Abstract
In June 2011, the Joint United Nations Programme on HIV/AIDS, the US President’s Emergency Plan for AIDS Relief (PEPFAR), and other collaborators outlined a transformative plan to virtually eliminate pediatric AIDS worldwide. The ambitious targets of this initiative included a 90% reduction in new pediatric HIV infections and a 50% reduction in HIV-related maternal mortality—all by 2015. PEPFAR has made an unprecedented commitment to the expansion and improvement of prevention of mother-to-child HIV transmission (PMTCT) services globally and is expected to play a critical role in reaching the virtual elimination target. To date, PEPFAR has been instrumental in the success of many national programs, including expanded coverage of PMTCT services, an enhanced continuum of care between PMTCT and HIV care and treatment, provision of more efficacious regimens for antiretroviral prophylaxis, design of innovative but simplified PMTCT approaches, and development of new strategies to evaluate program effectiveness. These accomplishments have been made through collaborative efforts with host governments, United Nations agencies, other donors (eg, the Global Fund for AIDS, Tuberculosis, and Malaria), nongovernmental organizations, and private sector partners. To successfully meet the ambitious global targets to prevent new infant HIV infections, PEPFAR must continue to leverage the existing PMTCT platform, while developing innovative approaches to rapidly expand quality HIV services. PEPFAR must also carefully integrate PMTCT into the broader combination prevention agenda for HIV, so that real progress can be made toward an “AIDS-free generation” worldwide.
Keywords: prevention of mother-to-child HIV transmission, PMTCT, PEPFAR, global response, HIV
Since President George W. Bush launched the President’s Emergency Plan for AIDS Relief (PEPFAR) in 2003, global efforts to prevent mother-to-child HIV transmission (PMTCT) have undergone a tremendous evolution. A number of affordable and effective interventions are now available to protect HIV-exposed infants from infection—the result of a remarkable chain of incremental discoveries in HIV prevention science. Service coverage has expanded rapidly, made possible by unprecedented donor and national government investments in health care delivery infrastructure and by the rapid adoption of new science into international policy. With increasing focus on integration of services, gains from PMTCT program investments have also begun to extend beyond the prevention of perinatal HIV transmission to broader improvements in maternal and child survival. These critical developments make it possible to virtually eliminate new pediatric HIV infections globally,1 a goal which seemed inconceivable only a decade ago. In this report, we review the scientific and public health advances made over the past decade and discuss the priority areas that PEPFAR and others will need to support to realize these ambitious PMTCT goals.
THE FRAMEWORK FOR PEPFAR’S PMTCT RESPONSE
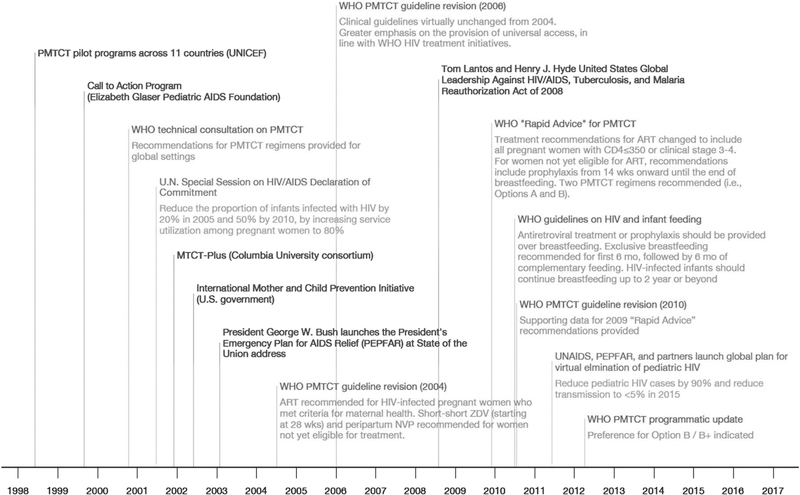
Although several programs—most notably, UNICEF PMTCT pilot programs (UNICEF), Call-to-Action (Elizabeth Glaser Pediatric AIDS Foundation), MTCT-Plus Initiative (Columbia University-led consortium), and the U.S. International Mother and Child HIV Prevention Initiative (US government)—began supporting PMTCT globally before 2003, the launch of PEPFAR accelerated these early efforts. Fifteen “focus countries” (chosen to represent the areas most severely affected by HIV) were supported under the original authorization, an emergency response designed to stem the tide of the epidemic. As part of PEPFAR’s 2008 re-authorization, support expanded to cover 36 countries and regions, as program priorities shifted from an emergency response to one that emphasizes strengthening health systems and fostering sustainability in the context of ongoing scale-up (Fig. 1).
FIGURE 1.
Important policy and program milestones in the PMTCT, 1999–2012. PMTCT, prevention of mother-to-child transmission of HIV.
PEPFAR’s comprehensive PMTCT response is aligned with the 4-pronged strategy articulated by the World Health Organization (WHO) as follows2: (1) prevention of HIV infection among women of childbearing age; (2) prevention of unintended pregnancies among those living with HIV; (3) prevention of HIV transmission from infected mothers to their infants; and (4) treatment, care, and support for infected mothers and children. Beginning in June 2011, PEPFAR’s efforts have been framed around the Global Plan Toward Elimination of New HIV Infections Among Children by 2015 and Keeping Their Mothers Alive.1 Co-led by the Joint United Nations Programme for HIV/AIDS (UNAIDS) and PEPFAR, this initiative seeks to reduce new pediatric infections by 90% and halve HIV-related maternal mortality by 2015.
