Abstract
Background
Squamous cell carcinoma of the anus (SCCA) incidence is rising in the United States. Study of incidence trends by stage at diagnosis, age-specific and birth cohort patterns, and trends in mortality could provide evidence for a true increase and etiological clues for the increase in incidence.
Methods
Using the US Cancer Statistics dataset, we examined trends in SCCA incidence (2001–2015) and mortality (2001–2016) rates. Join-point regression was used to compute annual and average annual percentage change (AAPC). Incidence patterns by 5-year age group and birth cohort were evaluated using incidence rate ratios (IRRs) and age-period-cohort modeling.
Results
SCCA incidence increased 2.7% per year (95% confidence interval [CI] = 2.1% to 3.3%), with pronounced increases in age groups 50 years and older. Distant-stage SCCA incidence tripled (AAPC = 8.6%, 95% CI = 5.4% to 12.0%, among men and AAPC = 7.5%, 95% CI = 4.8% to 10.2%, among women) and regional-stage SCCA incidence nearly doubled (AAPC = 4.7% for men and women) in both sexes; the AAPC for localized stage was 1.3% (95% CI = 0.6% to 2.0%) in men and 2.3% (95% CI = 1.8% to 2.8%) in women. Compared with adults born circa 1946, recently born black men (born circa 1986) had a nearly fivefold higher risk (IRR = 4.7, 95% CI = 2.1 to 10.2) of SCCA, and the risk doubled among white men (IRR = 2.0, 95% CI = 1.7 to 2.2) and white women (IRR = 2.1, 95% CI = 1.9 to 2.3) born after circa 1960. Anal cancer mortality rates increased 3.1% per year (95% CI = 2.6% to 3.5%) with statistically significant increases in age groups 50 years and older. SCCA incidence-based mortality increased 1.9% annually (95% CI = 0.5% to 3.4%), with a notable (4.9%, 95% CI = 2.4% to 7.3%, per year) rise in adults ages 60–69 years.
Conclusion
The increase in SCCA incidence, particularly advanced-stage disease, and a similar increase in mortality suggest a true increase in the occurrence of SCCA. Future research and improved prevention are urgently needed to mitigate the increasing disease burden.
Squamous cell carcinoma of the anus (SCCA) is the most common histologic subtype of anal cancer, and more than 90% of incident cancers are associated with human papillomavirus (HPV) (1). Adenocarcinoma is the second most common subtype that is very rare and etiologically different because HPV has been detected in a small fraction of cases (2,3). Previous studies have reported that SCCA incidence more than doubled between the late 1970s and early 2010s in the United States (4,5), whereas adenocarcinoma incidence has declined (4). To date, it is unclear whether the observed increase in SCCA incidence is attributable to an increase in early-stage tumors reflecting increased diagnostic scrutiny or whether the increase has occurred across tumor stages, supporting evidence for a real increase.
Because cervical cancer screening for analogous HPV-related tumors has resulted in a more than 50% decline in cervical cancer incidence (6), similar screening techniques for SCCA prevention have been implemented for some high-risk groups (7–9). However, the impact of screening on SCCA incidence remains unclear (10–12). Screening may also result in early-stage tumor diagnosis (13,14), but given its inconsistency and limited availability in most geographic regions (11,15), the increase in SCCA incidence is unlikely to be attributable to an artifact of detecting tumors as a result of increased diagnostic scrutiny. In addition, trends in SCCA mortality rates in the United States remain undescribed. Evidence of increasing SCCA deaths could confirm a true increase in the occurrence of SCCA.
Since the 1950s, dramatic changes in the risk factors for SCCA have occurred, including an ideological shift in sexual behaviors (normalization of receptive anal intercourse and increased number of sexual partners) and the emergence of the HIV epidemic (especially among men who have sex with men) (16–18), that may have influenced SCCA trends in contemporary birth cohorts. However, none of the prior studies simultaneously examined temporal SCCA incidence patterns by age, calendar period, and year of birth. Birth cohort analysis could provide etiological clues into the increase in SCCA rates by unraveling factors that equally affect all ages (period effects) from the factors that vary by generations (cohort effects) due to changes in exposures or behaviors.
Our objective was to describe contemporary SCCA incidence and mortality trends in the United States. To examine possible reasons for increasing SCCA incidence, we studied trends by age and stage at diagnosis and year of birth and evaluated mortality trends by SCCA diagnostic characteristics.
Methods
Data Sources
We analyzed the US Cancer Statistics dataset from the Centers for Disease Control and Prevention’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program on cases of SCCA diagnosed from 2001 to 2015 covering 99% of the US population for all 50 states and the District of Columbia (cases diagnosed in Mississippi during 2001–2002 were unavailable) (19).
Anal cancer death data were derived from information recorded in death certificates ascertained from the National Center for Health Statistics. We assessed trends in anal cancer death rates using nationwide data. To evaluate mortality by cancer diagnostic characteristics (ie, histology, age at diagnosis, and stage) that are not collected on death certificates, we used the incidence-based mortality file that links the SEER-13 cancer incidence file with death certificate information (20). To prevent underestimation of incidence-based mortality rates, we considered diagnoses during 1992–2016 and deaths during 2001–2016.
Demographic Characteristics
The demographic characteristics information submitted to each cancer registry were abstracted from patient medical records. We identified the age of cancer diagnosis and sex at birth. Race and ethnicity was classified into categories—non-Hispanic whites, non-Hispanic blacks, Hispanics, and non-Hispanic other races, including American Indian or Alaska Natives and Asian or Pacific Islanders.
Case Definitions and Tumor Characteristics
We identified anal cancer cases based on International Classification of Diseases for Oncology, 3rd edition (ICD-O-3; site codes C21.0-C21.2, C21.8, excluding histology codes 9050–9055, 9140, 9590–9992) (21). Anal cancer cases were classified by site (anal, not otherwise specified [C21.0], anal canal [C21.1], cloacogenic zone [C21.2], and overlapping lesion of rectum, anus, and anal canal [C21.8]). All cancers were malignant, and histological codes (8050–8076, 8083–8084, 8123–8124) were used to confirm SCCA. To classify stage at diagnosis, we used SEER Summary Stage 2000: localized (confined to anus), regional (spread outside the anal area to nearby structures or lymph nodes), distant (spread to distant parts of the body), and unknown stage (22). Persons of unknown age and sex and those whose cancer was diagnosed at autopsy or was first documented on the death certificate were excluded. The final analytical sample consisted of microscopically confirmed cases (Supplementary Figure 1, available online).
