Although myocardial ischaemia usually manifests as a consequence of atherosclerosis-dependent obstructive epicardial coronary artery disease, a significant percentage of patients suffer ischaemic events in the absence of epicardial coronary artery obstruction. Experimental and clinical evidence highlight the abnormalities of the coronary microcirculation as a main cause of myocardial ischaemia in patients with ‘normal or near normal’ coronary arteries on angiography. Coronary microvascular disturbances have been associated with early stages of atherosclerosis even prior to any angiographic evidence of epicardial coronary stenosis, as well as to other cardiac pathologies such as myocardial hypertrophy and heart failure. The main objectives of the manuscript are (i) to provide updated evidence in our current understanding of the pathophysiological consequences of microvascular dysfunction in the heart; (ii) to report on the current knowledge on the relevance of cardiovascular risk factors and comorbid conditions for microcirculatory dysfunction; and (iii) to evidence the relevance of the clinical consequences of microvascular dysfunction. Highlighting the clinical importance of coronary microvascular dysfunction will open the field for research and the development of novel strategies for intervention will encourage early detection of subclinical disease and will help in the stratification of cardiovascular risk in agreement with the new concept of precision medicine.
Keywords: Microvessels, Coronary microcirculation, Molecular and cellular targets, Risk factors, Ischaemic heart disease
This article is part of the Spotlight Issue on Coronary Microvascular Dysfunction.
1. Introduction
Myocardial infarction (MI) and myocardial ischaemia have been traditionally considered a ‘large vessel ’ disease caused by atherosclerosis and obstructive atherothrombotic events in epicardial coronary arteries that lead to the complete occlusion of the vessel at the ‘culprit site ’. While the coronary microcirculation is not affected by atherosclerosis, impairment of microvascular blood flow is an established cause of myocardial ischaemia.
Coronary microcirculation refers to different anatomically and functionally vascular compartments (<500 µm diameter) with a critical role in the physiological regulation of myocardial perfusion. Coronary microcirculation is responsible for the regulation of blood flow distribution to cover the metabolic demands of the myocardium and the modulation of peripheral vascular resistance. In healthy individuals, without significant epicardial atherosclerosis, increases in myocardial metabolic demand are met by progressive vasodilation of coronary arterioles, which can normally induce a five-fold increase of coronary blood flow.
Coronary microvascular dysfunction (CMD) refers to a term covering a wide spectrum of clinical situations in which the structure and function of the coronary microcirculation is affected. This leads to impaired responses of the coronary flow to vasodilator stimuli, being characterized by impaired coronary flow reserve (CFR) with cut-off values below 2.0–2.5 depending on the methodology, or abnormal high index of coronary microvascular resistance (IMR; e.g. IMR >25) and/or focal or diffuse vasoconstriction during acetylcholine provocation testing, in the absence of any significant epicardial coronary artery obstruction (>50% lumen stenosis at coronary angiography) or preserved fractional flow reserve (FFR, value ≥ 0.80).1,2
A wide spectrum of cardiac and systemic conditions can lead to myocardial ischaemia due to CMD without significant epicardial coronary obstruction1,3,4 These diseases are mainly those that cause left ventricular hypertrophy (hypertrophic cardiomyopathy, aortic stenosis, and hypertensive heart disease) or inflammation (myocarditis or vasculitis).1,3–6 However, often, it is in the absence of other diseases that functional and/or structural alterations in coronary microcirculation are responsible for myocardial ischaemia. This condition affects up to 50% of subjects with chronic coronary syndromes, up to 20% of those with acute coronary syndromes (ACS) and associates with an increase in event rates.
In light of these considerations, the objective of the present position paper of the European Society of Cardiology (ESC) Working Group on Coronary Pathophysiology and Microcirculation is to provide updated evidence and a critical view in our understanding of the pathophysiology of microvascular dysfunction in association with ischaemic heart disease. This includes a detailed discussion, based on clinical and experimental evidence, on the relevance of cardiovascular risk factors and comorbid conditions in CMD, and a critical view on the relevance of CMD in non-obstructive ischaemic heart disease and its clinical consequences in ST-segment elevation myocardial infarction (STEMI) patients.
2. Coronary microcirculation and ischaemic heart disease
CMD is a multifactorial disease, prevalent in a wide spectrum of clinical cardiovascular situations. Traditionally, CMD has been classified according to the clinical setting in which it may occur.5 Here, CMD is discussed in relation to the phenotype and severity of coronary artery disease (CAD) (non-obstructive chronic coronary syndrome, obstructive chronic coronary syndrome, non-obstructive ACS, obstructive ACS, and no-reflow), highlighting aspects from pathophysiology to treatment, including diagnosis, incidence, and prognosis.
2.1 CMD in chronic coronary syndrome
2.1.1 CMD in non-obstructive chronic coronary syndrome
A significant number of patients with symptoms and signs of ischaemic heart disease do not have a significant coronary artery stenosis (>50%).6–8 This phenomenon termed ‘ischaemia with non-obstructive coronary artery disease (INOCA)’ is associated with increased risk for major cardiovascular events compared with an asymptomatic reference population.9,10
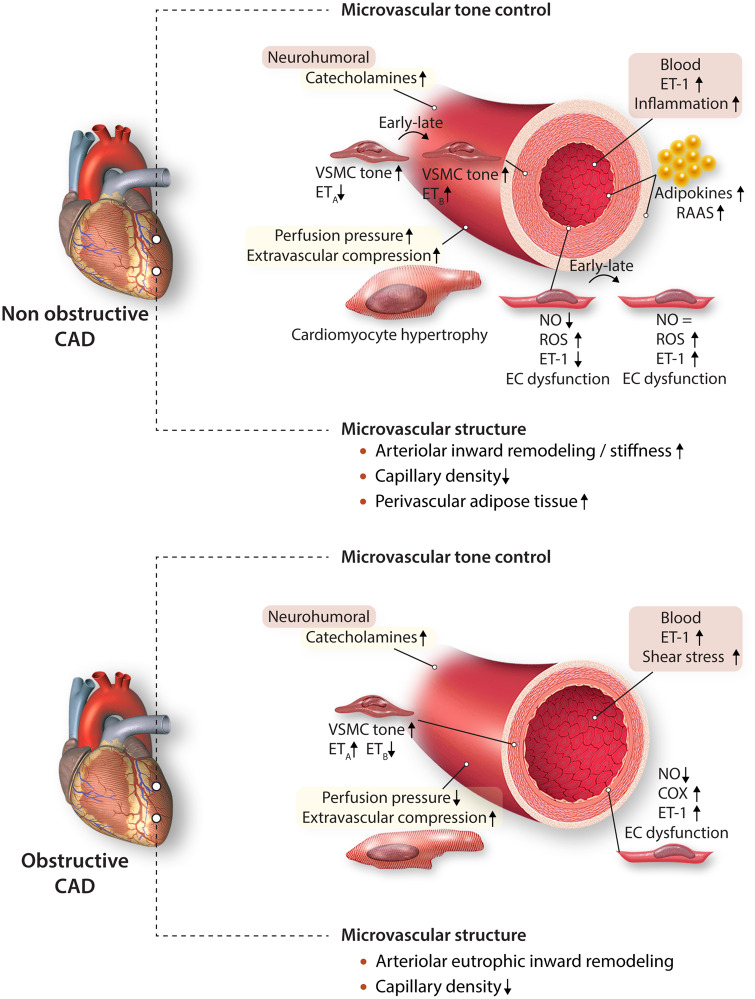
Major risk factors for INOCA include dyslipidaemia, obesity, metabolic syndrome, and diabetes.11 The pathophysiological link between metabolic dysregulation and INOCA most likely involves CMD (Figure 1). The mechanisms underlying CMD in non-obstructive CAD are still not completely understood, but they implicate both functional and structural changes. Thus, both experimental12,13 and clinical14 studies have shown that endothelial dysfunction is a key mediator in the pathogenesis of CMD. This includes attenuation of endothelium-dependent vasodilation resulting from reduced nitric oxide (NO) bioavailability12 and increased vasoconstrictor responses to endothelin-1 (ET-1), prostaglandin H2, and thromboxane A2,13 in swine models of metabolic dysregulation.
Figure 1.
Coronary microvascular dysfunction in non-obstructive and obstructive coronary artery disease. COX, cyclooxygenase; EC, endothelial cell; ET-1, endothelin-1; ETA, endothelin receptor A; ETB, endothelin receptor B; NO, nitric oxide; RAAS, renin–angiotensin–aldosterone system; ROS, reactive oxygen species; VSMC, vascular smooth muscle cell.
Increased sympathetic activity produces exaggerated alpha-adrenergic coronary vasoconstriction in patients with metabolic syndrome.15 Likewise, in patients with pre-hypertension and metabolic syndrome, activation of the renin– angiotensin–aldosterone system increases angiotensin II-mediated vasoconstriction in the coronary circulation.16 In addition, adipocyte-derived free fatty acids and leptin also lead to increased adrenergic tone.16 Experimental studies have shown that adipocytes and perivascular adipose tissue-derived adipokines such as leptin, resistin, IL-6, and tumour necrosis factor-α are potent pro-inflammatory molecules that can promote oxidative stress in the endothelium and impair endothelial function and NO bioavailability, either directly or via increased ET-1 production.11,17
Changes in microvascular structure also contribute to CMD. Ossabaw swine with MetS had a blunted response to adenosine along with a reduced microvascular density.18 In a similar model, hyperaemic flow was impaired with hypertrophic inward remodelling of coronary resistance arteries, capillary rarefaction, and augmented myogenic tone in isolated coronary arterioles.19 Taken together, microvascular dysfunction, inward remodelling of resistance arteries and reductions in vascular density can all reduce flow reserve and produce regional ischaemia in the absence of an epicardial stenosis in humans and in animal models with comorbidities.11,17
2.1.2 CMD in obstructive chronic coronary syndrome
Differing from the healthy heart, in which the coronary microcirculation is the principal site of vascular resistance, in the setting of a physiologically significant epicardial artery stenosis, this additional resistance limits maximal coronary blood flow. While some of this reduction is secondary to the pressure loss across the stenosis , 20 there is also evidence that abnormalities in the control of coronary microvascular tone and microvascular structure, can contribute to the reductions in CFR.21 Experimental studies have demonstrated that decreases in post-stenotic perfusion pressure can trigger structural and functional alterations in the distal microvasculature.20 These include inward remodelling of the coronary resistance arteries and coronary arteriolar and capillary rarefaction distal to a coronary stenosis. Capillary rarefaction is also seen in dysfunctional myocardium of patients undergoing coronary bypass surgery and is a predictor of poor functional recovery following revascularization.22 The concept that such structural microvascular abnormalities persist long after revascularization is supported by experimental studies in swine, in which weeks after revascularization of hibernating myocardium, resting flow normalizes but the flow response to metabolic stress (i.e. high-dose dobutamine infusion) remains blunted.23 Microvascular rarefaction occurs rather rapidly because it is already detected after 90 min left anterior descending artery occlusion in an experimental model of MI in swine and can be partially recovered by delivery of stem cells or stem cell-derived products.24 This is an area of active research because it may represent a novel therapeutic intervention to reduce CMD.
Interestingly, the recently presented ISCHEMIA trial (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches —recently presented at the American Heart Association - AHA- scientific session 2019) showed that revascularization was not better that optimal medical therapy, in stable patients with at least moderate ischaemia and ≥50% stenosis in a major epicardial vessel. In this very complicated and long trial, the reduction of the epicardial stenosis did not produce the expected benefits supporting the concept of the importance of mechanisms independent of epicardial arteries in ischaemia symptoms.
