Abstract
Gastrointestinal stromal tumors (GISTs) are the most common types of malignant mesenchymal tumors in the gastrointestinal tract, with an estimated incidence of 1.5/100.000 per year and 1–2% of gastrointestinal neoplasms. About 75–80% of patients have mutations in the KIT gene in exons 9, 11, 13, 14, 17, and 5–10% of patients have mutations in the platelet-derived growth factor receptor a (PDGFRA) gene in exons 12, 14, 18. Moreover, 10–15% of patients have no mutations and are classified as wild type GIST. The treatment for metastatic or unresectable GISTs includes imatinib, sunitinib, and regorafenib. So far, GIST therapies have raised great expectations and offered patients a better quality of life, but increased pharmacological resistance to tyrosine kinase inhibitors is often observed. New treatment options have emerged, with ripretinib, avapritinib, and cabozantinib getting approvals for these tumors. Nowadays, immune checkpoint inhibitors form a new landscape in cancer therapeutics and have already shown remarkable responses in various tumors. Studies in melanoma, non-small-cell lung cancer, and renal cell carcinoma are very encouraging as these inhibitors have increased survival rates. The purpose of this review is to present alternative approaches for the treatment of the GIST patients, such as combinations of immunotherapy and novel inhibitors with traditional therapies (tyrosine kinase inhibitors).
Keywords: GIST, immunotherapy, small molecules, imatinib
1. Introduction
Gastrointestinal Stromal Tumors (GISTs) are the most common types of mesenchymal tumors of the gastrointestinal tract and originate from interstitial Cajal cells [1]. GISTs are rare tumors, with an estimated incidence of 1.5/100.000 per year and account for 1–2% of gastrointestinal neoplasms. The disease’s median age is around 60–65 years old [2,3]. The most common localization is the stomach (60%) and the small intestine (20-30%), whereas GISTs are found less frequently in ortho-sigmoid and esophagus. The main clinical manifestations are not disease-specific, including hemorrhage, anemia, indigestion, and abdominal pain due to stressful events [4,5].
About 85% of the GISTs cases are associated with a known mutation. About 75–80% of patients have mutations in the KIT gene in exons 9, 11, 13, 14, leading to a truncated c-KIT/CD117 protein, which acts as a growth factor receptor located on normal cells’ surface. Mutations in the PDGFRA gene are identified in exons 12, 14, 18 to 5–10% of patients. Approximately 10–15% of patients have no mutations and are classified as wild type GIST [6,7]. Molecular characterization of GISTs has revealed novel mutations to BRAF, neurofibromatosis type 1 (NF1), and succinate dehydrogenase (SDH) in small percentages [8]. However, even after comprehensive analysis, a group of GISTs with no mutations is identified [9].
The NIH classification system categorizes patients into very low, low, intermediate, and high-risk groups taking into consideration the size of the lesion and the mitotic activity of the tumor. The conclusion is that tumors >5 cm (diameter), plus mitotic counter higher than 5/50 high power fields (HPF) and tumors >10 cm with any mitotic rate have a high risk of recurrence and indicate adjuvant chemotherapy [10]. It was shown that the risk of recurrence is more significant for non-gastric GISTs than for gastric [11,12].
Diagnostic imaging includes computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) has the advantage of displaying the thickness of the small bowel, leading to better visualization of deep ileal loops and mesentery [13]. Further, CT analysis facilitates the assessment of tyrosine kinase inhibitors (TKIs) response by using both RECIST and Choi criteria [14]. MRI can provide information about size, tumor perforation, or metastasis. PET scan with 2-(F-18)-fluoro-2-deoxy-d-glucose can be useful to identify areas of necrosis in tumors and differentiate benign versus malignant tumors and is used alone or with the CT for determinating the effectiveness of the adjuvant therapy [11].
Cytotoxic chemotherapy and radiation are not effective; thus, surgical resection appears as the only effective therapeutic intervention. Surgery in metastatic or recurrent is controversial, and case selection is essential. It can be helpful to patients whose neoplasm is responding to adjuvant therapy, to those with limited focal progression and as palliative surgery [15].
For metastatic or recurrent GIST, the first treatment line is the tyrosine kinase inhibitor, imatinib mesylate (IM). Patients with PDGFRA exon 18 mutation must receive avapritinib on the first line (Table 1). The therapeutic approach of GISTs for the second line of treatment contains sunitinib malate, and the third line regorafenib (with better outcomes if we choose personalized doses of regorafenib) [16,17,18].
Table 1.
Systemic Therapy for Resectable Gastrointestinal Stromal Tumors (GISTs) (NCCN Guidelines V.1.2021).
| Neo Adjuvant Therapy | Adjuvant Therapy |
|---|---|
| Imatinib (sensitive mutations) | Imatinib |
| Avapritinib (PDGFR exon 18) | - |
However, the addition of ripretinib and avapritinib to our therapeutic armamentarium challenges this algorithm [19,20] (Table 2). Imatinib is a TKI that works by binding to the ATP binding sites on CD117 and PDGFRA, which leads to a block of signal transduction. Patients who have c-KIT and PDGFRA are benefited from this therapy [21].
Table 2.
Systemic Therapy for Unresectable GISTs (NCCN Guidelines V.1.2021).
| First Line | Second Line | Third Line | Fourth Line | Additional Options |
|---|---|---|---|---|
| Imatinib | Sunitinib | Regorafenib | Ripretinib | Avapritinib |
| Avapritinib | - | - | - | Cabozantinib |
| - | - | - | - | Dasatinib |
| - | - | - | - | Everolimus |
| - | - | - | - | Nilotinib |
| - | - | - | Pazopanib | |
| - | - | - | - | Sorafenib |
| - | - | - | - | Larotrectinib |
Pediatric GISTs represent a clinically and molecularly distinct subset, characterized by the absence of c-KIT/PDGFRA mutations. Syndromes linked to GISTs are the Carney triad syndrome, Carney-Stratakis syndrome, and Neurofibromatosis type I [22].
2. Resistance to Imatinib
Despite the beneficial effect of tyrosine kinase inhibitors (TKIs) targeted therapy, due to the lack of complete elimination of GIST cells, eventually, 50% of the patients develop primary or secondary resistance against imatinib after two years. It should be mentioned that tumor progression is sometimes marked by an increase in density with or without an increase in the tumor size. Besides, cancer progression within the first six months after treatment with imatinib means develop of primary resistance for the patients [17,23,24].
Some GISTs lose expression of KIT oncoproteins and, therefore, become KIT-independent and, subsequently, resistant to KIT-inhibitor drugs [25]. Patients with CD117 mutations in exons 9, 11, 13, 14, 17 raise resistance to imatinib [7,26]. In 2014, patients with CD117 exon 9 mutations had better survival than patients with exon 11 mutations [27].
As primary resistance characterizes the tumor development through an initial imatinib challenge, as well as secondary resistance characterizes the tumor progresses after an initial period of response to imatinib [28]. Specific mutations and metastatic capability for specific regions have also been associated with secondary resistance (common location for GIST metastasis are liver (28%), mesentery and omentum (30%), lung (7%), subcutaneous tissues (4.7%), lymph nodes (4.7%), and bone (2.3%) [11]. Recent studies have shown that ligands from the fibroblast growth factors (FGF) family reduce imatinib’s effect on GIST cells, and FGF2 and FGFR1 are highly expressed in all primary GIST samples. Therefore, inhibitors for the FGF family constitute a potential therapy to overcome the resistance. Plenty of encouraging results exist for a number of neoplasms, e.g., bladder, digestive system, myeloma, and endometrial. These positive results give us the impetus for an extensive study of these molecules (e.g., SU5402, AZD4547, D173074, NDGA) and elucidate the pathways of GIST tumor development [29,30,31].
