Abstract
The 6 kDa early secreted antigen target (ESAT6) is a low molecular weight and highly immunogenic protein of Mycobacterium tuberculosis with relevance in the diagnosis of tuberculosis and subunit vaccine development. The gene encoding the ESAT6 protein is located in the M. tuberculosis-specific genomic region known as the region of difference (RD)1. There are 11 M. tuberculosis-specific RDs absent in all of the vaccine strains of BCG, and three of them (RD1, RD7, and RD9) encode immunodominant proteins. Each of these RDs has genes for a pair of ESAT6-like proteins. The immunological characterizations of all the possible proteins encoded by genes in RD1, RD7 and RD9 have shown that, besides ESAT-6 like proteins, several other proteins are major antigens useful for the development of subunit vaccines to substitute or supplement BCG. Furthermore, some of these proteins may replace the purified protein derivative of M. tuberculosis in the specific diagnosis of tuberculosis by using interferon-gamma release assays and/or tuberculin-type skin tests. At least three subunit vaccine candidates containing ESAT6-like proteins as antigen components of multimeric proteins have shown efficacy in phase 1 and phase II clinical trials in humans.
Keywords: M. tuberculosis, RD genomic segments, ESAT6-like proteins, diagnosis, vaccine
1. Introduction
Using the Bacillus Calmette Guerin (BCG) vaccine against tuberculosis (TB) has shown variable protective efficacy in different parts of the world [1,2,3,4,5]. In particular, BCG vaccination does not protect against pulmonary tuberculosis in adults [6,7,8,9,10,11], which is the major manifestation of the disease in humans as 85% of TB patients have pulmonary disease [12,13,14]. Since BCG has antigens cross-reactive with Mycobacterium tuberculosis and the non-tuberculous environmental mycobacteria, the low efficacy or the lack of protection by BCG vaccination is suggested to be due to masking and/or blocking effects [15,16,17,18]. According to the masking hypothesis, an early sensitization with environmental mycobacteria provides some degree of protection against TB that masks the effect of the BCG vaccine due to the presence of cross-reactive antigens [19,20]. Furthermore, the immune responses induced by cross-reactive antigens due to exposure to environmental mycobacteria lead to an early clearance of antigens in the BCG vaccine, which prevents an effective immune response from being generated, rendering it a failure, and hence causing a blocking effect [21,22,23,24]. The use of M. tuberculosis-specific antigens as subunit vaccines is expected to overcome the problems of blocking or masking effects [25,26,27,28].
BCG vaccination also faces a problem in the diagnosis of TB by using the widely used antigenic preparation of M. tuberculosis known as the purified protein derivative (PPD), in the tuberculin skin test, due to the presence of the cross-reactive antigens [28,29,30,31,32,33,34]. It is expected that M. tuberculosis-specific antigens may overcome the problem of diagnostic inaccuracy associated with the use of PPD in BCG-vaccinated people [35,36,37,38,39,40]. Hence, studies have been conducted to identify M. tuberculosis-specific antigens with vaccine and diagnostic potentials [41,42,43,44,45,46,47].
An M. tuberculosis-specific antigen was identified for the first time from the short-term culture filtrates of M. tuberculosis and was designated as the early secreted antigenic target of molecular mass 6 kDa (ESAT6) [48]. Immunological studies with ESAT6, biochemically purified from the short-term culture filtrates of M. tuberculosis, showed that it was a major antigen of M. tuberculosis recognized by T cells from mice infected with M. tuberculosis [49]. These results were further confirmed by using the recombinant antigen and synthetic peptides of ESAT6 [50,51,52,53,54,55,56]. ESAT6 also had epitopes recognized by B cells and antibodies [57]. Studies using overlapping synthetic peptides covering the entire sequence of ESAT6 also identified it as a major T cell antigen [58,59,60,61]. The results further showed that ESAT6 contained multiple T cell epitopes [62,63,64], which were recognized by T cells in association with several human leukocyte antigen (HLA) class II molecules that are frequently expressed in humans living in different countries and geographical locations [65,66]. These results suggested that ESAT6 could be a universally useful antigen in a subunit vaccine development and/or in diagnostic applications, and its use will not be limited due to variations in the expression of HLA molecules in different population groups [65]. Further studies confirmed the potential of ESAT6 for the specific diagnosis of TB [67,68,69,70], and its potential in the development of new subunit vaccines, either alone or in combination with other cross-reactive antigens [71,72,73,74,75].
To identify additional M. tuberculosis antigens and genomic regions, a subtractive genome hybridization approach was used by Mahairas et al. [76]. They identified three regions of differences (RDs), i.e., RD1, RD2, and RD3 between M. tuberculosis and BCG, and predicted genes in these regions for encoding 11 proteins from RD1, 13 proteins from RD2, and 12 proteins from RD3 [76]. Further analysis showed that RD1 and RD3 were absent in all BCG strains, whereas RD2 was absent from some BCG strains but present in others [76]. However, RD3 was also absent from most clinical isolates of M. tuberculosis [76]. Hence the antigens encoded by RD3 will not have any value in the vaccine or diagnostic applications. Among the proteins encoded by RD2, MPT64 (Rv1980c) has been identified as a dominant antigen having multiple epitopes and being presented to T cells in association with several HLA class II molecules [77,78,79,80,81]. Furthermore, MPT64 has been shown to have vaccine potential in animals either alone [82], or in combination with other M. tuberculosis antigens [83,84,85,86], However, MPT64 may not be useful in the specific diagnosis of TB because several BCG strains used for vaccination of people in different parts of the world express this antigen [87,88].
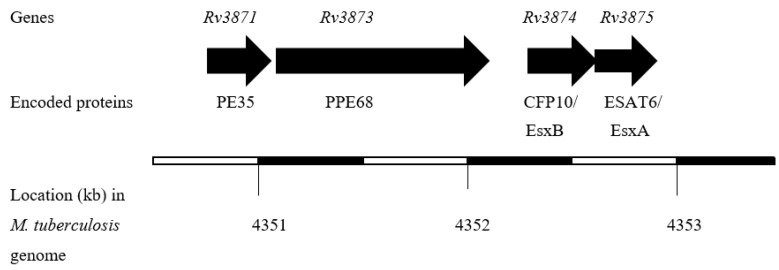
The proteins encoded by RD1 are considered more promising in vaccine applications because this region is present in all clinical M. tuberculosis isolates [89,90,91,92]. Furthermore, RD1 is absent in all sub-strains of BCG [93,94,95,96] because of its deletion during the attenuation of the parent BCG strain obtained by prolonged sub-culturing of pathogenic M. bovis in an artificial medium [97], and hence the application of RD1-encoded immunodominant antigens in the diagnosis of TB is not expected to have any effect due to BCG vaccination [98,99,100,101]. Interestingly, the ESAT6 gene is located in RD1, and the gene for another low molecular weight and immunodominant protein, known as the 10 kDa culture filtrate protein (CFP10), is also present in the RD1 region [102]. The genes for ESAT6 and CFP10 are located in close proximity in M. tuberculosis genome (Figure 1), and the two proteins are secreted as dimers [103]. Both of these proteins require the ESAT6 (ESX)-1 secretion system in order to be transported out of bacterial cells as dimers [104,105,106].
Figure 1.
The organization of the CFP10 and ESAT6 genes in M. tuberculosis genome.
The availability of the complete genome sequence of M. tuberculosis [107] and its comparison with BCG identified a total of 10 RDs, other than RD1 (Table 1), which are deleted in all strains of BCG that are being used for vaccination against TB in different parts of the world [108,109].
Table 1.
M. tuberculosis genomic regions deleted in all strains of Bacillus Calmette Guerin (BCG) and annotations of deleted genes in the lab-adopted virulent M. tuberculosis strain H37Rv.
| Region Deleted (RD) a | Annotations (Rv nos.) of Deleted Genes b |
|---|---|
| RD1 | Rv3871-Rv3879c |
| RD4 | Rv0221-Rv0223c |
| RD5 | Rv3117-Rv3121 |
| RD6 | Rv1506c-Rv1516c |
| RD7 | Rv2346c-Rv2353c |
| RD9 | Rv3617-Rv3623 |
| RD10 | Rv1255c-Rv1257c |
| RD11 | Rv3425-Rv3429 |
| RD12 | Rv2072c-Rv2075c |
| RD13 | Rv2645–Rv2660c |
| RD15 | Rv1963c-Rv1977 |
The experiments with the peptide pools of proteins encoded by all M. tuberculosis-specific RDs for determination of immunological reactivity showed that RD1, RD7, and RD9 contained the immunodominant antigens recognized by T cells from TB patients [110,111,112,113,114,115]. Further analysis of individual proteins encoded by genes present in RD1, RD7, and RD9 identified several major protein antigens of M. tuberculosis [116,117,118,119]. In this review, all the individual proteins encoded by the genes present in RD1, RD7, and RD9 have been identified and analyzed for their putative roles, including their immunological applications in the diagnosis of TB and vaccine developments.
2. RD1 Genes and Encoded Proteins
The RD1 region contains the genes for EsxA (ESAT6) and EsxB (CFP10) along with 11 other M. tuberculosis-specific genes predicted by Robertson and Thole corresponding to the open reading frames (ORFs) known as ORF2 to ORF14 [120], seven genes predicted by Mahairas et al. and designated as ORF1A to ORF1K [76], and nine genes predicted by Cole et al. and designated as Rv3871 to Rv3879 in M. tuberculosis H37Rv genome [107] (Table 2).
Table 2.
Gene annotation, gene name, and description of proteins encoded by genes in RD1.
| Amoudy et al. [120] | Mahairas et al. [76] | Cole et al. [107] | Gene Name | Description of Proteins |
|---|---|---|---|---|
| ORF2 | ORF1A | Rv3871 | Rv3871 | 591 aa, Probable conserved hypothetical protein |
| ORF3 | NP | Rv3872 | PE35 | 99 aa, PE family-related protein |
| ORF4 | NP | NP | orf4 | 139 aa, Hypothetical protein |
| ORF5 | ORF1B | Rv3873 | PPE68 | 368 aa, PPE family protein |
| ORF6 | NP | Rv3874 | esxB | 100 aa, 10 kDa culture filtrate antigen EsxB (LHP, CFP10, MTSA10) |
| ORF7 | ORF1C | Rv3875 | esxA | 95 aa, 6 kDa early secretory antigenic target EsxA (ESAT6) |
| ORF8 | NP | NP | orf8 | 140 aa, Hypothetical protein |
| ORF9 | ORF1D | Rv3876 | Rv3876 | 668 aa, Conserved hypothetical proline and alanine rich protein |
| ORF10 | ORF1E | Rv3877 | Rv3877 | 511 aa, Probable conserved transmembrane protein |
| ORF11 | ORF1F | Rv3878 | Rv3878 | 281 aa, Conserved hypothetical alanine-rich protein |
| ORF12 | ORF1G | none | orf12 | 564 aa, Hypothetical protein |
| ORF13 | ORF1K | Rv3879 | Rv38789 | 729 aa, Hypothetical alanine and proline rich protein |
| ORF14 | NP | NP | orf14 | 263 aa, Hypothetical protein, recognized by antibodies present in sera of TB patients |
| ORF15 | NP | NP | orf15 | 96 aa, Hypothetical protein |
NP = Not predicted.