To reach these goals, PEPFAR has worked closely with partners on multiple levels. First, PEPFAR has partnered with Ministries of Health to support country-led PMTCT efforts, working side-by-side with national staff to build local capacity and plan for program sustainability. Much of this collaborative work is formalized in so-called “Partnership Frameworks” that emphasize the shared goals and mutual responsibility of bilateral commitments.3 Second, PEPFAR has taken a key leadership role in the Global Plan’s implementation, which has included serving as co-chair for the Global Steering Group (with UNAIDS) and as an active partner in the UNICEF/WHO-led Interagency Task Team on Prevention and Treatment of HIV Infection in Pregnant Women, Mothers and Children. Third, PEPFAR has coordinated its activities with the Global Fund for AIDS, Tuberculosis, and Malaria, other donors, and multilateral agencies to minimize programmatic overlap and to maximize efficiencies on the ground. It has also developed active partnerships with the private sector to leverage financial resources and core competencies with an emphasis on laboratory system strengthening, improved commodity distribution, and management capacity building. Fourth, PEPFAR technical teams have helped to develop generic tools (eg, clinical curricula, job aids, and training manuals4) that have been adapted and implemented in many settings. Finally, PEPFAR has provided direct support for PMTCT program scale-up through the provision of technical assistance and financial resources to governments, nongovernmental organizations, and civil society organizations for implementation and evaluation of evidence-based PMTCT interventions. In fiscal years 2009–2011, PEPFAR’s investments in PMTCT totaled over $940 million, with annual budgets increasing each year. This total includes supplemental funds to accelerate PMTCT scale-up in 14 high-burden countries: $100 million in fiscal year 2010 and $180 in fiscal year 2011.
CHALLENGES AND OPPORTUNITIES IN PROGRAM IMPLEMENTATION
Increasing Coverage Through PMTCT Service Scale-Up
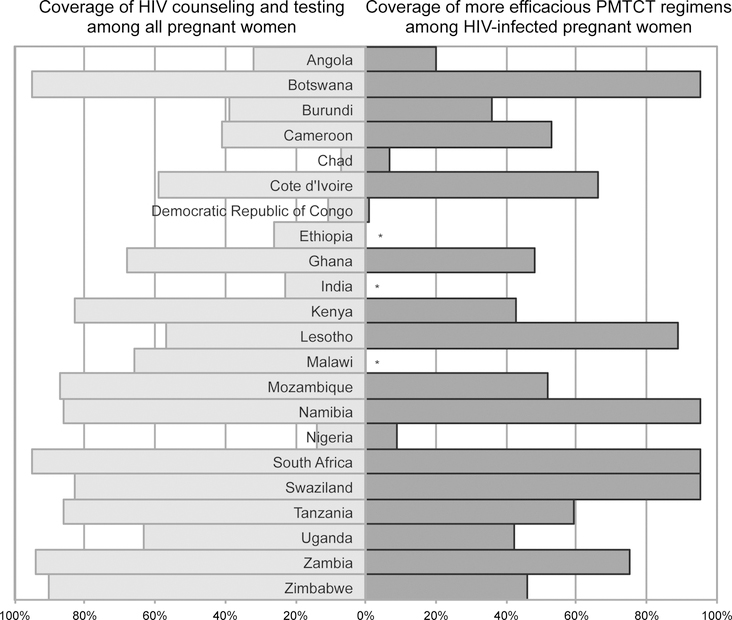
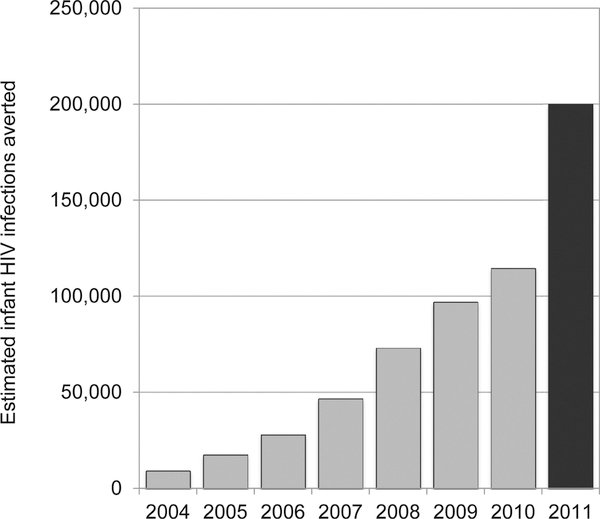
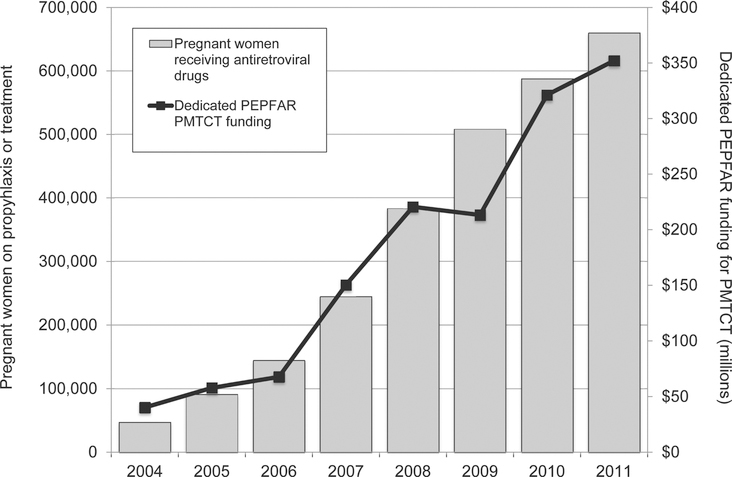
An important focus of most national programs has been the rapid expansion of services, in an effort to maximize population coverage of PMTCT interventions. The congressional authorization for PEPFAR sets explicit coverage targets for HIV testing and counseling of pregnant women (80%) and for providing antiretroviral drugs to those diagnosed with HIV (85%). To date, progress at the national level has varied in the 22 countries prioritized for virtual elimination of mother-to-child transmission (Fig. 2). Given the collaborative nature of PMTCT scale-up–in which PEPFAR is one donor partner among numerous actors—its direct contribution to these coverage targets is difficult to quantify. However, PEPFAR support has resulted in steadily decreasing numbers of new infant HIV infections (Fig. 3), and the program is on track to meet its target of directly supporting the provision of antiretroviral prophylaxis and/or therapy to 1.5 million HIV-infected pregnant women cumulatively in 2012 and 2013 (Fig. 4).