Epidermal growth factor receptor (EGFR) mutations identification is a rare finding in GISTs. In their publication, Shi et al. reported three EGFR mutated cases out of 323 GISTs tested, which accounted for 3.4% of the wild type GISTs of their cohort [32]. EGFR mutation was equally exclusive of any c-KIT, PDGFR, BRAF, and SDH mutations, indicating that the EGFR signaling pathway may phosphorylate and subsequently activate important downstream targets triggering proliferation and survival [32]. EGFR expression was shown from Cai et al. in GIST tumor samples, recommending an autocrine loop between transforming growth factor-α (TGF-α) and EGFR [33]. Furthermore, expression of EGFR and its ligands may be promoted by ADAMs activation, as was studied in GIST tumor samples, implying an important role of the EGFR pathway for GIST oncogenesis [34]. However, EGFR expression studies did not show any prognostic relevance to GIST patient outcomes [35]. In contrast, in their study, Zhao et al. showed that in high-risk GISTs, decreased expression of EGFR is associated with lower recurrent free survival (RFS) after treatment with imatinib [36].
The expression of EGFR has been further studied in several GIST cell lines. In a recent paper, Tu et al. have shown that in c-KIT independent GIST cell lines, EGFR was expressed, while in c-KIT positive cell lines, there was not EGFR expression [37]. Thus, it is reasonable to assume that EGFR expression is associated with imatinib resistance. However, treatment with gefitinib to c-KIT independent GIST cell lines did not impact their growth and any of the activated signals (AKT, MAPK), therefore implying that EGFR activation is not a key to imatinib resistance [37]. On the other hand, Nagata et al. showed that c-KIT and EGFR phosphorylation status is similar in imatinib-resistant GIST cell lines. In this case, the addition of gefitinib to standard imatinib treatment resulted in decreasing cell proliferation [38]. Conflicting data from in vivo experiments highlights the complexity of resistance to imatinib therapy and the unmet need to understand this process’s molecular mechanisms.
Novel Therapies
A literature search revealed 313 clinical trials for GISTs, including more than 86 molecules studied in order to discover new effective therapies. First-line drug therapy imatinib (STI571) has been used in 152 clinical trials, sunitinib in 74, and regorafenib (BAY73-4506) in 21. Ongoing clinical trials are presented in Table 3, Table 4 and Table 5. Through clinical trials of phases I, II, III, and IV, scientists try to target GISTs with new modalities and novel molecules or combine the classic therapeutic options (imatinib) with immunotherapy (anti-PDL1 or anti-PD1).
Table 3.
Imatinib ongoing clinical trials.
| Clinical Trial | Combination | Phase |
|---|---|---|
| NCT04006769 | Entacapone | I |
| NCT02924714 | none | N/A |
| NCT01991379 | MEK 162 | I/II |
| NCT02365441 | Regorafenib | II |
| NCT03609424 | PDR001 | I/II |
| NCT01541709 | none | II |
| NCT03862768 | Sunitinib | N/A |
| NCT02260505 | none | III |
| NCT04138381 | Selinexor | I/II |
| NCT04193553 | Lenvatinib | II |
| NCT03944304 | Paclitaxel | II |
| NCT01742299 | none | IV |
| NCT03381053 | none | IV |
| NCT02413736 | none | III |
| NCT01738139 | Ipilimumab | I |
Table 4.
Sunitinib ongoing clinical trials.
| Clinical Trial | Combination | Phase |
|---|---|---|
| NCT02164240 | Regorafenib | I |
| NCT04633122 | Ripretinib | II |
| NCT03673501 | Ripretinib | III |
| NCT04409223 | Famitinib | III |
| NCT01694277 | Masitinib | III |
| NCT03862768 | Imatinib | N/A |
| NCT00700258 | Axitinib/Temsirolimus | III |
Table 5.
Regorafenib ongoing clinical trials.
| Clinical Trial | Combination | Phase |
|---|---|---|
| NCT03465722 | Avapritinib | III |
| NCT02164240 | Sunitinib | I |
| NCT02638766 | none | II |
| NCT02365441 | imatinib | II |
| NCT01933958 | none | N/A |
| NCT03475953 | Avelumab | I/II |
| NCT03890731 | none | II |
3. Immunotherapy in GISTs
In recent years, there has been an increasing interest in immunotherapy-based therapeutic strategies against cancer, in attempt to incorporate all of the stepwise events required for tumor development and progression. It is well known that cancer cells, to evade the immune response, express numerous different molecules on the cell surface known, consequently leading to immune suppression. Vigorous research in the field of immunology has led to the development of a plethora of agents, also known as checkpoint inhibitors (i.e., anti-programmed death-1 (PD1)/PD-L1 and anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA4)), which are able to interrupt the inhibitory conjunction of cancer and T-cells. The checkpoint inhibitors are already used in a vast panel of cancer types [39].
The D842V mutation is the most common mutation associated with primary resistance to imatinib because it alters the kinase domain formation and, therefore, negatively affects imatinib association [40,41]. In fact, the progression free survival (PFS) of patients treated with imatinib carrying the D842V mutation compared to non-D842Vs is statistically significant (2.8 vs. 28.5 months, respectively) [42]. In another study of a cohort of patients with PDGFRA D842V-mutated GIST, imatinib resulted in progressive disease for most patients (58.9%), with PFS up to 8 months [43]. Moreover, resistance occurs due to this mutation in the sunitinib [40,41].
Many studies indicate that D824V mutation displays more immune cells with increased cytolytic activity [40,41,44,45,46,47]. In particular, express higher interferon levels and several chemokines, such as CXCL14; demonstrates additional driver-derived neoepitope-HLA binding proteins; and has more PD-1 and PD-L1 expressing tumors [40,41,47]. Differences in tumor microenvironment composition were also highlighted between the D824 mutation and the other GIST molecular subtypes. More specifically, there is an overexpression of T-regs and CD8 + T-cells and a lower CD4 + T-cell rate. Further, the T-cell-inflamed signature score for GISTs was at a similar rate with tumor types responsive to checkpoint inhibition [44,45]. All of the above data positively support the implementation of an immune-treatment approach in GIST and, in fact, in patients carrying the D824V mutation.
Seventeen clinical trials using immunotherapeutic agents in GISTs have been scheduled (Table 6).
Table 6.
Immunotherapy-clinical trials.
| Immunotherapeutic Agent | Clinical Trials | Active | Completed | Target |
|---|---|---|---|---|
| Ipilimumab | 6 | 5 | 1 | CTLA-4 |
| Nivolumab | 4 | 4 | 0 | PD-1 |
| Spartalizumab | 1 | 1 | 0 | PD-1 |
| Pembrolizumab | 3 | 2 | 1 | PD-1 |
| PDR001 | 1 | 1 | 0 | PD-1 |
| Avelumab | 2 | 2 | 0 | PD-1 |
However, how are the new drugs combined with the older ones in the clinical trials?
