Abstract
Simple Summary
Aside from its well-established role in prion disorders, in the last decades the significance of cellular prion protein (PrPC) expression in human cancers has attracted great attention. An extensive body of work provided evidence that PrPC contributes to tumorigenesis by regulating tumor growth, differentiation, and resistance to conventional therapies. In particular, PrPC over-expression has been related to the acquisition of a malignant phenotype of cancer stem cells (CSCs) in a variety of solid tumors, encompassing pancreatic ductal adenocarcinoma, osteosarcoma, breast, gastric, and colorectal cancers, and primary brain tumors as well. According to consensus, increased levels of PrPC endow CSCs with self-renewal, proliferative, migratory, and invasive capacities, along with increased resistance to anti-cancer agents. In addition, increasing evidence demonstrates that PrPc also participates in multi-protein complexes to modulate the oncogenic properties of CSCs, thus sustaining tumorigenesis. Therefore, strategies aimed at targeting PrPC and/or PrPC-organized complexes could be a promising approach for anti-cancer therapy.
Abstract
Cellular prion protein (PrPC) is seminal to modulate a variety of baseline cell functions to grant homeostasis. The classic role of such a protein was defined as a chaperone-like molecule being able to rescue cell survival. Nonetheless, PrPC also represents the precursor of the deleterious misfolded variant known as scrapie prion protein (PrPSc). This variant is detrimental in a variety of prion disorders. This multi-faceted role of PrP is greatly increased by recent findings showing how PrPC in its folded conformation may foster tumor progression by acting at multiple levels. The present review focuses on such a cancer-promoting effect. The manuscript analyzes recent findings on the occurrence of PrPC in various cancers and discusses the multiple effects, which sustain cancer progression. Within this frame, the effects of PrPC on stemness and differentiation are discussed. A special emphasis is provided on the spreading of PrPC and the epigenetic effects, which are induced in neighboring cells to activate cancer-related genes. These detrimental effects are further discussed in relation to the aberrancy of its physiological and beneficial role on cell homeostasis. A specific paragraph is dedicated to the role of PrPC beyond its effects in the biology of cancer to represent a potential biomarker in the follow up of patients following surgical resection.
Keywords: cellular prion protein, cancer stem cells, brain tumors, peripheral tumors, tumorigenesis, self-renewal, differentiation
1. Background
Prion protein is renowned for its causative role in the pathogenesis and transmission of prion diseases (Prusiner, 1998) [1]. Also known as transmissible spongiform encephalopathies (TSEs), prion diseases are progressive, irreversible, and fatal neurodegenerative disorders, affecting both humans and other mammals.
In humans, TSEs include Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker disease (GSS), fatal familial insomnia (FFI), kuru, and most recently variant of CJD (vCJD), whereas in animals TSEs comprise bovine spongiform encephalopathy (BSE) in cattle, scrapie in sheep and goats, and chronic wasting disease (CWD) in cervids [1]. Being either sporadic, inherited, or infectious [2], these disorders are characterized by spongiform degeneration of the central nervous system (CNS) [3], neuronal loss, and astrogliosis [4]. Independently from their origin, the neuropathological hallmark of prion disorders is the accumulation of protein aggregates within the brain containing a deleterious and transmittable isoform of the normal PrP, named PrP scrapie (PrPSc) [4]. PrPSc represents an altered and misfolded isoform of the cellular PrP (PrPC), which is normally expressed in eukaryotic cells [5,6]. Despite owning the same primary amino-acid sequence, PrPSc differs from the naturally occurring PrPC in its secondary structure. Compared with PrPc, PrPSc is mainly characterized by large β-sheets, which become predominant over the α-helical and coil structures [7]. Notably, the reduction in α-helices, which are refolded into β-sheets, results in profound changes in the physio-chemical properties of PrP. In fact, while PrPc is soluble and highly susceptible to protease digestion, PrPSc is insoluble and protease-resistant, thus forming protein aggregates that precipitate within the cell. This is consistent with the observation that β-sheet-enriched amyloid deposits of PrPSc are abundant and accumulate within prion-infected brains [2,8,9]. Furthermore, the disease-associated PrPSc can translate other normal PrPC into the pathological PrP isoform, acting as a seed that initiates protein refolding of a nascent PrPC molecule [10]. This, in turn, fosters intra-/extra-cellular accumulation of insoluble protein aggregates, which ultimately results in cellular dysfunction and neurotoxicity [11]. Again, any dysfunction in PrP removal increases the probability of spontaneous PrPSc generation. Although prion metabolism is partly dependent on the ubiquitin proteasome system, the clearance of PrPSc is tightly bound to the activity of the main protein degradation pathway, namely autophagy (ATG) [12,13,14]. When a high amount of PrPC occurs, this protein cannot be promptly degraded and its accumulation above a certain threshold over time enables PrP misfolding and aggregation, while clogging cell-clearing pathways [15]. Thus, when ATG is overwhelmed, PrP further accumulates within the cell, thereby exacerbating PrP pathogenic conversion, self-propagation, and spreading. As a proof of concept, ATG-inducing agents enhance misfolded PrP degradation thus preventing its aggregation into amyloids [12,16,17,18]. On the other hand, the clearance of aggregate-prone proteins is impaired when ATG is suppressed, either pharmacologically by known ATG inhibitors or genetically by siRNA targeting ATG genes [13,16].
Notwithstanding the recognition of the infectious potential of PrP, its significance extends way beyond prion diseases. In the last decades, PrPC has gathered great attention for its involvement in tumor cell biology. A growing body of evidence indicates that PrPc over-expression contributes to brain tumorigenesis by regulating tumor growth, invasiveness, and therapeutic resistance. Remarkably, PrPC over-expression occurs in various tumors of the nervous system, encompassing meningioma, medulloblastoma, schwannoma, and glioma, mostly glioblastoma multiforme (GBM) [19,20,21,22,23]. Among primary brain tumors, GBM represents the highest, most aggressive, and severe prognostic grade (WHO grade IV glioma). Intriguingly, increased PrPC expression in human glioma samples correlates with tumor grade and thus lower patients’ overall survival. In fact, human GBM samples feature higher levels of PrPC than low-grade glioma (LGG, grades I–II) and grade III astrocytoma [20,23].
It is worth mentioning that several lines of evidence indicate that PrPC plays a role not only in the nervous system but also throughout the human body. In fact, although being highly expressed within the CNS, PrPC also occurs in various human peripheral tissue and organs. Remarkably, the discovery of PrPC expression in different cell types joined the evidence of PrPC over-expression in various human cancers [24]. As recently reported, PrPC is highly expressed in a variety of solid tumors, including gastric and colorectal cancer [25,26,27,28,29], breast cancer [30,31,32], prostate cancer [33], pancreatic ductal adenocarcinoma (PDAC) [34,35,36], lung adenocarcinoma [37], head and neck squamous cell carcinoma (HNSCC) [38], osteosarcoma [39], and melanoma [40,41]. Again, PrPC over-expression is closely associated with tumor malignancy and poor prognosis [42,43,44,45].
Looking towards the comprehension of the role of PrPC in the biology of cancer, consistent evidence suggests that PrPC is involved in the proliferation, migration, invasion, and therapeutic resistance of cancer stem cells (CSCs). Regardless of the cell of origin, this applies to both hematopoietic and solid tumors. Virtually present in any kind of tumor, CSCs represent a small subset of cancer cells endowed with key features of normal stem cells, such as sustained self-renewal and proliferation [46]. Thus, CSCs are thought to be the driving force of tumorigenesis since they can initiate and sustain tumor growth and progression. Moreover, CSCs possess an enhanced disseminating capacity and increased therapeutic resistance enabling these cells to invade neighboring healthy tissues and/or metastasize to distant organs [46]. Consistent evidence indicates that PrPc over-expression is key to sustain CSC self-renewal, clonogenicity, and tumorigenic potential [35,47,48,49]. Conversely, its inhibition and/or down-regulation results in a more differentiated, less oncogenic CSC phenotype [22,29,50,51]. Again, PrPc down-regulation restores CSC sensitivity to chemo- and radiotherapy [52].
It is also noteworthy that, beyond its effects in cancer biology, emerging evidence suggests that PrPC expression may have a diagnostic value in various solid tumors [35,45,53]. Recently, PrPC emerged as a potential biomarker in the follow-up of patients following surgical resection of PDAC [36]. Thus, PrPC may become a useful tool in monitoring the therapeutic efficacy as well as predicting the outcome of cancer patients undergoing chemo- and radiotherapy [34,44,54,55,56].
Therefore, in the next paragraphs we provide evidence regarding the physiological and beneficial role of PrPC on cell homeostasis. Then we move forward to discuss recent data regarding the multiple effects of PrPC in sustaining cancer initiation, progression, and recurrence, with a special focus on the role of PrPC in CSC biology. These aspects, which are seminal in cancer research, may provide novel insights on the role of PrPC as both a prognostic biomarker and a potential therapeutic target to force CSC to shift towards more differentiated and therapy-sensitive cancer cells. The findings herein discussed may contribute to early diagnosis of cancer, while potentially improving future targeted interventions.
2. The Physiological Role of Cellular Prion Protein (PrPC)
2.1. Structure, Biogenesis, and Intracellular Trafficking of PrPC
The normal cellular prion protein (PrPC) is an endogenous, highly conserved cell-surface glycoprotein encoded by the PRNP gene [57]. In humans, PRNP transcripts are detected at a variable extent in various peripheral tissues (e.g., gastrointestinal tract, lung, heart, mammary glands), and to a higher level within the central and peripheral nervous system [58]. In fact, PrPc is most abundant within neurons and glia of selective brain areas, although they are also quite ubiquitously distributed in non-neuronal cell types [4].
The newly synthesized PrPC is a 253 amino acid polypeptide composed of an unstructured N-terminal domain and a globular C-terminal domain, which contains three α-helices and two β-sheets [59]. In particular, the N-terminal signal peptide is essential for the translocation of immature PrPC into the lumen of the endoplasmic reticulum (ER). However, some PrPC molecules fail to properly translocate into the ER, and thus, they are retained within the cytosol [60].
The biogenesis of PrPC requires a series of co- and post-translational modifications. In particular, the nascent protein enters into the ER lumen, where the N-terminal signal peptide is rapidly removed. Following the cleavage of the N-terminal flexible tail, PrPC undergoes a second cleavage at the carboxy-terminal globular domain along with a glycosyl-phosphatidyl-inositol (GPI) modification at residue 230. In detail, the C-terminal GPI anchor peptide signal sequence (GPI-PSS) is removed, while being rapidly replaced with a GPI anchor that tethers PrPC to the outer leaflet of the plasma membrane (PM) [61]. The removal of both signal peptides results in a mature PrPC, which consists of 208 amino acids. Then, the protein moves to the Golgi apparatus to undergo post-translational modifications [62]. In particular, PrPC undergoes N-glycosylation at two highly conserved sites in its C-terminal, namely Asn-181 and Asn-197 residues [61]. These latter may be occupied by sugar moieties at a variable extent, thus resulting in different glycosylated forms of PrPC (i.e., monoglycoylated, diglycosylated, and unglycosylated) [63]. This, in turn, stabilizes PrPC secondary structure, while favoring its correct localization to the PM [61,64].
However, in different human cancer cell lines and tissues such as PDAC and melanoma, PrP is incompletely processed and exists as a precursor form of normal PrPC [34,40,65]. This latter, known as pro-PrP, represents an alternative form of the mature, full-length PrPC. In particular, pro-PrP lacks the N-terminal signal peptide, the sugar moieties, and the GPI, while retaining the normally cleaved GPI-PSS [65]. Remarkably, the GPI-PSS contains several small hydrophobic amino acids, which in turn, are responsible for the unconventional insertion of pro-PrP into the phospholipid bilayer of the PM, rather than to the PM outer face, as occurring for mature PrPC.
