Abstract
Background
Since the early 1980s, papillary thyroid cancer (PTC) incidence rates and the prevalence of obesity, a risk factor for PTC, have increased substantially in the United States. We estimated the proportion of PTC incidence in the United States attributable to overweight and obesity during 1995–2015.
Methods
National Institutes of Health-AARP Diet and Health Study cohort data (n = 457 331 participants, 50–71 years and cancer-free at baseline) were used to estimate multivariable-adjusted hazard ratios (HRs) for PTC across body mass index categories. Population attributable fractions (PAFs) were calculated using estimated hazard ratios and annual overweight and obesity prevalence estimates from the National Health Interview Survey. PAF estimates were combined with Surveillance, Epidemiology, and End Results-13 data to calculate annual percent changes in PTC incidence rates attributable (and unrelated) to overweight and obesity.
Results
Overweight (25.0–29.0 kg/m2) and obesity (≥30.0 kg/m2) were associated with 1.26-fold (95% confidence interval [CI] = 1.05- to 1.52-fold) and 1.30-fold (95% CI = 1.05- to 1.62-fold) increased risks of PTC, respectively, and nearly threefold (HR = 2.93, 95% CI = 1.25 to 6.87) and greater than fivefold (HR = 5.42, 95% CI = 2.24 to 13.1) increased risks of large (>4 cm) PTCs compared with normal weight (18.5–24.9 kg/m2). During 1995–2015, PAF estimates for overweight and obesity increased from 11.4% to 16.2% for all PTCs and from 51.4% to 63.2% for large PTCs. Overweight or obesity accounted for 13.6% and 57.8% of the annual percent changes in total (5.9%/y) and large (4.5%/y) PTC incidence rates, respectively, during 1995–2015.
Conclusions
Overweight and obesity may have contributed importantly to the rapid rise in PTC incidence during 1995–2015. By 2015, we estimate that one of every six PTCs diagnosed among adults 60 years or older, including nearly two-thirds of large PTCs, were attributable to overweight and obesity.
US thyroid cancer incidence rates have increased substantially since the early 1980s, driven largely by an increase in papillary thyroid cancer (PTC), the most common histologic type (1). Similar trends have been observed internationally (2). Small, localized PTCs accounted for most of the increase, largely reflecting the more widespread use of diagnostic imaging procedures and improvements in diagnostic tools (3). However, there also have been statistically significant increases in the incidence rates for larger-sized (>2 cm) and advanced-stage PTC as well as in thyroid cancer mortality (1). These findings suggest that environmental or lifestyle risk factors also may have contributed to changing PTC incidence trends.
For decades, the etiology of thyroid cancer remained elusive, with few known modifiable risk factors apart from childhood and adolescent exposure to ionizing radiation and iodine deficiency (4,5). Early epidemiologic studies provided conflicting evidence linking greater body mass index (BMI), an indicator of general adiposity, with thyroid cancer risk (4–8); however, many of these studies were limited by retrospective exposure assessment, lack of data on potential confounding factors, and/or small numbers of cases, particularly among men. Within the last decade, several large prospective cohort studies and pooled analyses have emerged, showing consistent positive associations for BMI and other indicators of adiposity (eg, waist circumference and weight change) with both thyroid cancer incidence (all histologic types apart from medullary carcinoma) and, to a greater extent, thyroid cancer mortality (9–12). Based on the results from epidemiologic studies, the International Agency for Research on Cancer determined that there is sufficient evidence of a causal relationship between excess adiposity and thyroid cancer (13). Moreover, results from clinical studies have shown that obese PTC patients tend to present with more advanced-stage disease and clinically aggressive tumor characteristics (14–16), which may be attributable to a direct effect of excess adiposity on thyroid cancer growth and progression (17). Considering the tremendous increase in obesity prevalence among US adults since the early 1980s (from 15% in 1980 to nearly 40% in 2015–2016) (18,19), excess adiposity could have contributed appreciably to the observed temporal increases in US PTC incidence rates.
The overarching aim of the current study was to quantify the impact of the rising prevalence of overweight and obesity in the general US population on trends in PTC incidence rates (overall and by tumor characteristics at diagnosis) during 1995–2015. To estimate the risk of PTC associated with overweight and obesity, we analyzed data from a large US prospective cohort study and, for the first time in a cohort study to our knowledge, assessed this association according to tumor stage and size at diagnosis. We combined these results with national overweight and obesity prevalence and cancer registry data to estimate the proportions of PTCs diagnosed each year that were attributable to overweight and obesity.
Methods
Study Population and Case Definition
The National Institutes of Health-American Association of Retired Persons (NIH-AARP) is a large US cohort study that was established in 1995–1996 when 567 169 members of AARP (formerly known as the American Association of Retired Persons), aged 50–71 years, satisfactorily completed a mailed baseline questionnaire inquiring about health and lifestyle characteristics (20). For the analysis, we excluded proxy respondents, individuals who reported a prior diagnosis of cancer other than non-melanoma skin cancer; those with no follow-up information; individuals with missing data on smoking status, height, or weight; and cases with extreme values of BMI, yielding a total of 457 331 individuals (273 604 men and 183 727 women). Cancer diagnoses were obtained through 2011 by linking individual study participants with state and local cancer registry databases. Cases were individuals diagnosed with first primary PTC during follow-up (ICD-10 C73.9; ICD-O-3 8050, 8260, 8340-8344, 8350, 8450-8460). Cases were further classified by stage at diagnosis (Surveillance, Epidemiology and End Results Program [SEER] summary stage: localized, regional, distant) and tumor size [≤1.0, 1.1–2.0, 2.1–4.0, and >4 cm, corresponding to size thresholds used by the American Joint Committee on Cancer thyroid cancer staging system (21)]. A secondary analysis was restricted to first primary anaplastic thyroid cancers (ICD-O-3 8020-8035). The study protocol was approved by the Special Studies Institutional Review Board of the National Cancer Institute.
