Abstract
Background
Antineoplastic agents approved in recent decades are a marked advancement in cancer treatment, but they come at considerable cost. These drugs may widen survival disparities between patients who receive these agents and those who do not. We examine factors associated with the use of high-cost antineoplastic agents for the treatment of metastatic non–small cell lung cancer.
Methods
We conducted a retrospective observational study using 2007–2015 Surveillance, Epidemiology, and End-Results–Medicare data supplemented with the Area Health Resource File. Patients were aged 66 years and older, were enrolled in fee-for-service Medicare Part D, were diagnosed with a first primary diagnosis of metastatic non–small cell lung cancer, and had received an antineoplastic agent. “High-cost agents” were defined as agents costing $5000 or more per month. Independent variables include race/ethnicity, urban or rural residency, census tract poverty, and treatment facility type (eg, National Cancer Institute designation).
Results
Patients who lived in areas of high poverty were 4 percentage points less likely to receive high-cost agents (two-sided P < .001). Patients who were not treated at a National Cancer Institute–designated center were 10 percentage points less likely to receive these agents (two-sided P < .001). A 27 percentage-point increase in the likelihood of receiving a high-cost agent was observed in 2015, as compared to 2007, highlighting the rapid change in practice patterns (two-sided P < .001).
Conclusion
Potential policy and care delivery solutions involve outreach and support to community physicians who treat patients in remote areas. We estimate that widespread use of these agents conservatively cost approximately $3 billion per year for the treatment of metastatic non–small cell lung cancer alone.
New immunotherapies and other targeted agents are transforming cancer treatment. These agents—many of which are taken orally and through outpatient infusion—are considered guideline concordant for many cancers (1), but as with other cancer treatments such as surgery and radiation, utilization of these agents is unequal (2,3) and may vary systematically by patient groups. For example, rural residence and minority race/ethnicity are negatively associated with receipt of epidermal growth factor receptor tests—an important biomarker indicating whether targeted therapy is appropriate in metastatic non–small cell lung cancer patients (4). Receipt of these tests may increase the probability that targeted agents will be prescribed. Further complicating matters is the high cost of newly approved antineoplastic agents (averaging $13 000 per month in 2017) (5) that may be an additional hindrance to adoption for some physicians and their patients, particularly those with low income or with responsibility for a large portion of their medical care costs.
Disparities in cancer mortality across geographic areas in US counties is well documented (6,7). Rural residence in the United States has emerged as a potential detriment to cancer outcomes, with the age-adjusted mortality rate higher in rural areas compared to urban areas (8–10). Approximately one-fifth of the US population lives in rural areas. Racial/ethnic minority groups account for 80% of rural population growth, and, strikingly, more than 80% of rural counties where the majority of residents are African American or Hispanic are designated as Health Professional Shortage Areas, compared to 65% of rural counties overall (11). Thus, the confluence of poor access, higher risk factors, and unfavorable socioeconomic conditions exacerbate cancer disparities in rural areas. Moreover, only 3% of medical oncologists and 16% of radiation oncologists practice in rural areas, forcing residents to travel approximately 1 hour to get care, and longer for academic-based cancer care (12).
This study evaluates the relationship between use of high-cost antineoplastic treatments and factors such as residency in rural areas, living in a high-poverty area, and access to specialized care. We hypothesize that rural residency and high-poverty census tracts will be negatively associated with use of high-cost agents and access to specialized care will be positively associated with using these agents. We compare use of high-cost agents for metastatic non–small cell lung cancer in the Medicare fee-for-service population using 2007–2015 data from the Surveillance, Epidemiology, and End-Results (SEER) database. We study metastatic non–small cell lung cancer because of its prevalence and poor prognosis, and because new agents such as pemetrexed, erlotinib, and crizotinib were approved during our study period, allowing us to study the adoption of these agents shortly after their approval.
Methods
Data
We used the 2007 through 2015 SEER registry linked to Medicare claims data (13). The SEER registries are population based, ascertaining all incident cancers occurring in defined geographic areas that include 27.8% of the US population (14). For each patient, the SEER record contains demographic data, month and year of diagnosis, cancer site, and stage at diagnosis. The Medicare data include date of death (if applicable) and claims for beneficiaries with fee-for-service coverage. All claims include dates of service and codes for diagnoses, procedures, and medications using the International Classification of Diseases, Ninth Revision (ICD-9), Clinical Modification codes, Healthcare Common Procedure Coding System (HCPCS), or National Drug Code (NDC) number.
We linked these data from the Area Health Resource File on the presence of a hospital with oncology services, hospital with a medical school affiliation, and hospital with American Cancer Society cancer program within the county of residence (15). These variables are an indication of the availability of local specialty services. Only medical school–affiliated hospital was a statistically significant predictor of whether a patient received a high-cost agent. Therefore, it was retained in the model. Medical school–affiliated hospitals are more likely to conduct more diagnostic tests and use higher-cost treatments relative to other hospitals (16).