The GPI-anchored PrPC is strategically associated with lipid rafts, which implies that this protein is involved in signal transduction and cell-to-cell communication [66]. As well as its localization within cholesterol-rich lipid rafts, PrPC is also internalized through caveolin-dependent endocytosis and/or clathrin-coated pits [67,68]. In particular, recent studies demonstrate that PrPC moves towards the non-raft region of the PM to interact with the low-density lipoprotein receptor-related protein 1 (LPR1), thus undergoing receptor-mediated endocytosis [69]. Furthermore, increasing evidence in both neuronal and non-neuronal cell types demonstrates the association of PrPC with exosomes [70,71,72,73,74]. These latter are small extracellular vesicles (EVs) which participate in protein homeostasis and contribute to the maintenance of cellular fitness [75]. In particular, exosomes represent the smallest EVs (30–100 nm in diameter) and they originate from the endosomal system upon fusion of multivesicular bodies (MVBs) with the PM. In fact, most cell types remove unwanted and/or damaged material in a constitutive manner through the release of exosomes. Remarkably, exosomal functions extend way beyond the removal of waste material within the extracellular space. In fact, given that exosomes carry several proteins and nucleic acids that can be transferred to neighboring cells, these nanovesicles recently emerged as an unconventional mechanism of cell-to-cell communication, in normal physiological conditions as well as in pathological progression [76,77].
2.2. The Physiological and Beneficial Role of PrPC
In attempts to unravel the putative functions of PrPC, in the early 1990s several PRNP knock-out (KO) mice models were generated. Even though these experimental models helped to demonstrate the infectious potential of PrP, its physiological role still remained enigmatic. In fact, whilst PrPC KO prevented scrapie infection, these transgenic mice developed normally without any apparent CNS structural changes and/or behavioral alterations [78,79,80]. Similarly, pioneer studies in cattle and goats have indicated that PRNP KO animals do not show any developmental alterations or abnormal behavior when compared with controls [81,82]. However, as recently reported, the absence of a remarkable phenotype in these transgenic animals might be due to the compensatory role of two proteins belonging to the PrP family, namely Doppel and Shadoo [83,84]. Nonetheless, recent studies demonstrated that mice lacking PrPC or expressing a mutant PrPC isoform resulted in severe motor alterations due to impaired excitability and synaptic plasticity within cerebellar granule neurons [85,86].
Contrary to in vivo studies, in vitro models revealed a plethora of cellular functions that have been ascribed to PrPC. For instance, within the CNS, PrPC is involved in neurite extension, neuronal differentiation, and neuroprotection [84,87,88]. Moreover, albeit promoting differentiation of tissue-resident stem cells, PrPC may also promote stemness and cell proliferation, depending on specific conditions [84]. Intriguingly, recent studies pointed to a key role for PrPC in the transcriptional regulation of pluripotency and stemness genes of hematopoietic, mammary gland, mesenchymal, embryonic, and neural stem cells, but also influences stem cell fate and cell cycle [89,90,91,92]. More in general, PrPC is involved in copper metabolism, cell proliferation, adhesion, and migration [16].
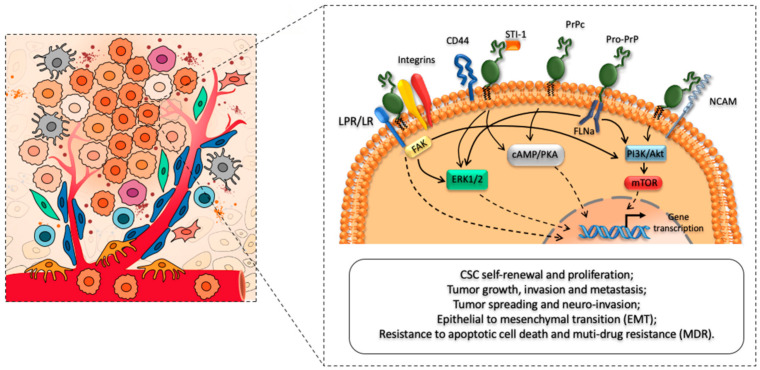
Remarkably, most PrPC biological functions appear to be linked to its binding partners [93]. Several lines of evidence are consistent with a role of PrPC as a molecular scaffold protein involved in signal transduction. In fact, PrPC activity depends on its specific localization within lipid rafts of the PM, where it interacts with a variety of receptors and molecules to transduce intracellular signals. Notably, recent investigations on PrP-interacting proteome (i.e., interactome) helped to disclose, at least in part, the elusive PrPC biology [94,95,96]. In particular, PrPC binds to several cell-surface components, such as the neural cell adhesion molecule (NCAM), LRP1, stress-inducible protein 1/Hsp70/Hsp90 organizing protein (STI1/HOP), 37-kDa/67-kDa laminin receptor precursor/laminin receptor (LPR/LR), filamin A (FLNa), and Notch 1 [35,97,98]. In this way, PrPc modulates the activity of various signaling pathways and/or signaling components including PI3K/Akt/mTOR, cAMP/PKA, MAPK/ERK, PKC, and Fyn kinase [87,99,100,101] For instance, PrPC interacts with the NCAM to promote PrP-dependent neurite outgrowth through activation of the cytosolic Fyn kinase [88]. Likewise, the interaction between several extracellular matrix components (ECM) and transmembrane receptors (i.e., laminin, integrin β1, LRP1, and EGFR) can elicit PrP-mediated neurotrophic effects [95,102,103,104,105].
2.3. PrPC Functions in Cell Survival and Stress Protection
Recent data relate the physiological role of PrPC in promoting cell survival, while rescuing the cell under stressful conditions [30,106,107,108]. In fact, it has been reported that PrPC exerts cytoprotective activity, particularly as regards protection against serum deprivation, DNA damage, and apoptotic and oxidative stress [93,106,109]. Furthermore, PrPC specifically prevents pathological protein aggregation in neuronal cells. For instance, PrPC confers neuroprotection against huntingtin (Htt)-induced toxicity, while its depletion contributes to a loss of function under stress conditions [110]. In fact, depletion of endogenous PrPC by RNA interference (RNAi) in neuronal cell lines results in a marked increase in Htt aggregation, along with a significant reduction in antioxidant enzymes and proteasome activity [110]. Thus, PrPC emerges as seminal in modulating a variety of baseline cell functions to grant cell homeostasis.
The classic role of such a protein was defined as a chaperone-like molecule being able to rescue cell survival. Notably, PrPC associates with several molecular chaperones and co-chaperones such as the heat shock protein (Hsp60) [111], Hsp40 and Hsps70 [112,113], BiP [112], STI1/HOP [22,101], and αβ-crystallin [114]. In particular, these molecular chaperones exhibit a cytoprotective function since they promote proper protein folding and facilitate correct refolding, while disassembling protein aggregates [115,116]. This, in turn, is essential for preserving cellular homeostasis under physiological conditions, while being crucial under stressful conditions to guarantee cell survival.
Mapping of PrPC peptide showed that this protein possesses a highly conserved binding site for Hsp60, a widely distributed and highly conserved molecular chaperone that mediates ATP-dependent folding of polypeptide chains [111,117]. Likewise, PrPc directly binds to αβ-crystallin, a member of small Hsp (sHsp) [114]. Moreover, STI1/HOP, a critical co-chaperone of the Hsp70/Hsp90 machinery [74,100] interacts with PrPC at the PM to trigger neuroprotective signals, while rescuing cells from apoptosis [99,100,118].
Interestingly, experimental evidence demonstrates that heat shock in human NT-2 cells results in the simultaneous induction of both PrPC and Hsp70 [119]. In particular, heat stress-induced PrP transcription and translation occurs through the interaction of heat-shock transcription factor (HSTF) on PRNP gene promoter, resulting in increased protein synthesis [119]. Furthermore, PrPC can act as an antioxidant chaperone to promote neural stem cells (NSC) survival and stemness. Upon stressful conditions (i.e., serum deprivation), an increase in intracellular reactive oxygen species (ROS) levels promotes β-mediated proteolytic cleavage of PrPC into a C-terminal and N-terminal fragment [120,121]. The latter is released extracellularly to trigger the MEK1 pathway, which sustains the antioxidant effects of PrPC [122]. Thus, it is conceivable that PrPC may act as an Hsp in protecting cells from the deleterious effects of heat, serum deprivation, and ROS.
3. The Multi-Faceted Role of PrPC in Cancer initiation and Progression
In addition to being implicated in the pathogenesis and transmission of prion diseases, increasing evidence demonstrates that PrPC contributes to tumorigenesis via multiple pathways by regulating tumor growth, differentiation, and resistance to conventional therapies [21,22,23,28,31,35,47] (Figure 1). Such a role may not be restricted to primary brain tumors, since PrPC is ubiquitously expressed throughout the human body. Given its widespread expression among different non-neuronal cell types, it is reasonable that PrPC plays a key role in various tumors, beyond CNS neoplasms [25,29,32,33,36,37,38]. In fact, mounting evidence indicates that PrPC is highly expressed in several cancer cell types and its over-expression is associated with increased tumor aggressiveness and invasiveness.
Figure 1.
Cellular prion protein (PrPC) as a main regulator of CSCs phenotype, biology, and functioning. The left panel of the cartoon depicts the cross-talk between cancer stem cells (CSCs) and major cellular components (i.e., endothelial cells, fibroblasts, pericytes, macrophages, and T cells) of the tumor micro-environment (TME). The right panel schematizes the role of PrPC in promoting CSC self-renewal, proliferation, and migration. In addition, PrPC interacts with several receptors and cell surface proteins to modulate CSCs’ tumor-initiating and metastatic capacities, while promoting increased therapeutic resistance. In this way, it modulates tumor growth, survival, infiltration, and multi-drug resistance.
Over the past few years, several lines of evidence raised the possibility that PrPC may act way beyond human embryonic and adult resident stem cells [123], thereby implicating PrPc in the biology of CSCs. These latter, which represent a fraction of tumor cells endowed with key feature of normal stem cells, have tumor-initiating potential and emerged as pivot to fuel tumor growth, infiltration, and relapse [124]. As a proof of concept, pioneer studies identified endogenous PrPC to be highly expressed within different subpopulations of CSCs encompassing colorectal, gastric, lung, breast, and GBM cancer cell lines [28,30,37,45,48,125,126,127,128]. Aside from PRNP, other members of the prion gene family were found to be up-regulated in various tumor tissues and cancer cell lines. For instance, an over-expression of the prion-like protein gene PRND (Doppel, Dpl) at both protein and mRNA levels occurs in high-grade astrocytoma and GBM [19,20,129]. Similarly, high levels of PRND are found in non-glial malignant tumors such as gastric adenocarcinoma, anaplastic meningioma, and osteosarcoma [19,39].
Interestingly, a specific analysis of PRNP gene mutations using the Cancer Genome Atlas (TCGA) database revealed a total of 48 somatic mutations in the PRNP gene in different cancers, encompassing lung adenocarcinoma, colorectal adenocarcinoma, endometrial carcinoma, head and neck squamous cell carcinoma, and melanoma [130]. Of note, five of these PRNP gene somatic mutations were considered to be pathogenic since they affect PrPC function, while four were identified as amyloid-prone PRNP somatic mutations [130]. Moreover, in pancreatic ductal adenocarcinoma cell line BxPC-3, Yang et al. [131] identified six missense mutations in four major genes involved in the GPI anchor modification pathway which contributes to increased tumor cell motility.
The multi-faceted role of PrPC in tumorigenesis is greatly increased by recent findings showing how PrPC in its folded conformation may foster tumor progression by regulating CSC oncogenic properties, such as self-renewal, pluripotency gene expression, and differentiation [21,23,132]. Remarkably, PrPC over-expression has been related to the acquisition of a malignant phenotype of CSCs in a variety of solid tumors. In fact, high levels of PrPC correlate with enhanced migratory and invasive capacities of CSCs [22,28,35,48], as well as increased resistance to anti-cancer agents [29,133,134]. Conversely, PrPC depression impairs CSCs proliferation, migration, and invasive potential [22,29,32,50,51], while restoring cell sensitivity to chemo- and radiotherapy [56,135].
In the next section, we discuss evidence on the role of PrPC as the main regulator of CSCs’ phenotype, biology, and functioning.
3.1. The Role of PrPC in CSC Stemness and Differentiation
One of the first indications concerning the role of PrPC in CSC biology dates back to the studies by Fan et al. [25,126], which provided strong evidence that over-expression of PrPC at both protein and mRNA levels occurs in gastric carcinomas and gastric cancer cell lines and correlates with increased invasive potential and therapeutic resistance.