Annual overweight and obesity prevalence data for adults aged 50 years or older during 1985–2005 were ascertained from the National Health Interview Survey (NHIS), a nationally representative survey of the health of the US civilian noninstitutionalized population (22). BMI was calculated from self-reported height and weight.
PTC incidence rates among those 60 years and older during 1995–2015 were estimated from data collected by 13 cancer registries as part of the SEER-13 (23). Cases were restricted to first primary thyroid cancers that were microscopically confirmed and classified using the histology codes and size categories described above.
Statistical Analysis
Using data from NIH-AARP, Cox proportional hazard regression models were used to estimate multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between BMI categories (<18.5 [underweight], 18.5–24.9 [normal weight], 25.0–29.9 [overweight], and ≥30.0 kg/m2 [obese]) and first primary PTC risk. Follow-up started at participants’ age at baseline questionnaire completion and ended at the age at diagnosis of any primary cancer other than non-melanoma skin cancer or right-censoring (loss to follow-up, or death, or end of follow-up on December 31, 2011). Additional models estimated associations between BMI and PTC by tumor stage and size at diagnosis. Models were adjusted for sex, race or ethnicity (non-Hispanic black, non-Hispanic white, Hispanic, other specified and unknown), education (college graduate, not college graduate, unknown), weekly alcohol consumption (continuous), and smoking status (never, former, and current). The proportionality assumption was assessed by estimating the associations between BMI and PTC stratified by follow-up time. All P values were two-sided with an alpha of .05. Interaction tests were conducted by including a cross-product term in the model and evaluating the Wald-based P value.
National prevalence estimates of overweight (BMI = 25.0–29.9 kg/m2) and obesity (BMI ≥ 30.0 kg/m2) in the NHIS were calculated using sample survey weights. Because height and weight data were unavailable in 1986, 1989, and 1996, prevalence estimates for these years were imputed as the average of the surrounding years.
Annual population attributable fractions (PAFs) (24,25) were calculated from the estimated hazard ratios (relative risks) derived from the NIH-AARP Diet and Health Study and prevalence estimates from NHIS as
In the above equation, wi denotes the survey weight, Zi the adjustment variables, Xi the variable of main interest (BMI category) for person i in the survey, and γ and β the log-relative risk parameters for Z and X, respectively, estimated from the NIH-AARP cohort. Assuming a 10-year latency period [based on a median time from questionnaire to PTC diagnosis in the NIH-AARP study of 8.9 years and available evidence regarding the latency period for other obesity–cancer associations (26)], PAFs based on overweight and obesity prevalence estimates for a given year were applied to PTC incidence rates 10 years later. To accommodate the variability of the estimated log-relative risk parameters, we computed the variance of PAF both by a bootstrap resampling approach and analytically. Results are based on the bootstrap variance estimates, which agreed well with the analytic estimates.
Estimated PAFs were then multiplied by annual age-standardized PTC rates for adults aged 60 years or older in SEER, partitioning out incidence rates attributable to (and unrelated to) overweight and obesity. Annual percent changes in PTC rates and for overweight- and obesity-attributable and overweight- and obesity-unrelated PTC incidence rates were estimated using Joinpoint software, which identifies calendar years where there is a statistically significant change in the slope of incidence rate trends over time. Average annual percent changes (AAPCs) also were estimated for the full 1995–2015 time period. The proportion of the AAPC driven by overweight- and obesity-attributable PTC incidence rates was estimated as 1 − [AAPC(overweight/obesity-unrelated) ÷ AAPCoverall].