FIGURE 2.
National coverage of HIV testing among all pregnant women and antiretroviral provision among HIV-infected pregnant women in 22 high priority countries for EMTCT in 2010. *Indicates that national coverage estimates not yet available. Adapted from the Joint United Nations Programme on HIV/AIDS (UNAIDS), Global HIV/AIDS update: epidemic update and health sector progress towards universal access, progress report 2011. EMTCT, eliminating mother-to-child HIV transmission.
FIGURE 3.
Modeled estimates of infant HIV infections averted through PEPFAR-supported programs, 2004–2011. Between 2004 and 2010 (shaded grey), modeling was based on the effectiveness of single-dose nevirapine regimen only because disaggregated data regarding prescribed regimens were not routinely collected. Starting in 2011, detailed data about prescribed regimens were collected across all supported countries. The estimate from 2011 (shaded black) takes into account the decreased risk of transmission with more efficacious regimens.
FIGURE 4.
Number of pregnant women placed on antiretroviral prophylaxis or treatment and the corresponding annual program funding across 22 high priority countries for EMTCT, 2004–2011. EMTCT, eliminating mother-to-child HIV transmission.
One critical obstacle to high population coverage is that many women may not seek antenatal services at health institutions and are therefore missed by facility-based interventions. In Ethiopia, for example, an estimated 72% of pregnant women never access antenatal care at a health facility. Even in countries where at least 1 antenatal clinic visit is common—including Zambia (94%), Malawi (92%), and Zimbabwe (90%)—rates of repeat clinic visits and institutional delivery decrease considerably.5 PEPFAR has supported several innovative approaches to increase uptake of PMTCT services in the face of such challenges, including extension of HIV testing into the community and engagement of community health workers and peer supporters to link patients to facilities and retain them in care.6–8 Such strategies can also improve overall maternal and child health, by broadening their scope outside of HIV and linking patients to other health services.
Reducing Mother-to-Child Transmission Through More Efficacious Regimens
Over the past decade, a steady stream of new discoveries has advanced the standards of HIV prophylaxis and treatment for pregnant and lactating women, and their breastfeeding infants. Since the original WHO PMTCT guidelines were published in 2000, the recommended duration and complexity of antiretroviral prophylaxis has steadily increased, with present recommendations now aimed specifically at the discrete antenatal, intrapartum, and postnatal risk periods. Initiating treatment-eligible women on antiretroviral treatment (ART) as early as possible in pregnancy has been incorporated as a priority intervention. With the demonstration of reduced postpartum transmission when mothers or infants are given antiretroviral regimens during breastfeeding,9–14 infant feeding guidance has moved toward longer breastfeeding durations to improve HIV-free survival. Properly implemented, the 2010 WHO guidelines for PMTCT could result in mother-to-child transmission rates of <5%, a figure that moves closer to the accomplishments of North America and Europe.15,16 However, implementation has been imperfect. Fragile health systems that struggled to provide the simplest PMTCT regimens (ie, peripartum single-dose nevirapine) have been asked to provide substantially more complex antiretroviral interventions. In this respect, many countries have grappled with how best to align expansion of PMTCT services to the rapid expansion of HIV treatment. PEPFAR’s investments in health systems—including human resources, supply chain, and laboratory capacity—have led to improved and more efficient clinical systems. Although data are limited, there is evidence that the quality of PMTCT services, including the provision of more efficacious regimens, has improved alongside increasing service coverage.17
Establishing a Continuum of Care: PMTCT to HIV Care and Treatment
PMTCT represents a unique and convenient entry point for HIV care and treatment services, not only for women and their infants but also for sexual partners and other family members. HIV prevalence among antenatal attendees is typically higher than that found in the general population and, when services are available, HIV testing is often accepted at near universal rates.18 The initiation of ART among treatment-eligible women in this context benefits both the mother and infant. Based on data from the Zambia Exclusive Breastfeeding Study (which was conducted before the widespread availability of ART), initiation of ART at the currently recommended CD4 threshold of ≤350 cells per microliter could reduce 88% of perinatal and postnatal infections and 92% of maternal deaths.19 Initiation of ART also has benefits in reducing horizontal HIV transmission to seronegative partners. Several studies have shown that, when their HIV-infected partner initiated ART at the <250 cells per microliter CD4 threshold, the risk of horizontal transmission to HIV-negative index patients was nearly eliminated.20–22 The pivotal HPTN 052 trial demonstrated similar results when ART was started at higher CD4 counts as well. The immediate ART initiation among individuals with CD4 cell counts in the 350–550 cells per microliter range resulted in a dramatic 96% reduction in horizontal HIV transmission among seronegative partners.23
Although the establishment of a continuum of care between PMTCT and HIV treatment services is a recognized priority, it has proven logistically difficult to link the services in many settings. In a review of 4 South African clinics, for example, Stinson et al24 found that only 51% of treatment-eligible patients initiated ART; the remaining received less effective antiretroviral prophylaxis (27%) or no regimen at all (22%). Successful linkages may be constrained by physical distances and transport costs, particularly when provision of ART is restricted to centralized facilities that are separate from antenatal services. Even when services are colocated in the same clinic or hospital, scheduling difficulties and lengthy waiting times may serve as disincentives for seeking care.
These bottlenecks can be at least partially addressed with integrated PMTCT-ART service models. Killam et al25 demonstrated a 2-fold increase in ART initiation among treatment-eligible women when services were integrated in the antenatal setting compared with a referral-based system. However, the absolute uptake within 60 days of CD4 screening was low in both comparison arms (32.9% vs. 14.4%; p<0.05).25 Promising strategies that could further boost performance of these integrated health systems include the incorporation of point-of-care CD4 assays and use of simplified treatment algorithms (eg, Option B or B+; below). Similar approaches could also improve the linkages between early infant diagnosis (which generally occurs in postnatal or well-child clinics, under the purview of PMTCT programs) and pediatric HIV care and treatment services.26 Results from the CHER study demonstrated the significant reduction in mortality associated with immediate initiation of ART for infants infected around the time of birth27; the tragedy of HIV transmission from mother to infant should not be compounded by missed opportunities to improve child survival.
