Abstract
Objective
Lymphopenia is a frequent clinical manifestation and risk factor for infections in SLE, but the underlying mechanisms are not fully understood. We previously identified novel roles for the RNA-binding protein serine arginine-rich splicing factor 1 (SRSF1) in the control of genes involved in signalling and cytokine production in human T cells. SRSF1 is decreased in T cells from patients with SLE and associates with severe disease. Because SRSF1 controls the expression of apoptosis-related genes, we hypothesized that SRSF1 controls T cell homeostasis and, when reduced, leads to lymphopenia.
Methods
We evaluated SRSF1 expression in T cells from SLE patients by immunoblots and analysed its correlation with clinical parameters. T cell conditional Srsf1 knockout mice were used to evaluate lymphoid cells and apoptosis by flow cytometry. Quantitative PCR and immunoblots were used to assess Bcl-xL mRNA and protein expression. SRSF1 overexpression was performed by transient transfections by electroporation.
Results
We found that low SRSF1 levels correlated with lymphopenia in SLE patients. Selective deletion of Srsf1 in T cells in mice led to T cell lymphopenia, with increased apoptosis and decreased expression of the anti-apoptotic Bcl-xL. Lower SRSF1 expression correlated with low Bcl-xL levels in T cells and lower Bcl-xL levels associated with lymphopenia in SLE patients. Importantly, overexpression of SRSF1 rescued survival of T cells from patients with SLE.
Conclusion
Our studies uncovered a previously unrecognized role for SRSF1 in the control of T cell homeostasis and its reduced expression as a molecular defect that contributes to lymphopenia in systemic autoimmunity.
Keywords: systemic lupus erythematosus, T cells, homeostasis, lymphopenia, SRSF1, Bcl-xL
Rheumatology key messages
Decreased SRSF1 levels correlate with lymphopenia in patients with SLE.
Deficiency of SRSF1 in T cells in mice leads to decreased Bcl-xL, increased apoptosis and lymphopenia.
SRSF1 is a novel regulator of T lymphocyte homeostasis and rescues survival of SLE T cells.
Introduction
SLE is a debilitating systemic autoimmune disease with no cure [1, 2] and a leading cause of mortality in young women [3]. Genetics, environment and hormones contribute to immune dysregulation, leading to pathology in multiple organs, including the skin, joints, kidneys and central nervous system. Although there is substantial clinical heterogeneity of disease, leading to difficulties in diagnosis, lymphopenia is a common clinical feature and also a diagnostic criteria [4, 5]. The cumulative prevalence of lymphopenia in lupus patients over the disease course is 15–93% [6–8] and predisposes to an increased risk of infections, which is a major cause of mortality [8].
In addition to its clinical repercussions, lymphopenia is strongly associated with the development of autoimmunity. Lymphocyte homeostasis is a highly regulated process with the lymphocyte population under strict physiologic control throughout life in mice and humans. Lymphopenia triggers homeostatic proliferation mediated by self-MHC/peptide and stromal-derived homeostatic cytokines and is associated with acquisition of an activated effector/memory cell phenotype [9]. Furthermore, lymphopenia-induced proliferation leads to the expansion of high-affinity, self-reactive T cell clones, contributing to autoimmunity [10, 11]. In humans, T cell recovery after lymphocyte depletion consisted of chronically activated, highly proliferative, oligoclonal, memory-like pro-inflammatory cytokine-producing CD4+ and CD8+ T cells with autoantibodies and clinical autoimmunity [12, 13]. Importantly, lymphopenia in SLE patients associates with worse disease outcomes, including leukopenia, severe disease activity and renal involvement [14]. Thus lymphopenia is an obviously important clinical feature of SLE and has a pathogenic role in the propagation of autoimmunity, yet the underlying mechanisms remain unclear.
Anti-lymphocyte antibodies have been reported in serum from some but not all SLE patients, suggesting that anti-lymphocyte antibodies are not the only cause of lymphopenia in SLE patients [6]. Apoptosis of peripheral blood T lymphocytes is shown to be increased in patients with SLE and was higher in patients with active disease and correlated with their SLE disease activity index (SLEDAI) [15]. However, the precise mechanisms underlying altered apoptosis of lymphocytes from SLE patients are still unclear. Furthermore, whereas patients with SLE displayed increased expression of anti-apoptotic molecules of the Bcl-2 and Fas apoptotic pathways in myeloid cells [16], the role of apoptosis-related genes in T cells in SLE remains unclear.
SLE T cells exhibit numerous molecular defects in signalling, gene regulation and function [2]. A key defect is the reduced expression of the TCR-associated CD3-ζ signalling chain. Aberrant alternative splicing of the CD3ζ 3′ untranslated region (UTR) contributes to its decreased expression in SLE [17]. Using discovery approaches, we identified serine arginine-rich splicing factor 1 (SRSF1) to bind the 3′ UTR of the CD3ζ mRNA and promote expression of its full-length isoform and thus enhance CD3ζ protein expression in human T cells [18, 19]. Importantly, SRSF1 expression levels decrease upon T cell activation [20] and are decreased in T cells from patients with SLE and associate with severe disease [21, 22]. We have recently shown that conditional deletion of Srsf1 in T cells in mice leads to a hyperactive T cell phenotype and systemic autoimmune disease through the phosphatase and tensin homologue (PTEN)–mechanistic target of rapamycin (mTOR) pathway [23]. In addition, overexpression of SRSF1 suppresses mTOR activity and reduces pro-inflammatory cytokine production in T cells from SLE patients [23].
SRSF1 is the prototype member of the serine arginine (SR) family of splicing regulators [24] and controls alternative splicing of genes of the Bcl and caspase family to promote the anti-apoptotic isoforms of genes including Bcl-x [25]. While our recent studies indicate that SRSF1 contributes to the control of T cell hyperactivity and systemic autoimmunity, and SRSF1 is known to control apoptosis-related genes in cancer cells and cell lines [26, 27], its role in immune cell homeostasis has not been studied. Here we show that low SRSF1 expression levels in T cells from SLE patients correlate with lymphopenia. Mechanistically, we show that conditional deletion of Srsf1 in T cells in mice leads to T cell lymphopenia and reduced expression levels of the anti-apoptotic gene Bcl-xL. Importantly, lower expression levels of Bcl-xL in T cells from SLE patients are associated with lymphopenia, and overexpression of SRSF1 in SLE T cells improves cell survival. Thus our studies showed a previously unrecognized role for SRSF1 in the control of T cell homeostasis in vivo and its reduced expression as an underlying molecular defect contributing to lymphopenia in SLE.
Methods
Human subjects
Patients with SLE, all fulfilling the American College of Rheumatology classification criteria [4], were recruited at the rheumatology clinic at Beth Israel Deaconess Medical Center (BIDMC). Age- (±5 years), race- and gender-matched healthy individuals were recruited as controls. Peripheral blood was drawn by venipuncture. For some studies, de-identified healthy volunteer donor blood samples were obtained from the blood donor centre at Boston Children’s Hospital. Written informed consent was obtained from all participants and all studies were approved by the institutional review board (Committee on Clinical Investigations) at BIDMC.