Functional coronary microvascular remodelling distal to a stenosis may reflect impaired vasodilator function as well as increased vasoconstrictor responses, as supported by experimental studies in dogs.25 In this respect, exaggerated ET-1 induced vasoconstriction was documented in isolated coronary arterioles from swine with a chronic stenosis (Figure 1).26 Surprisingly, vasodilator responses to bradykinin were preserved while ETB-mediated vasodilation was lost. The preservation of endothelial vasodilation to bradykinin may have reflected a shift in mechanism from NO to dilator endothelium-derived hyperpolarizing factor (EDHF). Indeed, the contribution of EDHF to the regulation of coronary microvascular tone in humans has been shown to accompany the loss of NO with the progression of CAD.27 Experimental studies have shown that structural inward remodelling of arterioles can be produced at low intraluminal pressure in vitro and is enhanced by ET-1 and prevented by the calcium-antagonist amlodipine.28
2.2 CMD in ACS
2.2.1 CMD in non-obstructive ACS
ACS with normal or near-normal coronary arteries on coronary angiography refers to a heterogeneous clinical entity with multiple potential causes that not always are apparent. Within this condition, MINOCA is defined as MI with non-obstructed coronary arteries (< 50% diameter stenosis in a major epicardial vessel) that refers to an ischaemic mechanism responsible for myocyte injury and troponin elevation. The concept of MINOCA has been recently included in the STEMI guidelines (2017) of the European Society of Cardiology.3 MINOCA is more common in women than in men and in patients presenting with non-STEMI (NSTEMI) than those presenting with STEMI.29
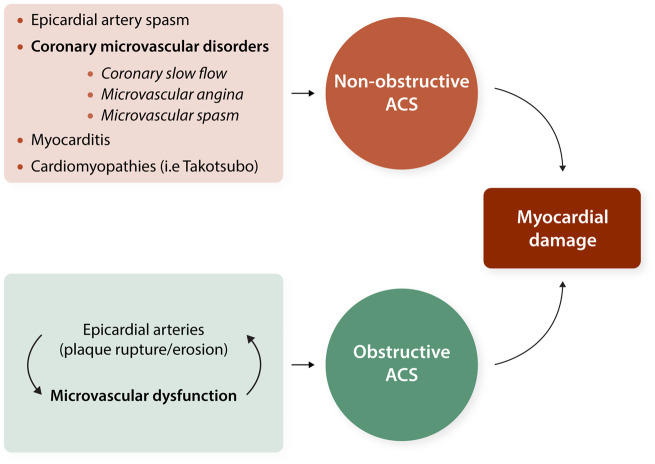
Pathophysiology of non-obstructive ACS may involve epicardial or microvascular causes (i.e. CMD) and cardiac non-ischaemic aetiologies (i.e. myocarditis or Takotsubo syndrome) (Figure 2).5,30 Myocardial disorders, including the Takotsubo cardiomyopathy, were initially included into the definition of MINOCA.31 However, according to the recently published Fourth Universal Definition of Myocardial Infarction, diagnosis of MINOCA, such as diagnosis of MI, refer to ischaemic mechanisms responsible for the myocyte injury, whereas non-ischaemic causes such as myocarditis have been excluded.32 Among MINOCA patients, coronary microvascular spasm is suggested to account for 16% of cases.33 Patients with non-obstructive ACS exhibit a significant coronary dysfunction, which seems to involve both an increased constrictor reactivity, likely mainly involving the coronary microcirculation, and a reduced microvascular dilator function both persisting at 12-month follow-up.31,34
Figure 2.
Microvascular dysfunction as underlying pathophysiological mechanism for acute coronary syndromes.
2.2.2 CMD in obstructive ACS
It is generally assumed that epicardial events precede and are cause of microvascular dysfunction. However, a novel concept of the mechanisms resulting in ACS takes into account the primary dysfunction of the microcirculation in addition to the vulnerable plaque as causal contributor to myocardial damage and infarct size (Figure 2). Thus, transient or permanent microvascular dysfunction limits coronary blood flow and leads to alterations of the shear stress affecting endothelial function and enhancing thrombus formation at epicardial level.35
A major challenge for considering the primary role of CMD is the lack of data on microvascular function prior to an ACS. Thus, much work remains to understand the mechanisms involving microcirculatory impairment prior, during and after ACS.
2.2.3 CMD and coronary no-reflow
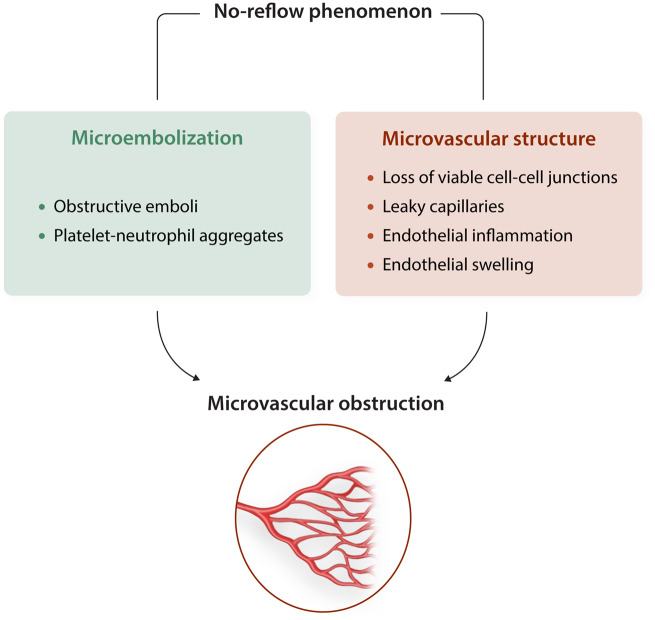
Timely reperfusion therapy after acute myocardial infarction (AMI) is a key factor in reducing infarct size and improving ventricular function and clinical outcome. Unfortunately, even in the absence of a residual epicardial stenosis, revascularization does not always re-establish effective microcirculatory perfusion due to the no-reflow phenomenon , 36 which occurs more frequently in women.37 Results in animal models suggest that suboptimal reperfusion after percutaneous coronary intervention (PCI) is related to microvascular disorders resulting from microvascular obstruction (MVO) secondary to endothelial injury and/or distal embolization.38 In addition, experimental studies define MVO as an independent predictor of adverse left ventricular remodelling.39 Moreover, a recent pooled analysis of individual data from seven randomized trials supports the notion that presence and extent of MVO after primary PCI in STEMI patients strongly associates with major adverse cardiovascular events occurring within a year.40
Coronary microvascular endothelial damage, resulting from either ischaemia–reperfusion and/or the associated comorbidities (metabolic dysfunction, hypertension, ageing, and dyslipidaemia), is thought to play a critical role in the no-reflow phenomenon.41 However, other mechanisms have also been proposed to contribute, including activation of inflammatory pathways, myocyte oedema, platelet activation, or leucocyte infiltration.38 Interruption of blood flow, followed by its acute restoration, results in endothelial dysfunction leading to alterations in the balance between the different vasomotor pathways, as summarized in Figure 3. With increasing severity of ischaemia–reperfusion injury, even more extensive microvascular injury occurs, with increased vascular permeability, thinning of the capillary wall and loss of viable cell–cell junctions, leading to oedema and even intramyocardial haemorrhage.42 Although there is no evidence that no-reflow contributes to secondary cardiomyocyte damage, it may affect infarct healing responses —i.e. lead to infarct thinning —and may thus aggravate post-infarct remodelling.43 Recent studies on P2Y12 inhibitors in a porcine model of ischaemia/reperfusion have shown a significant reduction in no-reflow in treated animals.44,45 Novel preclinical and clinical studies are needed to reduce MVO and improve clinical outcomes.
Figure 3.
Pathophysiological mechanisms of microvascular dysfunction associated to the non-reflow phenomenon.
2.3 CMD in reperfused acute MI
Moreover, there is clinical evidence showing that CMD may contribute to the persistence or recurrence of angina following successful revascularization after AMI.46,47 This represents an important unresolved clinical problem that affects from one-fifth to one-third of patients undergoing myocardial revascularization at 1-year follow-up. CMD in reperfused AMI is associated with adverse remodelling, lower ventricular function, and worse prognostic.48 Up to now, however, there is a recognized lack of knowledge of how ameliorate CMD and improve signs and symptoms of myocardial ischaemia in these patients, in part due to an insufficient understanding of the underlaying pathophysiology. Multiple underlying causes can contribute to CMD after successful PCI and stenting, from endothelial dysfunction, increased oxidative stress, reduced NO-release, to low shear stress conditions distal to the stenosis that may negatively influence microvascular function. In addition, a masked CMD pre-existing to PCI might also account for the prevalence of anginal symptoms and/or myocardial ischaemia after a successful PCI.49 Signs of ischaemia/reperfusion injury affecting coronary microcirculation go from reversible interstitial oedema that acts compressing coronary microcirculation to capillary destruction with intramyocardial haemorrhage. Interstitial iron deposition induces an inflammatory response that contributes to microvascular injury.50 Several mechanisms contributing to microvascular injury in reperfused AMI do not differ from those contributing to cardiomyocyte injury. To date, however, the temporal and causal relationship between both pathologic conditions needs to be clarified.50
3. Factors increasing the risk of CMD
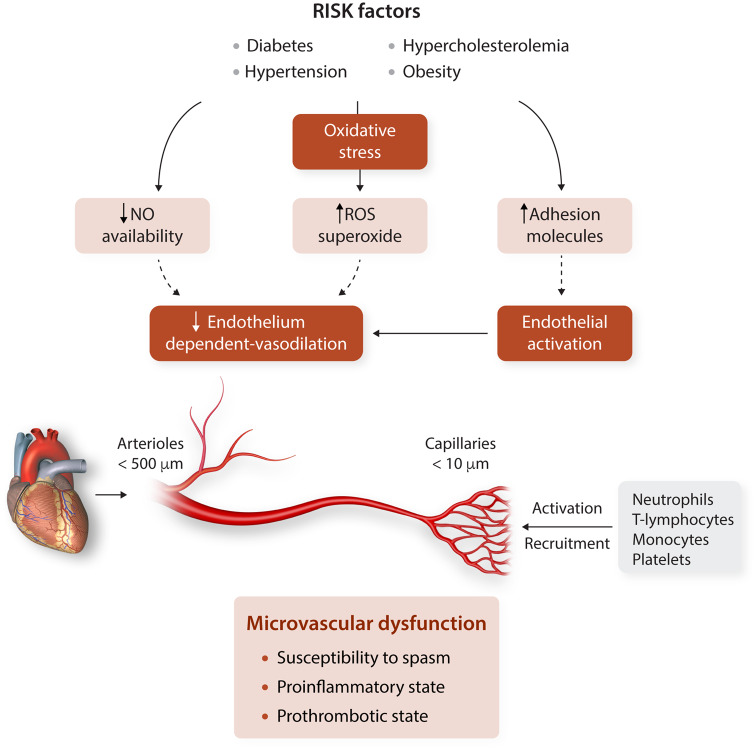
Risk factors for CMD do not differ from those for epicardial macrovascular arterial disease including diabetes mellitus, obesity, hypertension, hyperlipidaemia, smoking, and age, which, separately and synergistically, contribute to CMD (Figure 4). Thus, an increasing number of traditional cardiovascular risk factors associate with reduced values of myocardial perfusion reserve index and abnormal microvascular dilation leading to impaired CFR.
Figure 4.
Microvascular response to risk factors. NO, nitric oxide; ROS, reactive oxygen species.