Later on, several lines of evidence propose that high expression of PrPC contributes to cancer cell stemness and differentiation, which in turn became a hot topic in cancer research. For instance, Corsaro and colleagues (2016) provided evidence about a relevant role of PrPC in conferring distinct stem-like features to human GBM CSCs, such as self-renewal ability and pluripotency [47]. Often referred to as glioblastoma stem-like cells (GSCs), these cells display up-regulated levels of PrPC along with a higher expression of several stem cell markers including CD15, CD133, Nanog, Musashi-1, and Sox2 [20,22,47]. Remarkably, PrPC is co-expressed and co-localizes with CD133 on the plasma membrane of neurospheres enriched in stem-like cells, suggesting that these molecules act as a functional complex to sustain GSC proliferation and stemness, while restraining them from differentiation. Conversely, CD133 expression was markedly decreased in PrPC-depleted neurospheres. Again, PrPC down-regulation correlates with a marked suppression of stemness markers in GSCs, which in turn acquire a more differentiated, and thus less oncogenic, phenotype [47]. In fact, silencing PrPC results in the up-regulation of cell differentiation markers, while abrogating GSCs self-renewal and neurosphere formation [22,47]. These data are in line with the evidence that PrPC is key to maintain cancer stemness during colorectal cancer progression [125,133]. In fact, in human colorectal CSCs (CCSCs), PrPC regulates the expression of various stem cell markers (i.e., Nanog, Sox2, ALDH1A1) and particularly Oct4, which governs CSC self-renewal and pluripotency [136]. On the other hand, PRNP knockdown markedly decreases stem cell marker expression as well as sphere formation ability in human CCSCs [125,133]. Notably, high levels of PrPC expression identify a functionally distinct subpopulation of CD44-positive CCSCs which displays greater tumor-initiating and metastatic potential than PrPC-negative ones [48].
Collectively these data suggest that the presence of PrPC is critical to maintain CSC stemness and that its reduction could represent a strategy to force CSC to shift towards a more differentiated and chemotherapy-sensitive phenotype.
3.2. The Role of PrPC in CSC Growth and Proliferation
Recently, CSCs were reported to be highly dependent on PrPC to maintain their growth and proliferation [49,125,128]. In fact, PrPC promotes glucose uptake through Fyn-hypoxia-inducible factor (HIF)-2α-glucose transporter 1 (Glut1) signaling thus supporting CSC growth and survival [137].
Furthermore, consistent evidence indicates that PrPC expression correlates with in vitro proliferation rate and in vivo tumor-initiating activity of CSCs, whereas silencing PrPC strongly affects cell growth and clonogenic and tumorigenic potential [47,48]. For instance, in gastric cancer cells, PrPc up-regulation promotes CSC proliferation through PI3K/Akt activation and subsequent transcriptional activation of CyclinD1 to accelerate the G1/S phase transition [49]. Notably, pioneer studies revealed that endogenous PrPC production in human GBM cell lines is dependent on the cell cycle phase, with the highest expression during the G1-phase [127]. This, in turn, indicates that PrPC contributes to GSCs’ growth through G1/S phase transition and thus enhanced protein synthesis.
Again, PrPC expression correlates with increased cell proliferation and tumorigenesis of human PDAC cell lines by potentiating Notch1 signaling [35]. Remarkably, these effects are reverted by PrPC silencing through Notch1 down-regulation, and combining PrPC and Notch1 inhibition is more effective than targeting single pathways alone. From a mechanistic point of view, PrPC was shown to complex with Notch1 and to enhance Notch1 stability, thereby impairing proteasome-dependent Notch1 degradation. It is remarkable that down-regulation of Notch1, which occurs as a downstream effect of PrPC silencing [35], inhibits the PI3K/Akt/mTOR pathway to abolish CSC stemness, self-renewal, invasiveness, and in vivo tumor growth in GBM [84]. Notably, these effects are reproduced by the ATG inducers AZD8055 and rapamycin, which suppress GSC self-renewal and abolish GSC tumorigenicity through degradation and inhibition of Notch1 [84,138].
Interestingly, most PrPC functions appear to be linked to its binding partners [93]. For instance, PrPC-STI1/HOP binding induces GSC proliferation through the activation of PI3K/Akt and MAPK/ERK1/2 pathways [22,84,128]. Conversely, blockage of this interaction through a HOP peptide mimicking the PrPC-binding site (HOP230–245) impairs PI3K/Akt and Erk1/2 activation, thus affecting GCS proliferation [23]. Again, these effects are replicated in vivo where PrPC/HOP silencing has profound effects on tumor growth and animals’ survival. In fact, administration of HOP230–245 peptide to mice bearing GBM xenografts impairs in vivo tumorigenic potential of GSCs. This, in turn, results in a decreased tumor volume, while extending mice survival [23].
Collectively these data are in line with growing literature indicating that PrPC, either alone or through its binding partners, influences CSC stem cell characteristics, while affecting their growth, proliferation, and clonogenic ability. For example, in melanoma cells, the normal PrPC exists as a pro-PrP. This latter represents a PrP isoform retaining its GPI anchor peptide signal sequence (GPI-PSS) that contains an FLNa binding motif and binds FLNa. The engagement of pro-PrP with FLNa facilitates the recruitment of integrin β1, which ultimately regulates cell proliferation, migration, and spreading, thus providing a growth advantage to melanoma cells [40]. In addition to FLNa, PrPC interacts with Notch1, forming a PrPC/FLNa/Notch1 complex, which is associated with enhanced PDAC proliferation, invasiveness, and xenograft tumor growth [35,84]. Similarly, in gastric cancer cells, the interaction between PrPC and the MGr1-Ag/37 kDa laminin receptor precursor protein (37LRP) is associated with a high tumor proliferation rate [26]. On the other hand, PrPC-induced proliferation in gastric cancer cells is significantly attenuated by inhibition of PI3K/Akt following the knockdown of the MGr1-Ag/37LPR binding partner [26].
3.3. The Role of PrPC in CSC Migration and Invasion
Beyond its effects on CSC proliferation, stemness, and differentiation, it is also noteworthy that PrPC over-expression correlates with CSC enhanced cell migration and invasion, and thus tumor-spreading and metastasis [35,40,41,126,139]. In fact, CSCs are reported to be highly dependent on PrPC to maintain their increased invasive potential and metastatic abilities [32,35,48,126]. These, in turn, are among foremost key features of CSCs which enable them to disseminate within neighboring tissues and to metastasize to distant organs. Within this frame, several studies proposed that PrPC is involved in the regulation of cell adhesion-related proteins [47,132,134,140]. This is the case of the stem cell marker CD44, a cell surface adhesion receptor involved in cell adhesion, motility, and metastasis [141]. In line with this, CD44-positive colorectal CSCs expressing PrPC display enhanced disseminating capacity compared to PrPC-negative ones [48]. Remarkably, CD44 and PrPC are co-expressed and co-localize on the cell membrane in human breast cancer cell line MCF7/Adr [132]. Activation of EGFR by binding with CD44/PrPC results in increased CSC invasion and metastasis via up-regulation of cell adhesion-related proteins such as CD147, matrix metalloproteinase (MMP) 2 (MMP2), and MMP9 [132]. Conversely, siRNA-mediated PrPc depletion in MCF7/Adr cells significantly impairs CSC migration and invasiveness [47,132]. Notably, these effects are mimicked by monoclonal antibodies targeting PrPC which selectively abrogates in vivo PrPC pro-migratory functions, thereby restricting CSC to their primary tumor sites [48]. Similarly, RNAi-mediated PrPC down-regulation inhibits both in vitro and in vivo CCSC tumorigenicity and invasiveness by abrogating epithelial to mesenchymal transition (EMT) related to ERK2 (MAPK1) pathway [48,84]. Consistently, higher PrPC expression induces EMT in epithelial CRC cells through the modulation of E-cadherin and N-cadherin expression as well as β-catenin translocation from membrane to nucleus [139]. In schwannoma cells, PrPC contributes to cell-matrix adhesion by activating the 37/67 kDa non-integrin laminin receptor (LR/37/67 kDa) and downstream FAK signaling pathway [21,84]. Additionally, PrPC can bind to laminin, integrin, vitronectin, and/or HOP/STI1 to modulate CSC adhesion and metastasis formation [22,28,48,142].
Regarding lung adenocarcinoma, PrPC is key to promote cancer cell lamellipodia formation, migration, and invasion via JNK signaling [37]. In fact, knockdown of PrPC expression abrogates these in vitro effects, while decreasing in vivo experimental lung metastasis. This, in turn, is associated with reduced JNK phosphorylation and reduced protein levels of a transcriptional activator of the PRNP promoter, namely, the nuclear factor interleukin 3 (NFIL3) [37,84].
Furthermore, PrPC up-regulation promotes the adhesive, invasive, and in vivo metastatic abilities of gastric cancer cells, thus strongly affecting gastric cancer malignant phenotype. This occurs, at least in part, through PrPC-dependent activation of the MEK/ERK pathway and MMP11 transactivation [126]. As a proof of concept, silencing PrPC with siRNA produced a marked inhibition of in vitro invasive abilities in two gastric cancer cell lines, namely SGC7901 and MKN45 [126]. Similar results were reported in the human breast carcinoma cell line MCF7 [135]. In fact, PrPC over-expression promotes breast CSC invasiveness through ERK and NF-kB-dependent activation of MMP9 promoter, whereas silencing of PrPC inhibits breast cell migration and invasion [32]. Again, reducing PrPC expression alters the spatial distribution of FLNa as well as actin filament organization, thereby impairing cell spreading and migration in human A7 melanoma cell lines [40]. In PDAC cell lines, PrPC expression alters normal physiological functions of FLNa, thereby influencing in vitro CSC migration and invasiveness as well as in vivo tumor growth and infiltration [34].
Independently of its binding with FLNa, PrPC affects the cytoskeletal organization by modulating the Akt-hsp27-F-actin axis, thus enhancing cancer cell migration in M2 melanoma cells which lack FLNa expression [41]. In particular, in FLNa-deficient M2 melanoma cells, PrPC stabilizes Akt levels and its interaction with hsp27 to regulate hsp27 phosphorylation, actin polymerization, and thus cell migration [41].
3.3.1. The Role of PrPC in Tumor Spreading and Neuro-Invasion
Recently, it was suggested that PrPC might be involved in tumor spreading along the perineural pathway, also known as perineural invasion (PNI), and thus distant metastasis.
This is the case, for instance, of colorectal, gastric, and prostate cancer cells that use PNI as a route for tissue dissemination and tumor-spreading to distant organs [143,144]. Although the association between PrPC and PNI is yet poorly characterized, Zhou et al. (2014) report that in gastric cancer, PrPC is associated with several clinic-pathological parameters including depth of invasion and lymph node metastasis [54]. In line with this, increased levels of PrPC-positive cells are identified in the lymph nodes that are invaded by colorectal CSC [145]. Moreover, high levels of PrPC are present in colorectal CSC that are found invading tumor stroma and metastasis of liver and lung [145].
Again, extra-pancreatic PNI and nodal involvement along known peripancreatic neural plexuses occur in up to 100% of pancreatic cancers [36]. Being detected already at early stages, extra-pancreatic PNI negatively impact on the overall survival of PDAC patients [146]. Remarkably, preliminary data on surgically resected specimens of PDAC disclose a potential correlation between increased PrPC expression and PNI [36]. Although not significant so far, these preliminary data are encouraging since they evidence a trend towards a higher PrPC expression in PDAC patients with PNI [36]. Notably, the presence of PrPC within PDAC tissue might be a marker that could contribute to explaining the biology of the disease in terms of aggressiveness, explaining the uniquely preferred PNI of PDAC, based on the relationship of prions with neurotropism and neurodegenerative disease [36,147]. In this scenario, an analogous significance for PrPC in GBM has revealed how tumor diffusion is reminiscent of the spreading mechanisms in neurodegeneration. This implies a relationship between PrPC and neurotropism, which again is a biological peculiarity of PDAC [36,84].
Even in the cases eligible for surgical resection, the poor prognosis of most of these solid tumors is due to an extensive local infiltration and to an early lymphatic spreading [36]. In fact, after surgical resection, most of these solid tumors frequently recur both within the primary site and/or to distant, secondary sites. Thus, this aspect deserves particular mention and advances in understanding the biology of tumor PNI are urgently needed.
3.3.2. PrPC Spreading and PrPC-Mediated Epigenetic Effects
Recent findings suggest that PrPC can take part in cell-to-cell communication within the tumor microenvironment (TME) acting both as an autocrine and/or paracrine signaling molecule to foster CSC malignant phenotype. For instance, PrPC is abundantly released from schwannoma cells, either as a free cleaved peptide or via exosomes, to promote malignant cell growth in an autocrine fashion [21]. In addition, when functioning in a paracrine mode, secreted PrPC can disseminate within the TME to activate various downstream pathways (i.e., ERK1/2, PI3K/Akt, FAK) within neighboring cells, thus promoting tumor growth and infiltration [21,47]. In breast cancer, secreted PrPC can directly sequester chemotherapeutic drugs, blocking their cytotoxic activity, thus providing a growth advantage to breast CSCs [84,148]. Conversely, genetic depletion of PrPC prevents such an interaction, while sensitizing breast CSCs to chemotherapy [84,148].