Supporting Innovative But Simplified Policy Approaches to PMTCT
In its 2010 guidelines, the WHO endorsed 2 different strategies that seem to have similar efficacy for PMTCT among breastfeeding mothers not yet eligible for ART (defined as CD4 >350 cells/μL). Both approaches recommend initiation of prophylaxis as early as 14 weeks gestation and continuation until the end of breastfeeding. With “Option A,” HIV-infected pregnant women initiate zidovudine antenatally, receive single-dose nevirapine, zidovudine, and lamivudine during labor, and zidovudine and lamivudine for 1 week postpartum; their exposed infants then receive daily nevirapine through the breastfeeding period (or for 6 weeks if not breastfeeding).9–12 In contrast, “Option B” involves initiation of maternal triple antiretroviral prophylaxis during pregnancy with continuation until breastfeeding stops12–14,28–30; infants receive daily nevirapine or zidovudine for the first 6 weeks of life.
The 2010 WHO guidelines did not indicate a preference for either Option A or Option B, citing them as comparable strategies to be considered at the country level based on cost, feasibility, and acceptability. Certain national guideline modifications, however, have generated increased global interest in the use of maternal triple antiretroviral drug regimens during pregnancy and breastfeeding.31 In the newest Zambian national guidelines, HIV-infected members of serodiscordant couples are considered ART eligible, regardless of their CD4 cell count or disease stage.32 The Malawian Ministry of Health, recently followed by Haiti and Rwanda, have adopted policies to initiate life-long ART for all HIV-infected pregnant women, irrespective of CD4 count, in what has been termed “Option B+.”33,34 Without the need for CD4 screening to determine treatment eligibility—a step associated with significant delay and attrition35—this novel strategy is expected to allow initiation of therapy earlier in gestation and perhaps further reduce transmission rates. The initiation of ART earlier in the course of HIV disease will also reduce the risk of opportunistic infections among index patients and prevent horizontal HIV transmission to discordant partners.23,36 Given known association between maternal survival and infant well being,37,38 such strategies likely have important downstream benefits of reducing infant and child morbidity and mortality as well.
In April 2012, WHO issued a technical update to describe the possible clinical and programmatic advantages to the Option B or B+ approaches for PMTCT, particularly where laboratory infrastructure for antenatal CD4 testing is limited. These benefits include simplification and streamlining of antiretroviral regimens and service delivery, possible harmonization with ART programs, timely initiation of ART among treatment-eligible women, and potential to prevent sexual transmission to serodiscordant partners.39 However, concerns about adherence and retention, health system demands, and diversion of resources away from the sicker patient populations must be carefully studied and systematically addressed. Pharmacovigilance systems are needed to monitor the safety of in utero ART exposures in infants, particularly when efavirenz-based regimens are used. Animal models raised concern of possible teratogenicity with efavirenz in the first trimester. Although available data in humans does not demonstrate an increase in population-level risk, direct comparison has have not been made to unexposed populations. Finally, cost considerations must be carefully deliberated at the country level. Innovative policies like Option B+ have the potential for cost-savings over time, despite the large investments that may be required initially. Their implementation should leverage additional resources for HIV prevention, care, and treatment and not exacerbate funding gaps that may already exist in resource-constrained settings.
THE NEED FOR ROBUST PROGRAM EVALUATION
Evaluating Programs Using the PMTCT Cascade
Despite the numerous clinical advancements over the past decade, there remains a substantial gap between clinical trial efficacy and “real world” program effectiveness. To benefit optimally from proven interventions, patients must successfully navigate a critical path including (but not limited to): being offered and accepting HIV testing, receiving a positive result, being offered and agreeing to appropriate antiretroviral prophylaxis, and adhering to the prescribed regimen.40Ideally, all pregnant women seeking antenatal care would move along this “cascade” without complication; in reality, however, significant attrition occurs at each step. Barker et al41 demonstrated the potential impact of attrition on perinatal HIV transmission using a three-step model. Individuals who successfully navigated the cascade would receive optimal benefit (ie, greatest reduction in transmission risk). Depending on the point of attrition, patients would receive either partial or no benefit from the intervention itself, which would in turn lead to increases in infant HIV transmission at the population level. For example, at full coverage, provision for triple antiretroviral regimens antenatally (for either prophylaxis or treatment) resulted in an estimated 6-week infant HIV transmission of 1.0%. However when the “system efficiency” (ie, retention at each step) dropped to 80%—a more realistic estimate for most programs—infant transmission at the population level climbed to 6.8%.41 Ciaranello et al42 demonstrated similar changes in infant HIV transmission when different model estimates for uptake were incorporated across the antenatal and postpartum PMTCT cascade.
A detailed understanding of the PMTCT cascade—tailored to the local context—could prove useful to national program managers, because the identification of bottlenecks is an important first step for improving performance. Register-based indicators have been used to reconstruct steps along the cascade, but for a variety of reasons these data have typically proven unreliable.43,44 The PEARL Study included a facility-based cord blood surveillance methodology to evaluate nevirapine-based PMTCT programs in Zambia, South Africa, Cote d’Ivoire, and Cameroon.45–47 Its findings were notable for the significant heterogeneity that existed across facilities when the causes of failed coverage were considered. There is unlikely to be a “magic bullet” to improve program performance globally; instead, careful monitoring and quality improvement initiatives are needed, tailored to the specific challenges of each site.