Mice
C57BL/6J (stock 000664), B6.129S4-Srsf1-flox (stock 018020) and B6.dLck.Cre (stock 012837) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were maintained in a specific pathogen-free animal facility at BIDMC. All studies were approved by the Institutional Animal Care and Use Committee.
Antibodies and reagents
Flow cytometry antibodies anti-mouse CD4 (GK1.5), CD8a (53-6.7), CD90.2 (53-2.1), TCR-β (H57-597), purified anti-mouse CD3 (145-2C11), CD28 (37.51), CD16/32 (Fc block), anti-human CD3 (OKT3), CD4 (OKT4), CD8 (RPA-T8), 7-AAD and Annexin V/binding buffer were from BioLegend (San Diego, CA, USA), anti-SRSF1 (96) was from Invitrogen (Waltham, MA, USA), anti-Bcl-xL (54H6) was from Cell Signalling Technology (Danvers, MA, USA), β-actin (AC-74) antibody was from Sigma-Aldrich (St. Louis, MO, USA), goat anti-rabbit IgG-horseradish peroxidase (HRP) and goat anti-mouse IgG-HRP were from Thermo Fisher Scientific (Waltham, MA, USA) and Ammonium-Chloride-Potassium (ACK) lysing buffer was from Fisher Scientific (Pittsburgh, PA, USA).
Tissue processing and cell culture
Spleens and mesenteric lymph nodes (MLNs) were dissected from mice and homogenized using a syringe plunger and 70 μm mesh cell strainer. Red blood cell lysis was performed with ACK lysing buffer. Peripheral blood T cells were isolated using the RosetteSep human T cell enrichment cocktail (STEMCELL Technologies, Vancouver, BC, Canada). All cell cultures were in complete Roswell Park Memorial Institute (RPMI) medium (RPMI plus 10% foetal bovine serum plus penicillin and streptomycin antibiotics). For analysis of apoptosis, cells were stimulated with CD3 (2 μg/ml) and CD28 (2 μg/ml) antibodies.
Flow cytometry
Cells were washed with fluorescence-activated cell sorter (FACS) staining buffer (phosphate buffered saline plus 2% foetal bovine serum). Surface staining with fluorescent antibodies was performed in FACS staining buffer on ice for 20 min with Fc block. For apoptosis detection, cells were stained in Annexin V binding buffer (BioLegend) according to the manufacturer’s instructions. Flow cytometry data were acquired on a five-laser LSRII (BD Biosciences, San Jose, CA, USA) and CytoFLEX LX (Beckman Coulter, Indianapolis, IN, USA) and analysed with FlowJo software (BD, Ashland, OR, USA).
mRNA expression and RT-PCR
Total RNA was isolated using the RNeasy mini kit (Qiagen, Venlo, The Netherlands) and reverse transcribed into cDNA using the ecodry oligodT RNA to cDNA premix (Clontech, Takara Bio USA, Mountain View, CA, USA). Real-time quantitative PCR amplification was carried out with SYBR Green I mastermix using a LightCycler 480 (Roche, Basel, Switzerland) instrument and the following program: initial denaturation at 95°C for 5 min; 40 cycles of amplification (denaturation at 95°C for 15 s, annealing at 60°C for 15 s, extension at 72°C for 30 s); one cycle of melting curves (95°C for 15 s, 65°C for 2 min and 97°C continuous) and final cooling at 37°C. Threshold cycle values were used to calculate relative mRNA expression by the ΔCt relative quantification method. Primer sequences are Bcl-xL forward AACATCCCAGCTTCACATAACCCC, reverse GCGACCCCAGTTTACTCCATCC; Mcl-1 forward TGTAAGGACGAAACGGGACT, reverse AAAGCCAGCAGCACATTTCT; Bcl-2 forward CCTGGCTGTCTCTGAAGACC, reverse CTCACTTGTGGCCCAGGTAT; and cyclophilin A forward GGGTTCCTCCTTTCACAGAA, reverse GATGCCAGGACCTGTATGCT.
Western blots
Total protein extracts were prepared using with radioimmunoprecipitation assay buffer (Boston Bioproducts, Ashland, MA, USA), electrophoresed on NuPAGE 4–12% Bis-Tris gels (Life Technologies, Carlsbad, CA, USA) and transferred to polyvinylidene fluoride membranes. Membranes were blocked with 5% (wt/vol) non-fat milk in Tris-buffered saline with 0.05% Tween 20 (TBS-T) for 1 h, incubated with primary antibody (1:1000 or 1:10 000 for β-actin antibody) in 5% milk in TBS-T or Hikari solution A (Nacalai Tesque, Kyoto, Japan) at 4°C overnight or at room temperature for 2 h for β-actin antibody. Membranes were washed with TBS-T, incubated with HRP-conjugated secondary antibody for 1 h, washed with TBS-T, developed with enhanced chemiluminescence (ECL) reagents (1:2000 ECL or 1:4000 ECL prime; GE Healthcare, Chicago, IL, USA), and visualized by an LAS-4000 imager (Fujifilm, Tokyo, Japan) or a ChemiDoc XRS imager (Bio-Rad, Hercules, CA, USA). Densitometry was performed using Quantity One software (Bio-Rad). For the SRSF1 and Bcl-xL western blots, total protein was isolated from T cells from SLE patients and age- (±5 years), race- and gender-matched normal healthy control individuals. SLE and healthy control protein samples were electrophoresed on gels and transferred to membranes and immunoblots were performed for SRSF1, Bcl-xL and β-actin. Densitometric quantitation of blots was performed to obtain intensity values for these three proteins. Expression values of SRSF1 or Bcl-xL were first normalized to β-actin, then the relative SRSF1 or Bcl-xL value of each SLE patient was normalized to values from the respective matched healthy control sample run on the same gel (expression in healthy controls = 1).
Transfections
Human peripheral blood mononuclear cells (PBMCs) were transfected using the Amaxa Human T Cell Nucleofector Kit (Lonza, Cologne, Germany) following the manufacturer’s instructions. Briefly, 3–6 × 106 cells were resuspended in 100 μl of nucleofector solution. Plasmid DNA pcDNA3.1 empty vector (EV) or pcDNA3.1-Srsf1 (0.5 μg/106 cells) was added and cells were transferred into a cuvette and electroporated using the U-014 program in the nucleofector device. Cells were immediately rescued into prewarmed medium and cultured overnight.
Statistics
Statistical analyses were performed in GraphPad Prism (GraphPad Software, San Diego, CA, USA). Student’s two-tailed t test and linear regression were used to calculate statistical significance among groups. A P-value <0.05 was considered significant.