In line with this, the PrPC cell surface ligand STI1/HOP can be translocated to the cell surface and/or secreted outside the tumor cell, acting both as an autocrine and paracrine factor in promoting cell growth, proliferation, and migration of various tumor cells, encompassing ovarian [149,150], renal [151], and glioma (Erlich et al., 2007) [128] cancer cells. In the case of GBM, Erlich and colleagues (2007) report that STI1 is secreted by and induces tumor cell proliferation in human A172 GBM cell line through MAPK and PI3K pathways [128]. Moreover, Wang et al. (2017) demonstrate that STIP1/HOP promotes osteolytic bone metastasis in renal cell carcinoma (RCC) both in an autocrine and paracrine manner [151]. In particular, when exposed on the extracellular surface, STIP1 activates autocrine STIP1-ALK2-SMAD1/5 signaling in bone metastatic RCC tumor cells thus promoting cell proliferation, migration, and invasion [151]. In addition, STIP1 can be secreted within the extracellular space to activate the paracrine STIP1-PrPC-ERK1/2 pathway which promotes osteoclasts differentiation and aggravates osteolysis [151]. Importantly, this results in a vicious cycle between tumor cell and bone niche which contributes to the early osteolytic progression, a recurring feature of RCC bone metastasis [151,152]. Notably, treatment with anti-STIP1 and/or anti-PrPC antibody significantly abrogates these effects in both enriched bone-seeking RCC cell line OS-RC-2-BM5 and the murine preosteoclast cell line RAW264 [151].
Finally, it is worth of mentioning that emerging evidence suggests that PrPC can act as an epigenetic modulator of tumor invasiveness and metastatic potential. Remarkably, Wang et al. (2012) show that PrPC mediates colorectal cancer cell invasive and metastatic capacities by regulating SATB1 expression via epigenetic activation of the Fyn-SP1 pathway [139]. In particular, the authors demonstrate direct binding of SP1 to the SATB1 promoter, which is largely abolished by PrPC depletion [139]. On the other hand, PrPC overexpression accelerates tumor metastasis by upregulating SATB1 expression. This latter, which is a matrix attachment region (MARAR)-binding protein, is a master regulator of global gene expression conferring an aggressive, pro-metastatic phenotype in colorectal cancer cells [139].
3.4. Role of PrPC in Multidrug Resistance (MDR)
Compelling evidence support the view that PrPC may mediate protection of CSC from apoptosis, along with the acquisition of multidrug resistance (MDR), which in turn are responsible of treatment failure, tumor recurrence, and poor survival of cancer patients [30,48]. Remarkably, high PrPC expression correlates with MDR in CSCs as shown in gastric, breast, glioma, and colorectal cancers [25,29,31,49,126,153]. For instance, in gastric carcinoma, PrPC over-expression provides gastric cancer cells with increased resistance to doxorubicin [126]. In the case of GBM, PrPC has shown to exert a cytoprotective role in different glioma cell lines, conferring resistance to apoptotic cell death [154]. Similarly, Meslin et al., (2007) [31] demonstrate that PrPC over-expression in breast cancer cells is associated with resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. Conversely, down-regulation of PrPC by siRNA sensitizes breast cancer cells to adriamycin and TRAIL-mediated cell death [31].
In line with this, PrPC suppresses adriamycin-induced apoptosis and alters Bcl-2 and Bax expression, which may be another pathway contributing to PrPC-related MDR in SGC7901/ADR gastric cancer cell lines [25]. In particular, in these tumor cells, PrPC confers resistance of both P-glycoprotein (P-gp)-related and P-gp-non related drugs, which is accompanied by decreased accumulation and increased releasing amount of adriamycin in PrPC-overexpressing cells. Interestingly, Pan and colleagues (2005) [25] demonstrate that PrPC upregulates the expression of P-gp, an ATP-binding cassette (ABC) drug efflux pump, thus allowing the cell to evade cytotoxic attacks from a variety of chemotherapeutic agents via its anti-apoptotic activity. Similarly, in breast cancer, PrPC physically interacts and co-localizes with P-gp on the cell membrane of MDR breast cancer cell line MCF7/Adr [135]. Disruption of PrPC/P-gp complex has profound effects on tumor survival and aggressiveness, since it markedly reduces the anti-apoptotic activity, while promoting the reversal of PrPC-mediated drug resistance in MDR breast cancer cells [135]. Furthermore, the direct interaction between PrPC and EGFR is determinant for cisplatin/oxaliplatin resistance in colorectal cancer cells via FOXO3a-Krüppel-like factor 5 (KLF5) signaling, thus contributing to the development of metastases and poor outcome in CRC patients [155]. Again, PrPC/CD44 interaction promotes chemoresistance and tumor progression in MDR breast cancer cells [132].
It is important to remark that several beneficial effects including sensitization to chemotherapy are obtained by means of PrPC-targeted strategies resulting in PrPC inhibition/downregulation. In fact, both anti-PrPC antibodies and/or silencing PrPC expression by antisense of RNAi technology were shown to reverse, at least in part, the MDR phenotype of CSC in various solid tumors [25,51,84,126,132].
4. PrPC as a Potential Biomarker in Human Cancers
Beyond its effects on cancer cell biology and function, compelling evidence suggests that PrPC expression rate may be of clinical relevance in several malignancies. This issue is of particular interest in cancer research since it may provide early detection of high-risk pre-malignant and early-stage cancerous lesions, which in turn would facilitate early diagnosis and therapeutic intervention. For instance, Le Corre and colleagues (2019) [45] reported that plasma levels of PrPC are elevated in metastatic CRC patients compared with healthy subjects, thus suggesting that PrPC may serve as a potential biomarker for patient stratification in CRC [45]. Furthermore, high levels of circulating PrPC in metastatic CRC patients’ plasma is predictive of poor prognosis [45]. In line with this, high-resolution cell surface proteomic evaluation identified PrPC as a potential biomarker for colorectal adenoma-to-carcinoma progression, which could potentially discriminate normal colon and low-risk adenomas from high-risk adenoma and early-stage colorectal cancer patients [53].
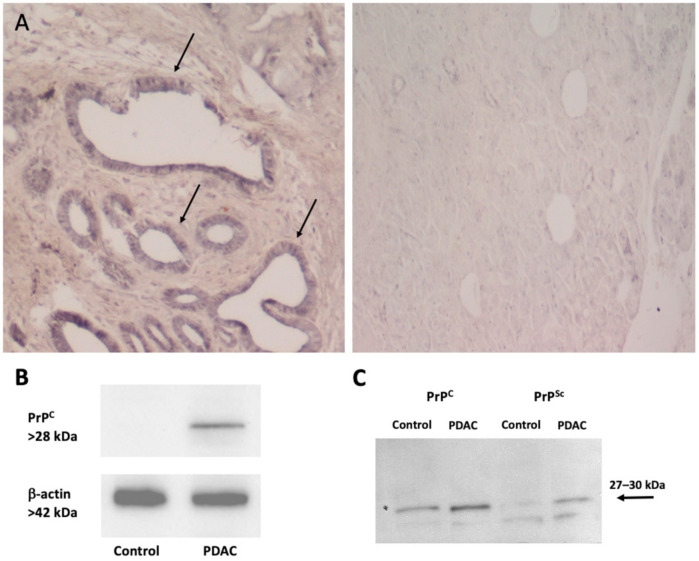
Notably, numerous lines of evidence support the potential role of PrPC in regulating cancer sensitivity to chemotherapy, and thus monitoring therapeutic efficacy and patients’ prognosis. For instance, Wang JH et al. (2011) [55] reported that a high PrPC expression in gastric cancer patients predicts both a poorer response to chemotherapy and a poorer prognosis. In fact, when compared with low PrPC expression group, patients with higher PrPC expression show increased resistance to chemotherapy, a lower 2-year survival rate and higher mortality rate [55]. Accordingly, PrPC over-expression in estrogen receptor (ER)-negative breast cancer patients is associated with a lower sensitivity to chemotherapy, thus suggesting that PrPC could be predictive for the benefit of adjuvant chemotherapy in ER-negative patients [56]. Expression of PrPc in human PDAC biopsies is associated with a worse survival than PrP-negative cases, supporting a critical tumor-promoting role of PrPC in PDAC [35]. Remarkably, western blot and immunohistochemical analyses from surgically resected specimens of PDAC demonstrate a marked difference between PDAC tissues compared to control. In detail, in PDAC specimens PrPC is overexpressed very selectively within the ductal compartment, whereas normal control tissues feature only few ductal epithelial cells with moderate PrP-staining [36] (Figure 2). Notably, PrPC expression was independent of the presence of dysplastic areas surrounding “healthy” pancreas, thus indicating that PrPC levels correlates with tumor invasiveness and aggressiveness, and not with preneoplastic lesions [36]. Although the mean patients’ follow-up is yet too short to correlate PrPC expression to clinical outcome, the authors report a significant difference between groups when analyzing the possible relationship between PrPC and cancer stage after resection based on pTNM [36]. Even if these data are yet preliminary in identifying PrPC as a potential biomarker in the follow-up of patients following surgical resection, they are nevertheless encouraging. However, further studies are needed to completely define a prognostic value of PrPC detection.
Figure 2.
PrPC expression in human pancreatic tissues. (A) The figure reports representative pictures of PrPC immunohistochemistry of pancreatic ductal adenocarcinoma (PDAC) tissue (left panel) compared with control tissue (right panel). Immunoperoxidase shows PrPC-specific labelling in ductal cells (arrows) from a human tumor sample (left panel). Remarkably, the increase in PrPc expression occurs along with a marked loss of cellular architecture within pancreatic tumoral tissue, which features enlarged and irregularly shaped ducts within abundant extracellular matrix. In contrast, normal human pancreas (right panel) possesses a well-preserved architecture of both acinar cells and ductal system, along with a weak PrPC-staining in the ductal epithelial cells (original magnification 10×). (B) The figure reports a representative immunoblot for PrPC and the house keeping protein β-actin in control and PDAC tissues. (C) The figure reports representative western blotting comparing scrapie prion protein (PrPSc) and PrPC (with or without proteinase K) in control and PDAC tissues as measured in Table 1. Images were obtained by an author of the manuscript (M.A. Giambelluca). Original western blot images (Figures S1 and S2) and densitometry readings (Tables S1 and S2) were provided in Supplemental Materials.
In addition, as reported by Du et al. (2005) [25], PrPC is ubiquitously expressed in gastric carcinoma cell lines and tissue, while negatively or weakly expressed in normal gastric mucosa and adjacent non-tumoral tissues. Remarkably, PrPC overexpression correlates with histopathological degree of differentiation and disease progression in gastric adenocarcinomas, being significantly higher in poorly-differentiated gastric carcinoma [25,54]. In line with this, pioneer studies in primary brain tumors identified endogenous PrPC as being highly expressed within human GBM cell lines constitutively [127,156]. Similarly, Comincini and colleagues [20] found that GBM tumor samples display overexpression in PrPC and prion-like protein Doppel, whilst PRND expression directly relates to tumor malignancy, thus being associated with a worse prognosis in high-grade astrocytoma [84]. Recently, an association between PrPC/STI-1(HOP) expression and lower patient survival has been confirmed in human astrocytoma samples. In detail, human GBM samples feature an increased expression in PrPC/STI-1(HOP) compared with low-grade astrocytoma (grade I–III) or non-tumoral tissue [23,84]. Again, higher PrPC-positive rates and stronger PrPC staining are found in breast [56], colorectal [42], gastric cancer [54,55], and head and neck squamous cell carcinoma (HNSCCs) [38]. Although the PrPC-positive rates may vary between different cancer cell types, collectively these data demonstrate that increased PrPC expression closely associates with the pathological degree and clinical progression of human cancers, while emphasizing a common molecular mechanism underlying the contributory role of PrPC in tumor progression. However, further studies with larger sample sizes and in different cohorts of patients are needed in order to completely define the diagnostic and prognostic value of PrPC detection. Nonetheless, it is fascinating that PrPC emerges as a kernel within a network to link the CNS to peripheral organs even in physiological conditions, which is paradoxically occurring in cancer.
Table 1.