Immunotherapeutic agents used in clinical trials are anti-PD-1/PDL 1 molecules and CTLA -4 (Table 7). An interim analysis of the randomized phase II clinical trial using nivolumab, a human immunoglobulin IgG4 monoclonal antibody, which is directed against the negative immunoregulatory human cell surface receptor programmed death-1 (PD-1, PDL-1) with immune checkpoint inhibitory and antineoplastic activities, with or without ipilimumab, a recombinant human immunoglobulin IgG1 monoclonal antibody, which is directed against the human T-cell receptor cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), with immune checkpoint inhibitory and antineoplastic activities, in 40 patients with metastatic or inoperable GIST, was recently published [48]. In the nivolumab arm, 7 out of the 15 patients showed SD as the best response. In the combination arm, 1 out of 12 patients showed PR and 2 of the 12 patients SD. There are three more clinical trials ongoing based on the same combination, with 164 and 60 patients without any results known [49,50,51] and one phase II clinical trial that will start recruiting patients in the next few months [52]. Moreover, ipilimumab was used with dasatinib treating patients with GISTs or other sarcomas that cannot be removed by surgery or are metastatic (phase I), unfortunately with no hopeful results. Pembrolizumab (MK3475) is a humanized monoclonal immunoglobulin IgG4 antibody directed against receptor PD-1 with potential immune checkpoint inhibitory antineoplastic activities, and was used with epacadostat in one clinical trial. However, this study’s enrollment was terminated early for insufficient evidence of clinical efficacy [53]. An ongoing phase II multicenter clinical trial PEMBROSARC combines pembrolizumab with metronomic cyclophosphamide in patients with advanced sarcomas (31 patients with GIST [54]. Spartalizumab a humanized monoclonal antibody. It is directed against the negative immunoregulatory human cell surface receptor programmed death-1 (PD-1, PCD-1), which is used in one phase Ib clinical trial, not yet recruiting patients, in combination with ribociclib with available results, until now [55]. Avelumab is a humanized monoclonal anti-PD1 agent. There is an ongoing clinical trial combining avelumab with axitinib in patients with unresectable or metastatic GIST after failure of standard therapy [56]. There is another ongoing phase I/II clinical trial combining avelumab with regorafenib in patients with solid tumors and GIST [57]. Unfortunately, we have no results available yet for both clinical trials. PDR001 is a monoclonal anti-PD-1 agent. There are two clinical trials with PDR001, the first phase I/II clinical trial is combining PDR001 with imatinib in patients with advanced GIST after failure of standard TKI therapies, without any results yet [58].
Table 7.
Immunotherapeutic Agents in ongoing clinical trials.
| Molecule | Clinical Trial | Phase |
|---|---|---|
| Ipilimumab/Nivolumab | NCT02880020 | II |
| Ipilimumab | NCT01738139 | I |
| Ipilimumab/Nivolumab | NCT02500797 | II |
| Ipilimumab/Nivolumab | NCT02834013 | II |
| Ipilimumab/Nivolumab | NCT02982486 | II |
| Spartalizumab | NCT04000529 | I |
| PDR001 | NCT03609424 | I/II |
| Avelumab + Regorafenib | NCT03475953 | I/II |
| Avelumab + Axitinib | NCT04258956 | II |
| Pembrolizumab + Epacadostat | NCT03291054 | II |
| NCT02406781 | II |
4. Molecules-Drugs Studied in GISTs and How are They Used in Clinical Trials Now
Nilotinib, a TKI inhibitor designed to overcome imatinib resistance resulting from Bcr-Abl kinase mutations, is also a PDGF-R, c-KIT inhibitor. Nilotinib has been used in 18 clinical trials, 15 completed, two not recruiting, and one active. An ongoing phase IV clinical trial with 300 patients with GIST and chronic myeloid leukemia (CML) is trying to find if nilotinib can be useful [59]. There are two more phase IV clinical trials, active but not recruiting patients yet [60,61]. Unfortunately, the other clinical trials that were completed did not show encouraging results.
Another molecule, Ripretinib, is a selective KIT and PDGFRA inhibitor. There is a phase III clinical trial, recruiting patients with advanced GIST after treatment with imatinib. Four hundred twenty-six patients are enrolled in two arms, one with ripretinib, and the other with sunitinib; the first results will be announced after June 2021 [19]. Another ongoing phase I clinical trial with 320 patients with advanced malignancies has no results as of yet [62]. Three more clinical trials are about to begin, recruiting patients with GIST in the next few months [63,64,65].
Avapritinib is a PDGFRa and mast/stem cell factor receptor c-KIT inhibitor. There is an ongoing phase I/II clinical trial with 87 participants with unresectable or metastatic GIST with no results yet [66]. Two more clinical trials with avapritinib will start recruiting patients in the next few months [67,68]. Avapritinib was approved in 2020 by the FDA for gastrointestinal stromal tumors with a mutation in exon 18 PDGFRA (NAVIGATOR study) [69], a multicenter, single-arm, open-label trial. Forty-three patients with GIST with PDGFRA exon 18 mutation, 38 of them with PDGFRA D842V mutations. The overall response rate (ORR) was 84% (95% CI: 69%, 93%), with 7% complete responses and 77% partial responses. For patients with PDGFRA D842V mutations, the ORR was 89% (95% CI: 75%, 97%), with 8% complete responses and 82% partial responses. The median response period was not reached with a median period of record for all patients of 10.6 months (range 0.3 to 24.9 months); 61% of the responding patients with exon 18 mutations had a response lasting at least six months (31% of patients with an ongoing response were followed for less than six months).
Cabozantinib in a phase II trial has shown antitumor activity in GIST patients treated with three or more previous lines of therapy. The CaboGIST EORTC 1317 trial met its primary endpoint with 30 of the 50 patients being progression free after 12 weeks. The mPFS was 5.5 months (3.6–6.9 months) [70]. As a result of this publication, cabozantinib was included in the recent GIST NCCN guidelines as an option after failure of other approved regimens.
Moreover, Pazopanib is a vascular endothelial growth factor receptor(VEGFR)-1, -2, and -3, c-KIT inhibitor and PDGF-R inhibitor. There is no ongoing clinical trial with pazopanib. Three trials have been completed. Clinical trial PAZOGIST, phase II, multicenter study with 81 patients with metastatic and/or locally advanced unresectable GIST resistant to imatinib and sunitinib had promising results [71].
Another drug, crenolanib is a PDGFRA inhibitor. There is an ongoing clinical trial phase III with 120 patients with advanced or metastatic GIST, with a D842V mutation in the PDGFRA gene, without any results as of yet [72].
Ponatinib is a TKI inhibitor1. It is used in an ongoing clinical trial phase II, with 81 participants with metastatic or unresectable GIST, as a second-line therapy after treatment with imatinib, without any results as of yet [73].
Moreover, Dasatinib is an inhibitor of the Src-family protein-tyrosine kinases. It was used in one clinical trial with ipilimumab and first-line treatment without good results [74]. Apart from that, there is no clinical trial using this molecule.
Furthermore, binimetinib is a MEK1/2 inhibitor. An ongoing phase Ib/II clinical trial with 62 patients with advanced GIST combines it with imatinib, without results as of yet [75]. Another clinical trial, which will start recruiting patients soon, will combine binimetinib with pexidartinib [76].