Results
From 1995 to 2011, 604 incident PTC cases occurred among men and women in the NIH-AARP Diet and Health Study (Table 1). Overweight (25.0–29.0 kg/m2) and obesity (≥30.0 kg/m2) were associated with 1.26-fold (95% CI = 1.05-fold to 1.52-fold) and 1.30-fold (95% CI = 1.05-fold to 1.62-fold) increased risks of PTC, respectively, compared with normal weight (18.5–24.9 kg/m2) (Table 2). Finer categorization of the top BMI categories showed consistently increasing risks of PTC with greater BMI, with hazard ratios for obese class I (30.0–34.9 kg/m2) of 1.21 (95% CI = 0.94 to 1.55), obese class II (35.0–39.9 kg/m2) of 1.40 (95% CI = 0.97 to 2.03), and obese class III (40+ kg/m2) of 1.93 (95% CI = 1.15 to 3.22) (Supplementary Figure 1, available online). Hazard ratios for continuous BMI were highest in the first 10 years of follow-up (0–4.9 years: HR = 1.08, 95% CI = 1.07 to 1.10; 5.0–9.9 years: HR = 1.05, 95% CI = 1.03 to 1.07; 10.0+ years: HR = 0.96, 95% CI = 0.95 to 0.98); the HR for continuous BMI over the full follow-up period was 1.03 (95% CI = 1.01 to 1.04). Associations were similar for localized versus regional and distant cases (Table 2). Overweight and obesity were associated with nearly threefold (HR = 2.93, 95% CI = 1.25 to 6.87) and greater than fivefold (HR = 5.42, 95% CI = 2.24 to 13.1) increased risks of large (>4 cm) PTCs compared with normal weight and were not associated with risk of smaller-sized PTCs. Hazard ratios by BMI category were similar for PTCs of less than 1 cm and those with missing or unknown size. Overall, BMI was more strongly associated with PTC risk in men than women (Supplementary Table 1, available online) (Pinteraction [using continuous BMI] = .04). Among men, associations were stronger for regional and distant PTC compared with localized PTC. Statistically significant positive associations were observed for obesity in both sexes after restricting to PTCs greater than 4 cm at diagnosis (men: HR = 7.62, 95% CI = 2.11 to 27.5; women: HR = 3.58, 95% CI = 1.02 to 12.5; Pinteraction = .36); no associations were observed for PTCs 4 cm or less in either men or women. No statistically significant interaction was observed between age at study entry (<60, 60+ years) and BMI (continuous) on PTC risk (Pinteraction = .07). Overweight and obesity were associated with increased risk of anaplastic thyroid cancer (n = 28; HRs = 1.74, 95% CI = 0.63 to 4.77 and 3.59, 95% CI = 1.31 to 9.83, respectively) compared with normal weight.
Table 1.
Baseline characteristics and diagnostic information for individuals diagnosed with PTC during follow-up (cases) and non-cases in the NIH-AARP Study, 1995–2011
| Baseline or diagnostic characteristic | Cases | Noncases |
|---|---|---|
| No. (%) | No. (%) | |
| Total | 604 (100) | 456 507 (100) |
| Sex | ||
| Male | 248 (41.1) | 273 242 (59.9) |
| Female | 356 (58.9) | 183 265 (40.2) |
| Age at baseline, y | ||
| 50–54 | 100 (16.6) | 62 586 (13.7) |
| 55–59 | 167 (27.6) | 104 002 (22.8) |
| 60–64 | 168 (27.8) | 128 418 (28.1) |
| 65–69 | 155 (25.7) | 145 482 (31.9) |
| ≥70 | 14 (2.3) | 16 019 (3.5) |
| Education | ||
| College graduate | 237 (39.2) | 178 887 (39.2) |
| Not college graduate | 340 (56.3) | 265 499 (58.2) |
| Unknown | 27 (4.5) | 12 121 (2.7) |
| Race/ethnicity | ||
| Non-Hispanic white | 551 (91.2) | 418 487 (91.7) |
| Non-Hispanic black | 26 (4.3) | 16 975 (3.7) |
| Hispanic | 8 (1.3) | 8504 (1.9) |
| Other | 11 (1.8) | 7423 (1.6) |
| Unknown | 8 (1.3) | 5118 (1.1) |
| Smoking status | ||
| Never | 268 (44.4) | 166 984 (36.6) |
| Former | 287 (47.5) | 233 011 (51.0) |
| Current | 49 (8.1) | 56 512 (12.4) |
| BMI, kg/m2 | ||
| <18.5 | 1 (0.2) | 3432 (0.8) |
| 18.5–24.9 | 196 (32.5) | 158 930 (34.8) |
| 25.0–29.9 | 262 (43.4) | 196 396 (43.0) |
| 30.0–34.9 | 96 (15.9) | 72 169 (15.8) |
| 35.0–39.9 | 33 (5.5) | 19 676 (4.3) |
| ≥40.0 | 16 (2.6) | 5904 (1.3) |
| Stage | ||
| Localized | 432 (71.5) | —* |
| Regional | 117 (19.4) | — |
| Distant | 36 (6.0) | — |
| Unknown | 19 (3.1) | — |
| Tumor size, cm | ||
| ≤1.0 | 185 (30.6) | — |
| 1.1–2.0 | 98 (16.2) | — |
| 2.1–4.0 | 86 (14.2) | — |
| >4.0 | 50 (8.3) | — |
| Unknown | 185 (30.6) | — |
Not applicable to noncases. BMI = body mass index; NIH-AARP = National Institutes of Health-American Association of Retired Persons; PTC = papillary thyroid cancer.
Table 2.