PrPC compared with PrPSc in control and PDAC tissues.
| Protein | Control | Tumor | Ratio T/C |
|---|---|---|---|
| PrPC | 32.80 ± 3.5 | 44.34 ± 5.4 | 1.35 ± 0.1 |
| PrPSc | 22.57 ± 4.1 | 50.36 ± 6.8 * | 2.26 ± 0.1 |
| Ratio PrPSc/PrPC | 0.68 ± 0.1 | 1.13 ± 0.1 * | 1.70 ± 0.1 |
Western blotting with (PrPSc) or without (PrPC) pre-treatment with proteinase K in control and PDAC tissues were compared and their ratio was provided. In PDAC tumor tissue, the increase in PrPSc surpasses at large that reported for PrPC. Values are given as the mean ± S.E.M. optical density * p < 0.05 compared with control.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/2/170/s1.
Author Contributions
Writing—original draft preparation, L.R. and F.F.; writing—review, editing, and artwork, representative pictures, L.R., F.B., C.L.B., and M.A.G.; conceptualization, L.R., L.M., A.F., and F.F.; supervision, F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Ministero della Salute (Ricerca Corrente 2020) and University of Pisa (Fondi di Ateneo).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prusiner S.B. Prions. Proc. Natl. Acad. Sci. USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fornai F., Ferrucci M., Gesi M., Di Poggio A.B., Giorgi F.S., Biagioni F., Paparelli A. A hypothesis on prion disorders: Are infectious, inherited, and sporadic causes so distinct? Brain Res. Bull. 2006;69:95–100. doi: 10.1016/j.brainresbull.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner S.B. Scrapie prions. Annu. Rev. Microbiol. 1989;43:345–374. doi: 10.1146/annurev.mi.43.100189.002021. [DOI] [PubMed] [Google Scholar]
- 4.Prusiner S.B., Scott M.R., Dearmond S.J., Cohen F.E. Prion Protein Biology Review. Cell. 1998;93:337–348. doi: 10.1016/S0092-8674(00)81163-0. [DOI] [PubMed] [Google Scholar]
- 5.Prusiner S.B. Molecular biology of prion diseases. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 6.Meyer R.K., McKinley M.P., Bowman K.A., Braunfeld M.B., Barry R.A., Prusiner S.B. Separation and properties of cellular and scrapie prion proteins. Proc. Natl. Acad. Sci. USA. 1986;83:2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan K.M., Baldwin M., Nguyen J., Gasset M., Serban A., Groth D., Mehlhorn I., Huang Z., Fletterick R.J., Cohen F.E., et al. Conversion of α-helices into β-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein F.H., Prusiner S.B. Prions and Neurodegenerative Diseases. N. Engl. J. Med. 1987;317:1571–1581. doi: 10.1056/NEJM198712173172505. [DOI] [PubMed] [Google Scholar]
- 9.Dearmond S.J., McKinley M.P., Barry R.A., Braunfeld M.B., McColloch J.R., Prusinert S.B. Identification of prion amyloid filaments in scrapie-infected brain. Cell. 1985;41:221–235. doi: 10.1016/0092-8674(85)90076-5. [DOI] [PubMed] [Google Scholar]
- 10.Ma J., Lindquist S. Conversion of PrP to a self-perpetuating PrPSc-like conformation in the cytosol. Science. 2002;298:1785–1788. doi: 10.1126/science.1073619. [DOI] [PubMed] [Google Scholar]
- 11.Corsaro A., Thellung S., Villa V., Nizzari M., Florio T. Role of prion protein aggregation in neurotoxicity. Int. J. Mol. Sci. 2012;13:8648–8669. doi: 10.3390/ijms13078648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heiseke A., Aguib Y., Riemer C., Baier M., Schätzl H.M. Lithium induces clearance of protease resistant prion protein in prion-infected cells by induction of autophagy. J. Neurochem. 2009;109:25–34. doi: 10.1111/j.1471-4159.2009.05906.x. [DOI] [PubMed] [Google Scholar]
- 13.Heiseke A., Aguib Y., Schatzl H.M. Autophagy, Prion Infection and their Mutual Interactions. Curr. Issues Mol. Biol. 2009;12:87–97. [PubMed] [Google Scholar]
- 14.Speldewinde S.H., Doronina V.A., Grant C.M. Autophagy protects against de novo formation of the [PSI+] prion in yeast. Mol. Biol. Cell. 2015;26:4541–4551. doi: 10.1091/mbc.E15-08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prusiner S.B. A unifying role for prions in neurodegenerative diseases. Science. 2012;336:1511–1513. doi: 10.1126/science.1222951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguib Y., Heiseke A., Gilch S., Riemer C., Baier M., Schätzl H.M., Ertmer A. Autophagy induction by trehalose counteracts cellular prion infection. Autophagy. 2009;5:361–369. doi: 10.4161/auto.5.3.7662. [DOI] [PubMed] [Google Scholar]
- 17.Lin C.F., Yu K.H., Jheng C.P., Chung R., Lee C.I. Curcumin reduces amyloid fibrillation of prion protein and decreases reactive oxidative stress. Pathogens. 2013;2:506–519. doi: 10.3390/pathogens2030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J.H., Moon J.H., Kim S.W., Jeong J.K., Nazim U.M.D., Lee Y.J., Seol J.W., Park S.Y. EGCG-mediated autophagy flux has a neuroprotection effect via a class III histone deacetylase in primary neuron cells. Oncotarget. 2015;6:9701–9717. doi: 10.18632/oncotarget.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comincini S., Facoetti A., Del Vecchio I., Peoc’h K., Laplanche J.L., Magrassi L., Ceroni M., Ferretti L., Nano R. Differential expression of the prion-like protein doppel gene (PRND) in astrocytomas: A new molecular marker potentially involved in tumor progression. Anticancer Res. 2004;24:1507–1517. [PubMed] [Google Scholar]
- 20.Comincini S., Ferrara V., Arias A., Malovini A., Azzalin A., Ferretti L., Benericetti E., Cardarelli M., Gerosa M., Passarin M.G., et al. Diagnostic value of PRND gene expression profiles in astrocytomas: Relationship to tumor grades of malignancy. Oncol. Rep. 2007;17:989–996. doi: 10.3892/or.17.5.989. [DOI] [PubMed] [Google Scholar]
- 21.Provenzano L., Ryan Y., Hilton D.A., Lyons-Rimmer J., Dave F., Maze E.A., Adams C.L., Rigby-Jones R., Ammoun S., Hanemann C.O. Cellular prion protein (PrP C) in the development of Merlin-deficient tumours. Oncogene. 2017;36:6132–6142. doi: 10.1038/onc.2017.200. [DOI] [PubMed] [Google Scholar]
- 22.Iglesia R.P., Prado M.B., Cruz L., Martins V.R., Santos T.G., Lopes M.H. Engagement of cellular prion protein with the co-chaperone Hsp70/90 organizing protein regulates the proliferation of glioblastoma stem-like cells. Stem Cell Res. Ther. 2017;8:76. doi: 10.1186/s13287-017-0518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopes M.H., Santos T.G., Rodrigues B.R., Queiroz-Hazarbassanov N., Cunha I.W., Wasilewska-Sampaio A.P., Costa-Silva B., Marchi F.A., Bleggi-Torres L.F., Sanematsu P.I., et al. Disruption of prion protein-HOP engagement impairs glioblastoma growth and cognitive decline and improves overall survival. Oncogene. 2015;34:3305–3314. doi: 10.1038/onc.2014.261. [DOI] [PubMed] [Google Scholar]
- 24.Castle A.R., Gill A.C. Physiological functions of the cellular prion protein. Front. Mol. Biosci. 2017;4:19. doi: 10.3389/fmolb.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du J., Pan Y., Shi Y., Guo C., Jin X., Sun L., Liu N., Qiao T., Fan D. Overexpression and significance of prion protein in gastric cancer and multidrug-resistant gastric carcinoma cell line SGC7901/ADR. Int. J. Cancer. 2005;113:213–220. doi: 10.1002/ijc.20570. [DOI] [PubMed] [Google Scholar]
- 26.Luo G., Wang W., Wu Q., Lu Y., Su T., Gu N., Li K., Wang J., Du R., Zhao X., et al. MGr1-Antigen/37 kDa laminin receptor precursor promotes cellular prion protein induced multi-drug-resistance of gastric cancer. Oncotarget. 2017;8:71630–71641. doi: 10.18632/oncotarget.17795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J.H., Du J.P., Li S.J., Zhai L.P., Yang X.Y., Wang Z.H., Wu Z.T., Han Y. Octarepeat peptides of prion are essential for multidrug resistance in gastric cancer cells. J. Dig. Dis. 2012;13:143–152. doi: 10.1111/j.1751-2980.2011.00563.x. [DOI] [PubMed] [Google Scholar]
- 28.de Lacerda T.C.S., Costa-Silva B., Giudice F.S., Dias M.V.S., de Oliveira G.P., Teixeira B.L., dos Santos T.G., Martins V.R. Prion protein binding to HOP modulates the migration and invasion of colorectal cancer cells. Clin. Exp. Metastasis. 2016;33:441–451. doi: 10.1007/s10585-016-9788-8. [DOI] [PubMed] [Google Scholar]
- 29.Lee J.H., Yun C.W., Lee S.H. Cellular prion protein enhances drug resistance of colorectal cancer cells via regulation of a survival signal pathway. Biomol. Ther. 2018;26:313–321. doi: 10.4062/biomolther.2017.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roucou X., Giannopoulos P.N., Zhang Y., Jodoin J., Goodyer C.G., LeBlanc A. Cellular prion protein inhibits proapoptotic Bax conformational change in human neurons and in breast carcinoma MCF-7 cells. Cell Death Differ. 2005;12:783–795. doi: 10.1038/sj.cdd.4401629. [DOI] [PubMed] [Google Scholar]
- 31.Meslin F., Hamaï A., Gao P., Jalil A., Cahuzac N., Chouaib S., Mehrpour M. Silencing of prion protein sensitizes breast adriamycin-resistant carcinoma cells to TRAIL-mediated cell death. Cancer Res. 2007;67:10910–10919. doi: 10.1158/0008-5472.CAN-07-0512. [DOI] [PubMed] [Google Scholar]
- 32.Gil M., Kim Y.K., Kim K.E., Kim W., Park C.S., Lee K.J. Cellular prion protein regulates invasion and migration of breast cancer cells through MMP-9 activity. Biochem. Biophys. Res. Commun. 2016;470:213–219. doi: 10.1016/j.bbrc.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 33.Sauer H., Dagdanova A., Hescheler J., Wartenberg M. Redox-regulation of intrinsic prion expression in multicellular prostate tumor spheroids. Free Radic. Biol. Med. 1999;27:1276–1283. doi: 10.1016/S0891-5849(99)00164-1. [DOI] [PubMed] [Google Scholar]
- 34.Li C., Yu S., Nakamura F., Yin S., Xu J., Petrolla A.A., Singh N., Tartakoff A., Abbott D.W., Xin W., et al. Binding of pro-prion to filamin A disrupts cytoskeleton and correlates with poor prognosis in pancreatic cancer. J. Clin. Investig. 2009;119:2725–2736. doi: 10.1172/JCI39542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y., Yu S., Huang D., Cui M., Hu H., Zhang L., Wang W., Parameswaran N., Jackson M., Osborne B., et al. Cellular Prion Protein Mediates Pancreatic Cancer Cell Survival and Invasion through Association with and Enhanced Signaling of Notch1. Am. J. Pathol. 2016;186:2945–2956. doi: 10.1016/j.ajpath.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bianchini M., Giambelluca M.A., Scavuzzo M.C., Di Franco G., Guadagni S., Palmeri M., Furbetta N., Gianardi D., Funel N., Pollina L.E., et al. The occurrence of prion protein in surgically resected pancreatic adenocarcinoma. Pancreatology. 2020;20:1218–1225. doi: 10.1016/j.pan.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Lin S.C., Lin C.H., Shih N.C., Liu H.L., Wang W.C., Lin K.Y., Liu Z.Y., Tseng Y.J., Chang H.K., Lin Y.C., et al. Cellular prion protein transcriptionally regulated by NFIL3 enhances lung cancer cell lamellipodium formation and migration through JNK signaling. Oncogene. 2020;39:385–398. doi: 10.1038/s41388-019-0994-0. [DOI] [PubMed] [Google Scholar]