Vandetanib is a VEGFR2 inhibitor. It is used in a phase II clinical trial in children and adults, with only nine participants with wild type GIST. It is a completed clinical trial and the results are about to be published [77].
The molecule famitinib is an receptor tyrosine kinase (RTK) inhibitor targeting c-Kit, VEGFR2, PDGFR, VEGFR3, Flt1, and Flt3. It is used in phase II clinical trial, 88 patients with advanced or metastatic GIST, as second-line therapy after imatinib, with no available results as of yet [78]. Another phase III clinical trial with advanced GIST patients, after imatinib’s failure, as a second-line therapy, compared to sunitinib [79].
Anlotinib is an RTK inhibitor VEGFR2 and VEGFR3 inhibitor. There is one ongoing phase III clinical trial with patients with advanced GIST after imatinib failure. No results are known as of yet [80].
Moreover, Axitinib is a PDGFR inhibitor. There is an ongoing single-arm, phase II (AXAGIST) clinical trial combining avelumab with axitinib in patients with unresectable or metastatic GIST, as a second-line treatment, with no known results as of yet [56]. Another recruiting observational clinical trial uses axitinib as second-line therapy for patients with metastatic renal cell carcinoma (mRCC), mantle cell Lymphoma (MCL), and GIST; no results are available [81].
Molecule alvocidib is a cyclin-dependent kinase (CDKs) inhibitor. A completed phase I clinical trial with 36 patients with metastatic or recurrent GISTs and sarcomas, combined with doxorubicin, with poor results, unfortunately [82].
Ribociclib is a CDK inhibitor. A recruiting Phase Ib, multicenter, open label study combines ribociclib with spartalizumab in patients with GIST, with no results yet [55].
Furthermore, Sorafenib is an RAF kinase inhibitor. A phase II clinical trial with imatinib and sunitinib treatment has failed, but there are no results yet [83]. Six clinical trials that have been completed in the past years have not shown encouraging results.
Moreover, pexidartinib is a TKI inhibitor. One clinical trial phase I is about to begin recruiting patients and will combine pexidartinib with binimetinib in patients with advanced GIST [76].
Olaratumab is a fully human IgG1 monoclonal antibody directed against PDGFRA, was given in one clinical trial, and the drug had an acceptable adverse events profile in patients with GIST. A phase II, open label, clinical trial, with 21 participants with previously treated unresectable or metastatic GIST. Although there was no apparent effect on PFS in patients without PDGFRA mutations, patients with PDGFRA-mutant GIST (all with D842V mutations) treated with olaratumab had longer disease control compared with historical data for this genotype [84].
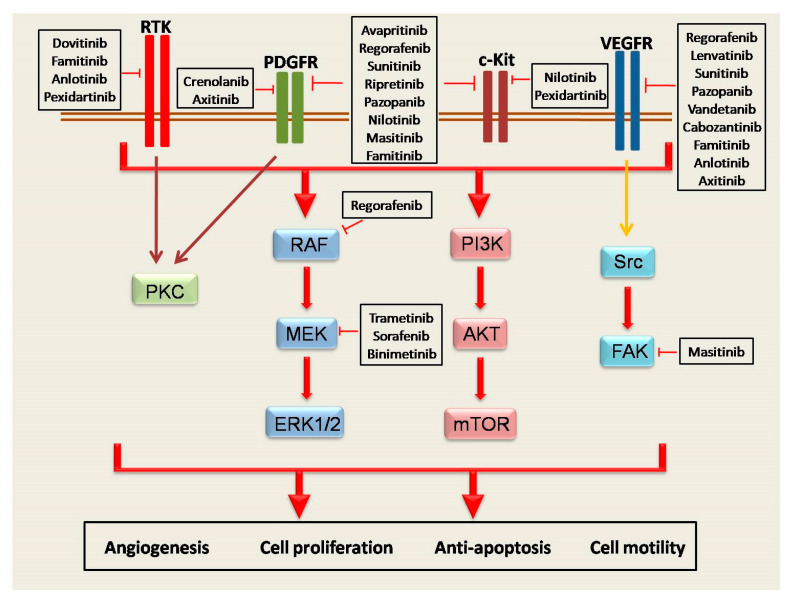
Other molecules used: Masitinib TKR inhibitor was used in clinical trials with poor results; trametinib, a MEK MAPK/ERK kinase, was used in one clinical trial, which was withdrawn [85]. HQP1351 was used in a phase I recruiting clinical trial with patients with GIST [86], PLX9486 is a TKR inhibitor, and was used with or without sunitinib in patients with advanced solid tumors and GIST, a completed clinical trial without any results known [87]. Temozolomide is an alkylating agent used in central nervous system (CNS) cancers. It was used in a phase II clinical trial in patients with SDH-mutant/deficient GIST, with no available results [88]. DS-6157 is a monoclonal antibody that targets the G-protein coupled receptor 20 (GPR20). It was used in a phase I clinical trial in patients with GIST as a single therapy to participants [89]. In Figure 1, the link between molecules and signaling pathways in GISTs is represented.
Figure 1.
The role of the receptors in the activation of signaling pathways in GISTs. The four major receptors groups (RTKs, PDGFR, c-KIT, and VEGFR) closely regulate the cellular process such as angiogenesis, cell proliferation and motility, and anti-apoptotic capability of cancer cells through the induction of RAF/MEK/ERK, PI3K/AKT/mTOR, PKC, and Src/FAK axis. In addition, a plethora of small agents that directly target the receptors or signaling pathways is presented in the figure.
5. Discussion
A very common problem with GIST therapy is resistance to imatinib. There have been a significant number of clinical trials trying to find new answers. Avapritinib is an effective solution, but only in PDGFRA D842V/exon 18 mutated GIST. Ripretinib challenges the therapeutic option in the second line and the results of the INTRIGUE trial are highly awaited. The other clinical trials performed are not able to give therapeutic solutions as of yet. There are 82 clinical trials, in all phases, active, and this is the first hopeful sign. The second hopeful sign is that the eight are using immunotherapeutic agents, such as nivolumab or ipilimumab, with or without chemotherapy. GISTs share some characteristics regarding the expression of PD1, PDL1, and immune checkpoint and membrane markers from immune cells, showing interesting data for the potential use of immunotherapy agents. Considering the impressive results immunotherapy has shown in other aspects of oncology, such as lung, renal, and melanoma, we are awaiting these studies’ results with great interest [90].
Resistance to imatinib, and in general TKI therapy, is a complex molecular process. Heterogeneity of secondary mutations is the principle mechanism of resistance and tumor progression. Several cellular signalings are involved, including Hippo pathway, MAPK, BET, PTEN, PI3K, PRKCQ, and JUN. Targeting these oncogenic mediators could potentially change the therapeutic approach of these untreatable tumors. Serrano et al., in their systematic approach, have suggested that a combination of TKIs might help improve the outcome of GIST tumors [25,26,91]. Further, Wozniak et al., in their review, recommend “liquid biopsy” as a potential mechanism of a personalized way to monitor resistance and response to TKI treatment [92].