Hazard ratios* and 95% confidence intervals for PTC, overall and by stage and tumor size, according to baseline categories of BMI in the NIH-AARP Diet and Health Study (1995–2011)
| Diagnostic characteristic | No. of cases | BMI, kg/m2 | |||
|---|---|---|---|---|---|
| HR (95% CI) | |||||
| <18.5 | 18.5–24.9 | 25.0–29.9 | ≥30.0 | ||
| Total | 604 | 0.22 (0.03 to 1.57) | 1.00 (Referent.) | 1.26 (1.05 to 1.52) | 1.30 (1.05 to 1.62) |
| Stage | |||||
| Localized | 432 | 0.30 (0.04 to 2.15) | 1.00 (Referent.) | 1.25 (1.00 to 1.56) | 1.28 (1.00 to 1.66) |
| Regional and distant | 153 | —† | 1.00 (Referent.) | 1.32 (0.90 to 1.93) | 1.39 (0.89 to 2.16) |
| Tumor size, cm | |||||
| ≤1 | 185 | — | 1.00 (Referent.) | 1.28 (0.92 to 1.79) | 1.20 (0.81 to 1.78) |
| 1.1–2.0 | 98 | — | 1.00 (Referent.) | 0.77 (0.48 to 1.23) | 1.09 (0.66 to 1.82) |
| 2.1–4.0 | 86 | — | 1.00 (Referent.) | 1.42 (0.87 to 2.32) | 1.04 (0.56 to 1.92) |
| >4.0 | 50 | — | 1.00 (Referent.) | 2.93 (1.25 to 6.87) | 5.42 (2.24 to 13.1) |
| Missing or unknown | 185 | 0.74 (0.10 to 5.35) | 1.00 (Referent.) | 1.30 (0.92 to 1.83) | 1.22 (0.82 to 1.82) |
Models used attained age as the time metric and were adjusted for sex, race or ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other specified, unknown), education (college graduate or not, unknown), number of drinks of alcohol per week (continuous), and cigarette smoking status (never, former, current). BMI = body mass index; CI = confidence interval; HR = hazard ratio; NIH-AARP = National Institutes of Health-American Association of Retired Persons; PTC = papillary thyroid cancer.
No cases contributed to the category.
The prevalence of obesity among NHIS survey respondents aged 50 years or older increased from 11.2% to 23.8% during 1985–2005, and the prevalence of overweight increased from 29.6% to 37.0% (Figure 1). In comparison, during the same calendar years, baseline (1995–1996) prevalence estimates of obesity and overweight among NIH-AARP participants were somewhat higher (21.4% and 43.0%, respectively; Table 1). Combining the NHIS prevalence estimates with the multivariable (including sex)-adjusted hazard ratios from the NIH-AARP study, we estimated that the PAF, or proportion attributable to overweight and obesity among US adults aged 50 years or older, increased from 11.4% (95% CI = 3.9% to 18.9%) to 16.2% (95% CI = 6.2% to 26.0%) during 1995–2015 (Figure 2). PAFs for the largest PTCs (>4.0 cm) increased from 51.4% (95% CI = 24.2% to 82.0%) to 63.2% (95% CI = 38.2% to 89.7%) during 1985–2005.
Figure 1.
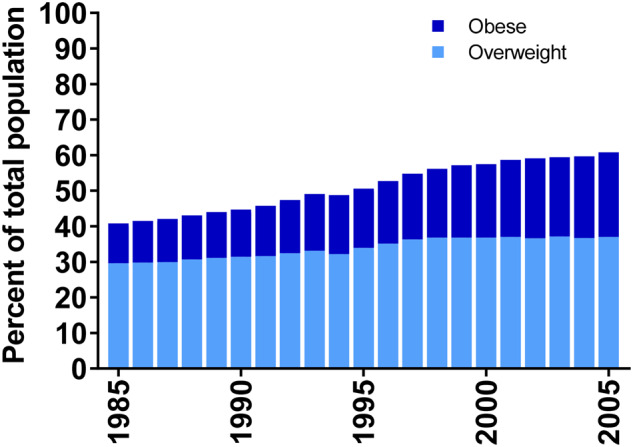
Prevalence of overweight and obesity among those 50 years and older in the United States from National Health Interview Survey, 1985–2005. Obesity and overweight prevalence estimates for 1986, 1989, and 1996 were estimated by averaging the surrounding prevalence estimates.
Figure 2.
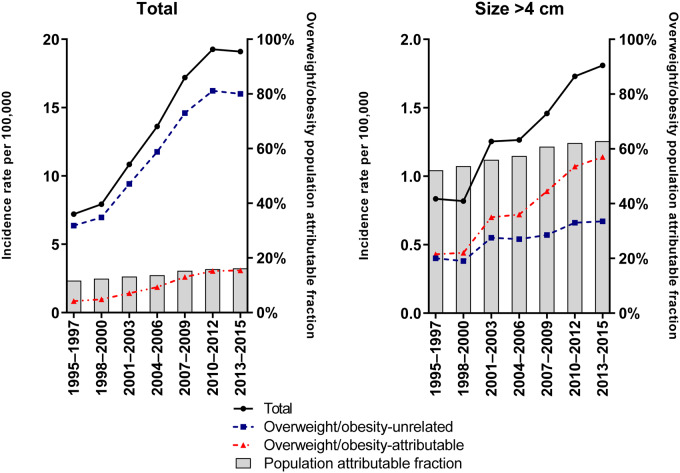
Age-standardized incidence rates of papillary thyroid cancer among those 60 years and older in Surveillance, Epidemiology, and End Results-13, 1995–2015. Rates presented overall and stratified by overweight- and obesity-attributable and overweight- and obesity-unrelated cases. Annual population attributable fractions are also presented. Please note the 10-fold difference in scale of the y-axis.
Age-standardized incidence rates of PTCs among US adults aged 60 years or older more than doubled from 1995–1997 to 2013–2015 (from 7.2 to 19.1 per 100 000 per year), with an average annual increase of 5.9% per year (95% CI = 5.0% to 6.7%) (Table 3; Figure 2). The average annual increase in incidence was greater for overweight- and obesity-attributable PTCs (AAPC = 7.1%, 95% CI = 5.7% to 8.4%) than for overweight- and obesity-unrelated PTCs (AAPC = 5.1%, 95% CI = 3.9% to 6.3%) during the study period. Similar patterns were observed for large (>4 cm) PTCs.