Statistical Analysis
SCCA incidence rates (overall and for 5-year calendar periods 2001–2005, 2006–2010, and 2011–2015) by subgroups of sex, age at diagnosis, race and ethnicity, and stage at diagnosis (localized, regional, distant, unknown) were estimated. Rate ratios and 95% confidence intervals (CI) were estimated by age to determine peak incidence over the three calendar periods. We used SEER*Stat version 8.3.5 to estimate incidence and mortality rates. Person-years were estimated by summing population sizes across calendar years. Incidence estimates were age-adjusted to the 2000 US standard population and were expressed per 100 000 person-years.
To quantify trends in incidence rates and mortality over time and to calculate annual percentage changes (APCs) and average APCs (AAPCs) (23), we used the National Cancer Institute’s Joinpoint Regression Analysis program (version 4.7.0). The APC characterizes trend, a single regression line on a log scale fitted over a fixed interval, whereas the AAPC is a weighted average of the APCs from the join-point model with the weights equal to the length of the APC interval. To determine whether the trends were different from 0, a t test was used for zero join-points, and a z test was used for one or more join-points. Statistical significance was assessed at an α level of P < .05, and all hypotheses were two-sided.
We used age-period-cohort models to simultaneously evaluate the effect of age, period, and birth cohort on SCCA incidence (24). For this, we grouped age (fourteen 5-year age groups [20 to ≥85 years]) and periods with 5-year interval to identify individuals who belong to approximately the same birth cohort (fifteen 5-year birth cohorts [1916–1990]). Birth cohort models were fitted using the National Cancer Institute’s Age Period Cohort web tool (25). Cohort effects are presented graphically as incidence rate ratios (IRRs) adjusted for age and calendar period effects. For comparison, we chose the reference year corresponding to the 1946 cohort (this arbitrary choice of reference year value does not affect result interpretation).
Results
Patient Characteristics
Between 2001 and 2015, 68 809 individuals met the case definition and were included in the incidence analysis. Women (64.9%), non-Hispanic whites (81.8%), those aged 50 years or older (79.5%), and individuals with localized stage disease (50.3%) comprised the majority of SCCA cases (Table 1).
Table 1.
SCCA incidence (2001–2015): NPCR and SEER Registry Databases
| Characteristic | Total |
Calendar years |
||||||
|---|---|---|---|---|---|---|---|---|
| 2001–2005 |
2006–2010 |
2011–2015 |
||||||
| Cases* No. (%) | Rate† (95% CI) | Cases* No. (%) | Rate† (95% CI) | Cases* No. (%) | Rate† (95% CI) | Cases* No. (%) | Rate† (95% CI) | |
| Overall | 68 809 | 1.41 (1.40 to 1.42) | 17 542 | 1.20 (1.18 to 1.22) | 23 031 | 1.42 (1.40 to 1.44) | 28 236 | 1.56 (1.54 to 1.58) |
| Sex | ||||||||
| Men | 24 141 (35.1) | 1.07 (1.06 to 1.08) | 6358 (36.2) | 0.94 (0.91 to 0.96) | 8185 (35.5) | 1.09 (1.06 to 1.11) | 9598 (34.0) | 1.15 (1.13 to 1.18) |
| Women | 44 668 (64.9) | 1.70 (1.69 to 1.72) | 11 184 (63.8) | 1.42 (1.39 to 1.44) | 14 846 (64.5) | 1.70 (1.67 to 1.73) | 18 638 (66.0) | 1.93 (1.90 to 1.95) |
| Race and ethnicity | ||||||||
| NH white | 56 013 (81.8) | 1.52 (1.51 to 1.54) | 14 418 (82.5) | 1.27 (1.25 to 1.29) | 18 710 (81.6) | 1.53 (1.51 to 1.55) | 22 885 (81.5) | 1.72 (1.70 to 1.74) |
| NH black | 6975 (10.2) | 1.38 (1.35 to 1.41) | 1746 (10.0) | 1.17 (1.12 to 1.23) | 2352 (10.3) | 1.39 (1.34 to 1.45) | 2877 (10.2) | 1.53 (1.47 to 1.59) |
| Hispanic | 4518 (6.6) | 1.05 (1.02 to 1.08) | 1085 (6.2) | 1.00 (0.94 to 1.07) | 1525 (6.7) | 1.06 (1.00 to 1.11) | 1908 (6.8) | 1.07 (1.02 to 1.12) |
| Other‡ | 987 (1.4) | 0.41 (0.39 to 0.44) | 237 (1.4) | 0.39 (0.34 to 0.44) | 343 (1.5) | 0.44 (0.39 to 0.49) | 407 (1.4) | 0.41 (0.37 to 0.45) |
| Age at diagnosis, y | ||||||||
| <40 | 2541 (3.7) | 0.11 (0.11 to 0.12) | 992 (5.7) | 0.13 (0.12 to 0.14) | 806 (3.5) | 0.11 (0.10 to 0.12) | 743 (2.6) | 0.10 (0.09 to 0.11) |
| 40–49 | 11 622 (16.9) | 1.76 (1.73 to 1.79) | 3665 (20.9) | 1.65 (1.60 to 1.71) | 4302 (18.7) | 1.91 (1.86 to 1.97) | 3655 (12.9) | 1.71 (1.65 to 1.76) |
| 50–59 | 20 486 (29.8) | 3.45 (3.40 to 3.50) | 4522 (25.8) | 2.60 (2.53 to 2.68) | 7142 (31.0) | 3.55 (3.47 to 3.63) | 8822 (31.2) | 4.04 (3.96 to 4.13) |
| 60–69 | 16 829 (24.5) | 4.17 (4.10 to 4.23) | 3534 (20.1) | 3.25 (3.14 to 3.36) | 5212 (22.6) | 3.93 (3.82 to 4.03) | 8083 (28.6) | 4.98 (4.87 to 5.09) |
| ≥70 | 17 331 (25.2) | 4.21 (4.14 to 4.27) | 4829 (27.5) | 3.75 (3.64 to 3.85) | 5569 (24.2) | 4.13 (4.02 to 4.24) | 6933 (24.6) | 4.68 (4.57 to 4.79) |
| Stage at diagnosis | ||||||||
| Localized | 34 592 (50.3) | 0.71 (0.70 to 0.72) | 9395 (53.6) | 0.64 (0.63 to 0.66) | 11 326 (49.2) | 0.70 (0.69 to 0.71) | 13 871 (49.1) | 0.77 (0.76 to 0.78) |
| Regional | 21 076 (30.6) | 0.43 (0.43 to 0.44) | 4844 (27.6) | 0.33 (0.32 to 0.34) | 7279 (31.6) | 0.45 (0.44 to 0.46) | 8953 (31.7) | 0.49 (0.48 to 0.51) |
| Distant | 7066 (10.3) | 0.14 (0.14 to 0.15) | 1423 (8.1) | 0.10 (0.09 to 0.10) | 2327 (10.1) | 0.14 (0.14 to 0.15) | 3316 (11.7) | 0.18 (0.18 to 0.19) |
| Unknown | 6075 (8.8) | 0.13 (0.12 to 0.13) | 1880 (10.7) | 0.13 (0.12 to 0.14) | 2099 (9.1) | 0.13 (0.12 to 0.13) | 2096 (7.4) | 0.12 (0.11 to 0.12) |
Cases included tumors that matched the selection criteria were microscopically confirmed and were not identified only from autopsy records or death certificates. AAPC = average annual percentage change; CI = confidence interval; NH = non-Hispanic; NPCR = National Program of Cancer Registries; SCCA = squamous cell carcinoma of the anus; SEER = Surveillance, Epidemiology, and End Results Program.