SEER-Medicare data contain a variable that indicates whether a patient received care at an NCI-designated cancer center. To achieve NCI designation, centers provide a substantial level of research, patient care, and access to clinical trials (17). Patients treated at these centers may be more likely to receive state-of-the-art care, regardless of their residence.
Sample
We selected beneficiaries aged 66 and older, diagnosed with a metastatic first and primary non–small cell lung cancer from 2007 through 2015, and treated with an antineoplastic agent, excluding those diagnosed with squamous cell non–small cell lung cancer. These patients have an unfavorable prognosis and fewer treatment options compared with adenocarcinoma non–small cell lung cancer patients. By limiting the sample to patients who are treated with an antineoplastic agent, we study a more homogeneous group and avoid potential confounding with factors that explain the decision to treat, regardless of agent prescribed.
Patients were excluded if they had more than one cancer, the month of diagnosis was unknown, or if cancer was identified through death certificate or autopsy. Patients were continuously enrolled in Medicare Parts A and B fee-for-service coverage. Because we study infusion and oral agents, patients had to be enrolled in the Medicare Part D prescription drug coverage program. Supplementary Figure 1 (available online) reports how the sample was derived.
Antineoplastic Agents
To identify high-cost agents, we designated a cutoff of $5000 per month, which corresponds to approximately the top-25th percentile of the monthly costs for the most common oral and infusion agents used to treat metastatic non–small cell lung cancer (Table 1). The average cost per month for drugs during our study period was $13 029 (SD = $10 201) (5). In sensitivity analyses, we used lower monthly cost cutoffs of $3000 and $4000. Findings were not sensitive to the choice of cutoff cost (available on request). Our follow-up period was 12 months after the month of diagnosis, which is the observation window for which we had complete data on all patients. The median survival of patients in the sample was 12 months; approximately 60% died during the observation period. The NDC and HCPCS codes used to define agents were obtained from the Cancer Medications Enquiry Database available in the SEER Observational Research in Oncology Toolbox (18).
Table 1.
Common oral and infusion agents used to treat metastatic non–small cell lung cancer (NSCLC), SEER-Medicare, 2007–2015
| Agent | Year approved | Monthly cost** | Frequency†† |
|---|---|---|---|
| Carboplatin | 1991 | $1495 | 7016 |
| Pemetrexed disodium | 2004 (second line)* | $6374 | 4467 |
| 2008 (first line)† | |||
| Paclitaxel | 1994 | $4176 | 3139 |
| Erlotinib | 2004 (second line)‡ | $5231 | 2195 |
| 2013 (first line for EGFR# positive)§ | |||
| Bevacizumab | 2006 (for NSCLC)‖ | $5551 | 2172 |
| Zoledronic acid | 2002 | $1159 | 1803 |
| Gemcitabine | 1996 | $3212 | 1341 |
| Docetaxel | 1996 | $3938 | 1163 |
| Cisplatin | 1978 | $454 | 679 |
| Etoposide phosphate | 1996 | $1034 | 594 |
| Vinorelbine | 1994 | $1653 | 421 |
| Nivolumab | 2014¶ | $12 435 | 269 |
Source for pemetrexed second line: http://theoncologist.alphamedpress.org/content/10/6/363.long. EGFR = epithelial growth factor receptor; SEER = Surveillance, Epidemiology, and End-Results.
Source for pemetrexed first line: http://theoncologist.alphamedpress.org/content/14/9/930.long.
Source for erlotinib second line: http://theoncologist.alphamedpress.org/content/10/7/461.full.
Source for erlotinib first line: https://www.asco.org/advocacy-policy/asco-in-action/fda-approves-erlotinib-first-line-treatment-metastatic-nsclc.
Source for bevacizumab: http://theoncologist.alphamedpress.org/content/12/6/713.full.
Source for nivolumab: http://theoncologist.alphamedpress.org/content/21/5/634.long.
Monthly costs are reported in 2014 dollars.
Frequency reports reflect number of patients in the study sample who received the drug.
Variables
We used the SEER-Medicare designation of patients’ residence into “Urban Commuting Area” vs “Not an Urban Commuting Area” based on the 2010 Rural-Urban Commuting Area (RUCA) designations by the US Department of Agriculture (19). RUCA codes classify census tracts using measures of population density, urbanization, and daily commuting flows. RUCA secondary codes 1.0, 1.1, 2.0, 2.1, 3.0, 4.1, 5.1, 7.1, 8.1, and 10.1 are classified as “Urban Commuting Areas,” and all other nonmissing codes are classified as “Not an Urban Commuting Area.” In sensitivity analyses, we used the Census Bureau’s Urban Rural Indicator Codes (URIC). The URIC classification is based on the percentage of a population living in an urban area without accounting for commuting flows. Our results were not sensitive to the choice of urban/rural classifications (Supplementary Table 1 and Supplementary Figure 2, available online).
