Abstract
Organic and inorganic nanoparticles (NPs) have shown promising outcomes in transdermal drug delivery. NPs can not only enhance the skin penetration of small/biomacromolecule therapeutic agents but can also impart control over drug release or target impaired tissue. Thanks to their unique optical, photothermal, and superparamagnetic features, NPs have been also utilized for the treatment of skin disorders, imaging, and biosensing applications. Despite the widespread transdermal applications of NPs, their delivery across the stratum corneum, which is the main skin barrier, has remained challenging. Microneedle array (MN) technology has recently revealed promising outcomes in the delivery of various formulations, especially NPs to deliver both hydrophilic and hydrophobic therapeutic agents. The present work reviews the advancements in the application of MNs and NPs for an effective transdermal delivery of a wide range of therapeutics in cancer chemotherapy and immunotherapy, photothermal and photodynamic therapy, peptide/protein vaccination, and the gene therapy of various diseases. In addition, this paper provides an overall insight on MNs’ challenges and summarizes the recent achievements in clinical trials with future outlooks on the transdermal delivery of a wide range of nanomedicines.
Keywords: microneedle arrays, drug delivery, immunotherapy, vaccination, gene delivery, nanomedicine
1. Introduction
Skin is responsible for enclosing and protecting the human body against the invasion of microorganisms, allergens, toxins, and UV irradiation [1]. Meanwhile, it serves as a pathway for administering therapeutics [2]. Compared to oral, nasal, intramuscular, and intravenous delivery routes, transdermal delivery (TDD) has immense advantages, such as pain-free administration over hypodermic injection, patients’ compliance, and self-administration [3]. Transdermal drug delivery systems (TDDSs) not only contribute to the continuous transport of therapeutic agents through the skin, but they also help the agent to overcome certain barriers (such as first-pass metabolism) and promote the transport of agents with low solubility and bioavailability [4]. However, passive skin transport is restricted to small lipophilic molecules.
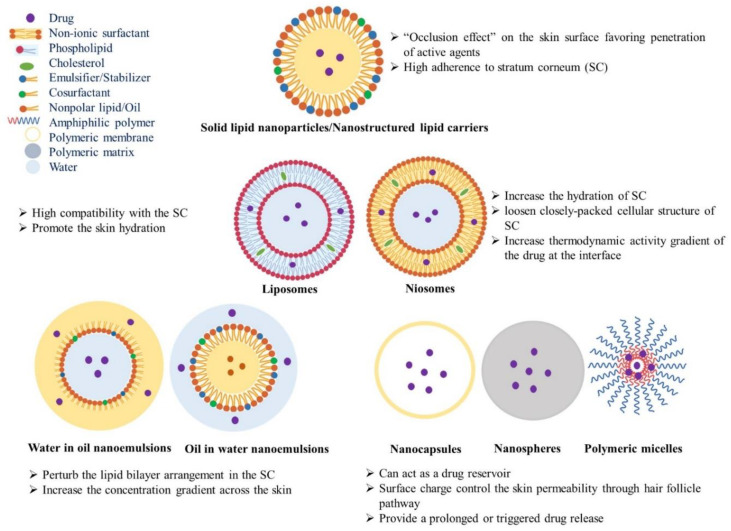
Nanotechnology has been explored in TDD to enhance the permeation of therapeutic agents through the skin. Due to their small size and large surface area, nanoparticles (NPs) can deliver drugs across the stratum corneum (SC) without disrupting the skin barrier function via transcellular or trans-appendageal pathways [5]. Various types of nanocarriers have been reported in transdermal drug delivery. Carrier-free nanomedicines have shown low toxicity, enhanced drug solubility, and dissolution [6]. Lipid vesicles have gained interests due to their ability to deliver drugs by “free drug mechanism”. The released drug molecules can permeate into the skin due to an effective penetration mechanism of carrier component(s) interacting with SC lipids. Thanks to their miscibility with SC lipids, liposomes have shown great promise in TDD. However, their adherence to corneocytes can result in the leakage of encapsulated therapeutic agents at the superficial layers of skin. Hence, several vesicular carriers, such as Niosomes, Ethosomes®, Transfersomes®, Invasomes®, Vesosomes®, Proliposomes, and Pharmacosomes® have been introduced as reviewed before [5]. Nanoemulsions, either water-in-oil (W/O) or oil-in-water (O/W) can also enhance TDD due to their small droplet size and the constituent surfactants, which destabilize the SC lipid bilayer (Figure 1) [5]. Lipid NPs, especially nanostructured lipid carriers (NLCs), can efficiently load insoluble drugs and thanks to their high adherence to the SC, they can facilitate the drug penetration into deeper layers of the skin [7,8]. Polymeric systems, such as a polymer–drug conjugate, nanospheres, dendrimers, polymeric micelles, polymeric vesicles and microcapsules can control the release and skin retention of therapeutic materials for a prolonged period (Figure 1) [9,10,11]. Inorganic NPs and carbon nanomaterials, such as superparamagnetic iron oxides (SPIONs), titanium dioxide (TiO2), zinc oxide (ZnO), gold (AuNPs), silver (AgNPs), quantum dots (QDs), mesoporous silica nanoparticles (MSNs), carbon nanotubes (CNTs), graphene oxide (GO), carbon nanofibers (CNFs), and fullerenes have been recently applied in not only TDD, but also in the diagnosis or treatment of skin disease [12,13]. Despite recent progress in nanomaterials, the safety and biological fate of these NPs should be explored to develop efficient TDDSs. To improve the skin penetration of NPs, various techniques, including physical methods as well as chemical permeation enhancers, have been explored. Iontophoresis [14], dermaportation [15], sonophoresis [16], and microneedle arrays (MNs) [17] have been applied in the delivery of not only small and large therapeutic agents, but also various NPs through the stratum corneum (SC), the outermost and nonviable barrier layer of the skin [12].
Figure 1.
Vesicular, lipid and polymer-based nanoparticles (NPs) currently used in nanomedicine and their underlying mechanisms for enhanced transdermal delivery (TDD).
With an emergence of the microfabrication techniques in mid-1990s, MNs have received enormous attention in TDD applications [18]. Since the advent of MNs, they have shown promising results due to their minimally invasive penetration into the SC, which promotes the delivery of therapeutic agents. In recent years, different types of MNs, such as solid, hollow, coated, dissolving, and hydrogel-forming MNs, have been developed for transdermal drug delivery and cosmetic applications [19,20]. This review highlights the significance and efforts made in the design of different MNs. In the following sections, the current advancement in the MN-assisted TDD of NPs will be discussed with a particular focus on cancer chemo- and immuno-therapy, photodynamic therapy (PDT), photothermal therapy (PTT), vaccination, therapeutic protein delivery, and gene therapy. Finally, current clinical trials as well as challenges and future outlook in this research area will be addressed.
2. MNs: An Advancement in Hypodermic Needles
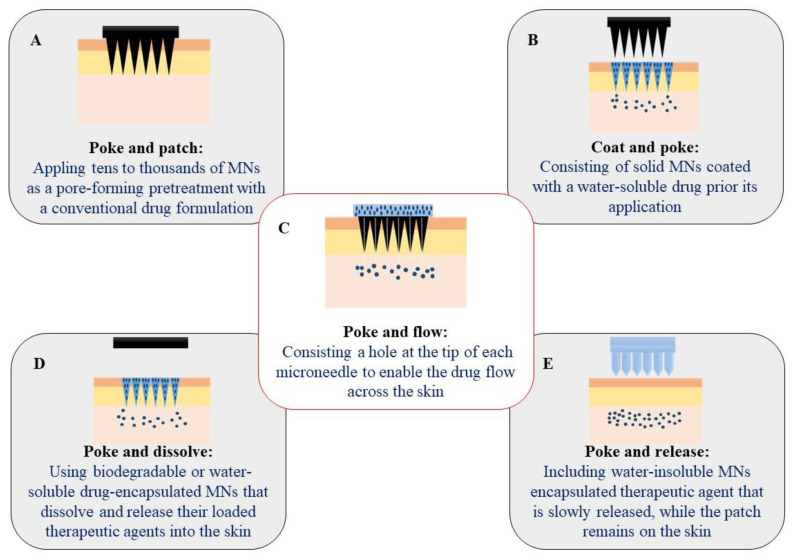
Even though hypodermic needles have been the gold standard for drug delivery and vaccination, they suffer from several drawbacks such as pain, needle phobia, hazardous sharp waste, and injury [21,22]. Hence, MNs have been developed to overcome the limitations of hypodermic needles. MNs are minimally invasive micron-sized needles, clustered on a solid base or patch. MNs have recently gained significant attention as a popular way of delivering macromolecules as well as small molecules across the skin due to their low cost, painlessness, and self-administration [23]. MNs can be classified according to their structures, delivery approaches, fabrication techniques, shapes, and constituent materials. Hence, they can be classified in more than one class. For example, MNs can be hollow based on their structure or delivery approach and at the same time they can be fabricated from metals, ceramics or biodegradable polymers [24]. The MNs can be classified based on delivery strategies as follows [19]:
-
(A)
Poke-and-patch approach to apply tens to tens of thousands of MNs as a pore-forming pretreatment. Afterward, a conventional drug formulation is applied on the skin surface.
-
(B)
Coat-and-poke approach, which consists of a water-soluble drug coating on solid MNs. Drug coating is dissolved and simply deposited within the skin during its administration.
-
(C)
Poke-and-release approach in which water-insoluble MNs are injected into the skin. The encapsulated therapeutic agent is slowly released, while the patch remains on the skin after its application.
-
(D)
Poke-and-flow approach, which is characterized by a hole in the structure (center or side) of each microneedle to enable the drug flow across the skin.
-
(E)
Poke-and-dissolve approach, which uses biodegradable or water-soluble drug-encapsulated MNs. The MNs dissolve and release their loaded therapeutic agents into the skin. Table 1 summarizes different delivery strategies, advantages, disadvantages, and applications of MNs.
Table 1.
Advantages, disadvantages, and applications used for different types of microneedles (MNs).
| Type of MNs | Advantages | Drawbacks | Applications |
|---|---|---|---|
| Solid | Technically simple, without any pump or loading/coating procedure, small doses can be administered | Two-step administration procedure, no exact dosing, drugs need to be reformulated | Skin pretreatment for the delivery of insulin [55], cosmetics [56], vaccines [57], potassium chloride [58], monitoring of lactate and glucose [59], urea sensing [60] |
| Coated | MN strength is retained after coating, without any patch or pump, precise dosing is possible | Appropriate coating technique is needed, limited to small doses, drugs need to be reformulated, coatings might lessen MN sharpness | Delivery of vaccines [61], insulin [62], proteins [63], desmopressin [64], parathyroid hormone [65], sampling, isolation and identification of biomarkers [66] |
| Hollow | Drug delivery rates can be controlled, delivery of a relatively high liquid volume is plausible, possible combination with lab-on-a-chip devices, precise dosing | Possible risk of clogging, reduced MN strength, possible risk of drug leakage, complex devices | Delivery of vaccines [67], insulin [68], cell therapy [69], delivery of mRNA [70], DNA (pDNA) [71], biofluid extraction and bio-signal detection [72,73], colorimetric detection of glucose [74] |
| Dissolving | No need for any pump or patch, precise dosing is possible, no sharp waste, low preparation costs | Small drug doses may be lost throughout the encapsulation/absorption procedure, low strength, low penetration ability, limited to small drug doses, drug reformulation is needed | Delivery of vaccines [75,76], insulin [77], therapeutic peptides [78], triamcinolone acetonide [79], doxorubicin [80], epidermal growth factor and ascorbic acid [81], adenosine [82], vitamin B12 [83], near-IR photosensitizer (Redaporfin™) [84], sodium nitroprusside in combination with sodium thiosulfate [85], DNA extraction [86] |
| Hydrogel-forming | No need for any pump or patch, precise dosing is possible, no sharp waste | Small drug doses may be lost throughout the encapsulation/absorption procedure, limited to small drug doses, low strength, and penetration ability, drug reformulation is needed | Delivery of vaccines [87], metformin hydrochloride [50], methotrexate [53], caffeine [46]. glucose-responsive insulin delivery [51], stimulus-responsive ibuprofen delivery [47], lithium monitoring [88], theophylline, caffeine and glucose monitoring [89] |
As shown in Figure 2, MNs can be classified based on delivery strategies as follows:
Figure 2.
A schematic representation of delivery approaches using various types of microneedle arrays (MNs): (A) poke-and-patch (solid MNs), (B) coat-and-poke (coated MNs), (C) poke-and-flow (hollow MNs), (D) poke-and-dissolve (dissolvable MNs), and (E) poke-and-release (hydrogel forming MNs).
Solid MNs—solid MNs use the “poke-and-patch” approach to deliver drugs or macromolecules [25]. The first MNs-based TDDS was fabricated based on silicon MNs to simplify in vitro calcein delivery across the excised human skin [26]. Silicon is a widely used material for micromachining MNs, as it can be easily processed to make MNs with different sizes and morphologies. Despite the great advantages of silicon in the fabrication of solid MNs, several limitations, such as cost, a long and complex manufacturing process, fragility, and the biocompatibility concerns have led to the development of novel materials for the fabrication of solid MNs. To date, various biocompatible, strong, and easily synthesized materials have been applied to facilitate the penetration of proteins, hormones, and vaccines into the skin, as presented in Table 1 [24]. For more detailed information, refer to excellent reviews on MN-assisted vaccination and the applications of solid MNs [27,28].
Hollow MNs—drug formulations that can be delivered by the “poke-and-flow” approach use hollow MNs with different height and geometries [29]. Silicon and metals are the main constituent materials to make hollow MNs (Table 1). Hollow MNs can deliver relatively high quantities of therapeutic agents by diffusion, pressure- or electrically- driven flow. Moreover, in combination with the iontophoresis technique, they can actively displace charged drugs or particles in a well-controlled manner [30]. To date, various therapeutic agents, such as proteins and vaccines as well as diagnostic agents have been delivered into the skin via hollow MNs (Table 1). Interested readers are directed to excellent reviews on this topic [27,28].
Coated MNs—coated MNs are fabricated by coating a drug layer on solid MNs [25]. For efficient drug delivery, the formulations should be uniformly coated, which highly depends on the wetting angle and spread of the drug formulation. Moreover, the drug formulation ought to be stable, water-soluble, and strong enough to maintain the coated material during the insertion into the skin. Layer-by-layer coated MNs have also been fabricated by the alternate dipping of metallic, polymeric, or silica MNs into a solution containing oppositely charged agents [31,32]. For example, Schipper et al. developed layer-by-layer pH-sensitive coated silicon MNs for the delivery of diphtheria toxoid (DT) as a promising vaccination strategy. First, the surface of MNs was chemically modified with pyridine functional groups to achieve a positive surface charge at a pH of 5.8. Then, multiple alternating layers of DT with a negative charge and positively charged N-trimethyl chitosan (TMC) were applied by a layer-by-layer coating procedure. It was shown that the increasing the number of DT/TMC bilayers resulted in a higher immune response with lower DT content [33]. As presented in Table 1, various formulations have been coated on MNs to facilitate the skin permeation of therapeutic agents, including vaccines, proteins, and hormones. More detailed information about coated MNs can be found in other publications [34,35].
Dissolvable/biodegradable MNs–nowadays, polymeric materials have been widely used in the fabrication of MNs, owning to their biocompatibility and low cost. Apart from their biocompatibility, some of them are biodegradable. Dissolvable MNs (dMNs) are completely dissolved in the skin, quickly leaving no biohazardous sharp wastes after use. They act based on a poke-and-dissolve approach in which the drugs are encapsulated in a safe, inert, and water-soluble polymer or sugar-based matrix [25]. Biodegradable MNs are formed using various types of biodegradable polymers, such as chitosan, hyaluronic acid (HA), polylactic acid (PLA), polyglycolic acid (PGA) or poly (lactide-co-glycolide) (PLGA) to form a matrix, that are degraded after the skin insertion [24]. The biodegradable MNs release the loaded cargos for months by diffusion and polymer erosion mechanisms [36]. To achieve high drug loading into dMNs and enhance their mechanical strength, several novel dMNs, such as double-layer, pedestal, and separable arrowhead dMNs have been fabricated [37]. Separable arrowhead MNs have also been developed on a metal support and a dissolvable tip to overcome the drawbacks associated with the low mechanical strength of dMNs and the biohazardous sharp waste of solid MNs [38,39]. The sharp-tipped polymer arrowhead MNs were able to insert into the skin and release their drug content after being dissolved in situ [40]. Among several advantages, dMNs do not require any pump or patch. In addition, they do not leave any sharp waste and their fabrication cost is fairly low. However, there are some issues related to dMNs, such as low mechanical strength and penetration ability, the delivery of small doses, and the need for drug reformulation. Interested readers are encouraged to refer to excellent reviews on MN-assisted drug delivery [41,42]. In addition to modifying the drug loading property of MNs, a drug release profile from MNs can be modified via the incorporation of bioresponsive NPs, resulting in the emergence of a novel class of MNs named “bioresponsive MNs”. The bioresponsive MNs can release the therapeutic agents in response to physiological signals, which in turn can modulate the therapeutic effectiveness and drug toxicity profiles. To date, some dissolvable or biodegradable polymers, such as polyvinylpyrrolidone (PVP) and modified HA have been reported in combination with NPs for the development of MNs responsive to pH, glucose, and enzymes [43,44,45].
Hydrogel-forming MNs—hydrogel-forming MNs are fabricated from polymers, which can rapidly swell upon insertion into the skin [46,47]. The MNs absorb the body fluid into their three-dimensional matrix, resulting in a controlled release of the loaded drug into the skin via their created microconduits without any measurable polymer residuals after removal [48,49]. To date, hydrogel-forming MNs, including PEG-crosslinked poly(methyl vinyl ether-co-maleic acid) [50], silk fibroin and phenylboronic acid/acrylamide [51], poly (methyl vinyl ether-co-maleic acid)/pectin [52], and poloxamer [53] have been intended for drug delivery and diagnostic applications. They are usually fabricated by the micro-molding process involving a light source [54].