Understanding the Population Impact of PMTCT Programs
Measuring the impact of PMTCT programs at the population level has proven challenging. UNAIDS, PEPFAR, and others have relied heavily on mathematical modeling to estimate trends in pediatric HIV incidence, using coverage rates of PMTCT services and other national indicators of the epidemic.48 Although this methodology has been refined over time, modeling approaches invariably fail to completely incorporate important individual-level covariates, such as maternal immunologic status, timing of initiation, and adherence. These factors contribute significantly to eventual infant outcomes but are largely unavailable at a population level.
Recognizing these limitations, PEPFAR and UN agencies have supported national governments to develop and implement approaches that directly measure the impact of PMTCT programs on HIV transmission rates and HIV-free survival at the population level.49 The South African PMTCT program, for example, completed its first national survey of PMTCT effectiveness on perinatal HIV transmission by testing 4–8 week old infants for HIV exposure and infection at immunization clinics across 9 provinces in 2010. Overall, 3.5% of HIV-exposed infants were perinatally infected at the population level.50 Although the initial evaluation was not designed to measure breastfeeding transmission, the same survey has been repeated in 2011 and 2012, and all HIV-exposed infants are being followed through 18 months of age to assess overall HIV transmission rates (through the breastfeeding period) and HIV-free survival at the population level. PEPFAR-funded projects have also studied the feasibility of community survey approaches to describe population impact of PMTCT programs. The PEARL Study included a community survey component that closely followed the Demographic and Health Surveys regularly conducted in many African countries, with the chief modification of linked maternal and infant HIV testing.40,51 When implemented in 25 communities across the 4 target countries, infant HIV-free survival was found to be 80% at 2 years of life.52 A similar approach has been used in Rwanda to evaluate its national PMTCT program.53 Although these approaches demonstrate great promise—and tools are now being developed to facilitate their implementation on a wider scale—work is needed to establish the accuracy and validity of the health outcome measurements, particularly at the national level.
TOWARD THE ELIMINATION OF NEW PEDIATRIC HIV INFECTIONS
The bold targets for new Global Plan have reframed the policy discussion around PMTCT,1 indicating that ending pediatric HIV is something that can and must be achieved. Although this vision is laudable—and in fact, has leveraged substantial political will and donor investments globally—the question remains as follows: are these goals attainable? Early mathematical models highlight the ambitious nature of these targets. Mahy et al54 used the SPECTRUM model to estimate overall reductions in mother-to-child HIV transmission using several comparison scenarios across 25 countries. Even under the most optimistic assumptions—90% coverage for the 2010 WHO guidelines, 50% reduction of incident HIV infection among all women, fulfillment of all unmet contraceptive need, and restriction of breastfeeding to 12 months—outcomes would still fall short of the goal set for virtual elimination. In this scenario, transmission globally would be 8% and new pediatric HIV infections would number 72,000 in 2015, representing a 79% reduction from 2009 estimates.54 Work by Ciaranello et al42 also estimates that, even with 95% uptake of the WHO guidelines, projected transmission risk with Option A or B (6.1%–7.7%) would still not meet the “virtual elimination” goal of <5%.42 These findings, like those of most mathematical models, are heavily reliant on key assumptions, which carry a degree of imprecision at the national and regional levels. These models also emphasize reductions in new pediatric HIV infections; however, the benefit associated with certain interventions (eg, restricted breastfeeding where nutritional supplementation may be limited) must be carefully weighed against their potential harm. If we are to make significant gains toward the unprecedented goal of virtual elimination—and at the same time maximize maternal and infant survival—a transformative, global approach to PMTCT and maternal-child health will be required.
We call for prioritization of 5 key areas. First, the 2010 WHO PMTCT guidelines must be implemented without delay. Novel strategies for the rapid and safe initiation of ART among eligible pregnant women require urgent evaluation. These may include point-of-care CD4 testing—which can reduce time between specimen collection, results reporting, and regimen initiation55—and treatment strategies (eg, Option B+) that reduce laboratory requirements before ART initiation, simplify regimens, and capitalize on antenatal care as an entry point into life-long care and treatment. (NB: although innovative approaches such Option B+ may remove the need for prerequisite CD4 screening, access to high quality laboratory services remains important for the long-term monitoring of treatment response.) The increased demand for antiretroviral prophylaxis and treatment will require further targeted support for health care infrastructure, and sustained investment in commodity distribution, human resource development, and monitoring and evaluation. Task shifting of routine HIV care and treatment to mid-level providers, and counseling and support services to community cadre or peer counselors, will play important roles in these efforts.56,57 Leveraging synergies between HIV services and key maternal, child, and neonatal care will be necessary to enhance PMTCT access and reduce mortality in these populations.56,57 Programs should also identify and evaluate innovative ways to extend programmatic reach into communities, so groups that do not typically access facility-based care gain access to PMTCT services.
Second, tailored approaches for patient retention and adherence are needed, for both women on prophylaxis and on life-long treatment. Program attrition has been recognized as an important threat to the long-term success of adult HIV treatment,58–60 and early studies suggest that rates of follow-up losses may be higher when women start ART during pregnancy.61,62 In a meta-analysis of 51 studies—including over 20,000 HIV-infected pregnant and postpartum women—74% had adequate adherence (defined as >80%) to their regimen; however, adherence was significantly lower in the postpartum than antepartum period (52% vs. 70%).63 Suboptimal adherence to medications and clinic visits will reduce the effectiveness of prophylaxis or treatment for PMTCT and could even lead to the transmission of resistant virus from mother to infant.64,65 Additionally, women who initiated ART for their own health, but interrupt treatment, face a more than 2-fold increased risk of disease progression.66 Novel models, such as partner-based support and community-based distribution of antiretroviral drugs, should be rigorously evaluated and considered for wider implementation.
