Abstract
Patients suffering from cholestasis often report experiencing a debilitating, unrelenting itch. In contrast to conditions, such as urticaria, in which histamine primarily drives itch (pruritus), cholestatic pruritus is multifactorial and more difficult to treat. Existing therapies are not always effective and have undesirable adverse effect profiles. Here, we conducted a systematic literature review to evaluate conventional treatment strategy, current pathophysiologic understanding, and the role of new therapies in the context of cholestatic pruritus. We discuss novel findings implicating bile acids, lysophosphatidic acid, and bilirubin as potential important mediators of cholestatic itch. New therapies that aim to remove or modulate pruritogens have been supported in observational cohort studies and randomized controlled trials. Although these new therapies show promise, further research is needed to confirm the pathophysiology of cholestatic pruritus so that targeted therapy can be developed.
Keywords: cholestasis, itch, PBC, primary biliary cholangitis, primary sclerosing cholangitis, pruritus, PSC
Pruritus is a debilitating symptom reported among 80% to 100% of patients with cholestatic liver disease, including primary biliary cholangitis, primary sclerosing cholangitis, and intrahepatic cholestasis of pregnancy.1 Cholestatic pruritus is severe. Although there is no primary rash associated with cholestatic pruritus, patients who have cholestatic itch often present with secondary lesions from scratching in an attempt to alleviate pruritus. Patients consider pruritus one of the most distressing symptoms of their cholestatic disease and report a significant decrease in quality of life as a result of pruritus.1,2 Refractory pruritus, experienced by 5% to 10% of patients with cholestatic disease, can lead to sleep deprivation, depression, and suicidal ideation.2,3 Managing cholestatic pruritus is challenging because the underlying pathogenic mechanisms are unclear, leading to few evidence-based guidelines.4
Recent research into the etiology of cholestatic pruritus offers novel therapeutic targets and strategies that may help patients who have refractory pruritus. The following review examines the current literature on the pathogenesis of cholestatic pruritus and evaluates available treatment options, which include cholestyramine, rifampin, opioid antagonists, and sertraline. This review will also cover experimental treatments for cholestatic pruritus such as phototherapy, cannabinoids, albumin dialysis, plasmapheresis, nasobiliary drainage, charcoal hemoperfusion, fibrate therapy, and ileal bile acide–transporter inhibitors (IBATs).
METHODS
We conducted a literature search with studies from the EMBASE, PubMed, Ovid MEDLINE, and Cochrane databases. Key words cholestasis, cholestatic, itch, and pruritus were used for primary initial review (Fig 1). All results were checked for relevance, and clinical studies were evaluated. Only studies focused on the treatment of pruritus in cholestatic disease were included in the review. We focused on novel treatments that are currently being applied to clinical settings. Randomized controlled trials (RCTs), observational cohort studies, and multi-patient case series were included in our analysis; single-patient case reports were excluded. None of the study or report authors was contacted for further data collection or confirmation. Relevant studies were evaluated based on the change in pruritus as measured by that study’s scale as the main outcome. Level of evidence and strength of recommendation were determined with the Strength of Recommendation Taxonomy system.5
Fig 1.
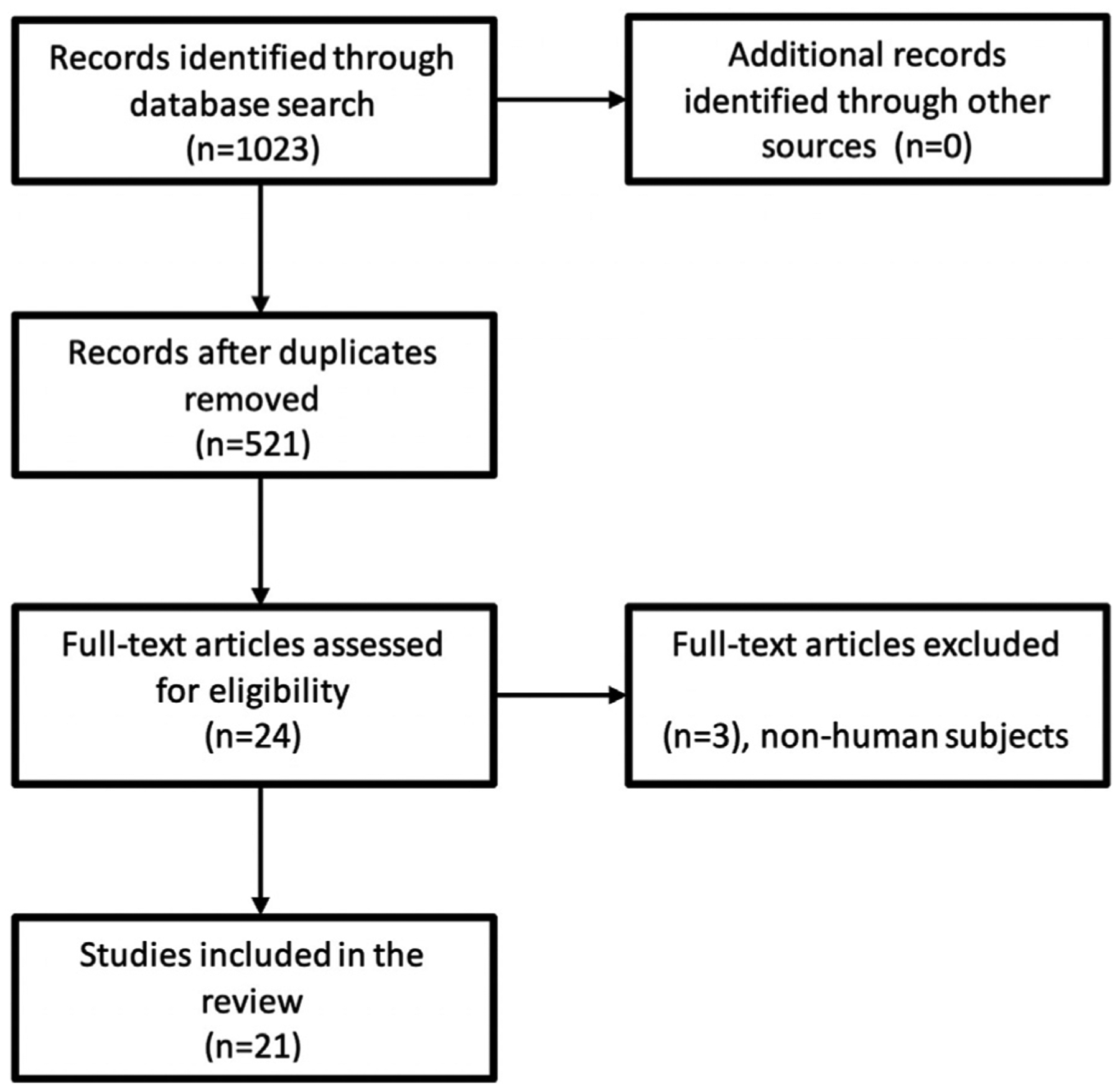
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram.
RESULTS
Our search yielded 521 reports containing our specific key words. Only primary sources addressing chronic cholestatic itch were included in the study. Ultimately, 21 primary sources were included to evaluate treatments for cholestatic pruritus. Traditional treatment methods, such as cholestyramine, rifampin, opioid antagonists, and sertraline, were the focus of 13 sources, whereas the other 8 sources presented outcomes on newer experimental therapies.
Pathogenesis