Rates were calculated as the number of cases per 100 000 person-years and age-adjusted to the 2000 US standard population.
Others include (non-Hispanic) American Indian or Alaskan Native, Asian or Pacific Islander, and other unspecified.
SCCA Incidence by Age, Sex, and Race/Ethnicity
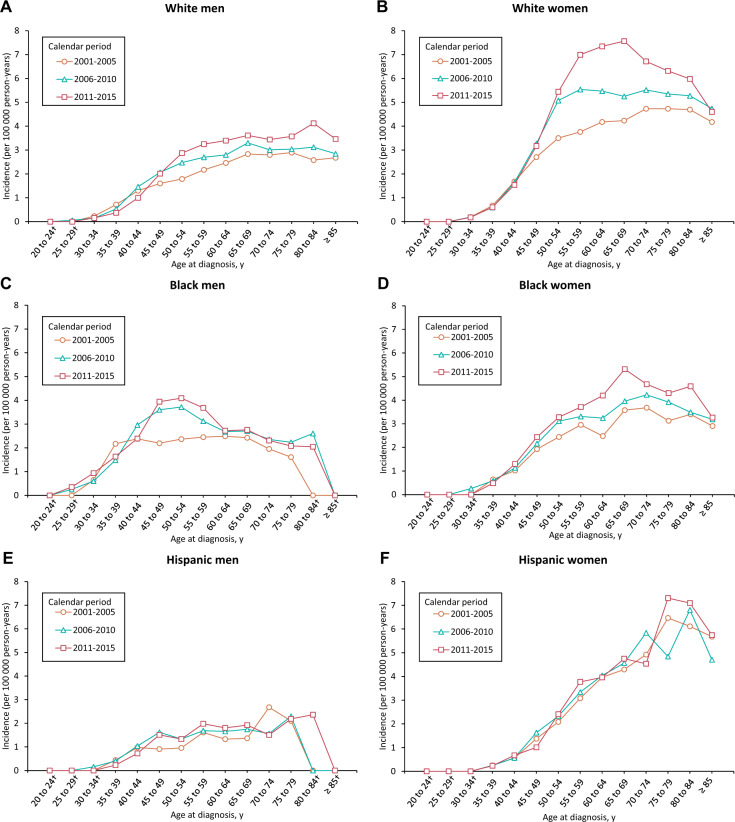
SCCA incidence rates as a function of age and race and ethnicity over time are presented in Figure 1; Rate ratios are available in Supplementary Tables 1 and 2 (available online). Between 2001 and 2015, among white men, the incidence increased steadily up to age 65–69 years. For white women, in 2001–2005, the incidence rates increased sharply from age 30 to 54 years and then gradually until age 70 to 74 years. In 2006–2010, the incidence increased sharply from 30 to 54 years of age and gradually up to age 59 years and it was more or less stable for the older age groups. Finally, between 2011 and 2015, the incidence rates increased sharply among white women aged 30–59 years and gradually up to age 69 years, whereas a decline was observed in older age groups. Notably, among black men, the incidence rates increased steeply at younger ages (35–39 years during 2001–2005, 40–44 years during 2006–2010, and 45–49 years during 2006–2015), whereas the increase in black women was observed until age 65–69 years. The incidence among Hispanic men increased until age 45–49 years and remained stable in older age groups. In contrast, incidence rates increased with advancing age in Hispanic women.
Figure 1.
Age- and race-specific incidence rates* of squamous cell carcinoma of the anus (SCCA): National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program (2001–2015). Age-specific SCCA incidence rates according to diagnosis during the calendar periods 2001–2005, 2006–2010, and 2011–2015. A–F) incidence rates among non-Hispanic white men, non-Hispanic white women, non-Hispanic black men, non-Hispanic black women, Hispanic men, and Hispanic women, respectively. *Rates were calculated as number of cases per 100 000 person-years and age adjusted to the 2000 US standard population. †Data suppressed because there were fewer than 16 cases in the time interval.
Trends in SCCA Incidence
Between 2001 and 2015, overall SCCA incidence increased 2.7% (95% CI = 2.1% to 3.3%) annually (Supplementary Table 3, available online); the increases among men and women were 2.2% (95% CI = 1.1% to 3.3%) (Supplementary Table 4, available online) and 3.1% (95% CI = 2.6% to 3.7%) (Supplementary Table 5, available online), respectively. Among men, the most pronounced increase was observed in those who were black (AAPC = 2.8%, 95% CI = 1.3% to 4.4%), whereas the increase among whites was 2.3% (95% CI = 1.7% to 3.0%) annually. Incidence increased rapidly among white women (3.7% per year, 95% CI = 3.1% to 4.3%) and black women (2.6% per year, 95% CI = 1.7% to 3.5%). No statistically significant changes were observed for Hispanics and other races or ethnicities.