The analysis of ORF genes for expression at mRNA and/or protein levels showed that at least 12/13 of them were expressed in M. tuberculosis [121,122,123,124,125,126]. In addition to ESAT6 (EsxA) and CFP10 (EsxB), Rv3871, PE35, PPE68, Rv3878, and Rv3879c have also been identified as major T cell antigens [127,128,129,130,131,132,133,134,135,136,137,138,139,140]. Concerning the functions, EsxA and EsxB are associated with deactivation of macrophage and dendritic cell functions and are involved in the virulence of M. tuberculosis [141,142,143,144,145]. Among the RD1 proteins, Rv3871, Rv3872 (PE35), Rv3873 (PPE68), ESAT6 (EsxA), CFP10 (EsxB) and Rv3879c have been suggested for use in the diagnosis of TB using T cell assays [146,147,148,149]. In fact, commercial tests developed to diagnose TB using interferon-gamma release assays include a cocktail of antigens including ESAT6 and CFP10 [150,151,152,153,154]. Furthermore, several RD1 antigens, i.e., ESAT6, CFP10, Rv3871, Rv3872 (PE35), Rv3873 (PPE68), Rv3872, Rv3876 Rv3879, and ORF14 have also been suggested for use in antibody assays for the specific diagnosis of TB [155,156,157,158,159,160,161,162,163]. Moreover, RD1 antigens have also been tested in tuberculin type response with encouraging results [164,165,166,167,168,169,170,171].
In addition to the role of the whole RD1 genomic segment in protective immunity [172,173], the evaluations of individual RD1 proteins for vaccine development in animals have shown the potential of PE35, PPE68, ESAT6, and CFP10 in the development of new subunit vaccines for TB [174,175,176,177,178,179,180,181]. Animal experiments with recombinant (r)BCG strains expressing RD1 antigens have shown the induction of protective type immune responses and protected the immunized animals after challenges with M. tuberculosis [182,183,184,185,186]. Furthermore, a rBCG vaccine candidate containing Ag85B, ESAT6, and CFP10 (GamTBvac) showed a strong protective effect as a BCG booster vaccine in mice and guinea pigs [187]. Phase 1 and Phase 2 clinical trials with GamTBvac have been conducted. In both types of clinical trials, the results after vaccination with GamTBvac showed that the vaccine had an acceptable safety profile and induced markers of protective immunity, i.e., antigen-specific interferon gamma release, Th1 cytokine-expressing CD4+ T cells, and IgG responses [188,189]. These results support further clinical evaluation of GamTBvac in Phase 3 trials to evaluate its efficacy in protecting against infection with M. tuberculosis and the development of the clinical disease.
Two other subunit vaccine candidates which have undergone clinical trials in humans are H1:IC31 and H56:IC31 [190,191]. The subunit vaccine H1:IC31 contains a fusion of Ag85B and ESAT6 and is given along with the adjuvant IC31 [192,193]. H56:IC31 contains the RD1 antigen ESAT6 and two other M. tuberculosis proteins, Ag85B and Rv2660c, as a fusion protein, and it is used for immunization along with the adjuvant IC31 [193]. Both Ag85B and Rv2660c are major antigens of M. tuberculosis and are cross-reactive with BCG and other mycobacteria [194,195,196]. The subunit vaccines H1:IC31 and H56:IC31 have induced protective type cellular immune responses in animals and protected them upon being challenged with the virulent M. tuberculosis [193,197,198].
Humans vaccinated with H1:IC31 vaccine did not show local or systemic adverse effects except transient soreness at the injection site, but there was induction of strong antigen-specific T cell responses, which persisted through 30 months of follow-up. This indicated the activation of a substantial memory response in the vaccinated subjects [199]. The H1:IC31 vaccine was also safe and well tolerated in HIV-infected adults with a CD4+ lymphocyte count greater than 350 cells/mm3 and induced a specific and long-lasting Th1 immune response [200]. The H1:IC31 vaccine was further tested in a phase 1, open-label trial in people living in a TB-endemic area. Healthy male participants aged 18–25 years were recruited into four groups. Participants in group 1 and group 2 were Tuberculin Skin Test (TST) negative and QuantiFERON-TB Gold in-tube test (QFT) negative (Mtb-naïve groups), participants in group 3 were TST positive and QFT negative (BCG group), and participants in group 4 were both TST and QFT positive (Mtb-infected group). The results showed that the vaccine was safe and generally well tolerated [201]. Immunogenicity assays showed a stronger response to TB antigens in the Mtb-naïve group [201]. H1:Ic31 has also undergone a phase 2 clinical trial in 240 healthy adolescents in South Africa including both M. tuberculosis-infected and non-infected subjects [202]. No noticeable safety events were observed in any group irrespective of the doses or vaccination schedule used [202]. Furthermore, the vaccine induced antigen-specific CD4+ T cell responses of protective phenotype in both the groups [202].
A double blind, placebo-controlled, dose selection trail in humans for dose optimization of H56:Ic31 in a tuberculosis-endemic population showed that two or three vaccinations at the lowest dose induced long-lasting antigen-specific CD4 T cell responses with acceptable safety profiles in both naïve and M. tuberculosis-infected subjects [203]. A phase 1b randomized study with the H56:IC31 vaccine showed that the vaccine had acceptable safety profiles in M. tuberculosis-uninfected adults and induced immunizing antigen-specific cellular and humoral immune responses [204]. This vaccine candidate is now being tested in phase 2a clinical trials, and recruitment has started for the phase 2b clinical trial [190].
3. RD7 Genes and Encoded Proteins
The M. tuberculosis-specific genomic region RD7 contains eight ORFs, and an equal number of genes are annotated in the M. tuberculosis H37Rv genome [205] (Table 3).
Table 3.
Open reading frame (ORF) code, gene annotation, gene name and description of proteins encoded by genes in RD7.
| ORF Code | Rv Gene Annotation | Gene Name | Description of Proteins |
|---|---|---|---|
| ORF1 | Rv2346c | EsxO | 94 aa, ESXO, MTB9.9E, ESAT6-like protein 6 |
| ORF2 | Rv2347c | EsxP | 98 aa, ESXP, QILSS, ESAT6-like protein 7 |
| ORF3 | Rv2348c | Rv2348c | 108 aa, Rv2348c, hypothetical unknown protein |
| ORF4 | Rv2349c | plcC | 508 aa, PLCC, probable phospholipase C 3 |
| ORF5 | Rv2350c | plcB | 512 aa, PLCB, probable membrane-associated phospholipase C 2 |
| ORF6 | Rv2351c | plcA | 512 aa, PPLCA, MTP40 antigen, probable membrane-associated phospholipase C 1 |
| ORF7 | Rv2352c | PPE38 | 392 aa, PPE38, PPE family protein |
| ORF8 | Rv2353c | PPE39 | 354 aa, PPE39, PPE family protein |
In human studies with TB patients, the mixture of peptides corresponding to all eight proteins showed that RD7 contains immunodominant antigens stimulating the immune response with a Th1-bias [110,111,114]. Two of the proteins encoded by genes in RD7 belong to the ESAT6 family and are known as EsxO (Rv2346c) and EsxP (Rv2347c) (Table 3). In tuberculin positive reactor cattle, EsxO (Rv2346c) and EsxP (Rv2347c) induced significant IFN-γ responses in vitro [206]. Further experiments with respect to the diagnostic value of ESAT6-like proteins showed that 57% TB reactor cattle responded to EsxO (Rv2346c) peptides in IFN-γ assays, without inducing positive responses in any of the BCG-vaccinated animals [207]. By using human T cell clones and a synthetic peptide library consisting of 15-mers overlapping by 11 aa, Lewinsohn et al. have shown that Rv2347c is an antigen capable of stimulating IFN-γ secreting CD8+ T cells [208]. In mice, immunizations with EsxO (Rv2346c) and EsxP (Rv2347c) using different delivery systems, i.e., chemical adjuvants, mycobacteria and naked plasmids, showed that both of these antigens induced protective Th1 responses but none of them induced pathologic Th2, Th17 and T regulatory cell responses [209]. However, none of these antigens have been tested in the diagnosis of TB in humans or in vaccine development.
4. RD9 Genes and Encoded Proteins
The M. tuberculosis-specific genomic region of RD9 contains seven ORFs and all of them are annotated as genes in the M. tuberculosis H37Rv genome [205] (Table 4).
Table 4.
ORF code, gene annotation, gene name and description of proteins encoded by genes in RD9.
| ORF Code | Rv Gene Annotation | Gene Name | Description of Proteins |
|---|---|---|---|
| ORF1 | Rv3617 | ephA | 322 aa, EPHA, probable epoxide hydrolase, (Epoxide hydratase) (Arene-oxide hydratase) |
| ORF2 | Rv3618 | Rv3618 | 395 aa, Rv3618, possible monooxygenase |
| ORF3 | Rv3619c | esxV | 94 aa, EsxV, ESAT6 family protein |
| ORF4 | Rv3620c | esxW | 98 aa, EsxW, ESAT6 family protein |
| ORF5 | Rv3621c | PPE65 | 414 aa, PPE65, PPE-family protein |
| ORF6 | Rv3622c | PE32 | 99 aa, PE32, PE family protein |
| ORF7 | Rv3623 | lpqG | 240 aa, LPQG, probable conserved lipoprotein |
The immunological evaluation of RD9 proteins using synthetic peptides showed that this region also encodes immunodominant proteins [110,111,114]. Among the RD9 proteins are included two ESAT6 family proteins, i.e., Rv3619c (EsxV) and Rv3620c (EsxW) (Table 4). Molecular modeling and docking studies predicted that the structure of Rv3619c-Rv3620c was similar to that of ESAT6-CFP10 [210]. Immunization with Rv3619c and/or Rv3620c proteins, either alone or in combination with other M. tuberculosis proteins, induced antigen-specific humoral and cellular immune responses in mice [174,175,210,211]. Immunizations of mice with Rv3619c protected against a challenge with M. tuberculosis [212], and allergic asthma [213].