Third, we must redouble our efforts to protect parents from HIV acquisition. HIV incidence remains unacceptably high in many settings,67–69 and a new maternal HIV infection during pregnancy poses greater risk for transmission to the infant.70,71 Partner-based HIV testing is an important first step in such efforts. Couples identified as discordant may benefit from several biomedical interventions such as vaginal microbicide prophylaxis, antiretroviral pre-exposure prophylaxis, male circumcision, and ART provided for the dual purposes of treatment and prevention (“treatment as prevention”).23,72–76 We must devise clinical care strategies that incorporate partners if PMTCT is to truly function as an entry point for family-based care.
Fourth, the unmet need for family planning must be addressed globally, particularly among HIV-infected women. Prevention of unintended pregnancies is a highly cost-effective tool for decreasing the pediatric HIV burden and for reducing maternal deaths, both related and unrelated to HIV disease.77–79 Strategies are needed to address barriers to access and to support its integration into HIV care and treatment.80,81 Further research is also needed to elucidate the complicated relationship between certain hormonal contraceptive methods (eg, injectable progestins), HIV acquisition, and HIV disease progression.82 Until definitive data are available, however, the magnitude of benefit from family planning clearly outweighs its potential risk, particularly when used in conjunction with barrier methods (ie, dual method use).
Finally, although access to PMTCT services should remain a goal of programs worldwide, we must recognize the limitations of the simple coverage metric in describing direct health impact. In parallel, countries should focus on promising strategies for measuring infant HIV infection and HIV-free survival at the population level, such as household surveys and/or immunization clinic-based evaluations with long-term infant follow-up. Such population-based methods must be evaluated for acceptability and feasibility, validated in their ability to reliably measure maternal and infant health outcomes, and implemented broadly and regularly to determine gains in PMTCT effectiveness. These methods will be critical to ensuring that interventions aimed at preventing transmission do not negatively impact AIDS-free maternal or child survival, as these are the ultimate goals of a comprehensive PMTCT program.
In conclusion, the incremental scientific discoveries of the past several years have culminated in the development of an evidence-based strategy for combination HIV prevention, one that has been supported by PEPFAR and others.83 Policymakers and program implementers now have a full armamentarium of interventions, including PMTCT, male circumcision, HIV treatment for eligible individuals, and ART for HIV-infected members of serodiscordant couples. If properly coordinated and fully implemented at scale, this “combination prevention package” can dramatically reduce the burden of HIV disease and lead us to an AIDS-free generation. The elimination of new pediatric HIV infections and the improvement of maternal and infant survival are cornerstones of this vision. In collaboration with national governments, multilateral organizations, donors, and civil society, PEPFAR’s sustained commitment and leadership are critical to realizing this ambitious goal in the years to come.
ACKNOWLEDGMENTS
The findings and conclusions included herein are solely the responsibility of the authors and do not necessarily represent the official position of the National Institutes of Health, Centers for Disease Control and Prevention, US Department of Health and Human Services, the Office of US Global AIDS Coordinator, or the US Agency for International Development.
Footnotes
The authors have no other funding or conflicts of interest to disclose.
Various authors have professional relationships with PEPFAR (either as employees of PEPFAR supported US Government agencies or as grantees/contractors) as outlined in the Copyright Transfer Agreement Forms.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the US Government, or the World Health Organization.
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS. Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive, 2011–2015. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/20110609_JC2137_Global-Plan-Elimination-HIV-Children_en.pdf. Accessed April 2, 2012.
- 2.World Health Organization. Strategic approaches to the prevention of HIV infection in infants: reporting a WHO meeting, Morges, Switzerland, March 20–22, 2002. Available at: http://www.who.int/hiv/pub/mtct/en/StrategicApproachesE.pdf. Accessed April 6, 2012. [Google Scholar]
- 3.US President’s Emergency Plan for AIDS Relief. Partnership frameworks. Available at: http://www.pepfar.gov/frameworks/index.htm. Accessed April 7, 2012.
- 4.University of California at San Francisco. Women, children, and HIV resource library. Available at: http://www.womenchildrenhiv.org/wchiv?page=wx-05. Accessed April 10, 2012.
- 5.Unicef. Statistics and monitoring: country statistics. Available at: http://www.unicef.org/statistics/index_countrystats.html. Accessed April 6, 2012.
- 6.Uwimana J, Zarowsky C, Hausler H, et al. Training community care workers to provide comprehensive TB/HIV/PMTCT integrated care in KwaZulu-Natal: lessons learnt. Trop Med Int Health. 2012. In press. [DOI] [PubMed] [Google Scholar]
- 7.Gwarinda S, Mwananyanda L, Pengele M, et al. Using mothers2mothersmentor mothers from communities to improve early infant diagnosis for HIV in urban and rural Zambia [abstract CDD172]. Paper presented at: 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 17–20, 2011; Rome, Italy. [Google Scholar]
- 8.Mwananyanda L, Pengele M, Chikampa D, et al. Mothers2mothers: role of trained lay community health workers in preventing mother to child transmission of HIV in Zambia [abstract CDD147]. Paper presented at: 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 17–20, 2011; Rome, Italy. [Google Scholar]
- 9.Bedri A, Gudetta B, Isehak A, et al. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–313. [DOI] [PubMed] [Google Scholar]
- 10.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008; 359:119–129. [DOI] [PubMed] [Google Scholar]
- 11.Coovadia HM, Brown ER, Fowler MG, et al. Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomised, double-blind, placebo-controlled trial. Lancet 2012;379:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362: 2271–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Vincenzi I Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–180. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362: 2282–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Townsend CL, Cortina-Borja M, Peckham CS, et al. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS 2008;22:973–981. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC). Achievements in public health. Reduction in perinatal transmission of HIV infection–United States, 1985–2005. MMWR Morb mortal Wkly Rep. 2006;55: 592–597. [PubMed] [Google Scholar]
- 17.Joint United Nations Programme on HIV/AIDS (UNAIDS). Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access, Progress Report 2011. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 18.Bolu OO, Allread V, Creek T, et al. Approaches for scaling up human immunodeficiency virus testing and counseling in prevention of mother-to-child human immunodeficiency virus transmission settings in resource-limited countries. Am J Obstet Gynecol. 2007;197: S83–S89. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn L, Aldrovandi GM, Sinkala M, et al. Potential impact of new WHO criteria for antiretroviral treatment for prevention of mother-to- child HIV transmission. AIDS. 2010;24:1374–1377. [PMC free article] [PubMed] [Google Scholar]
- 20.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds SJ, Makumbi F, Nakigozi G, et al. HIV-1 transmission amongHIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Romero J, Castilla J, Hernando V, et al. Combined antiretroviral treatment and heterosexual transmission of HIV-1: cross sectional and prospective cohort study. BMJ 2010;340:c2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stinson K, Boulle A, Coetzee D, et al. Initiation of highly active antiretroviral therapy among pregnant women in Cape Town, South Africa. Trop Med Int Health. 2010;15:825–832. [DOI] [PubMed] [Google Scholar]
- 25.Killam WP, Tambatamba BC, Chintu N, et al. Antiretroviral therapy in antenatal care to increase treatment initiation in HIV-infected pregnant women: a stepped-wedge evaluation. AIDS 2010;24:85–91. [DOI] [PubMed] [Google Scholar]
- 26.Ciaranello AL, Park JE, Ramirez-Avila L, et al. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359: 2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palombi L, Marazzi MC, Voetberg A, et al. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS. 2007;21(suppl 4): S65–S71. [DOI] [PubMed] [Google Scholar]
- 29.Thomas TK, Masaba R, Borkowf CB, et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding–the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8:e1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilewo C, Karlsson K, Ngarina M, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52:406–416. [DOI] [PubMed] [Google Scholar]
- 31.Panel on Antiretroviral Guidelines for Adults and. Adolescents, U.S. Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents, March 27, 2011 revision. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed June 16, 2012.