Results
Low SRSF1 levels correlate with lymphopenia in patients with SLE
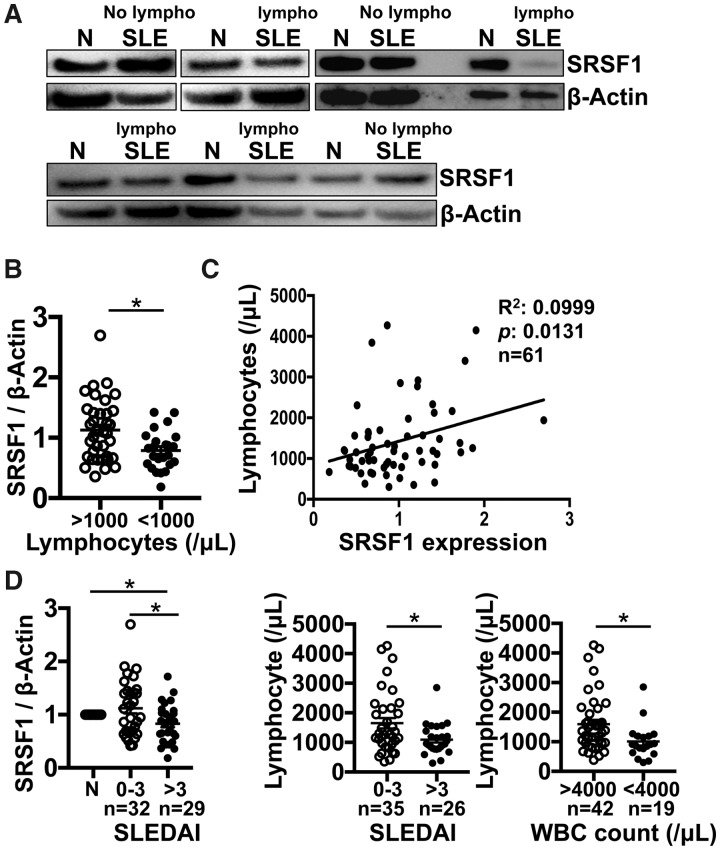
To assess the relationship between SRSF1 expression levels and T cell homeostasis in patients with SLE, T cells were isolated from peripheral blood from SLE patients and from age-, race- and gender-matched healthy control individuals (Table 1). We found that the expression levels of SRSF1 in T cells from SLE patients with lymphopenia (lymphocyte count <1000/μl) were significantly lower than those from patients without lymphopenia (Fig. 1A and B). Furthermore, there was a linear correlation between SRSF1 levels and lymphocyte counts in SLE patients (Fig. 1C). Consistent with our previous results [21, 22], lower SRSF1 protein expression levels associated with severe disease activity, as indicated by higher SLEDAI scores (Fig. 1D, left panel). In addition, lymphopenia correlated with higher SLEDAI scores (Fig. 1D, middle panel) and with leukopenia [white blood cell (WBC) count <4000/μl] (Fig. 1D, right panel) in SLE patients. There was no significant correlation between SRSF1 levels and other clinical features, including serum complement levels, anaemia, platelet counts, serum creatinine levels, proteinuria or treatment with prednisone or hydroxychloroquine (Supplementary Fig. 1A, available at Rheumatology online). Lymphopenia did not associate with other clinical features (Supplementary Fig. 1B, available at Rheumatology online) or with prednisone treatment in patients (Supplementary Fig. 1C, available at Rheumatology online). These data indicate that low expression levels of SRSF1 in T cells correlate with lymphopenia in SLE patients.
Table 1.
Demographics and clinical characteristics of SLE patients
| Demographic and clinical parameters | Values |
|---|---|
| Patients, N | 69 |
| Gender, n/n | |
| Female | 67 |
| Male | 2 |
| Race, n | |
| White | 31 |
| Black | 28 |
| Asian | 9 |
| Mixed | 1 |
| Age, years | 37.2 (10.5) (19–60) |
| SLEDAI | 3.17 (3.4) (0–16) |
| C3, mg/dl | 110.8 (37.4) |
| C4, mg/dl | 19.7 (12.1) |
| WBC, /μl | 6040 (3400) |
| Lymphocytes, /μl | 1470 (970) |
| Haemoglobin, g/dl | 12.6 (1.2) |
| Platelets, ×103/μl | 244 (78.6) |
| Creatinine, mg/dl | 0.72 (0.18) |
| Prednisone, mg/day | 6.7 (10.0) (0–50) |
| Hydroxychloroquine (n = 21) | 304 (93.5) (200–400) |
| Azathioprine (n = 6) | 100 (35.4) (50–150) |
| Mycophenolate mofetil (n = 5) | 2200 (1100) (1000–3000) |
Values are presented as mean (s.d.) (minimum–maximum).
Fig. 1.
Low SRSF1 levels correlate with lymphopenia in patients with SLE
Peripheral blood T cells were isolated from patients with SLE (n = 61) and healthy control individuals (n = 44). Total protein was immunoblotted for SRSF1 and β-actin. (A) Data are from one representative of eight independent experiments. (B) Densitometric quantitation of Western blots was performed and SRSF1 normalized to β-actin. Relative SRSF1 expression in SLE patients was normalized to matched healthy controls. (C) Graph shows linear correlation between relative SRSF1 protein levels and peripheral blood lymphocyte counts (n = 61). (D) Graphs show associations of relative SRSF1 with SLEDAI and lymphocyte counts with SLEDAI or WBC counts [B, D (middle and right): unpaired t test; D (left): one-way analysis of variance with Tukey’s correction; C: single linear regression, *P < 0.05].
T cell conditional deletion of Srsf1 in mice leads to lymphopenia and increased apoptosis
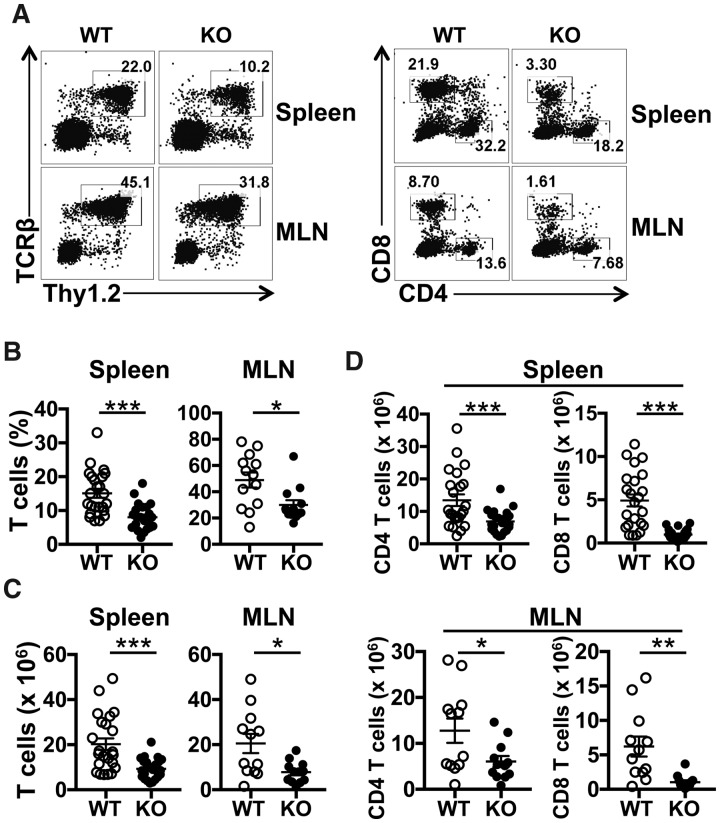
SRSF1 is known to be a pro-survival factor and its deletion in cell lines in vitro leads to apoptosis [28]. However, very little is known of the role of SRSF1 in the immune system and it is not known if SRSF1 controls immune cell homeostasis. We recently generated T cell restricted Srsf1flox/flox distal LckCreSrsf1-knockout (ko) mice, which exhibit T cell hyperactivity and develop systemic autoimmunity [23]. To evaluate the role of SRSF1 in T cell homeostasis, we wished to assess the central and peripheral lymphoid T cell compartments in the Srsf1-ko mice. Because the Cre recombinase is under control of the distal Lck promoter, which is expressed late during thymic development, the deletion of Srsf1 occurs mainly in single positive and mature T cells after thymic exit. Therefore thymic development and cellularity are normal in Srsf1-ko mice [23]. To evaluate the peripheral lymphoid compartments, we isolated cells from the spleen and MLNs from wild-type (WT) and Srsf1-ko mice and assessed the T cell proportions and absolute numbers by flow cytometry. We found that the frequency of T cells in peripheral lymphoid tissues, including spleen and MLNs from young ko mice (<20 weeks old), was significantly lower than that of WT mice (Fig. 2A and B). In addition, the absolute numbers of total T cells, CD4 T cells and CD8 T cells in the spleen and MLNs were also significantly decreased in Srsf1-ko mice (Fig. 2C and D). As we found previously, there was no significant difference in the frequencies or absolute numbers of other immune cell subsets, including B cells, dendritic cells and monocytes [23].