Census tract poverty is recorded in the SEER-Medicare data and measured as the percentage of the population within a census tract who live in poverty. A cutoff of greater than 20% poverty at the census tract level was used to distinguish low-income areas from those with more resources (20). Race and ethnicity were defined as non-Hispanic white, non-Hispanic African American, Hispanic, and other. We also controlled for patient age (66–75, 76–85, and 86 years and older), sex, and comorbidity burden in the estimations (21,22). We categorized Charlson comorbidities as 0, 1, or 2 or more based on comorbidities 1 year before diagnosis. Therefore, we study patients aged 66 years and older to have sufficient data following Medicare enrollment at age 65 years. We did not control for individual SEER registries because the registry indicators are correlated with rural and urban designations.
We calculated total Medicare spending per year by summing the paid claims across all service areas (inpatient, outpatient, professional, durable medical equipment, hospice, home health, and Part D prescription drugs) for the 12 months following diagnosis.
Dichotomous variables designated whether the county in which the patient resided had a hospital with a medical school affiliation and if the patient was treated at an NCI-designated cancer center. These variables indicate access to specialty services at the area and person level, respectively.
Statistical Analysis
We describe the characteristics of metastatic non–small cell lung cancer patients and use chi-square tests to test for statistical differences between patients residing in rural and urban areas and patients treated with high-cost agents compared with those treated with other therapies. Tests were two-sided, and a P value of less than .05 was considered statistically significant. Because there is considerable overlap between rurality and high poverty, we did not report sample characteristics stratified by poverty status. In addition, we graphed differences in prescribing patterns over time by year of diagnosis and rurality. Logistic regression was used to estimate the relationship between the independent variables and the probability of initiation of antineoplastic agents that cost greater than $5000 per month. Models that include area-level explanatory variables were adjusted for clustering to account for correlations between observations within a county. For ease of interpretation, we report marginal effects along with standard errors and P values. Marginal effects can be interpreted as average differences in the probability of prescribing high-cost antineoplastic between a category (eg, not treated at an NCI-designated center) and its reference (eg, treated at an NCI-designated center) (23). Stata 15 (College Station, TX) was used to analyze the data.
Results
Descriptive Statistics
Table 2 reports descriptive statistics for the sample. Women, white non-Hispanics, those who reside in urban areas or counties with less than 20% of the population living below the poverty level, and patients with fewer comorbid conditions were more likely to receive high-cost treatments. Treatment at an NCI-designated center or residing in a county with a medical school–affiliated hospital was also associated with a higher probability of getting a high-cost antineoplastic agent. Medicare spent more than $35 000 more on patients who received high-cost agents during the 12 months following diagnosis relative to those who did not receive these agents ($58 880 vs $93 953, P < .001). The difference in spending is due to higher payments for medications and outpatient and professional services.
Table 2.
Descriptive characteristics, metastatic non–small cell lung cancer, SEER-Medicare, 2007–2015, N = 10 655
| Variables | Monthly drug cost |
Residency |
||||
|---|---|---|---|---|---|---|
| < $5000 N = 3528 | ≥$5000 N = 7127 | P * | Rural N = 1287 | Urban N = 9354 | P * | |
| Age, y | ||||||
| 66–75 | 2118 (60.0) | 4292 (60.2) | .19 | 876 (68.1) | 5524 (59.1) | < .001 |
| 76–85 | 1254 (35.5) | 2466 (34.6) | 384 (29.8) | 3333 (35.6) | ||
| 86 and older | 156 (4.4) | 369 (5.2) | 27 (2.1) | 497 (5.3) | ||