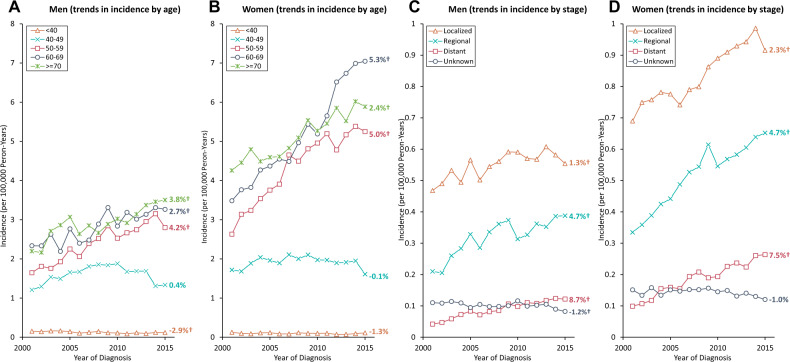
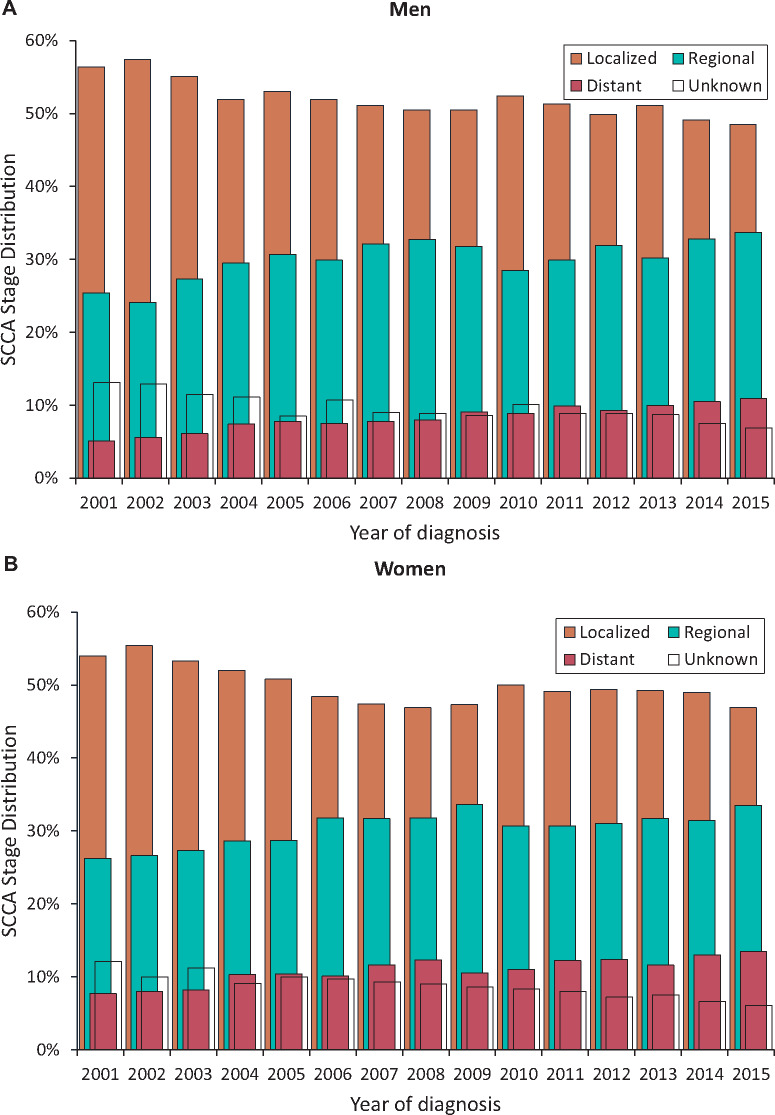
Trends in SCCA incidence rates by age and stage at diagnosis are shown graphically in Figure 2 with observed rates plotted with modeled join-point trends in Supplementary Tables 4 and 5 (available online). From 2001 to 2015, a statistically significant decline in SCCA rates was observed among men younger than 40 years (AAPC = −2.9%, P = .002) but not among women (AAPC = −1.3%, P = .16). In both sexes aged 40–49 years, a statistically significant increase was observed during 2001–2009 (APC = 5.5% for men and 2.3% for women) followed by a decline (APC of −8.8% for men and −3.1% for women during 2009–2015). The increase in incidence was statistically significant for all age groups 50 years and older: AAPCs for men 50–59, 60–69, and 70 years and older were 4.2%, 2.7%, and 3.8% and for women were 5.0%, 5.3%, and 2.5%, respectively. A statistically significant increase was observed for localized stage SCCA (AAPC = 1.3%, 95% CI = 0.6 to 2.0, for men and 2.3%, 95% CI = 1.8% to 2.8%, for women); the increase was rapid for distant-stage SCCA (AAPC = 8.6%, 95% CI = 5.4% to 12.0%, for men and 7.5%, 95% CI = 4.8 % to 10.2%, for women) and regional-stage SCCA (AAPC = 4.7%, P < .001, for men and women). As a result, the proportion of cases diagnosed with regional plus distant stage increased from 30.5% to 44.6% in men and 33.9% to 47% in women from 2001 to 2015 (Figure 3). Annual change for unknown stage was statistically significant for men (AAPC = −1.2%, P = .02) but not for women (AAPC = −1.0%, P = .18).
Figure 2.
Trends in the annual incidence rates* of squamous cell carcinoma of the anus (SCCA) according to age and stage at diagnosis among men and women: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program (2001–2015). Data markers represent the observed incidence rates (cases per 100 000 person-years) of SCCA. A and B) SCCA incidence trends according to age at diagnosis in men and women, respectively. C and D) SCCA incidence trends according to the stage at diagnosis in men and women, respectively. *Rates are age-adjusted to the 2000 US standard population. †Indicates statistically significant incidence trend (P < .05).
Figure 3.
Stage distribution of squamous cell carcinoma of the anus (SCCA) among men and women: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program (SEER) (2001–2015). Vertical bars represent proportion of SCCA cases diagnosed by SEER summary stage (localized, regional, distant, and unknown) among men (A) and women (B).
SCCA Incidence by Birth Cohort
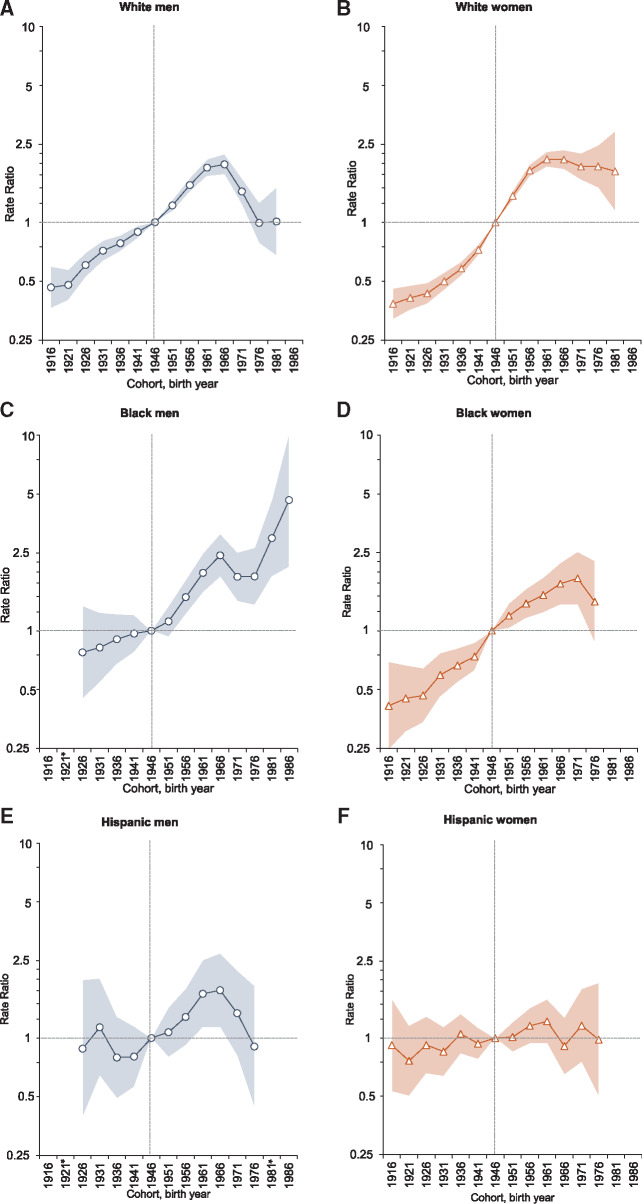
Age-period-cohort modeling, for which the 1946 cohort was the reference group, revealed distinct cohort patterns in SCCA incidence by race and ethnicity (Figure 4). Among white men, SCCA risk increased slowly among consecutive cohorts born from the 1910s to 1940s and then steeply until 1966 (IRR = 2.0, 95% CI = 1.7 to 2.2) followed by a moderation in the incidence among subsequent birth cohorts. In contrast, the risks increased for consecutive cohorts of white women, black men, and black women. Notably, compared with the reference cohort, the risk among black men increased for successive cohorts and was fivefold higher among those born circa 1986 (IRR = 4.7, 95% CI = 2.1 to 10.2). The risk doubled among white women (IRR = 2.1, 95% CI = 1.9 to 2.3) and black women (IRR = 1.9, 95% CI = 1.4 to 2.5), with peaks observed among those born circa 1961 and 1971, respectively. The risk generally remained stable in successive birth cohorts of Hispanic men and women. Incidence rates according to age and birth cohort presented in Supplementary Figure 2 (available online) confirm these findings. Additional output metrics are presented in Supplementary Figure 3 (available online).