Fig. 2.
T cell conditional deletion of Srsf1 in mice leads to lymphopenia
Spleen and MLN cells from WT and Srsf1-ko mice were stained with fluorescent antibodies and analysed by flow cytometry. (A) Plots show Thy1.2+ TCRβ+ T cells (left panel) and CD4+ and CD8+ T cells (right panel) gated on live cells. (B) Graphs show the percentage of T cells (spleen: n = 24 each, MLN: n = 13 each, mice <20 weeks old). (C) Graphs show absolute number of T cells (spleen: n = 23 each, MLN: n = 12 each, mice <20 weeks old). (D) Graphs show absolute numbers of CD4 and CD8 T cells (spleen: n = 23 each, MLNs: n = 12 each, mice <20 weeks old) (unpaired t test, *P < 0.05, **P < 0.005, ***P < 0.0005).
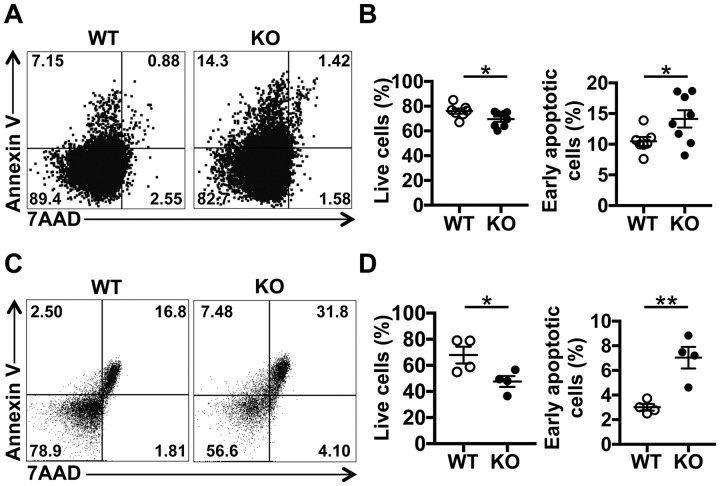
In cancer cells, SRSF1 is known to control apoptosis and promote cell survival [24], and overexpression of SRSF1 increased proliferation and delayed apoptosis in mammary epithelial cells [27]. Yet its role in immune cell apoptosis is unknown. We asked if T cells from Srsf1-ko mice exhibit increased apoptosis. Spleen cells were isolated from WT and Srsf1-ko mice and live T cells (Annexin V− 7AAD−) and early apoptosis (Annexin V+ 7AAD−) were assessed by flow cytometry. We found that ex vivo spleen T cells from Srsf1-ko mice exhibit lower frequencies of live T cells and higher frequencies of early apoptotic cells than those from WT mice (Fig. 3A and B). In addition, after TCR stimulation with anti-mouse CD3 and CD28 antibodies, a higher frequency of early apoptotic cells was observed among spleen T cells from Srsf1-ko mice compared with those from WT mice (Fig. 3C and D). These results showed that SRSF1 plays an important role in T cell homeostasis through the regulation of apoptosis.
Fig. 3.
T cell conditional deletion of Srsf1 in mice leads to increased apoptosis
Spleen cells were isolated from WT and Srsf1-ko mice, stained with fluorescent antibodies and analysed by flow cytometry. (A) Plots show 7AAD and Annexin V expression on ex vivo gated T cells. (B) Graph shows the percentage of live cells and early apoptotic cells (7AAD−Annexin V+) (WT, n = 7; ko, n = 8). (C) Spleen cells were stimulated with anti-CD3 (2 μg/ml) and anti-CD28 (2 μg/ml) antibodies for 48 h. Flow cytometry plots show 7AAD and Annexin V expression on T cells. (D) Graph shows the percentage of live cells and early apoptotic cells (7AAD−Annexin V+) (n = 4) (unpaired t test, *P < 0.05, **P < 0.005).
Bcl-xL expression is decreased in T cells from Srsf1-ko mice
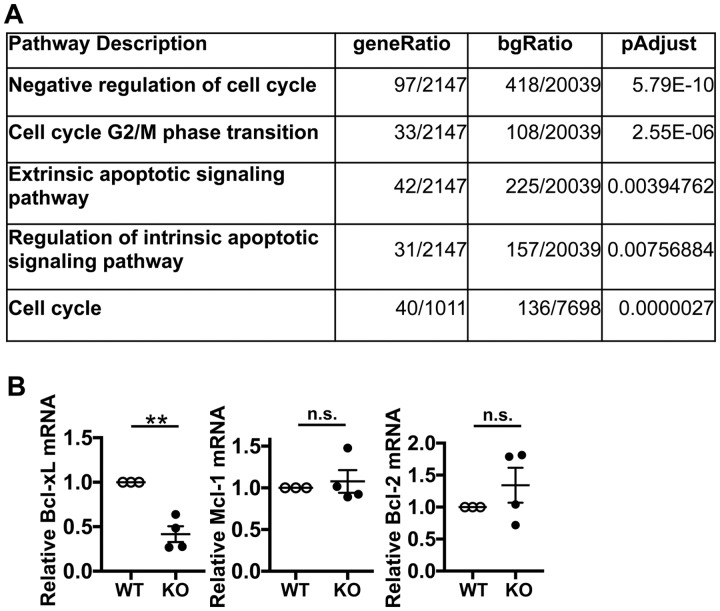
Because the Srsf1-ko mice develop T cell lymphopenia and exhibit increased apoptosis, we asked whether SRSF1 controls genes and pathways involved in apoptosis. We recently performed transcriptomics analysis by RNA sequencing of CD4 effector T (Teff) cells derived by stimulation of naïve CD4 T cells with anti-CD3 and anti-CD28 antibodies for 72 h [23] and found a number of genes differentially expressed. At fold change >1, P-value <0.05 and 1911 total genes, 890 were upregulated and 1021 were downregulated in the Srsf1-ko vs control mice [23]. Gene ontology enrichment and Kyoto Encyclopedia of Genes and Genomes pathway analyses revealed the aberrant pathways to include genes involved in the extrinsic apoptotic signalling pathway, regulation of the intrinsic apoptotic signalling pathway, negative regulation of the cell cycle, cell cycle G2/M transition and the cell cycle (Fig. 4A). These data suggest that deficiency of SRSF1 leads to the dysregulation of genes involved in apoptosis.