A multiprotein vaccine candidate, ID93, containing Rv2608, Rv3619c, Rv3620c, and Rv1813 M. tuberculosis antigens, combined with synthetic toll-like receptor 4 (TLR4) agonist glucopyranosyl lipid adjuvant (GLA) in a stable nano-emulsion (SE) has been developed and is known as ID93/GLA-SE [214]. Immunization of mice with ID93/GLA-SE did not induce sensitivity to the proteins present in PPD, hence it may not compromise the diagnostic efficacy of PPD in the diagnosis of TB [215]. In contrast, positive delayed-type hypersensitivity reactions to ID93 and its components were induced in ID93/GLA-SE-immunized animals, which indicated the induction of strong but specific cellular immune responses in the immunized animals [215].
Furthermore, immunizations with ID93/GLA-SE protected animals upon challenge with a clinical isolate of M. tuberculosis as well as the hyper-virulent Korean Beijing strain K of M. tuberculosis and induced long-lived immunity in mice [216,217]. Moreover, therapeutic immunizations with ID93/GLA-SE induced differential T cell immune responses over the course of infection that correlated with periods of enhanced bacterial control over that of drug treatment alone in mice [218]. In a BCG-prime boost regimen, the ID93/GLA-SE vaccine significantly reduced bacterial load at 16 weeks after challenge with the hyper-virulent Beijing strain of M. tuberculosis, while the BCG vaccine alone did not confer significant protection [219].
A randomized, double-blind, placebo-controlled phase 1 clinical trial with the ID93/GLA-SE vaccine has been conducted in HIV-negative and previously BCG-vaccinated adults in South Africa. The participants included M. tuberculosis infected and non-infected healthy subjects. The results using varying doses of the vaccine showed that it was well-tolerated and no severe or serious vaccine-related adverse events were observed [220]. Furthermore, different doses of the vaccine did not affect the frequency or severity of adverse events, but mild injection site adverse events and flu-like symptoms were common in M tuberculosis-infected group compared to non-infected group. Vaccination induced long-lasting antigen-specific IgG and T helper-1 type cellular immune responses, which peaked after administration of two doses of the vaccine [220]. The variations in the vaccine dose did not significantly affect the magnitude, kinetics, or profile of antibody and cellular immune responses [220]. When compared with vaccination with ID93 alone, vaccination with ID93 + GLA-SE induced higher titers of ID93-specific antibodies, a preferential increase in IgG1 and IgG3 antibody subclasses, and a multifaceted Fc-mediated effector function response [220]. The ID93/GLA-SE vaccine enhanced the magnitude and polyfunctional cytokine profile of CD4+ T cells, as compared to ID93 alone [221]. This vaccine is currently being tested in a phase 2 clinical trial [190].
5. Sequence Identities among ESAT6-Like Proteins Encoded by Genes in RD1, RD7 and RD9
All of the six ESAT6-like proteins encoded by genes in RD1, RD7 and RD9 are of low molecular weight, are approximately the same size and share a similar genomic organization [102], but they share minimum sequence identities (6 to 20%) with ESAT6 (EsxA) (Table 5) and CFP10 (EsxB) [102,168], suggesting that none of these proteins can replace ESAT6 (EsxA) and/or CFP10 (EsxB) either in diagnosis or vaccine applications.
Table 5.
The amino acid sequence identities between ESAT6, and ESAT6-like proteins encoded by RD1, RD7 and RD9.
| Protein | Comparison with | Protein Identity |
|---|---|---|
| ESAT6 (EsxA) | CFP10 (EsxB) | 15% |
| ESAT6 (EsxA) | EsxV (Rv3619c) | 20% |
| ESAT6 (EsxA) | EsxW (Rv3620c) | 6% |
| ESAT6 (EsxA) | EsxO (Rv2346c) | 18% |
| ESAT6 (EsxA) | EsxP (Rv2347c) | 6% |
Similarly, the individual ESAT6-like proteins encoded by genes in RD7 and RD9 also have minimum sequence identities with each other (Table 6). In contrast, the EsxV (Rv3619c) and EsxW (Rv3620c) encoded by RD7 have extensive sequence identities (93% and 97%) with EsxO (Rv2346c) and EsxP (Rv2347c) encoded by RD9, respectively (Table 6). This suggests that EsxV (Rv3619c) and EsxO (Rv2346c); and EsxW (Rv3620c) and EsxP (Rv2347c) have evolved through gene duplication. These high levels of sequence identities suggest that EsxV (Rv3619c) may replace EsxO (Rv2346c) and EsxW (Rv3620c) may replace EsxP (Rv2347c) in diagnostic and/or vaccine applications.
Table 6.
The amino acid sequence identities between ESAT6-like proteins encoded by RD7 and RD9.
| Protein | Comparison with | Percent Identity |
|---|---|---|
| EsxV (Rv3619c) | EsxW (Rv3620c) | 30% |
| EsxO (Rv2346c) | EsxP (Rv2347c) | <5% |
| EsxV (Rv3619c) | EsxO (Rv2346c) | 93% |
| EsxW (Rv3620c) | EsxP (Rv2347c) | 97% |
6. Summary
The M. tuberculosis-specific genomic regions RD1, RD7, and RD9 can potentially encode a total of 29 proteins. Among them, three pairs of proteins, i.e., EsxA (ESAT6) and EsxB (CFP10); EsxO (Rv2346c) and EsxP (Rv2347c); and EsxV (Rv3619c) and EsxW (3620c) belong to the family of ESAT6-like proteins. EsxO and EsxV share 93%, and EsxP and EsxW share 97% sequence identities, suggesting gene duplication. EsxA and EsxB have been widely used in the specific diagnosis of infection with M. tuberculosis in interferon-gamma release assays. Furthermore, both of these proteins have also been included as components of subunit vaccines that have been tested in human phase 1 and phase 2 clinical trials. However, EsxA and EsxB cannot be used both as vaccines and diagnostic reagents. Furthermore, EsxV and EsxW are also included in a vaccine preparation known as ID93, which is undergoing a phase 2a clinical trial in humans. Due to the extensive use of EsxA and EsxB in diagnostic applications, it is advisable to exclude them from vaccine preparations and focus on the use of the vaccine candidates containing EsxV and EsxW, i.e., ID93 for vaccination against TB.
Author Contributions
Conceptualization, A.S.M.; writing, A.S.M. The author has read and agreed to the published version of the manuscript.
Funding
This research was supported by Kuwait University Research Sector grants MI04/05 and SRUL02/13.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilson M.E. Applying experiences from trials of bacille Calmette-Guérin vaccine. Clin. Infect. Dis. 2000;30(Suppl. S3):S262–S265. doi: 10.1086/313886. [DOI] [PubMed] [Google Scholar]
- 2.Black G.F., Weir R.E., Floyd S., Bliss L., Warndorff D.K., Crampin A.C., Ngwira B., Sichali L., Nazareth B., Blackwell J.M., et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: Two randomised controlled studies. Lancet. 2002;359:1393–1401. doi: 10.1016/S0140-6736(02)08353-8. [DOI] [PubMed] [Google Scholar]
- 3.Barreto M.L., Pereira S.M., Ferreira A.A. BCG vaccine: Efficacy and indications for vaccination and revaccination. J. Pediatr. 2006;82(Suppl. S3):S45–S54. doi: 10.2223/JPED.1499. [DOI] [PubMed] [Google Scholar]
- 4.McShane H. Tuberculosis vaccines: Beyond bacille Calmette-Guerin. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2011;366:2782–2789. doi: 10.1098/rstb.2011.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin C., Aguilo N., Marinova D., Gonzalo-Asensio J. Update on TB vaccine pipeline. Appl. Sci. 2020;10:2632. doi: 10.3390/app10072632. [DOI] [Google Scholar]
- 6.Tafreshi S.H. BCG vaccine and pulmonary tuberculosis. Vaccine Res. 2016;3:36–40. doi: 10.29252/vacres.3.8.9.36. [DOI] [Google Scholar]
- 7.Dockrell H.M., Smith S.G. What have we learnt about BCG vaccination in the last 20 Years? Front. Immunol. 2017;8:1134. doi: 10.3389/fimmu.2017.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahasha P.W., Ndwandwe D.E., Mavundza E.J., Shey M., Wiysonge C.S. Systematic review protocol on Bacillus Calmette-Guerin (BCG) revaccination and protection against tuberculosis. BMJ Open. 2019;9:e027033. doi: 10.1136/bmjopen-2018-027033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez A.H., Flores-Valdez M.A. Can immunization with Bacillus Calmette-Guérin be improved for prevention or therapy and elimination of chronic Mycobacterium tuberculosis infection? Expert Rev. Vaccines. 2019;18:1219–1227. doi: 10.1080/14760584.2019.1704263. [DOI] [PubMed] [Google Scholar]
- 10.Young C., Walzl G., Du Plessis N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol. 2020;13:190–204. doi: 10.1038/s41385-019-0226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fatima S., Kumari A., Das G., Dwivedi V.P. Tuberculosis vaccine: A journey from BCG to present. Life Sci. 2020;252:117594. doi: 10.1016/j.lfs.2020.117594. [DOI] [PubMed] [Google Scholar]
- 12.Farer L.S., Lowel L.M., Meador M.P. Extrapulmonary tuberculosis in the United States. Am. J. Epidemiol. 1979;109:205–217. doi: 10.1093/oxfordjournals.aje.a112675. [DOI] [PubMed] [Google Scholar]
- 13.Wani R.L.S. Clinical manifestations of pulmonary and extra pulmonary tuberculosis. South Sudan Med. J. 2013;6:52–56. [Google Scholar]
- 14.Guler S.A., Bozkus F., Inci M.F., Kokoglu O.F., Ucmak H., Ozden S., Yuksel M. Evaluation of pulmonary and extrapulmonary tuberculosis in immunocompetent adults: A retrospective case series analysis. Med. Princ Pract. 2015;24:75–79. doi: 10.1159/000365511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer C.E., Long M.W. Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am. Rev. Respir. Dis. 1966;94:553–568. doi: 10.1164/arrd.1966.94.4.553. [DOI] [PubMed] [Google Scholar]
