Abstract
Background
Adolescent women treated for Hodgkin lymphoma (HL) are at increased risk of breast cancer (BC). We evaluate the cost-utility of eight high-risk BC surveillance strategies for this population, including the Children’s Oncology Group guideline of same-day annual mammography and magnetic resonance imaging (MRI) beginning at age 25 years.
Methods
A discrete event simulation model was used to simulate the life histories of a cohort of 500 000 25-year-old women treated for HL at age 15 years. We estimated BC incidence and mortality, life expectancy, quality-adjusted life-years (QALYs), health-care costs, and the relative cost-utility (incremental cost-utility ratio [ICUR]) under the eight assessed surveillance strategies. One-way sensitivity analysis enabled modeling of uncertainty evaluation. A publicly funded health-care payer perspective was adopted.
Results
Costs across the eight screening strategies ranged from $32 643 to $43 739, whereas QALYs ranged from 24.419 to 24.480. In an incremental cost-effectiveness analysis, annual mammography beginning at age 25 years was associated with an ICUR of $43 000/QALY gained, annual MRI beginning at age 25 years with a switch to annual mammography at age 50 years had an ICUR of $148 000/QALY, and annual MRI beginning at age 25 years had an ICUR of $227 222/QALY. Among all assessed surveillance strategies, the differences in life expectancy were small.
Conclusions
Current high-risk BC surveillance guidelines do not reflect the most cost-effective strategy in survivors of adolescent HL. The results suggest that groups at high risk of BC may require high-risk surveillance guidelines that reflect their specific risk profile.
Adolescent women treated with thoracic radiation therapy (RT) for Hodgkin lymphoma (HL) have an approximately 13.4-fold higher risk of developing breast cancer (BC) than the general population (1). BC surveillance guidelines therefore recommend that survivors of adolescent HL begin surveillance earlier than average-risk women. However, there is lack of consensus as to when early surveillance should be initiated or what it should consist of. The Children’s Oncology Group (COG) and the International Guideline Harmonization Group recommend initiating high-risk surveillance at age 25 years or 8 years after RT, whichever comes later, using annual same-day mammography and magnetic resonance imaging (MRI) (1,2). Conversely, the American Cancer Society (ACS) in the United States and Cancer Care Ontario (CCO) in Ontario, Canada’s most populous province, recommend initiating surveillance at age 30 years using same-day annual mammography and MRI, which reflects the surveillance recommendations for any woman at elevated BC cancer risk (3,4). This group includes survivors of pediatric HL and other pediatric cancers requiring chest irradiation, BRCA1/2 mutation carriers, first-degree relatives of a mutation carrier, and any other women who are determined to be at a 25% or greater lifetime risk of BC (4,5).
Despite limited evidence, the inclusion of MRI screening in addition to screening with mammography in HL survivors has been advocated for, based on expert consensus (6,7). A simulation study supported this recommendation by confirming a BC-specific survival benefit from early surveillance with MRI (8). This model found that initiating surveillance at age 25 years with MRI in addition to mammography reduced BC-specific mortality in the simulated population by 1.3%. This BC-specific mortality reduction was observed in comparison with annual mammography beginning at age 40 years, which reflects an outdated ACS guideline for average-risk screening (9). Whether there is an overall mortality benefit to initiating surveillance at age 25 years compared with age 30 years in survivors of pediatric HL is currently unknown. It is also unknown whether the incremental BC-specific mortality reduction of early surveillance with MRI results in an overall survival gain that can offset its higher costs in the long term.
The objective of this study was to evaluate the health and economic impact and the incremental cost-utility associated with eight distinct high-risk BC surveillance strategies for survivors of adolescent HL, which varied by age of screening initiation (25 or 30 years) and modality employed (MRI, mammography, or both), including the ACS, CCO, and COG guidelines.
Methods
Overview
We conducted a model-based economic evaluation to estimate the relative cost-utility of eight distinct BC surveillance protocols for women treated with thoracic RT for an HL diagnosis at age 15 years. A simulated cohort of 500 000 women were followed starting at age 25 years until death. All were assumed BC-free at age 25 years. Surveillance terminated at age 75 years, consistent with best practice guidelines (10,11). The economic and health outcomes of interest were lifetime probability of developing BC, overall life expectancy, quality-adjusted life-years (QALYs), and health sector costs under a publicly funded health-care payer perspective. Future costs and health outcomes were discounted by 1.5% (12). Discounting accounts for the effect of time preferences on the value of costs and QALYs, meaning that payoffs tend to be desired sooner rather than later (13). The incremental cost-utility ratio (ICUR) of each strategy was calculated as the additional cost per every QALY gained by surveillance per patient lifetime compared with the next best strategy.