Taken together, different types of MNs have shown promising results in the delivery of therapeutic agents; however, there are still some limitations for the efficient biomedical applications as presented in Table 1. Although MNs locally deliver therapeutic agents, there is still a need for precise control over drug release and the fate of MNs in vivo.
3. Potential Applications of MN Combination with NPs
Nowadays, numerous NP formulations have been synthesized and explored for drug delivery to carry therapeutic agents, such as proteins, vaccines, and nucleic acids [90,91,92]. NPs exhibit numerous benefits, including unique size-dependent physicochemical properties [24,93], the protection of payload against chemical or proteolytic degradation, controlled release over prolonged times [94] as well as targeted delivery to specific parts of the body; hence, reducing the side effects [95]. To date, various administration routes (e.g., oral, nasal, subcutaneous, and IV injections) have been applied for the delivery of nanomedicines. The IV injection benefits from the fast onset of operation and high plasma level. Nevertheless, injections are associated with pain, infections (due to the poor maintenance of sterile conditions), and require a skilled person for administration [96,97], thereby alternative administration routes are needed for the more efficient and controlled delivery of active agents. TDD is a promising administration route that can minimize the complications associated with oral or IV drug administration. NPs are designed to affect the SC barrier function by modulating their special physicochemical properties. Due to their small size and large surface area, NPs can deliver drugs across the SC without disrupting the skin barrier function via transcellular or trans-appendageal pathways [8]. Carrier-free nanomedicines have shown low toxicity, high drug loading, enhanced solubility, and a dissolution rate [9]. However, due to presence of the SC, the translocation of NPs across the skin is still an enormous challenge. Consequently, the emergence of new strategies or the combination of present techniques can lead to overcoming the mentioned limitations.
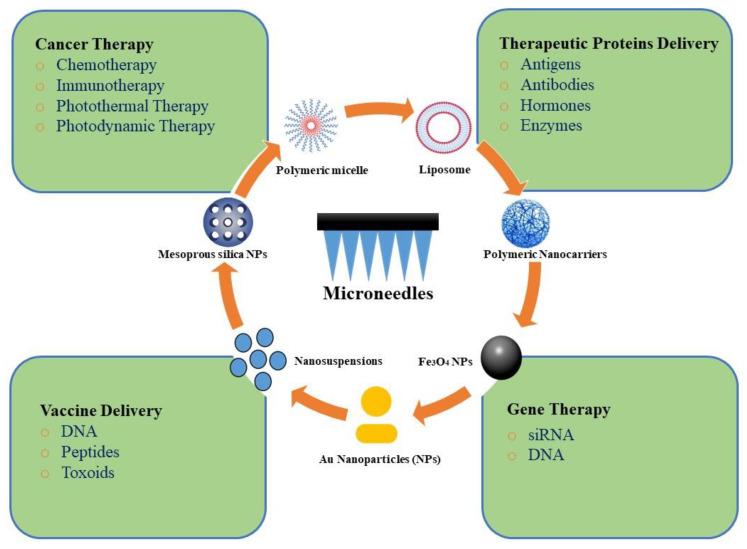
Currently, MN technology has provided a versatile platform to increase the TDD of NPs in a minimally invasive way (Figure 3) [98]. In recent years, the intradermal delivery of NPs using MNs has gained a significant interest due to their unique ability: (i) to facilitate transdermal administration, (ii) to deliver both hydrophilic and lipophilic therapeutic agents, (iii) to provide the homogeneous distribution of NP-based drug reservoirs into the skin, (iv) to combine therapeutic and diagnostic agents into one unique structure giving rise to a theranostic system. Table 2 summarizes a list of reports on MN’s applications in the TDD of NPs. A quick look at the research findings suggests a synergistic enhancement in skin permeation upon combining MNs with NPs due to the reversible barrier disruption function of MNs and subsequent controlled delivery of NPs [68]. For example, Ramadan et al. developed lamivudine-loaded polylactic-co-glycolic acid (PLGA) NPs and investigated their TDD in combination with stainless steel MNs. They showed that such a prolonged release system could overcome the short half-life of the drug. Furthermore, skin treatment with MNs can result in a two-fold enhancement in the steady-state flux, offering new transport pathways to increase the TDD of the drug-loaded NPs [99]. In another study, a layer of PLGA NPs containing vitamin D3 was coated on stainless steel MNs [100]. The MNs pierced the skin layer acting as a physical enhancer for drug molecules to be localized within the dermal region. Therefore, the dual delivery approach combining nanotechnology and MNs can improve TDD while promoting the sustained release of therapeutic agents. However, this action depends on the particle size, MN-induced pore size, and the skin ability to recover. Compared with drug-loaded NPs, a small-molecule drug is expected to penetrate through the pores left by MNs in the skin [101]. Moreover, compared with micron-sized particles, drug-loaded NPs can more easily pass the skin barrier through the MN pores. More recently, nanomedicine has received great attention for the treatment of various diseases using different approaches, such as chemotherapy, protein delivery, and gene therapy due to enhanced tumor accumulation, controlled cargo delivery, and increased intracellular uptake by NPs [102]. In the following sections, the MN applications in the TDD of NPs will be discussed in various fields including chemotherapy, immunotherapy, PTT, PDT, protein, vaccine, and gene delivery.
Figure 3.
Combinatorial applications of microneedles (MNs) with nanoparticles (NPs).
Table 2.
Microneedle-assisted transdermal delivery of nanoparticles (NPs).
| Type of MNs | Carrier | Drug | Outcome | Ref. |
|---|---|---|---|---|
| Solid (stainless steel) | Microemulsions | Propranolol | Enhanced permeation | [103] |
| Solid (silicon) | Dextran NPs | Rosiglitazone CL 316243 |
Localized and painless administration in a safe and effective manner | [104] |
| Solid (silicon) | Microemulsion | Tetramethyl pyrazine | Enhanced percutaneous absorption of drug-loaded microemulsions by MNs | [68] |
| Solid (rolling MNs) | Ethosomes | Paeoniflorin | Enhanced skin penetration, no synergistic effect in combination of MNs with ethosomes | [101] |
| Solid (not defined) | NLC | Alkaloids | Enhanced skin penetration with combination of MNs and NLCs, increased transdermal bioavailability of alkaloids, consistent blood drug concentrations | [105] |
| Solid (stainless steel) | Polymeric NPs (carboxymethyl chitosan) |
5-Fuorouracil | Localized and painless administration, low side effects | [106] |
| Dissolving (PVP) | PLGA hollow microspheres | Alexa 488 and Cy5 (model compounds) | Co-delivery into the skin | [43] |
| Dissolving (PEG-MGQD) | nanocomposites of chitosan and magnetic graphene quantum dot | Lidocaine hydrochloride | Delivery of small and large molecule therapeutics | [107] |
| Dissolving (PVP) | PLGA nano/microparticles | Vitamin D3 | Delivery to deep skin layers and sustained release | [108] |
| Dissolving (PVP) | Hollow mesoporous silica nanocomposites | Metformin | NIR-triggered TDD and photothermal-responsive delivery | [109] |
| Dissolving (PVP) | PLGA@chitosan and PCL@chitosan NPs | Doxycycline | Enhanced retention and improved dermatokinetic profile of doxycycline | [110] |
| Dissolving (PVP) | Nanosuspension | Doxycycline, Albendazole, and Ivermectin | Enhance retention in the dermis | [111] |
| Dissolving (PVA/PVP) | PCL NPs | Carvacrol | Enhanced skin retention, sustained therapeutic effect | [112] |
| Dissolving (HA) | HA NPs | Rhodamine B | Enhanced skin penetration, sustained release | [113] |
| Dissolving (PVA) | Nanosuspension | Curcumin | Improved intradermal delivery | [114] |
| Dissolving (HA) | MPEG-PCL NPs | 5-Fuorouracil and indocyanine green | NIR-responsive delivery, synergistic chemo-photothermal effect | [115] |
| Dissolving (HA) | Micelles | Curcumin | Enhanced transdermal permeation | [116] |
| Dissolving (PVA/PVP) | SLNs | Doxycycline, Diethylcarbamazine, and Albendazole | Enhanced retention in the dermis layer, high bioavailability, accumulation in lymph nodes | [117] |
| Dissolving (PVA) | Micelles | Rhodamine B | TDD of kidney targeting NPs | [118] |
| Hydrogel-forming (PLGA) | Hydrogel microparticles | Rhodamine B | Sustained release | [119] |
PCL: poly (ε-caprolactone); MPEG: monomethoxy-poly (ethylene glycol).
3.1. MN-Assisted NP Delivery in Cancer Chemotherapy
Despite the efforts made to develop new cancer treatment strategies, chemotherapy has remained an important therapeutic modality. Numerous nanomaterials have been investigated as drug carriers to enhance the therapeutic efficacy and bioavailability of anti-cancer agents. Nonetheless, the systemic administration of anti-cancer therapeutics can trigger serious side effects [120]. Using the local TDD of anti-cancer agent-loaded NPs, we can prevent systemic toxic effects. However, there are many challenges in TDD due to the SC layers preventing the penetration of NPs. This challenge can be addressed by MNs, which can offer a minimally invasive TDD method. For example, PLGA NPs encapsulating doxorubicin (DOX) was coated on stainless steel MNs for localized drug delivery to the oral cavity tumors [121]. As MNs does not involve fluid injection, this method minimizes drug clearance from the tumor site into the systemic circulation due to the convection process. After inserting MNs, PLGA NPs were deposited at the MNs’ insertion site. Unlike hypodermic needles, which resulted in a significant loss of the injected volume, MNs produced uniform drug distribution in a porcine cadaver buccal tissue. In another study, MNs mediated the TDD of cisplatin-loaded lipid NPs for cancer treatment [122]. In this work, tumor-targeting pH-responsive lipid NPs were applied as a carrier for cisplatin encapsulation. The encapsulation led to a substantial increase in the cisplatin solubility and improved in vitro antitumor efficiency. The antitumor efficiency of MNs embedding cisplatin NPs was compared with the cisplatin loaded MNs in a xenograft mice model of head and neck tumor. The result showed an enhancement in the treatment outcomes of the NP-embedded MNs, though no comparison was made with cisplatin-loaded NPs. Furthermore, no serum platinum, hepatotoxicity, pulmonary toxicity, and nephrotoxic effect were detected, indicating the biosafety of the MN-assisted delivery of cisplatin NPs. Therefore, the combination of MNs and NPs containing chemotherapy agents can offer promising cancer treatment opportunities by improving antitumor effects and minimizing the systemic toxicity.
3.2. MN-Assisted NP Delivery in Cancer Immunotherapy
As an immune reactive component, skin tissue could be a site for an efficient immune response against a wide range of antigens. Different immune cells including the dendritic cells (DCs), Langerhans cells (LCs), and antigen-presenting cells (APCs) are involved in an efficient immune response. A high density of these cells, as well as their accessibility, has highlighted skin as one of the most important vaccination sites [123]. In recent years, researchers have explored therapeutic vaccines and immunotherapies in the scope of cancer via intradermal administration. For instance, some studies in animal models and patients suggest that intradermal vaccination can improve the antitumor responses in melanoma and prostate cancers [124,125].
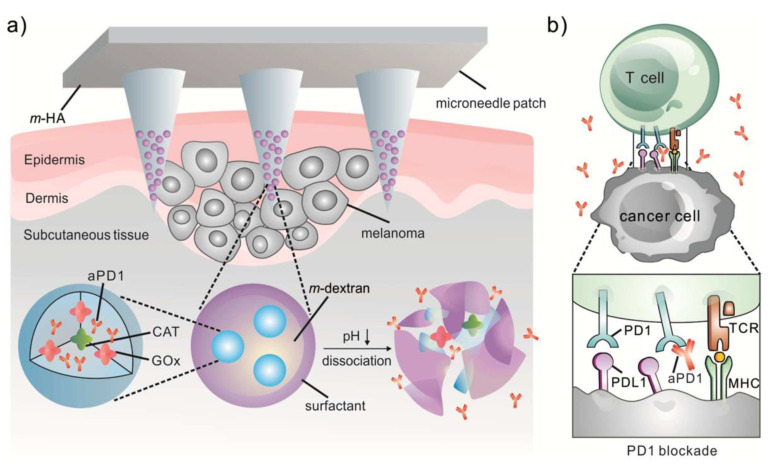
Considering the potential of MNs to improve percutaneous delivery, MN-mediated transcutaneous immunization can be an effective method for the stimulation of immune responses. For instance, dMNs consisting of poly (methyl vinyl ether) and maleic anhydride (PMVE/MA) laden with PLGA NPs-containing model antigen (ovalbumin, OVA) was utilized to target LCs. This approach has shown proper in vivo protection against melanoma (expressing B16 antigens) and para-influenza in a murine model by activating antigen-specific CD8+ T lymphocytes in tumors and viruses, respectively. Moreover, this study showed that antigen encapsulation in NPs can prolong the antigen’s retention time in the skin and enhance the antigen stability in MNs. Therefore, this approach could provide proper antigen protection in the case of OVA-expressed melanoma cells [126]. A few studies have also reported the application of MNs in combination with NPs for cancer vaccination. They have shown antitumor immunity in preclinical models applying MNs for the topical administration of cancer cell antigens, encoded in plasmids or obtained from tumor cell culture, which were loaded in polymeric NPs [127,128]. Indeed, a combination of MNs with NPs to deliver checkpoint inhibitors or immunosuppressive enzymes can prolong their retention time in the tumor and potentially reduce their side effects compared with the conventional administration [44]. To provide an enzyme-responsive drug release for cancer immunotherapy, Wang et al. integrated HA MNs with pH-sensitive dextran NPs containing anti-programmed death-1 (aPD1) and GOx. The blood glucose is catalyzed to gluconic acid by GOx, producing an acidic environment that causes the disintegration of NPs and subsequently, aPD1 release (Figure 4). It was shown that the single administration of the MN patch prevented the tumor’s proliferation in a mouse melanoma model, which was determined to be superior over the intratumoral injection of free aPD1 (at the same dose) or the aPD1-laden MNs without GOx-triggered degradation [45].
Figure 4.
Schematic preparation of the aPD1 containing hyaluronic acid microneedles (HA MNs) for the prevention of skin cancer: (a) MNs containing the pH-sensitive dextran nanoparticles encapsulating catalase/glucose oxidase (CAT/GOx) enzyme system that convert the blood glucose to gluconic acid and leading to aPD1 sustained release; (b) aPD1 released from the MNs blocks the PD-1 receptor, which resulted in activating the immune cells to kill skin cancer cells; CAT and GOx stand for the catalase and glucose oxidase, respectively. Reprinted with permission from [45].
3.3. MN-Assisted NP Delivery in Photothermal Therapy
Photothermal therapy (PTT) with near-infrared (NIR) irradiation is an interesting alternative technique for chemotherapy that can be combined with conventional chemotherapy to promote a synergistic effect. In PTT, the photothermal agents convert light to heat, which can induce thermal cell injury, membrane damage, and protein denaturation [129]. Furthermore, PTT not only inhibits solid tumor growth but also triggers the immune response. Compared to existing cancer therapies, PTT can be remotely controlled, leading to low systemic toxicity and adverse effects [130]. However, with an increase in the tissue’s depth, a decreasing dose of light can reach the photothermal agent, so PTT alone fails in a complete tumor elimination. Therefore, many delivery systems combining chemotherapy and PTT have been developed to obtain synergetic effects.
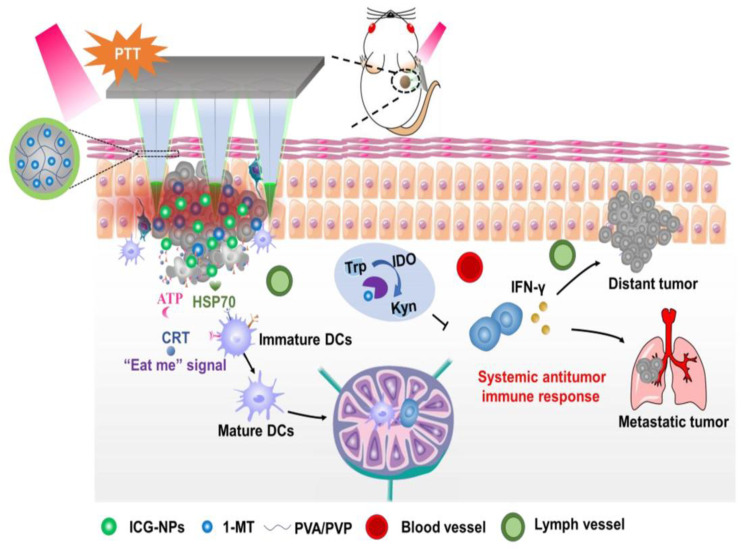
To achieve combined synergistic effects, chemotherapeutic drugs and photothermal agents should be simultaneously delivered precisely to the tumor site. However, finding an administration route for co-delivery has remained a big challenge that can be addressed by MNs. For this purpose, DOX-encapsulated dissolvable hyaluronic acid MNs (DOX-loaded HA MNs) containing a PEGylated gold nanorod (PEGylated-GNR) as a NIR light-responsive agent was developed for human epidermoid cancer therapy [131]. In this system, PEGylated-GNR acted as a photothermal agent, and hyaluronic MNs presented a high DOX loading capacity and good skin penetration capability. It was reported that the DOX release from HA MNs can be controlled through NIR light irradiation. Furthermore, the MN-treated animals revealed a notable antitumor efficacy and the tumor growth inhibition. Moreover, several studies have applied MNs for the transcutaneous co-delivery of chemotherapeutic drugs and photothermal agents into the tumor site, indicating MNs as a promising technique for the synergistic effects of PTT and chemotherapy [77,130,132,133]. A new MNs-based system was recently introduced for PTT featuring the co-delivery of a photosensitizer (indocyanine green) loaded into chitosan NPs (ICG-NPs) and 1-methyl-tryptophan (1-MT) as an indoleamine 2, 3-dioxygenase (IDO) blockade. The ICG-NPs were concentrated on the MNs’ tip and 1-MT was encapsulated into the cross-linked PVP and PVA gel as the MNs’ core. As shown in Figure 2, ICG-NPs converted the NIR laser irradiation into heat, which could destroy the tumor cells inducing the release of tumor-associated antigens, the maturation of dendritic cells, the secretion of the immune-stimulatory cytokines, and consequently the stimulation of systematic immune responses (Figure 5). The results indicated the potential application of MN-based systems for delivering NPs, which effectively combine PTT with immunotherapy in cancer treatment [134].