Many drugs have been tested as treatment for GISTs. However, only four molecules (imatinib, dasatinib, axitinib, and ribociclib) have been combined with immunotherapy in clinical trials, showing there is a broad field for more studies, and that effort is being made to understand the molecular pathways of GISTs. The answer to the complex and persistent question of effective treatment of GISTs seems to include combination therapies. New small molecules, new TKIs, and immunotherapy agents, combined, may be the right way to inhibit the molecular pathways used by GIST cells to progress. Our review sheds light on the current research efforts, in both clinical and basic research, and this combined strategy could open new research pathways in disease therapy, and lead to the development of therapies that offer better clinical outcomes and expand life expectancy.
Author Contributions
All authors contributed to data analysis, drafting, and revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work. C.V., P.S., A.K., E.K., and S.T. made substantial contributions to the acquisition, analysis, and interpretation of data. A.G.P. and M.V.K. made substantial contributions in the conception, and design of the data. C.V., P.S., A.G.P., and M.V.K. made substantial contributions in drafting the manuscript and revising it critically for valuable intellectual content. The manuscript has been read and approved by all named authors. There are no other persons who satisfied the criteria for authorship, but who are not listed. The order of authors listed in the manuscript has been approved by all of us. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kindblom L.G., Remotti H.E., Aldenborg F., Meis-Kindblom J.M. Gastrointestinal pacemaker cell tumor (GIPACT): Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am. J. Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 2.Amelio J.M.S., Cid-Ruzafa J., Desai K., Tzivelekis S., Muston D., Khalid J.M., Ashman P., Maguire A. Prevalence of gastrointestinal stromal tumour (GIST) in the United Kingdom at different therapeutic lines: An epidemiologic model. BMC Cancer. 2014;14:364. doi: 10.1186/1471-2407-14-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rammohan A. A gist of gastrointestinal stromal tumors: A review. World J. Gastrointest. Oncol. 2012;5:102–112. doi: 10.4251/wjgo.v5.i6.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishida T., Blay J.-Y., Hirota S., Kitagawa Y., Kang Y.-K. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3–14. doi: 10.1007/s10120-015-0526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corless C.L. Gastrointestinal stromal tumors: What do we know now? Mod. Pathol. 2014;27:S1–S16. doi: 10.1038/modpathol.2013.173. [DOI] [PubMed] [Google Scholar]
- 6.Wang W., Li H., Yu J., Li Y., Han X., Chen P., Yu W., Chen W., Jiao Z., Liu H. Identification of key genes and associated pathways in KIT/PDGFRA wild-type gastrointestinal stromal tumors through bioinformatics analysis. Mol. Med. Rep. 2018;18:4499–4515. doi: 10.3892/mmr.2018.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li K., Cheng H., Li Z., Pang Y., Jia X., Xie F., Hu G., Cai Q., Wang Y. Genetic progression in gastrointestinal stromal tumors: Mechanisms and molecular interventions. Oncotarget. 2017;8:60589–60604. doi: 10.18632/oncotarget.16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bempt I.V., Borght S.V., Sciot R., Spans L., Claerhout S., Brems H., Lehnert S., Dehaspe L., Fransis S., Neuville B., et al. Comprehensive targeted next-generation sequencing approach in the molecular diagnosis of gastrointestinal stromal tumor. Genes Chromosom. Cancer. 2020 doi: 10.1002/gcc.22923. [DOI] [PubMed] [Google Scholar]
- 9.Szucs Z., Thway K., Fisher C., Bulusu R., Constantinidou A., Benson C., Van Der Graaf W.T.A., Jones R.L. Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol. 2017;13:93–107. doi: 10.2217/fon-2016-0192. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher C.D., Berman J.J., Corless C., Gorstein F., Lasota J., Longley B., Miettinen M., O’Leary T.J., Remotti H., Rubin B.P., et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum. Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 11.Parab T.M., Derogatis M.J., Boaz A.M., Grasso S.A., Issack P.S., Duarte D.A., Urayeneza O., Vahdat S., Qiao J.-H., Hinika G.S. Gastrointestinal stromal tumors: A comprehensive review. J. Gastrointest. Oncol. 2018;10:144–154. doi: 10.21037/jgo.2018.08.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miettinen M., Lasota J. Gastrointestinal stromal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch. Pathol. Lab. Med. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 13.Ghanem N., Altehoefer C., Furtwängler A., Winterer J., Schäfer O., Springer O., Kotter E., Langer M. Computed tomography in gastrointestinal stromal tumors. Eur. Radiol. 2003;13:1669–1678. doi: 10.1007/s00330-002-1803-6. [DOI] [PubMed] [Google Scholar]
- 14.Choi H., Charnsangavej C., Faria S.C., Macapinlac H.A., Burgess M.A., Patel S.R., Chen L.L., Podoloff D.A., Benjamin R.S. Correlation of Computed Tomography and Positron Emission Tomography in Patients with Metastatic Gastrointestinal Stromal Tumor Treated at a Single Institution with Imatinib Mesylate: Proposal of New Computed Tomography Response Criteria. J. Clin. Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi H., Hiramatsu Y., Kamiya K., Morita Y., Sakaguchi T., Konno H., Takeuchi H. Surgery for metastatic gastrointestinal stromal tumor: To whom and how to? Transl. Gastroenterol. Hepatol. 2018;3:14. doi: 10.21037/tgh.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poveda A., Del Muro X.G., López-Guerrero J.A., Martínez V., Romero I., Valverde C., Cubedo R., Martín-Broto J. GEIS 2013 guidelines for gastrointestinal sarcomas (GIST) Cancer Chemother. Pharmacol. 2014;74:883–898. doi: 10.1007/s00280-014-2547-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzocca A., Napolitano A., Silletta M., Ceruso M.S., Santini D., Tonini G., Vincenzi B. New frontiers in the medical management of gastrointestinal stromal tumours. Ther. Adv. Med. Oncol. 2019;11:11. doi: 10.1177/1758835919841946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzo A., Nannini M., Novelli M., Ricci A.D., Di Scioscio V., Pantaleo M.A. Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020;12 doi: 10.1177/1758835920936932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemunaitis J., Bauer S., Blay J.-Y., Choucair K., Gelderblom H., George S., Schöffski P., Von Mehren M., Zalcberg J., Achour H., et al. Intrigue: Phase III study of ripretinib versus sunitinib in advanced gastrointestinal stromal tumor after imatinib. Futur. Oncol. 2020;16:4251–4264. doi: 10.2217/fon-2019-0633. [DOI] [PubMed] [Google Scholar]
- 20.Mohammadi M., Gelderblom H. Systemic therapy of advanced/metastatic gastrointestinal stromal tumors: An update on progress beyond imatinib, sunitinib, and regorafenib. Expert Opin. Investig. Drugs. 2020:1–10. doi: 10.1080/13543784.2021.1857363. [DOI] [PubMed] [Google Scholar]
