Abstract
Simple Summary
Organic poultry production should use only genetic lines and animals resistant to disease and well adapted to live outdoor, according to principles, rules, and requirements of organic farming systems. When broiler’s walking performance is reduced animals are not suitable for outdoor rearing. There is a straightforward relationship between bone health and growth rate in broilers. Body and breast weight play an important role in leg disorders. During the last decades, genetic selection has led to high producing broilers over the time. Unfortunately, fast growth may negatively influence correct leg development, reducing walking performance, and raising welfare issues. Leg abnormalities could represent a criterion for the choice of genetic lines suitable for organic production. A method for their early detection was developed in this study by means of Geometric Morphometrics (GM) that represents a tool for bone shape analysis and its correlation with walking capability. A valuable information emerged from the present study in relation to broiler intrinsic adaptability to organic production.
Abstract
In the present study, the conformation of the tibia of seven genetic lines of broilers was analyzed by Geometric Morphometrics and correlated to carcass weight and walking ability. The used chicken genetic lines were classified as fast, medium, or slow growing and ranked for their walking ability. Six chicken types were reared in an organic farm and slaughtered at 81 days of age while one slow-growing and highly walking line (Naked Neck) was reared in a commercial farm and used as external reference for moving activity and growth speed. A mixed landmarks and semi-landmarks model was applied to the study of tibia shape. Results of this study showed that: (i) body weight gain was positively correlated to the curvature of the antero-posterior axis of the tibia; (ii) the shape of the tibia and the active walking behavior were significantly correlated; (iii) walking and not-walking genetic lines could be discriminated in relation to the overall shape of the tibia; (iv) a prevalence of static behavior was correlated to a more pronounced curvature of the antero-posterior axis of the tibia. Results of this study revealed that the walking genetic types have a more functional and natural tibia conformation. This easy morphologic method for evaluating tibia shape could help to characterize the adaptability of genotypes to organic and outdoor rearing.
Keywords: bone shape, genetic lines, geometric morphometrics, organic, outdoor rearing, poultry
1. Introduction
According to EU Organic Regulations (Reg. EC N° 834/2007 and 889/2008), organic poultry production should be based on resistant genotypes able to live in outdoor conditions and to favorably exploit outdoor runs for foraging and exploring. When walking ability is reduced, animals are not suitable for rearing in open air; walking ability depends on genotype, weight, age, feeding, and housing [1]. It has been demonstrated that fast-growing broilers, if reared in organic system, are genetically prone to lameness and leg weakness [2]. There is a straightforward relationship between bone quality and growth rate in broilers [3]. Body weight plays a decisive role in lameness [1]. Genetic selection produced very efficient broilers, but fast growth can influence the isometric growth, causing leg problems [4] and changes in leg bones biomechanics [5]. Slow-growing broilers show less leg problems or lameness [1], but, as they have worse feed conversion rates and lower commercial weights, are often avoided by farmers who prefer most medium/fast genetic types. It should be noted that heavy weight is closely correlated with rotated tibia [6] and with the lateral curvature of the upper tibia [7]. Rotated tibiae can be also the result of too early rapid growth rate and low activity [8], or a genetic issue, since it was evidenced also in slow growing genotypes [9].
In this complex framework, the identification of genetic lines showing the tendency to develop leg anomalies, even at a non-pathological stage, is pivotal, in order to prevent welfare and health issues, in particular for organic poultry production.
This study was part of a project (TIPIBIO) focusing on poultry adaptability to organic systems from different point of view such as behavior, welfare, and metabolic status. One specific object of the project was to define a plausible adaptability index for the different genotypes. Leg anomalies (better if rapidly diagnosed) should be included in that index, given their strict relation with walking ability,
Many studies on broiler welfare in relation to leg problems used a gait score method, e.g., the Bristol scoring system, assessing in vivo ability to walk (from 0 to 5) [10]. Such inspective, non-invasive methods do not take into consideration bone pathologies [9] or bone anomalies but are very rapid and easy. Nevertheless, the scoring system is not fully objective, depending on the operator.
The morphological approach is a more objective method used to detect bone anomalies, cause of lameness, and bone defects. To the best of our knowledge, previous studies relating fast growing and body weight to bone health and morphology used classic morphometrical approaches, such as the measure of bone length, weight, diameter [2,3,11], or analysis of radiographic images [12]. Most of previous studies considered the occurrence of skeletal anomalies and pathologies [11,13,14,15]. Significant differences were found in morphometric traits of tibio-tarsus in two different broiler strains (Ross and Lohman Dual) [2]. Rapid growth genotypes showed more tibia deformities, lack of mineralization, and reduced strength.
In the present study, morphological changes in the tibia of different poultry genetic lines (meat type) were analyzed by the Geometric Morphometrics (GM) [16,17], a tool for shape analysis able to quantify changes in overall bone morphology and their relationship with other variables (e.g., genetic lines and body weight). GM easily permits localization of morphological changes and information about the magnitude of variation can be extracted [18] and visualized through deformation grids and vectors [19].