3.1 Diabetes and obesity
Diabetes is a strong inducer of microvascular dysfunction, but the relationship is bidirectional since microvascular dysfunction in muscle and adipose tissue also contributes to the pathogenesis of type 2 diabetes.51 As CMD precedes hyperglycaemia in the pathogenesis of type 2 diabetes, factors other than hyperglycaemia contribute to CMD in type 2 diabetes. CMD in diabetes is characterized by decreased NO activity, increased production of reactive oxygen species (ROS), increased endothelin synthesis, reduced endothelial barrier function, and elevated inflammatory activity oxidative stress, a basic pathological mechanism in ischaemic myocardial injury, is caused by an imbalance between excessive production and detoxification of free radicals, especially ROS, in the vascular endothelial cells resulting in the oxidative modification of cellular and extracellular components directly affecting cell function and viability. Diabetes is an endocrine multiorgan disease, and consequently, diabetes-associated CMD has been detected in multiple organs including the heart, retina, kidney, and skin. In pigs, diabetes decreases myocardial blood flow and capillary density (rarefaction ).52
Overweight and obesity, common in type 2 diabetes, are characterized by CMD even in the absence of signs and symptoms of cardiac ischaemia or dysfunction. In human obesity, and especially in abdominal obesity, perivascular and epicardial adipose tissue accumulate around the coronary vasculature and heart and become inflamed.53
3.2 Hypertension
Hypertension intensifies CMD through functional and structural alterations in the microcirculation. Clinical studies provide evidence that microvascular hallmarks of hypertension are inward remodelling of resistance arteries and microvascular rarefaction , 54 determinants of microvascular resistance reducing myocardial blood flow.
Chronic hypertension, obesity, and diabetes gradually reduce kidney function and are strong risk factors for chronic kidney disease (CKD), with high cardiovascular complications including myocardial hypertrophy, heart failure, and myocardial ischaemia, associated with CMD, as supported by the fact that most patients with CKD do not reach end-stage renal disease, but die from cardiovascular complications.55
The molecular connections between diabetes and hypertension are highlighted in Zucker rats, that show how elevated glucose concentrations activate Rho-kinase, which in turn inhibit internalization and facilitates recycling of angiotensin 1 type (AT1) receptors, leading to increased functional availability of AT1 receptors and sustained angiotensin II-induced arterial constriction.56
3.3 Dyslipidaemia
Dyslipidaemia is a major risk factor for microvascular dysfunction. Hypercholesterolaemic patients have clear evidence of reduced CFR , 57 from the early stages of atherosclerosis and before any angiographic evidence of coronary stenosis. Plasma levels of total cholesterol and LDL-C inversely correlate with the FFR and the IMR, independently of the severity of coronary atherosclerosis or the number of diseased vessels. Hypercholesterolaemia has been proven to lead to larger infarcts and adverse cardiac remodelling post-MI by compromising microvascular function in acute STEMI patients.58
CMD linked to hypercholesterolaemia primarily relates to impaired endothelium-dependent vasodilation in arterioles. This could be in part due to increased generation of ROS. Interestingly, Ox-LDL dependent changes in large conduit arteries are mirrored at the microvessels, as demonstrated by Hein et al.59 using an experimental model of isolated porcine coronary arterioles. The detrimental effect of OxLDL on endothelium-dependent dilation of arterioles is specifically mediated by the reduction in the expression and function of NO synthase (eNOS) and a low NO-bioavailability, whereas Ox-LDL does not affect the vasodilation of arterioles mediated by components such as cyclooxygenase and cytochromeP-450 monooxygenase nor mediated by endothelial hyperpolarization.59
In addition, the pathogenic mechanisms contributing to dyslipidaemia-induced CMD include inflammation, innate, and adaptive immune cell responses, and pro-thrombotic conditions.
4. Sex, microvascular dysfunction, and cardiovascular risk
Women with suspected or confirmed ischaemic heart disease , especially when young, have less atherosclerosis than men and less prevalence of obstructive CAD. However, women at young age have similar prevalence of STEMI than men.60 Women more often have unexplained higher mortality rates after STEMI, which may be due to smaller vessel size, less collateral flow, more vascular stiffness, and concurrent CMD.61 In addition, a disproportionate burden of coronary risk factors and comorbidities is a clear feature of women even after adjustment for age. Nevertheless, risk factors have a different impact on mortality depending on age and sex.37,61 Type 2 diabetes is more likely to be associated with endothelial dysfunction and CMD in women than in men. Similarly, smoking is a well-established cardiovascular risk factor for both sexes, but this is significantly increased in women under age 60, and more markedly in young women who combine the habit of smoking with the use of oral contraceptives.
Indeed, experimental data suggest complex oestrogen-related gender-specific differences in the regulation of NO-mediated microvessel vasomotor function in female and male. These findings may help to better understand the human observations showing significant gender-specific differences in the epidemiology, pathophysiology, clinical features, prognosis, and treatment success between women and men.62 It seems that sex differences of the coronary circulation are especially pronounced. Therefore, there is an urgent clinical unmet need to develop gender-specific therapeutic and preventive modalities.
In addition, high-risk pregnancies (e.g. gestational hypertension or diabetes) and other factors related to the female reproductive cycle (e.g. polycystic ovary syndrome) give women added vulnerability to develop cardiovascular disease, being recently referred to as sex-specific cardiovascular risk factors.63 However, their pathological relevance on the microcirculation has not been established.
5. Microvascular endothelial dysfunction and vasospastic angina
Maseri et al.64 suggested ‘coronary artery spasm ’ as the underlying pathogenic factor of variant angina and Bugiardini et al.65 defined ‘vasotonic angina ’ as a diffuse epicardial coronary constriction, usually ≥50% of lumen diameter, confined to the distal segments of the coronary arteries and limiting the blood flow supply to the myocardium, which produces myocardial ischaemia in the presence of normal smooth coronary arteries at angiography. In vasotonic angina, the microcirculation is still the major culprit as assessed by coronary blood flow measurements in the coronary sinus, suggesting a concurrent involvement of functional abnormalities in macro- and microvessels of the coronary tree. Thus, endothelial dysfunction is significantly associated with diffuse epicardial vasoconstrictor response following intracoronary infusion of acetylcholine with adverse cardiovascular events.66 Assessment of endothelial function may identify early changes in vasoactive functions relevant in the development of atherosclerosis rather than identifying atherosclerotic lesions per se, as documented in a study in women with chest pain and normal coronary angiograms.67
6. Mechanical, cellular, and molecular effectors of microvascular dysfunction
6.1 Haemodynamic forces: pressure and shear stress
The endothelial layer lining the interior of blood vessels is directly exposed to haemodynamic forces. High blood pressure accelerates development of atherosclerotic plaques in large epicardial coronaries and endothelial dysfunction of microvessels of the heart. High intraluminal pressure elicits constrictions of isolated small coronary arteries and arterioles in rats , 68 which serves —in part —to protect the distal microcirculation, including the capillary bed, and oedema development due to high hydraulic/filtration pressure. This, however, imposes higher blood flow velocity and thus shear stress on the endothelium of upstream large vessels, and at branching points.69
Haemodynamic forces have a wide spectrum of mechanical and molecular signalling effects on the endothelial cells, impacting their morphology and vasomotor function.68,70 Thus, the endothelium ‘senses ’ and ‘transduces ’ abnormal physical forces into cellular signalling events leading to vascular damage. However, major challenges remain in understanding how interactions between mechanotransduction and chemical signals—such as risk factors—play a pivotal role in eliciting the responses to shear stress in the different vascular beds.
6.2 Inflammation
Systemic inflammation may be a link between CMD and atherosclerosis. Inflammation-mediated endothelial cell activation is characterized by enhanced production of ROS, and platelet and leucocyte adhesion to the endothelium due to endothelial up-regulation of adhesion molecules (e.g. P-selectin) and loss of endothelial barrier function , 71 with detrimental effects on the epicardial coronary endothelium but also inducing CMD. Sources of ROS/superoxide are NADPH oxidase, xanthine oxidase, mitochondrial respiration, in addition to uncoupled eNOS.72 ROS/superoxide production plays a key role in the attenuation of NO bioavailability, as described above and it has been linked to the inflammatory response, possibly invoking stress-activated protein kinases such as c-Jun N-terminal kinases, in hypercholesterolaemic mice.73
The impact of inflammatory endothelial activation is less clear for other endothelial dilator mechanisms, specifically the endothelium-dependent dilator principle that relies on hyperpolarization (EDH-type dilation). The EDH-type dilation is most important in the microcirculation and therefore must be considered in the setting of CMD. Experimental studies using different animal models of cardiovascular risk reveal a shift of the endothelial-dilator mechanism from NO to EDH in arterioles rather than a global impairment of the response. Similarly, plasticity in endothelial signalling is found in the human microcirculation in disease , 74 suggesting involvement of further mechanisms.
6.3 Platelet activation
Clinical studies support the relevance of platelets at the microvascular level during ischaemia–reperfusion. Thus, platelets contribute to functional and structural coronary MVO occurring after PCI in a large proportion of patients.75
Platelets may compromise blood flow at the microvascular level by forming distal microemboli and by adhering to reperfused capillary or venular endothelium or to attached leucocytes, contributing to the release of vasoconstrictor or toxic molecules and a plethora of inflammatory mediators that further enhance the activation of the endothelial monolayer and the recruitment of circulating leucocytes.76
Platelet adhesion occurs in intact inflamed microvessels without the need of exposed extracellular matrix material. Oxidative stress, endothelial cell activation, and the accompanying recruitment of rolling and firmly adherent leucocytes are common features for platelet adhesion to the endothelium in the microcirculation. Molecules mediating this interaction are P-selectin and P-selectin glycoprotein ligand-1 or glycoprotein-Ib and von-Willebrand factor.77
Adhering to the endothelial cell lining turns platelets into effectors that boost the inflammatory process. They release a large number of proteins from preformed granules, synthetize bioactive molecules (ROS, thromboxane) or shed them from the membrane (e.g. CD40L). This promotes further activation of endothelial cells that express adhesion molecules such as intercellular adhesion molecule 1 fostering leucocyte–endothelial interaction. The interaction platelet CD40L with endothelial CD40-receptor is specifically important in the induction of inflammation-associated microvascular thrombosis, as demonstrated in venules and arterioles of wild type and CD40/CD40L deficient mice.78
In addition to the effects elicited through adhesion to the endothelium, activated platelets present HMGB1 (high mobility group box-1) that enhances production of neutrophil extracellular traps (NETs). A recent experimental study of ischaemia–reperfusion in rat demonstrates that NET-mediated microthrombosis significantly contributes to myocardial ‘no-reflow’.79
6.4 Autonomic dysfunction
Coronary microvascular tone, defined by the ratio between baseline and maximal vessel diameter, is regulated by several mechanisms including myogenic tone, metabolic control exerted by adjacent cells, endothelial function, and circulating factors, alongside autonomic innervation.80
Adrenergic innervation provides a mechanism for vessel tone regulation that is particularly important during exercise, or in the presence of endothelial dysfunction, but it has a negligible contribution to resting vascular tone in the healthy coronary circulation , 81 since coronary flow is mainly modulated by mechanisms of non-neural origin in normal conditions. Adrenergic receptors (α- and β-adrenergic receptors) regulate coronary circulation at the level of vascular endothelial and smooth muscle cells. Increased sympathetic activity results in stimulation of the β-adrenergic receptors, with β2 being the main adrenoceptors in the coronary microcirculation. Conversely, α-adrenergic stimulation produces vasoconstriction, with exception of the α2 receptors, which are abundantly distributed in the microvasculature and associate with vasodilation when located on endothelial cells.82
Chronic dysregulation of autonomic function is characterized by an imbalance between the sympathetic and parasympathetic systems. Autonomic dysfunction involves increased sympathetic activation, with increased vasoconstriction, and/or damage of the autonomic nerve fibres that innervate the heart and blood vessels, including the microvasculature. In general, adrenergic-derived vasoconstriction is relevant in clinical situations in which normal non-neural vasodilator mechanisms are impaired (i.e. dyslipidaemia, diabetes).83 The role of the parasympathetic innervation in the coronary microcirculation is debatable, even though activation of vascular endothelial M3 receptors results in increased NO synthesis and subsequent vasodilation.84
Impaired autonomic function resulting in vasoconstriction is associated to CMD after AMI and/or coronary reperfusion procedures. In this regards, an early study showed that left ventricular dysfunction (secondary to transient PCI-induced ischaemia) accompanied by increased coronary resistance and diffuse vasoconstriction was abolished by α-blockers, supporting the hypothesis that neural mechanisms elicit microvascular dysfunction.85
7. Assessment of the coronary microcirculation
Adequate methods for direct visualization in vivo of the coronary microcirculation both in human and animal are presently lacking. Assessment of CMD is performed with different diagnostic modalities, including non-invasive (positron emission tomography, myocardial contrast echocardiography, cardiac computed tomography, and cardiac magnetic resonance) and invasive (coronary angiography, Doppler-flow wire, CFR, and index of microvascular resistance) techniques, each one carrying specific strengths and weaknesses86,87 (see Supplementary material online, Table S3 for specifications).