The ICURs of all assessed surveillance strategies are evaluated via a cost-efficiency curve. Strategies that fall on the curve dominate those lying to its left, either directly or via extended dominance (14), because they are more effective and either cost less or have a more attractive cost-utility ratio than the next best strategy. In any cost-effectiveness analysis, the optimal strategy is that with the highest incremental cost-effectiveness ratio that falls at or below willingness to pay (WTP) for additional units of benefit (14). Thus, depending on the adopted WTP threshold, different optimal strategies are identified.
As a secondary analysis, we used the COG guideline of annual same-day mammography and MRI beginning at age 25 years as the reference strategy to estimate ICURs for the remaining seven surveillance strategies. This was done to ascertain which strategies, if any, specifically dominate over this widely accepted guideline. For this secondary analysis, the commonly used WTP threshold of $100 000/QALY was adopted (15). We use 2015 Canadian dollars throughout.
Overview of the Discrete Event Simulation Model
The discrete event simulation model was adapted from a model previously described in the literature (8) and comprised four components: BC incidence and natural history, BC surveillance and detection, BC diagnosis, and competing risk of other-cause mortality. These four components allowed for 12 states that simulated women could experience over time. A schematic of the model appears in Figure 1. Surveillance protocols were overlaid on individual women's BC natural history to ascertain when, how, and at which stage a tumor would be detected. A detailed description of these model components appears in the Supplementary Methods (available online). An overview of BC risk according to attained age appears in Supplementary Table 1 (available online).
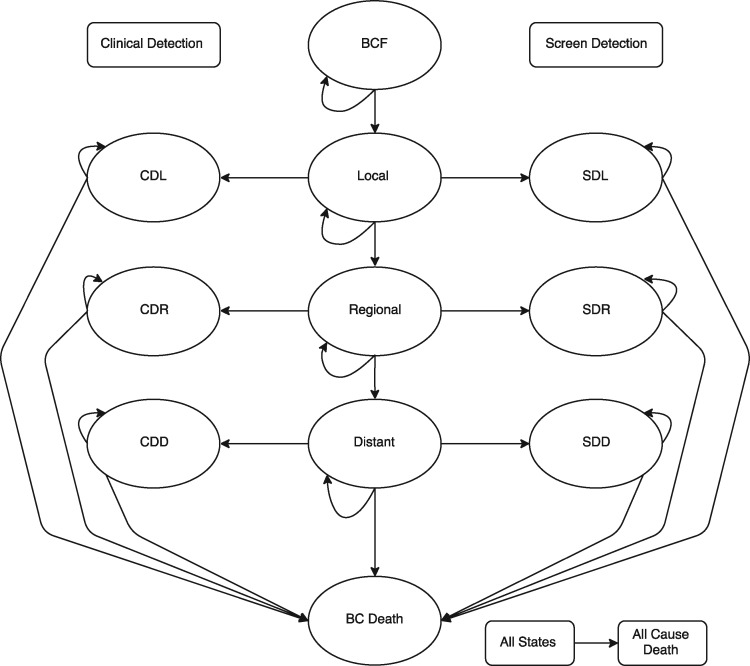
Figure 1.
Discrete-event simulation model. All simulated women start in a breast cancer-free stage and may move progressively through local, regional, and distant stages of breast cancer and death due to breast cancer. In each cycle they may also remain in their current stage. The progression between local, regional, and distant stages is conditional on a simulated woman’s natural history of BC, her propensity for compliance, and her tumor growth rate. Under the clinical detection arm, clinical detection may occur in the breast cancer stage to which a simulated woman has progressed. These are clinically detected local, regional, and distant stages. Correspondingly, under the screen detection arm, breast cancer may be detected via mammography or MRI in local, regional, or distant stages. At all stages, simulated women are also susceptible to death from all other nonbreast cancer causes. BC death = death due to breast cancer; BCF = breast cancer free; CDD = clinically detected breast cancer, distant stage; CDL = clinically detected breast cancer, local stage; CDR = clinically detected breast cancer, regional stage; SDD = screen-detected breast cancer, distant stage; SDL = screen-detected breast cancer, local stage; SDR = screen-detected breast cancer, regional stage.
The eight surveillance protocols of interest comprise four limited strategies of either annual mammography or annual MRI beginning at ages 25 and 30 years. We further consider same-day annual mammography and MRI beginning at both ages 25 and 30 years. Finally, we assess annual MRI beginning at age 25 years with same-day annual mammography beginning at age 50 years and annual MRI beginning at age 25 years with a switch to annual mammography beginning at age 50 years.
Tumor detection by MRI and mammography occurred according to a size-dependent threshold: at 5 mm, tumors were considered MRI detectable, whereas mammography was considered capable of detecting tumors with a median size of 10 mm (8,16). The sensitivity and specificity of the surveillance modalities were derived from the literature (8) and served as the base parameters to a diagnostic decision-tree component of the model invoked during each screening cycle. A schematic of the decision tree appears in Figure 2. The types and probabilities of the relevant diagnostic tests following a positive finding via mammography or MRI (Table 1) were derived from the literature (17). Clinical detection of a breast tumor was also possible. Further discussion of clinical detection, as well as surveillance adherence assumptions, appear in the Supplementary Methods (available online).