The aims of the present study were: (1) To assess the effects of genetic line on the shape of tibia in seven commercial lines of chickens; (2) to investigate the relationship between walking behavior and shape of tibia; (3) to provide useful information for selection of genetic lines suitable for organic production.
2. Materials and Methods
A total of 105 right tibiae were analyzed in this study; 90 samples were obtained from six poultry lines, from fast- to slow-growing ones (Table 1), reared in the experimental farm of the University of Perugia (Italy), within the TIPIBIO project, as fully described in a companion study [20]. Ninety male chickens from six commercial lines, Aviagen Ranger Classic (RC), Ranger Gold (RG) and Rowan Ranger (RR), Hubbard CY Gen5xJA87 (CY) M22xJA87 (M), and RedJA (C), were reared in an organic farm and slaughtered at 81 days of age (the minimal slaughtering age required for organic production, in compliance with Regulation CE 889/2008). The growth rate classification of the different lines was provided by the breeding companies. As for Aviagen strains, a previous study confirmed RC as fast-growing, RG as medium-growing, and RR as slow-growing [21]. For Hubbard lines, no comparative studies were available, but the breeder company ranking was applied as well (i.e., CY and M medium/fast growing and C slow growing). The remaining 15 tibiae were taken from Naked Neck (NN) chickens reared in an organic farm in Center Italy (Jesi, AN). NN were used as a benchmark, as it is normally used for free-range production (Label Rouge).
Table 1.
Genetic lines analyzed, walking behavior (W: Walking; NW: Not-Walking), and growth rate.
| Genetic Line | Acronym | Walking Behavior | Growth Rate |
|---|---|---|---|
| Aviagen Ranger Classic | RC | NW | Fast |
| Aviagen Ranger Gold | RG | W | Medium |
| Aviagen Rowan Ranger | RR | W | Slow |
| Hubbard CY Gen5xJA87 | CY | NW | Medium/Fast |
| Hubbard M22xJA87 | M | NW | Medium/Fast |
| Hubbard RedJA | C | W | Slow |
| Hubbard RedJA Naked Neck | NN | W | Slow |
Genetic lines have been so regrouped in Walking (W) and Not-Walking (NW) (Table 1), according to the results of a previous study [20], which characterized genetic lines depending on the time spent in active or static behaviors. Static behaviors were (1) rest (i.e., body in line with the ground, with erect head and open eyes) and (2) roost (i.e., be standing, no body movement, erect or relaxed head with open eyes). Walking (i.e., moving more than three steps in one direction with upright head) was classified as an active behavior. Carcass weight and breast yield were recorded (Table 2). Right tibiae were excised, fleshed out, and boiled in order to remove residual meat, ligaments, and tendons. The correlation of the measured traits with both carcass and breast yield was calculated, but being very similar, we decide to report only that with carcass weight.
Table 2.
Carcass weight (mean ± S.D.), length of the tibia (mean ± S.D.), and width of the arch (BH, mean ± S.D.) of the six genetic lines and of naked neck (NN). Different letters indicate significant differences among treatments (Tuckey test; p < 0.05); similar letters depict no significant differences.
| Acronym | Carcass Weight (g) | Tibia Length (cm) | BH (cm) |
|---|---|---|---|
| RC | 3616.0 ± 54.4 b | 13.4 ± 0.4 b | 1.3 ± 0.2 ab |
| CY | 3487.7 ± 117.6 b | 13.9 ± 1.3 b | 1.3 ± 0.2 ab |
| M | 3213.3 ± 121.2 c | 14.2 ± 0.8 b | 1.4 ± 0.1 a |
| RG | 3179.3 ± 71.7 c | 14.1 ± 0.5 b | 1.2 ± 0.1 bc |
| RR | 2859.33 ± 115.0 c | 13.6 ± 0.8 b | 1.1 ± 0.2 c |
| C | 2930.7 ± 77.2 c | 14.2 ± 0.6 b | 1.1 ± 0.2 c |
| NN | 2431.6 ±61.7 a | 12.0 ± 0.6 a | 0.9 ± 0.1 d |
All adopted procedures were in accordance with the EU legal framework relating to the protection of animals used for scientific purposes (Directive 2010/63/EU).