The pathogenesis of cholestatic pruritus is diverse, complex, and not clearly delineated. Although several groups have proposed a model in which a single systemic pruritogen mediates itch,6–12 it is more likely that several pruritogens are involved. A more comprehensive model of cholestatic pruritus would include independent contributions of bile acids, lysophosphatidic acid (LPA), endogenous opiates, and progesterone derivatives, all of which are often elevated in cholestasis.13 Notably, the circulating levels of these agents do not correlate well with itch severity, suggesting a complex interplay between multiple (known and unknown) pruritogens.14 In contrast, histamine—perhaps the most widely known and best understood pruritogen—does not appear to mediate cholestatic pruritus. Histaminergic itch is generally accompanied by edema and erythema, neither of which are observed with cholestatic pruritus.15,16 Additionally, patients do not benefit from widely used antihistamines, underscoring the challenges in treating cholestatic pruritus.4,17
Bile acids constitute a major component of bile and are, therefore, clear candidate pruritogens for cholestatic itch. During cholestasis, bile acids diffuse into both the systemic circulation and skin. Bile acids independently elicit itch when injected into mice and appear to act by binding and activating TGR5, a receptor expressed on itch-encoding sensory neurons.18 Treatments aimed at reducing bile acids can be effective in treating cholestatic pruritus.4,19 Serum bile acids are not always elevated in cholestatic pruritus, and pruritus does not correlate well with serum bile salt concentrations.13,20
LPA has also been identified as a potential causative pruritogen in cholestatic disease. Research into autotaxin (ATX), an enzyme that produces most LPA, supports the hypothesis that the ATX-LPA signaling axis contributes to cholestatic pruritus. Both LPA and ATX concentrations are often elevated in pruritic cholestatic patients compared with concentrations in nonpruritic cholestatic patients. The elevation of ATX is specific to cholestatic pruritus and is not observed in pruritus associated with noncholestatic diseases.2,13 Unique among proposed pruritogens, ATX activity correlates with cholestatic itch severity. Directly injecting LPA into mice induces itch.13 The pregnane X receptor agonist rifampin, among its many other effects, downregulates ATX transcription in vitro and appears to alleviate cholestatic itch.21 Despite these associations, there is still no clear consensus on the definitive etiology of cholestatic pruritus.4,13,22
Endogenous opioid peptides are widely considered to mediate a component of cholestatic itch. In the late 1980s, Thornton and Losowsky12 reported that the opiate antagonist nalmefene triggered opiate-like withdrawal in cholestatic patients. Subsequently, they observed elevated levels of Met-enkephalin in cholestatic plasma.23 In animal models, μ-opioid receptor agonists elicited scratching, whereas κ-opioid receptor agonists conversely decreased itch.24 The mechanisms by which opioid peptides elicit itch are not entirely clear but appear to influence sensation in part by modulating the activity of other pruritogens. For example, the opioid antagonist naloxone significantly decreased bile acid—associated itch despite having no known activity against TGR5.18
More recent evidence implicates the heme metabolite bilirubin as an additional potential pruritogen in cholestatic itch. Along with bile acids and LPA, bilirubin is a major component of bile and is the underlying cause of jaundice in patients with cholestasis, because bilirubin is itself yellow. Bilirubin appears to specifically bind and activate 2 members of the Mas-related G-protein—coupled receptor (Mrgpr) family of receptors, mouse Mrgpra1 and human MRGPRX4.25 Mrgrpr receptors are G-proteine coupled receptors expressed on itch-encoding sensory neurons and are major mediators of non-histaminergic pruritus.26,27 In several mouse models of cholestasis, specifically deleting either Mrgpra1 or Blυra, the gene that encodes the principal bilirubin-producing enzyme, attenuates itch. Whether bilirubin is itself a pruritogen has remained unclear because (as with other pruritogens) plasma bilirubin does not correlate well with itch severity. However, it appears that skin bilirubin is a stronger predictor of itch burden than plasma bilirubin.25 This finding is consistent with the anatomic distribution of itch sensory neurons and suggests that it might be worthwhile to explore whether skin concentrations of other potential pruritogens might similarly correlate better with itch severity than plasma concentrations.