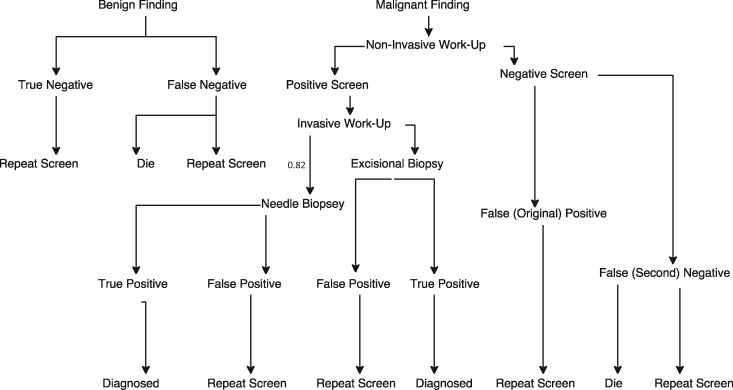
Figure 2.
Decision tree for screening and diagnosis. The decision-tree component is invoked during the screening process, prior to entering detection stage (represented by Figure 1). Each branch of the decision tree is associated with costs and probabilities found in Table 1. The probabilities of a true-positive or a true-negative finding are dependent on the sensitivity and specificity, respectively, of the screening modality, which differ between mammography and magnetic resonance imaging (MRI). The assumed sensitivity (at age <50y and ≥50y) and specificity (at age <50y and ≥50y) of mammography are 0.75 and 0.85 and 0.97 and 0.97, respectively. The assumed sensitivity (first two screens, all subsequent) and specificity (first two screens, all subsequent) of MRI are 0.92 and 0.90 and 0.91 and 0.93, respectively. The likelihood of a positive or negative screen depends, in part, on the detection capabilities of the screening modality, which differ between mammography and MRI; it also varies at each cycle, reflecting the natural history of the simulated woman’s unique breast tumors.
Table 1.
Discrete event simulation input parameters before diagnosis*
| Input parameters | Direct costs, $ | Probability | Time, d | Utility | Range | Sources |
|---|---|---|---|---|---|---|
| Screening costs | ||||||
| Mammography | 187 | N/A | 0.25 | — | — | Plevritis et al., 2006 (16), and Mittmann et al., 2015 (17) |
| Magnetic resonance imaging | 631 | N/A | 0.5 | — | — | Mittmann et al., 2015 (17), and Pataky et al., 2013 (30) |
| Clinical diagnosis | 229 | N/A | 0.5 | — | — | de Bock et al., 2013 (31) |
| Invasive work-up | 0.15 | — | — | Mittmann et al., 2014 (29) | ||
| Needle biopsy | 761 | 0.82 | 1 | — | — | Plevritis et al., 2006 (16), and Mittmann et al., 2014 (29) |
| Excisional biopsy | 1687 | 0.18 | 2 | — | — | Plevritis et al., 2006 (16), and Mittmann et al., 2014 (29) |
| Non invasive work-up | 455 | 1.0 | 0.25 | — | — | Plevritis et al., 2006 (16), and Mittmann et al., 2014 (29) |
| Assumed baseline utilities | ||||||
| Breast cancer free | NA | 1.0 | N/A | 0.84 | (0.76, 1.00) | Cappelli et al., 2001 (20), Antoniou et al., 2003 (21), and Ara and Wailoo, 2011 (22) |
| Undiagnosed breast cancer | NA | 1.0 | N/A | 0.84 | (0.76, 1.00) | Cappelli et al., 2001 (20), Antoniou et al., 2003 (21), and Ara and Wailoo, 2011 (22) |
*N/A = not applicable.
To capture the competing risk of death, we used 2011 Statistics Canada all-cause mortality data, adjusted to HL mortality using Surveillance, Epidemiology, and End Results (SEER) mortality rates for HL patients relative to non-HL patients. Assumptions around mortality rates appear in the Supplementary Methods (available online). Age at death for women with BC depended on whether death from all causes or death from BC occurred first in the model (8).
Costs
All costs were specific to Ontario. Ontario’s universal health insurance plan covers all medically necessary physician and hospital-based care, as well as home care and long-term care services. A detailed explanation of how costs were calculated as well as of the sources from which they were abstracted appears in the Supplementary Methods (available online). Every adherent woman incurred the cost of annual surveillance unless she was diagnosed with BC, at which point she entered a BC-specific state and remained in such, with associated costs, until death. Surveillance and diagnostic costs, as well as treatment costs by stage and phase, appear in Table 2. All costs are reported in 2015 Canadian dollars and were converted using the health and personal care portion of the consumer price index (CPI).