Each tibia (n = 105) was photographed in lateral aspect by a high-resolution digital camera (13 real MP) set on a tripod. The focal distance between the camera and the tibiae was 40 cm. For each tibia, length (measured between the ends of proximal and distal epiphyses) and measure of arch width (BH), a proxy of antero-posterior curvature [11,22] were collected using TpsDig2.0 [23], as illustrated in Figure 1. Landmarks (4) and semi-landmarks (16) were digitalized on each image using TpsDig2.0 (Figure 1). Landmarks were digitalized on the proximal anterior (1) and posterior (2) epiphyses and on the distal anterior (3) and posterior (4) epiphyses. As no other homologous landmarks should be identified on the tibia, in order to ensure shape coverage of the entire bone, two outline curves were recorded on each tibia. The first beginning from landmark 1 and ending at landmark 2 and the other beginning from landmark 3 and ending at landmark 4. On each of these two outlines, eight equally spaced semi-landmarks [18,24,25] were automatically digitalized, along each curve, using TpsDig2.0. Semi-landmarks could slide iteratively along the outlines curve using the spline relaxation procedure algorithm of Bookstein [26,27]. After relaxation, semi-landmarks can be treated in multivariate analyses as homologous points [26,27]. Landmarks and sliding landmarks were converted into shape coordinates using Procrustes superimposition [28], removing information about location and orientation from the raw coordinates and standardizing each specimen to a unit centroid size (CS—square root of the summed squared Euclidean distances from each landmark to the specimen’s centroid). Residuals were analyzed using the thin-plate spline (TPS) interpolating function [19], producing principal warps.
Figure 1.
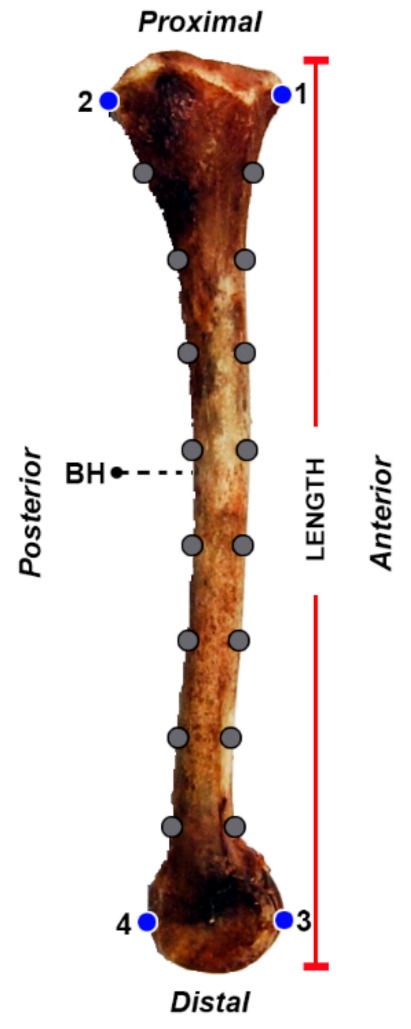
Landmarks (blue circles) and semi-landmarks (grey circles) collected on the tibia: (1) Proximal anterior and (2) proximal posterior epiphyses; (3) distal anterior and (4) distal posterior epiphyses. Biometrics collected along the tibia: Length and maximum width of the arch described by the bone (BH).
Morphometric software of the TPS series are freely available (http://life.bio.sunysb.edu/morph/).
Differences in carcass weight, tibia length, and tibia arch width among genetic lines were tested by the one-way analysis of variance (ANOVA, Welch’ test for unequal variances) with Tukey post-hoc pairwise test. Arch width and tibia length were significantly correlated (Spearman r = 0.5, p < 0.01), so ANOVA for arch width differences in genetic lines was performed on least square regression residuals.
To display tibia shape variation, principal component analysis (PCA) was performed on the covariance matrix. Spearman’s rank correlation coefficient was calculated between PC1 and PC2 scores and carcass weight. Individuals were distinguished in the plot according to their walking aptitude (as codified in Table 1).
Discriminant function analysis (DFA) was performed to separate a priori known groups in the data, providing an ordination that maximized the separation of the group means relative to the variation within groups. Genetic lines were separated into two categories: Walking and not-walking (Table 1). DFA procedure carries out a leave-one-out cross-validation to assess the reliability of classification [29].
Statistical tests were performed using PAST. PCA and DFA were performed using the software MorphoJ.
The pattern of covariation between the shape of the tibia and behavior (active or static behaviors, as measured by [20], was analyzed using partial least squares (PLS) analysis [18,30] only on the six genetic lines reared in the experimental section of the University of Perugia [20]. For details and computational aspects, see [19,30,31,32].
3. Results and Discussion
3.1. General Results
Average carcass weight, length of the tibiae, and width of the arch (BH) for each genotype are shown in Table 2. The six lines showed significant differences in final carcass weight (F = 35.5, p < 0.01): The reference group (NN) was different from all the other groups and showed the lowest carcass weight. These results are in line with the organic rearing conditions of NN. Two not-walking lines (RC and CY) showed higher carcass weight values, significantly different from walking ones (RG, RR, and C). Hubbard M22xJA87 (M), even though classified as a not-walking line, was not statistically different from W lines concerning the final carcass weight. The length of the tibia was significantly different among groups (F = 22.6, p < 0.01). The highest length of the tibia was measured in M and C strains, while the lowest in the reference strain (NN), followed by the RC. However, post-hoc Tuckey-pairwise comparisons were significant only between NN and all the other groups probably due to breed effect and different rearing conditions as well.