- 16.Andersen P., Doherty T.M. The success and failure of BCG—Implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 2005;3:656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 17.Barreto M.L., Pilger D., Pereira S.M., Genser B., Cruz A.A., Cunha S.S., Sant’Anna C., Hijjar M.A., Ichihara M.Y., Rodrigues L.C. Causes of variation in BCG vaccine efficacy: Examining evidence from the BCG REVAC cluster randomized trial to explore the masking and the blocking hypotheses. Vaccine. 2014;32:3759–3764. doi: 10.1016/j.vaccine.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 18.Arregui S., Sanz J., Marinova D., Martín C., Moreno Y. On the impact of masking and blocking hypotheses for measuring the efficacy of new tuberculosis vaccines. PeerJ. 2016;4:e1513. doi: 10.7717/peerj.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamala T., Paramasivan C.N., Herbert D., Venkatesan P., Prabhakar R. Immune response & modulation of immune response induced in the guinea-pigs by Mycobacterium avium complex (MAC) & M. fortuitum complex isolates from different sources in the south Indian BCG trial area. Indian J. Med. Res. 1996;103:201–211. [PubMed] [Google Scholar]
- 20.Brandt L., Cunha J.F., Olsen A.W., Chilima B., Hirsch P., Appelberg R., Andersen P. Failure of the Mycobacterium bovis BCG vaccine: Some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 2002;70:672–678. doi: 10.1128/IAI.70.2.672-678.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozes E., Denis O., Drowart A., Jurion F., Palfliet K., Vanonckelen A., De Bruyn J., De Cock M., Van Vooren J.P., Huygen K. Cross-reactive immune responses against Mycobacterium bovis BCG in mice infected with non-tuberculous mycobacteria belonging to the MAIS-Group. Scand. J. Immunol. 1997;46:16–26. doi: 10.1046/j.1365-3083.1997.d01-99.x. [DOI] [PubMed] [Google Scholar]
- 22.Buddle B.M., Wards B.J., Aldwell F.E., Collins D.M., de Lisle G.W. Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine. 2002;20:1126–1133. doi: 10.1016/S0264-410X(01)00436-4. [DOI] [PubMed] [Google Scholar]
- 23.de Lisle G.W., Wards B.J., Buddle B.M., Collins D.M. The efficacy of live tuberculosis vaccines after presensitization with Mycobacterium avium. Tuberculosis. 2005;85:73–79. doi: 10.1016/j.tube.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Tameris M., Mearns H., Penn-Nicholson A., Gregg Y., Bilek N., Mabwe S., Geldenhuys H., Shenje J., Luabeya A.K.K., Murillo I., et al. Live-attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: A randomised controlled, double-blind dose-escalation trial. Lancet Respir. Med. 2019;7:757–770. doi: 10.1016/S2213-2600(19)30251-6. [DOI] [PubMed] [Google Scholar]
- 25.Mustafa A.S., Al-Attiyah R. Tuberculosis: Looking beyond BCG vaccines. J. Postgrad. Med. 2003;49:129–140. [PubMed] [Google Scholar]
- 26.Hawn T.R., Day T.A., Scriba T.J., Hatherill M., Hanekom W.A., Evans T.G., Churchyard G.J., Kublin J.G., Bekker L.G., Self S.G. Tuberculosis vaccines and prevention of infection. Microbiol. Mol. Biol. Rev. 2014;78:650–671. doi: 10.1128/MMBR.00021-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davenne T., McShane H. Why don’t we have an effective tuberculosis vaccine yet? Expert Rev. Vaccines. 2016;15:1009–1013. doi: 10.1586/14760584.2016.1170599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brazier B., McShane H. Towards new TB vaccines. Semin. Immunopathol. 2020;42:315–331. doi: 10.1007/s00281-020-00794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno S., Blázquez R., Novoa A., Carpena I., Menasalvas A., Ramírez C., Guerrero C. The effect of BCG vaccination on tuberculin reactivity and the booster effect among hospital employees. Arch. Intern. Med. 2001;161:1760–1765. doi: 10.1001/archinte.161.14.1760. [DOI] [PubMed] [Google Scholar]
- 30.Arend S.M., Engelhard A.C., Groot G., de Boer K., Andersen P., Ottenhoff T.H., van Dissel J.T. Tuberculin skin testing compared with T-cell responses to Mycobacterium tuberculosis-specific and nonspecific antigens for detection of latent infection in persons with recent tuberculosis contact. Clin. Diagn. Lab. Immunol. 2001;8:1089–1096. doi: 10.1128/CDLI.8.6.1089-1096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farhat M., Greenaway C., Pai M., Menzies D. False-positive tuberculin skin tests: What is the absolute effect of BCG and non-tuberculous mycobacteria? Int. J. Tuberc. Lung Dis. 2006;10:1192–1204. [PubMed] [Google Scholar]
- 32.Latorre I., De Souza-Galvão M., Ruiz-Manzano J., Lacoma A., Prat C., Altet N., Ausina V., Domínguez J. Evaluating the non-tuberculous mycobacteria effect in the tuberculosis infection diagnosis. Eur. Respir. J. 2010;35:338–342. doi: 10.1183/09031936.00196608. [DOI] [PubMed] [Google Scholar]
- 33.Litwin C.M. In vitro gamma interferon tests for the detection of tuberculosis infection. J. Immunotoxicol. 2007;4:219–224. doi: 10.1080/15476910701385646. [DOI] [PubMed] [Google Scholar]
- 34.Sidibe A., Matteelli A., Menzies R., Getahun H. Differences in BCG vaccination and tuberculin skin-test positivity. Lancet Infect. Dis. 2015;15:1003. doi: 10.1016/S1473-3099(15)00233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunst H. Diagnosis of latent tuberculosis infection: The potential role of new technologies. Resp. Med. 2006;100:2098–2106. doi: 10.1016/j.rmed.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 36.Hill P.C., Jeffries D.J., Brookes R.H., Fox A., Jackson-Sillah D., Lugos M.D., Donkor S.A., de Jong B.C., Corrah T., Adegbola R.A., et al. Using ELISPOT to expose false positive skin test conversion in tuberculosis contacts. PLoS ONE. 2007;2:e183. doi: 10.1371/journal.pone.0000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Orainey I.O. Diagnosis of latent tuberculosis: Can we do better? Ann. Thorac. Med. 2009;4:5–9. doi: 10.4103/1817-1737.44778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slogotskaya L., Bogorodskaya E., Ivanova D., Sevostyanova T. Comparative sensitivity of the test with tuberculosis recombinant allergen, containing ESAT6-CFP10 protein, and Mantoux test with 2 TU PPD-L in newly diagnosed tuberculosis children and adolescents in Moscow. PLoS ONE. 2018;13:e0208705. doi: 10.1371/journal.pone.0208705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasempimolporn S., Areekul P., Thaveekarn W., Sutthisri R., Boonchang S., Sawangvaree A., Sitprija V. Application of transdermal patches with new skin test reagents for detection of latent tuberculosis. J. Med. Microbiol. 2019;68:1314–1319. doi: 10.1099/jmm.0.001037. [DOI] [PubMed] [Google Scholar]
- 40.Carranza C., Pedraza-Sanchez S., de Oyarzabal-Mendez E., Torres M. Diagnosis for latent tuberculosis infection: New alternatives. Front. Immunol. 2020;11:2006. doi: 10.3389/fimmu.2020.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cockle P.J., Gordon S.V., Lalvani A., Buddle B.M., Hewinson R.G., Vordermeier H.M. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect. Immun. 2002;70:6996–7003. doi: 10.1128/IAI.70.12.6996-7003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Black G.F., Weir R.E., Chaguluka S.D., Warndorff D., Crampin A.C., Mwaungulu L., Sichali L., Floyd S., Bliss L., Jarman E., et al. Gamma interferon responses induced by a panel of recombinant and purified mycobacterial antigens in healthy, non-mycobacterium bovis BCG-vaccinated Malawian young adults. Clin. Diagn. Lab Immunol. 2003;10:602–611. doi: 10.1128/CDLI.10.4.602-611.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mustafa A.S. What’s new in the development of tuberculosis vaccines. Med. Princ. Pract. 2012;21:195–196. doi: 10.1159/000337919. [DOI] [PubMed] [Google Scholar]
- 44.Mustafa A.S. In silico analysis and experimental validation of Mycobacterium tuberculosis-specific proteins and peptides of Mycobacterium tuberculosis for immunological diagnosis and vaccine development. Med. Princ. Pract. 2013;22(Suppl. S1):43–51. doi: 10.1159/000354206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meier N.R., Jacobsen M., Ottenhoff T.H.M., Ritz N. A systematic review on novel Mycobacterium tuberculosis antigens and their discriminatory potential for the diagnosis of latent and active tuberculosis. Front. Immunol. 2018;9:2476. doi: 10.3389/fimmu.2018.02476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coppola M., Ottenhoff T.H.M. Genome wide approaches discover novel Mycobacterium tuberculosis antigens as correlates of infection, disease, immunity and targets for vaccination. Sem. Immunol. 2018;39:88–101. doi: 10.1016/j.smim.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Kim A., Park K.J., Kim Y.S., Cho S.N., Dockrell H.M., Hur Y.G. Diagnostic potential of a PPE protein derived from Mycobacterium tuberculosis Beijing/K strain. Yonsei Med. J. 2020;61:789–796. doi: 10.3349/ymj.2020.61.9.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sørensen A.L., Nagai S., Houen G., Andersen P., Andersen A.B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 1995;63:1710–1717. doi: 10.1128/IAI.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersen P., Andersen A.B., Sørensen A.L., Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 50.Brandt L., Oettinger T., Holm A., Andersen A.B., Andersen P. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J. Immunol. 1996;157:3527–3533. [PubMed] [Google Scholar]
- 51.Mustafa A.S., Amoudy H.A., Wiker H.G., Abal A.T., Ravn P., Oftung F., Andersen P. Comparison of antigen-specific T-cell responses of tuberculosis patients using complex or single antigens of Mycobacterium tuberculosis. Scand. J. Immunol. 1998;48:535–543. doi: 10.1046/j.1365-3083.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 52.Pais T.F., Silva R.A., Smedegaard B., Appelberg R., Andersen P. Analysis of T cells recruited during delayed-type hypersensitivity to purified protein derivative (PPD) versus challenge with tuberculosis infection. Immunology. 1998;95:69–75. doi: 10.1046/j.1365-2567.1998.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lalvani A., Hill A.V. Cytotoxic T-lymphocytes against malaria and tuberculosis: From natural immunity to vaccine design. Clin. Sci. 1998;95:531–538. doi: 10.1042/cs0950531. [DOI] [PubMed] [Google Scholar]