Table 2.
Discrete event simulation input parameters after diagnosis
| Input parameters | Costs, $ |
Utility | Range | Sources | ||
|---|---|---|---|---|---|---|
| First year | Years 2 to 5 | Years ≥6 | ||||
| Local | 23 013 | 9862 | 1972 | 0.82 | (0.82, 0.84) | Plevritis et al., 2006 (16), Naik et al., 2015 (18), Cott Chubiz et al., 2013 (25), Mittmann et al., 2014 (29), and de Oliveira et al., 2016 (33) |
| Regional | 39 942 | 17 118 | 5135 | 0.77 | (0.74, 0.80) | Plevritis et al., 2006 (16), Naik et al., 2017 (19), Cott Chubiz et al., 2013 (25), Mittmann et al., 2014 (29), and de Oliveira et al., 2016 (33) |
| Distant | 51 215 | 21 949 | 21 949 | 0.75 | (0.72, 0.78) | Plevritis et al., 2006 (16), Naik et al., 2015 (18), Cott Chubiz et al., 2013 (25), Mittmann et al., 2014 (29), and de Oliveira et al., 2016 (33) |
| Remission* | N/A | N/A | N/A | 0.84 | (0.76, 1.00) | Assumed |
| Terminal stage† | N/A | N/A | 20 452 | 0.75 | (0.59, 0.78) | Plevritis et al., 2006 (16), Mittmann et al., 2014 (29), and Grann et al., 2002 (32) |
| Death | N/A | N/A | N/A | 0 | Not varied | Assumed |
Assumed return to baseline high risk. All costs fall to zero after 10 years. N/A = not applicable.
Equivalent cost of terminal stage regardless of phase determined by expert opinion. Death cost is the average hourly wage of employees in 2015 multiplied by a 40-hour work week and 52 weeks of work and mortality costs in first year.
Utilities
Utility values, used to adjust projected survival for quality of life, were derived from a Canadian study using time trade-off methods to extract utilities for local, regional, and distant stages of BC (18,19). We assumed a utility value for healthy, BC-free female HL survivors to be 0.84, a literature-derived value reflecting the disutility associated with the knowledge of increased BC risk (20,21). Additional assumptions around utilities can be found in the Supplementary Methods (available online). Utility values are reported in Tables 1 and 2. As per the National Institute for Health and Care Excellence (NICE) recommendations, the multiplicative method was used to determine the utility values used as inputs in the model, which enables the utility reduction relative to the baseline for each health state to be captured (22).
Sensitivity Analyses
We performed structural and one-way sensitivity analyses to assess the robustness of our results against variations in input parameters. Structural sensitivity analyses consisted of using an additive utility approach rather than multiplicative and decreasing utility linearly with age. One-way sensitivity analyses consisted of varying underlying BC risk to its lower and upper bound; varying surveillance stopping age to 65 and 85 years; setting BC treatment costs to both low and high ranges according to first- and third-quartile cost estimates, both across all stages and by individual stage; varying the all-cause mortality rate and BC mortality rate; varying the BC-free utility, tumor growth rate, and compliance rates; and increasing the discounting rate to 3.0%. We assess the impact of varying the underlying BC risk to lower and upper bounds because the study from which this underlying risk is derived included women treated with mantle RT (Supplementary Methods, available online). In sensitivity analyses, the COG guidelines of annual mammography and MRI beginning at age 25 years was used as the reference strategy.
Results
Outcomes
The cumulative incidence of clinically detected BC within the simulated cohort under the assessed surveillance strategies, as well as a full breakdown of BC detection by stage and by surveillance modality, can be found in Supplementary Tables 2 and 3 (available online). Lifetime BC mortality under the current ACS and CCO guideline of annual mammography and MRI beginning at age 30 years was 116.8 deaths per 1000 women, whereas lifetime BC mortality under the COG guideline of annual mammography and MRI beginning at age 25 years was 116.7 per 1000 women (Supplementary Table 4, available online).