Figure 4.
Incidence rate ratios (IRRs) by birth cohort for squamous cell carcinoma of the anus (SCCA; referent cohort = 1946): National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program (2001–2015). Shaded bands indicate 95% confidence interval. A–F) IRRs among non-Hispanic white men, non-Hispanic white women, non-Hispanic black men, non-Hispanic black women, Hispanic men, and Hispanic women, respectively. *Not reported because there were fewer than 16 cases in the time interval.
Observed Anal Cancer and SCCA Incidence-Based Mortality
A total 12 111 anal cancer deaths were identified in the US population during 2001–2016. Anal cancer mortality rates increased overall 3.1% (95% CI = 2.6% to 3.5%) annually with increases of 3.4% (95% CI = 2.6% to 4.2%) in men and 2.9% (95% CI = 2.3% to 3.6%) in women (Table 2, Supplementary Table 6 available online). A statistically significant increase in mortality rates was observed for all racial and ethnic groups (AAPCs, 3.3% for whites, 3.2% for blacks, and 2.5% for Hispanics) except “others” and all age groups aged 50 years and older (AAPCs, 3.6% for 50–59 years, 4.9% for 69–69 years, and 2.7% for 70 years).
Table 2.
Observed anal cancer and SCCA incidence-based mortality—number of cases, rates, and trends in rates (2001–2016): NCHS and SEER-13 Registry Databases
| Characteristics | Cases* No. (%) | Rate† (95% CI) | AAPC (95% CI) | P ‡ |
|---|---|---|---|---|
| Observed US anal cancer mortality, NCHS | ||||
| Overall | 12 111 | 0.23 (0.22 to 0.23) | 3.1 (2.6 to 3.5) | <.001 |
| Sex | ||||
| Men | 4720 (39.0) | 0.20 (0.19 to 0.20) | 3.4 (2.6 to 4.2) | <.001 |
| Women | 7391 (61.0) | 0.25 (0.25 to 0.26) | 2.9 (2.3 to 3.6) | <.001 |
| Race or ethnicity | ||||
| NH white | 9977 (82.6) | 0.24 (0.24 to 0.25) | 3.3 (2.8 to 3.8) | <.001 |
| NH black | 1312 (10.9) | 0.25 (0.23 to 0.26) | 3.2 (2.1 to 4.2) | <.001 |
| Hispanic | 581 (4.8) | 0.13 (0.12 to 0.14) | 2.5 (0.6 to 4.3) | .01 |
| Others§ | 207 (1.7) | 0.09 (0.08 to 0.10) | −0.15 (−5.2 to 5.1) | .95 |
| Age at death, y | ||||
| <40 | 284 (2.3) | 0.01 (0.01 to 0.01) | −0.8 (−3.5 to 2.0) | .55 |
| 40–49 | 1339 (11.1) | 0.19 (0.18 to 0.20) | 1.7 (−0.8 to 4.2) | .19 |
| 50–59 | 2766 (22.8) | 0.43 (0.41 to 0.44) | 3.6 (2.6 to 4.7) | <.001 |
| 60–69 | 2943 (24.3) | 0.66 (0.64 to 0.69) | 4.9 (4.0 to 5.8) | <.001 |
| ≥70 | 4779 (39.5) | 1.05 (1.02 to 1.08) | 2.7 (2.1 to 3.1) | <.001 |
| Incidence-based mortality, SEER-13‖ | ||||
| Anal cancer | 1206 | 0.18 (0.17 to 0.19) | 1.8 (0.5 to 3.1) | .01 |
| SCCA | 1055 | 0.16 (0.15 to 0.16) | 1.9 (0.5 to 3.4) | .01 |
| SCCA incidence-based mortality (according to patient characteristics) | ||||
| Sex | ||||
| Men | 446 (42.3) | 0.15 (0.13 to 0.16) | 2.7 (0.3 to 5.2) | .03 |
| Women | 609 (57.7) | 0.16 (0.15 to 0.18) | 1.3 (−0.6 to 3.2) | .16 |
| Race or ethnicity | ||||
| NH white | 807 (76.5) | 0.18 (0.17 to 0.20) | 2.1 (0.4 to 3.8) | .02 |
| NH black | 134 (12.7) | 0.21 (0.17 to 0.25) | 2.2 (−1.4 to 5.9) | .22 |
| Hispanic | 73 (6.9) | 0.08 (0.06 to 0.10) | 3.2 (−2.3 to 9.0) | .24 |
| Others§ | 41 (3.9) | 0.05 (0.03 to 0.06) | −0.6 (−6.7 to 5.9) | .85 |
| Age at diagnosis, y | ||||
| <40 | 45 (4.3) | 0.01 (0.01 to 0.01) | −2.5 (−8.5 to 4.0) | .42 |
| 40–49 | 174 (16.5) | 0.03 (0.02 to 0.03) | 1.2 (−2.6 to 5.0) | .52 |
| 50–59 | 272 (25.8) | 0.03 (0.03 to 0.04) | 2.9 (−0.5 to 6.4) | .09 |
| 60–69 | 241 (22.8) | 0.03 (0.03 to 0.04) | 4.9 (2.4 to 7.3) | .001 |
| ≥70 | 323 (30.6) | 0.05 (0.05 to 0.06) | 0.9 (−1.6 to 3.4) | .45 |
| Stage at diagnosis | ||||
| Localized | 298 (28.0) | 0.04 (0.04 to 0.05) | 2.6 (−1.0 to 6.5) | .15 |
| Regional | 419 (39.3) | 0.06 (0.06 to 0.07) | 3.3 (−1.9 to 8.8) | .22 |
| Distant | 263 (24.7) | 0.04 (0.03 to 0.04) | 1.6 (−1.2 to 4.5) | .25 |
| Unknown | 86 (8.1) | 0.01 (0.01 to 0.02) | −0.5 (−4.8 to 4.1) | .82 |
Cases included tumors that matched the selection criteria were microscopically confirmed and were not identified only from autopsy records or death certificates. AAPC = average annual percentage change; CI = confidence interval; NCHS = National Center for Health Statistics; NH = non-Hispanic; SCCA = squamous cell carcinoma of the anus; SEER = Surveillance, Epidemiology, and End Results Program.