Therapies
Studies of cholestatic pruritus are listed in in Table I with outcomes. Treatment doses and adverse effects are listed in Table II.
Table I.
Management of cholestatic pruritus
| Study | Intervention | Study design | Level of evidence | Pruritus scale | N | Dosage | Outcomes |
|---|---|---|---|---|---|---|---|
| Duncan et al, 198430 | Cholestyramine | Randomized crossover | Level 2 | 0–3 score | 8 | 4 g bid | Mean cumulative pruritus score significantly lower for cholestyramine compared with placebo (12.9 vs 20.3, P<..05) |
| Di Padova et al, 198429 | Cholestyramine | Parallel RCT | Level 2 | 0–100–mm VAS | 10 | 3 g tid | Significant decrease in pruritus compared with placebo (P<.01) |
| Woolf, 199060 | Rifampin | Randomized crossover | Level 2 | 0–100–mm VAS | 12 | 300 mg/d | No significant decrease in pruritus |
| Ghent and Carruthers, 198837 | Rifampin | Randomized crossover | Level 2 | 0–100–mm VAS | 9 | 300–450 mg/d | Significant decrease in pruritus compared with placebo (P<.002) |
| Podesta et al, 199138 | Rifampin | Randomized crossover | Level 2 | 0–100–mm VAS | 14 | 600 mg | Significant decrease in pruritus compared with placebo (P< .001) |
| Bachs et al, 198935 | Rifampin | Randomized crossover | Level 2 | 0–3 score | 22 | 10 mg/kg | Significant decrease in pruritus over study period (2.5 to 0.8, P<.001) compared with phenobarbitone |
| Juby et al, 199441 | Opioid antagonist (buprenorphine) | Randomized crossover | Level 2 | 0–10–cm VAS | 5 | 200 μg/d | Improvement in symptoms reported |
| Bergasa et al, 19998 | Opioid antagonist (nalmefene) | Parallel RCT | Level 2 | 0–10–cm VAS | 9 | 2–20 mg bid (titrated) | Significant decrease in pruritus, with mean 77% improvement in symptoms (P<.01) |
| Bergasa et al, 199542 | Opioid antagonist (naloxone) | Randomized crossover | Level 1 | 0–10–cm VAS | 29 | 0.4 mg bolus, 0.2 μg/ kg/min for 24 hours | Significant decrease in pruritus compared with placebo (P<.01) |
| Summerfield, 198043 | Opioid antagonist (naloxone) | Randomized crossover | Level 2 | 0–10–cm VAS | 20 | 2 mg IV bolus | Significant decrease in pruritus among placebo nonresponders (P<.05) |
| Terg et al, 200244 | Opioid antagonist (naltrexone) | Randomized crossover | Level 1 | 0–10–cm VAS | 20 | 50 mg qd | Significant decrease in pruritus compared with placebo (P<.0003) |
| Wolfhagen et al, 199745 | Opioid antagonist (naltrexone) | Parallel RCT | Level 2 | 0–100–mm VAS | 16 | 50 mg qd | Significant decrease in pruritus compared with placebo (P<.005) |
| Mayo et al, 200747 | Sertraline | RCT | Level 2 | 0–10–cm VAS | 21 | 75–100 mg qd | Significant decrease in pruritus compared with placebo (P<.01) |
| Decock, 201250 | Phototherapy | Observational case series | Level 3 | 0–10–cm VAS | 13 | 3 times a week | Significant decrease in pruritus from 8.0 to 2.0 (P<.001) |
| Neff, 200251 | Cannabinoids (dronabinol) | Observational case series | Level 3 | 3 | 2.5 mg qd to 5 mg tid | Reported improvements in pruritus, as well as sleep and depression | |
| Krawczyk et al, 201753 | Plasmapheresis | Prospective cohort study | Level 2 | 10-point numeric rating scale | 17 | Variable treatment schedule | Significant decrease in pruritus from 8.3 to 3.1 (P<.0001) |
| Pares, 201061 | Albumin dialysis | Prospective cohort study | Level 2 | 0–100–mm VAS | 20 | 1-time treatment | Significant decrease in pruritus from 70.2 to 20.1 (P<.001) |
| Hegade et al, 201654 | Nasobiliary drainage | Retrospective cohort study | Level 2 | 0–10–cm VAS | 27 | 1-time treatment | Significant decrease in pruritus from 10 to 0.3 (P <.0001) |