Figure 5.
Graphical presentation of the mechanism for the antitumor effect of microneedles in combination with indocyanine green-loaded chitosan NPs (ICG-NPs).
After inserting MNs into the tumor site, the MNs dissolved and released ICG NPs and 1-methyl-tryptophan (1-MT) as an indoleamine 2, 3-dioxygenase (IDO) blockade. Following NIR irradiation, the PTT effect of ICG-NPs can ablate tumor cells, warn the immune system, and promote interferon-γ (IFN-γ) secretion. Furthermore, the pre-apoptotic calreticulin (CRT) translocation starts generating an “eat me” signal to the cell surface that causes the uptake of death cancer cells by APCs. Other damage-mediated molecular patterns including some heat shock proteins (HSP70) can also help in the APCs’ maturation. Then, the released 1-MT can prevent the IDO activity to catalyze the tryptophan (Trp) degradation into kynurenine (Kyn). The local co-delivery of ICG-NPs with IDO blockade not only can destroy the primary tumor cells but also inhibit the growth of distant tumor cells and lung metastases. Reprinted with permission from [134].
3.4. MN-Assisted NP Delivery in Photodynamic Therapy
Photodynamic therapy (PDT) is a non-invasive approach to treat various tumors and non-neoplastic diseases [135]. PDT involves combining a photosensitizer and a specific light wavelength, which can promote cytotoxic reactive oxygen species (ROS) generation at the presence of tissue oxygen resulting in the death of the damaged cells. As the systemic administration of sensitizers suffers from poor selectivity and hence skin photosensitivity, local administration is more desirable as the photosensitization only occurs at the treatment tissue [136]. Various chemical and physical methods have been investigated to improve the TDD of photosensitizers, such as lipophilic derivatization [137], chemical enhancers [138], nanoemulsions [139], sonophoresis [140], iontophoresis [141], and MNs [142]. MNs have attracted widespread attention among these methods due to their exceptional performance and low side effects.
MN-mediated PDT has been investigated for its possible applications in tumor treatment. Donnelly et al. explored the ability of solid silicon MNs for tumor therapy utilizing the poke-and-patch approach [136]. They showed that this technique enhanced the photosensitizer penetration through the murine skin. In another study, Gill et al. indicated that 5-aminolevulinic acid-coated stainless MNs (ALA-coated MNs) can enhance the TDD of the photosensitizer. Interestingly, about 57% of the tumor inhibition was achieved with only one dose of ALA (1.75 mg), while the topical cream formulation of ALA (5 mg) failed in suppressing the tumor growth [143]. The potential application of dMNs (Gantrez®S-97 MNs) was recently explored for the skin delivery of the NIR photosensitizer, Redaporfin™ [84]. In vitro studies showed the successful delivery of a photosensitizer as it was detected at a 5 mm depth in the skin. The in vivo biodistribution results also demonstrated the fast-initial release and localized delivery of the photosensitizer. Nevertheless, some migration away from the application site was reported by day 7, showing that widespread skin photosensitivity in patients would be improbable. In another work, the ALA tip-encapsulated fast-dissolving HA MNs (ALA@HA MNs) patch was fabricated by Zhao et al. [132]. Notably, the efficacy of PDT in xenografted tumor mice treated with ALA@HA MNs was much higher than the free ALA injection group. About 97% tumor inhibition was attained by ALA@HA MNs group (dose of 0.61 mg) while the rate was only 66% in the ALA injection group treated with a dose of 1.65 mg.
As mentioned before, the delivery of highly potent photosensitizers to the tumor site is vital for the excellent therapeutic efficacy of the PDT approach. Some recent studies reported photosensitizer-loaded NP formulations for not only controlled delivery but also for combinational therapy incorporating two or more drugs [144,145]. For this purpose, Tham et al. established a mesoporous nanocarrier with a dual loading ability to encapsulate a photosensitizer and a clinically relevant therapeutic agent for combination therapy [146]. They also utilized metal MNs to promote nanocarrier penetration into the deep skin layers (Figure 6). This strategy demonstrated a synergistic killing effect on skin cancer cells and prevented the growth of the tumor cells in a 3D spheroid model in vitro. This system enhanced the skin penetration of the NPs to reach deep-seated cancer cells, which resulted in superior therapeutic efficacy through PDT in combination with the targeted therapy. Altogether, these studies suggest that the photosensitizer loaded MNs are a promising platform for enhanced and safer PDT.
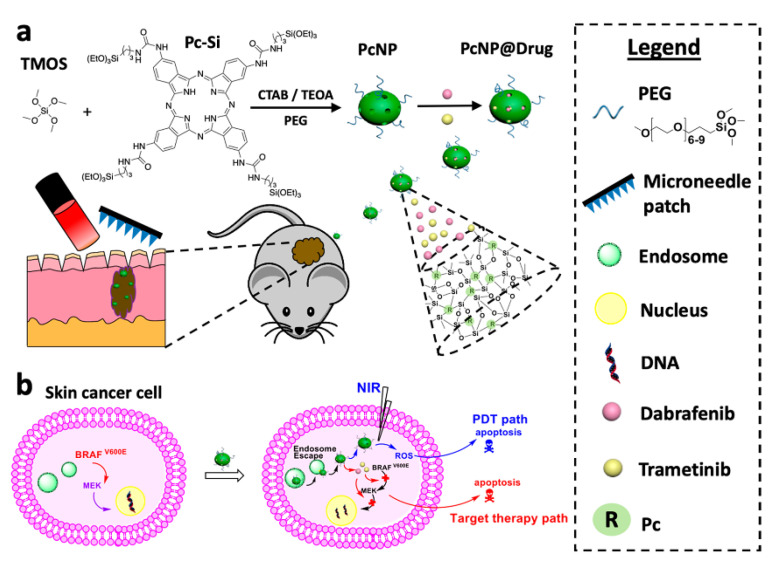
Figure 6.
(a) PcNP@Drug synthesis and its skin penetration. Phthalocyanine was modified with four silicate units (Pc-Si), Pc units excited by far-red light and acting as photosensitizers, then the Pc-conjugated mesoporous organosilica nanoparticle (PcNP) was fabricated via silane co-condensation and hydrolysis using Pc-Si. In this process, hexadecyltrimethylammonium bromide (CTAB) acts as the structure-directing agent, triethanolamine (TEOA) acts as a basic catalyst and tetramethyl orthosilicate (TMOS) acts as the inorganic silica agent. To prepare PcNP@Drug, dabrafenib and trametinib, as small inhibitor drugs, were encapsulated into the PcNP pores. PcNP@Drug was then delivered to mice skin through a microneedle patch. (b) PcNP@Drug cellular uptake: under NIR light irradiation, PcNP@Drug produced reactive oxygen species (ROS) in-vivo and the drugs’ release of PcNP@Drug destroyed cancer cells. Reprinted with permission from [146].
When used alone, the PDT approach suffers from several challenges, such as low efficacy and systemic phototoxicity [147,148]. To address these issues, an MN-assisted delivery system was recently developed combining PDT with immunotherapy for the treatment of focal cancer. To do so, HA-MNs were incorporated with the pH-sensitive dextran NPs to deliver zinc phthalocyanine as PS and CTLA4 antibody (aCTLA4) as a checkpoint inhibitor. The in vivo study in the 4T1 mouse model showed the accumulation of zinc phthalocyanine at the tumor vicinity. PDT was first applied to kill the tumor cells, which activated the immune responses, leading to improved immunotherapy with aCTLA4. This result indicated that an MNs-assisted system in combination with NPs can be regarded as a promising co-delivery platform for the treatment of focal cancer [149].
3.5. MN-Assisted NP Delivery in Delivery of Therapeutic Proteins
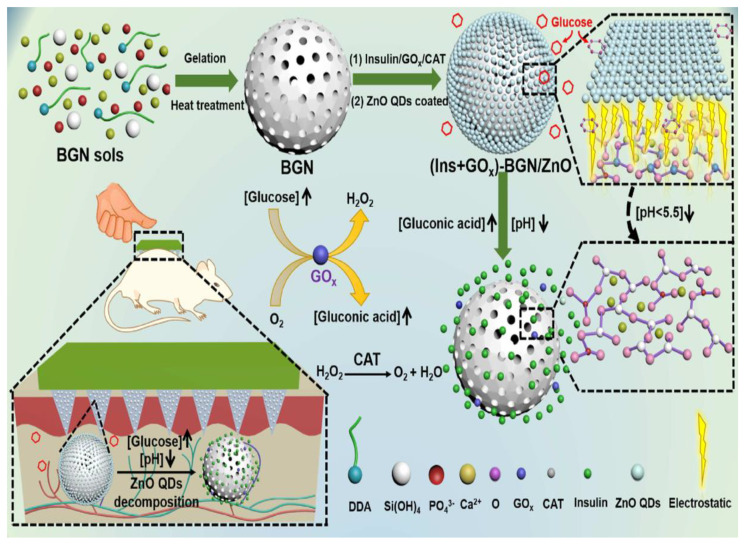
MN technology has offered a revolutionary platform for protein delivery. There are generally serious challenges for the TDD of therapeutic proteins such as their susceptibility to degradation and relatively large molecule size [150]. Concerning this issue, MN technology could be an interesting delivery system capable of smoothly passing SC and delivering proteins into the systemic circulation [150]. MN technology has been widely applied in recent years for the effective delivery of proteins, including antigen [151], antibody [152], insulin [153], exendin-4 [154], and lysozyme [155]. For example, introducing MN technology to insulin delivery has been used as a non-invasive, painless, and easy-to-handle administration technique in tuning the glucose level in diabetic patients. MN-based insulin delivery systems mostly employ hollow MNs, which are currently in clinical trials [156]. As an alternative approach, dMNs were used for insulin delivery. However, the fabrication of insulin-loaded dMNs with appropriate mechanical strength and stability is a challenging task suffering from difficult skin penetration and rapid payloads release [157]. Interestingly, integrating MNs with NPs can improve mechanical properties and stability. For example, dissolving the MN composite based on PVP and insulin-encapsulated CaCO3 microparticles showed notable mechanical strength and sustained release in comparison with pure PVP MNs [158]. This study suggests that the combination of MNs with microparticles can lead to constant insulin release and improved therapeutic efficiency. Programmable MNs can also reduce the hypoglycemia risk associated with unnecessary drug release, thereby avoiding the side effects [159]. For this purpose, mesoporous bioactive glasses (MBGs) capped with ZnO quantum dots (ZnO QDs) were integrated with PVP dMNs exhibiting smart pH-triggered ability for the glucose-mediated TDD of insulin [109]. Glucose oxidase/catalase (GOx/CAT) were loaded as insulin and glucose-responsive factors into the MBGs’ pores that were electrostatically sealed by ZnO QDs as a pH-responsive switch. GOx/CAT content of MBGs’ pores could catalyze glucose to gluconic acid, resulting in a local pH drop leading to the dissolution of ZnO QD caps from MBGs and the insulin release from the MBGs’ pores. In vivo studies in the diabetic model showed a glucose-mediated release of insulin, along with proper blood glucose lowering action, and a lower risk of hypoglycemia (Figure 7). In another programmable MN, the surface of the insulin-loaded MBGs was modified with a glucose-sensitive layer composed of poly ethyleneimine (PEI), GOx, and CAT. Insulin release was achieved through converting the body fluids’ glucose to gluconic acid by GOx/CAT, resulting in a drop in pH and the subsequent destruction of the gatekeeping surficial layer [160]. Altogether, these studies demonstrate that the MNs-based, glucose-responsive and pH-triggered TDD can be a successful strategy in the diabetes treatment.
Figure 7.
Schematic presentation of the ZnO quantum dots QDs which capped the pores of mesoporous bioactive glass NPs (MBGNs) integrated with PVP MNs for the glucose mediated TDD of insulin. After penetration into the skin and participation in the body circulation, GOx and CAT in the MBGs convert glucose to gluconic acid, resulting in decreasing the local pH and dis-assembly of ZnO QD caps, leading to the opening of MBGs’ nanopores and then releasing the insulin molecules. Reprinted with permission from [109].
The ongoing development of smart insulin delivery based on MN technology in combination with NPs has shown the potential to improve the quality of life of diabetic patients. In recent works, the MNs integrated with insulin-loaded, H2O2-responsive NPs were designed to obtain fast and painless administration [161,162]. In this system, GOx was co-loaded into the NPs to catalyze blood glucose to gluconic acid and generate H2O2 as a reaction byproduct resulting in NPs disintegration and insulin release. Therefore, the NPs act as both the glucose-sensing agent moieties and the insulin release actuator. NPs can provide basal insulin release in a controllable manner. Moreover, they facilitate insulin release in response to a hyperglycemic condition [163]. These results indicate that stimuli-responsive glucose-mediated TDDSs based on MNs in combination with NPs have potential applications in diabetes management.
MNs have been utilized for transdermal vaccination and the immunotherapy of skin tumors. Zhou et al. developed MNs containing transfersomes co-encapsulated anti-PD1 (antigen) and polyinosinic:polycytidylic acid (adjuvant) which were functionalized with αCD40 (DCs targeting ligand). The accumulation of the transferosomes in tumor-draining lymph nodes enhanced the maturation of DCs and improved Th1 immune responses. An improved T cell activation and infiltration was reported in a mouse melanoma model. Moreover, the activity of regulatory T cells decreased in the tumor site, reverting the immunosuppressive tumor microenvironment [164]. This study indicated the potential application of MNs in combination with transfersomes as a promising platform for cancer immunotherapy. In another study, MicronJet600 hollow MNs were used to deliver an auto-antigen peptide conjugated with AuNPs. In vitro investigations showed AuNPs uptake by DCs and the activation of naïve T cells. AuNPs can also facilitate the temporal and spatial delivery of peptides. Therefore, AuNP–peptide formulations, which are currently under clinical investigation, can be incorporated into MNs for immunotherapy applications [165].
3.6. MN-Assisted NP Delivery in Vaccine Delivery
Most vaccines are currently administered using hypodermic needles, necessitating expert administration, cold chain storage, and the transportation of liquid formulations. In addition, most vaccines are formulated in liquid necessitating close temperature control during transport, storage, and distribution (i.e., cold chain) [166]. The issues of poor vaccine transport through the skin barrier, patient compliance, and cold chain can be addressed by MNs. MNs can painlessly bore the SC and canalize the epidermis to improve the vaccination as well as dependency on the cold chain and the need for reconstitution [167]. Additionally, MNs should be regarded as a unique strategy for the delivery of antigen to immune cells such as DCs within the skin, which is an essential problem in vaccine delivery. To date, MN reports have demonstrated similar or even higher immunogenicity and dose sparing [168]. Several investigations have reported the application of different MN strategies and their use in various vaccine formulations, including influenza, and Human papillomavirus (HPV). For example, inactivated influenza virus vaccine was encapsulated in dMNs, and sucrose or trehalose was used to stabilize the antigen. The results indicated that the stabilization and vaccine drying through lyophilization can lead to greater vaccine stability and in vivo immunogenicity compared with the conventional vaccine preserved for 1 month at 45 °C [169]. Accordingly, dMNs can reduce the dependency on the cold chain, improve the thermostability, eliminate the need for reconstitution, and simplifying vaccine distribution. For more detailed information regarding MNs-based vaccination systems, please refer to several excellent reviews [25,170,171].
As mentioned earlier, MNs have contributed to the delivery of many NPs into the skin that can be extended to vaccine delivery. The main reason behind the use of NPs in combination with MNs to deliver the antigen is the improved stability and controlled release of antigen for inducing higher immunogenicity. For example, chicken OVA was encapsulated into PLGA-NPs, which was then incorporated in dMNs. Using this approach, MNs slowly released the antigen to lymph nodes occupied with DCs. This strategy led to the successful in vivo immune system activation against influenza and melanoma tumors [126]. In another study, monophosphoryl lipid A, OVA, imiquimod, and Toll-like receptor (TLR) agonists were encapsulated in PLGA NPs, which were then intradermally delivered through hollow MNs. Unlike intramuscular injection, the MNs generated higher levels of IgG2a antibody and IFN-γ-producing lymphocyte [172]. Guangsheng et al. compared different types of nanocarriers to modulate the immune response by hollow MNs. To do so, OVA was loaded into MSNs, liposomes, PLGA, or gelatin NPs with or without polyinosinic:polycytidylic acid as an immunostimulant. Liposome and PLGA induced significantly higher IgG2a response. Moreover, liposomes led to CD4+ and CD8+ T cell activation [173]. Table 3 summarizes the studies on the MNs’ application in the transcutaneous delivery of antigen-loaded NPs.
Table 3.