Rates for observed anal cancer and SCCA incidence-based mortality were calculated as a number of deaths per 100 000 person-years and age-adjusted to the 2000 US standard population.
Statistical significance was determined when the APCs and AAPCs were different from zero at the α = 0.05 level. A t test was used for zero join-points, and a z test was used for one or more join-points. Statistical significance was assessed at an α level of P < .05, and all hypotheses were two-sided.
Others include American Indian or Alaskan Native, Asian or Pacific Islander, other unspecified.
Incidence-based mortality was estimated for SCCA cases diagnosed during 1992–2016.
In SEER-13 regions, there were 1055 deaths among eligible SCCA cases during 2001–2016 (Table 2). SCCA incidence-based mortality increased 1.9% (95% CI = 0.5% to 3.4%) annually (Table 2; Supplementary Table 7, available online); the increase was statistically significant in men (AAPC = 2.7%, 95% CI = 0.3% to 5.2%) but not in women. Positive AAPCs were observed for all age groups at SCCA diagnosis except for age at diagnosis of 40 years or younger but were statistically significant only for patients aged 60–69 years (AAPC = 4.9%, 95% CI = 2.4% to 7.3%). AAPCs were positive but not statistically significant for localized, regional, and distant stage at diagnosis and negative but not statistically significant for unknown stage.
Discussion
Our study is the first to our knowledge to characterize and systematically compare contemporary national SCCA incidence trends by stage at diagnosis, birth year, and mortality. Birth cohort analysis revealed that SCCA incidence more than doubled among recent-born white women and white men, and a nearly fivefold increase was observed among young black men relative to those born in the mid-1940s. In addition, the proportion of cases diagnosed with distant-stage SCCA has doubled, and incidence-based mortality rates have increased by 1.5-fold. These findings suggest that the increase in SCCA incidence rates is real and not driven by increased screening in some populations.
Our findings are consistent with a previous study from the United Kingdom that projected SCCA to be the fastest accelerating cancer (3.1% per year) and cause of mortality (3.7% per year) of 26 cancer sites in women (26). SCCA risk is associated with sexual behaviors, including number of sexual partners and receptive anal intercourse, reflecting HPV acquisition (3). Therefore, this increase may have been caused by the increased number of sexual partners following the “sexual revolution” of the 1950s–1960s and the increased prevalence of anal sex in recent birth cohorts of women (27–30). However, reasons for distinct incidence patterns by race and ethnicity remain unclear given the lack of studies determining racial and ethnic differences in anal HPV natural history in women (31). Attributable to screening, rates of cervical cancer have declined more than 65% from 1975 to 2016 among women aged 50 years or older (6,32). If increasing SCCA incidence trends continue in older women and cervical cancer incidence continues to decline (33), SCCA may surpass cervical cancer during the next 10–15 years to become the leading HPV-associated cancer in elderly US women. Even if SCCA incidence stabilizes (which is unlikely), with the anticipated 150% growth (from 56 million in 2019 to 86 million in 2050) in the US elderly during the next 30 years, with older women continuing to outnumber older men (33), the burden of SCCA will continue to increase.
Diagnosis of anal HPV infection and anal cancer has also been reported among men and women who do not report receptive anal intercourse (34–37), supporting the contribution of risk factors other than sexual behaviors to SCCA carcinogenesis. One group at enhanced risk is women with prior lower genital tract dysplasia or malignancy (38–41). Notably, a recent collaborative pooled analysis of women with paired cervical and anal samples showed that age and detection of high-risk cervical HPV infection may collectively help determine a woman’s anal cancer risk profile for investigation of risk-based screening approaches (41). An association between current smoking and SCCA risk has also been reported (3,36,42); however, smoking prevalence in the United States has declined in the past decades, which does not correlate with the recent increase in SCCA incidence rates (43). Recent data from the HPV Infection in Men study revealed that increasing levels of body mass index were associated with greater persistence of oncogenic anal HPV infection, particularly among heterosexual men (44), suggesting that the recent obesity epidemic in the United States may have contributed to the increase in SCCA incidence.
We found a steep increase in SCCA incidence in young black men (ie, those born during 1971–1986 who are currently aged 30–49 years) compared with those born circa 1950, whereas the increase in incidence (twofold increase in those born in the 1960s) was moderated among similarly aged white men. This distinct pattern supports a dominant role for a birth cohort effect and also suggests that changes in key exposures may have influenced the risk over time. HIV-related immunosuppression is a major risk factor for SCCA that has evolved over successive birth cohorts (45). The HIV epidemic has disproportionately affected young black men compared with whites since the beginning of the epidemic (46). Notably, the rates of AIDS diagnoses stabilized (from 1.0 to 1.5 per 100 000) among young (ages 13–24 years) white men between 1999 and 2013, whereas a greater than twofold increase (from 13.8 to 31.0 per 100 000) was observed in similarly aged black men. The distinct pattern in SCCA incidence seems to have mirrored the differences in AIDS diagnosis trends by age and race among birth cohorts. Consistent with our findings, a previous study reported that in the United States during 2001–2005, 84% of anal cancer cases in black men aged 20–49 years and 51% in white men were HIV infected (47).