- 54.Ravn P., Demissie A., Eguale T., Wondwosson H., Lein D., Amoudy H.A., Mustafa A.S., Jensen A.K., Holm A., Rosenkrands I., et al. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 1999;179:637–645. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 55.Mustafa A.S., Oftung F., Amoudy H.A., Madi N.M., Abal A.T., Shaban F., Rosen Krands I., Andersen P. Multiple epitopes from the Mycobacterium tuberculosis ESAT-6 antigen are recognized by antigen-specific human T cell lines. Clin. Infect. Dis. 2000;30(Suppl. S3):S201–S205. doi: 10.1086/313862. [DOI] [PubMed] [Google Scholar]
- 56.Al-Attiyah R., Mustafa A.S., Abal A.T., El-Shamy A.S., Dalemans W., Skeiky Y.A. In vitro cellular immune responses to complex and newly defined recombinant antigens of Mycobacterium tuberculosis. Clin. Exp. Immunol. 2004;138:139–144. doi: 10.1111/j.1365-2249.2004.02609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harboe M., Malin A.S., Dockrell H.S., Wiker H.G., Ulvund G., Holm A., Jørgensen M.C., Andersen P. B-cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect. Immun. 1998;66:717–723. doi: 10.1128/IAI.66.2.717-723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arend S.M., Geluk A., van Meijgaarden K.E., van Dissel J.T., Theisen M., Andersen P., Ottenhoff T.H.M. Antigenic equivalence of human T-cell responses to Mycobacterium tuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect Immun. 2000;68:3314–3321. doi: 10.1128/IAI.68.6.3314-3321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vordermeier H.M., Whelan A., Cockle P.J., Farrant L., Palmer N., Hewinson R.G. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 2001;8:571–578. doi: 10.1128/CDLI.8.3.571-578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Attiyah R., Mustafa A.S., Abal A.T., Madi N.M., Andersen P. Restoration of mycobacterial antigen-induced proliferation and interferon-gamma responses in peripheral blood mononuclear cells of tuberculosis patients upon effective chemotherapy. FEMS Immunol. Med. Microbiol. 2003;38:249–256. doi: 10.1016/S0928-8244(03)00166-4. [DOI] [PubMed] [Google Scholar]
- 61.Brock I., Weldingh K., Leyten E.M., Arend S.M., Ravn P., Andersen P. Specific T-cell epitopes for immunoassay-based diagnosis of Mycobacterium tuberculosis infection. J. Clin Microbiol. 2004;42:2379–2387. doi: 10.1128/JCM.42.6.2379-2387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mustafa A.S., Shaban F.A. ProPred analysis and experimental evaluation of promiscuous T-cell epitopes of three major secreted antigens of Mycobacterium tuberculosis. Tuberculosis. 2006;86:115–124. doi: 10.1016/j.tube.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Mustafa A.S. Recombinant and synthetic peptides to identify Mycobacterium tuberculosis antigens and epitopes of diagnostic and vaccine relevance. Tuberculosis. 2005;85:367–376. doi: 10.1016/j.tube.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Aagaard C.S., Hoang T.T., Vingsbo-Lundberg C., Dietrich J., Andersen P. Quality and vaccine efficacy of CD4+ T cell responses directed to dominant and subdominant epitopes in ESAT-6 from Mycobacterium tuberculosis. J. Immunol. 2009;183:2659–2668. doi: 10.4049/jimmunol.0900947. [DOI] [PubMed] [Google Scholar]
- 65.Mustafa A.S., Shaban F.A., Al-Attiyah R., Abal A.T., El-Shamy A.M., Andersen P., Oftung F. Human Th1 cell lines recognize the Mycobacterium tuberculosis ESAT-6 antigen and its peptides in association with frequently expressed HLA class II molecules. Scand. J. Immunol. 2003;57:125–134. doi: 10.1046/j.1365-3083.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 66.Ong E., He Y., Yang Z. Epitope promiscuity and population coverage of Mycobacterium tuberculosis protein antigens in current subunit vaccines under development. Infect. Genet. Evol. 2020;80:104186. doi: 10.1016/j.meegid.2020.104186. [DOI] [PubMed] [Google Scholar]
- 67.Pollock J.M., Andersen P. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J. Infect Dis. 1997;175:1251–1254. doi: 10.1086/593686. [DOI] [PubMed] [Google Scholar]
- 68.Buddle B.M., Parlane N.A., Keen D.L., Aldwell F.E., Pollock J.M., Lightbody K., Andersen P. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin. Diagn. Lab. Immunol. 1999;6:1–5. doi: 10.1128/CDLI.6.1.1-5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson P.D., Stuart R.L., Grayson M.L., Olden D., Clancy A., Ravn P., Andersen P., Britton W.J., Rothel J.S. Tuberculin-purified protein derivative-, MPT-64-, and ESAT-6-stimulated gamma interferon responses in medical students before and after Mycobacterium bovis BCG vaccination and in patients with tuberculosis. Clin. Diagn. Lab. Immunol. 1999;6:934–937. doi: 10.1128/CDLI.6.6.934-937.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ulrichs T., Anding P., Porcelli S., Kaufmann S.H., Munk M.E. Increased numbers of ESAT-6- and purified protein derivative-specific gamma interferon-producing cells in subclinical and active tuberculosis infection. Infect. Immun. 2000;68:6073–6076. doi: 10.1128/IAI.68.10.6073-6076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brandt L., Elhay M., Rosenkrands I., Lindblad E.B., Andersen P. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 2000;68:791–795. doi: 10.1128/IAI.68.2.791-795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mustafa A.S. Mycobacterial gene cloning and expression, comparative genomics, bioinformatics and proteomics in relation to the development of new vaccines and diagnostic reagents. Med. Princ. Pract. 2005;14(Suppl. S1):27–34. doi: 10.1159/000086182. [DOI] [PubMed] [Google Scholar]
- 73.Khan A., Singh S., Galvan G., Jagannath C., Sastry K.J. Prophylactic sublingual immunization with Mycobacterium tuberculosis subunit vaccine incorporating the natural killer T cell agonist alpha-galactosylceramide enhances protective immunity to limit pulmonary and extra-pulmonary bacterial burden in mice. Vaccines. 2017;6:47. doi: 10.3390/vaccines5040047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi H.G., Kwon K.W., Choi S., Back Y.W., Park H.S., Kang S.M., Choi E., Shin S.J., Kim H.J. Antigen-specific IFN-γ/IL-17-co-producing CD4+ T-cells are the determinants for protective efficacy of tuberculosis subunit vaccine. Vaccines. 2020;8:300. doi: 10.3390/vaccines8020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clemmensen H.S., Knudsen N.P.H., Billeskov R., Rosenkrands I., Jungersen G., Aagaard C., Andersen P., Mortensen R. Rescuing ESAT-6 specific CD4 T cells from terminal differentiation is critical for long-term control of murine Mtb infection. Front. Immunol. 2020;11:585359. doi: 10.3389/fimmu.2020.585359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahairas G.G., Sabo P.J., Hickey M.J., Singh D.C., Stover C.K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 1996;178:1274–1282. doi: 10.1128/JB.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roche P.W., Feng C.G., Britton W.J. Human T-cell epitopes on the Mycobacterium tuberculosis secreted protein MPT64. Scand. J. Immunol. 1996;43:662–670. doi: 10.1046/j.1365-3083.1996.d01-260.x. [DOI] [PubMed] [Google Scholar]
- 78.Sable S.B., Kumar R., Kalra M., Verma I., Khuller G.K., Dobos K., Belisle J.T. Peripheral blood and pleural fluid mononuclear cell responses to low-molecular-mass secretory polypeptides of Mycobacterium tuberculosis in human models of immunity to tuberculosis. Infect. Immun. 2005;73:3547–3558. doi: 10.1128/IAI.73.6.3547-3558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mustafa A.S. HLA-promiscuous Th1-cell reactivity of MPT64 (Rv1980c), a major secreted antigen of Mycobacterium tuberculosis, in healthy subjects. Med. Princ. Pract. 2009;18:385–392. doi: 10.1159/000226293. [DOI] [PubMed] [Google Scholar]
- 80.Mustafa A.S., Shaban F. Mapping of Th1-cell epitope regions of Mycobacterium tuberculosis protein MPT64 (Rv1980c) using synthetic peptides and T-cell lines from M. tuberculosis-infected healthy humans. Med. Princ. Pract. 2010;19:122–128. doi: 10.1159/000273073. [DOI] [PubMed] [Google Scholar]
- 81.Mustafa A.S. In silico binding predictions for identification of HLA-DR-promiscuous regions and epitopes of Mycobacterium tuberculosis protein MPT64 (Rv1980c) and their recognition by human Th1 cells. Med. Princ. Pract. 2010;19:367–372. doi: 10.1159/000316375. [DOI] [PubMed] [Google Scholar]
- 82.Kamath A.T., Feng C.G., Macdonald M., Briscoe H., Britton W.J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 1999;67:1702–1707. doi: 10.1128/IAI.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morris S., Kelley C., Howard A., Li Z., Collins F. The immunogenicity of single and combination DNA vaccines against tuberculosis. Vaccine. 2000;18:2155–2163. doi: 10.1016/S0264-410X(99)00540-X. [DOI] [PubMed] [Google Scholar]
- 84.Kalra M., Grover A., Mehta N., Singh J., Kau J., Sable S.B., Behera D., Sharma P., Verma I., Khuller G.K. Supplementation with RD antigens enhances the protective efficacy of BCG in tuberculous mice. Clin. Immunol. 2007;125:173–183. doi: 10.1016/j.clim.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 85.Wang C., Chen Z., Fu R., Zhang Y., Chen L., Huang L., Li J., Shi C., Fan X. A DNA vaccine expressing CFP21 and MPT64 fusion protein enhances BCG-induced protective immunity against Mycobacterium tuberculosis infection in mice. Med. Microbiol. Immunol. 2011;200:165–175. doi: 10.1007/s00430-011-0188-z. [DOI] [PubMed] [Google Scholar]
- 86.Bai C., He J., Niu H., Hu L., Luo Y., Liu X., Peng L., Zhu B. Prolonged intervals during Mycobacterium tuberculosis subunit vaccine boosting contributes to eliciting immunity mediated by central memory-like T cells. Tuberculosis. 2018;110:104–111. doi: 10.1016/j.tube.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 87.Wu X., Yang Y., Zhang J., Li B., Liang Y., Zhang C., Dong M. Comparison of antibody responses to seventeen antigens from Mycobacterium tuberculosis. Clin. Chim. Acta. 2010;411:1520–1528. doi: 10.1016/j.cca.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 88.Yan Z.H., Yi L., Wei P.J., Jia H.Y., Wang J., Wang X.J., Yang B., Gao X., Zhao Y.L., Zhang H.T. Evaluation of panels of Mycobacterium tuberculosis antigens for serodiagnosis of tuberculosis. Int. J. Tuberc. Lung Dis. 2018;22:959–965. doi: 10.5588/ijtld.18.0060. [DOI] [PubMed] [Google Scholar]