| Reig et al, 201856 | Fibrates (bezafibrate) | Prospective cohort study | Level 2 | 0–10–cm VAS | 48 | 400 mg qd | Significant decrease in pruritus from 3.7 to 0 (P<.001) |
| Kittanamongkolchai et al, 201755 | Charcoal hemoperfusion | Retrospective cohort study | Level 2 | 10–point numeric rating scale | 13 | 4 hours, 3 times a week | Significant decrease in pruritus from 9 to 4 (P<.005) |
| Hegade, 201758 | Ilealbile acid –transporter inhibitor | RCT | Level 1 | 10–point numeric rating scale | 22 | 45–90 mg twice daily | Significant decrease in pruritus compared with placebo (P<.05) |
bid, 2 times a day; IV, intravenous; qd, 4 times a day; RCT, randomized controlled trial; tid, 3 times a day; VAS, visual analog scale.
Table II.
Adverse effects of treatments
| Therapy | Recommended dosage/treatment schedule | Adverse effects | References |
|---|---|---|---|
| Cholestyramine | 4–12 g/d | Constipation, diarrhea, bloating | 28,29,31,32 |
| Rifampin | 300–600 mg/d | Hepatotoxicity, nephrotoxicity, hemolysis, drug interactions | 34–36,38,39 |
| Opioid antagonist | Naltrexone 50 mg/d Naloxone 2 mg IV bolus |
Opioid withdrawal symptoms, tolerance | 31,40,46 |
| Sertraline | 75–100 mg/d | Dizziness, nausea, diarrhea | 47–49 |
| Ultraviolet B phototherapy | 3 times a week | Erythema, paresthesia | 50 |
| Cannabinoid | Dronabinol 5 mg tid | Ataxia | 51 |
| Albumin dialysis | 1-time treatment* | Bleeding | 20,52 |
| Plasmapheresis | Variable treatment schedule | Bleeding | 53 |
| Nasobiliary drainage | 1-time treatment* | Pancreatitis | 54 |
| Charcoal hemoperfusion | 4 hours, 3 times a week | Bleeding, fever | 55 |
| Fibrates | 400 mg/d | Myalgia | 56,59 |
| Ileal bile acid-transporter inhibitor | 45–90 mg twice daily | Diarrhea | 33,58 |
IV, Intravenous; tid, 3 times a day.
Additional treatment sessions can be considered if the first treatment is effective but symptoms return..
Cholestyramine.
Cholestyramine is the current first-line therapy for cholestatic pruritus. An anion exchange resin, cholestyramine alleviates symptoms by binding and sequestering systemic bile salts. Various case series and RCTs found that cholestyramine is successful in significantly reducing pruritus symptoms and serum bile acids, with 75% of patients reporting symptom relief.28–30 However, a meta-analysis of RCTs found insufficient data to confirm cholestyramine’s efficacy.31 There are also conflicting data as to whether bile acids are the etiologic pruritogen in these patients. Nevertheless, cholestyramine is still considered the first-line medication to manage pruritus of cholestasis. Adverse effects include gastrointestinal symptoms such as constipation, diarrhea, and bloating.30,32,33
Rifampin.
Second-line therapy aims to alter the metabolism of specific pruritogens. Rifampin is considered when cholestyramine is contraindicated or insufficiently effective. As a pregnane X agonist, rifampin downregulates ATX, leading to less formation of the potential pruritogen LPA.34,35 Various RCTs have found significant improvements in pruritus with rifampin (300–600 mg/d).35–37 A meta-analysis of 5 RCTs with short-term rifampin therapy indicated that 77% of patients reported complete relief from pruritus and 20% of patients reported partial relief.34 Long-term therapy beyond 6 months can be effective but carries an increased risk of hepatotoxicity. In fact, hepatotoxicity was found in 13% of treated patients within 2 months.38 Thus, liver function tests and blood counts should be monitored consistently with rifampin therapy.39 Other adverse effects include nephrotoxicity, hemolysis, and orange discoloration of body fluids.35,36,38
Opioid antagonists.