We found noteworthy increases in SCCA mortality rates among the elderly population. This finding is likely to be associated with the increasing rates of advanced-stage SCCA among people older than 50 years. The increased incidence of advanced stage may reflect an increasing prevalence of immunosuppressed adults in the United States. A recent US study reported that 2.7% of US adults (3.5% women and 1.8% men) were immunosuppressed because of medical conditions or treatments, with peak prevalence (4.4%) observed at ages 50–59 years (48). One hypothesis is that comorbid and iatrogenic immunosuppression may have their greatest impact in the elderly, impairing both HPV clearance and immunologic cancer surveillance and thereby potentially contributing to the growth of advanced-stage SCCA. Contrary to this hypothesis, the shift toward local-stage anal cancers has been observed among HIV-infected people and transplant recipients (49), which may be attributable to heightened medical surveillance among men who have sex with men with HIV and similar close follow-up for organ transplant recipients. Nonetheless, future research is needed to understand risk factors underlying the increase in tumor progression to advanced-stage disease and the impact of screening on stage shift.
SCCA is preventable through HPV vaccination; however, vaccination coverage (50% in 2017) remains suboptimal in the United States, and less than 30% vaccine-eligible individuals or their family members received recommendation for HPV vaccination from their health-care professionals (50,51). Even if high HPV vaccine coverage is attained in the next 5 years, the benefits of vaccination may not be evident for at least 15–20 years given the lengthy time between initial HPV infection and development of SCCA. Furthermore, no evidence-based SCCA screening guidelines exist except for expert opinion-based recommendations for persons with HIV. The Anal Cancer/HSIL Outcomes Research study is currently underway to determine if treating anal cancer precursors in HIV-infected persons will reduce SCCA incidence. Further investigation of the harms and benefits of screening considering the impact of anal precancer treatment on SCCA incidence and mortality could determine an optimal screening regimen (10,52).
Our study has certain limitations. First, individual-level risk factors (eg, HPV, HIV, smoking, and organ transplantation) are not captured in cancer registries; therefore, the impact of these risk factors on the increasing incidence of and mortality from SCCA cannot be directly measured, limiting us to speculate on potential etiologies. Second, the SCCA cases with unknown tumor stage may have influenced secular trends by stage at diagnosis; however, no statistically significant decline in unknown stage for women and a small decline in men suggest that such an impact is likely to be minimal. Finally, to ensure data completeness, Centers for Disease Control and Prevention’s National Program of Cancer Registries and SEER allow an interval of 23 months after the close of the diagnosis year for submission; however, reporting of SCCA cases diagnosed in an outpatient facility may still be delayed. As a result, the trends in incident SCCA cases may erroneously appear to have decreased in the most recent year (19). Despite these limitations, the principal strength of our study is the use of nationwide, high-quality, population-based registries that allowed us to report the most comprehensive and contemporary data on SCCA incidence and mortality trends in the United States.
SCCA incidence has increased dramatically in elderly women and young black men. Advanced-stage SCCA incidence tripled, with a prominent increase in SCCA mortality. Our findings call for future studies to identify reasons for the increase in SCCA incidence and mortality. Improved prevention strategies are urgently needed to mitigate the increasing SCCA burden among a rapidly growing number of aging US adults.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers R01CA232888, UM1CA121947, and the intramural research program of the National Cancer Institute.
Notes
Affiliations of authors: Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (MSS); Center for Health Services Research (AAD, RS, KSo) and Center for Healthcare Data (KSo), Department of Management, Policy, and Community Health, UTHealth School of Public Health, Houston, TX; Clinical Cancer Center/Center for AIDS Intervention Research, Medical College of Wisconsin, Milwaukee, WI (AGN); Department of Medicine, University of California San Francisco, San Francisco, CA (JMP); Department of Pathology (YL) and Division of Infectious Diseases, Department of Medicine (MMG) and Department of General Internal Medicine, Department of Medicine (KSi), Mt. Sinai Icahn School of Medicine, New York, NY.
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr Deshmukh has received consulting fees from Merck for unrelated projects. Dr Palefsky has received grants and nonfinancial support from Merck. He also has received grants, personal fees, and other support from Vir Biotechnologies, Ubiome, and Antiva Biosciences. He has received personal fees from Janssen Pharmaceuticals, Novan, and Vaccitech and nonfinancial support from Virion Therapeutics.
We thank Eric A. Engels, MD, MPH, for feedback on the manuscript.
Supplementary Material
References
- 1. Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107(6):djv086.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoots BE, Palefsky JM, Pimenta JM, et al. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009;124(10):2375–2383. [DOI] [PubMed] [Google Scholar]
- 3. Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101(2):270–280. [DOI] [PubMed] [Google Scholar]
- 4. Shiels MS, Kreimer AR, Coghill AE, et al. Anal cancer incidence in the United States, 1977-2011: distinct patterns by histology and behavior. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nelson RA, Levine AM, Bernstein L, et al. Changing patterns of anal canal carcinoma in the United States. J Clin Oncol. 2013;31(12):1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Islami F, Fedewa SA, Jemal A.. Trends in cervical cancer incidence rates by age, race/ethnicity, histological subtype, and stage at diagnosis in the United States. Prev Med. 2019;123:316–323. [DOI] [PubMed] [Google Scholar]
- 7.American Cancer Society. Anal cancer: early detection, diagnosis, and staging; screening in people at high risk. https://www.cancer.org/cancer/anal-cancer/detection-diagnosis-staging/detection.html. Accessed October 1, 2019.
- 8. Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(1):e1–e34. [DOI] [PubMed] [Google Scholar]
- 9. Stewart DB, Gaertner WB, Glasgow SC, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for Anal Squamous Cell Cancers (revised 2018). Dis Colon Rectum. 2018;61(7):755–774. [DOI] [PubMed] [Google Scholar]
- 10. Deshmukh AA, Chiao EY, Cantor SB, et al. Management of precancerous anal intraepithelial lesions in human immunodeficiency virus-positive men who have sex with men: clinical effectiveness and cost-effectiveness. Cancer. 2017;123(23):4709–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palefsky JM. Screening to prevent anal cancer: current thinking and future directions. Cancer Cytopathol. 2015;123(9):509–510. [DOI] [PubMed] [Google Scholar]
- 12. Wentzensen N, Clarke MA.. From clinical epidemiology to practice recommendations: knowledge gaps and uncertainty in the management of anal precancers. Cancer. 2017;123(23):4530–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berry JM, Jay N, Cranston RD, et al. Progression of anal high-grade squamous intraepithelial lesions to invasive anal cancer among HIV-infected men who have sex with men. Int J Cancer. 2014;134(5):1147–1155. [DOI] [PubMed] [Google Scholar]
- 14. Palefsky JM. Practising high-resolution anoscopy. Sex Health. 2012;9(6):580–586. [DOI] [PubMed] [Google Scholar]
- 15. Albuquerque A. High-resolution anoscopy: unchartered territory for gastroenterologists? World J Gastrointest Endosc. 2015;7(13):1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finer LB. Trends in premarital sex in the United States, 1954-2003. Public Health Rep. 2007;122(1):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hess KL, Crepaz N, Rose C, et al. Trends in sexual behavior among men who have sex with men (MSM) in high-income countries, 1990-2013: a systematic review. AIDS Behav. 2017;21(10):2811–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rossi AS. Sexuality Across the Life Course. Chicago, IL: University of Chicago Press; 1994. [Google Scholar]
- 19.National Cancer Institute Surveillance, Epidemiology, and End Results Program SEER 18 Regs Research Data, Nov 2017 Sub (1973-2015). National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. http://www.seer.cancer.gov. Published 2017. Accessed October 1, 2019.