- 32.Zambian Ministry of Health. ART Program Update, December 2010. Lusaka, Zambia: Printech Press; 2010. [Google Scholar]
- 33.Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet 2011;378:282–284. [DOI] [PubMed] [Google Scholar]
- 34.Zolfo M, De Weggheleire A, Schouten E, et al. Time for “test and treat” in prevention of mother-to-child transmission programs in low- and middleincome countries. J Acquir Immune Defic Syndr. 2010;55:287–289. [DOI] [PubMed] [Google Scholar]
- 35.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grinsztejn B, Ribaudo H, Cohen MS, et al. Effects of early versus delayed initiation of antiretroviral therapy (ART) on HIV clinical outcomes: results from the HPTN 052 randomized clinical trial [abstract MOAX0105]. Paper presented at: 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 17–20, 2011; Rome, Italy. [Google Scholar]
- 37.Crampin AC, Floyd S, Glynn JR, et al. The long-term impact of HIV and orphanhood on the mortality and physical well-being of children in rural Malawi. AIDS. 2003;17:389–397. [DOI] [PubMed] [Google Scholar]
- 38.Mermin J, Were W, Ekwaru JP, et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. Lancet. 2008;371:752–759. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Use of antiretroviral drugs for treating pregnant women and prevention HIV infection in infants: executive summary, April 2012. Available at: http://www.who.int/hiv/PMTCT_update.pdf. Accessed April 5, 2012.
- 40.Stringer EM, Chi BH, Chintu N, et al. Monitoring effectiveness of programmes to prevent mother-to-child HIV transmission in lower-income countries. Bull World Health Organ. 2008;86:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barker PM, Mphatswe W, Rollins N. Antiretroviral drugs in the cupboard are not enough: the impact of health systems’ performance on mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011;56:E45–E48. [DOI] [PubMed] [Google Scholar]
- 42.Ciaranello AL, Perez F, Keatinge J, et al. What will it take to eliminate pediatric HIV? Reaching WHO target rates of mother-to-child HIV transmission in Zimbabwe: a model-based analysis. PLoS Med. 2012;9:e1001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferguson L, Grant AD, Ong’ech JO, et al. Prevention of mother-to-child transmission of HIV: assessing the accuracy of routinely collected data on maternal antiretroviral prophylaxis coverage in Kenya. Sex Transm Infect. 2012;88:120–124. [DOI] [PubMed] [Google Scholar]
- 44.Mate KS, Bennett B, Mphatswe W, et al. Challenges for routine health system data management in a large public programme to prevent motherto-child HIV transmission in South Africa. PLoS One. 2009;4:e5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stringer EM, Ekouevi DK, Coetzee D, et al. Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA. 2010;304:293–302. [DOI] [PubMed] [Google Scholar]
- 46.Coffie PA, Kanhon SK, Toure H, et al. Nevirapine for the prevention of mother-to-child transmission of HIV: a nation-wide coverage survey in Cote d’Ivoire. J Acquir Immune Defic Syndr. 2011;57(suppl 1):S3–S8. [DOI] [PubMed] [Google Scholar]
- 47.Stinson K, Boulle A, Smith PJ, et al. Coverage of the prevention of mother-to-child transmission programme in the Western Cape, South Africa using cord blood surveillance. J Acquir Immune Defic Syndr. 2012;60:199–204. [DOI] [PubMed] [Google Scholar]
- 48.Joint United Nations Programme on HIV/AIDS. Spectrum/EPP 2011. Available at: http://www.unaids.org/en/dataanalysis/tools/spectrumepp2011/ Accessed April 10, 2012.
- 49.Hayashi C International guidance on methods to measure PMTCT impact [Session SUSA15]. Paper presented at: 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 17–20, 2011; Rome, Italy. [Google Scholar]
- 50.Goga AE, Dinh TH, Jackson DJ, for the SAPMTCTE study group. Evaluation of the effectiveness of the national prevention of mother-to-child transmission (PMTCT) programme measured at six weeks postpartum in South Africa, 2010. Available at: http://www.doh.gov.za/docs/reports/2012/pmtcteffectiveness.pdf. Accessed June 16, 2012.