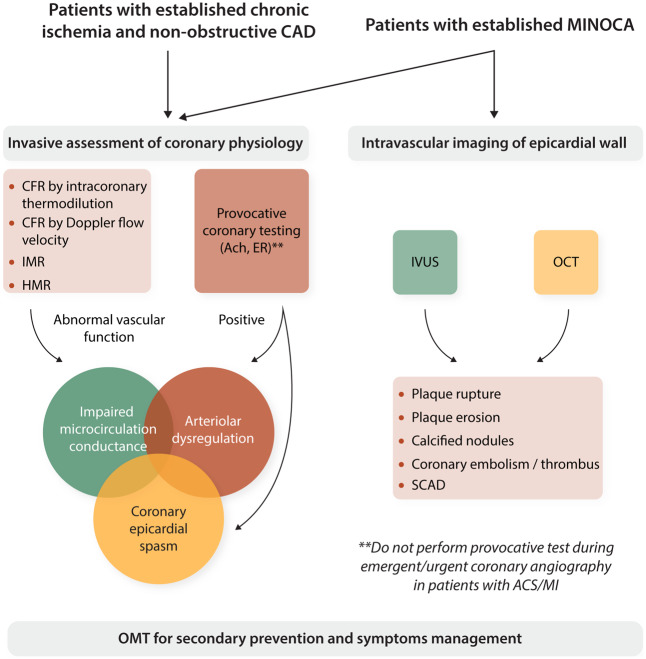
The ESC has proposed an assessment procedure to reach a more precise identification and diagnosis of microvascular angina (Figure 5). In fact, preliminary data show that impaired microcirculatory conductance and arteriolar dysregulation may benefit from different treatments.1
Figure 5.
Invasive assessment of coronary physiology and intravascular imaging for patients with non-obstructive CAD. Ach, acetylcholine; ACS, acute coronary syndromes; CAD, coronary artery disease; CFR, coronary flow reserve; ER, ergonovine; HMR, hyperaemic microvascular resistance index; IMR, index of microcirculatory resistance; IVUS, intravascular ultrasounds; MI, myocardial infarction; MINOCA, myocardial infarction with non-obstructed coronary arteries; OCT, optical coherence tomography; OMT, optimal medical therapy; SCAD, spontaneous coronary artery dissection. **Contraindications to provocative coronary testing include emergent coronary angiography in patients with ACS/MI, pregnancy, severe hypertension, severe left ventricular dysfunction, heart failure (New York Heart Association Class III or IV), moderate/severe aortic stenosis, left main coronary stenosis >50%, multivessel CAD, spontaneous spasm during angiography; uncontrolled ventricular arrhythmia, renal insufficiency, severe bronchial asthma; use of isosorbide dinitrate (shortly before).
Similarly, assessment of CMD in animal models basically include in vivo non-invasive imaging, invasive catheter based physiological coronary testing and ex vivo investigation based on contemporary histology. Haemodynamic indexes, such CFR, IMR, and zero-flow pressure, have been proved to be the most reliable to assess coronary microcirculation in swine, a good preclinical model of heart disease.88
8. Coronary microvascular ischaemia: the size of the problem
In the last two decades, with the increasing widespread use of imaging techniques for assessing myocardial perfusion, there has been a growing awareness of the fact that many patients suffer myocardial ischaemia and MI , without any atherosclerotic coronary lesion limiting blood flow.
In the literature, the rate of such patients varies widely, since it depends on the definition used for ‘non-obstructive CAD ’. Some analyses refer to smooth coronary arteries or coronary arteries with minimal lumen irregularities (<20% lumen stenosis),10,89 others (most studies) to the absence of a significant (≥50%) coronary artery lumen stenosis,90–93 while others to the absence of any severe (>70%) lumen stenosis.94,95 Moreover, the estimated prevalence is influenced by gender, clinical syndrome (stable or acute), ethnicity, and other characteristics of the study population, for example veterans with respect to the general population.94,95
INOCA: The rate of patients with lumen stenosis <50% in any major coronary arteries, which is the most common definition for INOCA, is approximately 47% in women (from 34% to 65%) and 30% in men (from 14% to 36%).10,89,96Supplementary material online, Table S1 shows data of 10 large cohort studies with angiograms of more than 530 000 patients. One out of two women with chest pain of suspected cardiac origin has non-obstructive CAD compared with one out of three men. However, the concept of INOCA as a woman’s disease is an oversimplification, since the absolute number of women and men with non-obstructive CAD is similar.
MINOCA and acute coronary syndromes: Compared with chronic coronary syndrome, a lower but still important percentage of patients with non-obstructive CAD is documented in ACS.30,90–93,97Supplementary material online, Table S2 reports data of 30 international cohorts. The incidence of non-obstructive CAD, defined by lumen stenosis <50% in any major coronary artery, ranges from 2.2% to 21.8%, being influenced by changes in the definition of AMI over the time (i.e. incorporation of high-sensitivity troponins) and by the clinical context: MINOCA, STEMI, non-STEMI, non ST-elevation ACS, or unstable angina. The incidence is generally lower in STEMI (3%, range 2.2–11.6%) than in NSTEMI (10%, range 8.1–17%) or non ST-elevation ACS (10%, range 9.2–12.1%). MINOCA has a mean rate of 8% (range 2.9–13.8%), with most of the patients NSTEMI. The disease affects both sexes, but women appear more vulnerable and the rate of women with MINOCA (or other ACSs) is from 2 to 3 times higher than that of men.
No-reflow phenomenon: This is a relatively common complication, especially in the setting of STEMI.41,98–100 The size of the problem depends on its definition. Based on the use of thrombolysis in myocardial infarction (TIMI) grade flow, the occurrence seems to be restricted to less than 5% of the population undergoing primary angioplasty. However, evaluation of myocardial rather than merely epicardial perfusion reveals a much higher prevalence. Thus, despite optimal restoration of TIMI 3 grade blood flow, suboptimal reperfusion is still observed in 20–40% of the STEMI population.100 Factors associated with impaired reperfusion —despite optimal epicardial reperfusion are advanced age, diabetes, late presentation (>4 h), preprocedural low TIMI flow, and advanced Killip class at presentation.101–106 Recently, it has been shown that there is a significant sex difference in post-PCI slow flow, being women more affected than men.37
8.1 Prognostic relevance of microvascular ischaemia
For a long time, ischaemia with non-obstructive CAD was considered a clinical condition affecting almost exclusively women and not associated with any serious risk of adverse cardiac events. However, several studies published in the early 2000s showed controversial results.107,108 Al Suwaidi et al.108 reported 4.8% of death, 2.4% of AMI, and 14% of coronary revascularization during 2 years of observation, in 42 stable patients with coronary lumen stenosis <40% and severe endothelial dysfunction. Subsequent analysis of a large cohort of patients with ACS and <50% lumen stenosis, revealing a rate >12% for major cardiovascular events (death, AMI, stroke, revascularization or severe angina) at 1-year , 107 focused attention on the unfavourable prognosis of these patients (women and men) and stimulated further studies in the acute and stable clinical context.
INOCA: The Women’s Ischemia Syndrome Evaluation study (WISE) was designed to assess the outcome of women undergoing cardiac catheterization for suspected angina. A major strength of the study was the use of a blinded core-lab for evaluating angiograms by quantitative coronary analysis; a weakness was the enrolment of women only. Sharaf et al.109 observed that cardiovascular death or AMI at 10 years had occurred in 6.7%, 12.8%, and 25.9% of women with no, non-obstructive (>20% but <50% lumen stenosis), and obstructive CAD, respectively. Data from a Danish cohort of 11 223 patients with stable angina also showed an association between non-obstructive CAD and major adverse cardiovascular events. This study found a 1.52-fold and 1.85-fold increased risk of major adverse cardiac events for patients with normal coronary arteries and non-obstructive CAD, respectively, compared to healthy individuals.10 Furthermore, an increase in risk—regardless of sex—was observed in association with the degree of CAD (normal, non-obstructive and obstructive). Reported mortality rates from 2.4% at 2.5 years , 10 to 5.3% at 6.5 years.110
MINOCA: Several studies reported that the outcome of AMI patients with non-obstructive CAD was not favourable as previously thought. Thus, in-hospital death occurs in up to 2% of MINOCA patients , 30,92,111 while at 1-year mortality ranges from 3.1% to 6.4%,92,112 rising to 10.9% at 5-year.92 Data from the literature also indicate that during hospital stay, patients with MINOCA often experience left ventricular failure , 93,111,113 while major cardiovascular events (death, AMI, hospital readmission, revascularization, or stroke) occur in 12–14% of these patients at 6–12 months.90,107
Vasospastic angina: Perfusion-imaging studies provide evidence that patients with chest pain may actually have reduced CFR in the absence of flow-limiting coronary stenoses. However, impaired CFR does not necessarily imply endothelial vascular dysfunction because the defect can reside in endothelium-independent responses. Abnormalities in coronary microvascular responses to adenosine (which is principally endothelium-independent) do not appear to be predictive of adverse outcomes. Conversely, when impaired CFR is accompanied by coronary endothelial dysfunction, as assessed by acetylcholine testing, it predicts an unfavourable outcome. A number of studies have addressed the long-term prognostic value of endothelial function testing in patients with non-obstructive CAD and demonstrate that endothelial dysfunction is associated with significantly more adverse cardiovascular events.66,108,114 An investigation of 42 women demonstrated that 30% of those women with chest pain, ‘normal ’ angiograms, and severe endothelial dysfunction developed CAD during a 10-year follow-up.67 Another study in 163 patients with ‘normal ’ coronary angiography and abnormal endothelial function showed an overall event rate of 14% at 48 months. Outcome data included increased rates of cardiovascular death (10% of adverse events), AMI, congestive heart failure, or stroke (21% of adverse events), and angina, revascularization, or other vascular events (69% of adverse events).114 Loss of endothelium-dependent vasodilation in response to acetylcholine is an early sign of vascular injury ultimately leading to atherosclerosis, and may thus account for the prognostic value of acetylcholine testing.