- 20. Chu KC, Miller BA, Feuer EJ, et al. A method for partitioning cancer mortality trends by factors associated with diagnosis: an application to female breast cancer. J Clin Epidemiol. 1994;47(12):1451–1461. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. International Classification of Diseases for Oncology. 3rd ed Geneva: World Health Organization; 2000. [Google Scholar]
- 22. Ruhl J, Callaghan C, Hurlbut A, et al. Summary Stage 2018: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute; 2018. [Google Scholar]
- 23. Clegg LX, Hankey BF, Tiwari R, et al. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keyes KM, Li G.. A multiphase method for estimating cohort effects in age-period contingency table data. Ann Epidemiol. 2010;20(10):779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenberg PS, Check DP, Anderson WF.. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smittenaar CR, Petersen KA, Stewart K, et al. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016;115(9):1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mosher WD, Chandra A, Jones J.. Sexual behavior and selected health measures: men and women 15-44 years of age. Adv Data. 2002;2005(362):1–55. [PubMed] [Google Scholar]
- 28. Copen CE, Chandra A, Febo-Vazquez I.. Sexual Behavior, Sexual Attraction, and Sexual Orientation Among Adults Aged 18-44 in the United States: Data From the 2011-2013 National Survey of Family Growth National Health Statistics Report; 2016:1–14. [PubMed]
- 29. Benson LS, Martins SL, Whitaker AK.. Correlates of heterosexual anal intercourse among women in the 2006-2010 National Survey of Family Growth. J Sex Med. 2015;12(8):1746–1752. [DOI] [PubMed] [Google Scholar]
- 30. Leichliter JS, Chandra A, Liddon N, et al. Prevalence and correlates of heterosexual anal and oral sex in adolescents and adults in the United States. J Infect Dis. 2007;196(12):1852–1859. [DOI] [PubMed] [Google Scholar]
- 31. Moscicki AB, Darragh TM, Berry-Lawhorn JM, et al. Screening for anal cancer in women. J Low Genit Tract Dis. 2015;19(3 suppl 1):S27–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers—United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67(33):918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vespa J, Armstrong DM, Medina L.. Demographic Turning Points for the United States: Population Projections for 2020 to 2060: Population Estimates and Projections Washington, DC: Department of Commerce Economics and Statistics Administration; 2018:25–1144.
- 34. Piketty C, Darragh TM, Da Costa M, et al. High prevalence of anal human papillomavirus infection and anal cancer precursors among HIV-infected persons in the absence of anal intercourse. Ann Intern Med. 2003;138(6):453–459. [DOI] [PubMed] [Google Scholar]
- 35. Holly EA, Whittemore AS, Aston DA, et al. Anal cancer incidence: genital warts, anal fissure or fistula, hemorrhoids, and smoking. J Natl Cancer Inst. 1989;81(22):1726–1731. [DOI] [PubMed] [Google Scholar]
- 36. Tseng HF, Morgenstern H, Mack TM, et al. Risk factors for anal cancer: results of a population-based case--control study. Cancer Causes Control. 2003;14(9):837–846. [DOI] [PubMed] [Google Scholar]
- 37. Hernandez BY, McDuffie K, Zhu X, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiol Biomarkers Prev. 2005;14(11):2550–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suk R, Mahale P, Sonawane K, et al. Trends in risks for second primary cancers associated with index human papillomavirus-associated cancers. JAMA Netw Open. 2018;1(5):e181999.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fokom Domgue J, Messick C, Milbourne A, et al. Prevalence of high-grade anal dysplasia among women with high-grade lower genital tract dysplasia or cancer: results of a pilot study. Gynecol Oncol. 2019;153(2):266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Edgren G, Sparen P.. Risk of anogenital cancer after diagnosis of cervical intraepithelial neoplasia: a prospective population-based study. Lancet Oncol. 2007;8(4):311–316. [DOI] [PubMed] [Google Scholar]
- 41. Lin C, Slama J, Gonzalez P, et al. Cervical determinants of anal HPV infection and high-grade anal lesions in women: a collaborative pooled analysis. Lancet Infect Dis. 2019;19(8):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coffey K, Beral V, Green J, et al. Lifestyle and reproductive risk factors associated with anal cancer in women aged over 50 years. Br J Cancer. 2016;114(12):e16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holford TR, Levy DT, McKay LA, et al. Patterns of birth cohort-specific smoking histories, 1965–2009. Am J Prev Med. 2014;46(2):e31– e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nyitray AG, Peng F, Day RS, et al. The association between body mass index and anal canal human papillomavirus prevalence and persistence: the HIM study. Hum Vaccin Immunother. 2019;15(7–8):1911–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Colón-López V, Shiels MS, Machin M, et al. Anal cancer risk among people with HIV infection in the United States. J Clin Oncol. 2018;36(1):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centers for Disease C, Prevention. Disparities in diagnoses of HIV infection between blacks/African Americans and other racial/ethnic populations--37 states, 2005-2008. MMWR Morb Mortal Wkly Rep. 2011;60(4):93–98. [PubMed] [Google Scholar]
- 47. Shiels MS, Pfeiffer RM, Chaturvedi AK, et al. Impact of the HIV epidemic on the incidence rates of anal cancer in the United States. J Natl Cancer Inst. 2012;104(20):1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harpaz R, Dahl RM, Dooling KL.. Prevalence of immunosuppression among US adults, 2013. JAMA. 2016;316(23):2547–2548. [DOI] [PubMed] [Google Scholar]
- 49. Shiels MS, Copeland G, Goodman MT, et al. Cancer stage at diagnosis in patients infected with the human immunodeficiency virus and transplant recipients. Cancer. 2015;121(12):2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years-United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(33):909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suk R, Montealegre JR, Nemutlu GS, et al. Public knowledge of human papillomavirus and receipt of vaccination recommendations [published online ahead of print September 16, 2019]. JAMA Pediatr. doi:10.1001/jamapediatrics.2019.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deshmukh AA, Cantor SB, Fenwick E, et al. Adjuvant HPV vaccination for anal cancer prevention in HIV-positive men who have sex with men: the time is now. Vaccine. 2017;35(38):5102–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.