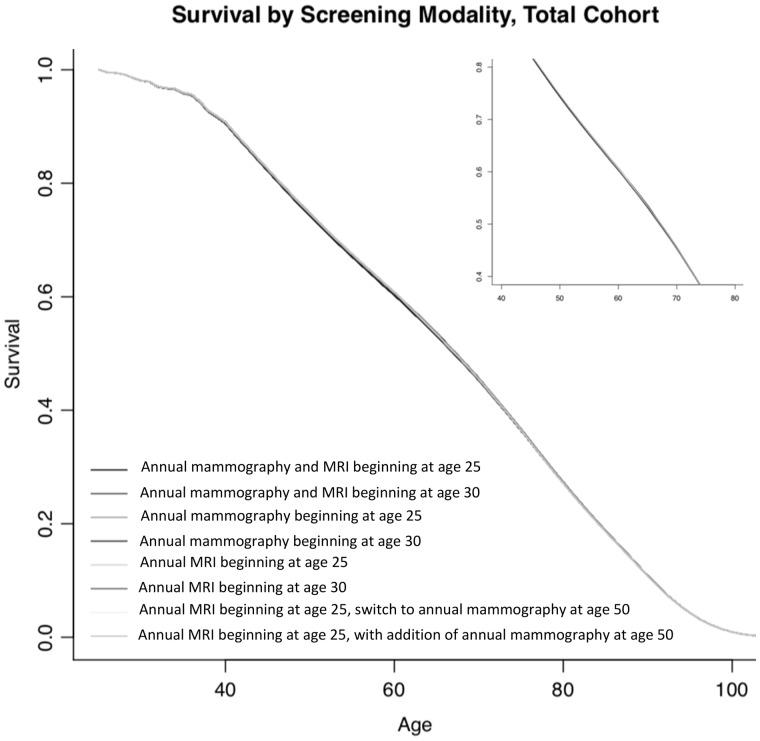
Survival under each surveillance protocol appears in Figure 3. Across the surveillance strategies, conditional life expectancy ranged from 45.73 years (annual mammography beginning at age 30 years) to 45.90 years (annual mammography and MRI beginning at age 25 years) after simulation start at age 25 years (Table 3). Number of BC-related deaths in the simulated population by surveillance strategy are reported in Supplementary Figure 1 (available online).
Figure 3.
Survival by screening modality (total cohort). Survival according to age under the eight surveillance strategies for the entire cohort of simulated women. MRI = magnetic resonance imaging.
Table 3.
Life expectancy, discounted costs, discounted QALYS and ICURs for all surveillance strategies*
| Strategy | Life expectancy, y | Discounted costs, $ | Discounted QALYs | Comparator | Cost difference, $ | QALY difference | ICUR, $ |
|---|---|---|---|---|---|---|---|
| Annual MAM beginning at age 30 years | 45.73 | 32 643 | 24.419 | — | — | — | — |
| Annual MAM beginning at age 25 years | 45.76 | 33 331 | 24.435 | Annual mammography beginning at age 30 years | 688 | 0.016 | 43 000 |
| Annual MRI beginning at age 30 years | 45.86 | 38 494 | 24.458 | Annual mammography beginning at age 25 years | 5163 | 0.023 | 224 478† |
| Annual MRI beginning at age 25 years, switch to annual MAM at age 50 years | 45.86 | 38 659 | 24.471 | Annual mammography beginning at age 25 years | 5328 | 0.036 | 148 000 |
| Annual MRI beginning at age 25 years | 45.90 | 40 704 | 24.480 | Annual MRI beginning at age 25, switch to annual mammography at age 50 years | 2045 | 0.009 | 227 222 |
| Annual MAM and MRI beginning at age 30 years | 45.88 | 40 932 | 24.467 | Annual MRI beginning at age 25 years | 229 | −0.013 | Dominated‡ |
| Annual MRI beginning at age 25 years, addition of annual MAM at age 50 years | 45.89 | 41 475 | 24.472 | Annual MRI beginning at age 25 years | 771 | −0.008 | Dominated‡ |
| Annual MAM and MRI beginning at age 25 years | 45.90 | 43 739 | 24.477 | Annual MRI beginning at age 25 years | 3035 | −0.003 | Dominated‡ |
All values are discounted to time period 1, when simulated women are aged 25 years. QALYs and Discounted QALYs report QALYs remaining after age 25 years. ICUR = incremental cost utility ratio; QALY = quality-adjusted life-year; MAM = mammography; MRI = magnetic resonance imaging.
Indicates strategies that can be eliminated via the principle of extended dominance. Extended dominance is applied in incremental cost-effectiveness analysis to eliminate from consideration strategies whose costs and benefits are improved by a mixed strategy of two other alternatives.
”Strongly dominated” indicates a strategy that is more expensive and produces less benefit than its comparator, resulting in a negative ICUR, which is not standardly reported.
When QALYs were considered and discounting incorporated, annual mammography beginning at age 30 years was the least effective surveillance strategy, with 24.419 QALYs. Annual MRI beginning at age 25 years was the most effective strategy, with 24.480 QALYs. The ACS and CCO guideline of annual mammography and MRI beginning at age 30 years was associated with 24.467 QALYs, whereas the COG guideline of annual mammography and MRI beginning at age 25 years was associated with 24.477 QALYs (Table 3).
The total discounted cost associated with the ACS and CCO guideline of annual mammography and MRI beginning at age 30 years was estimated at $40 932 over the lifetime. Annual mammography and MRI beginning at age 25 years was the costliest surveillance strategy, at $43 739 over the lifetime. The least costly surveillance strategy was annual mammography beginning at age 30 years, at $32 643 (Table 3).