Fig. 4.
Bcl-xL is decreased in T cells from Srsf1-ko mice
(A) RNA sequencing data analysis of CD4 effector T cells from WT and Srsf1-ko mice shows pathways identified by the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and gene ontology (GO) analyses of differentially expressed genes. (B) Conventional CD4 T cells (CD4+CD25−) were sorted by flow cytometry and stimulated with anti-CD3 (5 μg/ml) and anti-CD28 (5 μg/ml) antibodies for 24 h. Total RNA was isolated and reverse transcribed. Expression levels of apoptosis-associated genes were measured by real-time quantitative PCR and normalized to housekeeping gene cyclophilin A (n = 4 each, unpaired t test, **P < 0.005, n.s. not significant).
SRSF1 controls the alternative splicing of apoptosis-related genes including Bcl-x [27]. Bcl-x belongs to the Bcl-2 family of proteins, and alternative splicing leads to synthesis of two isoforms with antagonistic activities, anti-apoptotic Bcl-xL (long isoform) and pro-apoptotic Bcl-xS (short isoform). In cell lines, the knockdown of SRSF1 decreases the anti-apoptotic isoform of Bcl-xL [25], and overexpression of SRSF1 promotes the generation of Bcl-xL [29]. In addition, SRSF1 controls the expression levels of other Bcl-2 family genes, including Mcl-1 [30]. We analysed the expression levels of these genes in T cells from Srsf1-ko mice. Importantly, the expression levels of anti-apoptotic isoform Bcl-xL were significantly decreased in T cells from Srsf1-ko mice compared with those from WT mice (Fig. 4B). There was no difference in the expression levels of Mcl-1 or Bcl-2 (Fig. 4B). These data indicate that the loss of Srsf1 in T cells leads to increased apoptosis through the regulation of anti-apoptotic Bcl-xL expression.
Low Bcl-xL levels correlate with SRSF1 expression in T cells and associate with lymphopenia in SLE patients
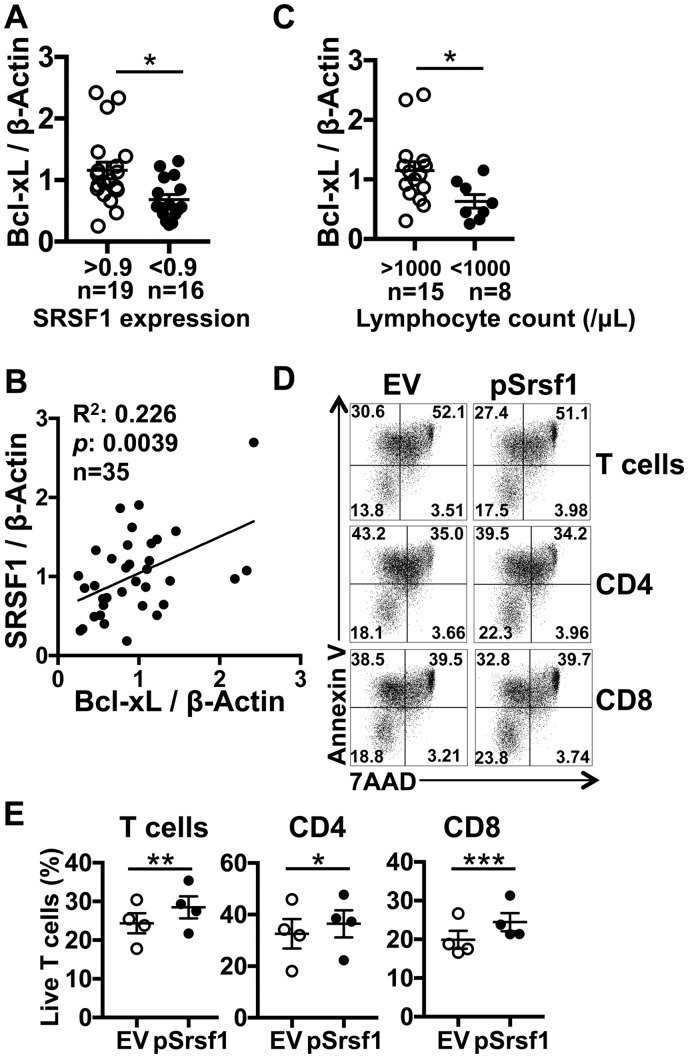
Because we found that lower expression levels of SRSF1 in T cells from SLE patients are associated with lymphopenia, and the deletion of Srsf1 in T cells in mice leads to lower expression levels of Bcl-xL, we asked if there was a correlation between the expression levels of Bcl-xL with SRSF1 and lymphopenia in SLE patients. We isolated T cells from peripheral blood of SLE patients and assessed protein levels of Bcl-xL and SRSF1 by western blotting. Interestingly, the expression levels of Bcl-xL were significantly lower in T cells from SLE patients with lower SRSF1 expression (Fig. 5A). In addition, we observed a linear correlation between Bcl-xL and SRSF1 expression levels in T cells from SLE patients (R2 = 0.226, P = 0.0039; Fig. 5B). We observed a similar linear correlation between SRSF1 and Bcl-xL protein levels in T cells from normal healthy control individuals (Supplementary Fig. 2, available at Rheumatology online). More importantly, we found that the expression levels of Bcl-xL were significantly lower in T cells from SLE patients with lymphopenia compared with those from patients without lymphopenia (Fig. 5C). These results suggest that SRSF1 plays an important role in lymphopenia in SLE patients through the regulation of Bcl-xL expression.
Fig. 5.
Low Bcl-xL levels correlate with SRSF1 expression in T cells and associate with lymphopenia in SLE patients
SRSF1 overexpression rescues survival of SLE T cells. Peripheral blood T cells were isolated from patients with SLE and healthy control individuals. Total protein was immunoblotted for SRSF1, Bcl-xL and β-actin. (A) Graph shows the relative quantitation of Bcl-xL/β-actin by densitometry (n = 35). (B) The graph shows the linear correlation between Bcl-xL and SRSF1 (n = 35). (C) The graph shows the relative quantitation of Bcl-xL/β-actin by densitometry (n = 23) in association with lymphocyte counts grouped by lymphopenia. (D and E) PBMCs were isolated from peripheral blood from patients with SLE and transfected with empty vector (EV) or Srsf1 overexpression plasmid (pSrsf1). At 16–18 h after transfection, cells were analysed by flow cytometry. Plots show 7AAD and Annexin V expression on gated T cells, CD4 T cells and CD8 T cells (D). Graphs show the percentage of live (7AAD−Annexin V−) cells (E, n = 4) (A and C: unpaired t test, B: single linear regression, E: paired t test; *P < 0.05, **P < 0.005, ***P < 0.0005).