Table 3.
Age-standardized incidence rates and APCs of PTC among those 60 years or older in SEER-13, 1995–2015, overall and stratified by overweight- and obesity-attributable and -unrelated cases*
| Characteristic of cases | Age-standardized rates |
Trend, 1995–2015 | Segment 1 |
Segment 2 |
Segment 3 |
||||
|---|---|---|---|---|---|---|---|---|---|
| 1995–1997 | 2013–2015 | AAPC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | |
| Papillary | |||||||||
| Overall (SEER-13) | 7.20 | 19.1 | 5.9 (5.0 to 6.7) | 1995–2009 | 8.2 (7.3 to 9.2) | 2009–2015 | 0.6 (–1.5 to 2.7) | ||
| Overweight- and obesity-attributable | 0.83 | 3.06 | 7.1 (5.7 to 8.4) | 1995–1998 | 3.8 (–3.5 to 11.6) | 1998–2009 | 11.2 (10.0 to 12.4) | 2009–2015 | 1.4 (–0.9 to 3.8) |
| Overweight- and obesity-unrelated | 6.37 | 16.0 | 5.1 (3.9 to 6.3) | 1995–2011 | 7.1 (6.4 to 7.8) | 2011–2015 | –2.4 (–7.6 to 3.2) | ||
| Papillary, >4 cm | |||||||||
| Overall (SEER-13) | 0.84 | 1.81 | 4.5 (3.4 to 5.6) | 1995–2015 | 4.5 (3.4 to 5.6) | ||||
| Overweight- and obesity-attributable | 0.43 | 1.14 | 4.5 (–1.1 to 10.3) | 1995–1998 | –13.8 (–30.8 to 7.5) | 1998–2002 | 20.9 (–5.1 to 54.1) | 2002–2015 | 4.4 (2.6 to 6.3) |
| Overweight- and obesity-unrelated | 0.40 | 0.67 | 1.9 (–2.6 to 6.5) | 1995–1998 | –15.1 (–28.4 to 0.7) | 1998–2002 | 16.6 (–4.8 to 42.9) | 2002–2015 | 1.9 (0.00 to 3.8) |
AAPC = average annual percent change; APC = annual percent change; CI = confidence interval; PTC = papillary thyroid cancer; SEER = Surveillance, Epidemiology, and End Results.
The rising prevalence of overweight and obesity in the United States accounted for 13.6% and 57.8% of the increase in the total (5.9% per year) and large (>4 cm, 4.5% per year) PTC incidence rates during 1995–2015, respectively. In the absence of overweight and obesity, we would have observed an estimated 0.77, 1.9, and 3.1 fewer PTCs per 100 000 individuals 60 years or older in 1995, 2005, and 2015, respectively.
Combining our PAF estimates for overweight and obesity in 2015 with projected estimates of thyroid cancer diagnoses in 2019 by the American Cancer Society (27), we estimate that approximately 7500 of the 47 000 expected PTC diagnoses in the United States in 2019 will be attributable to overweight and obesity [and 2350 of the 3700 large [>4 cm] PTCs, assuming 8% of all diagnosed PTCs are >4 cm (28)].
Discussion
Excess adiposity is an important risk factor for PTC (29), and both the prevalence of overweight and obesity and the incidence of PTC have increased substantially over the past four decades in the United States (1,19). Our study used an innovative approach that combined relative risk estimates with annual data from national health surveys and cancer registries to estimate the impact of overweight and obesity on US trends in PTC incidence rates, overall and stratified by stage and tumor size. Assuming a causal relationship, we estimated that one in six PTCs and two in three large PTCs diagnosed among US adults aged 60 years or older in 2015 may have been attributable to overweight and obesity. Furthermore, we estimated that in the absence of overweight and obesity, the increase in PTC incidence rates [particularly for the larger tumors, which require more aggressive clinical management (30)] would have been substantially lower.
Some have argued that the link between obesity and thyroid cancer may be at least partly explained by greater diagnostic scrutiny among patients with an underlying thyroid disorder (eg, hypothyroidism) or other obesity-related chronic conditions (31,32). For this to be the sole explanation for our findings, however, we would have expected overweight and obesity to be associated with an increased risk of small PTCs, which are more likely to be incidentally detected and indolent compared with larger PTCs. Instead, we found that the positive association between BMI and PTC risk was restricted to large (>4 cm) tumors. The lack of an association for small PTCs could reflect the greater challenges in detecting nodules of this size in obese patients through palpation or imaging (33,34). Greater delays in diagnosis among the obese participants (thus allowing for greater tumor growth) and/or more incidental detection of small PTCs among the normal-weight participants could also explain the patterns observed. On the other hand, a recent study found no difference in the method of initial detection of differentiated thyroid cancer (palpation, imaging, incidental) by obesity status (35). Without individual-level information on the pathways through which PTCs were detected and ultimately diagnosed in the NIH-AARP study, however, we could not fully evaluate or control for the effects of healthcare access and utilization on our results.