Opioid antagonists such as naltrexone and naloxone are therapeutic options that significantly reduce pruritus. Both oral and intravenous administration of opioid receptor antagonists have been effective in reducing pruritus severity in patients with cholestasis in RCTs, with all patients reporting some symptom relief.8,31,40–45 However, opioid antagonists are associated with significant adverse effects mimicking opioid withdrawal symptoms such as tachycardia, hypertension, piloerection, and abdominal pain. Furthermore, tolerance can result from increased expression of opioid receptors decreasing the efficacy of antipruritic therapy over time. Long-term use and increasing doses have the potential to lead to chronic pain syndrome, so appropriate patient risks need to be evaluated before opioid antagonist therapy is prescribed.31,40,46
Sertraline.
When all preceding therapies fail to alleviate symptoms, sertraline is commonly administered. Sertraline, a selective serotonin reuptake inhibitor, is an antidepressant that also alters potential pruritic pathways involving serotonin. An RCT reported antipruritic effects independent of the antidepressant effect, with 91% of patients reporting symptom improvement.47,48 Sertraline is generally considered to have a favorable adverse effect profile, which includes dizziness, insomnia, nausea, and gastrointestinal symptoms.49
Phototherapy.
Ultraviolet B phototherapy was found to alleviate pruritus in an observational case series of 13 patients with symptoms refractory to the aforementioned traditional treatments. Patients were treated 3 times a week, with treatment cessation if there was no improvement after 13 treatments. With an average of 23 treatments of 8 weeks, 92% of patients reported reduced levels of pruritus. Reported adverse effects included 1 case of erythema and 1 case of paresthesia at the treatment site upon retreatment.50
Dronabinol.
Cannabinoid treatment has been reported to be efficacious in a case series for patients with refractory pruritus secondary to cholestatic liver disease.51 Three patients who had failed to gain relief from the aforementioned treatments had decreased pruritus, improvement in sleep, and overall improvement in quality of life with dronabinol treatment. One patient experienced ataxia, which resolved when doses were reduced. However, frequent dosing may be necessary because the antipruritic effects of dronabinol are limited to 4 to 6 hours.51
Albumin dialysis.
Albumin dialysis is a relatively invasive therapy option, but it may provide relief by binding and sequestering pruritogens, such as bile acids and bilirubin.20 A prospective cohort study found a significant improvement in pruritus in 95% of participants.52 Only 1 treatment was required for 75% of participants, although others received up to 4 treatments over 4 years, depending on their symptoms. Studies reported no significant infections, bleeding, or other adverse effects. Platelet count and hemoglobin level were temporarily reduced after treatment, but levels were found to return to baseline within a month without any clinical consequences.52Patients also reported feeling cold during the first hour of treatment, but this was also temporary.20
Plasmapheresis.
Plasmapheresis therapy provides relief by removing systemic pruritogens. Although this is generally well tolerated and effective in patients for a considerable period of time, it is an invasive therapy.13,53 A prospective cohort study reported significant improvements in pruritus, with patient-rated scores out of 10 dropping from 8.3 to3.1 (P < .0001).53 Patients had 1 to 6 hospital admissions and received 2 to 4 plasmapheresis treatments at each admission. The mean time between admissions was 9.8 months. There were no adverse effects reported in that study apart from difficulty inserting the central venous catheter in 1 patient.53
Nasobiliary drainage.
Nasobiliary drainage can be used to reduce serum ATX levels and alleviate pruritus by endoscopically placing a nasobiliary catheter into the common bile duct during endoscopic cholangiopancreatography, allowing bile to drain through the nose into a bag. An RCT found that drainage alleviated symptoms in 90% of patients, with significant reductions in pruritus; however, symptom relief was temporary.54 Only 1 treatment was required for 93% of participants; 2 patients had a second treatment. The mean treatment duration was 7 days. Pancreatitis was a common complication of nasobiliary drainage experienced by 31% of patients, because this treatment entailed having endoscopic cholangiopancreatography.54
Charcoal hemoperfusion.
Charcoal hemoperfusion is an invasive option that involves extracorporeal filtration of the blood, similar to dialysis. It provides significant, but temporary, relief that can be accompanied by dialyzer reactions including pain, fever, nausea, and hypotension.55 In an RCT,55 69% of participants experienced significant symptom improvement, but 15% did not complete the study because of the dialyzer reactions. Sessions were administered 3 times a week for 3.5 to 4 hours, with a median of 5 treatment sessions.
Fibrate therapy.
Fibrate therapy has also been found to have an antipruritic effect in patients with cholestatic disease, although the mechanism of action is still unclear.56,57 A prospective cohort study found significant improvements in pruritus with complete or partial symptom relief in 98% of participants.56 However, this relief is temporary, and pruritus quickly returns with treatment cessation.56
IBAT inhibitors.
IBAT inhibition is also being explored as a treatment for cholestatic pruritus because it reduces the enterohepatic recirculation of bile acids.33 An RCT found that pruritus was significantly alleviated with IBAT inhibitors compared with placebo, which correlated with decreased levels of bile acids and decreased ATX activity.58 Diarrhea is a common adverse effect of IBAT inhibitors experienced by 33% of patients, which may limit their clinical use for many patients.33
DISCUSSION
Once pruritus is determined to be secondary to cholestatic disease, a stepwise strategy should be used for pharmacologic therapy. Pruritus can be alleviated in most patients through pharmacologic therapy, but novel and invasive options are also available for refractory pruritus. Multimodal approaches combining therapies may be effective in refractory cases, although further research is needed to evaluate this. Regardless of therapy choice, patients affected by pruritus should be counseled to use moisturizing ointments and take steps to minimize additional complications from scratching. Despite various medical options, complete symptom relief may be hard to attain, in part because of the adverse effects associated with many therapies (Table II). Invasive therapy options may be effective in some patients, but they also are accompanied by increased costs and risks.