- 21.Din O.S., Woll P.J. Treatment of gastrointestinal stromal tumor: Focus on imatinib mesylate. Ther. Clin. Risk Manag. 2008;4:149–162. doi: 10.2147/tcrm.s1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricci R. Syndromic gastrointestinal stromal tumors. Hered. Cancer Clin. Pract. 2016;14:1–15. doi: 10.1186/s13053-016-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bamboat Z.M., DeMatteo R.P. Updates on the Management of Gastrointestinal Stromal Tumors. Surg. Oncol. Clin. N. Am. 2012;21:301–316. doi: 10.1016/j.soc.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid T. Reintroduction of Imatinib in GIST. J. Gastrointest. Cancer. 2013;44:385–392. doi: 10.1007/s12029-013-9532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ou W.-B., Ni N., Zuo R., Zhuang W., Zhu M., Kyriazoglou A., Wu D., Eilers G., Demetri G.D., Qiu H., et al. Cyclin D1 is a mediator of gastrointestinal stromal tumor KIT-independence. Oncogene. 2019;38:6615–6629. doi: 10.1038/s41388-019-0894-3. [DOI] [PubMed] [Google Scholar]
- 26.Serrano C., Mariño-Enríquez A., Tao D.L., Ketzer J., Eilers G., Zhu M., Yu C., Mannan A.M., Rubin B.P., Demetri G.D., et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br. J. Cancer. 2019;120:612–620. doi: 10.1038/s41416-019-0389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wozniak A., Rutkowski P., Schöffski P., Ray-Coquard I., Hostein I., Schildhaus H.-U., Le Cesne A., Bylina E., Limon J., Blay J.-Y., et al. Tumor Genotype Is an Independent Prognostic Factor in Primary Gastrointestinal Stromal Tumors of Gastric Origin: A European Multicenter Analysis Based on ConticaGIST. Clin. Cancer Res. 2014;20:6105–6116. doi: 10.1158/1078-0432.ccr-14-1677. [DOI] [PubMed] [Google Scholar]
- 28.Li G.Z., Raut C.P. Targeted therapy and personalized medicine in gastrointestinal stromal tumors: Drug resistance, mechanisms, and treatment strategies. Onco-Targets Ther. 2019;12:5123–5133. doi: 10.2147/OTT.S180763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boichuk S., Galembikova A., Dunaev P., Valeeva E., Shagimardanova E., Gusev O., Khaiboullina S.F. A Novel Receptor Tyrosine Kinase Switch Promotes Gastrointestinal Stromal Tumor Drug Resistance. Molecules. 2017;22:2152. doi: 10.3390/molecules22122152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li F., Huynh H., Li X., Ruddy D., Wang Y., Ong R., Chow P., Qiu S., Tam A., Rakiec D.P., et al. FGFR-Mediated Reactivation of MAPK Signaling Attenuates Antitumor Effects of Imatinib in Gastrointestinal Stromal Tumors. Cancer Discov. 2015;5:438–451. doi: 10.1158/2159-8290.CD-14-0763. [DOI] [PubMed] [Google Scholar]
- 31.Boichuk S., Dunaev P., Galembikova A., Bikinieva F., Nurgatina I., Mustafin I.G., Aukhadieva A., Kurtasanov R., Natalia A., Shagimardanova E., et al. Inhibition of FGFR2-Signaling Attenuates a Homology-Mediated DNA Repair in GIST and Sensitizes Them to DNA-Topoisomerase II Inhibitors. Int. J. Mol. Sci. 2020;21:352. doi: 10.3390/ijms21010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi S.-S., Wu N., He Y., Wei X., Xia Q.-Y., Wang X., Ye S.-B., Li R., Rao Q., Zhou X.-J. EGFR gene mutation in gastrointestinal stromal tumours. Histopathol. 2017;71:553–561. doi: 10.1111/his.13251. [DOI] [PubMed] [Google Scholar]
- 33.Cai Y.-C., Jiang Z., Vittimberga F., Xu X., Savas L., Woda B., Callery M., Banner B. Expression of transforming growth factor-α and epidermal growth factor receptor in gastrointestinal stromal tumours. Virchows Archiv. 1999;435:112–115. doi: 10.1007/s004280050407. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa M., Nabeshima K., Asano S., Hamasaki M., Uesugi N., Tani H., Yamashita Y., Iwasaki H. Up-regulated expression of ADAM17 in gastrointestinal stromal tumors: Coexpression with EGFR and EGFR ligands. Cancer Sci. 2009;100:654–662. doi: 10.1111/j.1349-7006.2009.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang J., Jin M.-S., Suo J., Wang Y.-P., He L., Cao X.-Y. Evaluation of malignancy using Ki-67, p53, EGFR and COX-2 expressions in gastrointestinal stromal tumors. World J. Gastroenterol. 2012;18:2569–2575. doi: 10.3748/wjg.v18.i20.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao W.-Y., Zhuang C., Xu J., Wang M., Zhang Z.-Z., Tu L., Wang C.-J., Ling T.-L., Cao H., Zhang Z.-G. HER4 is a novel prognostic biomarker in gastrointestinal stromal tumor specifically originated from stomach. Am. J. Cancer Res. 2014;4:838–849. [PMC free article] [PubMed] [Google Scholar]
- 37.Tu Y., Zuo R., Ni N., Eilers G., Wu D., Pei Y., Nie Z., Wu Y., Wu Y., Ou W.-B. Activated tyrosine kinases in gastrointestinal stromal tumor with loss of KIT oncoprotein expression. Cell Cycle. 2018;17:2577–2592. doi: 10.1080/15384101.2018.1553335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagata K., Kawakami T., Kurata Y., Kimura Y., Suzuki Y., Nagata T., Sakuma Y., Miyagi Y., Hirano H. Augmentation of multiple protein kinase activities associated with secondary imatinib resistance in gastrointestinal stromal tumors as revealed by quantitative phosphoproteome analysis. J. Proteom. 2015;115:132–142. doi: 10.1016/j.jprot.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Sarantis P., Koustas E., Papadimitropoulou A., Papavassiliou A.G., Karamouzis M.V. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020;12:173–181. doi: 10.4251/wjgo.v12.i2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Indio V., Ravegnini G., Astolfi A., Urbini M., Saponara M., De Leo A., Gruppioni E., Tarantino G., Angelini S., Pession A., et al. Gene Expression Profiling of PDGFRA Mutant GIST Reveals Immune Signatures as a Specific Fingerprint of D842V Exon 18 Mutation. Front. Immunol. 2020;11:851. doi: 10.3389/fimmu.2020.00851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Indio V., Astolfi A., Tarantino G., Urbini M., Patterson J., Nannini M., Saponara M., Gatto L., Santini D., Valle I.F.D., et al. Integrated Molecular Characterization of Gastrointestinal Stromal Tumors (GIST) Harboring the Rare D842V Mutation in PDGFRA Gene. Int. J. Mol. Sci. 2018;19:732. doi: 10.3390/ijms19030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cassier P.A., Fumagalli E., Rutkowski P., Schöffski P., Van Glabbeke M., Debiec-Rychter M., Emile J.F., Duffaud F., Martin-Broto J., Landi B., et al. Outcome of Patients with Platelet-Derived Growth Factor Receptor Alpha–Mutated Gastrointestinal Stromal Tumors in the Tyrosine Kinase Inhibitor Era. Clin. Cancer Res. 2012;18:4458–4464. doi: 10.1158/1078-0432.CCR-11-3025. [DOI] [PubMed] [Google Scholar]