- 89.Talbot E.A., Williams D.L., Frothingham R. PCR identification of Mycobacterium bovis BCG. J. Clin. Microbiol. 1997;35:566–569. doi: 10.1128/JCM.35.3.566-569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mostowy S., Cousins D., Brinkman J., Aranaz A., Behr M.A. Genomic deletions suggest a phylogeny for the Mycobacterium tuberculosis complex. J. Infect. Dis. 2002;186:74–80. doi: 10.1086/341068. [DOI] [PubMed] [Google Scholar]
- 91.Mostowy S., Cousins D., Behr M.A. Genomic interrogation of the dassie bacillus reveals it as a unique RD1 mutant within the Mycobacterium tuberculosis complex. J. Bacteriol. 2004;186:104–109. doi: 10.1128/JB.186.1.104-109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mustafa A.S. Vaccine potential of Mycobacterium tuberculosis-specific genomic regions: In vitro studies in humans. Expert Rev. Vaccines. 2009;8:1309–1312. doi: 10.1586/erv.09.93. [DOI] [PubMed] [Google Scholar]
- 93.Teo J.W., Cheng J.W., Jureen R., Lin R.T. Clinical utility of RD1, RD9 and hsp65 based PCR assay for the identification of BCG in vaccinated children. BMC Res. Notes. 2013;6:434. doi: 10.1186/1756-0500-6-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parsons L.M., Brosch R., Cole S.T., Somoskövi A., Loder A., Bretzel G., Van Soolingen D., Hale Y.M., Salfinger M. Rapid and simple approach for identification of Mycobacterium tuberculosis complex isolates by PCR-based genomic deletion analysis. J. Clin. Microbiol. 2002;40:2339–2345. doi: 10.1128/JCM.40.7.2339-2345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brosch R., Gordon S.V., Marmiesse M., Brodin P., Buchrieser C., Eiglmeier K., Garnier T., Gutierrez C., Hewinson G., Kremer K., et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. USA. 2002;99:3684–3689. doi: 10.1073/pnas.052548299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huard R.C., Lazzarini L.C., Butler W.R., van Soolingen D., Ho J.L. PCR-based method to differentiate the subspecies of the Mycobacterium tuberculosis complex on the basis of genomic deletions. J. Clin. Microbiol. 2003;41:1637–1650. doi: 10.1128/JCM.41.4.1637-1650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh S., Kumar M., Singh P. Evolution of M. bovis BCG vaccine: Is niacin production still a valid biomarker? Tuberc. Res. Treat. 2015;2015:957519. doi: 10.1155/2015/957519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mustafa A.S. Biotechnology in the development of new vaccines and diagnostic reagents against tuberculosis. Curr. Pharm. Biotechnol. 2001;2:157–173. doi: 10.2174/1389201013378707. [DOI] [PubMed] [Google Scholar]
- 99.Mustafa A.S., Al-Attiyah R. Mycobacterium tuberculosis antigens and peptides as new vaccine candidates and immunodiagnostic reagents against tuberculosis. Kuwait Med. J. 2004;36:171–176. [Google Scholar]
- 100.Pai M. Alternatives to the tuberculin skin test: Interferon-gamma assays in the diagnosis of Mycobacterium tuberculosis infection. Indian J. Med. Microbiol. 2005;23:151–158. doi: 10.4103/0255-0857.16585. [DOI] [PubMed] [Google Scholar]
- 101.Mustafa A.S. Cell mediated immunity assays identify proteins of diagnostic and vaccine potential from genomic regions of difference of Mycobacterium tuberculosis. Kuwait Med. J. 2010;42:98–105. [Google Scholar]
- 102.Berthet F.X., Rasmussen P.B., Rosenkrands I., Andersen P., Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144:3195–3203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- 103.Guinn K.M., Hickey M.J., Mathur S.K., Zakel K.L., Grotzke J.E., Lewinsohn D.M., Smith S., Sherman D.R. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 2004;51:359–370. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brodin P., Majlessi L., Marsollier L., de Jonge M.I., Bottai D., Demangel C., Hinds J., Neyrolles O., Butcher P.D., Leclerc C., et al. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect. Immun. 2006;74:88–98. doi: 10.1128/IAI.74.1.88-98.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu J., Laine O., Masciocchi M., Manoranjan J., Smith J., Du S.J., Edwards N., Zhu X., Fenselau C., Gao L.Y. A unique Mycobacterium ESX-1 protein co-secretes with CFP-10/ESAT-6 and is necessary for inhibiting phagosome maturation. Mol. Microbiol. 2007;66:787–800. doi: 10.1111/j.1365-2958.2007.05959.x. [DOI] [PubMed] [Google Scholar]
- 106.Teutschbein J., Schumann G., Möllmann U., Grabley S., Cole S.T., Munder T. A protein linkage map of the ESAT-6 secretion system 1 (ESX-1) of Mycobacterium tuberculosis. Microbiol. Res. 2009;164:253–259. doi: 10.1016/j.micres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 107.Cole S.T., Brosch R., Parkhill J., Garnier T., Churcher C., Harris D., Gordon S.V., Eiglmeier K., Gas S., Barry C.E., et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 108.Behr M.A., Wilson M.A., Gill W.P., Salamon H., Schoolnik G.K., Rane S., Small P.M. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 109.Gordon S.V., Brosch R., Billault A., Garnier T., Eiglmeier K., Cole S.T. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol. Microbiol. 1999;32:643–655. doi: 10.1046/j.1365-2958.1999.01383.x. [DOI] [PubMed] [Google Scholar]
- 110.Al-Attiyah R., Mustafa A.S. Characterization of human cellular immune responses to novel Mycobacterium tuberculosis antigens encoded by genomic regions absent in Mycobacterium bovis BCG. Infect. Immun. 2008;76:4190–4198. doi: 10.1128/IAI.00199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mustafa A.S., Al-Attiyah R. Identification of Mycobacterium tuberculosis-specific genomic regions encoding antigens inducing protective cellular immune responses. Indian J. Exp. Biol. 2009;47:498–504. [PubMed] [Google Scholar]
- 112.Al-Attiyah R.J., Mustafa A.S. Mycobacterial antigen-induced T helper type 1 (Th1) and Th2 reactivity of peripheral blood mononuclear cells from diabetic and non-diabetic tuberculosis patients and Mycobacterium bovis bacilli Calmette-Guérin (BCG)-vaccinated healthy subjects. Clin. Exp. Immunol. 2009;158:64–73. doi: 10.1111/j.1365-2249.2009.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Al-Attiyah R., Mustafa A.S. Characterization of human cellular immune responses to Mycobacterium tuberculosis proteins encoded by genes predicted in RD15 genomic region that is absent in Mycobacterium bovis BCG. FEMS Immunol. Med. Microbiol. 2010;59:177–187. doi: 10.1111/j.1574-695X.2010.00677.x. [DOI] [PubMed] [Google Scholar]
- 114.Mustafa A.S., Al-Saidi F., El-Shamy A.S., Al-Attiyah R. Cytokines in response to proteins predicted in genomic regions of difference of Mycobacterium tuberculosis. Microbiol. Immunol. 2011;55:267–278. doi: 10.1111/j.1348-0421.2011.00307.x. [DOI] [PubMed] [Google Scholar]
- 115.Al-Attiyah R., El-Shazly A., Mustafa A.S. Comparative analysis of spontaneous and mycobacterial antigen-induced secretion of Th1, Th2 and pro-inflammatory cytokines by peripheral blood mononuclear cells of tuberculosis patients. Scand. J. Immunol. 2012;75:623–632. doi: 10.1111/j.1365-3083.2012.02692.x. [DOI] [PubMed] [Google Scholar]
- 116.Mustafa A.S., Cockle P.J., Shaban F., Hewinson R.G., Vordermeier H.M. Immunogenicity of Mycobacterium tuberculosis RD1 region gene products in infected cattle. Clin. Exp. Immunol. 2002;130:37–42. doi: 10.1046/j.1365-2249.2002.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ahmad S., Ali M.M., Mustafa A.S. Construction of a modified vector for efficient purification of recombinant Mycobacterium tuberculosis proteins expressed in Escherichia coli. Protein Expr. Purif. 2003;29:167–175. doi: 10.1016/S1046-5928(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 118.Mustafa A.S., Al-Attiyah R., Hanif S.N., Shaban F.A. Efficient testing of large pools of Mycobacterium tuberculosis RD1 peptides and identification of major antigens and immunodominant peptides recognized by human Th1 cells. Clin. Vaccine Immunol. 2008;15:916–924. doi: 10.1128/CVI.00056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hanif S.N., El-Shammy A.M., Al-Attiyah R., Mustafa A.S. Whole blood assays to identify Th1 cell antigens and peptides encoded by Mycobacterium tuberculosis-specific RD1 genes. Med. Princ. Pract. 2008;17:244–249. doi: 10.1159/000117800. [DOI] [PubMed] [Google Scholar]
- 120.Amoudy H.A., Al-Turab M.B., Mustafa A.S. Identification of transcriptionally active open reading frames within the RD1 genomic segment of Mycobacterium tuberculosis. Med. Princ. Pract. 2006;15:137–144. doi: 10.1159/000090919. [DOI] [PubMed] [Google Scholar]
- 121.Ahmad S., Amoudy H.A., Thole J.E., Young D.B., Mustafa A.S. Identification of a novel protein antigen encoded by a Mycobacterium tuberculosis-specific RD1 region gene. Scand. J. Immunol. 1999;49:515–522. doi: 10.1046/j.1365-3083.1999.00531.x. [DOI] [PubMed] [Google Scholar]
- 122.Daugelat S., Kowall J., Mattow J., Bumann D., Winter R., Hurwitz R., Kaufmann S.H. The RD1 proteins of Mycobacterium tuberculosis: Expression in Mycobacterium smegmatis and biochemical characterization. Microbes Infect. 2003;5:1082–1095. doi: 10.1016/S1286-4579(03)00205-3. [DOI] [PubMed] [Google Scholar]
- 123.Amoudy H.A., Ahmad S., Thole J.E., Mustafa A.S. Demonstration of in vivo expression of a hypothetical open reading frame (ORF-14) encoded by the RD1 region of Mycobacterium tuberculosis. Scand. J. Immunol. 2007;66:422–425. doi: 10.1111/j.1365-3083.2007.01961.x. [DOI] [PubMed] [Google Scholar]
- 124.Amoudy H.A., Mustafa A.S. Amplification of six putative RD1 genes of Mycobacterium tuberculosis for cloning and expression in Escherichia coli and purification of expressed proteins. Med. Princ. Pract. 2008;17:378–384. doi: 10.1159/000141501. [DOI] [PubMed] [Google Scholar]
- 125.Hanif S.N.M., Al-Attiyah R., Mustafa A.S. The natural expression of genes encoding major antigens of RD1 and RD9 in M. tuberculosis and other mycobacteria. Mycobact. Dis. 2011;1:105. [Google Scholar]