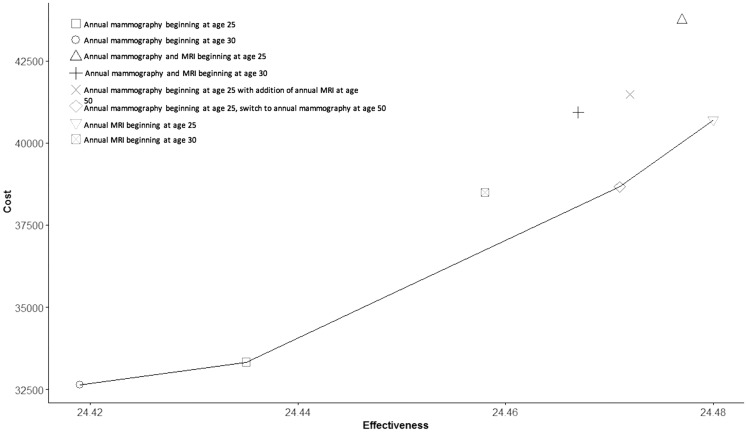
As can be seen in the cost-efficiency frontier (Figure 4) and in Table 3, four of the eight surveillance strategies lie on the frontier. These are annual mammography beginning at age 30 years; annual mammography beginning at age 25 years (ICUR = $43 000/QALY); annual MRI beginning at age 25 years with a switch to annual mammography at age 50 years (ICUR = $148 000/QALY); and annual MRI beginning at age 25 years (ICUR = $227 222/QALY).
Figure 4.
Cost-effectiveness frontier. Costs by effectiveness (ie, quality-adjusted life-years) are plotted for each surveillance strategy. Each point on the graph corresponds to the incremental cost-utility ratio for each respective surveillance strategy. Strategies that lie to the left of the frontier are dominated by those that form the frontier, either directly or via extended dominance. MRI = magnetic resonance imaging.
In our secondary analysis, annual MRI beginning at age 25 years is a cost-saving strategy over the COG guideline with negligible difference in life expectancy and QALY gains (Supplementary Table 5). A full list of ICURs computed relative to annual mammography and MRI beginning at age 25 years appears in Supplementary Table 5 (available online).
Sensitivity Analyses
Across the structural sensitivity analyses, annual MRI beginning at age 25 years remained a cost-saving strategy when compared to the COG guideline under the additive utility adjustment scenario. Conversely, under the age-adjusted utility scenario, annual MRI beginning at age 25 years was no longer dominant compared to the COG guideline. In one-way sensitivity analyses, compared with the current COG guideline, annual MRI beginning at age 25 years remains cost saving across all assessed scenarios. The average incremental QALY difference between the COG guideline and MRI beginning at age 25 years across all sensitivity analyses ranges from −0.009 to 0.05, with an average value around zero, indicating that MRI beginning at age 25 years has effectively the same estimated QALY as the COG guideline (ie, the same screening strategy with the addition of mammography). ICURs for structural sensitivity specifications and results from the sensitivity analyses appear in Supplementary Table 6 and Figures 2 and 3 (available online).
Discussion
This study found small differences in survival and QALY benefit over the broad range of high-risk surveillance protocols assessed within the analysis. This is common for screening in low-incidence disorders (23,24). However, this study provides the first quantitative evidence that there are more cost-effective surveillance strategies for this population than the current COG guideline, namely annual MRI beginning at age 25 years.
In cost-effectiveness analyses, different optimal strategies are identified based on different WTP thresholds. At WTP thresholds of less than $50 000/QALY, annual mammography beginning at age 25 years is the cost-effective strategy; conversely, if the public payer is willing to pay $150 000/QALY gained, annual MRI beginning at age 25 years with a switch to mammography at age 50 years becomes a more costly but more effective surveillance strategy compared with mammography beginning at age 25 years. For a WTP threshold of more than $150 000/QALY gained, annual MRI beginning at age 25 years becomes the most cost-effective strategy.
A mortality benefit to earlier MRI-based surveillance, in addition to mammography, in HL survivors treated in adolescence has been described previously (8). Although our results substantiate the mortality benefit of early surveillance (ie, beginning at age 25 years), they also suggest that from a health-utility perspective, inclusion of mammographic screening in addition to MRI in the HL survivor population does not produce a clinically meaningful benefit (Supplementary Figure 1, available online). This is likely because of the decreased sensitivity of mammography in women younger than age 50 years. Because women simulated in this study had an average life expectancy of 65 years, the benefits conferred by mammography past the age of 50 years were minimal.
Our findings indicate that the current surveillance guidelines for high-risk populations may require reevaluation to reflect specific risk profiles. For instance, previous work has demonstrated that among BRCA1/2 mutation carriers, combining annual MRI and mammography is cost effective in BRCA1 women at a willingness-to-pay threshold of $100 000 per QALY gained (16,25). In our simulated population, neither screening with both MRI and mammography beginning at age 25 years nor at age 30 years is cost effective at this same threshold.