- 38.Wei W., Shi Q., Zhang N.S., Xiao K., Chen L.N., Yang X.D., Ji J.F., Dong X.P. Expression of prion protein is closely associated with pathological and clinical progression and abnormalities of p53 in head and neck squamous cell carcinomas. Oncol. Rep. 2016;35:817–824. doi: 10.3892/or.2015.4425. [DOI] [PubMed] [Google Scholar]
- 39.Sollazzo V., Galasso M., Volinia S., Carinci F. Prion proteins (PRNP and PRND) are over-expressed in osteosarcoma. J. Orthop. Res. 2012;30:1004–1012. doi: 10.1002/jor.22034. [DOI] [PubMed] [Google Scholar]
- 40.Li C., Yu S., Nakamura F., Pentikäinen O.T., Singh N., Yin S., Xin W., Sy M.S. Pro-prion binds filamin A, facilitating its interaction with integrin β1, and contributes to melanomagenesis. J. Biol. Chem. 2010;285:30328–30339. doi: 10.1074/jbc.M110.147413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ke J., Wu G., Zhang J., Li H., Gao S., Shao M., Gao Z., Sy M.S., Cao Y., Yang X., et al. Melanoma migration is promoted by prion protein via Akt-hsp27 signaling axis. Biochem. Biophys. Res. Commun. 2020;523:375–381. doi: 10.1016/j.bbrc.2019.12.042. [DOI] [PubMed] [Google Scholar]
- 42.Antonacopoulou A.G., Palli M., Marousi S., Dimitrakopoulos F.I., Kyriakopoulou U., Tsamandas A.C., Scopa C.D., Papavassiliou A.G., Kalofonos H.P. Prion protein expression and the M129V polymorphism of the PRNP gene in patients with colorectal cancer. Mol. Carcinog. 2010;49:693–699. doi: 10.1002/mc.20642. [DOI] [PubMed] [Google Scholar]
- 43.Déry M.A., Jodoin J., Ursini-Siegel J., Aleynikova O., Ferrario C., Hassan S., Basik M., LeBlanc A.C. Endoplasmic reticulum stress induces PRNP prion protein gene expression in breast cancer. Breast Cancer Res. 2013;15:R22. doi: 10.1186/bcr3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang Z., Ma J., Zhang W., Gong C., He J., Wang Y., Yu G., Yuan C., Wang X., Sun Y., et al. The role of prion protein expression in predicting gastric cancer prognosis. J. Cancer. 2016;7:984–990. doi: 10.7150/jca.14237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Corre D., Ghazi A., Balogoun R., Pilati C., Aparicio T., Martin-Lannerée S., Marisa L., Djouadi F., Poindessous V., Crozet C., et al. The cellular prion protein controls the mesenchymal-like molecular subtype and predicts disease outcome in colorectal cancer. EBioMedicine. 2019;46:94–104. doi: 10.1016/j.ebiom.2019.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L., Shi P., Zhao G., Xu J., Peng W., Zhang J., Zhang G., Wang X., Dong Z., Chen F., et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020;5:8. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corsaro A., Bajetto A., Thellung S., Begani G., Villa V., Nizzari M., Pattarozzi A., Solari A., Gatti M., Pagano A., et al. Cellular prion protein controls stem cell-like properties of human glioblastoma tumor-initiating cells. Oncotarget. 2016;7:38638–38657. doi: 10.18632/oncotarget.9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du L., Rao G., Wang H., Li B., Tian W., Cui J., He L., Laffin B., Tian X., Hao C., et al. CD44-positive cancer stem cells expressing cellular prion protein contribute to metastatic capacity in colorectal cancer. Cancer Res. 2013;73:2682–2694. doi: 10.1158/0008-5472.CAN-12-3759. [DOI] [PubMed] [Google Scholar]
- 49.Liang J., Pan Y., Zhang D., Guo C., Shi Y., Wang J., Chen Y., Wang X., Liu J., Guo X., et al. Cellular prion protein promotes proliferation and G1/S transition of human gastric cancer cells SGC7901 and AGS. FASEB J. 2007;21:2247–2256. doi: 10.1096/fj.06-7799com. [DOI] [PubMed] [Google Scholar]
- 50.McEwan J.F., Windsor M.L., Cullis-Hill S.D. Antibodies to prion protein inhibit human colon cancer cell growth. Tumor Biol. 2009;30:141–147. doi: 10.1159/000225243. [DOI] [PubMed] [Google Scholar]
- 51.Yun C.W., Yun S., Lee J.H., Han Y.S., Yoon Y.M., An D., Lee S.H. Silencing prion protein in ht29 human colorectal cancer cells enhances anticancer response to fucoidan. Anticancer Res. 2016;36:4449–4458. doi: 10.21873/anticanres.10989. [DOI] [PubMed] [Google Scholar]
- 52.Zhuang D., Liu Y., Mao Y., Gao L., Zhang H., Luan S., Huang F., Li Q. TMZ-induced PrPc/par-4 interaction promotes the survival of human glioma cells. Int. J. Cancer. 2012;130:309–318. doi: 10.1002/ijc.25985. [DOI] [PubMed] [Google Scholar]
- 53.De Wit M., Jimenez C.R., Carvalho B., Belien J.A.M., Delis-Van Diemen P.M., Mongera S., Piersma S.R., Vikas M., Navani S., Pontén F., et al. Cell surface proteomics identifies glucose transporter type 1 and prion protein as candidate biomarkers for colorectal adenoma-to-carcinoma progression. Gut. 2012;61:855–864. doi: 10.1136/gutjnl-2011-300511. [DOI] [PubMed] [Google Scholar]
- 54.Zhou L., Shang Y., Liu C., Li J., Hu H., Liang C., Han Y., Zhang W., Liang J., Wu K. Overexpression of PrPc, combined with MGr1-Ag/37LRP, is predictive of poor prognosis in gastric cancer. Int. J. Cancer. 2014;135:2329–2337. doi: 10.1002/ijc.28883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J.H., Du J.P., Zhang Y.H., Zhao X.J., Fan R.Y., Wang Z.H., Wu Z.T., Han Y. Dynamic changes and surveillance function of prion protein expression in gastric cancer drug resistance. World J. Gastroenterol. 2011;17:3986–3993. doi: 10.3748/wjg.v17.i35.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meslin F., Conforti R., Mazouni C., Morel N., Tomasic G., Drusch F., Yacoub M., Sabourin J.C., Grassi J., Delaloge S., et al. Efficacy of adjuvant chemotherapy according to Prion protein expression in patients with estrogen receptor-negative breast cancer. Ann. Oncol. 2007;18:1793–1798. doi: 10.1093/annonc/mdm406. [DOI] [PubMed] [Google Scholar]
- 57.Oesch B., Westaway D., Wälchli M., McKinley M.P., Kent S.B.H., Aebersold R., Barry R.A., Tempst P., Teplow D.B., Hood L.E., et al. A cellular gene encodes scrapie PrP 27–30 protein. Cell. 1985;40:735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- 58.Makrinou E., Collinge J., Antoniou M. Genomic characterization of the human prion protein (PrP) gene locus. Mamm. Genome. 2002;13:696–703. doi: 10.1007/s00335-002-3043-0. [DOI] [PubMed] [Google Scholar]
- 59.Acevedo-Morantes C.Y., Wille H. The structure of human prions: From biology to structural models—Considerations and pitfalls. Viruses. 2014;6:3875–3892. doi: 10.3390/v6103875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rane N.S., Yonkovich J.L., Hegde R.S. Protection from cytosolic prion protein toxicity by modulation of protein translocation. EMBO J. 2004;23:4550–4559. doi: 10.1038/sj.emboj.7600462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scheckel C., Aguzzi A. Prions, prionoids and protein misfolding disorders. Nat. Rev. Genet. 2018;19:405–418. doi: 10.1038/s41576-018-0011-4. [DOI] [PubMed] [Google Scholar]
- 62.Stahl N., Borchelt D.R., Hsiao K., Prusiner S.B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987;51:229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- 63.Wiseman F.K., Cancellotti E., Piccardo P., Iremonger K., Boyle A., Brown D., Ironside J.W., Manson J.C., Diack A.B. The Glycosylation Status of PrP C Is a Key Factor in Determining Transmissible Spongiform Encephalopathy Transmission between Species. J. Virol. 2015;89:4738–4747. doi: 10.1128/JVI.02296-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yi C.W., Wang L.Q., Huang J.J., Pan K., Chen J., Liang Y. Glycosylation Significantly Inhibits the Aggregation of Human Prion Protein and Decreases Its Cytotoxicity. Sci. Rep. 2018;8:12603. doi: 10.1038/s41598-018-30770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li C., Xin W., Sy M.S. Binding of pro-prion to filamin A: By design or an unfortunate blunder. Oncogene. 2010;29:5329–5345. doi: 10.1038/onc.2010.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis V., Hooper N.M. The role of lipid rafts in prion protein biology. Front. Biosci. 2011;16:151–168. doi: 10.2741/3681. [DOI] [PubMed] [Google Scholar]
- 67.Hooper N.M., Taylor D.R., Watt N.T. Mechanism of the metal-mediated endocytosis of the prion protein. Biochem. Soc. Trans. 2008;36:1272–1276. doi: 10.1042/BST0361272. [DOI] [PubMed] [Google Scholar]
- 68.Peters P.J., Mironov A., Peretz D., Van Donselaar E., Leclerc E., Erpel S., DeArmond S.J., Burton D.R., Williamson R.A., Vey M., et al. Trafficking of prion proteins through a caveolae-mediated endosomal pathway. J. Cell Biol. 2003;162:703–717. doi: 10.1083/jcb.200304140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor D.R., Hooper N.M. The low-density lipoprotein receptor-related protein 1 (LRP1) mediates the endocytosis of the cellular prion protein. Biochem. J. 2007;402:17–23. doi: 10.1042/BJ20061736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fevrier B., Vilette D., Archer F., Loew D., Faigle W., Vidal M., Laude H., Raposo G. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. USA. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robertson C., Booth S.A., Beniac D.R., Coulthart M.B., Booth T.F., McNicol A. Cellular prion protein is released on exosomes from activated platelets. Blood. 2006;107:3907–3911. doi: 10.1182/blood-2005-02-0802. [DOI] [PubMed] [Google Scholar]
- 72.Vella L.J., Sharples R.A., Lawson V.A., Masters C.L., Cappai R., Hill A.F. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J. Pathol. 2007;211:582–590. doi: 10.1002/path.2145. [DOI] [PubMed] [Google Scholar]
- 73.Abdulrahman B.A., Abdelaziz D.H., Schatzl H.M. Autophagy regulates exosomal release of prions in neuronal cells. J. Biol. Chem. 2018;293:8956–8968. doi: 10.1074/jbc.RA117.000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hajj G.N.M., Arantes C.P., Dias M.V.S., Roffé M., Costa-Silva B., Lopes M.H., Porto-Carreiro I., Rabachini T., Lima F.R., Beraldo F.H., et al. The unconventional secretion of stress-inducible protein 1 by a heterogeneous population of extracellular vesicles. Cell. Mol. Life Sci. 2013;70:3211–3227. doi: 10.1007/s00018-013-1328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baixauli F., López-Otín C., Mittelbrunn M., Maria Merino A. Exosomes and autophagy: Coordinated mechanisms for the maintenance of cellular fitness. Front. Immunol. 2014;5:403. doi: 10.3389/fimmu.2014.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abels E.R., Breakefield X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryskalin L., Biagioni F., Lenzi P., Frati A., Fornai F. mTOR Modulates Intercellular Signals for Enlargement and Infiltration in Glioblastoma Multiforme. Cancers (Basel) 2020;12:2486. doi: 10.3390/cancers12092486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Büeler H., Fischer M., Lang Y., Bluethmann H., Lipp H.P., Dearmond S.J., Prusiner S.B., Aguet M., Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 79.Büeler H., Aguzzi A., Sailer A., Greiner R.A., Autenried P., Aguet M., Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 80.Sailer A., Büeler H., Fischer M., Aguzzi A., Weissmann C. No propagation of prions in mice devoid of PrP. Cell. 1994;77:967–968. doi: 10.1016/0092-8674(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 81.Richt J.A., Kasinathan P., Hamir A.N., Castilla J., Sathiyaseelan T., Vargas F., Sathiyaseelan J., Wu H., Matsushita H., Koster J., et al. Production of cattle lacking prion protein. Nat. Biotechnol. 2007;25:132–138. doi: 10.1038/nbt1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu G., Chen J., Xu Y., Zhu C., Yu H., Liu S., Hongying S., Chen J., Xu X., Wu Y., et al. Generation of goats lacking prion protein. Mol. Reprod. Dev. 2009;76:3. doi: 10.1002/mrd.20960. [DOI] [PubMed] [Google Scholar]