| Sex | ||||||
| Female | 1735 (49.2) | 3897 (54.7) | < .001 | 621 (48.3) | 5006 (53.5) | < .001 |
| Male | 1793 (50.8) | 3230 (45.3) | 666 (51.7) | 4348 (46.5) | ||
| Race/Ethnicity | ||||||
| White non-Hispanic | 2895 (82.1) | 5620 (78.9) | < .001 | 1184 (92.0) | 7321 (78.3) | < .001 |
| Black non-Hispanic | 291 (8.2) | 474 (6.7) | 47 (3.7) | 717 (7.7) | ||
| Hispanic | 195 (5.5) | 383 (5.4) | 31 (2.4) | 544 (5.8) | ||
| Other/unknown | 147 (4.2) | 650 (9.1) | 25 (1.9) | 772 (8.3) | ||
| Census tract percentage below poverty† | ||||||
| < 20% poverty | 2630 (74.9) | 5662 (79.6) | < .001 | 787 (61.3) | 7498 (80.4) | < .001 |
| .≥20% poverty | 882 (25.1) | 1452 (20.4) | 497 (38.7) | 1831 (19.6) | ||
| Rurality‡ | ||||||
| Urban | 3012 (85.5) | 6342 (89.1) | < .001 | N/A | N/A | |
| Rural | 510 (14.5) | 777 (10.9) | ||||
| Comorbidities | ||||||
| 0 | 1243 (35.2) | 2961 (41.5) | < .001 | 488 (37.9) | 3707 (39.6) | .30 |
| 1 | 1039 (29.5) | 2131 (29.9) | 406 (31.5) | 2762 (29.5) | ||
| 2 or more | 1246 (35.3) | 2035 (28.6) | 393 (30.5) | 2885 (30.8) | ||
| Treated at an NCI-designated center | ||||||
| No/unknown | 3029 (85.9) | 5452 (76.5) | < .001 | 1087 (84.5) | 7381 (78.9) | < .001 |
| Yes | 499 (14.1) | 1675 (23.5) | 200 (15.5) | 1973 (21.1) | ||
| Medical school–affiliated hospital in county | ||||||
| No | 1276 (36.2) | 2061 (28.9) | < .001 | 1064 (82.7) | 2264 (24.2) | < .001 |
| Yes | 2250 (63.8) | 5064 (71.1) | 223 (17.3) | 7086 (75.8) | ||
| High-cost drug | ||||||
| < $5000/month | N/A | N/A | 510 (39.6) | 3012 (32.2) | < .001 | |
| ≥ $5000/month | 777 (60.4) | 6342 (67.8) | ||||
| Year of diagnosis | ||||||
| 2007 | 544 (15.4) | 500 (7.0) | < .001 | 128 (9.9) | 915 (9.8) | .24 |
| 2008 | 473 (13.4) | 574 (8.1) | 140 (10.9) | 901 (9.6) | ||
| 2009 | 407 (11.5) | 701 (9.8) | 125 (9.7) | 980 (10.5) | ||
| 2010 | 354 (10.0) | 677 (9.5) | 129 (10.0) | 901 (9.6) | ||
| 2011 | 362 (10.3) | 734 (10.3) | 128 (9.9) | 967 (10.3) | ||
| 2012 | 366 (10.4) | 885 (12.4) | 176 (13.7) | 1, 075 (11.5) | ||
| 2013 | 361 (10.2) | 1020 (14.3) | 163 (12.7) | 1218 (13.0) | ||
| 2014 | 344 (9.8) | 1033 (14.5) | 149 (11.6) | 1228 (13.1) | ||
| 2015 | 317 (9.0) | 1003 (14.1) | 149 (11.6) | 1169 (12.5) | ||
| Total Medicare spending 12 months following diagnosis§ | 58 880 | 93 953 | < .001 | 67 580 | 84 394 | < .001 |
| Inpatient | 27 946 | 25 400 | < .001 | 19 812 | 27 122 | < .001 |
| Outpatient hospital | 8885 | 22 273 | <.001 | 19 188 | 17 666 | < .001 |
| Professional | 14 877 | 29 701 | < .001 | 18 966 | 25 601 | < .001 |
| Durable medical equipment | 585 | 560 | .59 | 610 | 562 | .26 |
| Prescription medications | 2 264 | 11 980 | < .001 | 5 576 | 9 212 | < .001 |
| Hospice | 2807 | 2125 | < .001 | 2157 | 2380 | .11 |
| Home health | 1516 | 1913 | < .001 | 1271 | 1851 | < .001 |
Two-sided P values were calculated using the chi-square test. Percentage of sample size is shown in parentheses. N/A = not applicable; NCI = National Cancer Institute; SEER = Surveillance, Epidemiology, and End-Results.
Area-level poverty is defined at the census tract when available. For observations missing the census tract, area-level poverty is defined at the zip code. Twenty-eight observations were missing census tract data.
Urban/rural classification is based on the U.S. Department of Agriculture’s Rural Urban Commuting Area Codes.
Medicare spending is for 12 months following the month of diagnosis.
The three right-hand columns of Table 2 report patterns by urban and rural residency. Rural patients are more likely to be younger, male, and non-Hispanic white. A greater proportion live in high-poverty areas, and fewer patients are treated at an NCI-designated center relative to their urban counterparts (15.5% vs 21.1%). Only 17.3% live in a county with a hospital affiliated with a medical school relative to 75.8% of urban patients who live in a county with a hospital that has a medical school affiliation. Sixty percent (60.4%) of rural patients received high-cost agents compared to 67.8% of urban patients. Medicare spending during the 12 months following diagnosis is lower for rural patients by about $17 000 per patient.