Vaccine delivery studies with different MNs regarding the type of vaccine and NPs.
| Type of MNs | Vaccine Type | Carrier | Outcome | Ref. |
|---|---|---|---|---|
| Solid | Fluvax vaccine + CXCL1-specific siRNA | Liposome | Effective silencing of CXCL1 gene in skin | [174] |
| Solid | HPV-16 E6/E7 DNA | PVP | More potent vaccination than intramuscular administration, delayed TC-1 tumor growth | [175] |
| Solid | Ovalbumin | Hyaluronan | Humoral and mucosal immune activation, strong immune-recall responses, strong immunization | [176] |
| Solid | Tetanus toxoid | Chitosan | Higher IgG2a level in comparison to commercial vaccine, balanced Th1/Th2 ratio | [177,178] |
| Coated | HIV-1 p24 Gag peptide | Polypropylene sulfide | Efficient uptake without adjuvant, potent HIV-1specific CD4+ T-cell responses | [179] |
| Coated | pH1N1 DNA | Polyplex containing PLGA/poly ethyleneimine (PEI) | Rapid dissolution of polyplex which induced potent humoral immune response | [180] |
| Hollow | H1N1 virus | Virus like nanoparticles (VLPs) | New vaccination approach | [181] |
| Hollow | Ovalbumin | PLGA | Protective T cell-mediated immunity | [182] |
| Hollow | Plasmid DNA | Niosome | High level of IgG titer | [183] |
| Hollow | Diphtheria toxoid | Liposome | High level of IgG2a | [184] |
| Hollow | Peptide/ Human papillomavirus | Liposome | Strong T helper responses | [184] |
| Dissolving | Ovalbumin | Liposome | High IgG level, enhanced immune response | [185] |
| Dissolving | Ovalbumin | Liposome | Effective mucosal immunization via oral route vaccination | [186] |
| Dissolving | pEGFP-N1 plasmid DNA | PVP and PVA | Superior DNA preservation by PVA than PVP | [187] |
| Dissolving | Ovalbumin | Liposome | Enhanced immune response, balanced Th1 and Th2 humoral immune responses | [188] |
| Dissolving | Ovalbumin | Transfersomes | Increased IgG2a/IgG1 ratio, specific Th1 antigen-specific immunizations Immune response in lymph nodes |
[189] |
| Dissolving | Hepatitis B virus | Liposome | Strong cell-mediated immune response HBV, CD8+ T cells population increase significantly | [186] |
| Dissolving | Ovalbumin Plasmid | OSM-(PEG-PAEU) * | Humoral and cellular immunity, activation of cytotoxic CD8+ T cells | [190] |
| Dissolving | DNA | Pluronic P123/PEI | Higher humoral and cellular immunity compared to IM administration | [177,178] |
| Dissolving | SIINFEKL peptide | Cubosome | Efficient local delivery. | [191] |
* Oligo sulfamethazine conjugated poly (β-amino ester urethane).
3.7. MN-Assisted NP Delivery for Gene Therapy
Gene therapy, or replacing a malfunctioning gene with therapeutic nucleic acids [192], can be applied in the treatment of a wide variety of genetic skin disorders, cutaneous cancers, and wound healing. Transdermal DNA delivery has several advantages, including localized delivery, large surface area, and evading first-pass metabolism [193]. However, the SC limits the TDD of macromolecules, particularly nucleic acids. To date, several physical strategies, such as the gene gun, iontophoresis, sonophoresis, needle-free liquid jet injections intradermal injection, electroporation, and MNs have been employed to deliver DNA therapeutics across the skin [193]. The application of MNs in gene delivery has been explored with different delivery approaches, particularly with solid MN arrays, which have been shown for bare DNA or its combination with NPs [71,194]. For the first time, the bare plasmid DNA (pDNA) encoding firefly luciferase was applied topically to introduce DNA into the disrupted skin of the shaved dorsum of BALB/c mice by blunt-tipped silicon MNs. The results showed a 2800-fold increase in gene expression when the solid MN was used in scraping the SC barrier instead of a topical application. In another study, the pDNA encoding the hepatitis B surface antigen induced a robust and high antibody titer via silicon MNs compared to intradermal injection. Furthermore, the combinatorial use of NPs enables us to appropriately control the release kinetics, gene loading, and cellular uptake of genetic materials delivered by MNs. Ruan et al. developed MNs coated with BRAF siRNA (siBraf) complexes with cell-penetrating peptide octaarginine (R8) for melanoma treatment. In vivo experiments exhibited the significant inhibition of melanoma development, apoptosis, and the suppression of cell proliferation in mice bearing melanoma [195].
MNs are especially considered in the transcutaneous delivery of DNA vaccines. For example, the double conjugate of PEI to mannose (Man) and cell-penetrating peptide (CPP: a sequence of TAT: RRRQRRKKRC-SH) were used to condense the DNA vaccine, which was then applied in transcutaneous immunization using solid MNs. This system effectively activated the Trp2-specific response, leading to effective immune system activation against B16 melanoma in BALB/c mice [128]. In this same research performed by Xu et al., MNs were applied co-delivery of DNA vaccine and low-dose paclitaxel (PTX) encapsulated in polymeric nanocomplex for cancer immunotherapy. In this system, polymeric nanocomplex was constructed using low-dose PTX as a neoadjuvant that was physically loaded in sulfobutylether-cyclodextrin (SBE), and the PTX/SBE further acted as an anionic crosslinker to self-assemble with the cationic mannosylated N, N, N-trimethyl chitosan/DNA polyplexes. MNs assisted in the co-delivery of the DNA vaccine and PTX to DCs, which resulted in synergistic effects on the DCs maturation and antigen-presenting function, consequently enhancing the immune stimulation and decreasing the immune escape [196].
4. Advances in Clinical Trials and Commercialization of MNs
As mentioned earlier, the applicability of MNs in TDD has been investigated since 1998 [26]. To date, numerous pre-clinical and clinical trials have been conducted to examine the safety and efficacy of MNs for cosmeceutical, diagnosis, and therapeutic applications [18]. As presented in Table 4 and Table 5, advances in MN fabrication technology have led to the commercialization of MNs for clinical applications. During the last decade, clinical trials have been aimed at using MNs for vaccination and insulin delivery. The results from the clinical trials on cosmeceutical applications have shown that MN rollers, as a type of solid MNs, are the most attractive choice. According to Table 4, the application of MNs combined with NPs for TDD is limited to the proof-of-concept pre-clinical studies and there is only one report of a phase 1 clinical trial of the intradermal administration of human C19A3 proinsulin peptide coupled to gold NPs using MNs in type 1 diabetes [197]. Due to the significant features of NPs and their promising clinical outcomes, it is expected that a combination of NPs’ and MNs’ application grow fast in the future, but further studies are required to exploit the therapeutic and diagnostic potentials of smart MNs.
Table 4.
Clinical trials and their current status [198].
| Field | Title | Type of MNs | Condition/Disease | Phase | Status | Clinical Trial Registry Number |
|---|---|---|---|---|---|---|
| Therapeutic | 2010/2011 trivalent influenza vaccination | Solid (MicronJet) | Influenza | Not provided | Completed | NCT01304563 |
| The effect of microneedle pretreatment on topical anesthesia | Solid (MN roller) | Pain | Not provided | Completed | NCT02596750 | |
| The use of microneedles to expedite treatment time in photodynamic therapy | Solid (MN roller) | Keratosis, actinic | Not provided | Completed | NCT02594644 | |
| The use of microneedles with topical botulinum toxin for treatment of palmar hyperhidrosis | Solid (Sham MN) | Hyperhidrosis | I | Completed | NCT03203174 | |
| A study to determine the patient preference between Zosano Pharma parathyroid hormone (ZP-PTH) patch and Forteo pen | Coated titanium (ZP-PTH MN patch) | Postmenopausal Osteoporosis | I | Completed | NCT02478879 | |
| Phase 2 study of BA058 (Abaloparatide) transdermal delivery in postmenopausal women with osteoporosis | Coated 3 M microstructured transdermal system | Post-menopausal osteoporosis | II | Completed | NCT01674621 | |
| Dose sparing intradermal H1N1 influenza vaccination device | Hollow (MicronJet 600™) | Influenza infection | Not provided | Completed | NCT01049490 | |
| Immunogenicity of inactivated and live polio vaccines | Hollow (MicronJet 600™) | Poliomyelitis | III | Unknown | NCT01813604 | |
| Safety study of suprachoroidal triamcinolone acetonide via microneedle to treat uveitis | Hollow | Uveitis | I/II | Completed | NCT01789320 | |
| Routes of immunization and flu immune responses | MN injection (not defined) | Influenza | I/II | Completed | NCT01707602 | |
| Comparison of 4 influenza vaccines in seniors | Hollow (Becton Dickinson (BD) MN) | Influenza | IV | Completed | NCT01368796 | |
| Immunogenicity of the inactivated split-virion influenza vaccine in renal transplant subjects | Hollow (BD Soluvia™) | Influenza Orthomyx-oviridae infection |
II | Completed | NCT00606359 | |
| Atopic dermatitis research network (adrn) influenza vaccine study | Pre-filled or hollow (Fluzone®) | Dermatitis, atopic | Not provided | Completed | NCT01737710 | |
| Immunogenicity study of the influenza vaccine in adults | Hollow (BD MN injection) | Orthomyx-oviridae infection Influenza |
II | Completed | NCT00258934 | |
| Study of inactivated, split-virion influenza vaccine compared with the reference vaccine Vaxigrip® in the elderly | Hollow (BD MN injection) | Orthomyx-oviridae infection Influenza Myxovirus infection |
III | Completed | NCT00383526 | |
| Intradermal versus intramuscular polio vaccine booster in HIV-infected subjects | Hollow (MicronJet 600™) | Polio immunity | II | Completed | NCT01686503 | |
| Varicella zoster virus (VZV) vaccine for hematopoietic stem cell transplantation | Hollow (MN syringe) | Varicella zoster infection | II/III | Completed | NCT02329457 | |
| Insulin delivery using microneedles in type 1 diabetes | Hollow (glass) | Type 1 Diabetes Mellitus | II/III | Completed | NCT00837512 | |
| A pilot study to assess the safety, pharmacokinetics/ pharmacodynamics (PK/PD) of insulin injected via MicronJet™ or conventional needle | Hollow (MicronJet™) | Intradermal injections | Early phase I | Completed | NCT00602914 | |
| Pharmacokinetic comparison of intradermal versus sub-cutaneous insulin and glucagon delivery in type 1 diabetes | Hollow (MicronJet™) | Type 1 Diabetes | II | Unknown | NCT01684956 | |
| Multi-day (3) in-patient evaluation of intradermal versus subcutaneous basal and bolus insulin infusion | Hollow (BD Research Catheter) | Diabetes | I/II | Completed | NCT01557907 | |
| Safety and efficacy of ZP-glucagon to injectable glucagon for hypoglycemia | Not defined | Hypoglycemia | I | Completed | NCT02459938 | |
| Study on the effects on blood glucose following intradermal and subcutaneous dosing of insulin in diabetic patients | Hollow (BD Research Catheter) | Diabetes | I/II | Completed | NCT01120444 | |
| Pharmacokinetics/dynamics of basal (continuous) insulin infusion administered either intradermally or subcutaneously | Hollow (BD Research Catheter) | Diabetes Mellitus, Type 1/2 | I/II | Completed | NCT01061216 | |
| A study to assess the safety and efficacy of a microneedle device for local anesthesia | Hollow (MicronJet™) | Local anesthesia intradermal injections |
Not provided | Completed | NCT00539084 | |
| Safety demonstration of microneedle insertion | Gold/silver coated or uncoated hollow MNs | Allergic reaction to nickel | NA (safety demonstration) | Completed | NCT02995057 | |
| Microneedle patch study in healthy infants/young children | Dissolvable | Vaccination skin absorption |
Not provided | Completed | NCT03207763 | |
| Microneedle array–doxorubicin (MNA-D) in patients with cutaneous T-cell lymphoma (CTCL) | Dissolvable | Cutaneous T cell lymphoma | I | Recruiting | NCT02192021 | |
| Inactivated influenza vaccine delivered by microneedle patch or by hypodermic needle | Dissolvable | Influenza | I | Completed | NCT02438423 | |
| Microneedle patch for psoriatic plaques | Dissolvable (MN-HA patch) | Psoriasis | Not provided | Unknown | NCT02955576 | |
| Safety and efficacy of ZP-zolmitriptan intracutaneous microneedle systems for the acute treatment of migraine | MN patch (not defined) | Acute migraine | II/III | Completed | NCT02745392 | |
| Cosmetic | Microneedling plus the universal peel for acne scarring | Solid (MicroPen) | Acne scarring | Not provided | Completed | NCT02174393 |
| Comparison of efficacy between fractional microneedling radiofrequency and bipolar radiofrequency for acne scar | Solid (Microneedling radiofrequency device) | Acne scarring | Not provided | Completed | NCT02207738 | |
| Comparison of treatments for atrophic acne scars | Solid (Dermaroller) | Acne scarring | Not provided | Unknown | NCT02025088 | |
| Comparison of the efficacy of micro-holes versus laser-assisted dermabrasion, for repigmenting in vitiligo skin | Solid (Dermaroller) | Vitiligo-macular depigmentation | Not provided | Unknown | NCT02660320 | |
| Transplantation of Basal Cell Layer Suspension Using Derma-rolling System in Vitiligo | Solid (Dermaroller) | Vitiligo | Not provided | Unknown | NCT02962180 | |
| Safety and efficacy of the EndyMed Pro system using RF microneedles fractional skin remodeling | Solid (EndyMed Pro™ RF Microneedles) | Aging | Not provided | Unknown | NCT02368626 | |
| Evaluating the efficacy of microneedling in the treatment of androgenetic alopecia | Solid (Microneedling) | Androgenetic alopecia | I | Unknown | NCT02154503 | |
| Performance of the ePrime System for Cellulite | Solid (ePrime Syneron Candela) | Cellulite | Not provided | Unknown | NCT02489994 | |
| Tolerability study of the application of a 3M microstructure transdermal system | Solid (Transdermal Microchannel Skin System) | Healthy | I | Completed | NCT01257763 | |
| Teosyal® PureSense redensity [I] injection using Micronjet® needle in the treatment of crow’s feet wrinkles | Hollow (MicronJet™) | Crow’s feet wrinkles | IV | Completed | NCT02497846 | |
| Diagnostic | Physiological study to determine the allergic skin activity after different skin preparation | Solid (Micro Skin System, 3M) | Birch pollen allergy | I | Completed | NCT01628484 |
| Minimally Invasive Sensing of Beta-lactam Antibiotics (MISBL) | Solid | Healthy volunteers | I | Completed | NCT03847610 | |
| Analysis of non-invasively collected microneedle device samples from mild plaque psoriasis for use in transcriptomics profiling | Solid MNs as sampling device | Psoriasis vulgaris | Cohort, prospective | Completed | NCT03795402 | |
| Optimization of tuberculosis intradermal skin test | Hollow (BD Research Catheter) | Healthy volunteers | Not provided | Completed | NCT01611844 | |
| Glucose measurement using microneedle patches | Hydrogel forming | Diabetes (diagnostic) | Not provided | Completed | NCT02682056 |
Unknown: study has passed its completion date and status has not been verified in more than two years.
Table 5.
Commercially available MNs.
| Type of MNs | Product Name | Company Name | Description of the Product | Use |
|---|---|---|---|---|
| Solid | Dermapen | Dermaroller GmbH | An array of 12 needles loaded on an electric motor unit fitted with a spring that punches skin (412–700 cycle/min) | Treatment of isolated scars, skin lesions and wrinkles, appropriate for smaller areas of the skin |
| Dermaroller | Whitelotusbeauty | Contains 192 titanium needles (0.5 mm long) in cylindrical assembly | Cosmetic application and skin care with cream and serum, appropriate for larger areas of the skin | |
| Dermastamp | Whitelotusbeauty | An array of 40 needles loaded on an electric motor unit with controlled motion back and forth like stamp | Collagen induction therapy for skin scars, age spots, varicella scars and wrinkles, appropriate for smaller areas of the skin | |
| DermaFrac | Dermafrac.co | Very small stainless steel MNs roller equipped with electric power source and instrument for serum infusion, also contains light emitting devices | Wrinkles, skin ageing, hyperpigmentation, acne, uneven skin tone | |
| Onvax | Becton Dickinson | An array of silicon or plastic micro-projections on a hand-held applicator | Vaccine delivery | |
| LiteClear | Nanomed skincare | Silicon MNs pen for skin pretreatment | Treatment of acne and skin blemishes | |
| h-patch | Valeritas | Small adhesive MNs, hydrolytically regulated | Basal and bolus delivery of insulin | |
| Beauty Mouse | Dermaroller GmbH | Three rollers of 50 mm width and a total of 480 MNs, creating fine microchannels for enhanced penetration | Increasing the skin’s sensitivity towards anti-cellulite creams | |
| NanoCare | NanoPass Inc. | A small hand-held device for the rejuvenation of skin and to boost the cosmetic effect of topical applications | Cosmetic | |
| Adminstamp | AdminMed | MNs array attached to the applicator with six stainless steel screws (1400 µm-long MNs on 1 cm2 circular array), compatible with all sterilization methods | Transdermal drug delivery through the skin with excellent skin sensation and cosmetics | |
| Coated | MacroFLUX™ | Zosano pharma | PTH-coated titanium MN patch | Osteoporosis |
| Nanopatch | Vaxxas | Silicon patch (1 cm2) made up thousands of coated micro-projections | Polio vaccine | |
| Hollow | 3M MTS | 3M Corp | MN patch containing 351 needles/cm2 (650 µm length) | Skin treatment before dermatological application |
| FLUARIX | GlaxoSmithKline Biologicals | Three MNs (600 µm length) attached to a syringe | Influenza vaccine delivery | |
| Micronjet | Nanopass | Four silicon MNs (450µm length) attached to the tip of a plastic adapter | Intradermal vaccine delivery | |
| Fluzone | Sanofi Pasteur Inc Becton Dickinson |
A hand syringe gun unit that injects 1.5 mL vaccine solution by a micro-injector in intradermal site by a 1.5 mm MN tip | Influenza vaccine | |
| Soluvia | Sanofi-Aventis Becton Dickinson |
MNs (length 1.5 mm) for intradermal delivery | Influenza vaccine | |
| Microinfusor | Becton Dickinson | An automated hands-free system consists of an electrical pump connected to a hollow MN patch (capacity 0.2–15 mL) | Influenza vaccine, insulin, and highly viscose biotech drug | |
| Nanoject | Debiotech | Based on MEMS technology, hollow MN patch (length 300 to 1000 µm), featuring side holes which prevent sticking in the MN channels during skin penetration | Intradermal drug delivery and injection of diagnostic fluid | |
| Micro-Trans | Valeritas Inc. | Hollow MNs constructed with metal or biodegradable polymers | Intradermal drug delivery | |
| Intanza/IDflu | Sanofi-Aventis Becton Dickinson |
MNs (length 1.5 mm) combined with a needle shielding system and a 0.1 mL injection volume | Influenza vaccine | |
| AdminPen | AdminMed | Forty-three needles (length 1100 µm) on 1 cm2 circular microneedle array, stainless steel liquid injector device attached to a standard syringe | Liquid formulations (vaccines, drug) | |
| Microinject | Nanopass | Four hollow silicon MNs (length 250 µm), fitted with a syringe for intradermal injection | Influenza vaccine | |
| DebioJect | Debiotech | One or several hollow silicon MNs with a length ranging from 350 to 900 µm and a side protected delivery holes, injections up to 0.5 mL in a few seconds | Vaccine delivery | |
| Dissolving | Drugmat® | Theraject Inc. | Sumatriptan-loaded dMN patch made from a sugar polysaccharide | Migraine |
| Vaxmat® | Theraject Inc. | Sumatriptan-load dMNs | Migraine | |
| MicroCor | Coriumintl | PTH-loaded dissolvable peptide MN patch | Osteoporosis | |
| MicroHyala | CosMed | MN patch made of biocompatible hyaluronic acid | Wrinkle treatment, influenza vaccine |
PTH: parathyroid hormone, microstructured transdermal system technology.
