between 1997 and 2017. 2019. [Google Scholar]
- 41.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. Aids. 2009;23(11):1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 42.Antinori A, Cozzi Lepri A, Ammassari A. Low-level viremia ranging from 50 to 500 copies/mL is associated to an increased risk of AIDS events in the Icona Foundation Cohort. [Abstract PS4/2]. 15th European AIDS Conference. 2015. [Google Scholar]
- 43.Mackie NE, Phillips AN, Kaye S, Booth C, Geretti A-M. Antiretroviral drug resistance in HIV-1-infected patients with low-level viremia. J Infect Dis. 2010;201(9):1303–1307. doi: 10.1086/651618. [DOI] [PubMed] [Google Scholar]
- 44.Li JZ, Gallien S, Do TD, Martin JN, Deeks S, Kuritzkes DR, et al. Prevalence and significance of HIV-1 drug resistance mutations among patients on antiretroviral therapy with detectable low-level viremia. Antimicrob Agents Chemother. 2012;56(11):5998–6000. doi: 10.1128/AAC.01217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delaugerre C, Gallien S, Flandre P, Mathez D, Amarsy R, Ferret S, et al. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PLoS One. 2012;7(5):e36673. doi: 10.1371/journal.pone.0036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collaboration ATC Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS. 2015;29(3):373–383. doi: 10.1097/QAD.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 47.Laprise C, de Pokomandy A, Baril J-G, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis. 2013;57(10):1489–1496. doi: 10.1093/cid/cit529. [DOI] [PubMed] [Google Scholar]
- 48.Greub G, Cozzi-Lepri A, Ledergerber B, Staszewski S, Perrin L, Miller V, et al. Intermittent and sustained low-level HIV viral rebound in patients receiving potent antiretroviral therapy. Aids. 2002;16(14):1967–1969. doi: 10.1097/00002030-200209270-00017. [DOI] [PubMed] [Google Scholar]
- 49.Ryscavage P, Kelly S, Li JZ, Harrigan PR, Taiwo B. Significance and clinical management of persistent low level viremia and very low level viremia in HIV-1 infected patients. Antimicrobial agents and chemotherapy. 2014;AAC:00076–00014. doi: 10.1128/AAC.00076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vourli G, Pharris A, Cazein F, Costagliola D, Dabis F, Amo JD, et al. Assessing the representativeness of European HIV cohort participants as compared to HIV surveillance data. 2017. [Google Scholar]
- 51.Vourli G, Pharris A, Cazein F, Costagliola D, Dabis F, Del Amo J, et al. Are European HIV cohort data within EuroCoord representative of the diagnosed HIV population? AIDS (London, England) 2019;33(1):133. doi: 10.1097/QAD.0000000000002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jansen K, Thamm M, Bock C-T, Scheufele R, Kücherer C, Muenstermann D, et al. High prevalence and high incidence of coinfection with hepatitis B, hepatitis C, and syphilis and low rate of effective vaccination against hepatitis B in HIV-positive men who have sex with men with known date of HIV seroconversion in Germany. PLoS One. 2015;10(11):e0142515. doi: 10.1371/journal.pone.0142515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to data protection and confidentiality but are available from the corresponding author on reasonable request.