Arch width, which could be considered a proxy of the curvature of the tibia, was significantly different among groups (F = 3.9; p < 0.01). The reference group (NN) was significantly different from all other groups, with the lowest curvature. A cluster including walking genetic lines (RG, RR, and C) showed intermediate values, while higher values were recorded for not-walking ones (RC, CY, and M).
The arch width (BH) was correlated with antero-posterior curvature and chicken growth. Shim et al. (2012) found that fast-growing lines show higher risk of tibia breakage due to lower bone density, confirmed by a decrease in mechanical strength and ash content, when compared to slow-growing ones. In a previous study, authors observed a higher antero-posterior curvature in chickens affected by varus limb [22]. Other authors reported a tendence to higher antero-posterior curvature in broilers with leg disorders [11]. In our study, antero-posterior curvature simply measured as the arch width (BH) was not enough to discriminate between walking and not-walking groups, rising the need of a more comprehensive multiparametric indicator (e.g., bone shape).
3.2. Shape Analysis
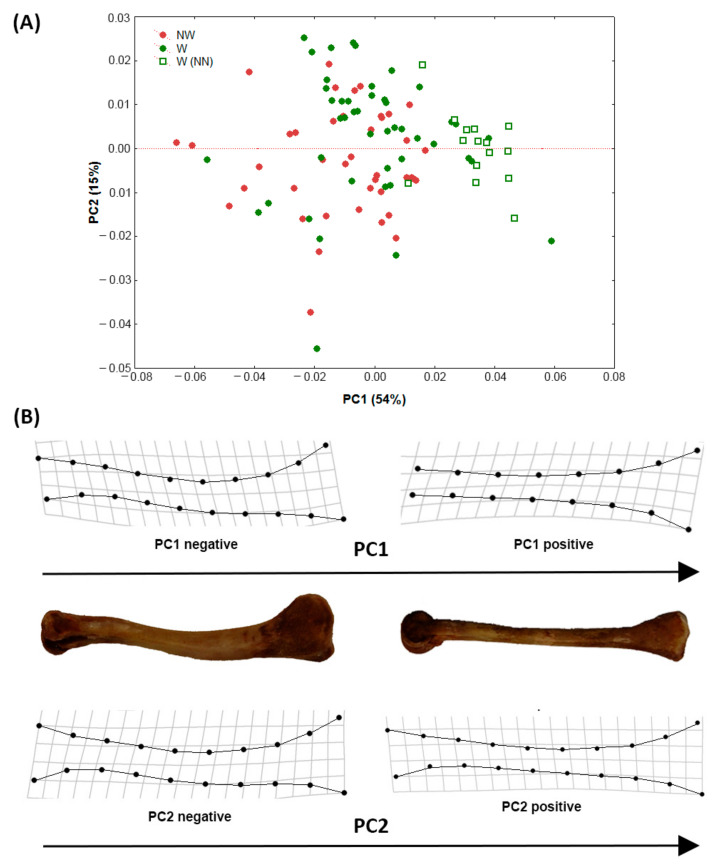
The first two axes of PCA accounted for 69% of total variance (Figure 2). Even if the two groups (W: Walking; NW: Not-walking) were partially overlapped, observations related to the W group were mostly located in the positive part of PC1 (54% of variance explained), while NW observations were in the negative portion. The reference group (NN) was mainly distributed in the very right side of the axis PC1. Along PC2 (15% of variance explained) the two categories were almost completely overlapped. Negative scores on PC1 corresponded to a more evident curvature of the tibia antero-posterior axis, compared to the straighter configuration of this bone in specimens positioned in the positive region of the same axis. Scores along PC1 were negatively correlated with carcass weight (Spearman r = −0.44, p < 0.05), thus weight increase was correlated to a more evident curvature of the tibia.
Figure 2.
(A) Principal component analysis plot. Grouping variable: Walking (W)/not-walking (NW)/walking naked neck (W-NN). (B) Shape variation along PC1 and PC2 was represented by splines relative to positive and negative extremes of the axes. For shape variation along PC1, tibiae of two extreme individuals were reported.
Walking (W) and not-walking (NW) genetic lines were significantly discriminated by discriminant analysis (T2 = 106.5; p < 0.05). Groups were slightly overlapped and classification was very reliable (Figure 3; Table 3). The percentage of correct classification to a priori defined groups after cross-validation was up to 80%. The highest error occurred in the correct attribution of individuals to not-walking class (16.7%).
Figure 3.