5. Current Challenges and Future Perspectives
Given the thorough research performed in recent years on various types of MNs, there are several critical issues to address for their widespread applications in clinical settings. MN applications may lead to short- and long-term safety concerns in patients. It is generally believed that there is no major complication with the short-term use of MNs; nevertheless, the long-term application of MNs can cause erythema (skin redness) as well as pain based on their size and the number of needles. In addition, the effectiveness of MNs as a pore-forming pretreatment depends on the availability of their open micropores. The skin type can also affect the micropore closure time following the MN’s application. Recently, it was reported that the micropore closure time can vary in human subjects of different ethnic/racial backgrounds, with a longer micropore closure time in colored skin. These findings indicate that further research is warranted to verify the safety and efficacy of MNs in a variety of human populations [199]. Another issue is the large-scale production which may hinder their clinical translation. There are also some other limitations including (i) a lack of specific regulatory guidelines for quality control tests of MNs (e.g. geometry, mechanical strength, and in vitro/in vivo correlation), (ii) human safety requirements for the clinical use of MNs, (iii) pharmacokinetic and pharmacodynamic evaluations of MNs are vital to confirm their safety and efficacy, (iv) manufacturing sterile MNs in aseptic conditions or using final sterilization processes are expected to significantly increase production costs, (v) specialized industrial machinery, several fabrication steps, particularly for the coated MNs, and clean room facility requirements upfront investment, and (vi) preserving MNs against moisture and microbial contamination through using suitable packaging [24]. Hence, the complex and costly production of MNs as well as several application-related concerns can hinder their clinical translation. New fabrication methods especially micromachining and 3D printing technologies are expected to reduce the cost and fabrication steps in the future.
Due to the emerging features of NPs and their promising clinical outcomes, rapid developments in NP-laden MNs are expected, but further research is required to exploit their therapeutic and diagnostic potentials. Apart from the efficacy of NPs, the safety of their use via the transdermal route should be addressed. Furthermore, incorporating NPs into the MN matrix can change their mechanical strength. There are a variety of modes resulting in the MNs’ mechanical failure, such as base-plate fracturing, MNs folding, and warping. Consequently, the mechanical properties of MNs should be characterized using different mechanical tests including axial and transverse force, base-plate strength, and shear strength analysis [24]. Moreover, it may change the NPs stability and dispersibility which can deteriorate their function. In addition, more investigations are still warranted to elucidate the therapeutic action of NPs in combination with MNs because most studies have only reported pharmacological and clinical outcomes in animal models without necessarily addressing the molecular basis of their observation. There is also no reliable preclinical model to precisely investigate the pharmacokinetics and biodistribution of NPs when applied using MNs, hindering the clinical translation of these systems. Last but not the least, the aseptic, large scale production, and appropriate storage conditions should be concerned.
6. Conclusions
Minimally invasive TDD has remained a challenge requiring more advanced delivery systems to address the limitations associated with conventional therapies. Among numerous TDD systems, NPs have attracted significant attention in recent years due to their potential features such as effective delivery carriers, not only to reduce the side effects associated with conventional formulations, but also to enhance skin permeation, depot drug action, and targeted delivery to impaired skin cells. Moreover, due to the interesting characteristics of certain NPs, including optical, photothermal, and superparamagnetic properties, novel therapeutic, imaging, and biosensing applications are emerging. MNs-based delivery systems have attracted much attention in improving the function of NPs in TDD due to their non-invasive and pain-free delivery characteristics, without a first-pass effect as well as infection and safety problems. Interestingly, the MNs-array technology can shuttle NPs directly into the skin layers. In recent years, MNs, in combination with NPs containing therapeutic agents, have advanced TDD to a new level to effectively treat skin disorders. A combination of MNs and NPs has shown numerous benefits over conventional systems, such as the improved skin penetration of NPs, prolonged or controlled drug release, and the possibility of new add-on therapeutics (e.g., PTT and PDT). Although not fully explored, NPs might play a vital role in cancer chemotherapy using MNs. Moreover, these combined systems have been especially explored for vaccination, immunotherapy, and gene delivery. For a successful delivery of NPs in combination with MNs, several features are warranted, such as the type, physicochemical, pharmacokinetic, and biologic features of NPs as well as the strategy used for delivery by MNs. Overall, MNs can be applied in the TDD of NPs, either drug-loaded or alone, by properly developing and reliably evaluating their practical aspects in clinical trials. These combined systems would have a significant impact on the future of nanomedicine to effectively treat skin disorders as well as systemic applications.
Acknowledgments
The authors acknowledge Center for Nanotechnology in Drug Delivery (SUMS) and Terasaki Institute for Biomedical Innovation for their support.
Abbreviations
5-Aminolevulinic acid (ALA); aminolevulinic acid (ALA)-coated stainless MNs (ALA-coated MNs); antibody-dependent cell-mediated cytotoxicity (ADCC); antigen-presenting cells (APCs); anti-the programmed death-1 (aPD1); bubble MNs (BMNs); carbon nanotubes (CNTs); carbon nanofibers (CNFs); cell-penetrating peptide (CPP); dissolvable MNs (dMNs); DOX-loaded dissolvable hyaluronic acid MNs (DOX-loaded HA MNs); graphene oxide (GO); glucose oxidase/catalase, (GOx/CAT); gold NPs (AuNPs); hafnium oxide (HfO2); hexadecyltrimethylammonium bromide (CTAB); hydrogen peroxide (H2O2); hyaluronic acid (HA); hydroxyethyl methacrylate (HEMA); dendritic cells (DCs); ethylene glycol dimethacrylate (EGDMA); hydroxyethyl methacrylate (HEMA); indocyanine green loaded into chitosan NPs (ICG-NPs); indoleamine 2,3-dioxygenase (IDO); Langerhans cells (LCs); magnetic graphene quantum dot (MGQD); magnetic resonance imaging (MRI); mannose (Man); mesoporous bioactive glasses (MBGs); mesoporous silica nanoparticles (MSNs); 1-methyl-tryptophan (1-MT); microneedle arrays (MNs); microneedles (MNs); monomethoxy-poly (ethylene glycol) (MPEG); multivesicular vesicles (MVV); nanocarriers (NCs); nanoparticles (NPs); nanostructured lipid carriers (NLCs); near-infrared (NIR); oil-in-water (O/W); oligo sulfamethazine conjugated poly (β-amino ester urethane (OSM-(PEG-PAEU)); organosilica nanoparticle (PcNP); ovalbumin (OVA); paclitaxel (PTX); PEGylated gold nanorod (PEGylated-GNR); photodynamic therapy (PDT); photodynamic therapy (PDT); photothermal ablation therapy (PTT); photothermal therapy (PTT); phthalocyanine (Pc); plasmid DNA (pDNA); siRNA (siBraf); stratum corneum (SC); octaarginine (R8); poly(ε-caprolactone) (PCL); polyethyleneimine (PEI); poly methyl vinyl ether and maleic anhydride (PMVE/MA); polyethylene glycols (PEGs); poly-lactic-acid (L-PLA); polylactic-co-glycolic acid (PLGA); polyvinylpyrrolidone (PVP); quantum dots (QDs); reactive oxygen species (ROS); silver nanoparticles (AgNPs); transdermal drug delivery systems (TDDSs); tumor-draining lymph nodes (tdLN); zinc oxide (ZnO); scanning electron microscopy (SEM), solid lipid nanoparticles (SLNs); stratum corneum (SC); sulfobutylether-cyclodextrin (SBE); superparamagnetic iron oxide (SPIONs); surface plasmon resonance (SPR); surface-enhanced Raman scattering (SERS); tetramethyl orthosilicate (TMOS); titanium dioxide (TiO2); transdermal delivery (TDD); virus like nanoparticles (VLPs); water-in-oil (W/O).
Author Contributions
V.A. conceived the subject of the work. V.A., Z.R., and M.A. wrote the manuscript. S.S.A., G.Y., A.T., and S.A. provided the theoretical support and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This article is a part of Vahid Alimardani thesis funded by Shiraz University of Medical Sciences under supervision of Samira Sadat Abolmaali.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhou X., Hao Y., Yuan L., Pradhan S., Shrestha K., Pradhan O., Liu H., Li W. Nano-formulations for transdermal drug delivery: A review. Chin. Chem. Lett. 2018;29:1713–1724. doi: 10.1016/j.cclet.2018.10.037. [DOI] [Google Scholar]
- 2.Cevc G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 2004;56:675–711. doi: 10.1016/j.addr.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Szunerits S., Boukherroub R. Heat: A Highly Efficient Skin Enhancer for Transdermal Drug Delivery. Front. Bioeng. Biotechnol. 2018;6:15. doi: 10.3389/fbioe.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prausnitz M.R., Langer R. Transdermal drug delivery. Nat. Biotechnol. 2008;26:1261. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moradi L., Javanmardi S., Abolmaali S., Mohammadi Samani S. Passive Enhancement of Transdermal Drug Delivery: Lipid-Based Colloidal Carriers as an Emerging Pharmaceutical Technology Platform. TiPS. 2019;5:25–40. [Google Scholar]
- 6.Yang M.-Y., Zhao R.-R., Fang Y.-F., Jiang J.-L., Yuan X.-T., Shao J.-W. Carrier-free nanodrug: A novel strategy of cancer diagnosis and synergistic therapy. Int. J. Pharm. 2019;570:118663. doi: 10.1016/j.ijpharm.2019.118663. [DOI] [PubMed] [Google Scholar]
- 7.Qin Z., Chen F., Chen D., Wang Y., Tan Y., Ban J. Transdermal permeability of triamcinolone acetonide lipid nanoparticles. Int. J. Nanomed. 2019;14:2485–2495. doi: 10.2147/IJN.S195769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapoor H., Aqil M., Imam S.S., Sultana Y., Ali A. Formulation of amlodipine nano lipid carrier: Formulation design, physicochemical and transdermal absorption investigation. J. Drug Deliv. Sci. Technol. 2019;49:209–218. doi: 10.1016/j.jddst.2018.11.004. [DOI] [Google Scholar]
- 9.Mangalathillam S., Rejinold N.S., Nair A., Lakshmanan V.-K., Nair S.V., Jayakumar R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale. 2012;4:239–250. doi: 10.1039/C1NR11271F. [DOI] [PubMed] [Google Scholar]
- 10.Abolmaali S., Tamaddon A., Salmanpour M., Mohammadi S., Dinarvand R. Block ionomer micellar nanoparticles from double hydrophilic copolymers, classifications and promises for delivery of cancer chemotherapeutics. Eur. J. Pharm. Sci. 2017;104:393–405. doi: 10.1016/j.ejps.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Abolmaali S.S., Tamaddon A.M., Dinarvand R. Nano-hydrogels of methoxy polyethylene glycol-grafted branched polyethyleneimine via biodegradable cross-linking of Zn2+-ionomer micelle template. J. Nanopart. Res. 2013;15:2134. doi: 10.1007/s11051-013-2134-z. [DOI] [Google Scholar]
- 12.Labouta H.I., Schneider M. Interaction of inorganic nanoparticles with the skin barrier: Current status and critical review. Nanomed. Nanotechnol. 2013;9:39–54. doi: 10.1016/j.nano.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Taghizadeh S., Alimardani V., Roudbali P.L., Ghasemi Y., Kaviani E. Gold nanoparticles application in liver cancer. Photodiagn. Photodyn. Ther. 2019;25:389–400. doi: 10.1016/j.pdpdt.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Dhal S., Pal K., Giri S. Transdermal Delivery of Gold Nanoparticles by a Soybean Oil-Based Oleogel under Iontophoresis. ACS Appl. Bio Mater. 2020;3:7029–7039. doi: 10.1021/acsabm.0c00893. [DOI] [PubMed] [Google Scholar]
- 15.Larese Filon F., Crosera M., Adami G., Bovenzi M., Rossi F., Maina G. Human skin penetration of gold nanoparticles through intact and damaged skin. Nanotoxicology. 2011;5:493–501. doi: 10.3109/17435390.2010.551428. [DOI] [PubMed] [Google Scholar]
- 16.Rangsimawong W., Opanasopit P., Rojanarata T., Panomsuk S., Ngawhirunpat T. Influence of sonophoresis on transdermal drug delivery of hydrophilic compound-loaded lipid nanocarriers. Pharm. Dev. Technol. 2017;22:597–605. doi: 10.1080/10837450.2016.1221428. [DOI] [PubMed] [Google Scholar]
- 17.Zhang P., Zhang Y., Liu C.-G. Polymeric nanoparticles based on carboxymethyl chitosan in combination with painless microneedle therapy systems for enhancing transdermal insulin delivery. RSC Adv. 2020;10:24319–24329. doi: 10.1039/D0RA04460A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatnagar S., Dave K., Venuganti V.V.K. Microneedles in the clinic. J. Control. Release. 2017;260:164–182. doi: 10.1016/j.jconrel.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Alimardani V., Abolmaali S.S., Tamaddon A.M., Ashfaq M. Recent advances on microneedle arrays-mediated technology in cancer diagnosis and therapy. Drug Deliv. Transl. Res. 2020:1–29. doi: 10.1007/s13346-020-00819-z. [DOI] [PubMed] [Google Scholar]
- 20.Dsouza L., Ghate V.M., Lewis S.A. Derma rollers in therapy: The transition from cosmetics to transdermal drug delivery. Biomed. Microdevices. 2020;22:1–11. doi: 10.1007/s10544-020-00530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAllister D.V., Wang P.M., Davis S.P., Park J.-H., Canatella P.J., Allen M.G., Prausnitz M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA. 2003;100:13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Indermun S., Luttge R., Choonara Y.E., Kumar P., Du Toit L.C., Modi G., Pillay V. Current advances in the fabrication of microneedles for transdermal delivery. J. Control. Release. 2014;185:130–138. doi: 10.1016/j.jconrel.2014.04.052. [DOI] [PubMed] [Google Scholar]
- 23.Serrano-Castañeda P., Escobar-Chavez J.J., Rodríguez-Cruz I.M., Melgoza L.M., Martinez-Hernandez J. Microneedles as Enhancer of Drug Absorption Through the Skin and Applications in Medicine and Cosmetology. J. Pharm. Pharm. Sci. 2018;21:73–93. doi: 10.18433/jpps29610. [DOI] [PubMed] [Google Scholar]
- 24.Larraneta E., Lutton R.E., Woolfson A.D., Donnelly R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016;104:1–32. doi: 10.1016/j.mser.2016.03.001. [DOI] [Google Scholar]
- 25.Prausnitz M.R. Engineering microneedle patches for vaccination and drug delivery to skin. Annu. Rev. Chem. Biomol. Eng. 2017;8:177–200. doi: 10.1146/annurev-chembioeng-060816-101514. [DOI] [PubMed] [Google Scholar]
- 26.Henry S., McAllister D.V., Allen M.G., Prausnitz M.R. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharm. Sci. 1998;87:922–925. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- 27.Waghule T., Singhvi G., Dubey S.K., Pandey M.M., Gupta G., Singh M., Dua K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharm. 2019;109:1249–1258. doi: 10.1016/j.biopha.2018.10.078. [DOI] [PubMed] [Google Scholar]
