Learning point for clinicians
Many cases of kidney disease with tubulopathy might not have an obvious etiology. However, increased staining for megalin (in appropriate cases) may reveal anti-brush border antibody disease more frequently. Prompt recognition can lead to earlier and more effective treatment, as well encourage providers to investigate for underlying malignancies.
Introduction
Anti-brush border antibody (ABBA) renal disease is a relatively under-reported cause of severe tubular injury in elderly patients. In ABBA, immune complexes deposit within the tubular basement membrane (TBM) of the proximal convoluted tubule (PCT), and Bowman’s capsule.1,2 A recent study reported that anti-low-density lipoprotein receptor-related protein 2 (LRP2, also known as megalin) is an auto-antibody in ABBA.1 The disease course often rapidly progresses to end stage kidney disease (ESKD). To date, only 11 cases have been reported.1–3
Case report
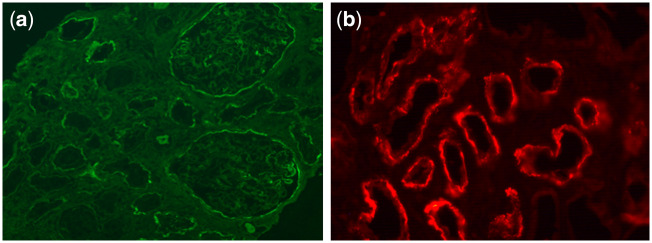
A 67-year-old male presented for a 3-week history of weakness and abdominal pain. He had no significant personal medical history, but a positive familial history of Crohn’s disease. He had empirically taken ibuprofen and Mylanta (containing simethicone, aluminum hydroxide and magnesium hydroxide). On arrival, his sodium was 117 mEq/l and potassium 2.6 mEq/l. Creatinine was 20.5 mg/dl (unknown baseline), blood urea nitrogen 203 mg/dl and magnesium was 4.6 mEq/dl. Urinalysis showed microscopic hematuria and 30 (+) protein. Catheter placement returned 300 ml of urine. Intravenous fluids were started and renal ultrasound showed a 9.3-cm mass of the right kidney. Urology was consulted and began work-up and staging for likely renal cell carcinoma. Despite hydration, renal function did not improve and he was started on hemodialysis. Renal biopsy of the right kidney showed no evidence of glomerulonephritis, but immunofluorescence showed immunoglobulin G (IgG) staining of the Bowman’s capsule and TBM, severe tubulointerstitial fibrosis by light microscopy and immune complex deposits in the TBM and segmental subepithelial regions of the glomerular basement membrane (GBM) by electron microscopy (Figure 1a). C3 was low, but anti-neutrophil cytoplasmic antibody, anti-GBM and serum protein electrophoresis were negative. Prednisone was stopped after 8 days due to a lack of renal response. Final staining of the renal biopsy returned positive for LRP2/megalin, with normal IgG4, confirming a diagnosis of anti-brush border disease (Figure 1b). Ultimately, urology performed a radical right nephrectomy, with a final diagnosis of clear cell carcinoma. During work-up for his cancer, he was incidentally found to have a primary atypical carcinoid tumor of his right lung, which was also surgically removed.
Figure 1.
(a) Immunofluorescence showing IgG staining segmentally in the glomerular capillary walls, Bowman capsules and TBMs. (b) Immunofluorescence staining with anti-LRP2/megalin antibody highlights tubular basement membranes.
Discussion
ABBA renal disease typically occurs in males over the age of 60, who present with acute kidney injury (AKI) and subnephrotic proteinuria, and typically progress rapidly to ESKD.1,2 The disease process is thought to occur from IgG antibodies developing against anti-LRP2 and, together with C3, deposit as immune complexes within the TBM of the PCT, and to a lesser degree, the subepithelial membrane of the glomerulus.1,2 LRP2 normally functions to reabsorb albumin and other proteins within the PCT and is a major autoantigen in systemic autoimmune diseases, such as rheumatoid arthritis.4 To date, only 11 cases of ABBA renal disease have been reported, the first report in 2016.1–3 The demographics of patients previously reported to have ABBA have not shown any correlation to the presence of malignancy. However, there are reports of ABBAs present in the sera of patients with Crohn’s disease.5 Renal biopsies in patients with ABBA disease show extensive tubular damage, with electron microscopy highlighting immune complex deposits along the TBM and GBM. These deposits stain positive for IgG and anti-LRP2/megalin via immunofluorescence. Optimal treatment remains unclear. A retrospective analysis of 10 patients noted that only one patient had serologic remission following treatment with prednisone and cyclophosphamide.1 The other patients that were treated with observation, rituximab or prednisone had various outcomes; five required renal replacement therapy.1 One patient received a transplant and had recurrence of the disease.1,2 Our case highlights a rare, yet important, cause of severe and progressive AKI. Future work is needed to determine the best detection methods and treatment options.
Ethical approval
Our institution does not require ethical approval for reporting individual cases or case series.
Consent
Consent was obtained from the patient. A completed consent form can be made available to the journal editor if specifically requested.
Conflicts of interest. None declared.
References
- 1. Larsen CP, Trivin-Avillach C, Coles P, Collins AB, Merchant M, Ma H, et al. LDL receptor-related protein 2 (Megalin) as a target antigen in human kidney anti-brush border antibody disease. J Am Soc Nephrol 2018; 29:644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosales IA, Collins AB, do Carmo PA, Tolkoff-Rubin N, Smith RN, Colvin RB.. Immune complex tubulointerstitial nephritis due to autoantibodies to the proximal tubule brush border. J Am Soc Nephrol 2016; 27:380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dinesh KP, Raniele D, Michels K, Avasare RS, Larsen CP, Kayton R, et al. Anti-LRP2 Nephropathy with abundant IgG4-positive plasma cells: a case report. Am J Kidney Dis 2019; 74:132–7. [DOI] [PubMed] [Google Scholar]
- 4. Ooka S, Matsui T, Nishioka K, Kato T.. Autoantibodies to low-density-lipoprotein-receptor-related protein 2 (LRP2) in systemic autoimmune diseases. Arthritis Res Ther 2003; 5:R174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skogh T, Heuman R, Tagesson C.. Anti-brush border antibodies (ABBA) in Crohn’s disease. J Clin Lab Immunol 1982; 9:147–50. [PubMed] [Google Scholar]