No-reflow phenomenon: Several studies —using different techniques —have evaluated the prognostic impact of myocardial no-reflow (below TIMI 3 flow), demonstrating an impact on cardiac remodelling and survival.37,115–119 Among 1548 STEMI patients, impaired reperfusion was associated with a three-fold increase in the relative risk of mortality at 1- year follow-up99 and similar results were observed at 5 years of follow-up.116 The prognostic impact of impaired reperfusion on survival and left ventricular remodelling has been demonstrated using myocardial contrast echocardiography (MCE) 117 or cardiac magnetic resonance (CMR).118
8.2 Preventive and conventional strategies
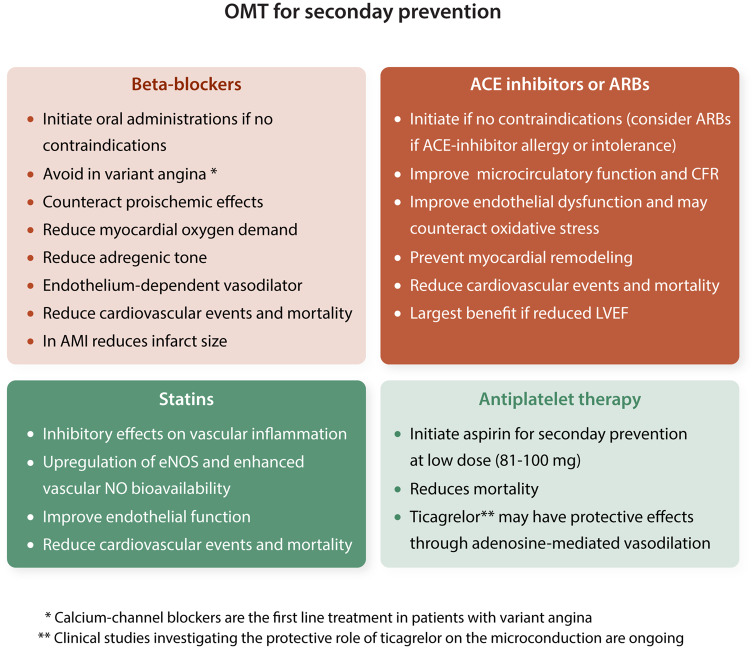
To date, no randomized trials comparing therapies for the reduction of adverse cardiac events in patients with angina and ‘normal ’ coronary arteries have been conducted, and available adverse outcome data are limited to cohort studies. However, the presence of non-obstructive CAD at coronary angiography is increasingly recognized as pathological. Lifestyle changes and risk factor management should be considered essential components of any therapeutic approach for patients with traditional cardiac risk factors, with or without evidence of coronary atherosclerosis. For patients with cardiac chest pain and evidence of ischaemia by perfusion testing, beta-blockers may reduce myocardial oxygen consumption and symptoms. Aggressive therapy with statins and angiotensin-converting enzyme (ACE) inhibitors should be used for patients who qualify for this treatment by the presence of cardiac risk factors and have evidence of atherosclerosis or evidence of endothelial dysfunction (Figure 6).
Figure 6.
Secondary prevention strategies in patients with microvascular dysfunction or MINOCA. (i) Contraindications to beta-blockers include decompensated heart failure, symptomatic bradycardia and atrioventricular blocks, hypotension, and asthma acute bronchospasm. In case of AMI all the aforementioned with the inclusion of Killip Classes III (acute pulmonary oedema) and IV (cardiogenic shock). (ii) Contraindications to ACE inhibitors or ARBs include acute and worsening renal failure, advanced chronic kidney disease, bilateral renal artery stenosis, hyperkaliaemia, hypotension, history of angio-oedema and hereditary or idiopathic angio-oedema, and pregnancy due to teratogenicity. AMI, acute myocardial infarction; LVEF, left ventricular ejection fraction; MINOCA, myocardial infarction with non-obstructive coronary arteries; OMT, optimal medical therapy. aCalcium-channel blockers are the first line treatment in patients with varian angina. bClinical studies investigating the protective role of ticagrelor on the microconduction are ongoing.
Research into novel treatments for CMD is an unmet clinical need. One novel strategy, not yet proven, has been to inhibit Rho-kinase in order to ameliorate CMD and vasospastic angina.120 In addition, therapeutic targeting of perivascular adipose tissue to stimulate the production of vasoactive, vasorelaxing factors such as adiponectin121 or hydrogen sulphide122 could be of benefit.
Platelet inhibitors: Data on the use of platelet inhibitors including aspirin for the treatment of CMD are insufficiently established to provide clinical recommendations. Nonetheless, in patients referred for CAD diagnostic assessment, documentation of CMD often justifies the use of aspirin since typically these patients have also non-obstructive CAD.123 Ticagrelor, may protect the microcirculation through its adenosine-mediated vasodilator effects.44,45 Clinical studies investigating its protective role on the microcirculation are ongoing.
ACE inhibitors: Inhibition of the renin-angiotensin axis elicits beneficial vasoprotective effects by improving microcirculatory function and CFR .124 ACE inhibitors and statin improve endothelial dysfunction, may counteract oxidative stress, and may be of benefit in patients with CMD.125 Data from EMMACE-2 registry91 showed that ACE inhibitor therapy was associated with reduced 6-month incidence of mortality in patients presenting with ACS and non-obstructive CAD. This study however, has limitations, including its observational nature, lack of randomization, short duration of follow-up, and inability to assess MI , heart failure, and stroke as outcomes.
Statins: Beyond reducing cholesterol levels, statins have inhibitory effects on vascular inflammation, up-regulate eNOS and enhance vascular NO bioavailability. In patients with normal angiograms and inducible myocardial ischaemia several small randomized trials and case–control studies have shown positive effects of statins on exercise tolerance, exercise-induced reversible perfusion defects , 125–128 endothelial function , 125–127 and quality of life.125 In the setting of ACS, statin therapy prior to revascularization improves coronary microvascular perfusion in patients with and without STEMI, as assessed by contrast echocardiography in a 30 days of follow-up period.129 The analysis of eight TIMI trials reported lower rate of death or reinfarction at 30 days in patients with NSTE-ACS and non-obstructive CAD on statin treatment.130 Moreover, during long-term (>4 years) follow-up, data from SWEDEHEART, which enrolled 9136 patients with MINOCA surviving at least 30 days after the index event, confirmed the beneficial effects of statins on major cardiovascular events (all-cause mortality, MI , ischaemic stroke, and heart failure).97
Beta-blockers: Beta-blockers have been shown to be highly effective for reduction of chest pain episodes during daily life. There are several potential mechanisms by which beta-blockers may act in reducing chest pain recurrences as reducing myocardial oxygen demand and inducing endothelium-dependent vasodilation.131 Exercise training, which increase parasympathetic activity, has shown to be of benefit, indicating the impact of adrenergic modulation.132 In patients with variant angina beta-blockers should be avoided; whereas calcium-channel blockers are the first line treatment.
Nitrates: Nitrates are effective in inducing vasodilation and relieve angina symptoms, but without consistent findings for patients with non-obstructive CAD.133
L-arginine: Long-term, 6-month supplementation of L-arginine, the precursor of NO, improved endothelial function, coronary blood flow, and symptoms in patients with non-obstructive CAD, but not CFR.134
9. Concluding remarks
CMD is an intricate part of ischaemic heart disease. It arises from changes in both microvascular function and structure and is most strongly associated with endothelial dysfunction. The dominant underlying functional mechanisms include a loss of NO bioavailability and increased ET-1 vasoconstrictor tone. The structural mechanisms identified to date include inward arteriolar remodelling and vascular rarefaction. Together these increase minimal coronary vascular resistance and reduce maximum myocardial perfusion. As a result, CFR decreases promoting myocardial ischaemia during increases in myocardial oxygen demand. With increasing sophistication to assess coronary physiology in humans, it has become apparent that CMD is highly prevalent and developing therapeutic interventions to reverse functional abnormalities in coronary resistance vessel control could become an important approach to treat ischaemic heart disease.
10. Recommendations and future perspectives
Both structural and functional defects contribute and interact to cause a progressive impairment of coronary microvascular blood flow. There is a need to clarify the relative impact of each one in the diverse forms of presentation of CMD.
There are no therapeutic strategies focused on specifically treating CMD and the microvasculature. There is an urgent need to identify novel and specific targets for therapy.
There is a need to better understand the anatomic and physiologic features that predispose a higher burden of microvascular dysfunction in women.
There is a need of new research to improve CMD assessment and the diagnostic methodologies now available.
The management of patients with non-obstructive CAD and ACS or inducible myocardial ischaemia does not currently have a consensus strategy. These patients should be studied for the evaluation of endothelial and CMD , since they may influence outcome.
Clinical studies and double-blind randomized clinical trials in patients with non-obstructive disease specifically designed to assess the effect of conventional and novel anti-ischaemic therapies are required.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the Spanish Ministry of Economy and Competitiveness of Science [PNS2016-76819-R] to L.B., Institute of Health Carlos III, ISCIII [FIS PI16/01915] to T.P. and Red Terapia Celular TerCel- [RD16/0011/0018] cofounded by FEDER 'Una Manera de Hacer Europa' to L.B. Secretaria d'Universitats i Recerca del Departament d'Empresa i Coneixement de la Generalitat de Catalunya [2017 SGR 1480] to L.B. Grant from the Netherlands Cardiovascular Research Initiative (CVON2014-11), an initiative with financial support from the Dutch Heart Foundation to D.J.D. Scientific Excellence Program 2019. National Research, Development and Innovation Fund, OTKA K 132596 to A.K. and Scientific Excellence Program at the University of Physical Education, Innov. and Tech. Ministry, Hungary [TUDFO/51757/2019-ITM] to A.K.