- 28.Lim D.-J., Vines J.B., Park H., Lee S.-H. Microneedles: A versatile strategy for transdermal delivery of biological molecules. Int. J. Biol. Macromol. 2018;110:30–38. doi: 10.1016/j.ijbiomac.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 29.van der Maaden K., Jiskoot W., Bouwstra J. Microneedle technologies for (trans) dermal drug and vaccine delivery. J. Control. Release. 2012;161:645–655. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 30.Jung J.H., Chiang B., Grossniklaus H.E., Prausnitz M.R. Ocular drug delivery targeted by iontophoresis in the suprachoroidal space using a microneedle. J. Control. Release. 2018;277:14–22. doi: 10.1016/j.jconrel.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeMuth P.C., Moon J.J., Suh H., Hammond P.T., Irvine D.J. Releasable layer-by-layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano. 2012;6:8041–8051. doi: 10.1021/nn302639r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeMuth P.C., Su X., Samuel R.E., Hammond P.T., Irvine D.J. Nano-layered microneedles for transcutaneous delivery of polymer nanoparticles and plasmid DNA. Adv. Mater. 2010;22:4851–4856. doi: 10.1002/adma.201001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schipper P., van der Maaden K., Groeneveld V., Ruigrok M., Romeijn S., Uleman S., Oomens C., Kersten G., Jiskoot W., Bouwstra J. Diphtheria toxoid and N-trimethyl chitosan layer-by-layer coated pH-sensitive microneedles induce potent immune responses upon dermal vaccination in mice. J. Control. Release. 2017;262:28–36. doi: 10.1016/j.jconrel.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Ingrole R.S., Gill H.S. Microneedle coating methods: A review with a perspective. J. Pharmacol. Exp. Ther. 2019;370:555–569. doi: 10.1124/jpet.119.258707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatnagar S., Gadeela P.R., Thathireddy P., Venuganti V.V.K. Microneedle-based drug delivery: Materials of construction. J. Chem. Sci. 2019;131:90. doi: 10.1007/s12039-019-1666-x. [DOI] [Google Scholar]
- 36.Park J.-H., Allen M.G., Prausnitz M.R. Polymer Microneedles for Controlled-Release Drug Delivery. Pharm. Res. 2006;23:1008–1019. doi: 10.1007/s11095-006-0028-9. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q.L., Zhu D.D., Liu X.B., Chen B.Z., Guo X.D. Microneedles with controlled bubble sizes and drug distributions for efficient transdermal drug delivery. Sci. Rep. 2016;6:38755. doi: 10.1038/srep38755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu L.Y., Prausnitz M.R. Separable arrowhead microneedles. J. Control. Release. 2011;149:242–249. doi: 10.1016/j.jconrel.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue P., Zhang X., Chuah Y.J., Wu Y., Kang Y. Flexible PEGDA-based microneedle patches with detachable PVP–CD arrowheads for transdermal drug delivery. RSC Adv. 2015;5:75204–75209. doi: 10.1039/C5RA09329E. [DOI] [Google Scholar]
- 40.Zhu D.D., Wang Q.L., Liu X.B., Guo X.D. Rapidly separating microneedles for transdermal drug delivery. Acta Biomater. 2016;41:312–319. doi: 10.1016/j.actbio.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Juster H., van der Aar B., de Brouwer H. A review on microfabrication of thermoplastic polymer-based microneedle arrays. Polym. Eng. Sci. 2019;59:877–890. doi: 10.1002/pen.25078. [DOI] [Google Scholar]
- 42.Singh P., Carrier A., Chen Y., Lin S., Wang J., Cui S., Zhang X. Polymeric microneedles for controlled transdermal drug delivery. J. Control. Release. 2019;315:97–113. doi: 10.1016/j.jconrel.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 43.Ke C.-J., Lin Y.-J., Hu Y.-C., Chiang W.-L., Chen K.-J., Yang W.-C., Liu H.-L., Fu C.-C., Sung H.-W. Multidrug release based on microneedle arrays filled with pH-responsive PLGA hollow microspheres. Biomaterials. 2012;33:5156–5165. doi: 10.1016/j.biomaterials.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 44.Ye Y., Wang J., Hu Q., Hochu G.M., Xin H., Wang C., Gu Z. Synergistic transcutaneous immunotherapy enhances antitumor immune responses through delivery of checkpoint inhibitors. ACS Nano. 2016;10:8956–8963. doi: 10.1021/acsnano.6b04989. [DOI] [PubMed] [Google Scholar]
- 45.Wang C., Ye Y., Hochu G.M., Sadeghifar H., Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16:2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- 46.Larrañeta E., Lutton R.E.M., Brady A.J., Vicente-Pérez E.M., Woolfson A.D., Thakur R.R.S., Donnelly R.F. Microwave-Assisted Preparation of Hydrogel-Forming Microneedle Arrays for Transdermal Drug Delivery Applications. Macromol. Mater. Eng. 2015;300:586–595. doi: 10.1002/mame.201500016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardy J.G., Larrañeta E., Donnelly R.F., McGoldrick N., Migalska K., McCrudden M.T., Irwin N.J., Donnelly L., McCoy C.P. Hydrogel-forming microneedle arrays made from light-responsive materials for on-demand transdermal drug delivery. Mol. Pharm. 2016;13:907–914. doi: 10.1021/acs.molpharmaceut.5b00807. [DOI] [PubMed] [Google Scholar]
- 48.Hong X., Wu Z., Chen L., Wu F., Wei L., Yuan W. Hydrogel microneedle arrays for transdermal drug delivery. Nanomicro Lett. 2014;6:191–199. doi: 10.1007/BF03353783. [DOI] [Google Scholar]
- 49.Sharma S., Hatware K., Bhadane P., Sindhikar S., Mishra D.K. Recent advances in microneedle composites for biomedical applications: Advanced drug delivery technologies. Mater. Sci. Eng. C. 2019;103:109717. doi: 10.1016/j.msec.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Migdadi E.M., Courtenay A.J., Tekko I.A., McCrudden M.T.C., Kearney M.-C., McAlister E., McCarthy H.O., Donnelly R.F. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J. Control. Release. 2018;285:142–151. doi: 10.1016/j.jconrel.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen S., Matsumoto H., Moro-oka Y., Tanaka M., Miyahara Y., Suganami T., Matsumoto A. Smart Microneedle Fabricated with Silk Fibroin Combined Semi-interpenetrating Network Hydrogel for Glucose-Responsive Insulin Delivery. ACS Biomater. Sci. Eng. 2019;11:5781–5789. doi: 10.1021/acsbiomaterials.9b00532. [DOI] [PubMed] [Google Scholar]
- 52.Demir Y.K., Metin A.Ü., Şatıroğlu B., Solmaz M.E., Kayser V., Mäder K. Poly (methyl vinyl ether-co-maleic acid)-Pectin based hydrogel-forming systems: Gel, film, and microneedles. Eur. J. Pharm. Biopharm. 2017;117:182–194. doi: 10.1016/j.ejpb.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 53.Sivaraman A., Banga A.K. Novel in situ forming hydrogel microneedles for transdermal drug delivery. Drug Deliv. Transl. Res. 2017;7:16–26. doi: 10.1007/s13346-016-0328-5. [DOI] [PubMed] [Google Scholar]
- 54.Garland M.J., Singh T.R.R., Woolfson A.D., Donnelly R.F. Electrically enhanced solute permeation across poly (ethylene glycol)–crosslinked poly (methyl vinyl ether-co-maleic acid) hydrogels: Effect of hydrogel crosslink density and ionic conductivity. Int. J. Pharm. 2011;406:91–98. doi: 10.1016/j.ijpharm.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Li Q.Y., Zhang J.N., Chen B.Z., Wang Q.L., Guo X.D. A solid polymer microneedle patch pretreatment enhances the permeation of drug molecules into the skin. RSC Adv. 2017;7:15408–15415. doi: 10.1039/C6RA26759A. [DOI] [Google Scholar]
- 56.Kim J.K., Roh M.R., PARK G.h., Kim Y.J., Jeon I.K., Chang S.E. Fractionated microneedle radiofrequency for the treatment of periorbital wrinkles. J. Dermatol. 2013;40:172–176. doi: 10.1111/1346-8138.12046. [DOI] [PubMed] [Google Scholar]
- 57.de Groot A.M., Platteel A., Kuijt N., van Kooten P.J., Vos P.J., Sijts A.J., Van Der Maaden K. Nanoporous microneedle arrays effectively induce antibody responses against diphtheria and tetanus toxoid. Front. Immunol. 2017;8:1789. doi: 10.3389/fimmu.2017.01789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abiandu I., Ita K. Transdermal delivery of potassium chloride with solid microneedles. J. Drug Deliv. Sci. Technol. 2019;53:101216. doi: 10.1016/j.jddst.2019.101216. [DOI] [Google Scholar]
- 59.Bollella P., Sharma S., Cass A.E.G., Antiochia R. Minimally-invasive Microneedle-based Biosensor Array for Simultaneous Lactate and Glucose Monitoring in Artificial Interstitial Fluid. Electroanalysis. 2019;31:374–382. doi: 10.1002/elan.201800630. [DOI] [Google Scholar]
- 60.Senel M., Dervisevic M., Voelcker N.H. Gold microneedles fabricated by casting of gold ink used for urea sensing. Mater. Lett. 2019;243:50–53. doi: 10.1016/j.matlet.2019.02.014. [DOI] [Google Scholar]
- 61.Meyer B.K., Kendall M.A., Williams D.M., Bett A.J., Dubey S., Gentzel R.C., Casimiro D., Forster A., Corbett H., Crichton M. Immune Response and Reactogenicity of an Unadjuvanted Intradermally Delivered Human Papillomavirus Vaccine Using a First Generation Nanopatch™ in Rhesus Macaques: An Exploratory, Pre-Clinical Feasibility Assessment. Vaccine: X. 2019;2:100030. doi: 10.1016/j.jvacx.2019.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ross S., Scoutaris N., Lamprou D., Mallinson D., Douroumis D. Inkjet printing of insulin microneedles for transdermal delivery. Drug Deliv. Transl. Res. 2015;5:451–461. doi: 10.1007/s13346-015-0251-1. [DOI] [PubMed] [Google Scholar]
- 63.van der Maaden K., Yu H., Sliedregt K., Zwier R., Leboux R., Oguri M., Kros A., Jiskoot W., Bouwstra J.A. Nanolayered chemical modification of silicon surfaces with ionizable surface groups for pH-triggered protein adsorption and release: Application to microneedles. J. Mater. Chem. B. 2013;1:4466–4477. doi: 10.1039/c3tb20786b. [DOI] [PubMed] [Google Scholar]
- 64.Cormier M., Johnson B., Ameri M., Nyam K., Libiran L., Zhang D.D., Daddona P. Transdermal delivery of desmopressin using a coated microneedle array patch system. J. Control. Release. 2004;97:503–511. doi: 10.1016/S0168-3659(04)00171-3. [DOI] [PubMed] [Google Scholar]
- 65.Daddona P.E., Matriano J.A., Mandema J., Maa Y.-F. Parathyroid Hormone (1-34)-Coated Microneedle Patch System: Clinical Pharmacokinetics and Pharmacodynamics for Treatment of Osteoporosis. Pharm. Res. 2011;28:159–165. doi: 10.1007/s11095-010-0192-9. [DOI] [PubMed] [Google Scholar]
- 66.Al Sulaiman D., Chang J.Y.H., Bennett N.R., Topouzi H., Higgins C.A., Irvine D.J., Ladame S. Hydrogel-Coated Microneedle Arrays for Minimally Invasive Sampling and Sensing of Specific Circulating Nucleic Acids from Skin Interstitial Fluid. ACS Nano. 2019;13:9620–9628. doi: 10.1021/acsnano.9b04783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Maaden K., Heuts J., Camps M., Pontier M., van Scheltinga A.T., Jiskoot W., Ossendorp F., Bouwstra J. Hollow microneedle-mediated micro-injections of a liposomal HPV E743–63 synthetic long peptide vaccine for efficient induction of cytotoxic and T-helper responses. J. Control. Release. 2018;269:347–354. doi: 10.1016/j.jconrel.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 68.Zu Q., Yu Y., Bi X., Zhang R., Di L. Microneedle-Assisted Percutaneous Delivery of a Tetramethylpyrazine-Loaded Microemulsion. Molecules. 2017;22:2022. doi: 10.3390/molecules22112022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iliescu F.S., Teo J.C.M., Vrtacnik D., Taylor H., Iliescu C. Cell therapy using an array of ultrathin hollow microneedles. Microsyst.Technol. 2018;24:2905–2912. doi: 10.1007/s00542-017-3631-2. [DOI] [Google Scholar]
- 70.Golombek S., Pilz M., Steinle H., Kochba E., Levin Y., Lunter D., Schlensak C., Wendel H.P., Avci-Adali M. Intradermal Delivery of Synthetic mRNA Using Hollow Microneedles for Efficient and Rapid Production of Exogenous Proteins in Skin. Mol. Ther. Nucleic Acids. 2018;11:382–392. doi: 10.1016/j.omtn.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dul M., Stefanidou M., Porta P., Serve J., O’Mahony C., Malissen B., Henri S., Levin Y., Kochba E., Wong F.S., et al. Hydrodynamic gene delivery in human skin using a hollow microneedle device. J. Control. Release. 2017;265:120–131. doi: 10.1016/j.jconrel.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 72.Yu L., Tay F., Guo D., Xu L., Yap K. A microfabricated electrode with hollow microneedles for ECG measurement. Sens. Actuators A. 2009;151:17–22. doi: 10.1016/j.sna.2009.01.020. [DOI] [Google Scholar]
- 73.Daugimont L., Baron N., Vandermeulen G., Pavselj N., Miklavcic D., Jullien M.-C., Cabodevila G., Mir L.M., Préat V. Hollow microneedle arrays for intradermal drug delivery and DNA electroporation. J. Membr. Biol. 2010;236:117–125. doi: 10.1007/s00232-010-9283-0. [DOI] [PubMed] [Google Scholar]
- 74.Nicholas D., Logan K.A., Sheng Y., Gao J., Farrell S., Dixon D., Callan B., McHale A.P., Callan J.F. Rapid paper based colorimetric detection of glucose using a hollow microneedle device. Int. J. Pharm. 2018;547:244–249. doi: 10.1016/j.ijpharm.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y.-H., Lai K.-Y., Chiu Y.-H., Wu Y.-W., Shiau A.-L., Chen M.-C. Implantable microneedles with an immune-boosting function for effective intradermal influenza vaccination. Acta Biomater. 2019;97:230–238. doi: 10.1016/j.actbio.2019.07.048. [DOI] [PubMed] [Google Scholar]
- 76.Duong H.T.T., Kim N.W., Thambi T., Giang Phan V.H., Lee M.S., Yin Y., Jeong J.H., Lee D.S. Microneedle arrays coated with charge reversal pH-sensitive copolymers improve antigen presenting cells-homing DNA vaccine delivery and immune responses. J. Control. Release. 2018;269:225–234. doi: 10.1016/j.jconrel.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 77.Dong L., Li Y., Li Z., Xu N., Liu P., Du H., Zhang Y., Huang Y., Zhu J., Ren G. Au nanocage-strengthened dissolving microneedles for chemo-photothermal combined therapy of superficial skin tumors. ACS Appl. Mater. Interfaces. 2018;10:9247–9256. doi: 10.1021/acsami.7b18293. [DOI] [PubMed] [Google Scholar]
- 78.Dillon C., Hughes H., O’Reilly N.J., Allender C.J., Barrow D.A., McLoughlin P. Dissolving microneedle based transdermal delivery of therapeutic peptide analogues. Int. J. Pharm. 2019;565:9–19. doi: 10.1016/j.ijpharm.2019.04.075. [DOI] [PubMed] [Google Scholar]
- 79.Lin S., Quan G., Hou A., Yang P., Peng T., Gu Y., Qin W., Liu R., Ma X., Pan X., et al. Strategy for hypertrophic scar therapy: Improved delivery of triamcinolone acetonide using mechanically robust tip-concentrated dissolving microneedle array. J. Control. Release. 2019;306:69–82. doi: 10.1016/j.jconrel.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen H.X., Bozorg B.D., Kim Y., Wieber A., Birk G., Lubda D., Banga A.K. Poly (vinyl alcohol) microneedles: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur. J. Pharm. Biopharm. 2018;129:88–103. doi: 10.1016/j.ejpb.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 81.Huh I., Kim S., Yang H., Jang M., Kang G., Jung H. Effects of two droplet-based dissolving microneedle manufacturing methods on the activity of encapsulated epidermal growth factor and ascorbic acid. Eur. J. Pharm. Sci. 2018;114:285–292. doi: 10.1016/j.ejps.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 82.Yang H., Kim S., Jang M., Kim H., Lee S., Kim Y., Eom Y.A., Kang G., Chiang L., Baek J.H., et al. Two-phase delivery using a horse oil and adenosine-loaded dissolving microneedle patch for skin barrier restoration, moisturization, and wrinkle improvement. J. Cosmet. Dermatol. 2019;18:936–943. doi: 10.1111/jocd.12768. [DOI] [PubMed] [Google Scholar]
- 83.Ramöller I.K., Tekko I.A., McCarthy H.O., Donnelly R.F. Rapidly dissolving bilayer microneedle arrays–A minimally invasive transdermal drug delivery system for vitamin B12. Int. J. Pharm. 2019;566:299–306. doi: 10.1016/j.ijpharm.2019.05.066. [DOI] [PubMed] [Google Scholar]
- 84.Hamdan I.M.N., Tekko I.A., Matchett K.B., Arnaut L.G., Silva C.S., McCarthy H.O., Donnelly R.F. Intradermal Delivery of a Near-Infrared Photosensitizer Using Dissolving Microneedle Arrays. J. Pharm. Sci. 2018;107:2439–2450. doi: 10.1016/j.xphs.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 85.Li Y., Liu F., Su C., Yu B., Liu D., Chen H.-J., Lin D.-a., Yang C., Zhou L., Wu Q., et al. Biodegradable Therapeutic Microneedle Patch for Rapidly Antihypertensive Treatment. ACS Appl. Mater. Interfaces. 2019;11:30575–30584. doi: 10.1021/acsami.9b09697. [DOI] [PubMed] [Google Scholar]
- 86.Oerke E.C. Crop Losses to Pests. J. Agric. Sci. 2006;144:31. doi: 10.1017/S0021859605005708. [DOI] [Google Scholar]
- 87.Courtenay A.J., Rodgers A.M., McCrudden M.T.C., McCarthy H.O., Donnelly R.F. Novel Hydrogel-Forming Microneedle Array for Intradermal Vaccination in Mice Using Ovalbumin as a Model Protein Antigen. Mol. Pharm. 2019;16:118–127. doi: 10.1021/acs.molpharmaceut.8b00895. [DOI] [PubMed] [Google Scholar]