Treatment Patterns over Time
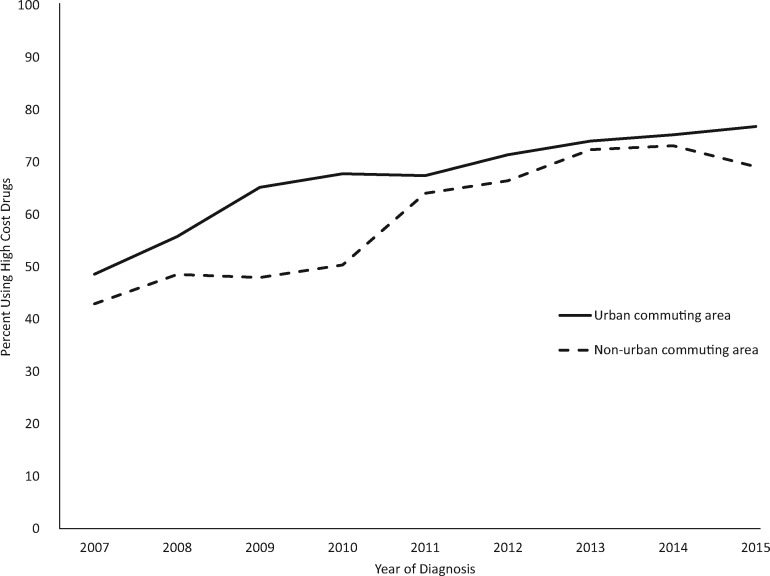
Between 2007 and 2015, high-cost agents were more frequently prescribed each year, with the frequency of use lagging in rural areas (Figure 1). Use increased from 48.6% to 76.8% and 43.0% to 69.1% in urban and rural areas, respectively. The yearly percentage point difference in use of high-cost agents between urban and rural areas ranges from a low of 1.7 percentage points (74.1% and 72.4% in urban and rural areas, respectively) in 2013 and 17.4 percentage points (67.8% and 50.4% in urban and rural areas, respectively), with an average of 7.5 percentage points across the years we study.
Figure 1.
Trends in prescribing agents with a cost of $5000 per month or greater for the treatment of metastatic non–small cell lung cancer, Surveillance, Epidemiology, and End-Results–Medicare, 2007–2015.
Use of High-Cost Agents
Table 3 reports marginal effects of logistic models estimating the likelihood of receiving a high-cost agents. In column 1, we control for year of diagnosis, patient demographic characteristics, comorbid conditions, and rurality. Compared to patients who resided in urban areas, patients in rural areas were approximately 6 percentage points less likely to receive a high-cost agent (P < .001). When we added a variable for whether the patient resided in an area with 20% or greater poverty (column 2), the effect of rural residence remained statistically significant and diminished only slightly. Patients who lived in areas of high poverty were 4 percentage points less likely to receive high-cost agents (P < .001). No statistically significant coefficients were observed in terms of race or ethnicity after controlling for poverty, other than for patients characterized as other or unknown.
Table 3.
Likelihood of receiving a drug at a cost of $5000 per month or greater, marginal effects reported, for the treatment of metastatic non–small cell lung cancer, SEER-Medicare, 2007–2015*
| Independent variables | Base model, poverty and access excluded |
Poverty included |
Access to specialized care |
|||
|---|---|---|---|---|---|---|
| Marginal effects (95% CI) | P † | Marginal effects (95% CI) | P † | Marginal effects (95% CI) | P † | |
| Total, No. | 10 641 | 10 613 | 10 612 | |||
| Age category, y | ||||||
| 66–75 | (Referent) | (Referent) | (Referent) | |||
| 76–85 | −0.010 | .28 | −0.012 | .21 | −0.009 | .37 |
| (−0.029 to 0.008) | (−0.031 to 0.007) | (−0.029 to 0.11) | ||||
| 86 and older | 0.011 | .59 | 0.009 | .65 | 0.013 | .63 |
| (−0.030 to 0.053) | (−0.032 to 0.051) | (−0.039 to 0.064) | ||||
| Sex | ||||||
| Female | (Referent) | (Referent) | (Referent) | |||
| Male | −0.041 | < .001 | −0.040 | < .001 | −0.039 | < .001 |
| (−0.059 to −0.024) | (−0.058 to −0.023) | (−0.059 to −0.019) | ||||
| Race/Ethnicity | ||||||
| White non-Hispanic | (Referent) | (Referent) | (Referent) | |||
| Black non-Hispanic | −0.035 | .05 | −0.016 | .38 | −0.020 | .23 |
| (−0.070 to −0.00049) | (−0.052 to 0.020) | (−0.054 to 0.013) | ||||
| Hispanic | 0.004 | .82 | 0.013 | .51 | 0.008 | .72 |
| (−0.035 to 0.044) | (−0.026 to 0.052) | (−0.034 to 0.049) | ||||
| Other | 0.151 | < .001 | 0.154 | < .001 | 0.139 | < .001 |
| (0.122 to 0.180) | (0.125 to 0.183) | (0.102 to 0.177) | ||||
| Charlson comorbidities | ||||||
| 0 | (Referent) | (Referent) | (Referent) | |||
| 1 | −0.028 | .008 | −0.027 | .01 | −0.024 | .03 |
| (−0.049 to −0.007) | (−0.048 to −0.006) | (−0.045 to −0.003) | ||||
| 2 or more | −0.077 | < .001 | −0.075 | < .001 | −0.069 | < .001 |
| (−0.098 to −0.056) | (−0.096 to −0.054) | (−0.091 to −0.048) | ||||
| Census tract percentage below poverty‡ | ||||||
| <20% | N/A | (Referent) | (Referent) | |||
| ≥20% | N/A | −0.040 | < .001 | −0.029 | .004 | |
| (−0.063 to −0.018) | (−0.049 to −0.009) | |||||
| Rurality | ||||||
| Urban | (Referent) | (Referent) | (Referent) | |||
| Rural | −0.059 | < .001 | −0.051 | < .001 | −0.024 | .14 |
| (−0.087 to −0.032) | (−0.079 to −0.023) | (−0.056 to 0.008) | ||||
| Treated at an NCI-designated center | ||||||