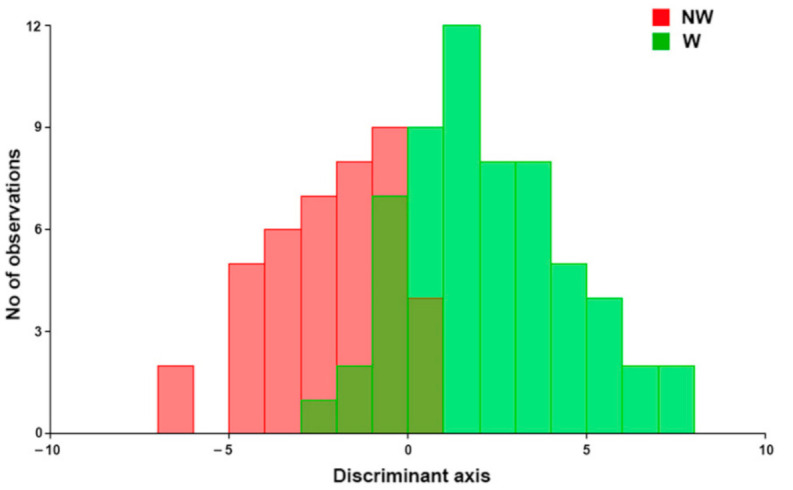
Discriminant analysis histogram. Grouping variable: Walking (W)/not-walking (NW).
Table 3.
Discriminant analysis classification/misclassification scores.
| True | Allocated to | Total | % | |
|---|---|---|---|---|
| W | NW | |||
| W | 37 | 4 | 41 | 90.2 |
| NW | 10 | 50 | 60 | 83.3 |
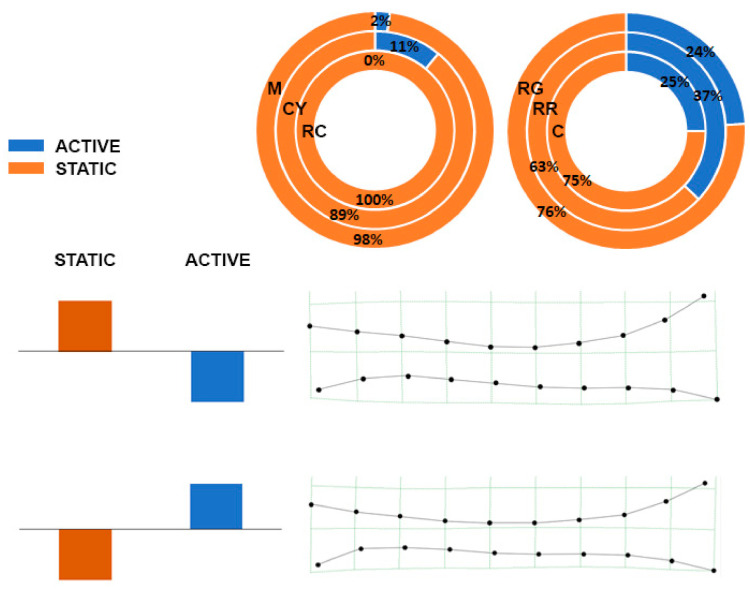
The shape of tibia and chicken behavior (active/static) (Figure 4) were significantly correlated (r = 0.40; p < 0.05). Genetic types classified as walking had active behaviors ranging between 0% and 11%; in those classified as not-walking, active behaviors ranged between 24% and 37% and the highest values was observed for RR. A prevalence of static behavior was correlated to a more pronounced curvature of the antero-posterior axis of the tibia.
Figure 4.
Partial least squares (PLS) showing the morphological relationship between the tibia and the walking/resting behavior described as percentage of time spent in two main activities (Walking W and Not Walking—NW). Percentages for each genotype are represented in pie charts. The splines depict tibia shape configuration corresponding to opposite patterns of behavior.
These results are consistent with the above-mentioned studies evidencing a strong relationship between leg deformities, lameness, and consequently walking activity. A positive correlation between time spent lying and lameness was found in broilers [33] and most cases of lameness can be attributed to leg anomalies. One of the main causes of leg diseases in commercial broilers is the selection for rapid growth and high body weight, which affect chick skeletal development and conformation, causing bone anomalies [34]. Bone anomalies, therefore, present a certain degree of heritability [35], making some genetic lines more prone to develop lameness than others [36], even if the relationship between leg disorders and genetic factors is not yet clarified. Morphological changes in fast-growing broilers, such as the increased weight of breast muscles, may cause changes in bone structure and morphology, moving forward the center of gravity of the animal [34]. High incidence of leg pathologies is related to growth rate and breast muscles yield and negatively affected the normal chicken anatomy and physiology [37]. Broilers affected by valgus-varus disease had impaired walking ability and lower body weight, probably due to the difficulty of drinking and eating [11]. In contrast with these last findings, in the present study, carcass weight was positively correlated to antero-posterior curvature of tibia. Anyway, results are not comparable, as all the chickens analyzed are healthy and apparently free of leg diseases. In support of this claim, [38] found that fast-growing broiler are more subjected to tibio-tarsus mineralization disorders and bone fractures, being the bones overcharged for high body weight [39,40]. Bone quality (i.e., mineralization and bone density) of slow-growing lines was higher than that of fast-growing ones [3,41,42,43]. The rigidity and consequently the bending resistance, of tibiotarsus in the Lohman Dual slow-growing line was greater than in Ross 308, a highly selected fast-growing line [2], confirming that skeleton stability is affected by weight gain.