- 88.Eltayib E., Brady A.J., Caffarel-Salvador E., Gonzalez-Vazquez P., Zaid Alkilani A., McCarthy H.O., McElnay J.C., Donnelly R.F. Hydrogel-forming microneedle arrays: Potential for use in minimally-invasive lithium monitoring. Eur. J. Pharm. Biopharm. 2016;102:123–131. doi: 10.1016/j.ejpb.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 89.Caffarel-Salvador E., Brady A.J., Eltayib E., Meng T., Alonso-Vicente A., Gonzalez-Vazquez P., Torrisi B.M., Vicente-Perez E.M., Mooney K., Jones D.S., et al. Hydrogel-Forming Microneedle Arrays Allow Detection of Drugs and Glucose In Vivo: Potential for Use in Diagnosis and Therapeutic Drug Monitoring. PLoS ONE. 2016;10:e0145644. doi: 10.1371/journal.pone.0145644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kennedy J., Larrañeta E., McCrudden M.T., McCrudden C.M., Brady A.J., Fallows S.J., McCarthy H.O., Kissenpfennig A., Donnelly R.F. In vivo studies investigating biodistribution of nanoparticle-encapsulated rhodamine B delivered via dissolving microneedles. J. Control. Release. 2017;265:57–65. doi: 10.1016/j.jconrel.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abedi M., Abolmaali S.S., Abedanzadeh M., Borandeh S., Samani S.M., Tamaddon A.M. Citric acid functionalized silane coupling versus post-grafting strategy for dual pH and saline responsive delivery of cisplatin by Fe3O4/carboxyl functionalized mesoporous SiO2 hybrid nanoparticles: A-synthesis, physicochemical and biological characterization. Mater. Sci. Eng. C. 2019;104:109922. doi: 10.1016/j.msec.2019.109922. [DOI] [PubMed] [Google Scholar]
- 92.Abedi M., Abolmaali S.S., Abedanzadeh M., Farjadian F., Samani S.M., Tamaddon A.M. Core–Shell Imidazoline–Functionalized Mesoporous Silica Superparamagnetic Hybrid Nanoparticles as a Potential Theranostic Agent for Controlled Delivery of Platinum (II) Compound. Int. J. Nanomed. 2020;15:2617. doi: 10.2147/IJN.S245135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Navya P., Daima H.K. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 2016;3:1–14. doi: 10.1186/s40580-016-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prow T.W., Grice J.E., Lin L.L., Faye R., Butler M., Becker W., Wurm E.M.T., Yoong C., Robertson T.A., Soyer H.P., et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011;63:470–491. doi: 10.1016/j.addr.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 95.Patravale V., Dandekar P., Jain R. Nanoparticulate Drug Delivery: Perspectives on the Transition from Laboratory to Market. Elsevier; Amsterdam, The Netherlands: 2012. [Google Scholar]
- 96.Xie S., Li Z., Yu Z. Microneedles for transdermal delivery of insulin. J. Drug Deliv. Sci. Technol. 2015;28:11–17. doi: 10.1016/j.jddst.2015.04.008. [DOI] [Google Scholar]
- 97.Chu L.-Y. Controlled release systems for insulin delivery. Expert Opin. Ther. Pat. 2005;15:1147–1155. doi: 10.1517/13543776.15.9.1147. [DOI] [Google Scholar]
- 98.Larrañeta E., Stewart S., Fallows S.J., Birkhäuer L.L., McCrudden M.T.C., Woolfson A.D., Donnelly R.F. A facile system to evaluate in vitro drug release from dissolving microneedle arrays. Int. J. Pharm. 2016;497:62–69. doi: 10.1016/j.ijpharm.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramadan E., Borg T., Abdelghani G., Saleh N.M. Transdermal microneedle-mediated delivery of polymeric lamivudine-loaded nanoparticles. J. Pharm. Tech. Drug Res. 2016;5:1. doi: 10.7243/2050-120X-5-1. [DOI] [Google Scholar]
- 100.Kim H.-G., Gater D.L., Kim Y.-C. Development of transdermal vitamin D3 (VD3) delivery system using combinations of PLGA nanoparticles and microneedles. Drug Deliv. Transl. Res. 2018;8:281–290. doi: 10.1007/s13346-017-0460-x. [DOI] [PubMed] [Google Scholar]
- 101.Cui Y., Mo Y., Zhang Q., Tian W., Xue Y., Bai J., Du S. Microneedle-Assisted Percutaneous Delivery of Paeoniflorin-Loaded Ethosomes. Molecules. 2018;23:3371. doi: 10.3390/molecules23123371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boulaiz H., Alvarez P.J., Ramirez A., Marchal J.A., Prados J., Rodríguez-Serrano F., Perán M., Melguizo C., Aranega A. Nanomedicine: Application areas and development prospects. Int. J. Mol. Sci. 2011;12:3303–3321. doi: 10.3390/ijms12053303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kelchen M.N., Brogden N.K. In Vitro Skin Retention and Drug Permeation through Intact and Microneedle Pretreated Skin after Application of Propranolol Loaded Microemulsions. Pharm. Res. 2018;35:228. doi: 10.1007/s11095-018-2495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y., Liu Q., Yu J., Yu S., Wang J., Qiang L., Gu Z. Locally Induced Adipose Tissue Browning by Microneedle Patch for Obesity Treatment. ACS Nano. 2017;11:9223–9230. doi: 10.1021/acsnano.7b04348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guo T., Zhang Y., Li Z., Zhao J., Feng N. Microneedle-mediated transdermal delivery of nanostructured lipid carriers for alkaloids from Aconitum sinomontanum. Artif. Cells Nanomed. Biotechnol. 2018;46:1541–1551. doi: 10.1080/21691401.2017.1376676. [DOI] [PubMed] [Google Scholar]
- 106.Park J., Kim Y.-C. Topical delivery of 5-fluorouracil-loaded carboxymethyl chitosan nanoparticles using microneedles for keloid treatment. Drug Deliv. Transl. Res. 2020 doi: 10.1007/s13346-020-00781-w. [DOI] [PubMed] [Google Scholar]
- 107.Justin R., Chen B. Multifunctional chitosan–magnetic graphene quantum dot nanocomposites for the release of therapeutics from detachable and non-detachable biodegradable microneedle arrays. Interface Focus. 2018;8:20170055. doi: 10.1098/rsfs.2017.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vora L.K., Donnelly R.F., Larrañeta E., González-Vázquez P., Thakur R.R.S., Vavia P.R. Novel bilayer dissolving microneedle arrays with concentrated PLGA nano-microparticles for targeted intradermal delivery: Proof of concept. J. Control. Release. 2017;265:93–101. doi: 10.1016/j.jconrel.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 109.Xu B., Cao Q., Zhang Y., Yu W., Zhu J., Liu D., Jiang G. Microneedles Integrated with ZnO Quantum-Dot-Capped Mesoporous Bioactive Glasses for Glucose-Mediated Insulin Delivery. ACS Biomater. Sci. Eng. 2018;4:2473–2483. doi: 10.1021/acsbiomaterials.8b00626. [DOI] [PubMed] [Google Scholar]
- 110.Permana A.D., Mir M., Utomo E., Donnelly R.F. Bacterially sensitive nanoparticle-based dissolving microneedles of doxycycline for enhanced treatment of bacterial biofilm skin infection: A proof of concept study. Int. J. Pharm. 2020;2:119220. doi: 10.1016/j.ijpharm.2020.119220. [DOI] [PubMed] [Google Scholar]
- 111.Permana A.D., McCrudden M.T., Donnelly R.F. Enhanced intradermal delivery of nanosuspensions of antifilariasis drugs using dissolving microneedles: A proof of concept study. Pharmaceutics. 2019;11:346. doi: 10.3390/pharmaceutics11070346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mir M., Permana A.D., Ahmed N., Khan G.M., ur Rehman A., Donnelly R.F. Enhancement in site-specific delivery of carvacrol for potential treatment of infected wounds using infection responsive nanoparticles loaded into dissolving microneedles: A proof of concept study. Eur. J. Pharm. Biopharm. 2020;147:57–68. doi: 10.1016/j.ejpb.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 113.Kim D.-S., Choi J.-T., Kim C.B., Shin Y.-R., Park P.-G., Kim H., Lee J.M., Park J.-H. Microneedle Array Patch (MAP) Consisting of Crosslinked Hyaluronic Acid Nanoparticles for Processability and Sustained Release. Pharm. Res. 2020;37:50. doi: 10.1007/s11095-020-2768-3. [DOI] [PubMed] [Google Scholar]
- 114.Abdelghany S., Tekko I.A., Vora L., Larrañeta E., Permana A.D., Donnelly R.F. Nanosuspension-based dissolving microneedle arrays for intradermal delivery of curcumin. Pharmaceutics. 2019;11:308. doi: 10.3390/pharmaceutics11070308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hao Y., Chen Y., He X., Yang F., Han R., Yang C., Li W., Qian Z. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioact. Mater. 2020;5:542–552. doi: 10.1016/j.bioactmat.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cheng Z., Lin H., Wang Z., Yang X., Zhang M., Liu X., Wang B., Wu Z., Chen D. Preparation and characterization of dissolving hyaluronic acid composite microneedles loaded micelles for delivery of curcumin. Drug Deliv. Transl. Res. 2020:1–11. doi: 10.1007/s13346-020-00735-2. [DOI] [PubMed] [Google Scholar]
- 117.Permana A.D., Tekko I.A., McCrudden M.T., Anjani Q.K., Ramadon D., McCarthy H.O., Donnelly R.F. Solid lipid nanoparticle-based dissolving microneedles: A promising intradermal lymph targeting drug delivery system with potential for enhanced treatment of lymphatic filariasis. J. Control. Release. 2019;316:34–52. doi: 10.1016/j.jconrel.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 118.Tripathy N., Wang J., Tung M., Conway C., Chung E. Transdermal Delivery of Kidney Targeting Nanoparticles Using Dissolvable Microneedles. Cell. Mol. Bioeng. 2020;13:475–486. doi: 10.1007/s12195-020-00622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim M., Jung B., Park J.-H. Hydrogel swelling as a trigger to release biodegradable polymer microneedles in skin. Biomaterials. 2012;33:668–678. doi: 10.1016/j.biomaterials.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 120.Girma W.M., Tzing S.-H., Tseng P.-J., Huang C.-C., Ling Y.-C., Chang J.-Y. Synthesis of cisplatin (IV) prodrug-tethered CuFeS2 nanoparticles in tumor-targeted chemotherapy and photothermal therapy. ACS Appl. Mater. Interfaces. 2018;10:4590–4602. doi: 10.1021/acsami.7b19640. [DOI] [PubMed] [Google Scholar]
- 121.Ma Y., Boese S.E., Luo Z., Nitin N., Gill H.S. Drug coated microneedles for minimally-invasive treatment of oral carcinomas: Development and in vitro evaluation. Biomed. Microdevices. 2015;17:44. doi: 10.1007/s10544-015-9944-y. [DOI] [PubMed] [Google Scholar]
- 122.Lan X., She J., Lin D.-A., Xu Y., Li X., Yang W.-F., Lui V.W.Y., Jin L., Xie X., Su Y.-X. Microneedle-Mediated Delivery of Lipid-Coated Cisplatin Nanoparticles for Efficient and Safe Cancer Therapy. ACS Appl. Mater. Interfaces. 2018;10:33060–33069. doi: 10.1021/acsami.8b12926. [DOI] [PubMed] [Google Scholar]
- 123.Romani N., Flacher V., Tripp C., Sparber F., Ebner S., Stoitzner P. Intradermal Immunization. Springer; Berlin/Heidelberg, Germany: 2011. Targeting skin dendritic cells to improve intradermal vaccination; pp. 113–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bol K.F., Aarntzen E.H., Pots J.M., Nordkamp M.A.O., van de Rakt M.W., Scharenborg N.M., de Boer A.J., van Oorschot T.G., Croockewit S.A., Blokx W.A. Prophylactic vaccines are potent activators of monocyte-derived dendritic cells and drive effective anti-tumor responses in melanoma patients at the cost of toxicity. Cancer Immunol. Immunother. 2016;65:327–339. doi: 10.1007/s00262-016-1796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eriksson F., Tötterman T., Maltais A.-K., Pisa P., Yachnin J. DNA vaccine coding for the rhesus prostate specific antigen delivered by intradermal electroporation in patients with relapsed prostate cancer. Vaccine. 2013;31:3843–3848. doi: 10.1016/j.vaccine.2013.06.063. [DOI] [PubMed] [Google Scholar]
- 126.Zaric M., Lyubomska O., Touzelet O., Poux C., Al-Zahrani S., Fay F., Wallace L., Terhorst D., Malissen B., Henri S. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D, L-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano. 2013;7:2042–2055. doi: 10.1021/nn304235j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kumar A., Wonganan P., Sandoval M.A., Li X., Zhu S., Cui Z. Microneedle-mediated transcutaneous immunization with plasmid DNA coated on cationic PLGA nanoparticles. J. Control. Release. 2012;163:230–239. doi: 10.1016/j.jconrel.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu Y., Xu B., Xu J., Shou D., Liu E., Gao J., Liang W., Huang Y. Microneedle-assisted dendritic cell-targeted nanoparticles for transcutaneous DNA immunization. Polym. Chem. 2015;6:373–379. doi: 10.1039/C4PY01394H. [DOI] [Google Scholar]
- 129.Liao J., Li W., Peng J., Yang Q., Li H., Wei Y., Zhang X., Qian Z. Combined cancer photothermal-chemotherapy based on doxorubicin/gold nanorod-loaded polymersomes. Theranostics. 2015;5:345. doi: 10.7150/thno.10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen M.-C., Lin Z.-W., Ling M.-H. Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and photothermal therapy. ACS Nano. 2015;10:93–101. doi: 10.1021/acsnano.5b05043. [DOI] [PubMed] [Google Scholar]
- 131.Hao Y., Chen Y., Lei M., Zhang T., Cao Y., Peng J., Chen L., Qian Z. Near-Infrared Responsive PEGylated Gold Nanorod and Doxorubicin Loaded Dissolvable Hyaluronic Acid Microneedles for Human Epidermoid Cancer Therapy. Adv. Ther. 2018;1:1800008. doi: 10.1002/adtp.201800008. [DOI] [Google Scholar]
- 132.Pei P., Yang F., Liu J., Hu H., Du X., Hanagata N., Zhao S., Zhu Y. Composite-dissolving microneedle patches for chemotherapy and photothermal therapy in superficial tumor treatment. Biomater. Sci. 2018;6:1414–1423. doi: 10.1039/C8BM00005K. [DOI] [PubMed] [Google Scholar]
- 133.Moreira A.F., Rodrigues C.F., Jacinto T.A., Miguel S.P., Costa E.C., Correia I.J. Poly (vinyl alcohol)/chitosan layer-by-layer microneedles for cancer chemo-photothermal therapy. Int. J. Pharm. 2020;576:118907. doi: 10.1016/j.ijpharm.2019.118907. [DOI] [PubMed] [Google Scholar]
- 134.Chen M., Quan G., Wen T., Yang P., Qin W., Mai H., Sun Y., Lu C., Pan X., Wu C. Cold to Hot: Binary Cooperative Microneedle Array Amplified Photo-Immunotherapy for Eliciting Antitumor Immunity and Abscopal Effect. ACS Appl. Mater. Interfaces. 2020 doi: 10.1021/acsami.0c05090. [DOI] [PubMed] [Google Scholar]
- 135.Dolmans D.E.J.G.J., Fukumura D., Jain R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 136.Donnelly R.F., Morrow D.I.J., McCarron P.A., Woolfson A.D., Morrissey A., Juzenas P., Juzeniene A., Iani V., McCarthy H.O., Moan J. Microneedle-mediated intradermal delivery of 5-aminolevulinic acid: Potential for enhanced topical photodynamic therapy. J. Control. Release. 2008;129:154–162. doi: 10.1016/j.jconrel.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 137.Szeimiesa R., Karrera S., Radakovic-Fijanb S., Tanewb A., Calzavara-Pintonc P., Zanec C., Sidoroffd A., Hempele M., Ulrichf J., Proebstleg T. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: A prospective, randomized study. J. Am. Acad. Dermatol. 2002;47:258–262. doi: 10.1067/mjd.2002.119649. [DOI] [PubMed] [Google Scholar]
- 138.Morrow D.I.J., McCarron P.A., Woolfson A.D., Juzenas P., Juzeniene A., Iani V., Moan J., Donnelly R.F. Influence of penetration enhancers on topical delivery of 5-aminolevulinic acid from bioadhesive patches. J. Pharm. Pharmacol. 2010;62:685–695. doi: 10.1211/jpp.62.06.0004. [DOI] [PubMed] [Google Scholar]
- 139.Zhang L.-W., Al-Suwayeh S.A., Hung C.-F., Chen C.-C., Fang J.-Y. Oil components modulate the skin delivery of 5-aminolevulinic acid and its ester prodrug from oil-in-water and water-in-oil nanoemulsions. Int. J. Nanomed. 2011;6:693. doi: 10.2147/IJN.S17524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Krishnan G., Grice J.E., Roberts M.S., Benson H.A.E., Prow T.W. Enhanced sonophoretic delivery of 5-aminolevulinic acid: Preliminary human ex vivo permeation data. Skin Res. Technol. 2013;19:e283–e289. doi: 10.1111/j.1600-0846.2012.00640.x. [DOI] [PubMed] [Google Scholar]
- 141.Fallows S.J., Garland M.J., Cassidy C.M., Tunney M.M., Singh T.R.R., Donnelly R.F. Electrically-responsive anti-adherent hydrogels for photodynamic antimicrobial chemotherapy. J. Photochem. Photobiol. B. 2012;114:61–72. doi: 10.1016/j.jphotobiol.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 142.Moothanchery M., Seeni R.Z., Xu C., Pramanik M. In vivo studies of transdermal nanoparticle delivery with microneedles using photoacoustic microscopy. Biomed. Opt. Express. 2017;8:5483–5492. doi: 10.1364/BOE.8.005483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jain A.K., Lee C.H., Gill H.S. 5-Aminolevulinic acid coated microneedles for photodynamic therapy of skin tumors. J. Control. Release. 2016;239:72–81. doi: 10.1016/j.jconrel.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 144.Tham H.P., Chen H., Tan Y.H., Qu Q., Sreejith S., Zhao L., Venkatraman S.S., Zhao Y. Photosensitizer anchored gold nanorods for targeted combinational photothermal and photodynamic therapy. Chem. Commun. 2016;52:8854–8857. doi: 10.1039/C6CC03076A. [DOI] [PubMed] [Google Scholar]