| Yes | N/A | N/A | (Referent) | |||
| No | N/A | N/A | −0.095 | < .001 | ||
| (−0.118 to −0.073) | ||||||
| Medical school–affiliated hospital in county | ||||||
| Yes | N/A | N/A | (Referent) | |||
| No | N/A | N/A | −0.042 | .002 | ||
| (−0.068 to −0.016) | ||||||
| Year of diagnosis | ||||||
| 2007 | (Referent) | (Referent) | (Referent) | |||
| 2008 | 0.070 | .001 | 0.072 | < .001 | 0.074 | < .001 |
| (0.028 to 0.112) | (0.029 to 0.114) | (0.035 to 0.112) | ||||
| 2009 | 0.148 | < .001 | 0.149 | < .001 | 0.147 | < .001 |
| (0.107 to 0.189) | (0.108 to 0.190) | (0.108 to 0.187) | ||||
| 2010 | 0.176 | < .001 | 0.180 | < .001 | 0.176 | < .001 |
| (0.135 to 0.218) | (0.138 to 0.221) | (0.137 to 0.214) | ||||
| 2011 | 0.191 | < .001 | 0.191 | < .001 | 0.186 | < .001 |
| (0.150 to 0.231) | (0.150 to 0.232) | (0.144 to 0.228) | ||||
| 2012 | 0.227 | < .001 | 0.227 | < .001 | 0.223 | < .001 |
| (0.188 to 0.266) | (0.188 to 0.266) | (0.183 to 0.262) | ||||
| 2013 | 0.259 | < .001 | 0.258 | < .001 | 0.251 | < .001 |
| (0.221 to 0.297) | (0.220 to 0.296) | (0.212 to 0.289) | ||||
| 2014 | 0.270 | < .001 | 0.268 | < .001 | 0.261 | < .001 |
| (0.233 to 0.308) | (0.231 to 0.306) | (0.223 to 0.299) | ||||
| 2015 | 0.276 | < .001 | 0.275 | < .001 | 0.266 | < .001 |
| (0.238 to 0.314) | (0.237 to 0.312) | (0.227 to 0.305) | ||||
Two-sided P values were calculated for logistic regression marginal effects. Confidence intervals are reported in parentheses. N/A = not applicable; NCI = National Cancer Institute; SEER = Surveillance, Epidemiology, and End-Results.
Because of missing data, sample size was reduced by 11 observations when poverty was included in estimation.
In column 1, we control for year of diagnosis, patient demographic characteristics, comorbid conditions, and rurality. In column 2, we add variables for poverty, and in column 3, variables for access to specialized care such as treatment at an NCI–designated center and residing in a county with a medical school–affiliated hospital.
In column 3, we report coefficients after adding variables for whether the patient was treated in an NCI-designated center or lived in a county with a medical school–affiliated hospital. Patients who were not treated at an NCI-designated center were 9.5 percentage points less likely to receive high-cost agents (P < .001). Likewise, patients who resided in a county without a medical school–affiliated hospital were 4.2 percentage points less likely to receive high-cost agents (P = .002). The coefficient for poverty diminishes but remains statistically significant (2.9 lower percentage points, P = .004). The coefficient for rural residency is no longer statistically significant. We observe a 27 percentage point increase in the likelihood of receiving a high-cost agent in 2015 compared to 2007, highlighting the rapid change in practice patterns (P < .001).
We also tested interaction terms and found no statistically significant interactions between rurality and poverty (results not shown) but found a statistically significant interaction between rurality and receiving care at an NCI-designated facility. Urban patients were 10 percentage points more likely receive a high-cost agent if the patient visited an NCI-designated facility, whereas for rural patients, visiting an NCI-designated facility increased the likelihood of receiving a high-cost agent by only a 2 percentage-point difference, suggesting that barriers remain for rural patients treated at an NCI-designated facility.
Discussion
Unequal prescribing of new and often high-cost antineoplastic agents could widen survival disparities between groups of patients (6). We explored whether groups of patients, rural residents and low-income patients in particular, experience widening disparities in receipt of high-cost treatments for metastatic non–small cell lung cancer. The evidence suggested a modest disparity attributable to rurality and poverty, whereas treatment at an NCI-designated center was a strong predictor of receiving a high-cost agent, as was living in a county with a medical school–affiliated hospital.