- 126.Amoudy H.A., Safar H.A., Mustafa A.S. Development of Escherichia coli and Mycobacterium smegmatis recombinants expressing major Mycobacterium tuberculosis-specific antigenic proteins. Int. J. Mycobacteriol. 2016;5(Suppl. S1):S84–S85. doi: 10.1016/j.ijmyco.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 127.Okkels L.M., Brock I., Follmann F., Agger E.M., Arend S.M., Ottenhoff T.H.M., Oftung F., Rosenkrands I., Andersen P. PPE protein (Rv3873) from DNA segment RD1 of Mycobacterium tuberculosis: Strong recognition of both specific T-cell epitopes and epitopes conserved within the PPE family. Infect. Immun. 2003;71:6116–6123. doi: 10.1128/IAI.71.11.6116-6123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Agger E.M., Brock I., Okkels L.M., Arend S.M., Aagaard C.S., Weldingh K.N., Andersen P. Human T-cell responses to the RD1-encoded protein TB27.4 (Rv3878) from Mycobacterium tuberculosis. Immunology. 2003;110:507–512. doi: 10.1111/j.1365-2567.2003.01763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Demangel C., Brodin P., Cockle P.J., Brosch R., Majlessi L., Leclerc C., Cole S.T. Cell envelope protein PPE68 contributes to Mycobacterium tuberculosis RD1 immunogenicity independently of a 10-kilodalton culture filtrate protein and ESAT-6. Infect. Immun. 2004;72:2170–2176. doi: 10.1128/IAI.72.4.2170-2176.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu X.Q., Dosanjh D., Varia H., Ewer K., Cockle P., Pasvol G., Lalvani A. Evaluation of T-cell responses to novel RD1- and RD2-encoded Mycobacterium tuberculosis gene products for specific detection of human tuberculosis infection. Infect. Immun. 2004;72:2574–2581. doi: 10.1128/IAI.72.5.2574-2581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mustafa A.S., Skeiky Y., Al-Attiyah R., Alderson M.R., Hewinson R.G., Vordermeier H.M. Immunogenicity of Mycobacterium tuberculosis antigens in Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle. Infect. Immun. 2006;74:4566–4572. doi: 10.1128/IAI.01660-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fox A., Jeffries D.J., Hill P.C., Hammond A.S., Lugos M.D., Jackson-Sillah D., Donkor S.A., Owiafe P.K., McAdam K.P., Brookes R.H. ESAT-6 and CFP-10 can be combined to reduce the cost of testing for Mycobacterium tuberculosis infection, but CFP-10 responses associate with active disease. Trans. R. Soc. Trop. Med. Hyg. 2007;101:691–698. doi: 10.1016/j.trstmh.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 133.Mustafa A.S., El-Shamy A.M., Madi N.M., Amoudy H.A., Al-Attiyah R. Cell-mediated immune responses to complex and single mycobacterial antigens in tuberculosis patients with diabetes. Med. Princ. Pract. 2008;17:325–330. doi: 10.1159/000129614. [DOI] [PubMed] [Google Scholar]
- 134.Mustafa A.S. Th1 cell reactivity and HLA-DR binding prediction for promiscuous recognition of MPT63 (Rv1926c), a major secreted protein of Mycobacterium tuberculosis. Scand. J. Immunol. 2009;69:213–222. doi: 10.1111/j.1365-3083.2008.02221.x. [DOI] [PubMed] [Google Scholar]
- 135.Hinks T.S., Dosanjh D.P., Innes J.A., Pasvol G., Hackforth S., Varia H., Millington K.A., Liu X.Q., Bakir M., Soysal A., et al. Frequencies of region of difference 1 antigen-specific but not purified protein derivative-specific gamma interferon-secreting T cells correlate with the presence of tuberculosis disease but do not distinguish recent from remote latent infections. Infect. Immun. 2009;77:5486–5495. doi: 10.1128/IAI.01436-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hanif S.N., Al-Attiyah R., Mustafa A.S. Molecular cloning, expression, purification and immunological characterization of three low-molecular weight proteins encoded by genes in genomic regions of difference of Mycobacterium tuberculosis. Scand. J. Immunol. 2010;71:353–361. doi: 10.1111/j.1365-3083.2010.02388.x. [DOI] [PubMed] [Google Scholar]
- 137.Dosanjh D.P.S., Bakir M., Millington K.A., Soysal A., Aslan Y., Efee S., Deeks J.J., Lalvani A. Novel M tuberculosis antigen-specific T-cells are early markers of infection and disease progression. PLoS ONE. 2011;6:e28754. doi: 10.1371/journal.pone.0028754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mustafa A.S. Comparative evaluation of MPT83 (Rv2873) for T helper-1 cell reactivity and identification of HLA-promiscuous peptides in Mycobacterium bovis BCG-vaccinated healthy subjects. Clin. Vaccine Immunol. 2011;18:1752–1759. doi: 10.1128/CVI.05260-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mustafa A.S. Characterization of a cross-reactive, immunodominant and HLA-promiscuous epitope of Mycobacterium tuberculosis-specific major antigenic protein PPE68. PLoS ONE. 2014;9:e103679. doi: 10.1371/journal.pone.0103679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aguilo N., Gonzalo-Asensio J., Alvarez-Arguedas S., Marinova D., Gomez A.B., Uranga S., Spallek R., Singh M., Audran R., Spertini F., et al. Reactogenicity to major tuberculosis antigens absent in BCG is linked to improved protection against Mycobacterium tuberculosis. Nat. Commun. 2017;8:16085. doi: 10.1038/ncomms16085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pym A.S., Brodin P., Brosch R., Huerre M., Cole S.T. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 2002;46:709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 142.Lewis K.N., Liao R., Guinn K.M., Hickey M.J., Smith S., Behr M.A., Sherman D.R. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guérin attenuation. J. Infect. Dis. 2003;187:117–123. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McLaughlin B., Chon J.S., MacGurn J.A., Carlsson F., Cheng T.L., Cox J.S., Brown E.J. A mycobacterium ESX-1-secreted virulence factor with unique requirements for export. PLoS Pathog. 2007;3:e105. doi: 10.1371/journal.ppat.0030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ganguly N., Siddiqui I., Sharma P. Role of M. tuberculosis RD-1 region encoded secretory proteins in protective response and virulence. Tuberculosis. 2008;88:510–517. doi: 10.1016/j.tube.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 145.Sun J., Champion P.A., Bigi F. Editorial: Cellular and molecular mechanisms of Mycobacterium tuberculosis virulence. Front. Cell. Infect. Microbiol. 2019;9:331. doi: 10.3389/fcimb.2019.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brock I., Munk M.E., Kok-Jensen A., Andersen P. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 2001;5:462–467. [PubMed] [Google Scholar]
- 147.Mahmoudi S., Pourakbari B., Mamishi S. Interferon Gamma Release Assay in response to PE35/PPE68 proteins: A promising diagnostic method for diagnosis of latent tuberculosis. Eur. Cytokine Netw. 2017;28:36–40. doi: 10.1684/ecn.2017.0391. [DOI] [PubMed] [Google Scholar]
- 148.Mamishi S., Pourakbari B., Sadeghi R.H., Marjani M., Mahmoudi S. Diagnostic accuracy of monocyte chemotactic protein (MCP)-2 as biomarker in response to PE35/PPE68 proteins: A promising diagnostic method for the discrimination of active and latent tuberculosis. Protein Pept. Lett. 2019;26:281–286. doi: 10.2174/0929866526666190119165805. [DOI] [PubMed] [Google Scholar]
- 149.Liu Z., Qie S., Li L., Xiu B., Yang X., Dai Z., Zhang X., Duan C., Que H., Zhao P., et al. Identification of novel RD1 antigens and their combinations for diagnosis of sputum smear-/culture+ TB Patients. Biomed. Res. Int. 2016;2016:7486425. doi: 10.1155/2016/7486425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pollock N.R., Campos-Neto A., Kashino S., Napolitano D., Behar S.M., Shin D., Sloutsky A., Joshi S., Guillet J., Wong M., et al. Discordant QuantiFERON-TB Gold test results among US healthcare workers with increased risk of latent tuberculosis infection: A problem or solution? Infect. Control Hosp. Epidemiol. 2008;29:878–886. doi: 10.1086/590262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chiappini E., Bonsignori F., Accetta G., Boddi V., Galli L., Biggeri A., De Martino M. Interferon-γ release assays for the diagnosis of Mycobacterium tuberculosis infection in children: A literature review. Int. J. Immunopathol. Pharmacol. 2012;25:335–343. doi: 10.1177/039463201202500203. [DOI] [PubMed] [Google Scholar]
- 152.Park I.N., Shim T.S. Qualitative and quantitative results of interferon-γ release assays for monitoring the response to anti-tuberculosis treatment. Korean J. Intern. Med. 2017;32:302–308. doi: 10.3904/kjim.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu M., Zhu Z., Yang J., Hu K. Performance evaluation of IGRA-ELISA and T-SPOT.TB for diagnosing tuberculosis infection. Clin. Lab. 2019;65 doi: 10.7754/Clin.Lab.2019.181109. [DOI] [PubMed] [Google Scholar]
- 154.Zellweger J.P., Sotgiu G., Corradi M., Durando P. The diagnosis of latent tuberculosis infection (LTBI): Currently available tests, future developments, and perspectives to eliminate tuberculosis (TB) Med. Lav. 2020;111:170–183. doi: 10.23749/mdl.v111i3.9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mukherjee P., Dutta M., Datta P., Dasgupta A., Pradhan R., Pradhan M., Kundu M., Basu J., Chakrabarti P. The RD1-encoded antigen Rv3872 of Mycobacterium tuberculosis as a potential candidate for serodiagnosis of tuberculosis. Clin. Microbiol. Infect. 2007;13:146–152. doi: 10.1111/j.1469-0691.2006.01660.x. [DOI] [PubMed] [Google Scholar]