The results of this study must be interpreted in the context of real-life survivor behavior. Among eligible survivors in the United States and Canada, it has been estimated that 55% to 85% do not undergo BC screening (26,27). In testing incrementally worse adherence assumptions, the optimal surveillance strategy, annual MRI beginning at age 25 years, becomes less cost saving. Based on these results, early screening (ie, starting at age 25 years), combined with initiatives to increase adherence among the HL survivor population, strengthen the cost savings of our results.
The results must also be interpreted in conjunction with the study’s limitations. Neither the costs nor health implications of a ductal carcinoma in situ (DCIS) state and its prevention were considered, as little is known about DCIS progression in populations previously exposed to RT (8). As approximately 25% of diagnosed BCs are DCIS, its inclusion would affect our cost estimates (28). Treatment cost estimates also reflect estrogen receptor positive cases only, which are associated with cost-intensive hormonal therapy (29). Finally, in the study used to populate the estimates of treatment costs, the average age of women in the study was 61 years (29). No adjustments to costs were made for younger women in our model who developed BC and who may accumulate more costs over time. These factors would likely result in differing estimations of BC costs across all assessed surveillance strategies. Although we cannot test for direct impact of factors such as inclusion of DCIS on cost, we tested the impact of cost reductions and increases in individual BC stages and across all BC stages in sensitivity analyses. We find these cost changes do not affect our overall interpretation.
The sensitivity of the model to changing costs of technology, namely MRI, over time was also not assessed. This would likely decrease the incremental cost for all strategies incorporating MRI. A final limitation of this analysis is that we consider only a population treated at age 15 years with thoracic RT for HL. Variations in radiation dose as well as the possibility of additional risk factors for BC were not considered. However, using discrete-event simulation modeling does allow for different natural pathways of tumor progression and other causes of death across HL survivors in our simulated population. This allows for higher or lower BC risk across simulated individuals, along with other causes of death. HL survivors treated with the highest dose of RT or with added risk, for example, in the form of a BRCA1/2 mutation, may however benefit from even higher intensity screening. We elucidate this further through testing the impact of higher and lower overall standardized incidence ratio of BC and BC- and all-cause mortality overall. We find no change to overall results interpretation. We do not report results based on substrata because the aim of this study is to inform population-level decisions across all HL survivors rather than targeted population screening.
Annual screening with MRI beginning at age 25 years constitutes a dominant alternative to the current COG guideline for survivors of adolescent HL treated with thoracic RT. Importantly, this finding may be relevant to other pediatric cancer populations with similar treatment, morbidity, and mortality profiles to the pediatric HL population. In the context of systems that place great emphasis on bending the health-care cost curve and health system sustainability, the potential to improve resource use, while increasing QALYs, constitutes a desirable course of action.
Funding
No funding was provided for this work.
Notes
The authors have no conflicts of interest to declare.
Supplementary Material
References
- 1. Veit-Rubin N, Rapiti E, Usel M, et al. Risk, characteristics, and prognosis of breast cancer after Hodgkin’s lymphoma. Oncologist. 2012;17(6):783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mulder RL, Kremer LCM, Hudson MM, et al. Recommendations for breast cancer surveillance for female childhood, adolescent and young adult cancer survivors treated with chest radiation: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2013;14(13):e621–e629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breast Cancer Diagnosis Early Detection And Diagnosis. How is breast cancer detected? American Cancer Society website. https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection.html. Accessed September 17, 2017.
- 4.OBSP screening for women at high risk. Cancer Care Ontario website. 2017. https://www.cancercare.on.ca/pcs/screening/breastscreening/OBSP/highrisk/. Accessed September 17, 2017.
- 5. Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32(21):2217–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saslow D, Boetes C, Burke W, et al. American Cancer Society Guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. [DOI] [PubMed] [Google Scholar]
- 7. Warner E, Messersmith H, Causer P, et al. Magnetic resonance imaging screening of women at high risk for breast cancer. Cancer Care Ontario; 2012. https://www.cancercareontario.ca/sites/ccocancercare/files/guidelines/full/pebc15-11f_1.pdf. Accessed September 17, 2017.
- 8. Hodgson DC, Cotton C, Crystal P, Nathan PC.. Impact of early breast cancer screening on mortality among young survivors of childhood Hodgkin’s lymphoma. J Natl Cancer Inst. 2016;108(7):djw010.. [DOI] [PubMed] [Google Scholar]
- 9. Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin. 2003;53(3):141–169. [DOI] [PubMed] [Google Scholar]
- 10.Ontario Breast Screening Program (OBSP). Cancer Care Ontario website. https://www.cancercareontario.ca/en/cancer-care-ontario/programs/screening-programs/ontario-breast-obsp. Accessed December 1, 2017.