A correlation between growing rate and behavioral repertoire as well as welfare conditions have been evidenced [44]: Slow-growing lines showed lower gait scores than fast-growing and spent more time in active behaviors, assuming the same diet and stocking density.
4. Conclusions
The correlation between growth rate and bone health in broilers has been previously documented [3]. Leg disorders are serious concern for poultry industry and cost over $100 million per year to producers [45]. In the present study, a Geometric Morphometric approach was applied to the analysis of shape variation of the tibia in broiler lines with different growth rates. Relationship between tibia shape and walking attitude was investigated. The conclusions are:
Tibia shape could represent a reference/starting point for studies on chicken walking behavior. Statistical analysis evidenced a clear and straightforward correlation between the curvature of this bone and the tendency of the animal to spend more time in static activities, such as resting and roosting;
Degree of curvature of the tibia is positively related to carcass weight and to growth rate. Fast-growing genotypes showed a more pronounced curvature of the tibia;
Ranger Gold, Rowan Ranger, and RedJA, previously classified as walking poultry lines [20], showed a less curved tibia, similar to that of the reference line (the slow-growing Naked Neck).
Besides, the results of this study suggest that walking lines are associated to favorable tibia shape and characteristics. The easy and cheap (mainly based on open-source software) method used for tibia shape and curvature definition is ideal for defining an early index of the adaptability of specific genotypes to organic and outdoor rearing.
All animals were apparently healthy and none of them showed evident leg pathologies all over the experiment, but it is rational that heavier chickens, characterized by a curved tibia, could be more prone to deformities or pathologies and more exposed to lameness risk [2,3].
Further studies are necessary to validate these results by the inclusion of left leg in the analysis (right is supposed to be more sensitive to deformation). A larger number of individuals and genetic lines included in the study could help to have a more faithful representation of the reality and would balance anatomic markers also.
Acknowledgments
Thanks to Aviagen and Hubbard for providing genetic lines.
Author Contributions
Conceptualization, D.P. and M.G.A.; methodology, D.P.; software, D.P.; validation, M.G.A.; formal analysis, D.P. and F.C.; data curation, D.P. and F.C.; writing—original draft preparation, D.P.; writing—review and editing M.G.A., D.M.Z., and C.C.; supervision, M.G.A.; project management, D.M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Italian Ministry of Agriculture—Project TIPIBIO.
Institutional Review Board Statement
Ethical review and approval were waived for this study, as animals were reared according to the organic regulation rules (Reg. EC 889/2008) with standard practice, without compromising animal welfare. Animals were slaughtered according to commercial authorized practice.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kestin S.C., Gordon S., Su G., Sørensen P. Relationships in broiler chickens between lameness, liveweight, growth rate and age. Vet. Rec. 2001;148:195–197. doi: 10.1136/vr.148.7.195. [DOI] [PubMed] [Google Scholar]
- 2.Harash G., Richardson K.C., Alshamy Z., Hünigen H., Hafez H.M., Plendl J., Al Masri S. Basic morphometry, microcomputed tomography and mechanical evaluation of the tibiotarsal bone of a dual-purpose and a broiler chicken line. PLoS ONE. 2020;15:e0230070. doi: 10.1371/journal.pone.0230070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shim M.Y., Karnuah A.B., Mitchell A.D., Anthony N.B., Pesti G.M., Aggrey S.E. The effects of growth rate on leg morphology and tibia breaking strength, mineral density, mineral content, and bone ash in broilers. Poult. Sci. 2012;91:1790–1795. doi: 10.3382/ps.2011-01968. [DOI] [PubMed] [Google Scholar]
- 4.Tickle P.C., Paxton H., Rankin J.W., Hutchinson J.R., Codd J.R. Anatomical and biomechanical traits of broiler chickens across ontogeny. Part I. Anatomy of the musculoskeletal respiratory apparatus and changes in organ size. PeerJ. 2014;2:e432. doi: 10.7717/peerj.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang S., Kong A., Cao Q., Tong Z., Wang X. The role of blood vessels in broiler chickens with tibial dyschondroplasia. Poult. Sci. 2019;98:6527–6532. doi: 10.3382/ps/pez497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kestin S.C., Knowles T.G., Tinch A.F., Gregory N.G. The prevalence of leg weakness in broiler chickens and its relationship with genotype. Vet. Rec. 1992;131:190–194. doi: 10.1136/vr.131.9.190. [DOI] [PubMed] [Google Scholar]