Supplementary Material
References
- 1. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, Neumann F-J, Sechtem U, Banning AP, Bonaros N, Bueno H, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407-477. [DOI] [PubMed] [Google Scholar]
- 2. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 3. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 4. Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio ALP, De Caterina R, Zimarino M, Roffi M, Kjeldsen K, Atar D, Kaski JC, Sechtem U, Tornvall P; WG on Cardiovascular Pharmacotherapy. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017;38:143–153. [DOI] [PubMed] [Google Scholar]
- 5. Crea F, Camici PG, Bairey Merz CN.. Coronary microvascular dysfunction: an update. Eur Heart J 2014;35:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bugiardini R, Bairey Merz CN.. Angina with “normal” coronary arteries: a changing philosophy. JAMA 2005;293:477–484. [DOI] [PubMed] [Google Scholar]
- 7. Herscovici R, Sedlak T, Wei J, Pepine CJ, Handberg E, Bairey Merz CN.. Ischemia and no obstructive coronary artery disease (INOCA): what is the risk? J Am Heart Assoc 2018;7:e008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levy BI, Heusch G, Camici PG.. The many faces of myocardial ischaemia and angina. Cardiovasc Res 2019;115:1460–1470. [DOI] [PubMed] [Google Scholar]
- 9. Bradley SM, Maddox TM, Stanislawski MA, O’Donnell CI, Grunwald GK, Tsai TT, Ho PM, Peterson ED, Rumsfeld JS.. Normal coronary rates for elective angiography in the Veterans Affairs Healthcare System: insights from the VA CART program (veterans affairs clinical assessment reporting and tracking). J Am Coll Cardiol 2014;63:417–426. [DOI] [PubMed] [Google Scholar]
- 10. Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E.. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 11. Badimon L, Bugiardini R, Cenko E, Cubedo J, Dorobantu M, Duncker DJ, Estruch R, Milicic D, Tousoulis D, Vasiljevic Z, Vilahur G, de Wit C, Koller A.. Position paper of the European Society of Cardiology-working group of coronary pathophysiology and microcirculation: obesity and heart disease. Eur Heart J 2017;38:1951–1958. [DOI] [PubMed] [Google Scholar]
- 12. Bender SB, de Beer V, Tharp DL, Bowles DK, Laughlin MH, Merkus D, Duncker DJ.. Severe familial hypercholesterolemia impairs the regulation of coronary blood flow and oxygen supply during exercise. Basic Res Cardiol 2016;111:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berwick ZC, Dick GM, Tune JD.. Heart of the matter: coronary dysfunction in metabolic syndrome. J Mol Cell Cardiol 2012;52:848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ford TJ, Rocchiccioli P, Good R, McEntegart M, Eteiba H, Watkins S, Shaukat A, Lindsay M, Robertson K, Hood S, Yii E, Sidik N, Harvey A, Montezano AC, Beattie E, Haddow L, Oldroyd KG, Touyz RM, Berry C.. Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur Heart J 2018;39:4086–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grassi G, Seravalle G, Quarti-Trevano F, Scopelliti F, Dell’Oro R, Bolla G, Mancia G.. Excessive sympathetic activation in heart failure with obesity and metabolic syndrome: characteristics and mechanisms. Hypertension 2007;49:535–541. [DOI] [PubMed] [Google Scholar]
- 16. Kachur S, Morera R, Schutter AD, Lavie CJ.. Cardiovascular risk in patients with prehypertension and the metabolic syndrome. Curr Hypertens Rep 2018;20:15. [DOI] [PubMed] [Google Scholar]
- 17. Bagi Z, Feher A, Cassuto J.. Microvascular responsiveness in obesity: implications for therapeutic intervention. Br J Pharmacol 2012;165:544–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Z-L, Woollard JR, Ebrahimi B, Crane JA, Jordan KL, Lerman A, Wang S-M, Lerman LO.. Transition from obesity to metabolic syndrome is associated with altered myocardial autophagy and apoptosis. Arterioscler Thromb Vasc Biol 2012;32:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trask AJ, Katz PS, Kelly AP, Galantowicz ML, Cismowski MJ, West TA, Neeb ZP, Berwick ZC, Goodwill AG, Alloosh M, Tune JD, Sturek M, Lucchesi PA.. Dynamic micro- and macrovascular remodeling in coronary circulation of obese Ossabaw pigs with metabolic syndrome. J Appl Physiol 2012;113:1128–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duncker DJ, Koller A, Merkus D, Canty JM.. Regulation of coronary blood flow in health and ischemic heart disease. Prog Cardiovasc Dis 2015;57:409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson NP, Kirkeeide RL, Gould KL.. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc Imaging 2012;5:193–202. [DOI] [PubMed] [Google Scholar]
- 22. Shimoni S, Frangogiannis NG, Aggeli CJ, Shan K, Quinones MA, Espada R, Letsou GV, Lawrie GM, Winters WL, Reardon MJ, Zoghbi WA.. Microvascular structural correlates of myocardial contrast echocardiography in patients with coronary artery disease and left ventricular dysfunction. Circulation 2002;106:950–956. [DOI] [PubMed] [Google Scholar]
- 23. Kelly RF, Cabrera JA, Ziemba EA, Crampton M, Anderson LB, McFalls EO, Ward HB.. Continued depression of maximal oxygen consumption and mitochondrial proteomic expression despite successful coronary artery bypass grafting in a swine model of hibernation. J Thorac Cardiovasc Surg 2011;141:261–268. [DOI] [PubMed] [Google Scholar]
- 24. Vilahur G, Oñate B, Cubedo J, Béjar MT, Arderiu G, Peña E, Casaní L, Gutiérrez M, Capdevila A, Pons-Lladó G, Carreras F, Hidalgo A, Badimon L.. Allogenic adipose-derived stem cell therapy overcomes ischemia-induced microvessel rarefaction in the myocardium: systems biology study. Stem Cell Res Ther 2017;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heusch G, Deussen A.. The effects of cardiac sympathetic nerve stimulation on perfusion of stenotic coronary arteries in the dog. Circ Res 1983;53:8–15. [DOI] [PubMed] [Google Scholar]
- 26. Sorop O, Merkus D, de Beer VJ, Houweling B, Pistea A, McFalls EO, Boomsma F, van Beusekom HM, van der Giessen WJ, VanBavel E, Duncker DJ.. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circ Res 2008;102:795–803. [DOI] [PubMed] [Google Scholar]
- 27. Beyer AM, Zinkevich N, Miller B, Liu Y, Wittenburg AL, Mitchell M, Galdieri R, Sorokin A, Gutterman DD.. Transition in the mechanism of flow-mediated dilation with aging and development of coronary artery disease. Basic Res Cardiol 2017;112:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van den Akker J, Schoorl MJ, Bakker EN, Vanbavel E.. Small artery remodeling: current concepts and questions. J Vasc Res 2010;47:183–202. [DOI] [PubMed] [Google Scholar]
- 29. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF.. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015;131:861–870. [DOI] [PubMed] [Google Scholar]
- 30. Smilowitz NR, Mahajan AM, Roe MT, Hellkamp AS, Chiswell K, Gulati M, Reynolds HR.. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines). Circ Cardiovasc Qual Outcomes 2017;10:e003443. [DOI] [PubMed] [Google Scholar]
- 31. Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, Reynolds HR, Geda M, Bueno H, Dziura JD, Krumholz HM, D'Onofrio G.. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO Study. J Am Heart Assoc 2018;7:e009174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Circulation 2018;138:e618–e651. [DOI] [PubMed] [Google Scholar]
- 33. Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Cammà G, Lanza GA, Crea F.. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J 2018;39:91–98. [DOI] [PubMed] [Google Scholar]
- 34. De Vita A, Manfredonia L, Lamendola P, Villano A, Ravenna SE, Bisignani A, Niccoli G, Lanza GA, Crea F.. Coronary microvascular dysfunction in patients with acute coronary syndrome and no obstructive coronary artery disease. Clin Res Cardiol 2019;108:1364-1370. [DOI] [PubMed] [Google Scholar]
- 35. Lerman A, Holmes DR, Herrmann J, Gersh BJ.. Microcirculatory dysfunction in ST-elevation myocardial infarction: cause, consequence, or both? Eur Heart J 2007;28:788–797. [DOI] [PubMed] [Google Scholar]
- 36. Krug A, Mesnil de Rochemont D, Korb G.. Blood supply of the myocardium after temporary coronary occlusion. Circ Res 1966;19:57–62. [DOI] [PubMed] [Google Scholar]
- 37. Cenko E, van der Schaar M, Yoon J, Kedev S, Valvukis M, Vasiljevic Z, Ašanin M, Miličić D, Manfrini O, Badimon L, Bugiardini R.. Sex-specific treatment effects after primary percutaneous intervention: a study on coronary blood flow and delay to hospital presentation. J Am Heart Assoc 2019;8:e011190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jaffe R, Charron T, Puley G, Dick A, Strauss BH.. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 2008;117:3152–3156. [DOI] [PubMed] [Google Scholar]
- 39. Kali A, Cokic I, Tang R, Dohnalkova A, Kovarik L, Yang H-J, Kumar A, Prato FS, Wood JC, Underhill D, Marbán E, Dharmakumar R.. Persistent microvascular obstruction after myocardial infarction culminates in the confluence of ferric iron oxide crystals, proinflammatory burden, and adverse remodeling. Circ Cardiovasc Imaging 2016;9:e004996 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Waha S, Patel MR, Granger CB, Ohman EM, Maehara A, Eitel I, Ben-Yehuda O, Jenkins P, Thiele H, Stone GW.. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur Heart J 2017;38:3502–3510. [DOI] [PubMed] [Google Scholar]
- 41. Cenko E, Ricci B, Kedev S, Kalpak O, Câlmâc L, Vasiljevic Z, Knežević B, Dilic M, Miličić D, Manfrini O, Koller A, Dorobantu M, Badimon L, Bugiardini R.. The no-reflow phenomenon in the young and in the elderly. Int J Cardiol 2016;222:1122–1128. [DOI] [PubMed] [Google Scholar]
- 42. de Waard GA, Hollander MR, Teunissen PF, Jansen MF, Eerenberg ES, Beek AM, Marques KM, van de Ven PM, Garrelds IM, Danser AH, Duncker DJ, van Royen N.. Changes in coronary blood flow after acute myocardial infarction: insights from a patient study and an experimental porcine model. JACC Cardiovasc Interv 2016;9:602–613. [DOI] [PubMed] [Google Scholar]
- 43. Kloner RA, King KS, Harrington MG.. No-reflow phenomenon in the heart and brain. Am J Physiol Heart Circ Physiol 2018;315:H550–H562. [DOI] [PubMed] [Google Scholar]
- 44. Vilahur G, Gutiérrez M, Casani L, Varela L, Capdevila A, Pons-Lladó G, Carreras F, Carlsson L, Hidalgo A, Badimon L.. Protective effects of ticagrelor on myocardial injury after infarction. Circulation 2016;134:1708–1719. [DOI] [PubMed] [Google Scholar]
- 45. Vilahur G, Gutiérrez M, Casani L, Lambert C, Mendieta G, Ben-Aicha S, Capdevila A, Pons-Lladó G, Carreras F, Carlsson L, Hidalgo A, Badimon L.. P2Y12 antagonists and cardiac repair post-myocardial infarction: global and regional heart function analysis and molecular assessments in pigs. Cardiovasc Res 2018;114:1860–1870. [DOI] [PubMed] [Google Scholar]
- 46. Hess CN, Kaltenbach LA, Doll JA, Cohen DJ, Peterson ED, Wang TY.. Race and sex differences in post-myocardial infarction angina frequency and risk of 1-year unplanned rehospitalization. Circulation 2017;135:532–543. [DOI] [PubMed] [Google Scholar]