Other published evidence confirms that teaching hospitals are early adopters of new technology and approaches for cancer treatment (24). Patients treated at teaching hospitals are also more likely to get genetic testing to guide treatment selection (25). Ample evidence supports that NCI-designated centers provide equitable care (26–29), but some patients (eg, Hispanic, rural) travel farther than others to receive this care (30,31). Although we do not directly measure distance, RUCA codes take into account daily commuting patterns when designating rural areas.
Investments that support outreach from teaching and research and NCI-designated centers to patients in remote and low-income areas may mediate treatment disparities. Outreach may include telehealth, education for community physicians, closer collaborations with community physicians, patient travel support such as transportation and lodging to NCI–designated centers, and patient navigators for those who live in rural or low-income areas. Access and referrals, rather than distance and poverty, may be the main contributors to disparate treatment for Medicare-insured patients diagnosed with metastatic non–small cell lung cancer.
We cannot ignore the costs associated with widespread use of these agents. Nationally, there are approximately 228 000 new cases of non–small cell lung cancer annually (32). Of these, approximately 53%, or 120 840 new cases, will be metastatic (33). If we exclude squamous cell patients (about 40%), who are usually not prescribed high-cost agents, although some might, we conservatively estimate that there are 72 504 metastatic non–small cell lung cancer patients per year who are potential candidates for an antineoplastic agent. Approximately 61% will receive conventional chemotherapy and/or one or more newly approved high-cost agents. Assuming that use of high-cost agents increases at the same average rate as in the years 2013 through 2015 (approximately 1% per year), a conservative use rate of high-costs agents for 2020 is approximately 80%, leading to an annual Medicare cost for providing care to these patients alone exceeding $3 billion.
The study has limitations. Although we use the most recently available SEER-Medicare data, the last year for diagnosis was 2015, and the last year for vital status was 2017, which does not reflect agents approved in recent years. We do not have information on patients’ preferences, functional status, or quality of life, or toxicities and morbidity associated with treatment. We measure use, which reflects submission of a claim for reimbursement and does not reflect access or whether agents were prescribed but not taken. We select the sample based on the receipt of any antineoplastic agents and thereby eliminate patients who do not receive these agents. As a result, patients who live in rural or low-poverty areas were disproportionately excluded. For example, this exclusion applied to 42% of rural patients compared to 38% of urban patients. Furthermore, we do not present estimates beyond 12 months. When we extended the study period to 18 months, the results were unchanged. In addition, we do not know if the treatments prescribed were appropriate as indicated through a biomarker test. Finally, information about oral agents is available only for patients enrolled in Medicare Part D. Therefore, the generalizability of the estimates to patients who pay for prescription drugs through other means is unknown. Likewise, enrollment in Medicare Advantage may also result in prescribing patterns systematically different from those observed for the fee-for-service sample.
Our study provides a population-based assessment of the receipt of high-cost treatments in metastatic non–small cell lung cancer and indicates disparities in use patterns. The disparities are attributable to treatment at NCI centers and residing in a county with a medical school–affiliated hospital more so than residence in rural or low-income areas. Access considerations for all patients must include benefits balanced against cost, which are escalating rapidly as new agents become available.
Funding
Bradley and Eguchi’s research was supported by National Cancer Institute grant number P30CA046934, “University of Colorado Cancer Center Core Support Grant.” Bradley, Eguchi, and Perraillon were also supported by Data Science to Patient Value (D2V) initiative – University of Colorado School of Medicine ID2016.17 and a grant from the National Cancer Institute “Addressing Urban-Rural Disparities in Cancer: The Case for Registry Expansion” (R01CA22599, Bradley and Perraillon, principal investigators). All analyses were conducted by the Population Health Shared Resource (P30CA046934).
Notes
Affiliations of authors: University of Colorado Cancer Center and the Department of Health, Systems, Management, and Policy, Aurora, CO (CJB); Population Health Shared Resource, University of Colorado Cancer Center, Aurora, CO (ME); Department of Health Systems, Management and Policy, University of Colorado, Aurora, CO (MCP).
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors have no conflicts of interest to report.
Supplementary Material
References
- 1. Gotwals P, Cameron S, Cipolletta D, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17(5):286–301. [DOI] [PubMed] [Google Scholar]
- 2. Green AK, Aviki EM, Matsoukas K, Patil S, Korenstein D, Blinder V.. Racial disparities in chemotherapy administration for early-stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2018;172(2):247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23(27):6639–6646. [DOI] [PubMed] [Google Scholar]
- 4. Lynch JA, Berse B, Rabb M, et al. Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010-2013. BMC Cancer. 2018;18(1):306.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bach P. Monthly and median costs of cancer drugs at the time of FDA approval 1965-2016. https://www.mskcc.org/research-programs/health-policy-outcomes/cost-drugs. Accessed February 28, 2019.