Our findings, particularly the strong associations between BMI and risks of larger-sized PTCs and anaplastic thyroid cancer, may also reflect a direct influence of excess adiposity on differentiated thyroid cancer growth and progression. Larger tumor size is associated with disease-specific survival and recurrence (36,37), and anaplastic thyroid cancers are highly lethal undifferentiated tumors that appear to evolve as part of the natural course of untreated differentiated thyroid cancer (38). Although the biological mechanisms underlying the relationship between adiposity and thyroid cancer are not fully understood, there have been tremendous advancements in knowledge regarding the complex mechanisms by which excess adiposity influences the induction and progression of cancer more generally (39–41). Many mechanisms, including hyperinsulinemia, chronic inflammation, and alterations in circulating adipokines (including leptin and adiponectin), appear to be shared across multiple cancer types, whereas others are site specific (39–41). Prolonged hyperinsulinemia promotes a favorable cellular environment for tumor progression, in part, through reduced production of insulin-like growth factor (IGF) binding protein-1 and -2 and elevated levels of free IGF-I. Insulin also directly influences tumor development through promotional and antiapoptotic effects. Among men and postmenopausal women, adipose tissue is the major source of estrogen exposure, and both obesity and circulating estrogen levels have been clearly linked to risks of postmenopausal breast and endometrial cancers (41). With regard to differentiated thyroid cancer specifically, recent epidemiologic and molecular studies support a possible mediating role of insulin and IGF-I, inflammatory, and leptin signaling pathways (17,42–48). Additional research is needed to better elucidate these and other plausible biological mechanisms underlying the obesity–thyroid cancer association, including estrogen- and thyroid hormone-mediated pathways.
A major limitation of our study was the reliance on self-reported height and weight data in both the NIH-AARP study and NHIS (49). Random and systematic errors in the reporting of weight and/or height may have contributed to the stronger hazard ratio estimates for men compared with women based on NIH-AARP study data. Previous studies suggested that women tend to underreport (and men overreport) their weight, and women and, to a greater extent, men tend to overreport height (50). Although random errors would have attenuated the hazard ratios, systematic errors could lead to either over- or underestimated hazard ratios (with estimates of PAF following in the same direction). NHIS data may have underestimated the prevalence of overweight and obesity prevalence in the United States, thus contributing to an underestimation of the PAFs (50). Unlike other national surveillance data, however, NHIS captured height and weight on an annual basis over the calendar period of interest, which enabled us to calculate annual estimates of PAF. The NIH-AARP study also offered several strengths, including the very large size, long length of follow-up, capture of detailed cancer diagnosis data (including histology, stage, and size), and individual-level information on several potential confounding factors. Nonetheless, if possible, our findings should be replicated in studies using measured height and weight data.
Our hazard ratio estimates directly assessed the relationship between overweight and obesity and PTC risk among middle-to-older-aged adults in the United States. Thus, our results may have limited generalizability to other populations. Similar analyses should be conducted using data from younger populations, because PTC risk may be more strongly associated with excess adiposity in adolescence and young adulthood (9,51), possibly reflecting greater susceptibility to the effects of metabolic and hormonal influences during periods of development and/or greater lifetime exposure to such effects (4).
Because PTCs are often slow-growing and there is a potentially large reservoir of undiagnosed indolent disease in the population, PTC incidence rates are highly sensitive to changes in clinical recommendations affecting detection and diagnosis, including those involving guidelines for the management and diagnostic work-up of thyroid nodules, thyroid cancer screening, medical surveillance and diagnostic imaging, and thyroid cancer coding and terminology (29,31,52–54). In the current study, we did not attempt to quantify the impact on PTC incidence trends of changes in medical practice, which appear to affect mostly small localized PTCs (29,31), or other environmental and lifestyle factors for PTC (4). Several clinical recommendations recently have been introduced or changed in response to concerns about thyroid cancer overdiagnosis and overtreatment (30,52), which may have contributed to the plateauing of PTC incidence rates since 2009 (1). In the future, PTC incidence rates may continue to remain stable or even decline because of these developments. If the prevalence of overweight and obesity continues to climb at a steady pace (19), the fraction of PTCs attributable to excess body weight also will increase, particularly if the impact of overdiagnosis on rising incidence rates subsides.
In summary, our results suggested that overweight and obesity may have contributed importantly to the rapid rise in PTC incidence during 1995–2015. By 2015, we estimated that one of every six PTCs diagnosed, including nearly two-thirds of large PTCs, among adults aged 60 years and older were attributable to overweight and obesity. Thus, a substantial number of PTCs, especially larger PTCs, could potentially be avoided by implementing public health interventions targeting overweight and obesity in the population.
Funding
This work was supported by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health.
Notes
Affiliations of authors: Radiation Epidemiology Branch, Division of Cancer Epidemiology and Genetics (CMK) and Biostatistics Branch, Division of Cancer Epidemiology and Genetics (RMP), National Cancer Institute, Rockville, MD; Department of Surgery, University of California San Francisco, San Francisco, CA (JAS); Infections and Immunoepidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (MSS).
The funder had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures
JAS is a member of the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry supported by GlaxoSmithKline, Novo Nordisk, Astra Zeneca, and Eli Lilly. CMK, RMP, and MSS have no conflicts of interest to disclose.
Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia. Cancer incidence data from California were collected by the California Cancer Registry, California Department of Public Health’s Cancer Surveillance and Research Branch, Sacramento, California. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, Lansing, Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (Miami, Florida) under contract with the Florida Department of Health, Tallahassee, Florida. The views expressed herein are solely those of the authors and do not necessarily reflect those of the Florida Cancer Data System and Florida Department of Health. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Health Sciences Center School of Public Health, New Orleans, Louisiana. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, the Rutgers Cancer Institute of New Jersey, New Brunswick, New Jersey. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry, Raleigh, North Carolina. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services, Phoenix, Arizona. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, Texas. Cancer incidence data from Nevada were collected by the Nevada Central Cancer Registry, Division of Public and Behavioral Health, State of Nevada Department of Health and Human Services, Carson City, Nevada.
We thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management and Leslie Carroll, Dave Campbell, Jr, Bill Wheeler, and Michael Spriggs at Information Management Services for data support and analysis.
Supplementary Material
References
- 1. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM.. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317(13):1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. James BC, Mitchell JM, Jeon HD, Vasilottos N, Grogan RH, Aschebrook-Kilfoy B.. An update in international trends in incidence rates of thyroid cancer, 1973-2007. Cancer Causes Control. 2018;29(4–5):465–473. [DOI] [PubMed] [Google Scholar]
- 3. Davies L, Welch HG.. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164–2167. [DOI] [PubMed] [Google Scholar]
- 4. Kitahara CM, Schneider A, Brenner AV.. Thyroid cancer In: Thun MJ, Linet MS, Cerhan JR, Haiman CA, Schottefeld D, eds. Cancer Epidemiology and Prevention. 4th ed New York, NY: Oxford University Press; 2018:839–860. [Google Scholar]
- 5. Dal Maso L, Bosetti C, La Vecchia C, Franceschi S.. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20(1):75–86. [DOI] [PubMed] [Google Scholar]
- 6. Dal Maso L, La Vecchia C, Franceschi S, et al. A pooled analysis of thyroid cancer studies. V. Anthropometric factors. Cancer Causes Control. 2000;11(2):137–144. [DOI] [PubMed] [Google Scholar]
- 7. Iribarren C, Haselkorn T, Tekawa IS, Friedman GD.. Cohort study of thyroid cancer in a San Francisco Bay area population. Int J Cancer. 2001;93(5):745–750. [DOI] [PubMed] [Google Scholar]
- 8. Engeland A, Tretli S, Akslen LA, Bjorge T.. Body size and thyroid cancer in two million Norwegian men and women. Br J Cancer. 2006;95(3):366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kitahara CM, McCullough M, Franceschi S, et al. Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies. Thyroid. 2016;26(2):306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rinaldi S, Lise M, Clavel-Chapelon F, et al. Body size and risk of differentiated thyroid carcinomas: findings from the EPIC study. Int J Cancer. 2012;131(6):E1004–E1014. [DOI] [PubMed] [Google Scholar]
- 11. Son H, Lee H, Kang K, Lee I.. The risk of thyroid cancer and obesity: a nationwide population-based study using the Korea National Health Insurance Corporation cohort database. Surg Oncol. 2018;27(2):166–171. [DOI] [PubMed] [Google Scholar]
- 12. Schmid D, Ricci C, Behrens G, Leitzmann MF.. Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev. 2015;16(12):1042–1054. [DOI] [PubMed] [Google Scholar]
- 13. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K; International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer--viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim HJ, Kim NK, Choi JH, et al. Associations between body mass index and clinico-pathological characteristics of papillary thyroid cancer. Clin Endocrinol. 2013;78(1):134–140. [DOI] [PubMed] [Google Scholar]
- 15. Tresallet C, Seman M, Tissier F, et al. The incidence of papillary thyroid carcinoma and outcomes in operative patients according to their body mass indices. Surgery. 2014;156(5):1145–1152. [DOI] [PubMed] [Google Scholar]
- 16. Choi JS, Kim EK, Moon HJ, Kwak JY.. Higher body mass index may be a predictor of extrathyroidal extension in patients with papillary thyroid microcarcinoma. Endocrine. 2015;48(1):264–271. [DOI] [PubMed] [Google Scholar]
- 17. Kim WG, Cheng SY.. Mechanisms linking obesity and thyroid cancer development and progression in mouse models. Horm Canc. 2018;9(2):108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ogden CL, Yanovski SZ, Carroll MD, Flegal KM.. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–2102. [DOI] [PubMed] [Google Scholar]
- 19. Hales CM, Carroll MD, Fryar CD, Ogden CL.. Prevalence of obesity among adults and youth: United States. NCHS Data Brief. 2015-2016;2017(288):1–8. [PubMed] [Google Scholar]
- 20. Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. [DOI] [PubMed] [Google Scholar]
- 21. Tuttle RM, Haugen B, Perrier ND.. Updated American Joint Committee on Cancer/Tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): what changed and why? Thyroid. 2017;27(6):751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics. National Health Interview Survey. Atlanta, GA. https://www.cdc.gov/nchs/nhis/index.htm. Accessed January 17, 2019.
- 23.Surveillance, Epidemiology, and End Results Program (SEER). Registry Groupings in SEER Data and Statistics. Bethesda, MD. https://seer.cancer.gov/registries/terms.html. Accessed January 17, 2019.