- 145.Liu X., Yang G., Zhang L., Liu Z., Cheng Z., Zhu X. Photosensitizer cross-linked nano-micelle platform for multimodal imaging guided synergistic photothermal/photodynamic therapy. Nanoscale. 2016;8:15323–15339. doi: 10.1039/C6NR04835H. [DOI] [PubMed] [Google Scholar]
- 146.Tham H.P., Xu K., Lim W.Q., Chen H., Zheng M., Thng T.G.S., Venkatraman S.S., Xu C., Zhao Y. Microneedle-Assisted Topical Delivery of Photodynamically Active Mesoporous Formulation for Combination Therapy of Deep-Seated Melanoma. ACS Nano. 2018;12:11936–11948. doi: 10.1021/acsnano.8b03007. [DOI] [PubMed] [Google Scholar]
- 147.MacCormack M.A. Photodynamic therapy. Adv. Dermatol. 2006;22:219–258. doi: 10.1016/j.yadr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 148.Calzavara-Pinton P., Venturini M., Sala R. Photodynamic therapy: Update 2006 Part 1: Photochemistry and photobiology. Eur. Acad. Dermatol. Venereol. 2007;21:293–302. doi: 10.1111/j.1468-3083.2006.01902.x. [DOI] [PubMed] [Google Scholar]
- 149.Chen S.-X., Ma M., Xue F., Shen S., Chen Q., Kuang Y., Liang K., Wang X., Chen H. Construction of microneedle-assisted co-delivery platform and its combining photodynamic/immunotherapy. J. Control. Release. 2020;324:218–227. doi: 10.1016/j.jconrel.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 150.Ye Y., Yu J., Wen D., Kahkoska A.R., Gu Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 2018;127:106–118. doi: 10.1016/j.addr.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mistilis M.J., Joyce J.C., Esser E.S., Skountzou I., Compans R.W., Bommarius A.S., Prausnitz M.R. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv. Transl. Res. 2017;7:195–205. doi: 10.1007/s13346-016-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mönkäre J., Reza Nejadnik M., Baccouche K., Romeijn S., Jiskoot W., Bouwstra J.A. IgG-loaded hyaluronan-based dissolving microneedles for intradermal protein delivery. J. Control. Release. 2015;218:53–62. doi: 10.1016/j.jconrel.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 153.Seong K.-Y., Seo M.-S., Hwang D.Y., O’Cearbhaill E.D., Sreenan S., Karp J.M., Yang S.Y. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J. Control. Release. 2017;265:48–56. doi: 10.1016/j.jconrel.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 154.Lahiji S.F., Jang Y., Huh I., Yang H., Jang M., Jung H. Exendin-4–encapsulated dissolving microneedle arrays for efficient treatment of type 2 diabetes. Sci. Rep. 2018;8:1170. doi: 10.1038/s41598-018-19789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Caudill C.L., Perry J.L., Tian S., Luft J.C., DeSimone J.M. Spatially controlled coating of continuous liquid Interface production microneedles for transdermal protein delivery. J. Control. Release. 2018;284:122–132. doi: 10.1016/j.jconrel.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 156.Jin X., Zhu D.D., Chen B.Z., Ashfaq M., Guo X.D. Insulin delivery systems combined with microneedle technology. Adv. Drug Deliv. Rev. 2018;127:119–137. doi: 10.1016/j.addr.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 157.Jung D., Rejinold N.S., Kwak J.-E., Park S.-H., Kim Y.-C. Nano-patterning of a stainless steel microneedle surface to improve the dip-coating efficiency of a DNA vaccine and its immune response. Colloids Surf. B Biointerfaces. 2017;159:54–61. doi: 10.1016/j.colsurfb.2017.07.059. [DOI] [PubMed] [Google Scholar]
- 158.Liu D., Yu B., Jiang G., Yu W., Zhang Y., Xu B. Fabrication of composite microneedles integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats. Mater. Sci. Eng. C. 2018;90:180–188. doi: 10.1016/j.msec.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 159.Wang J., Ye Y., Yu J., Kahkoska A.R., Zhang X., Wang C., Sun W., Corder R.D., Chen Z., Khan S.A. Core–Shell Microneedle Gel for Self-Regulated Insulin Delivery. ACS Nano. 2018;12:2466–2473. doi: 10.1021/acsnano.7b08152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jiang G., Xu B., Zhu J., Zhang Y., Liu T., Song G. Polymer microneedles integrated with glucose-responsive mesoporous bioactive glass nanoparticles for transdermal delivery of insulin. Biomed. Phys. Eng. Express. 2019;5:045038. doi: 10.1088/2057-1976/ab3202. [DOI] [Google Scholar]
- 161.Tong Z., Zhou J., Zhong J., Tang Q., Lei Z., Luo H., Ma P., Liu X. Glucose- and H2O2-Responsive Polymeric Vesicles Integrated with Microneedle Patches for Glucose-Sensitive Transcutaneous Delivery of Insulin in Diabetic Rats. ACS Appl. Mater. Interfaces. 2018;10:20014–20024. doi: 10.1021/acsami.8b04484. [DOI] [PubMed] [Google Scholar]
- 162.Xu B., Jiang G., Yu W., Liu D., Zhang Y., Zhou J., Sun S., Liu Y. H2O2-Responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin. J. Mater. Chem. B. 2017;5:8200–8208. doi: 10.1039/C7TB02082A. [DOI] [PubMed] [Google Scholar]
- 163.Hu X., Yu J., Qian C., Lu Y., Kahkoska A.R., Xie Z., Jing X., Buse J.B., Gu Z. H2O2-Responsive Vesicles Integrated with Transcutaneous Patches for Glucose-Mediated Insulin Delivery. ACS Nano. 2017;11:613–620. doi: 10.1021/acsnano.6b06892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou Z., Pang J., Wu X., Wu W., Chen X., Kong M. Reverse immune suppressive microenvironment in tumor draining lymph nodes to enhance anti-PD1 immunotherapy via nanovaccine complexed microneedle. Nano Res. 2020;13:1509–1518. doi: 10.1007/s12274-020-2737-5. [DOI] [Google Scholar]
- 165.Dul M., Nikolic T., Stefanidou M., McAteer M., Williams P., Mous J., Roep B., Kochba E., Levin Y., Peakman M. Conjugation of a peptide autoantigen to gold nanoparticles for intradermally administered antigen specific immunotherapy. Int. J. Pharm. 2019;562:303–312. doi: 10.1016/j.ijpharm.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 166.Dumpa N., Goel K., Guo Y., McFall H., Pillai A.R., Shukla A., Repka M., Murthy S.N. Stability of Vaccines. AAPS PharmSciTech. 2019;20:42. doi: 10.1208/s12249-018-1254-2. [DOI] [PubMed] [Google Scholar]
- 167.Rodgers A.M., Cordeiro A.S., Kissenpfennig A., Donnelly R.F. Microneedle arrays for vaccine delivery: The possibilities, challenges and use of nanoparticles as a combinatorial approach for enhanced vaccine immunogenicity. Expert Opin. Drug Deliv. 2018;15:851–867. doi: 10.1080/17425247.2018.1505860. [DOI] [PubMed] [Google Scholar]
- 168.Suh H., Shin J., Kim Y.-C. Microneedle patches for vaccine delivery. Clin. Exp. Vaccine Res. 2014;3:42–49. doi: 10.7774/cevr.2014.3.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chu L.Y., Ye L., Dong K., Compans R.W., Yang C., Prausnitz M.R. Enhanced stability of inactivated influenza vaccine encapsulated in dissolving microneedle patches. Pharm. Res. 2016;33:868–878. doi: 10.1007/s11095-015-1833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rodgers A.M., Cordeiro A.S., Donnelly R.F. Technology update: Dissolvable microneedle patches for vaccine delivery. Med. Devices. 2019;12:379. doi: 10.2147/MDER.S198220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.He X., Sun J., Zhuang J., Xu H., Liu Y., Wu D. Microneedle System for Transdermal Drug and Vaccine Delivery: Devices, Safety, and Prospects. Dose-Response. 2019;17:1559325819878585. doi: 10.1177/1559325819878585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Niu L., Chu L.Y., Burton S.A., Hansen K.J., Panyam J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J. Control. Release. 2019;294:268–278. doi: 10.1016/j.jconrel.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 173.Du G., Hathout R.M., Nasr M., Nejadnik M.R., Tu J., Koning R.I., Koster A.J., Slütter B., Kros A., Jiskoot W., et al. Intradermal vaccination with hollow microneedles: A comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J. Control. Release. 2017;266:109–118. doi: 10.1016/j.jconrel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 174.Haigh O., Depelsenaire A.C.I., Meliga S.C., Yukiko S.R., McMillan N.A.J., Frazer I.H., Kendall M.A.F. CXCL1 gene silencing in skin using liposome-encapsulated siRNA delivered by microprojection array. J. Control. Release. 2014;194:148–156. doi: 10.1016/j.jconrel.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 175.Ali A.A., McCrudden C.M., McCaffrey J., McBride J.W., Cole G., Dunne N.J., Robson T., Kissenpfennig A., Donnelly R.F., McCarthy H.O. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomed. Nanotechnol. 2017;13:921–932. doi: 10.1016/j.nano.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 176.Kim K.S., Kim H., Park Y., Kong W.H., Lee S.W., Kwok S.J.J., Hahn S.K., Yun S.H. Noninvasive Transdermal Vaccination Using Hyaluronan Nanocarriers and Laser Adjuvant. Adv. Funct. Mater. 2016;26:2512–2522. doi: 10.1002/adfm.201504879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Siddhapura K., Harde H., Jain S. Immunostimulatory effect of tetanus toxoid loaded chitosan nanoparticles following microneedles assisted immunization. Nanomed. Nanotechnol. 2016;12:213–222. doi: 10.1016/j.nano.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 178.Yin D., Liang W., Xing S., Gao Z., Zhang W., Guo Z., Gao S. Hepatitis B DNA Vaccine-Polycation Nano-Complexes Enhancing Immune Response by Percutaneous Administration with Microneedle. Biol. Pharm. Bull. 2013;36:1283–1291. doi: 10.1248/bpb.b13-00050. [DOI] [PubMed] [Google Scholar]
- 179.Caucheteux S.M., Mitchell J.P., Ivory M.O., Hirosue S., Hakobyan S., Dolton G., Ladell K., Miners K., Price D.A., Kan-Mitchell J., et al. Polypropylene Sulfide Nanoparticle p24 Vaccine Promotes Dendritic Cell-Mediated Specific Immune Responses against HIV-1. J. Investig. Dermatol. 2016;136:1172–1181. doi: 10.1016/j.jid.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 180.Seok H., Noh J.Y., Lee D.Y., Kim S.J., Song C.S., Kim Y.C. Effective humoral immune response from a H1N1 DNA vaccine delivered to the skin by microneedles coated with PLGA-based cationic nanoparticles. J. Control. Release. 2017;265:66–74. doi: 10.1016/j.jconrel.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 181.Pearton M., Pirri D., Kang S.-M., Compans R.W., Birchall J.C. Host Responses in Human Skin After Conventional Intradermal Injection or Microneedle Administration of Virus-Like-Particle Influenza Vaccine. Adv. Healthc. Mater. 2013;2:1401–1410. doi: 10.1002/adhm.201300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.de Groot A.M., Du G., Mönkäre J., Platteel A.C.M., Broere F., Bouwstra J.A., Sijts A.J.A.M. Hollow microneedle-mediated intradermal delivery of model vaccine antigen-loaded PLGA nanoparticles elicits protective T cell-mediated immunity to an intracellular bacterium. J. Control. Release. 2017;266:27–35. doi: 10.1016/j.jconrel.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 183.Pamornpathomkul B., Niyomtham N., Yingyongnarongkul B.-E., Prasitpuriprecha C., Rojanarata T., Ngawhirunpat T., Opanasopit P. Cationic Niosomes for Enhanced Skin Immunization of Plasmid DNA-Encoding Ovalbumin via Hollow Microneedles. AAPS PharmSciTech. 2018;19:481–488. doi: 10.1208/s12249-017-0855-5. [DOI] [PubMed] [Google Scholar]
- 184.Du G., Leone M., Romeijn S., Kersten G., Jiskoot W., Bouwstra J.A. Immunogenicity of diphtheria toxoid and poly(I:C) loaded cationic liposomes after hollow microneedle-mediated intradermal injection in mice. Int. J. Pharm. 2018;547:250–257. doi: 10.1016/j.ijpharm.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 185.Guo L., Chen J., Qiu Y., Zhang S., Xu B., Gao Y. Enhanced transcutaneous immunization via dissolving microneedle array loaded with liposome encapsulated antigen and adjuvant. Int. J. Pharm. 2013;447:22–30. doi: 10.1016/j.ijpharm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 186.Zhen Y., Wang N., Gao Z., Ma X., Wei B., Deng Y., Wang T. Multifunctional liposomes constituting microneedles induced robust systemic and mucosal immunoresponses against the loaded antigens via oral mucosal vaccination. Vaccine. 2015;33:4330–4340. doi: 10.1016/j.vaccine.2015.03.081. [DOI] [PubMed] [Google Scholar]
- 187.Cole G., McCaffrey J., Ali A.A., McBride J.W., McCrudden C.M., Vincente-Perez E.M., Donnelly R.F., McCarthy H.O. Dissolving microneedles for DNA vaccination: Improving functionality via polymer characterization and RALA complexation. Hum. Vaccines Immunother. 2017;13:50–62. doi: 10.1080/21645515.2016.1248008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhao J.-H., Zhang Q.-B., Liu B., Piao X.-H., Yan Y.-L., Hu X.-G., Zhou K., Zhang Y.-T., Feng N.-P. Enhanced immunization via dissolving microneedle array-based delivery system incorporating subunit vaccine and saponin adjuvant. Int. J. Nanomed. 2017;12:4763–4772. doi: 10.2147/IJN.S132456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wu X., Li Y., Chen X., Zhou Z., Pang J., Luo X., Kong M. A surface charge dependent enhanced Th1 antigen-specific immune response in lymph nodes by transfersome-based nanovaccine-loaded dissolving microneedle-assisted transdermal immunization. J. Mater. Chem. B. 2019;7:4854–4866. doi: 10.1039/C9TB00448C. [DOI] [PubMed] [Google Scholar]
- 190.Duong H.T.T., Yin Y., Thambi T., Nguyen T.L., Giang Phan V.H., Lee M.S., Lee J.E., Kim J., Jeong J.H., Lee D.S. Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials. 2018;185:13–24. doi: 10.1016/j.biomaterials.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 191.Rattanapak T., Birchall J., Young K., Ishii M., Meglinski I., Rades T., Hook S. Transcutaneous immunization using microneedles and cubosomes: Mechanistic investigations using Optical Coherence Tomography and Two-Photon Microscopy. J. Control. Release. 2013;172:894–903. doi: 10.1016/j.jconrel.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 192.Mulligan R.C. The basic science of gene therapy. Science. 1993;260:926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- 193.Chen X. Current and future technological advances in transdermal gene delivery. Adv. Drug Deliv. Rev. 2018;127:85–105. doi: 10.1016/j.addr.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 194.Kim N.W., Lee M.S., Kim K.R., Lee J.E., Lee K., Park J.S., Matsumoto Y., Jo D.-G., Lee H., Lee D.S. Polyplex-releasing microneedles for enhanced cutaneous delivery of DNA vaccine. J. Control. Release. 2014;179:11–17. doi: 10.1016/j.jconrel.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 195.Ruan W., Zhai Y., Yu K., Wu C., Xu Y. Coated microneedles mediated intradermal delivery of octaarginine/BRAF siRNA nanocomplexes for anti-melanoma treatment. Int. J. Pharm. 2018;553:298–309. doi: 10.1016/j.ijpharm.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 196.Xu J., Xu B., Tao J., Yang Y., Hu Y., Huang Y. Microneedle-Assisted, DC-Targeted Codelivery of pTRP-2 and Adjuvant of Paclitaxel for Transcutaneous Immunotherapy. Small. 2017;13:1700666. doi: 10.1002/smll.201700666. [DOI] [PubMed] [Google Scholar]
- 197.Roep B.O., Wheeler D.C.S., Peakman M. Antigen-based immune modulation therapy for type 1 diabetes: The era of precision medicine. Lancet Diabetes Endocrinol. 2019;7:65–74. doi: 10.1016/S2213-8587(18)30109-8. [DOI] [PubMed] [Google Scholar]
- 198.Clinicaltrials.gov. [(accessed on 31 December 2020)]; Available online: https://clinicaltrials.gov/
- 199.Ogunjimi A.T., Carr J., Lawson C., Ferguson N., Brogden N.K. Micropore closure time is longer following microneedle application to skin of color. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-75246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.