Overexpression of SRSF1 rescues survival of T cells from SLE patients
SLE patients commonly suffer from lymphopenia, which is shown to affect T cells more than B cells [31]. Given that the SRSF1 levels in T cells correlate with lymphocyte counts and with the expression of anti-apoptotic Bcl-xL, we asked whether overexpression of SRSF1 into SLE T cells could rescue T cell survival. To confirm that SRSF1 plays an important role in the homeostasis of lupus T cells, SRSF1 was overexpressed by transient transfections in PBMCs from patients with SLE and cell survival measured by flow cytometry staining. We found that the proportion of live T cells was increased by the overexpression of SRSF1 (Fig. 5D and E), therefore SRSF1 overexpression rescued survival of the SLE T cells. These results indicate that decreased SRSF1 expression contributes to increased apoptosis in T cells from SLE patients through low Bcl-xL expression and that overexpression of SRSF1 can rescue the lymphopenic phenotype.
Discussion
In this study we report a number of interesting findings. We show that lower expression levels of SRSF1 in T cells associate with lymphopenia in patients with SLE (Fig. 1). Consistent with this finding, T cell–conditional Srsf1-ko mice exhibit T cell lymphopenia and increased apoptosis (Figs 2 and 3). At a mechanistic level, we find that the expression levels of anti-apoptotic isoform Bcl-xL are decreased in Srsf1-deficient T cells (Fig. 4). Furthermore, T cells from SLE patients with lymphopenia had decreased levels of Bcl-xL, which correlate with decreased expression levels of SRSF1 (Fig. 5). Finally, overexpression of SRSF1 in T cells from SLE patients rescues the lymphopenic phenotype (Fig. 5).
Immunodeficiency and autoimmunity are closely linked and result from a dysfunctional immune system and are considered to be two sides of the same coin [32]. SRSF1 is known to play an important role in cellular homeostasis, and the deletion of SRSF1 induces cell cycle arrest and apoptosis in cell lines [28]. In this study we found that young T cell–conditional Srsf1-ko mice exhibit T cell lymphopenia. We have previously shown that the Srsf1-ko mice develop systemic autoimmunity through activation of the mTOR pathway via decreased expression of PTEN, a negative regulator of the mTOR pathway [23]. In addition to this pathway, the deficiency of SRSF1 may drive autoimmunity through lymphopenia-induced proliferation in these mice. This lymphoproliferation is evident by the increased cellularity of spleens in a number of mice >12–18 months of age [23]. Importantly, the expression levels of SRSF1 in T cells from SLE patients with lymphopenia were significantly lower than in those without lymphopenia, suggesting that SRSF1 is a previously unrecognized factor, reduced levels of which contribute to lymphopenia and autoimmune disease pathogenesis in SLE.
The mTOR pathway plays important and varied roles in T cell proliferation and has recently emerged as a key pathogenic mechanism in autoimmune disease [33, 34]. mTOR-deficient CD4 T cells proliferate less in response to activation [35], and the deletion of RAPTOR, an essential signalling adaptor for mTORC1, abrogated the generation of plasma cells [36]. In turn, rapamycin inhibits the mTORC1 pathway in Tregs from SLE patients and promotes their expansion in vitro [37]. In addition, rapamycin improves the quantity and quality of memory CD8 T cells induced by viral infection [38]. However, mTOR blockade with rapamycin/sirolimus did not change leucocyte counts in SLE patients [39], suggesting that additional mechanisms contribute to lymphopenia in SLE patients. Our finding that SRSF1 controls the expression of the anti-apoptotic molecule Bcl-xL indicates that this may be a plausible mechanism underlying lymphopenia independent of its role in the mTOR pathway (Supplementary Fig. 3, available at Rheumatology online).
Recently we showed that the deficiency of SRSF1 in T cells leads to a hyperactive T cell phenotype and systemic autoimmunity in mice through activation of the mTOR pathway [23]. Importantly, rapamycin administration alleviates features of autoimmunity in these mice. Recent studies have shown the value of sirolimus/rapamycin in improving disease parameters such as arthritis, new rash and pyuria in SLE patients [39]. These in vivo and clinical studies suggest that the mTOR pathway is one of the underlying molecular mechanisms that connects low SRSF1 expression levels with T cell abnormalities and thus with disease activity in SLE. However, further studies with larger cohorts are required to determine how SRSF1 expression levels may stratify clinical subgroups of SLE patients.
The underlying pathogenesis of lymphopenia in SLE remains unclear. Autoantibodies including antilymphocyte antibodies have long been considered to contribute to lymphopenia [8]. Recently, lymphocyte apoptosis has been recognized as another mechanism underlying lymphopenia in patients with SLE [40]. Higher expression of the apoptosis-inducing Fas ligand was found both in naïve and memory T cells from SLE patients, which negatively correlated with the peripheral lymphocyte counts [41]. Another study found that the expression levels of Bcl-xL were decreased in T cells from patients with SLE compared with healthy individuals [42]. However, little is known of the role of apoptosis-related genes, and specifically Bcl-xL, in lymphopenia in SLE patients. In this study we observed that the expression levels of Bcl-xL were significantly lower in T cells from SLE patients and correlated with SRSF1 expression. Thus we uncovered the aberrantly low expression of Bcl-xL in SLE T cells as a potential underlying molecular defect in lymphopenia.
Excessive apoptosis and impaired clearance of apoptotic debris leads to an overload of self-antigens in the pathogenesis of SLE [43]. Pristane administration, which leads to a lupus-like autoimmune syndrome in mice, induces apoptosis, and the nuclear autoantigens thus created may be the initiating events in the development of autoimmunity [44]. Deficiency of caspase-activated DNase, which is responsible for DNA degradation during apoptosis, results in increased anti-dsDNA antibodies in lupus-prone mice [45]. In addition, the intrinsic apoptotic pathway mediated by the Bcl-2 genes are remarkably disordered in SLE [46, 47]. In this study we showed that SRSF1 plays an important role in control of apoptosis of T cells via regulation of Bcl-xL expression in mice and in patients with SLE.
A limitation is that our current study of SLE patients is cross-sectional and it is not known how Bcl-xL and SRSF1 change in patients over time and how they relate to disease activity, lymphopenia and treatment during the disease course. Furthermore, due to the small cohort size, we were unable to assess correlations with specific clinical features of lupus that might enable further stratification of patients. Another limitation is that our studies of SRSF1 expression in SLE are in total T cells and it will be important to assess these in CD4 or CD8 T cells and the subsets therein. Furthermore, the available clinical lab parameters of recruited patients included total WBC counts and total lymphocyte counts but not T lymphocyte counts or subsets. Therefore, in future studies, we plan to assess the T cell and CD4/CD8 subset distributions and correlations with SRSF1 expression. Interestingly, it was reported that Bcl-xL is required for the development of functional regulatory CD4 T cells in lupus-prone mice [48]. Further studies are required to assess the role of Bcl-xL and SRSF1 in regulatory T cells in autoimmunity.