- 43.Farag S., Somaiah N., Choi H., Heeres B., Wang W.-L., Van Boven H., Nederlof P., Benjamin R., Van Der Graaf W.T.A., Grunhagen D., et al. Clinical characteristics and treatment outcome in a large multicentre observational cohort of PDGFRA exon 18 mutated gastrointestinal stromal tumour patients. Eur. J. Cancer. 2017;76:76–83. doi: 10.1016/j.ejca.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Pantaleo M.A., Tarantino G., Agostinelli C., Urbini M., Nannini M., Saponara M., Castelli C., Stacchiotti S., Fumagalli E., Gatto L., et al. Immune microenvironment profiling of gastrointestinal stromal tumors (GIST) shows gene expression patterns associated to immune checkpoint inhibitors response. Oncoimmunology. 2019;8:e1617588. doi: 10.1080/2162402X.2019.1617588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Indio V., Astolfi A., Urbini M., Nannini M., Pantaleo M.A. Genetics and treatment of gastrointestinal stromal tumors with immune checkpoint inhibitors: What do we know? Pharmacogenomics. 2020;21:231–234. doi: 10.2217/pgs-2019-0173. [DOI] [PubMed] [Google Scholar]
- 46.Vitiello G.A., Bowler T.G., Liu M., Medina B.D., Zhang J.Q., Param N.J., Loo J.K., Goldfeder R.L., Chibon F., Rossi F., et al. Differential immune profiles distinguish the mutational subtypes of gastrointestinal stromal tumor. J. Clin. Investig. 2019;129:1863–1877. doi: 10.1172/JCI124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun X., Sun J., Yuan W., Gao X., Fu M., Xue A., Li H., Shu P., Fang Y., Hou Y., et al. Immune Cell Infiltration and the Expression of PD-1 and PD-L1 in Primary PDGFRA-Mutant Gastrointestinal Stromal Tumors. J. Gastrointest. Surg. 2020:1–10. doi: 10.1007/s11605-020-04860-8. [DOI] [PubMed] [Google Scholar]
- 48.Singh A.S., Chmielowski B., Hecht J.R., Rosen L.S., Chow W.A., Wang X., Brackert S., Adame C., Bovill J., Schink E., et al. A randomized phase II study of nivolumab monotherapy versus nivolumab combined with ipilimumab in advanced gastrointestinal stromal tumor (GIST) J. Clin. Oncol. 2019;37:11017. doi: 10.1200/JCO.2019.37.15_suppl.11017. [DOI] [Google Scholar]
- 49.Reilley M.J., Bailey A., Subbiah V., Janku F., Naing A., Falchook G., Karp D.D., Piha-Paul S.A., Tsimberidou A.-M., Fu S., et al. Phase I clinical trial of combination imatinib and ipilimumab in patients with advanced malignancies. J. Immunother. Cancer. 2017;5:35. doi: 10.1186/s40425-017-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D’Angelo S.P., Mahoney M.R., Van Tine B.A., Atkins J., Milhem M.M., Jahagirdar B.N., Antonescu C.R., Horvath E., Tap W.D., Schwartz G.K., et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19:416–426. doi: 10.1016/S1470-2045(18)30006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nivolumab and Ipilimumab in Treating Patients with Rare Tumors. [(accessed on 23 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02834013?term=Ipilimumab&cond=GIST&draw=2&rank=5.
- 52.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Epacadostat and Pembrolizumab in Patients with GIST. [(accessed on 23 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03291054?term=pembrolizumab&cond=gist&draw=2&rank=1.
- 54.Le Cesne A., Marec-Bérard P., Blay J.-Y., Gaspar N., Bertucci F., Penel N., Bompas E., Cousin S., Toulmonde M., Bessede A., et al. Programmed cell death 1 (PD-1) targeting in patients with advanced osteosarcomas: Results from the PEMBROSARC study. Eur. J. Cancer. 2019;119:151–157. doi: 10.1016/j.ejca.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 55.Phase Ib Study of TNO155 in Combination with Spartalizumab or Ribociclib in Selected Malignancies. [(accessed on 23 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04000529?term=spartalizumab&cond=gist&draw=2&rank=1.
- 56.A Study of Avelumab In Combination with Axitinib in Patients with Unresectable/Metastatic Gastrointestinal Stromal Tumor After Failure of Standard Therapy. [(accessed on 14 December 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04258956?term=avelumab&cond=gist&draw=2&rank=1.
- 57.A Phase I/II Study of Regorafenib Plus Avelumab in Solid Tumors. [(accessed on 14 December 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03475953?term=avelumab&cond=gist&draw=2&rank=2.
- 58.PDR001 Plus Imatinib for Metastatic or Unresectable GIST. [(accessed on 14 December 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03609424?term=PDR001&cond=gist&draw=2&rank=1.
- 59.Study to Allow Access to Nilotinib for Patients Who Are on Nilotinib Treatment in a Novartis-sponsored Study. [(accessed on 22 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01735955?term=nilotinib&recrs=abdf&cond=gist&draw=2&rank=2.
- 60.Treatment of Patients with Metastatic or Unresectable Gastrointestinal Stromal Tumors in First Line with Nilotinib. [(accessed on 23 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00756509?term=nilotinib&recrs=abdf&cond=gist&draw=2&rank=1.
- 61.Nilotinib Roll-over Protocol for Patients in Novartis-sponsored Nilotinib Study and Benefiting From Nilotinib Treatment. [(accessed on 22 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01863745?term=nilotinib&recrs=abdf&cond=gist&draw=2&rank=3.
- 62.Janku F., Razak A.R.A., Chi P., Heinrich M.C., Von Mehren M., Jones R.L., Ganjoo K., Trent J., Gelderblom H., Somaiah N., et al. Switch Control Inhibition of KIT and PDGFRA in Patients with Advanced Gastrointestinal Stromal Tumor: A Phase I Study of Ripretinib. J. Clin. Oncol. 2020;38:3294–3303. doi: 10.1200/JCO.20.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.A Drug-Drug Interaction Study to Evaluate the Effect of Ripretinib on the Pharmacokinetics of a CYP2C8 Probe Substrate in Patients with Advanced GIST. [(accessed on 22 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04530981?term=ripretinib&recrs=abdf&cond=gist&draw=2&rank=1.
- 64.A Study of DCC-2618 (Ripretinib) Evaluating Efficacy, Safety, and Pharmacokinetics In Patients with Advanced Gastrointestinal Stromal Tumors (GIST) [(accessed on 22 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04282980?term=ripretinib&recrs=abdf&cond=gist&draw=2&rank=2.
- 65.Blay J.-Y., Serrano C., Heinrich M.C., Zalcberg J., Bauer S., Gelderblom H., Schöffski P., Jones R.L., Attia S., D’Amato G., et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:923–934. doi: 10.1016/S1470-2045(20)30168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.A Study of CS3007 in Subjects with Gastrointestinal Stromal Tumor. [(accessed on 22 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04254939?term=avapritinib&recrs=abdf&cond=gist&draw=2&rank=3.
- 67.Gebreyohannes Y.K., Wozniak A., Zhai M.-E., Wellens J., Cornillie J., Vanleeuw U., Evans E.K., Gardino A.K., Lengauer C., Debiec-Rychter M., et al. Robust Activity of Avapritinib, Potent and Highly Selective Inhibitor of Mutated KIT, in Patient-derived Xenograft Models of Gastrointestinal Stromal Tumors. Clin. Cancer Res. 2019;25:609–618. doi: 10.1158/1078-0432.ccr-18-1858. [DOI] [PubMed] [Google Scholar]
- 68.Early Access Program (EAP) for Avapritinib in Patients with Locally Advanced Unresectable or Metastatic GIST. [(accessed on 22 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03862885?term=avapritinib&cond=gist&draw=2&rank=2.