- 24. Graubard BI, Flegal KM, Williamson DF, Gail MH.. Estimation of attributable number of deaths and standard errors from simple and complex sampled cohorts. Statist Med. 2007;26(13):2639–2649. [DOI] [PubMed] [Google Scholar]
- 25. Rust KF, Rao J.. Variance estimation for complex surveys using replication techniques. Stat Methods Med Res. 1996;5(3):283–310. [DOI] [PubMed] [Google Scholar]
- 26. Renehan AG, Soerjomataram I, Tyson M, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer. 2010;126(3):692–702. [DOI] [PubMed] [Google Scholar]
- 27.American Cancer Society. Cancer Statistics Center. Atlanta, GA. https://cancerstatisticscenter.cancer.org. Accessed January 17, 2019.
- 28. Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev. 2017;26(4):632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitahara CM, Sosa JA.. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davies L, Morris LG, Haymart M, et al. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: the increasing incidence of thyroid cancer. Endocr Pract. 2015;21(6):686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L.. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614–617. [DOI] [PubMed] [Google Scholar]
- 33. Uppot RN, Sahani DV, Hahn PF, Gervais D, Mueller PR.. Impact of obesity on medical imaging and image-guided intervention. Am J Roentgenol. 2007;188(2):433–440. [DOI] [PubMed] [Google Scholar]
- 34. Cham S, Zanocco K, Sturgeon C, Yeh MW, Harari A.. Risk-based ultrasound screening for thyroid cancer in obese patients is cost-effective. Thyroid. 2014;24(6):975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zagzag J, Malone MK, Lopresti MA, Ogilvie JB, Patel KN, Heller KS.. Method of detection of well-differentiated thyroid cancers in obese and non-obese patients. PLoS One. 2016;11(4):e0152768.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tam S, Amit M, Boonsripitayanon M, et al. Effect of tumor size and minimal extrathyroidal extension in patients with differentiated thyroid cancer. Thyroid. 2018;28(8):982–990. [DOI] [PubMed] [Google Scholar]
- 37. Machens A, Holzhausen HJ, Dralle H.. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005;103(11):2269–2273. [DOI] [PubMed] [Google Scholar]
- 38.Wiseman SM, Loree TR, Hicks WL, et al. Anaplastic thyroid cancer evolved from papillary carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129(1):96–100. [DOI] [PubMed] [Google Scholar]
- 39. Colditz GA, Peterson LL.. Obesity and cancer: evidence, impact, and future directions. Clin Chem. 2018;64(1):154–162. [DOI] [PubMed] [Google Scholar]
- 40. van Kruijsdijk RCM, van der Wall E, Visseren F.. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2569–2578. [DOI] [PubMed] [Google Scholar]
- 41. Roberts DL, Dive C, Renehan AG.. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61(1):301–316. [DOI] [PubMed] [Google Scholar]
- 42. Kim WG, Park JW, Willingham MC, Cheng SY.. Diet-induced obesity increases tumor growth and promotes anaplastic change in thyroid cancer in a mouse model. Endocrinology. 2013;154(8):2936–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dossus L, Franceschi S, Biessy C, et al. Adipokines and inflammation markers and risk of differentiated thyroid carcinoma: the EPIC study. Int J Cancer. 2018;142(7):1332–1342. [DOI] [PubMed] [Google Scholar]
- 44. Schmidt JA, Allen NE, Almquist M, et al. Insulin-like growth factor-i and risk of differentiated thyroid carcinoma in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2014;23(6):976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Celano M, Maggisano V, Lepore SM, et al. Expression of leptin receptor and effects of leptin on papillary thyroid carcinoma cells. Int J Endocrinol. 2019;2019:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uddin S, Bavi P, Siraj AK, et al. Leptin-R and its association with PI3K/AKT signaling pathway in papillary thyroid carcinoma. Endocr Relat Cancer. 2010;17(1):191–202. [DOI] [PubMed] [Google Scholar]
- 47. Park S, Willingham MC, Qi J, Cheng SY.. Metformin and JQ1 synergistically inhibit obesity-activated thyroid cancer. Endocr Relat Cancer. 2018;25(10):865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Park JW, Han CR, Zhao L, Willingham MC, Cheng SY.. Inhibition of STAT3 activity delays obesity-induced thyroid carcinogenesis in a mouse model. Endocr Relat Cancer. 2016;23(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Preston SH, Fishman E, Stokes A.. Effects of categorization and self-report bias on estimates of the association between obesity and mortality. Ann Epidemiol. 2015;25(12):907–911; e901–e902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stommel M, Schoenborn CA.. Accuracy and usefulness of BMI measures based on self-reported weight and height: findings from the NHANES & NHIS 2001-2006. BMC Public Health. 2009;9:421.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kitahara CM, Gamborg M, Berrington de González A, Sørensen TI, Baker JL.. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res. 2014;74(1):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haugen BR, Sawka AM, Alexander EK, et al. American Thyroid Association guidelines on the management of thyroid nodules and Differentiated Thyroid Cancer Task Force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2017;27(4):481–483. [DOI] [PubMed] [Google Scholar]
- 53. Cooper DS, Doherty GM, Haugen BR, et al. ; American Thyroid Association Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. [DOI] [PubMed] [Google Scholar]
- 54.US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for thyroid cancer: US Preventive Services Task Force recommendation statement. JAMA. 2017;317(18):1882–1887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.