Our study has uncovered the role of a new molecule SRSF1 in the control of T cell homeostasis and its reduced levels to contribute to lymphopenia in SLE patients. Given these findings, it is important to investigate the underlying molecular mechanisms of altered SRSF1 levels in SLE. We have previously shown that ubiquitin-induced proteasomal degradation contributes to the downregulation of SRSF1 in T cells from SLE patients [20]. Furthermore, SRSF1 was decreased in muscle biopsies from patients with autoimmune inflammatory myositis and TNF-α downregulated SRSF1 protein levels in differentiated C2C12 myotubes [49]. The activity of SRSF1 and other SR proteins is partially dependent on phosphorylation and these proteins are dephosphorylated by ceramide-induced activation of protein phosphatase 1. Ceramides are lipid metabolites generated by sphingomyelin hydrolysis induced by various environmental triggers such as ultraviolet radiation, inflammatory cytokines including TNF and cytotoxic drugs, and this dephosphorylation may alter the splicing of Bcl-xL [26, 50]. Therefore these factors may influence the expression and/or activity of SRSF1. In addition, epigenetic modifications including microRNA (miR)-mediated regulation are known to control the expression of SRSF1 [51]. In addition, hormones are integral to lupus pathogenesis, and oestrogen is known to control not only transcriptional activity of immune-related genes but also post-transcriptional gene expression via miR-mediated regulation [52] and post-translational expression via the ubiquitin-proteasome protein turnover pathway [53]. Further studies are needed to delineate the precise role of these factors in regulating SRSF1 expression in SLE.
In conclusion, we have uncovered a previously unidentified role of SRSF1 in T cell homeostasis and demonstrated the association between the low expression levels of SRSF1 and Bcl-xL in T cells and lymphopenia in patients with SLE. Our results implicate the decreased SRSF1 levels as a molecular defect in the underlying mechanisms of lymphopenia in SLE.
Supplementary Material
Acknowledgements
The authors acknowledge Dr George C. Tsokos for providing access to patient samples and critical reading of the manuscript and Kotaro Iida for excellent technical assistance. The authors thank Dr Roberto Caricchio for helpful suggestions. TK and VRM conceptualized the study and designed the experiments. TK, IJM and VRM performed the experiments. SK and VK contributed patient samples and clinical information. TK, IJM, VK and VRM analysed and interpreted the data. All authors were involved in drafting the manuscript or revising it critically and approved the final version of the manuscript.
Funding: This work was supported by grants from the National Institutes of Health (R01AR067894 to VRM and R01AI042269 to GCT).
Disclosure statement: VK has a grant from Exagen Diagnostics and is conducting clinical trials funded by AbbVie and Takeda. The other authors have declared no conflicts of interest.
References
- 1. Tsokos GC. Systemic lupus erythematosus. N Engl J Med 2011;365:2110–21. [DOI] [PubMed] [Google Scholar]
- 2. Katsuyama T, Tsokos GC, Moulton VR.. Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front Immunol 2018;9:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yen EY, Singh RR.. Brief report: lupus—an unrecognized leading cause of death in young females: a population-based study using nationwide death certificates, 2000–2015. Arthritis Rheumatol 2018;70:1251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan EM, Cohen AS, Fries JF. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 5. Tedeschi SK, Johnson SR, Boumpas D. et al. Developing and refining new candidate criteria for systemic lupus erythematosus classification: an international collaboration. Arthritis Care Res 2018;70:571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li C, Mu R, Lu X. et al. Antilymphocyte antibodies in systemic lupus erythematosus: association with disease activity and lymphopenia. J Immunol Res 2014;2014:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carli L, Tani C, Vagnani S, Signorini V, Mosca M.. Leukopenia, lymphopenia, and neutropenia in systemic lupus erythematosus: prevalence and clinical impact—a systematic literature review. Semin Arthritis Rheum 2015;45:190–4. [DOI] [PubMed] [Google Scholar]
- 8. Fayyaz A, Igoe A, Kurien BT. et al. Haematological manifestations of lupus. Lupus Sci Med 2015;2:e000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sprent J, Surh CD.. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol 2011;12:478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baccala R, Theofilopoulos AN.. The new paradigm of T-cell homeostatic proliferation-induced autoimmunity. Trends Immunol 2005;26:5–8. [DOI] [PubMed] [Google Scholar]
- 11. Datta S, Sarvetnick N.. Lymphocyte proliferation in immune-mediated diseases. Trends Immunol 2009;30:430–8. [DOI] [PubMed] [Google Scholar]
- 12. Jones JL, Thompson S, Loh P, Davies JL. et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc Natl Acad Sci USA 2013;110:20200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merayo-Chalico J, Rajme-López S, Barrera-Vargas A. et al. Lymphopenia and autoimmunity: a double-edged sword. Hum Immunol 2016;77:921–9. [DOI] [PubMed] [Google Scholar]
- 14. Vilá LM, Alarcón GS, McGwin G, Bastian HM, Fessler BJ, Reveille JD.. Systemic lupus erythematosus in a multiethnic US cohort, XXXVII: association of lymphopenia with clinical manifestations, serologic abnormalities, disease activity, and damage accrual. Arthritis Rheum 2006;55:799–806. [DOI] [PubMed] [Google Scholar]
- 15. Dhir V, Singh AP, Aggarwal A. et al. Increased T-lymphocyte apoptosis in lupus correlates with disease activity and may be responsible for reduced T-cell frequency: a cross-sectional and longitudinal study. Lupus 2009;18:785–91. [DOI] [PubMed] [Google Scholar]
- 16. Hutcheson J, Scatizzi JC, Siddiqui AM. et al. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity 2008;28:206–17. [DOI] [PubMed] [Google Scholar]
- 17. Chowdhury B, Tsokos CG, Krishnan S. et al. Decreased stability and translation of T cell receptor ζ mRNA with an alternatively spliced 3′-untranslated region contribute to ζ chain down-regulation in patients with systemic lupus erythematosus. J Biol Chem 2005;280:18959–66. [DOI] [PubMed] [Google Scholar]
- 18. Moulton VR, Kyttaris VC, Juang Y-T, Chowdhury B, Tsokos GC.. The RNA-stabilizing protein HuR regulates the expression of ζ chain of the human T cell receptor-associated CD3 complex. J Biol Chem 2008;283:20037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moulton VR, Tsokos GC.. Alternative splicing factor/splicing factor 2 regulates the expression of the ζ subunit of the human T cell receptor-associated CD3 complex. J Biol Chem 2010;285:12490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moulton VR, Gillooly AR, Tsokos GC.. Ubiquitination regulates expression of the serine/arginine-rich splicing factor 1 (SRSF1) in normal and systemic lupus erythematosus (SLE) T cells. J Biol Chem 2014;289:4126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moulton VR, Grammatikos AP, Fitzgerald LM, Tsokos GC.. Splicing factor SF2/ASF rescues IL-2 production in T cells from systemic lupus erythematosus patients by activating IL-2 transcription. Proc Natl Acad Sci USA 2013;110:1845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kono M, Kurita T, Yasuda S. et al. Decreased expression of serine/arginine-rich splicing factor 1 in T cells from patients with active systemic lupus erythematosus accounts for reduced expression of RasGRP1 and DNA methyltransferase 1. Arthritis Rheumatol 2018;70:2046–56. [DOI] [PubMed] [Google Scholar]