If the standard therapies fail to provide relief of pruritus symptoms, experimental approaches should be considered. When possible, these patients should be transferred to centers with specialists focused on experimental salvage therapies aimed at relieving cholestatic pruritus. Given the uncertain evidence for many newer therapies, they should be only used as a last resort after thorough discussion of risks and benefits with the patient.
Finally, in severely debilitating refractory cases of pruritus, liver transplantation may be an option. Transplantation improves pruritus and fatigue, but it should be considered only if pruritus is refractory to all standard and experimental therapies.39
CONCLUSION
Given the debilitating nature of cholestatic pruritus, it is imperative that clinicians work with patients to manage their symptoms and prevent severe sequelae. Many of the challenges associated with managing cholestatic pruritus result from the lack of understanding of how pruritogenic pathways lead to symptoms. As new developments in the pathogenesis elucidate the etiology of cholestatic pruritus, more effective treatment strategies can be developed. Although further investigation is necessary before experimental therapies can be incorporated into the primary guidelines, these options should be considered for patients with refractory pruritus. As options continue to grow, additional RCTs will be necessary.
CAPSULE SUMMARY.
Chronic, intractable pruritus is often reported as the most debilitating symptom of cholestatic disease.
Emerging evidence-based therapies, including cholestyramine, rifampin, opioid antagonists, sertraline, and phototherapy, can be used clinically to aid in the management of refractory cholestatic pruritus.
Abbreviations used:
- ATX
autotaxin
- IBAT
ileal bile acidetransporter
- LPA
lysophosphatidic acid
- RCT
randomized controlled trial
Footnotes
Disclosure: Dr Kwatra is an advisory board member for Menlo Therapeutics. He is also the recipient of a Dermatology Foundation Medical Dermatology Career Development Award. Dr Dong is on the advisory board of Escient Pharmaceuticals. Mr Meixiong is a consultant for Escient Pharmaceuticals. Mr Patel, Mr Vasavda, and Dr Ho have no conflicts of interest to declare.
REFERENCES
- 1.Weisshaar E, Dalgard F. Epidemiology of itch: adding to the burden of skin morbidity. Acta Derm Venereol. 2009;89(4):339–350. [DOI] [PubMed] [Google Scholar]
- 2.Kremer AE, Beuers U, Oude-Elferink RPJ, Pusl T. Pathogenesis and treatment of pruritus in cholestasis. Drugs. 2008;68(15): 2163–2182. [DOI] [PubMed] [Google Scholar]
- 3.Mells GF, Pells G, Newton JL, et al. Impact of primary biliary cirrhosis on perceived quality of life: The UK-PBC national study. Hepatology. 2013;58(1):273–283. [DOI] [PubMed] [Google Scholar]
- 4.Bolier R, Elferink RPO, Beuers U. Advances in pathogenesis and treatment of pruritus. Clin Liver Dis. 2013;17(2):319–329. [DOI] [PubMed] [Google Scholar]
- 5.Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69(3):548–556. [PubMed] [Google Scholar]
- 6.Carey JB Jr. Bile acids in the serum of jaundiced patients. Gastroenterology. 1961;41:285. [PubMed] [Google Scholar]
- 7.Gittlen SD, Schulman ES, Maddrey WC. Raised histamine concentrations in chronic cholestatic liver disease. Gut. 1990; 31(1):96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergasa NV, Alling DW, Talbot TL, Wells MC, Jones EA. Oral nalmefene therapy reduces scratching activity due to the pruritus of cholestasis: a controlled study. J Am Acad Dermatol. 1999;41(3 Pt 1):431–434. [DOI] [PubMed] [Google Scholar]
- 9.Bergasa NV, Thomas DA, Vergalla J, Turner ML, Jones EA. Plasma from patients with the pruritus of cholestasis induces opioid receptor-mediated scratching in monkeys. Life Sci. 1993;53(16):1253–1257. [DOI] [PubMed] [Google Scholar]
- 10.Kremer AE, Martens JJ, Kulik W, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139(3):1008–1018. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto T, Ohata H, Momose K. Itch-scratch responses induced by lysophosphatidic acid in mice. Pharmacology. 2004;72(1):51–56. [DOI] [PubMed] [Google Scholar]
- 12.Thornton JR, Losowsky MS. Opioid peptides and primary biliary cirrhosis. BMJ. 1988;297(6662):1501–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kremer AE, Namer B, Bolier R, Fischer MJ, Elferink RPO, Beuers U. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(Suppl. 2):164–175. [DOI] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver. EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51(2):237–267. [DOI] [PubMed] [Google Scholar]