- 47. Maria GD, Cuculi F, Patel N, Dawkins S, Fahrni G, Kassimis G, Choudhury RP, Forfar JC, Prendergast BD, Channon KM, Kharbanda RK, Banning AP.. How does coronary stent implantation impact on the status of the microcirculation during primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction? Eur Heart J 2015;36:3165–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Borlotti A, Jerosch-Herold M, Liu D, Viliani D, Bracco A, Alkhalil M, Maria GD OxAMI Study Investigators OSChannon KM, Banning AP, Choudhury RP, Neubauer S, Kharbanda RK, Dall’Armellina E.. Acute microvascular impairment post-reperfused STEMI is reversible and has additional clinical predictive value: a CMR OxAMI study. JACC Cardiovasc Imaging 2019;12:1783–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Niccoli G, Montone RA, Lanza GA, Crea F.. Angina after percutaneous coronary intervention: the need for precision medicine. Int J Cardiol 2017;248:14–19. [DOI] [PubMed] [Google Scholar]
- 50. Heusch G. Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res Cardiol 2019;114:45. [DOI] [PubMed] [Google Scholar]
- 51. Wasserman DH, Wang TJ, Brown NJ.. The vasculature in prediabetes. Circ Res 2018;122:1135–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hinkel R, Howe A, Renner S, Ng J, Lee S, Klett K, Kaczmarek V, Moretti A, Laugwitz K-L, Skroblin P, Mayr M, Milting H, Dendorfer A, Reichart B, Wolf E, Kupatt C.. Diabetes mellitus–induced microvascular destabilization in the myocardium. J Am Coll Cardiol 2017;69:131–143. [DOI] [PubMed] [Google Scholar]
- 53. Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y.. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003;108:2460–2466. [DOI] [PubMed] [Google Scholar]
- 54. Triantafyllou A, Anyfanti P, Triantafyllou G, Zabulis X, Aslanidis S, Douma S.. Impaired metabolic profile is a predictor of capillary rarefaction in a population of hypertensive and normotensive individuals. J Am Soc Hypertens 2016;10:640–646. [DOI] [PubMed] [Google Scholar]
- 55. Shah NR, Charytan DM, Murthy VL, Skali Lami H, Veeranna V, Cheezum MK, Taqueti VR, Kato T, Foster CR, Hainer J, Gaber M, Klein J, Dorbala S, Blankstein R, Carli MD.. Prognostic value of coronary flow reserve in patients with dialysis-dependent ESRD. J Am Soc Nephrol 2016;27:1823–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bagi Z, Feher A, Cassuto J, Akula K, Labinskyy N, Kaley G, Koller A.. Increased availability of angiotensin AT 1 receptors leads to sustained arterial constriction to angiotensin II in diabetes—role for Rho-kinase activation. Br J Pharmacol 2011;163:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alonso R, Mata P, De Andres R, Villacastin BP, Martı´nez-González J, Badimon L.. Sustained long-term improvement of arterial endothelial function in heterozygous familial hypercholesterolemia patients treated with simvastatin. Atherosclerosis 2001;157:423–429. [DOI] [PubMed] [Google Scholar]
- 58. Reindl M, Reinstadler SJ, Feistritzer H-J, Theurl M, Basic D, Eigler C, Holzknecht M, Mair J, Mayr A, Klug G, Metzler B.. Relation of low-density lipoprotein cholesterol with microvascular injury and clinical outcome in revascularized ST-elevation myocardial infarction. J Am Heart Assoc 2017;6: e006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hein TW, Liao JC, Kuo L.. ox LDL specifically impairs endothelium-dependent, NO-mediated dilation of coronary arterioles. Am J Physiol Circ Physiol 2000;278:H175–H183. [DOI] [PubMed] [Google Scholar]
- 60. Ricci B, Cenko E, Vasiljevic Z, Stankovic G, Kedev S, Kalpak O, Vavlukis M, Zdravkovic M, Hinic S, Milicic D, Manfrini O, Badimon L, Bugiardini R.. Acute Coronary Syndrome: the Risk to Young Women. J Am Heart Assoc 2017;6:e007519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cenko E, Yoon J, Kedev S, Stankovic G, Vasiljevic Z, Krljanac G, Kalpak O, Ricci B, Milicic D, Manfrini O, M van der S, Badimon L, Bugiardini R.. Sex differences in outcomes after STEMI: effect modification by treatment strategy and age. JAMA Intern Med 2018;178:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Koller A. Perspectives: microvascular endothelial dysfunction and gender. Eur Heart J Suppl 2014;16:A16–A19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vaccarino V, Badimon L, Corti R, de Wit C, Dorobantu M, Hall A, Koller A, Marzilli M, Pries A, Bugiardini R; Working Group on Coronary Pathophysiology and Microcirculation. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc Res 2011;90:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maseri A, Severi S, Nes MD, L’Abbate A, Chierchia S, Marzilli M, Ballestra AM, Parodi O, Biagini A, Distante A.. “Variant” angina: one aspect of a continuous spectrum of vasospastic myocardial ischemia. Pathogenetic mechanisms, estimated incidence and clinical and coronary arteriographic findings in 138 patients. Am J Cardiol 1978;42:1019–1035. [DOI] [PubMed] [Google Scholar]
- 65. Bugiardini R, Pozzati A, Ottani F, Morgagni GL, Puddu P.. Vasotonic angina: a spectrum of ischemic syndromes involving functional abnormalities of the epicardial and microvascular coronary circulation. J Am Coll Cardiol 1993;22:417–425. [DOI] [PubMed] [Google Scholar]
- 66. Halcox JPJ, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KRA, Quyyumi AA.. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002;106:653–658. [DOI] [PubMed] [Google Scholar]
- 67. Bugiardini R, Manfrini O, Pizzi C, Fontana F, Morgagni G.. Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms. Circulation 2004;109:2518–2523. [DOI] [PubMed] [Google Scholar]
- 68. Szekeres M, Nádasy GL, Dörnyei G, Szénási A, Koller A.. Remodeling of wall mechanics and the myogenic mechanism of rat intramural coronary arterioles in response to a short-term daily exercise program: role of endothelial factors. J Vasc Res 2018;55:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Siasos G, Tsigkou V, Zaromytidou M, Sara JD, Varshney A, Coskun AU, Lerman A, Stone PH.. Role of local coronary blood flow patterns and shear stress on the development of microvascular and epicardial endothelial dysfunction and coronary plaque. Curr Opin Cardiol 2018;33:638–644. [DOI] [PubMed] [Google Scholar]
- 70. Hodges GJ, Stewart DG, Davison PJ, Cheung SS.. The role of shear stress on cutaneous microvascular endothelial function in humans. Eur J Appl Physiol 2017;117:2457–2468. [DOI] [PubMed] [Google Scholar]
- 71. Mundi S, Massaro M, Scoditti E, Carluccio MA, van Hinsbergh VWM, Iruela-Arispe ML, De Caterina R.. Endothelial permeability, LDL deposition, and cardiovascular risk factors—a review. Cardiovasc Res 2018;114:35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Förstermann U, Xia N, Li H.. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res 2017;120:713–735. [DOI] [PubMed] [Google Scholar]
- 73. Osto E, Matter CM, Kouroedov A, Malinski T, Bachschmid M, Camici GG, Kilic U, Stallmach T, Boren J, Iliceto S, LüScher TF, Cosentino F.. c-Jun N-terminal kinase 2 deficiency protects against hypercholesterolemia-induced endothelial dysfunction and oxidative stress. Circulation 2008;118:2073–2080. [DOI] [PubMed] [Google Scholar]
- 74. Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM.. The human microcirculation. Circ Res 2016;118:157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Niccoli G, Scalone G, Lerman A, Crea F.. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J 2016;37:1024–1033. [DOI] [PubMed] [Google Scholar]
- 76. Stokes KY, Granger DN.. Platelets: a critical link between inflammation and microvascular dysfunction. J Physiol 2012;590:1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tailor A, Cooper D, Granger DN.. Platelet–vessel wall interactions in the microcirculation. Microcirculation 2005;12:275–285. [DOI] [PubMed] [Google Scholar]
- 78. Gavins FNE, Li G, Russell J, Perretti M, Granger DN.. Microvascular thrombosis and CD40/CD40L signaling. J Thromb Haemost 2011;9:574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ge L, Zhou X, Ji W-J, Lu R-Y, Zhang Y, Zhang Y-D, Ma Y-Q, Zhao J-H, Li Y-M.. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: therapeutic potential of DNase-based reperfusion strategy. Am J Physiol Heart Circ Physiol 2015;308:H500–H509. [DOI] [PubMed] [Google Scholar]
- 80. Pries AR, Badimon L, Bugiardini R, Camici PG, Dorobantu M, Duncker DJ, Escaned J, Koller A, Piek JJ, de Wit C.. Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur Heart J 2015;36:3134–3146. [DOI] [PubMed] [Google Scholar]
- 81. Heusch G, Baumgart D, Camici P, Chilian W, Gregorini L, Hess O, Indolfi C, Rimoldi O.. α-adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation 2000;101:689–694. [DOI] [PubMed] [Google Scholar]
- 82. Heusch G. The paradox of α-adrenergic coronary vasoconstriction revisited. J Mol Cell Cardiol 2011;51:16–23. [DOI] [PubMed] [Google Scholar]
- 83. Yun J-S, Park Y-M, Cha S-A, Ahn Y-B, Ko S-H.. Progression of cardiovascular autonomic neuropathy and cardiovascular disease in type 2 diabetes. Cardiovasc Diabetol 2018;17:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Miyashita T, Takeishi Y, Takahashi H, Miyamoto T, Fujii S, Yoshimura T, Tomoike H, Kato S, Kubota I.. Comparison of nitric oxide production in response to carbachol between macrovascular and microvascular cardiac endothelial cells. Circ J 2002;66:511–511. [DOI] [PubMed] [Google Scholar]
- 85. Gregorini L, Marco J, Palombo C, Kozàkovà M, Anguissola GB, Cassagneau B, Bernies M, Distante A, Marco I, Fajadet J, Zanchetti A.. Postischemic left ventricular dysfunction is abolished by alpha-adrenergic blocking agents. J Am Coll Cardiol 1998;31:992–1001. [DOI] [PubMed] [Google Scholar]
- 86. Feher A, Sinusas AJ.. Quantitative assessment of coronary microvascular function: dynamic single-photon emission computed tomography, positron emission tomography, ultrasound, computed tomography, and magnetic resonance imaging. Circ Cardiovasc Imaging 2017;10:e006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Camici PG, d'Amati G, Rimoldi O.. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 2015;12:48–62. [DOI] [PubMed] [Google Scholar]
- 88. Zhang Z, Takarada S, Molloi S.. Assessment of coronary microcirculation in a swine animal model. Am J Physiol Heart Circ Physiol 2011;301:H402–H408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sharaf BL, Pepine CJ, Kerensky RA, Reis SE, Reichek N, Rogers WJ, Sopko G, Kelsey SF, Holubkov R, Olson M, Miele NJ, Williams DO, Merz C.. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] study angiographic core laboratory). Am J Cardiol 2001;87:937–941. [DOI] [PubMed] [Google Scholar]
- 90. Dey Sfor the GRACE investigatorsFlather MD, Devlin G, Brieger D, Gurfinkel EP, Steg PG, Fitzgerald G, Jackson EA, Eagle KA; Global Registry of Acute Coronary Events investigators. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart 2008;95:20–26. [DOI] [PubMed] [Google Scholar]
- 91. Manfrini O, Morrell C, Das R, Barth JH, Hall AS, Gale CP, Cenko E, Bugiardini R; Evaluation of Methods and Management of Acute Coronary Events Study Group. Effects of angiotensin-converting enzyme inhibitors and beta blockers on clinical outcomes in patients with and without coronary artery obstructions at angiography (from a Register-Based Cohort Study on Acute Coronary Syndromes). Am J Cardiol 2014;113:1628–1633. [DOI] [PubMed] [Google Scholar]
- 92. Bainey KR, Welsh RC, Alemayehu W, Westerhout CM, Traboulsi D, Anderson T, Brass N, Armstrong PW, Kaul P.. Population-level incidence and outcomes of myocardial infarction with non-obstructive coronary arteries (MINOCA): insights from the Alberta contemporary acute coronary syndrome patients invasive treatment strategies (COAPT) study. Int J Cardiol 2018;264:12–17. [DOI] [PubMed] [Google Scholar]