- 83.Moore R.C., Lee I.Y., Silverman G.L., Harrison P.M., Strome R., Heinrich C., Karunaratne A., Pasternak S.H., Chishti M.A., Liang Y., et al. Ataxia in prion protein (PrP)-deficient mice is associated with upregulation of the novel PrP-like protein doppel. J. Mol. Biol. 1999;292:797–817. doi: 10.1006/jmbi.1999.3108. [DOI] [PubMed] [Google Scholar]
- 84.Ryskalin L., Busceti C.L., Biagioni F., Limanaqi F., Familiari P., Frati A., Fornai F. Prion protein in glioblastoma multiforme. Int. J. Mol. Sci. 2019;20:5107. doi: 10.3390/ijms20205107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prestori F., Rossi P., Bearzatto B., Lainé J., Necchi D., Diwakar S., Schiffmann S.N., Axelrad H., D’Angelo E. Altered neuron excitability and synaptic plasticity in the cerebellar granular layer of juvenile prion protein knock-out mice with impaired motor control. J. Neurosci. 2008;28:7091–7103. doi: 10.1523/JNEUROSCI.0409-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Senatore A., Colleoni S., Verderio C., Restelli E., Morini R., Condliffe S.B., Bertani I., Mantovani S., Canovi M., Micotti E., et al. Mutant PrP Suppresses Glutamatergic Neurotransmission in Cerebellar Granule Neurons by Impairing Membrane Delivery of VGCC α 2δ-1 Subunit. Neuron. 2012;74:300–313. doi: 10.1016/j.neuron.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roffé M., Beraldo F.H., Bester R., Nunziante M., Bach C., Mancini G., Gilch S., Vorberg I., Castilho B.A., Martins V.R., et al. Prion protein interaction with stress-inducible protein 1 enhances neuronal protein synthesis via mTOR. Proc. Natl. Acad. Sci. USA. 2010;107:13147–13152. doi: 10.1073/pnas.1000784107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Santuccione A., Sytnyk V., Leshchyns’ka I., Schachner M. Prion protein recruits its neuronal receptor NCAM to lipid rafts to activate p59fyn and to enhance neurite outgrowth. J. Cell Biol. 2005;169:341–354. doi: 10.1083/jcb.200409127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liao M.J., Cheng C.Z., Zhou B., Zimonjic D.B., Mani S.A., Kaba M., Gifford A., Reinhardt F., Popescu N.C., Guo W., et al. Enrichment of a population of mammary gland cells that form mammospheres and have in vivo repopulating activity. Cancer Res. 2007;67:8131–8138. doi: 10.1158/0008-5472.CAN-06-4493. [DOI] [PubMed] [Google Scholar]
- 90.Miranda A., Pericuesta E., Ramírez M., Gutierrez-Adan M. Prion Protein Expression Regulates Embryonic Stem Cell Pluripotency and Differentiation. PLoS ONE. 2011;6:e18422. doi: 10.1371/journal.pone.0018422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang C.C., Steele A.D., Lindquist S., Lodish H.F. Prion protein is expressed on long-term repopulating hematopoietic stem cells and its important for their self-renewal. Proc. Natl. Acad. Sci. USA. 2006;103:2184–2189. doi: 10.1073/pnas.0510577103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Santos T.G., Silva I.R., Costa-Silva B., Lepique A.P., Martins V.R., Lopes M.H. Enhanced neural progenitor/stem cells self-renewal via the interaction of stress-inducible protein 1 with the prion protein. Stem Cells. 2011;29:1126–1136. doi: 10.1002/stem.664. [DOI] [PubMed] [Google Scholar]
- 93.Chen S., Mangé A., Dong L., Lehmann S., Schachner M. Prion protein as trans-interacting partner for neurons is involved in neurite outgrowth and neuronal survival. Mol. Cell. Neurosci. 2003;22:227–233. doi: 10.1016/S1044-7431(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 94.Schmitt-Ulms G., Legname G., Baldwin M.A., Ball H.L., Bradon N., Bosque P.J., Crossin K.L., Edelman G.M., DeArmond S.J., Cohen F.E., et al. Binding of neural cell adhesion molecules (N-CAMs) to the cellular prion protein. J. Mol. Biol. 2001;314:1209–1225. doi: 10.1006/jmbi.2000.5183. [DOI] [PubMed] [Google Scholar]
- 95.Gauczynski S., Peyrin J.M., Haïk S., Leucht C., Hundt C., Rieger R., Krasemann S., Deslys J.P., Dormont D., Lasmézas C.I., et al. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J. 2001;20:5863–5875. doi: 10.1093/emboj/20.21.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Linden R., Cordeiro Y., Lima L.M.T.R. Allosteric function and dysfunction of the prion protein. Cell. Mol. Life Sci. 2012;69:1105–1124. doi: 10.1007/s00018-011-0847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Linden R., Martins V.R., Prado M.A., Cammarota M., Izquierdo I., Brentani R.R. Physiology of the prion protein. Physiol. Rev. 2008;88:673–728. doi: 10.1152/physrev.00007.2007. [DOI] [PubMed] [Google Scholar]
- 98.Martins V.R., Beraldo F.H., Hajj G.N., Lopes M.H., Lee K.S., Prado M.A., Linden R. Prion protein: Orchestrating neurotrophic activities. Curr. Issues Mol. Biol. 2010;12:63–86. [PubMed] [Google Scholar]
- 99.Chiarini L.B., Freitas A.R.O., Zanata S.M., Brentani R.R., Martins V.R., Linden R. Cellular prion protein transduces neuroprotective signals. EMBO J. 2002;21:3317–3326. doi: 10.1093/emboj/cdf324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zanata S.M., Lopes M.H., Mercadante A.F., Hajj G.N.M., Chiarini L.B., Nomizo R., Freitas A.R.O., Cabral A.L.B., Lee K.S., Juliano M.A., et al. Stress-inducible protein 1 is a cell surface ligand for cellular prion that triggers neuroprotection. EMBO J. 2002;21:3307–3316. doi: 10.1093/emboj/cdf325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lopes M.H., Hajj G.N.M., Muras A.G., Mancini G.L., Castro R.M.P.S., Ribeiro K.C.B., Brentani R.R., Linden R., Martins V.R. Interaction of cellular prion and stress-inducible protein 1 promotes neuritogenesis and neuroprotection by distinct signaling pathways. J. Neurosci. 2005;25:11330–11339. doi: 10.1523/JNEUROSCI.2313-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Graner E., Mercadante A.F., Zanata S.M., Forlenza O.V., Cabral A.L.B., Veiga S.S., Juliano M.A., Roesler R., Walz R., Minetti A., et al. Cellular prion protein binds laminin and mediates neuritogenesis. Mol. Brain Res. 2000;76:85–92. doi: 10.1016/S0169-328X(99)00334-4. [DOI] [PubMed] [Google Scholar]
- 103.Rushworth J.V., Griffiths H.H., Watt N.T., Hooper N.M. Prion protein-mediated toxicity of amyloid-β oligomers requires lipid rafts and the transmembrane LRP1. J. Biol. Chem. 2013;288:8935–8951. doi: 10.1074/jbc.M112.400358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Loubet D., Dakowski C., Pietri M., Pradines E., Bernard S., Callebert J., Ardila-Osorio H., Mouillet-Richard S., Launay J., Kellermann O., et al. Neuritogenesis: The prion protein controls β1 integrin signaling activity. FASEB J. 2012;26:678–690. doi: 10.1096/fj.11-185579. [DOI] [PubMed] [Google Scholar]
- 105.Llorens F., Carulla P., Villa A., Torres J.M., Fortes P., Ferrer I., del Río J.A. PrPC regulates epidermal growth factor receptor function and cell shape dynamics in Neuro2a cells. J. Neurochem. 2013;127:124–138. doi: 10.1111/jnc.12283. [DOI] [PubMed] [Google Scholar]
- 106.Kuwahara C., Takeuchi A.M., Nishimura T., Haraguchi K., Kubosaki A., Matsumoto Y., Saeki K., Matsumoto Y., Yokoyama T., Itohara S., et al. Prions prevent neuronal cell-line death. Nature. 1999;400:225–226. doi: 10.1038/22241. [DOI] [PubMed] [Google Scholar]
- 107.Roucou X., Guo Q., Zhang Y., Goodyer C.G., LeBlanc A.C. Cytosolic Prion Protein Is Not Toxic and Protects against Bax-mediated Cell Death in Human Primary Neurons. J. Biol. Chem. 2003;278:40877–40881. doi: 10.1074/jbc.M306177200. [DOI] [PubMed] [Google Scholar]
- 108.Vassallo N., Herms J., Behrens C., Krebs B., Saeki K., Onodera T., Windl O., Kretzschmar H.A. Activation of phosphatidylinositol 3-kinase by cellular prion protein and its role in cell survival. Biochem. Biophys. Res. Commun. 2005;332:75–82. doi: 10.1016/j.bbrc.2005.04.099. [DOI] [PubMed] [Google Scholar]
- 109.Rachidi W., Vilette D., Guiraud P., Arlotto M., Riondel J., Laude H., Lehmann S., Favier A. Expression of prion protein increases cellular copper binding and antioxidant enzyme activities but not copper delivery. J. Biol. Chem. 2003;278:9064–9072. doi: 10.1074/jbc.M211830200. [DOI] [PubMed] [Google Scholar]
- 110.Lee K.J., Panzera A., Rogawski D., Greene L.E., Eisenberg E. Cellular prion protein (PrPc) protects neuronal cells from the effect of huntingtin aggregation. J. Cell Sci. 2007;120:2663–2671. doi: 10.1242/jcs.004598. [DOI] [PubMed] [Google Scholar]
- 111.Edenhofer F., Rieger R., Famulok M., Wendler W., Weiss S., Winnacker E.L. Prion protein PrPc interacts with molecular chaperones of the Hsp60 family. J. Virol. 1996;70:4724–4728. doi: 10.1128/JVI.70.7.4724-4728.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ma J., Lindquist S. Wild-type PrP and a mutant associated with prion disease are subject to retrograde transport and proteasome degradation. Proc. Natl. Acad. Sci. USA. 2001;98:14955–14960. doi: 10.1073/pnas.011578098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rambold A.S., Miesbauer M., Rapaport D., Bartke T., Baier M., Winklhofer K.F., Tatzelt J. Association of Bcl-2 with misfolded prion protein is linked to the toxic potential of cytosolic PrP. Mol. Biol. Cell. 2006;17:3356–3368. doi: 10.1091/mbc.e06-01-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun G., Guo M., Shen A., Mei F., Peng X., Gong R., Guo D., Wu J., Tien P., Xiao G. Bovine PrPC directly interacts with αB-crystalline. FEBS Lett. 2005;579:5419–5424. doi: 10.1016/j.febslet.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 115.Hartl F.U., Hayer-Hartl M. Protein folding. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 116.Camberg J.L., Doyle S.M., Johnston D.M., Wickner S. Brenner’s Encyclopedia of Genetics. 2nd ed. Elsevier Inc.; Amsterdam, The Netherlands: 2013. Molecular Chaperones; pp. 456–460. [Google Scholar]
- 117.Satoh J., Onoue H., Arima K., Yamamura T. The 14-3-3 Protein Forms a Molecular Complex with Heat Shock Protein Hsp60 and Cellular Prion Protein. J. Neuropathol. Exp. Neurol. 2005;64:858–868. doi: 10.1097/01.jnen.0000182979.56612.08. [DOI] [PubMed] [Google Scholar]
- 118.Mahabadi H.M., Taghibiglou C. Cellular prion protein (Prpc): Putative interacting partners and consequences of the interaction. Int. J. Mol. Sci. 2020;21:7058. doi: 10.3390/ijms21197058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shyu W.C., Harn H.J., Saeki K., Kubosaki A., Matsumoto Y., Onodera T., Chen C.J., Hsu Y.D., Chiang Y.H. Molecular modulation of expression of prion protein by heat shock. Mol. Neurobiol. 2002;26:1–12. doi: 10.1385/MN:26:1:001. [DOI] [PubMed] [Google Scholar]