- 69.Heinrich M.C., Jones R.L., von Mehren M., Schöffski P., Serrano C., Kang Y.K., Cassier P.A., Mir O., Eskens F., Tap W.D., et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): A multicentre, open-label, phase 1 trial. Lancet Oncol. 2020;21:935–946. doi: 10.1016/S1470-2045(20)30269-2. [DOI] [PubMed] [Google Scholar]
- 70.Schöffski P., Mir O., Kasper B., Papai Z., Blay J.-Y., Italiano A., Benson C., Kopeckova K., Ali N., Dileo P., et al. Activity and safety of the multi-target tyrosine kinase inhibitor cabozantinib in patients with metastatic gastrointestinal stromal tumour after treatment with imatinib and sunitinib: European Organisation for Research and Treatment of Cancer phase II trial 1317 ‘CaboGIST’. Eur. J. Cancer. 2020;134:62–74. doi: 10.1016/j.ejca.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 71.Grover P.K., Cummins A.G., Price T.J., Roberts-Thomson I.C., Hardingham J.E. Circulating tumour cells: The evolving concept and the inadequacy of their enrichment by EPCAM-based methodology for baskc clinical cancer research. Ann. Oncol. 2014:1506–1516. doi: 10.1093/annonc. [DOI] [PubMed] [Google Scholar]
- 72.Randomized Trial of Crenolanib in Subjects with D842V Mutated GIST. [(accessed on 22 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02847429?term=Crenolanib&cond=gist&draw=2&rank=2.
- 73.Cortes J., Kantarjian H., Shah N.P., Bixby D., Mauro M.J., Flinn I., O’Hare T., Hu S., Narasimhan N.I., Rivera V.M., et al. Ponatinib in Refractory Philadelphia Chromosome–Positive Leukemias. N. Engl. J. Med. 2012;367:2075–2088. doi: 10.1056/nejmoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dasatinib and Ipilimumab in Treating Patients with Gastrointestinal Stromal Tumors or Other Sarcomas That Cannot Be Removed by Surgery or Are Metastatic. [(accessed on 22 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01643278?term=Ipilimumab%2Cdasatinib&cond=gist&draw=2&rank=1.
- 75.MEK162 in Combination with Imatinib Mesylate in Patients with Untreated Advanced Gastrointestinal Stromal Tumor (GIST) [(accessed on 22 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01991379?term=Binimetinib&cond=gist&draw=2&rank=2.
- 76.Rosenbaum E., Kelly C., D’Angelo S.P., Dickson M.A., Gounder M., Keohan M.L., Movva S., Condy M., Adamson T., McFadyen C.R., et al. A Phase I Study of Binimetinib (MEK162) Combined with Pexidartinib (PLX3397) in Patients with Advanced Gastrointestinal Stromal Tumor. Oncol. 2019;24:1309-e983. doi: 10.1634/theoncologist.2019-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phase II Trial of Vandetanib in Children and Adults with Wild-Type Gastrointestinal Stromal Tumors. [(accessed on 22 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02015065?term=Vandetanib&cond=gist&draw=2&rank=1.
- 78.A Study of Famitinib in Patients with Gastrointestinal Stromal Tumor. [(accessed on 22 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02336724?term=Famitinib&cond=gist&draw=2&rank=1.
- 79.Efficacy and Safety of Famitinib Versus Sunitinib in the Treatment of Advanced Gastrointestinal Stromal Tumour Patients After Failure of Imatinib. [(accessed on 22 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04409223?term=Famitinib&cond=gist&draw=2&rank=2.
- 80.Efficacy and Safety of Anlotinib in Patients with Advanced Gastrointestinal Stromal Tumor After Failure of Imatinib: A Prospective, Single Arm and Multicenter Trial. [(accessed on 22 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04106024?term=anlotinib&cond=gist&draw=2&rank=1.
- 81.Boegemann M., Schlack K., Rink M., Bernhardt S., Moran M., Hubbe M., Bergmann L., Schmid M., Strauss A. Effect of comorbidities/comedications on sunitinib outcomes for metastatic renal cell carcinoma: The STAR-TOR registry. Future Oncol. 2020;16:2939–2948. doi: 10.2217/fon-2020-0548. [DOI] [PubMed] [Google Scholar]
- 82.Doxorubicin Hydrochloride and Alvocidib in Treating Patients with Metastatic or Recurrent Sarcoma That Cannot Be Removed By Surgery. [(accessed on 23 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT00098579?term=Alvocidib&cond=gist&draw=2&rank=1.
- 83.Sorafenib in Treating Patients with Malignant Gastrointestinal Stromal Tumor That Progressed During or After Previous Treatment with Imatinib Mesylate and Sunitinib Malate. [(accessed on 23 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00265798?term=sorafenib&recrs=abdf&cond=gist&draw=2&rank=1.
- 84.Wagner A.J., Kindler H., Gelderblom H., Schöffski P., Bauer S., Hohenberger P., Kopp H.-G., Lopez-Martin J.A., Peeters M., Reichardt P., et al. A phase II study of a human anti-PDGFRα monoclonal antibody (olaratumab, IMC-3G3) in previously treated patients with metastatic gastrointestinal stromal tumors. Ann. Oncol. 2017;28:541–546. doi: 10.1093/annonc/mdw659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.SARC029: Trametinib and Pazopanib in Patients with GIST (Gastrointestinal Stromal Tumor) [(accessed on 23 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02342600?term=TRAMETINIB&cond=GIST&draw=2&rank=1.
- 86.A Study of HQP1351 in Patients with GIST or Other Solid Tumors. [(accessed on 23 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03594422?term=HQP1351&cond=gist&draw=2&rank=1.
- 87.PLX9486 as a Single Agent and in Combination with PLX3397 or PLX9486 with Sunitinib in Patients with Advanced Solid Tumors. [(accessed on 23 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02401815?term=PLX9486&cond=gist&draw=2&rank=1.
- 88.Temozolomide (TMZ) In Advanced Succinate Dehydrogenase (SDH)-Mutant/Deficient Gastrointestinal Stromal Tumor (GIST) [(accessed on 14 December 2020)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT03556384?term=imatinib&recrs=abdf&cond=GIST&draw=4.
- 89.DS-6157a in Participants with Advanced Gastrointestinal Stromal Tumor (GIST) [(accessed on 14 December 2020)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04276415?term=imatinib&recrs=abdf&cond=GIST&draw=5.
- 90.Koustas E., Sarantis P., Papavassiliou A.G., Karamouzis M.V. The Resistance Mechanisms of Checkpoint Inhibitors in Solid Tumors. Biomolecules. 2020;10:666. doi: 10.3390/biom10050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hemming M.L., Lawlor M., Andersen J.L., Hagan T., Chipashvili O., Scott T.G., Raut C.P., Sicinska E., Armstrong S.A., Demetri G.D., et al. Enhancer Domains in Gastrointestinal Stromal Tumor Regulate KIT Expression and Are Targetable by BET Bromodomain Inhibition. Cancer Res. 2019;79:994–1009. doi: 10.1158/0008-5472.CAN-18-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wozniak A., Gebreyohannes Y.K., Debiec-Rychter M., Schöffski P. New targets and therapies for gastrointestinal stromal tumors. Expert Rev. Anticancer. Ther. 2017;17:1117–1129. doi: 10.1080/14737140.2017.1400386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.