- 93. Gehrie ER, Reynolds HR, Chen AY, Neelon BH, Roe MT, Gibler WB, Ohman EM, Newby LK, Peterson ED, Hochman JS.. Characterization and outcomes of women and men with non-ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early. Am Heart J 2009;158:688–694. [DOI] [PubMed] [Google Scholar]
- 94. Shaw LJ, Shaw RE, Merz CNB, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, Block PC, Hewitt K, Weintraub WS, Peterson ED; American College of Cardiology-National Cardiovascular Data Registry Investigators. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation 2008;117:1787–1801. [DOI] [PubMed] [Google Scholar]
- 95. Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, Leon B, Bhatt DL, Fihn SD, Rumsfeld JS.. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 2014;312:1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Johnston N, Jönelid B, Christersson C, Kero T, Renlund H, Schenck-Gustafsson K, Lagerqvist B.. Effect of gender on patients with ST-elevation and non-ST-elevation myocardial infarction without obstructive coronary artery disease. Am J Cardiol 2015;115:1661. [DOI] [PubMed] [Google Scholar]
- 97. Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjöld A, Gard A, Jernberg T.. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation 2017;135:1481–1489. [DOI] [PubMed] [Google Scholar]
- 98. Gibson CM, Cannon CP, Daley WL, Dodge JT, Alexander B, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, Braunwald E.. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation 1996;93:879–888. [DOI] [PubMed] [Google Scholar]
- 99. De Luca G, van 't Hof AW, Ottervanger JP, Hoorntje JC, Gosselink AT, Dambrink JH, Zijlstra F, de Boer MJ, Suryapranata H.. Unsuccessful reperfusion in patients with ST-segment elevation myocardial infarction treated by primary angioplasty. Am Heart J 2005;150:557–562. [DOI] [PubMed] [Google Scholar]
- 100. Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, McCabe CH, Van De, Werf F, Braunwald E.. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation 2000;101:125–130. [DOI] [PubMed] [Google Scholar]
- 101. De Luca G, van 't Hof AW, de Boer MJ, Hoorntje JC, Gosselink AT, Dambrink JH, Ottervanger JP, Zijlstra F, Suryapranata H.. Impaired myocardial perfusion is a major explanation of the poor outcome observed in patients undergoing primary angioplasty for ST-segment-elevation myocardial infarction and signs of heart failure. Circulation 2004;109:958–961. [DOI] [PubMed] [Google Scholar]
- 102. De Luca G, van 't Hof AW, de Boer MJ, Ottervanger JP, Hoorntje JC, Gosselink AT, Dambrink JH, Zijlstra F, Suryapranata H.. Time-to-treatment significantly affects the extent of ST-segment resolution and myocardial blush in patients with acute myocardial infarction treated by primary angioplasty. Eur Heart J 2004;25:1009–1013. [DOI] [PubMed] [Google Scholar]
- 103. Timmer JR, van der Horst IC, de Luca G, Ottervanger JP, Hoorntje JC, de Boer MJ, Suryapranata H, Dambrink JH, Gosselink M, Zijlstra F, van 't Hof AW; Zwolle Myocardial Infarction Study Group. Comparison of myocardial perfusion after successful primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction with versus without diabetes mellitus. Am J Cardiol 2005;95:1375–1377. [DOI] [PubMed] [Google Scholar]
- 104. De Luca G, Ernst N, van 't Hof AW, Ottervanger JP, Hoorntje JC, Dambrink JH, Gosslink AT, de Boer MJ, Suryapranata H.. Preprocedural thrombolysis in myocardial infarction (TIMI) flow significantly affects the extent of ST-segment resolution and myocardial blush in patients with acute anterior myocardial infarction treated by primary angioplasty. Am Heart J 2005;150:827–831. [DOI] [PubMed] [Google Scholar]
- 105. Iwakura K, Ito H, Kawano S, Shintani Y, Yamamoto K, Kato A, Ikushima M, Tanaka K, Kitakaze M, Hori M, Higashino Y, Fujii K.. Predictive factors for development of the no-reflow phenomenon in patients with reperfused anterior wall acute myocardial infarction. J Am Coll Cardiol 2001;38:472–477. [DOI] [PubMed] [Google Scholar]
- 106. Ndrepepa G, Tiroch K, Keta D, Fusaro M, Seyfarth M, Pache J, Mehilli J, Schömig A, Kastrati A.. Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Interv 2010;3:27–33. [DOI] [PubMed] [Google Scholar]
- 107. Bugiardini R, Manfrini O, Ferrari GMDe.. Unanswered questions for management of acute coronary syndrome. Arch Intern Med 2006;166:1391. [DOI] [PubMed] [Google Scholar]
- 108. Suwaidi JAl, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A.. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000;101:948–954. [DOI] [PubMed] [Google Scholar]
- 109. Sharaf B, Wood T, Shaw L, Johnson BD, Kelsey S, Anderson RD, Pepine CJ, Bairey Merz CN.. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic core lab. Am Heart J 2013;166:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kissel CK, Chen G, Southern DA, Galbraith PD, Anderson TJ; for the APPROACH investigators. Impact of clinical presentation and presence of coronary sclerosis on long-term outcome of patients with non-obstructive coronary artery disease. BMC Cardiovasc Disord 2018;18:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. André R, Elbaz M, Simon T, Khalife K, Lim P, Ennezat P-V, Coste P, Breton HLe, Bataille V, Ferrières J, Danchin N.. Prevalence, clinical profile and 3-year survival of acute myocardial infarction patients with and without obstructive coronary lesions: the FAST-MI 2005 registry. Int J Cardiol 2014;172:e247–9. [DOI] [PubMed] [Google Scholar]
- 112. Larsen AI, Galbraith PD, Ghali WA, Norris CM, Graham MM, Knudtson ML; APPROACH Investigators. Characteristics and outcomes of patients with acute myocardial infarction and angiographically normal coronary arteries. Am J Cardiol 2005;95:261–263. [DOI] [PubMed] [Google Scholar]
- 113. Patel MR, Chen AY, Peterson ED, Newby LK, Pollack CV, Brindis RG, Gibson CM, Kleiman NS, Saucedo JF, Bhatt DL, Gibler WB, Ohman EM, Harrington RA, Roe MT.. Prevalence, predictors, and outcomes of patients with non-ST-segment elevation myocardial infarction and insignificant coronary artery disease: Results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early. Am Heart J 2006;152:641–647. [DOI] [PubMed] [Google Scholar]
- 114. von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA; National Heart, Lung, and Blood Institute. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:722–725. [DOI] [PubMed] [Google Scholar]
- 115. De Luca G, Maas AC, van 't Hof AW, Ottervanger JP, Hoorntje JC, Gosselink AT, Dambrink JH, de Boer MJ, Suryapranata H.. Impact of ST-segment depression resolution on mortality after successful mechanical reperfusion in patients with ST-segment elevation acute myocardial infarction. Am J Cardiol 2005;95:234–236. [DOI] [PubMed] [Google Scholar]
- 116. Ndrepepa G, Tiroch K, Fusaro M, Keta D, Seyfarth M, Byrne RA, Pache J, Alger P, Mehilli J, Schömig A, Kastrati A.. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol 2010;55:2383–2389. [DOI] [PubMed] [Google Scholar]
- 117. Bolognese L, Carrabba N, Parodi G, Santoro GM, Buonamici P, Cerisano G, Antoniucci D.. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation 2004;109:1121–1126. [DOI] [PubMed] [Google Scholar]
- 118. Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JAC.. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 1998;97:765–772. [DOI] [PubMed] [Google Scholar]
- 119. Niccoli G, Montone RA, Ibanez B, Thiele H, Crea F, Heusch G, Bulluck H, Hausenloy DJ, Berry C, Stiermaier T, Camici PG, Eitel I.. Optimized treatment of ST-elevation myocardial infarction. Circ Res 2019;125:245–258. [DOI] [PubMed] [Google Scholar]
- 120. Ohyama K, Matsumoto Y, Takanami K, Ota H, Nishimiya K, Sugisawa J, Tsuchiya S, Amamizu H, Uzuka H, Suda A, Shindo T, Kikuchi Y, Hao K, Tsuburaya R, Takahashi J, Miyata S, Sakata Y, Takase K, Shimokawa H.. Coronary adventitial and perivascular adipose tissue inflammation in patients with vasospastic angina. J Am Coll Cardiol 2018;71:414–425. [DOI] [PubMed] [Google Scholar]
- 121. Antonopoulos AS, Margaritis M, Verheule S, Recalde A, Sanna F, Herdman L, Psarros C, Nasrallah H, Coutinho P, Akoumianakis I, Brewer AC, Sayeed R, Krasopoulos G, Petrou M, Tarun A, Tousoulis D, Shah AM, Casadei B, Channon KM, Antoniades C.. Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-γ/adiponectin signalling. Circ Res 2016;118:842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wójcicka G, Jamroz-Wiśniewska A, Atanasova P, Chaldakov GN, Chylińska-Kula B, Bełtowski J.. Differential effects of statins on endogenous H2S formation in perivascular adipose tissue. Pharmacol Res 2011;63:68–76. [DOI] [PubMed] [Google Scholar]
- 123. Samim A, Nugent L, Mehta PK, Shufelt C, Bairey Merz CN.. Treatment of angina and microvascular coronary dysfunction. Curr Treat Options Cardiovasc Med 2010;12:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hiller K-H, Ruile P, Kraus G, Bauer WR, Waller C.. Tissue ACE inhibition improves microcirculation in remote myocardium after coronary stenosis: MR imaging study in rats. Microvasc Res 2010;80:484–490. [DOI] [PubMed] [Google Scholar]
- 125. Pizzi C, Manfrini O, Fontana F, Bugiardini R.. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac Syndrome X: role of superoxide dismutase activity. Circulation 2004;109:53–58. [DOI] [PubMed] [Google Scholar]
- 126. Kayikcioglu M. Benefits of statin treatment in cardiac syndrome-X. Eur Heart J 2003;24:1999–2005. [DOI] [PubMed] [Google Scholar]
- 127. Fábián E, Varga A, Picano E, Vajo Z, Rónaszéki A, Csanády M.. Effect of simvastatin on endothelial function in cardiac syndrome X patients. Am J Cardiol 2004;94:652–655. [DOI] [PubMed] [Google Scholar]
- 128. Manfrini O, Pizzi C, Morgagni G, Fontana F, Bugiardini R.. Effect of pravastatin on myocardial perfusion after percutaneous transluminal coronary angioplasty. Am J Cardiol 2004;93:1391–1393, A6. [DOI] [PubMed] [Google Scholar]
- 129. Paraskevaidis IA, Iliodromitis EK, Ikonomidis I, Rallidis L, Hamodraka E, Parissis J, Andoniadis A, Tzortzis S, Anastasiou-Nana M.. The effect of acute administration of statins on coronary microcirculation during the pre-revascularization period in patients with myocardial infraction. Atherosclerosis 2012;223:184–189. [DOI] [PubMed] [Google Scholar]
- 130. De Ferrari GM, Fox KA, White JA, Giugliano RP, Tricoci P, Reynolds HR, Hochman JS, Gibson CM, Théroux P, Harrington RA, Van de Werf F, White HD, Califf RM, Newby LK.. Outcomes among non-ST-segment elevation acute coronary syndromes patients with no angiographically obstructive coronary artery disease: observations from 37,101 patients. Eur Hear J Acute Cardiovasc Care 2014;3:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, Jankowski M, Martyniec L, Angielski S, Malinski T.. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation 2003;107:2747–2752. [DOI] [PubMed] [Google Scholar]
- 132. Eriksson BE, Tyni-Lennè R, Svedenhag J, Hallin R, Jensen-Urstad K, Jensen-Urstad M, Bergman K, Selvén C.. Physical training in Syndrome X: physical training counteracts deconditioning and pain in Syndrome X. J Am Coll Cardiol 2000;36:1619–1625. [DOI] [PubMed] [Google Scholar]
- 133. Bugiardini R, Borghi A, Pozzati A, Ottani F, Morgagni GL, Puddu P.. The paradox of nitrates in patients with angina pectoris and angiographically normal coronary arteries. Am J Cardiol 1993;72:343–347. [DOI] [PubMed] [Google Scholar]
- 134. Lerman A, Burnett JC, Higano ST, McKinley LJ, Holmes DR.. Long-term l-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation 1998;97:2123–2128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.