- 7.Julian R.J. Rapid Growth Problems: Ascites and Skeletal Deformities in Broilers. Poult. Sci. 1998;77:1773–1780. doi: 10.1093/ps/77.12.1773. [DOI] [PubMed] [Google Scholar]
- 8.Duff S.R.I. Diseases of the musculoskeletal system. In: Jordan F.T.W., editor. Poultry Diseases. Balliere Tindall; London, UK: 1990. pp. 254–283. [Google Scholar]
- 9.Bradshaw R.H., Kırkden R.D., Broom R.H. A review of the aetiology and pathology of leg weakness in broilers in relation to welfare. Avian Poult. Biol. Rev. 2002;13:45–103. doi: 10.3184/147020602783698421. [DOI] [Google Scholar]
- 10.Corr S.A., Mccorquodale C.C., Gentle M.J. Gait Analysis of Poultry. Res. Vet. Sci. 1998;65:233–238. doi: 10.1016/S0034-5288(98)90149-7. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y., Tang H., Wang X., Li W., Wang Y., Yan F., Kang X., Li H.R. Clinical assessment of growth performance, bone morphometry, bone quality, and serum indicators in broilers affected by valgus-varus deformity. Poult. Sci. 2019;98:4433–4440. doi: 10.3382/ps/pez269. [DOI] [PubMed] [Google Scholar]
- 12.Hocking P.M., Sandercock D.A., Wilson S., Fleming R.H. Quantifying genetic (co)variation and effects of genetic selection on tibial bone morphology and quality in 37 lines of broiler, layer and traditional chickens. Br. Poult. Sci. 2009;50:443–450. doi: 10.1080/00071660903110927. [DOI] [PubMed] [Google Scholar]
- 13.Randall C.J., Mills C.P. Observations on leg deformity in broilers with particular reference to the intertarsal joint. Avian Pathol. 1981;10:407–431. doi: 10.1080/03079458108418492. [DOI] [PubMed] [Google Scholar]
- 14.Vestergaard K.S., Sanotra G.S. Relationships between leg disorders and changes in the behaviour of broiler chickens. Vet. Rec. 1999;144:205–209. doi: 10.1136/vr.144.8.205. [DOI] [PubMed] [Google Scholar]
- 15.Sherlock L., Demmers T.G.M., Goodship A.E., Mccarthy I.D., Wathes C.M. The relationship between physical activity and leg health in the broiler chicken. Br. Poult. Sci. 2010;51:22–30. doi: 10.1080/00071660903460637. [DOI] [PubMed] [Google Scholar]
- 16.Rohlf F.J., Marcus L.F. Geometric morphometrics: Reply to M. Corti. Trends Ecol. Evol. 1993;8:339. doi: 10.1016/0169-5347(93)90244-J. [DOI] [PubMed] [Google Scholar]
- 17.Marcus L.F., Corti M., Loy A., Naylor G.J.P., Slice D.E. Advances in Morphometrics. Plenum Press; New York, NY, USA: 1996. [Google Scholar]
- 18.Zelditch M.L., Swiderki D.L., Sheets H.D., Fink W.L. Geometric Morphometrics for Biologists: A Primer. Elsevier/Academic Press; San Diego, CA, USA: 2004. [Google Scholar]
- 19.Bookstein F.L. Morphometric Tools for Landmark Data: Geometry and Biology. Cambridge University Press; Cambridge, UK: 1991. [Google Scholar]
- 20.Cartoni Mancinelli A., Mattioli S., Dal Bosco A., Aliberti A., Guarino Amato M., Castellini C. Performance, behavior, and welfare status of six different organically reared poultry genotypes. Animals. 2020;10:550. doi: 10.3390/ani10040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louton H., Keppler C., Erhard M., van Tuijl O., Bachmeier J., Damme K., Reese S., Rauch E. Animal-based welfare indicators of 4 slow-growing broiler genotypes for the approval in an animal welfare label program. Poult. Sci. 2019;98:2326–2337. doi: 10.3382/ps/pez023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leterier C., Nys Y. Clinical and anatomical differences in varus and valgus deformities of chick limbs suggest different aetio-pathogenesis. Avian Pathol. 1992;21:429–442. doi: 10.1080/03079459208418861. [DOI] [PubMed] [Google Scholar]
- 23.Rohlf F.J. TpsDig Version 2.10, Digitalized Landmarks and Outlines. Department of Ecology and Evolution, State University of New York; Stony Book, NY, USA: 2006. [Google Scholar]
- 24.Perez S.I., Bernal V., Gonzalez P.N. Differences between sliding semilandmark methods in geometric morphometrics, with an application to human craniofacial and dental variation. J. Anat. 2006;208:769–784. doi: 10.1111/j.1469-7580.2006.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulcini D., Russo T., Reale P., Massa-Gallucci A., Brennan G., Cataudella S. Rainbow trout (Oncorhynchus mykiss, Walbaum 1792) develop a more robust body shape under organic rearing. Aquac. Res. 2014;45:397–409. doi: 10.1111/j.1365-2109.2012.03236.x. [DOI] [Google Scholar]