- 120.Mangé A., Béranger F., Peoc’h K., Onodera T., Frobert Y., Lehmann S. Alpha- and beta- cleavages of the amino-terminus of the cellular prion protein. Biol. Cell. 2004;96:125–132. doi: 10.1016/j.biolcel.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 121.Watt N.T., Taylor D.R., Gillott A., Thomas D.A., Perera W.S.S., Hooper N.M. Reactive oxygen species-mediated β-clevage of the prion protein in the cellular response to oxidative stress. J. Biol. Chem. 2005;280:35914–35921. doi: 10.1074/jbc.M507327200. [DOI] [PubMed] [Google Scholar]
- 122.Haigh C.L., McGlade A.R., Collins S.J. MEK1 transduces the prion protein N2 fragment antioxidant effects. Cell. Mol. Life Sci. 2015;72:1613–1629. doi: 10.1007/s00018-014-1777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martin-Lannerée S., Hirsch T.Z., Hernandez-Rapp J., Halliez S., Vilotte J.L., Launay J.M., Mouillet-Richard S. PrP C from stem cells to cancer. Front. Cell Dev. Biol. 2014;2:55. doi: 10.3389/fcell.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Adorno-Cruz V., Kibria G., Liu X., Doherty M., Junk D.J., Guan D., Hubert C., Venere M., Mulkearns-Hubert E., Sinyuk M., et al. Cancer stem cells: Targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. Cancer Res. 2015;75:924–929. doi: 10.1158/0008-5472.CAN-14-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Go G., Yun C.W., Yoon Y.M., Lim J.H., Lee J.H., Lee S.H. Role of PrPC in cancer stem cell characteristics and drug resistance in colon cancer cells. Anticancer Res. 2020;40:5611–5620. doi: 10.21873/anticanres.14574. [DOI] [PubMed] [Google Scholar]
- 126.Pan Y., Zhao L., Liang J., Liu J., Shi Y., Liu N., Zhang G., Jin H., Gao J., Xie H., et al. Cellular prion protein promotes invasion and metastasis of gastric cancer. FASEB J. 2006;20:1886–1888. doi: 10.1096/fj.06-6138fje. [DOI] [PubMed] [Google Scholar]
- 127.Kikuchi Y., Kakeya T., Yamazaki T., Takekida K., Nakamura N., Matsuda H., Takatori K., Tanimura A., Tanamoto K.I., Sawada J.I. G1-dependent prion protein expression in human glioblastoma cell line T98G. Biol. Pharm. Bull. 2002;25:728–733. doi: 10.1248/bpb.25.728. [DOI] [PubMed] [Google Scholar]
- 128.Erlich R.B., Kahn S.A., Lima F.R.S., Muras A.G., Martins R.A.P., Linden R., Chiarini L.B., Martins V.R., Neto V.M. STI1 promotes glioma proliferation through MAPK and PI3K pathways. Glia. 2007;55:1690–1698. doi: 10.1002/glia.20579. [DOI] [PubMed] [Google Scholar]
- 129.Azzalin A., Sbalchiero E., Barbieri G., Palumbo S., Muzzini C., Comincini S. The doppel (Dpl) protein influences in vitro migration capability in astrocytoma-derived cells. Cell. Oncol. 2008;30:491–501. doi: 10.3233/CLO-2008-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim Y.C., Won S.Y., Jeong B.H. Identification of Prion Disease-Related Somatic Mutations in the Prion Protein Gene (PRNP) in Cancer Patients. Cells. 2020;9:1480. doi: 10.3390/cells9061480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang L., Gao Z., Hu L., Wu G., Yang X., Zhang L., Zhu Y., Wong B.S., Xin W., Sy M.S., et al. Glycosylphos-phatidylinositol anchor modification machinery deficiency is responsible for the formation of pro-prion protein (PrP) in BxPC-3 protein and increases cancer cell motility. J. Biol. Chem. 2016;291:3905–3917. doi: 10.1074/jbc.M115.705830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cheng Y., Tao L., Xu J., Li Q., Yu J., Jin Y., Chen Q., Xu Z., Zou Q., Liu X. CD44/Cellular prion protein interact in multidrug resistant breast cancer cells and correlate with responses to neoadjuvant chemotherapy in breast cancer patients. Mol. Carcinog. 2014;53:686–697. doi: 10.1002/mc.22021. [DOI] [PubMed] [Google Scholar]
- 133.Lee J.H., Yun C.W., Han Y.S., Kim S.M., Jeong D., Kwon H.Y., Kim H., Baek M.J., Lee S.H. Melatonin and 5-fluorouracil co-suppress colon cancer stem cells by regulating cellular prion protein-Oct4 axis. J. Pineal Res. 2018;65:e12519. doi: 10.1111/jpi.12519. [DOI] [PubMed] [Google Scholar]
- 134.Chieng C.K.-L., Say Y.-H. Cellular prion protein contributes to LS 174T colon cancer cell carcinogenesis by increasing invasiveness and resistance against doxorubicin-induced apoptosis. Tumor Biol. 2015;36:8107–8120. doi: 10.1007/s13277-015-3530-z. [DOI] [PubMed] [Google Scholar]
- 135.Li Q.Q., Cao X.X., Xu J.D., Chen Q., Wang W.J., Tang F., Chen Z.Q., Liu X.P., Xu Z.D. The role of P-glycoprotein/cellular prion protein interaction in multidrug-resistant breast cancer cells treated with paclitaxel. Cell. Mol. Life Sci. 2009;66:504–515. doi: 10.1007/s00018-008-8548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Esch D., Vahokoski J., Groves M.R., Pogenberg V., Cojocaru V., Vom Bruch H., Han D., Drexler H.C.A., Araúzo-Bravo M.J., Ng C.K.L., et al. A unique Oct4 interface is crucial for reprogramming to pluripotency. Nat. Cell Biol. 2013;15:295–301. doi: 10.1038/ncb2680. [DOI] [PubMed] [Google Scholar]
- 137.Li Q.Q., Sun Y.P., Ruan C.P., Xu X.Y., Ge J.H., He J., De Xu Z., Wang Q., Gao W.C. Cellular prion protein promotes glucose uptake through the Fyn-HIF-2α-Glut1 pathway to support colorectal cancer cell survival. Cancer Sci. 2011;102:400–406. doi: 10.1111/j.1349-7006.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- 138.Tao Z., Li T., Ma H., Yang Y., Zhang C., Hai L., Liu P., Yuan F., Li J., Yi L., et al. Autophagy suppresses self-renewal ability and tumorigenicity of glioma-initiating cells and promotes Notch1 degradation. Cell Death Dis. 2018;9:1063. doi: 10.1038/s41419-018-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Q., Qian J., Wang F., Ma Z. Cellular prion protein accelerates colorectal cancer metastasis via the Fyn-SP1-SATB1 axis. Oncol. Rep. 2012;28:2029–2034. doi: 10.3892/or.2012.2025. [DOI] [PubMed] [Google Scholar]
- 140.Mangé A., Milhavet O., Umlauf D., Harris D., Lehmann S. PrP-dependent cell adhesion in N2a neuroblastoma cells. FEBS Lett. 2002;514:159–162. doi: 10.1016/S0014-5793(02)02338-4. [DOI] [PubMed] [Google Scholar]
- 141.Senbanjo L.T., Chellaiah M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017;5:18. doi: 10.3389/fcell.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Muras A.G., Hajj G.N.M., Ribeiro K.B., Nomizo R., Nonogaki S., Chammas R., Martins V.R. Prion protein ablation increases cellular aggregation and embolization contributing to mechanisms of metastasis. Int. J. Cancer. 2009;125:1523–1531. doi: 10.1002/ijc.24425. [DOI] [PubMed] [Google Scholar]
- 143.Ayala G.E., Dai H., Tahir S.A., Li R., Timme T., Ittmann M., Frolov A., Wheeler T.M., Rowley D., Thompson T.C. Stromal antiapoptotic paracrine loop in perineural invasion of prostatic carcinoma. Cancer Res. 2006;66:5159–5164. doi: 10.1158/0008-5472.CAN-05-1847. [DOI] [PubMed] [Google Scholar]
- 144.Skancke M., Arnott S.M., Amdur R.L., Siegel R.S., Obias V.J., Umapathi B.A. Lymphovascular invasion and perineural invasion negatively impact overall survival for stage II adenocarcinoma of the colon. Dis. Colon Rectum. 2019;62:181–188. doi: 10.1097/DCR.0000000000001258. [DOI] [PubMed] [Google Scholar]
- 145.Lee J.H., Han Y.S., Yoon Y.M., Yun C.W., Yun S.P., Kim S.M., Kwon H.Y., Jeong D., Baek M.J., Lee H.J., et al. Role of HSPA1L as a cellular prion protein stabilizer in tumor progression via HIF-1α/GP78 axis. Oncogene. 2017;36:6555–6567. doi: 10.1038/onc.2017.263. [DOI] [PubMed] [Google Scholar]
- 146.Patel B.N., Olcott E., Jeffrey R.B. Extrapancreatic perineural invasion in pancreatic adenocarcinoma. Abdom. Radiol. 2018;43:323–331. doi: 10.1007/s00261-017-1343-9. [DOI] [PubMed] [Google Scholar]
- 147.Piro J.R., Harris B.T., Nishina K., Soto C., Morales R., Rees J.R., Supattapone S. Prion Protein Glycosylation Is Not Required for Strain-Specific Neurotropism. J. Virol. 2009;83:5321–5328. doi: 10.1128/JVI.02502-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wiegmans A.P., Saunus J.M., Ham S., Lobb R., Kutasovic J.R., Dalley A.J., Miranda M., Atkinson C., Foliaki S.T., Ferguson K., et al. Secreted cellular prion protein binds doxorubicin and correlates with anthracycline resistance in breast cancer. JCI Insight. 2019;4:e124092. doi: 10.1172/jci.insight.124092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tsai C.L., Tsai C.N., Lin C.Y., Chen H.W., Lee Y.S., Chao A., Wang T.H., Wang H.S., Lai C.H. Secreted Stress-Induced Phosphoprotein 1 Activates the ALK2-SMAD Signaling Pathways and Promotes Cell Proliferation of Ovarian Cancer Cells. Cell Rep. 2012;2:283–293. doi: 10.1016/j.celrep.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 150.Wang T.H., Chao A., Tsai C.L., Chang C.L., Chen S.H., Lee Y.S., Chen J.K., Lin Y.J., Chang P.Y., Wang C.J., et al. Stress-induced phosphoprotein 1 as a secreted biomarker for human ovarian cancer promotes cancer cell proliferation. Mol. Cell. Proteomics. 2010;9:1873–1884. doi: 10.1074/mcp.M110.000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang J., You H., Qi J., Yang C., Ren Y., Cheng H. Autocrine and paracrine STIP1 signaling promote osteolytic bone metastasis in renal cell carcinoma. Oncotarget. 2017;8:17012–17026. doi: 10.18632/oncotarget.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sahi C., Knox J.J., Clemons M., Joshua A.M., Broom R. Renal cell carcinoma bone metastases: Clinical advances. Ther. Adv. Med. Oncol. 2010;2:75–83. doi: 10.1177/1758834009358417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao Y., You H., Liu F., An H., Shi Y., Yu Q., Fan D. Differentially expressed gene profiles between multidrug resistant gastric adenocarcinoma cells and their parental cells. Cancer Lett. 2002;185:211–218. doi: 10.1016/S0304-3835(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 154.Barbieri G., Palumbo S., Gabrusiewicz K., Azzalin A., Marchesi N., Spedito A., Biggiogera M., Sbalchiero E., Mazzini G., Miracco C., et al. Silencing of cellular prion protein (PrPc) expression by DNA-antisense oligonucleotides induces autophagy-dependent cell death in glioma cells. Autophagy. 2011;7:840–853. doi: 10.4161/auto.7.8.15615. [DOI] [PubMed] [Google Scholar]
- 155.Atkinson C.J., Kawamata F., Liu C., Ham S., Győrffy B., Munn A.L., Wei M.Q., Möller A., Whitehall V., Wiegmans A.P. EGFR and Prion protein promote signaling via FOXO3a-KLF5 resulting in clinical resistance to platinum agents in colorectal cancer. Mol. Oncol. 2019;13:725–737. doi: 10.1002/1878-0261.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kikuchi Y., Kakeya T., Sakai A., Takatori K., Nakamura N., Matsuda H., Yamazaki T., Tanamoto K.I., Sawada J.I. Propagation of a protease-resistant form of prion protein in long-term cultured human glioblastoma cell line T98G. J. Gen. Virol. 2004;85:3449–3457. doi: 10.1099/vir.0.80043-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.