- 23. Katsuyama T, Li H, Comte D, Tsokos GC, Moulton VR.. Splicing factor SRSF1 controls T cell hyperactivity and systemic autoimmunity. J Clin Invest 2019;129:5411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Das S, Krainer AR.. Emerging functions of SRSF1, splicing factor and oncoprotein, in RNA metabolism and cancer. Mol Cancer Res 2014;12:1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bielli P, Bordi M, Di Biasio V, Sette C.. Regulation of BCL-X splicing reveals a role for the polypyrimidine tract binding protein (PTBP1/hnRNP I) in alternative 5′ splice site selection. Nucleic Acids Res 2014;42:12070–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chalfant CE, Rathman K, Pinkerman RL. et al. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem 2002;277:12587–95. [DOI] [PubMed] [Google Scholar]
- 27. Anczuków O, Rosenberg AZ, Akerman M. et al. The splicing factor SRSF1 regulates apoptosis and proliferation to promote mammary epithelial cell transformation. Nat Struct Mol Biol 2012;19:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li X, Wang J, Manley JL.. Loss of splicing factor ASF/SF2 induces G2 cell cycle arrest and apoptosis, but inhibits internucleosomal DNA fragmentation. Genes Dev 2005;19:2705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leu S, Lin Y-M, Wu C-H, Ouyang P.. Loss of Pnn expression results in mouse early embryonic lethality and cellular apoptosis through SRSF1-mediated alternative expression of Bcl-xS and ICAD. J Cell Sci 2012;125:3164–72. [DOI] [PubMed] [Google Scholar]
- 30. Kędzierska H, Piekiełko-Witkowska A.. Splicing factors of SR and hnRNP families as regulators of apoptosis in cancer. Cancer Lett 2017;396:53–65. [DOI] [PubMed] [Google Scholar]
- 31. Wouters CH, Diegenant C, Ceuppens JL, Degreef H, Stevens EA.. The circulating lymphocyte profiles in patients with discoid lupus erythematosus and systemic lupus erythematosus suggest a pathogenetic relationship. Br J Dermatol 2004;150:693–700. [DOI] [PubMed] [Google Scholar]
- 32. Grimbacher B, Warnatz K, Yong PFK, Korganow A-S, Peter H-H.. The crossroads of autoimmunity and immunodeficiency: lessons from polygenic traits and monogenic defects. J Allergy Clin Immunol 2016;137:3–17. [DOI] [PubMed] [Google Scholar]
- 33. Perl A. mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Ann NY Acad Sci 2015;1346:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perl A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol 2016;12:169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Delgoffe GM, Kole TP, Zheng Y. et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009;30:832–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones DD, Gaudette BT, Wilmore JR. et al. mTOR has distinct functions in generating versus sustaining humoral immunity. J Clin Invest 2016. 01;126:4250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kato H, Perl A.. Mechanistic target of rapamycin complex 1 expands Th17 and IL-4+ CD4−CD8− double-negative T cells and contracts regulatory T cells in systemic lupus erythematosus. J Immunol 2014;192:4134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Araki K, Turner AP, Shaffer VO. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 2009;460:108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lai Z-W, Kelly R, Winans T. et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet 2018;391:1186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silva LM, Garcia AB, Donadi EA.. Increased lymphocyte death by neglect-apoptosis is associated with lymphopenia and autoantibodies in lupus patients presenting with neuropsychiatric manifestations. J Neurol 2002;249:1048–54. [DOI] [PubMed] [Google Scholar]
- 41. Amasaki Y, Kobayashi S, Takeda T. et al. Up-regulated expression of Fas antigen (CD95) by peripheral naive and memory T cell subsets in patients with systemic lupus erythematosus (SLE): a possible mechanism for lymphopenia. Clin Exp Immunol 2008;99:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee W-S, Sung M-S, Lee E-G. et al. A pathogenic role for ER stress-induced autophagy and ER chaperone GRP78/BiP in T lymphocyte systemic lupus erythematosus. J Leukoc Biol 2015;97:425–33. [DOI] [PubMed] [Google Scholar]
- 43. Sanford AN, Suriano AR, Herche D, Dietzmann K, Sullivan KE.. Abnormal apoptosis in chronic granulomatous disease and autoantibody production characteristic of lupus. Rheumatology (Oxford) 2006;45:178–81. [DOI] [PubMed] [Google Scholar]
- 44. Calvani N, Caricchio R, Tucci M. et al. Induction of apoptosis by the hydrocarbon oil pristane: implications for pristane-induced lupus. J Immunol 2005;175:4777–82. [DOI] [PubMed] [Google Scholar]
- 45. Jog NR, Frisoni L, Shi Q. et al. Caspase-activated DNase is required for maintenance of tolerance to lupus nuclear autoantigens. Arthritis Rheum 2012;64:1247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Badillo-Almaráz I, Daza L, Avalos-Díaz E, Herrera-Esparza R.. Glomerular expression of Fas ligand and Bax mRNA in lupus nephritis. Autoimmunity 2001;34:283–9. [DOI] [PubMed] [Google Scholar]
- 47. Liphaus BL, Kiss MHB, Carrasco S, Goldenstein-Schainberg C.. Increased Fas and Bcl-2 expression on peripheral blood T and B lymphocytes from juvenile-onset systemic lupus erythematosus, but not from juvenile rheumatoid arthritis and juvenile dermatomyositis. Clin Dev Immunol 2006;13:283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sharabi A, Lapter S, Mozes E.. Bcl-xL is required for the development of functional regulatory CD4 cells in lupus-afflicted mice following treatment with a tolerogenic peptide. J Autoimmun 2010;34:87–95. [DOI] [PubMed] [Google Scholar]
- 49. Xiong Z, Shaibani A, Li Y-P. et al. Alternative splicing factor ASF/SF2 is down regulated in inflamed muscle. J Clin Pathol 2006;59:855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pettus BJ, Chalfant CE, Hannun YA.. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta 2002;1585:114–25. [DOI] [PubMed] [Google Scholar]
- 51. Sokół E, Kędzierska H, Czubaty A. et al. microRNA-mediated regulation of splicing factors SRSF1, SRSF2 and hnRNP A1 in context of their alternatively spliced 3′UTRs. Exp Cell Res 2018;363:208–17. [DOI] [PubMed] [Google Scholar]
- 52. Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol 2018;9:2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rider V, Abdou NI, Kimler BF. et al. Gender bias in human systemic lupus erythematosus: a problem of steroid receptor action? Front Immunol 2018;9:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.