- 11. Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidelines for the economic evaluation of health technologies: Canada, 4th edition. CADTH website. https://www.cadth.ca/guidelines-economic-evaluation-health-technologies-canada-4th-edition. Accessed September 17, 2017.
- 13.CADTH. Discounting and the evaluation of health care programs. Published 2016. https://www.cadth.ca/sites/default/files/pdf/CP0008_RiB_e.pdf. Accessed January 13, 2019.
- 14. Suen S, Goldhaber-Fiebert JD.. An efficient, non-iterative method of identifying the cost-effectiveness frontier. Med Decis Making. 2016;36(1):132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neumann PJ, Cohen JT, Weinstein MC.. Updating cost-effectiveness—the curious resilience of the $50 000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. [DOI] [PubMed] [Google Scholar]
- 16. Plevritis SK, Kurian AW, Sigal BM, et al. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA. 2006;295(20):2374–2384. [DOI] [PubMed] [Google Scholar]
- 17. Mittmann N, Stout NK, Lee P, et al. Total cost-effectiveness of mammography screening strategies. Statistics Canada; 2015. http://www.statcan.gc.ca/pub/82-003-x/2015012/article/14295-eng.htm. Accessed December 4, 2016. [PMC free article] [PubMed]
- 18. Naik H, Howell D, Su J, et al. Stage specific health utility index scores of Canadian Cancer Patients. J Clin Oncol. 2015; 33 Abstract 6614. Presented at the ASCO Annual Meeting in Chicago, IL, USA on June 1, 2015. http://meetinglibrary.asco.org/content/150991-156. Accessed December 4, 2016. [Google Scholar]
- 19. Naik H, Howell D, Su S, et al. EQ-5D health utility scores: data from a comprehensive Canadian Cancer Centre. Patient. 2017;10(1):105–115. [DOI] [PubMed] [Google Scholar]
- 20. Cappelli M, Surh L, Humphreys L, et al. Measuring women’s preferences for breast cancer treatments and BRCA1/BRCA2 testing. Qual Life Res. 2001;10(7):595–607. [DOI] [PubMed] [Google Scholar]
- 21. Antoniou A, Pharoah PDP, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ara R, Wailoo A The use of health state utility values in decision models. Decision Support Unit; 2011. https://www.ncbi.nlm.nih.gov/books/NBK425824/pdf/Bookshelf_NBK425824.pdf. Accessed December 4, 2016.
- 23. Mittmann N, Stout NK, Tosteson ANA, Trentham-Dietz A, Alagoz O, Yaffe MJ.. Cost-effectiveness of mammography from a publicly funded health care system perspective. CMAJ Open. 2018;6(1):E77–E86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goede SL, Rabeneck L, van Ballegooijen M, et al. Harms, benefits and costs of fecal immunochemical testing versus guaiac fecal occult blood testing for colorectal cancer screening. PloS One. 2017;12(3):e0172864.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cott Chubiz JE, Lee JM, Gilmore ME, et al. Cost-effectiveness of alternating MRI and digital mammography screening in BRCA1 and BRCA2 gene mutation carriers. Cancer. 2013;119(6):1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hodgson DC, Grunfeld E, Gunraj N, Del Giudice L.. A population-based study of follow-up care for Hodgkin lymphoma survivors: opportunities to improve surveillance for relapse and late effects. Cancer. 2010;116(14):3417–3425. [DOI] [PubMed] [Google Scholar]
- 27. Nathan PC, Ness KK, Mahoney MC, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study (CCSS). Ann Intern Med. 2010;153(7):442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins LC, Laronga C, Wong JS. Breast ductal carcinoma in situ: epidemiology, clinical manifestations, and diagnosis. UpToDate. 2016. http://www.uptodate.com/contents/breast-ductal-carcinoma-in-situ-epidemiology-clinical-manifestations-and-diagnosis. Accessed December 6, 2016.
- 29. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pataky R, Armstrong L, Chia S, et al. Cost-effectiveness of MRI for breast cancer screening in BRCA1/2 mutation carriers. BMC Cancer. 2013;13(339):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Bock GH, Vermeulen KM, Jansen L, et al. Which screening strategy should be offered to women with BRCA1 or BRCA2 mutations? A simulation of comparative cost-effectiveness. Br J Cancer. 2013;108(8):1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grann VR, Jacobson JS, Thomason D, Hershman D, Heitjan DF, Neugut AI.. Effect of prevention strategies on survival and quality-adjusted survival of women with BRCA1/2 mutations: An updated decision analysis. J Clin Oncol. 2002;20(10):2520–2529. [DOI] [PubMed] [Google Scholar]
- 33. de Oliveira C, Pataky R, Bremner KE, et al. Phase-specific and lifetime costs of cancer care in Ontario, Canada. BMC Cancer. 2016;16(1):809.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.