- 51.Anonymous. Measure DHS: Demographic and Health Surveys. Available at: http://www.measuredhs.com. Accessed May 30, 2006.
- 52.Tih P, Coetzee D, Ekouevi DK, et al. Population HIV-free survivalamong HIV-exposed children in four African countries: the PEARL community survey [abstract TUAB0205]. Paper presented at: 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 17–20, 2011; Rome, Italy. [Google Scholar]
- 53.Ruton H, Mugwaneza P, Shema N, et al. HIV-free survival among nine-to 24-month-old children born to HIV-positive mothers in the Rwandan national PMTCT programme: a community-based household survey. J Int AIDS Soc. 2012;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahy M, Stover J, Kiragu K, et al. What will it take to achieve virtual elimination of mother-to-child transmission of HIV? An assessment of current progress and future needs. Sex Transm Infect. 2010;86(suppl 2): ii48–ii55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jani IV, Sitoe NE, Alfai ER, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011; 378:1572–1579. [DOI] [PubMed] [Google Scholar]
- 56.Youngleson MS, Nkurunziza P, Jennings K, et al. Improving a mother tochild HIV transmission programme through health system redesign: quality improvement, protocol adjustment and resource addition. PLoS One. 2010;5:e13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nabudere H, Asiimwe D, Mijumbi R. Task shifting in maternal and child health care: an evidence brief for Uganda. Int J Technol Assess Health care. 2011;27:173–179. [DOI] [PubMed] [Google Scholar]
- 58.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15(suppl 1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myer L, Cornell M, Fox M, et al. Loss to follow-up and mortality among pregnant and non-pregnant women initiating ART: South Africa[abstract 22]. Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; March 5–8, 2012; Seattle, WA. [Google Scholar]
- 62.Clouse K, Maskew M, Bassett J, et al. Delayed diagnosis of HIV and high rates of lost to follow-up among pregnant women attending antenatal services at a primary health clinic: Johannesburg, South Africa [abstract 1004]. Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012; Seattle, WA. [Google Scholar]
- 63.Nachega J, Uthman C, Mills E, et al. Adherence to antrietroviral therapy(ART) during and after pregnancy in low, middle, and high income countries: a systematic review and meta-analysis [abstract 1006]. Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; March 5–8, 2012; Seattle, WA. [Google Scholar]
- 64.Fogel J, Li Q, Taha TE, et al. Initiation of antiretroviral treatment in women after delivery can induce multiclass drug resistance in breastfeeding HIV-infected infants. Clin Infect Dis. 2011;52:1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dross S, Beck I, Micek M, et al. Transmission of NVP-resistant HIV-1 to infants by breastfeeding observed after maternal sdNVP [abstract 917]. Paper presented at: 17th Conference on Retroviruses and Opportunistic Infections; 2010; San Francisico, CA. [Google Scholar]
- 66.Fixed duration interruptions are inferior to continuous treatment in African adults starting therapy with CD4 cell counts < 200 cells/microl. AIDS. 2008;22:237–247. [DOI] [PubMed] [Google Scholar]
- 67.Moodley D, Esterhuizen TM, Pather T, et al. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009;23: 1255–1259. [DOI] [PubMed] [Google Scholar]
- 68.Kieffer MP, Nhlabatsi B, Mahdi M, et al. Improved detection of incident HIV infection and uptake of PMTCT services in labor and delivery in a high HIV prevalence setting. J Acquir Immune Defic Syndr. 2011;57: E85–E91. [DOI] [PubMed] [Google Scholar]
- 69.Kharsany AB, Hancock N, Frohlich JA, et al. Screening for ‘window-period’ acute HIV infection among pregnant women in rural South Africa. HIV Med. 2010;11:661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taha TE, James MM, Hoover DR, et al. Association of recent HIV infection and in-utero HIV-1 transmission. AIDS 2011;25:1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Humphrey JH, Marinda E, Mutasa K, et al. Mother to child transmission of HIV among Zimbabwean women who seroconverted postnatally: prospective cohort study. BMJ. 2010;341:C6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329: 1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010; 363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Auvert B, Taljaard D, Lagarde E, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. [DOI] [PubMed] [Google Scholar]
- 76.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007; 369:657–666. [DOI] [PubMed] [Google Scholar]
- 77.Sweat MD, O’Reilly KR, Schmid GP, et al. Cost-effectiveness of nevirapine to prevent mother-to-child HIV transmission in eight African countries. AIDS. 2004;18:1661–1671. [DOI] [PubMed] [Google Scholar]
- 78.Reynolds HW, Janowitz B, Wilcher R, et al. Contraception to prevent HIV-positive births: current contribution and potential cost savings in PEPFAR countries. Sex Transm Infect. 2008;84(suppl 2): ii49–ii53. [DOI] [PubMed] [Google Scholar]
- 79.Halperin DT, Stover J, Reynolds HW. Benefits and costs of expanding access to family planning programs to women living with HIV. AIDS. 2009;23(suppl 1):S123–S130. [DOI] [PubMed] [Google Scholar]
- 80.Chibwesha CJ, Li MS, Matoba CK, et al. Modern contraceptive and dual method use among HIV-infected women in Lusaka, Zambia. Infect Dis Obstet Gynecol. 2011;2011:261453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rutenberg N, Baek C. Field experiences integrating family planning into programs to prevent mother-to-child transmission of HIV. Stud Fam Plann. 2005;36:235–245. [DOI] [PubMed] [Google Scholar]
- 82.World Health Organization. Hormonal contraception and HIV: technical statement, 16 February 2012. Available at: http://whqlibdoc.who.int/hq/2012/WHO_RHR_12.08_eng.pdf. Accessed April 2, 2012. [PubMed]
- 83.US Department of State. Hillary Rodham Clinton, Remarks on “Creatingan AIDS-Free Generation,” National Institutes of Health’s Masur Auditorium; 2011. Available at: http://www.state.gov/secretary/rm/2011/11/176810.htm. Accessed April 7, 2012. [Google Scholar]