- 15.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE.Specific C-receptors for itch in human skin. J Neurosci. 1997; 17(20):8003–8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenniger LM, Beuers U. Bile salts and cholestasis. Dig Liver Dis.2010;42(6):409–418. [DOI] [PubMed] [Google Scholar]
- 17.Greaves MW. Antihistamines in dermatology. Skin Pharmacol Physiol. 2005;18(5):220–229. [DOI] [PubMed] [Google Scholar]
- 18.Alemi F, Kwon E, Poole DP, et al. The TGR5 receptor mediates bile acid—induced itch and analgesia. J Clin Invest. 2013;123(4): 1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirby J, Heaton KW, Burton JL. Pruritic effect of bile salts. Br Med J. 1974;4(5946):693–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leckie P, Tritto G, Mookerjee R, Davies N, Jones D, Jalan R. ‘Out-patient’ albumin dialysis for cholestatic patients with intractable pruritus. Aliment Pharmacol Ther. 2012;35(6):696–704. [DOI] [PubMed] [Google Scholar]
- 21.Kremer AE, van Dijk R, Leckie P, et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012; 56(4):1391–1400. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Zhang W, Evans JF, et al. Autotaxin, pruritus and primary biliary cholangitis (PBC). Autoimmun Rev. 2016;15(8): 795–800. [DOI] [PubMed] [Google Scholar]
- 23.Thornton JR, Losowsky MS. Plasma leucine enkephalin is increased in liver disease. Gut. 1989;30(10):1392–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi N, Tominaga M, Kosaka R, et al. Involvement of μ-opioid Receptors and κ-opioid receptors in itch-related scratching behaviour of imiquimod-induced psoriasis-like dermatitis in mice. Acta Derm Venereol. 2017;97(8–9):928–933. [DOI] [PubMed] [Google Scholar]
- 25.Meixiong J, Vasavda C, Green D, et al. Identification of a bilirubin receptor may mediate a component of cholestatic itch. Elife. 2019;8:e44116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q, Tang Z, Surdenikova L, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139(7):1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106(5):619–632. [DOI] [PubMed] [Google Scholar]
- 28.Oster ZH, Rachmilewitz EA, Moran E, Stein Y. Relief of pruritus by cholestyramine in chronic liver disease. Isr J Med Sci. 1965; 1(4):599–606. [PubMed] [Google Scholar]
- 29.Di Padova C, Tritapepe R, Rovagnati P, Rossetti S. Double-blind placebo-controlled clinical trial of microporous cholestyramine in the treatment of intra-and extra-hepatic cholestasis: relationship between itching and serum bile acids. Methods Find Exp Clin Pharmacol. 1984;6(12):773–776. [PubMed] [Google Scholar]
- 30.Duncan JS, Kennedy HJ, Triger DR. Treatment of pruritus due to chronic obstructive liver disease. Br Med J (Clin Res Ed). 1984;289(6436):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tandon P, Rowe BH, Vandermeer B, Bain VG. The efficacy and safety of bile acid binding agents, opioid antagonists, or rifampin in the treatment of cholestasis-associated pruritus. Am J Gastroenterol. 2007;102(7):1528–1536. [DOI] [PubMed] [Google Scholar]
- 32.Pusl T, Beuers U. Extrahepatic manifestations of cholestatic liver diseases. Clin Rev Allergy Immunol. 2005;28(2):147–157. [DOI] [PubMed] [Google Scholar]
- 33.Hegade VS, Kendrick SFW, Dobbins RL, et al. BAT117213: ileal bile acid transporter (IBAT) inhibition as a treatment for pruritus in primary biliary cirrhosis: study protocol for a randomised controlled trial. BMC Gastroenterol. 2016;16(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khurana S, Singh P. Rifampin is safe for treatment of pruritus due to chronic cholestasis: a meta-analysis of prospective randomized-controlled trials. Liver Int. 2006;26(8):943–948. [DOI] [PubMed] [Google Scholar]
- 35.Bachs L, Elena M, Parés A, Piera C, Rodés J. Comparison of rifampicin with phenobarbitone for treatment of pruritus in biliary cirrhosis. Lancet. 1989;333(8638):574–576. [DOI] [PubMed] [Google Scholar]
- 36.Podesta A, Lopez P, Terg R, et al. Treatment of pruritus of primary biliary cirrhosis with rifampin. Dig Dis Sci. 1991;36(2): 216–220. [DOI] [PubMed] [Google Scholar]
- 37.Ghent CN, Carruthers SG. Treatment of pruritus in primary biliary cirrhosis with rifampin. Results of a double-blind, crossover, randomized trial. Gastroenterology. 1988;94(2):488–493. [DOI] [PubMed] [Google Scholar]
- 38.Bachs L, Parés A, Elena M, Piera C, Rodés J. Effects of long-term rifampicin administration in primary biliary cirrhosis. Gastroenterology. 1992;102(6):2077–2080. [DOI] [PubMed] [Google Scholar]