- 26.Gunz P., Mitteroecker P., Bookstein F.L. Semilandmarks in three dimensions. In: Slice D.E., editor. Modern Morphometrics in Physical Anthropology. Kluwer Academic/Plenum Publishers; New York, NY, USA: 2005. pp. 73–98. [Google Scholar]
- 27.Gunz P., Harvati K. The Neanderthal ‘chignon’: Variation, integration, and homology. J. Hum. Evol. 2007;52:262–274. doi: 10.1016/j.jhevol.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Rohlf F.J., Slice D.E. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Zool. 1990;39:40–59. doi: 10.2307/2992207. [DOI] [Google Scholar]
- 29.Weiss S.M., Kulikowski C.A. Computer Systems That Learn. Morgan Kaufmann; San Mateo, CA, USA: 1991. [Google Scholar]
- 30.Bookstein F.L., Gunz P., Mitteroecker P., Prossinger H., Schaefer K., Seidler H. Cranial integration in Homo: Singular warps analysis of the midsagittal plane in ontogeny and evolution. J. Hum. Evol. 2003;44:167–187. doi: 10.1016/S0047-2484(02)00201-4. [DOI] [PubMed] [Google Scholar]
- 31.Rohlf F.J., Corti M. The use of two-block partial least squares to study covariation in shape. Syst. Biol. 2000;49:740–753. doi: 10.1080/106351500750049806. [DOI] [PubMed] [Google Scholar]
- 32.Fadda C., Corti M. Three-dimensional geometric morphometrics of Arvicanthis: Implications for systematics and taxonomy. J. Zool. Syst. Evol. Res. 2001;39:235–245. doi: 10.1046/j.1439-0469.2001.00169.x. [DOI] [Google Scholar]
- 33.Aydin A. Leg Weaknesses and Lameness Assessment Methods in Broiler Chickens. AAHDS. 2018;1:AAHDS.MS.ID.000506. doi: 10.33552/AAHDS.2018.01.000506. [DOI] [Google Scholar]
- 34.Corr S.A., Gentle M.J., Mccorquodale D., Bennet D. The effect of morphology on walking ability in the modern broiler: A gait analysis study. Anim. Welf. 2003;12:159–171. [Google Scholar]
- 35.Gonzálezcerón F., Rekaya R., Anthony N.B., Aggrey S.E. Genetic analysis of leg problems and growth in a random mating broiler population. Poult. Sci. 2015;94:162–168. doi: 10.3382/ps/peu052. [DOI] [PubMed] [Google Scholar]
- 36.Kjaer J.B., Su G., Nielsen B.L., Sorensen P. Foot pad dermatitis and hock burn in broiler chickens and degree of inheritance. Poult. Sci. 2006;85:1342–1348. doi: 10.1093/ps/85.8.1342. [DOI] [PubMed] [Google Scholar]
- 37.Paxton H., Tickle P.J., Rankin J.W., Codd J.R., Hutchinson J.R. Anatomical and biomechanical traits of broiler chickens across ontogeny. Part II. Body segment inertial properties and muscle architecture of the pelvic limb. PeerJ. 2014;2:e473. doi: 10.7717/peerj.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charuta A., Dzierzęcka M., Majchrzak T., Czerwiński E., Cooper R.G. Computer-generated radiological imagery of the structure of the spongius substance in the postnatal development of the tibiotarsal bones of the Peking domestic duck (Anas platyrhynchos var. domestica) Poult. Sci. 2011;90:830–835. doi: 10.3382/ps.2010-01031. [DOI] [PubMed] [Google Scholar]
- 39.Woo S.L., Kuei S.C., Amiel D., Gomez M.A., Hayes W.C., White F.C., Akeson W.H. The effect of prolonged physical training on the properties of long bone: A study of Wolff’s Law. J. Bone Jt. Surg. Am. 1981;63:780–787. doi: 10.2106/00004623-198163050-00013. [DOI] [PubMed] [Google Scholar]
- 40.Chen J.H., Liu C., You L., Simmons C.A. Boning up on Wolff’s Law: Mechanical regulation of the cells that make and maintain bone. J. Biomech. 2010;43:108–118. doi: 10.1016/j.jbiomech.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Yair R., Cahaner A., Uni Z., Shahar R. Maternal and genetic effects on broiler bone properties during incubation period. Poult. Sci. 2017;96:2301–2311. doi: 10.3382/ps/pex021. [DOI] [PubMed] [Google Scholar]
- 42.Williams B., Solomon S., Waddington D., Thorp B., Farquharson C. Skeletal development in the meat-type chicken. Br. Poult Sci. 2000;41:141–149. doi: 10.1080/713654918. [DOI] [PubMed] [Google Scholar]
- 43.Williams B., Waddington D., Murray D.H., Farquharson C. Bone strength during growth: Influence of growth rate on cortical porosity and mineralization. Calcif. Tissue Int. 2004;74:236–245. doi: 10.1007/s00223-002-2124-0. [DOI] [PubMed] [Google Scholar]
- 44.Rayner A.C., Newberry R.C., Vas J., Mullan S. Slow‑growing broilers are healthier and express more behavioural indicators of positive welfare. Sci. Rep. 2020;10:15151. doi: 10.1038/s41598-020-72198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cook M.E. Skeletal deformities and their causes: Introduction. Poult. Sci. 2000;79:982–984. doi: 10.1093/ps/79.7.982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.