- 6. O’Connor JM, Sedghi T, Dhodapkar M, Kane MJ, Gross CP.. Factors associated with cancer disparities among low-, medium-, and high-income US counties. JAMA Netw Open. 2018;1(6):e183146.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Snyder BM, Mounessa JS, Fazzari M, et al. Greater distance to an academic medical center is associated with poorer melanoma prognostic factors: the University of Colorado Experience. Dermatol Online J. 2017;23(11):1–6. [PubMed] [Google Scholar]
- 8. Mokdad AH, Dwyer-Lindgren L, Fitzmaurice C, et al. Trends and patterns of disparities in cancer mortality among US counties, 1980-2014. JAMA. 2017;317(4):388–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. US county-level trends in mortality rates for major causes of death, 1980-2014. JAMA. 2016;316(22):2385–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henley SJ, Anderson RN, Thomas CC, Massetti GM, Peaker B, Richardson LC.. Invasive cancer incidence, 2004-2013, and deaths, 2006-2015, in nonmetropolitan and metropolitan counties - United States. Mmwr Surveill Summ. 2017;66(14):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Probst JC, Moore CG, Glover SH, Samuels ME.. Person and place: the compounding effects of race/ethnicity and rurality on health. Am J Public Health. 2004;94(10):1695–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Onega T, Alford-Teaster J, Wang F.. Population-based geographic access to parent and satellite National Cancer Institute Cancer Center Facilities. Cancer. 2017;123(17):3305–3311. [DOI] [PubMed] [Google Scholar]
- 13.SEER Medicare. SEER-Medicare: Brief description of the SEER-Medicare database. https://healthcaredelivery.cancer.gov/seermedicare/overview/. Accessed February 28, 2019.
- 14.NCI SEER. Number of persons by race and Hispanic ethnicity for SEER participants. https://seer.cancer.gov/registries/data.html. Accessed March 7, 2019.
- 15.Health and human services. https://data.hrsa.gov/data/download. Accessed February 28, 2019.
- 16. Dranitsaris G, Zhu X, Adunlin G, Vincent MD.. Cost effectiveness vs. affordability in the age of immuno-oncology cancer drugs. Expert Rev Pharmacoecon Outcomes Res. 2018;18(4):351–357. [DOI] [PubMed] [Google Scholar]
- 17.NCI Designated Cancer Centers. https://www.cancer.gov/research/nci-role/cancer-centers. Accessed February 28, 2019.
- 18.SEER. Observational research in oncology toolbox. https://seer.cancer.gov/oncologytoolbox. Accessed February 28, 2019.
- 19.US Department of Agriculture. 2010 Rural-Urban Community Area Codes Documentation. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/. 2019. Accessed November 25, 2019.
- 20.US Census Bureau. Number of people living in “Poverty Areas” up. https://www.census.gov/newsroom/press-releases/2014/cb14-123.html. 2014. Accessed November 25, 2019.
- 21. Klabunde CN, Potosky AL, Legler JM, Warren JL.. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 22. Deyo RA, Cherkin DC, Ciol MA.. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 23. Norton EC, Dowd BE, Maciejewski ML.. Marginal effects—Quantifying the effect of changes in risk factors in logistic regression models. JAMA. 2019;321(13):1304–1305. [DOI] [PubMed] [Google Scholar]
- 24. Makarov DV, Li H, Lepor H, Gross CP, Blustein J.. Teaching hospitals and the disconnect between technology adoption and comparative effectiveness research: the case of the surgical robot. Med Care Res Rev. 2017;74(3):369–376. [DOI] [PubMed] [Google Scholar]
- 25. Seymour EK, Ruterbusch JJ, Beebe-Dimmer JL, Schiffer CA.. Real-world testing and treatment patterns in chronic lymphocytic leukemia: a SEER patterns of care analysis. Cancer. 2019;125(1):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tao L, Schwab RB, San Miguel Y, et al. Breast cancer mortality in older and younger patients in California. Cancer Epidemiol Biomarkers Prev. 2019;28(2):303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ho G, Wun T, Muffly L, et al. Decreased early mortality associated with the treatment of acute myeloid leukemia at National Cancer Institute-designated cancer centers in California. Cancer. 2018;124(9):1938–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parsons HM, Lathrop KI, Schmidt S, et al. Breast cancer treatment delays in a majority minority community: is there a difference? J Oncol Pract. 2015;11(2):e144–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fouad MN, Lee JY, Catalano PJ, et al. Enrollment of patients with lung and colorectal cancers onto clinical trials. J Oncol Pract. 2013;9(2):e40–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang RL, Wapnir I.. Hispanic breast cancer patients travel further for equitable surgical care at a comprehensive cancer center. Health Equity. 2018;2(1):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Temkin SM, Fleming SA, Amrane S, Schluterman N, Terplan M.. Geographic disparities amongst patients with gynecologic malignancies at an urban NCI-designated cancer center. Gynecol Oncol. 2015;137(3):497–502. [DOI] [PubMed] [Google Scholar]
- 32.American Cancer Society. How common is lung cancer? https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/key-statistics.html. Accessed February 28, 2019.
- 33.SEER. Compare stats by data type. https://seer.cancer.gov/faststats/selections.php?#Output. Accessed February 28, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.