- 39.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50(1): 291–308. [DOI] [PubMed] [Google Scholar]
- 40.Carson KL, Tran TT, Cotton P, Sharara AI, Hunt CM. Pilot study of the use of naltrexone to treat the severe pruritus of cholestatic liver disease. Am J Gastroenterol. 1996;91(5):1022–1023. [PubMed] [Google Scholar]
- 41.Juby LD, Wong VS, Losowsky MS. Buprenorphine and hepatic pruritus. Br J Clin Pract. 1994;48(6):331. [PubMed] [Google Scholar]
- 42.Bergasa NV, Alling DW, Talbot TL, et al. Effects of naloxone infusions in patients with the pruritus of cholestasis. A double-blind, randomized, controlled trial. Ann Intern Med. 1995; 123(3):161–167. [DOI] [PubMed] [Google Scholar]
- 43.Summerfield JA. Naloxone modulates the perception of itch in man. Br J Clin Pharmacol. 1980;10(2):180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terg R, Coronel E, Sorda J, Munoz AE, Findor J. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717–722. [DOI] [PubMed] [Google Scholar]
- 45.Wolfhagen FH, Sternieri E, Hop WC, Vitale G, Bertolotti M, Van Buuren HR. Oral naltrexone treatment for cholestatic pruritus: a double-blind, placebo-controlled study. Gastroenterology. 1997;113(4):1264–1269. [DOI] [PubMed] [Google Scholar]
- 46.Mcrae CA, Prince MI, Hudson M, Day CP, James OFW, Jones DEJ. Pain as a complication of use of opiate antagonists for symptom control in cholestasis. Gastroenterology. 2003; 125(2):591–596. [DOI] [PubMed] [Google Scholar]
- 47.Mayo MJ, Handem I, Saldana S, Jacobe H, Getachew Y, Rush AJ. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology. 2007;45(3):666–674. [DOI] [PubMed] [Google Scholar]
- 48.Browning J, Combes B, Mayo MJ. Long-term efficacy of sertraline as a treatment for cholestatic pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 2003;98:2736. [DOI] [PubMed] [Google Scholar]
- 49.Thébaut A, Habes D, Gottrand F, et al. Sertraline as an additional treatment for cholestatic pruritus in children. J Pediatr Gastroenterol Nutr. 2017;64(3):431–435. [DOI] [PubMed] [Google Scholar]
- 50.Decock S, Roelandts R, Van Steenbergen W, et al. Cholestasis-induced pruritus treated with ultraviolet B photo-therapy: an observational case series study. J Hepatol. 2012;57(3):637–641. [DOI] [PubMed] [Google Scholar]
- 51.Neff GW, O’Brien CB, Reddy KR, et al. Preliminary observation with dronabinol in patients with intractable pruritus secondary to cholestatic liver disease. Am J Gastroenterol. 2002;97(8): 2117–2119. [DOI] [PubMed] [Google Scholar]
- 52.Parés A, Herrera M, Avilés J, Sanz M, Mas A. Treatment of resistant pruritus from cholestasis with albumin dialysis: combined analysis of patients from three centers. J Hepatol. 2010; 53(2):307–312. [DOI] [PubMed] [Google Scholar]
- 53.Krawczyk M, Liebe R, Wasilewicz M, Wunsch E, Raszeja-Wyszomirska J, Milkiewicz P. Plasmapheresis exerts a long-lasting antipruritic effect in severe cholestatic itch. Liver Int. 2017;37(5):743–747. [DOI] [PubMed] [Google Scholar]
- 54.Hegade VS, Krawczyk M, Kremer AE, et al. The safety and efficacy of nasobiliary drainage in the treatment of refractory cholestatic pruritus: a multicentre European study. Aliment Pharmacol Ther. 2016;43(2):294–302. [DOI] [PubMed] [Google Scholar]
- 55.Kittanamongkolchai W, El-Zoghby ZM, Eileen Hay J, et al. Charcoal hemoperfusion in the treatment of medically refractory pruritus in cholestatic liver disease. Hepatol Int. 2017; 11(4):384–389. [DOI] [PubMed] [Google Scholar]
- 56.Reig A, Sese P, Pares A. Effects of bezafibrate on outcome and pruritus in primary biliary cholangitis with suboptimal ursodeoxycholic acid response. Am J Gastroenterol. 2018;113(1):49–55. [DOI] [PubMed] [Google Scholar]
- 57.Bolier R, de Vries ES, Pares A, et al. Fibrates for the treatment of cholestatic itch (FITCH): study protocol for a randomized controlled trial. Trials. 2017;18(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017; 389(10074):1114–1123. [DOI] [PubMed] [Google Scholar]
- 59.Corpechot C, Chazouilleres O, Rousseau A, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med. 2018;378(23):2171–2181. [DOI] [PubMed] [Google Scholar]
- 60.Woolf GM, Reynolds TB. Failure of rifampin to relieve pruritus in chronic liver disease. J Clin Gastroenterol. 1990; 12:174–177. [DOI] [PubMed] [Google Scholar]
- 61.Parés A, Herrera M, Avilés J, Sanz M, Mas A. Treatment of resistant pruritus from cholestasis with albumin dialysis: combined analysis of patients from three centers. J Hepatol. 2010; 53(2):307–312. [DOI] [PubMed] [Google Scholar]