- 156.Rao P.V., Murthy M.K., Basirudeen S., Sharma P., Swaminathan S., Raja A. Improved diagnosis of tuberculosis in HIV-positive patients using RD1-encoded antigen CFP-10. Int. J. Infect. Dis. 2009;13:613–622. doi: 10.1016/j.ijid.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 157.Al-Khodari N.Y., Al-Attiyah R., Mustafa A.S. Identification, diagnostic potential, and natural expression of immunodominant seroreactive peptides encoded by five Mycobacterium tuberculosis-specific genomic regions. Clin. Vaccine Immunol. 2011;18:477–482. doi: 10.1128/CVI.00405-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu J.N., Chen J.P., Chen D.L. Serodiagnosis efficacy and immunogenicity of the fusion protein of Mycobacterium tuberculosis composed of the 10-kilodalton culture filtrate protein, ESAT-6, and the extracellular domain fragment of PPE68. Clin. Vaccine Immunol. 2012;19:536–544. doi: 10.1128/CVI.05708-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang X., Su Z., Zhang X., Hu C., Yu J., Gao Q., Wang H. Generation of Mycobacterium tuberculosis-specific recombinant antigens and evaluation of the clinical value of antibody detection for serological diagnosis of pulmonary tuberculosis. Int. J. Mol. Med. 2013;31:751–757. doi: 10.3892/ijmm.2013.1254. [DOI] [PubMed] [Google Scholar]
- 160.Legesse M., Ameni G., Medhin G., Mamo G., Franken K.L., Ottenhoff T.H., Bjune G., Abebe F. IgA response to ESAT-6/CFP-10 and Rv2031 antigens varies in patients with culture-confirmed pulmonary tuberculosis, healthy Mycobacterium tuberculosis-infected and non-infected individuals in a tuberculosis endemic setting, Ethiopia. Scand. J. Immunol. 2013;78:266–274. doi: 10.1111/sji.12080. [DOI] [PubMed] [Google Scholar]
- 161.Tebianian M., Mosavari N., Taghizadeh M., Ebrahimi S.M. Evaluation of specific antibodies against Mycobacterium tuberculosis recombinant antigens for detection of recent infection. Int. J. Mycobacteriol. 2016;5(Suppl. S1):S254. doi: 10.1016/j.ijmyco.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 162.Ren N., JinLi J., Chen Y., Zhou X., Wang J., Ge P., Khan F.A., Zhang L., Hu C., Robertson I.D., et al. Identification of new diagnostic biomarkers for Mycobacterium tuberculosis and the potential application in the serodiagnosis of human tuberculosis. Microb. Biotechnol. 2018;11:893–904. doi: 10.1111/1751-7915.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dewi D.N.S.S., Mertaniasih N.M., Ozeki Y., Artama W.T., Fihiruddin, Niki M., Tateishi Y., Ato M., Matsumoto S. Characteristic profile of antibody responses to PPD, ESAT-6, and CFP-10 of Mycobacterium tuberculosis in pulmonary tuberculosis suspected cases in Surabaya, Indonesia. Braz. J. Infect. Dis. 2019;23:246–253. doi: 10.1016/j.bjid.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Van Pinxteren L.A., Ravn P., Agger E.M., Pollock J., Andersen P. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 2000;7:155–160. doi: 10.1128/CDLI.7.2.155-160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Arend S.M., Franken W.P., Aggerbeck H., Prins C., van Dissel J.T., Thierry-Carstensen B., Tingskov P.N., Weldingh K., Andersen P. Double-blind randomized Phase I study comparing rdESAT-6 to tuberculin as skin test reagent in the diagnosis of tuberculosis infection. Tuberculosis. 2008;88:249–261. doi: 10.1016/j.tube.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 166.Weldingh K., Andersen P. ESAT-6/CFP10 skin test predicts disease in M. tuberculosis-infected guinea pigs. PLoS ONE. 2008;3:e1978. doi: 10.1371/journal.pone.0001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hanif S.N., Al-Attiyah R., Mustafa A.S. Species-specific antigenic Mycobacterium tuberculosis proteins tested by delayed-type hypersensitivity response. Int. J. Tuberc. Lung Dis. 2010;14:489–494. [PubMed] [Google Scholar]
- 168.Mustafa A.S. Diagnostic and vaccine potentials of ESAT-6 family proteins encoded by M. tuberculosis genomic regions absent in M. bovis BCG. J. Mycobac. Dis. 2013;3:2. [Google Scholar]
- 169.Mustafa A.S. The future of Mycobacterium tuberculosis-specific antigens/peptides in tuberculin skin testing for the diagnosis of tuberculosis. J. Mycobac. Dis. 2014;4:3. [Google Scholar]
- 170.Ruhwald M., Aggerbeck H., Gallardo R.V., Hoff S.T., Villate J.I., Borregaard B., Martinez J.A., Kromann I., Penas A., Anibarro L.L. TESEC Working Group. Safety and efficacy of the C-Tb skin test to diagnose Mycobacterium tuberculosis infection, compared with an interferon γ release assay and the tuberculin skin test: A phase 3, double-blind, randomised, controlled trial. Lancet Respir. Med. 2017;5:259–268. doi: 10.1016/S2213-2600(16)30436-2. [DOI] [PubMed] [Google Scholar]
- 171.Zhang H., Wang L., Li F., Lu S., Xia J. Induration or erythema diameter not less than 5 mm as results of recombinant fusion protein ESAT6-CFP10 skin test for detecting M. tuberculosis infection. BMC Infect. Dis. 2020;20:685. doi: 10.1186/s12879-020-05413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pym A.S., Brodin P., Majlessi L., Brosch R., Demangel C., Williams A., Griffiths K.E., Marchal G., Leclerc C., Cole S.T. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 2003;9:533–539. doi: 10.1038/nm859. [DOI] [PubMed] [Google Scholar]
- 173.Demangel C., Garnier T., Rosenkrands I., Cole S.T. Differential effects of prior exposure to environmental mycobacteria on vaccination with Mycobacterium bovis BCG or a recombinant BCG strain expressing RD1 antigens. Infect. Immun. 2005;73:2190–2196. doi: 10.1128/IAI.73.4.2190-2196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hanif S.N., Al-Attiyah R., Mustafa A.S. DNA vaccine constructs expressing Mycobacterium tuberculosis-specific genes induce immune responses. Scand. J. Immunol. 2010;72:408–415. doi: 10.1111/j.1365-3083.2010.02452.x. [DOI] [PubMed] [Google Scholar]
- 175.Hanif S.N., Al-Attiyah R., Mustafa A.S. Cellular immune responses in mice induced by M. tuberculosis PE35-DNA vaccine construct. Scand. J. Immunol. 2011;74:554–560. doi: 10.1111/j.1365-3083.2011.02604.x. [DOI] [PubMed] [Google Scholar]
- 176.Shaban K., Amoudy H.A., Mustafa A.S. Cellular immune responses to recombinant Mycobacterium bovis BCG constructs expressing major antigens of region of difference 1 of Mycobacterium tuberculosis. Clin. Vaccine Immunol. 2013;20:1230–1237. doi: 10.1128/CVI.00090-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dai F.Y., Wang J.F., Gong X.L., Bao L. Immunogenicity and protective efficacy of recombinant Bacille Calmette-Guerin strains expressing mycobacterium antigens Ag85A, CFP10, ESAT-6, GM-CSF and IL-12p70. Hum. Vaccin. Immunother. 2017;13:1–8. doi: 10.1080/21645515.2017.1279771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ellis A., Balgeman A., Rodgers M., Updike C., Tomko J., Maiello P., Scanga C.A., O’Connor S.L. Characterization of T cells specific for CFP-10 and ESAT-6 in Mycobacterium tuberculosis-infected Mauritian Cynomolgus macaques. Infect Immun. 2017;85:e01009-16. doi: 10.1128/IAI.01009-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mustafa A.D., Kalyanasundram J., Sabidi S., Song A.A., Abdullah M., Abdul Rahim R., Yusoff K. Proof of concept in utilizing in-trans surface display system of Lactobacillus plantarum as mucosal tuberculosis vaccine via oral administration in mice. BMC Biotechnol. 2018;18:63. doi: 10.1186/s12896-018-0461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.GarcIa E.A., Blanco F.C., Muñiz X.F., Eirin M.E., Klepp L.I., Bigi F. Elimination of ESAT-6 and CFP-10 from a candidate vaccine against bovine tuberculosis impaired its protection efficacy in the BALBc mouse model. Int. J. Mycobacteriol. 2020;9:417–421. doi: 10.4103/ijmy.ijmy_180_20. [DOI] [PubMed] [Google Scholar]
- 181.Nadolinskaia N.I., Karpov D.S., Goncharenko A.V. Vaccines against tuberculosis: Problems and prospects (Review) Appl. Biochem. Microbiol. 2020;56:497–504. doi: 10.1134/S0003683820050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dey B., Jain R., Khera A., Rao V., Dhar N., Gupta U.D., Katoch V.M., Ramanathan V.D., Tyagi A.K. Boosting with a DNA vaccine expressing ESAT-6 (DNAE6) obliterates the protection imparted by recombinant BCG (rBCGE6) against aerosol Mycobacterium tuberculosis infection in guinea pigs. Vaccine. 2009;28:63–70. doi: 10.1016/j.vaccine.2009.09.121. [DOI] [PubMed] [Google Scholar]
- 183.Bottai D., Frigui W., Clark S., Rayner E., Zelmer A., Andreu N., de Jonge M.I., Bancroft G.J., Williams A., Brodin P., et al. Increased protective efficacy of recombinant BCG strains expressing virulence-neutral proteins of the ESX-1 secretion system. Vaccine. 2015;33:2710–2718. doi: 10.1016/j.vaccine.2015.03.083. [DOI] [PubMed] [Google Scholar]
- 184.Groschel M.I., Sayes F., Shin S.J., Frigui W., Pawlik A., Orgeur M., Canetti R., Honoré N., Simeone R., van der Werf T.S., et al. Recombinant BCG expressing ESX-1 of Mycobacterium marinum combines low virulence with cytosolic immune signaling and improved TB protection. Cell Rep. 2017;18:2752–2765. doi: 10.1016/j.celrep.2017.02.057. [DOI] [PubMed] [Google Scholar]
- 185.Chen Y., Lin C., Huang W., Chang J., Su I., Hsu C., Chemg H., Hsu S., Dou H. Recombinant bacille Calmette-Guerin coexpressing Ag85b, CFP10, and interleukin-12 elicits effective protection against Mycobacterium tuberculosis. J. Microbiol. Immunol. Infect. 2017;50:90–96. doi: 10.1016/j.jmii.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 186.Mustafa A.S. BCG as a vector for novel recombinant vaccines against infectious diseases and cancers. Vaccines. 2020;8:736. doi: 10.3390/vaccines8040736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tkachuk A.P., Gushchin V.A., Potapov V.D., Demidenko A.V., Lunin V.G., Gintsburg A.L. Multi-subunit BCG booster vaccine GamTBvac: Assessment of immunogenicity and protective efficacy in murine and guinea pig TB models. PLoS ONE. 2017;12:e0176784. doi: 10.1371/journal.pone.0176784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vasina D.V., Kleymenov D.A., Manuylov V.A., Mazunina E.P., Koptev E.Y., Tukhovskaya E.A., Murashev A.N., Gintsburg A.L., Gushchin V.A., Tkachuk A.P. First-in-human trials of GamTBvac, a recombinant subunit tuberculosis vaccine candidate: Safety and immunogenicity assessment. Vaccines. 2019;7:166. doi: 10.3390/vaccines7040166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tkachuk A.P., Bykonia E.N., Popova L.I., Kleymenov D.A., Semashko M.A., Chulanov V.P., Fitilev S.B., Maksimov S.L., Smolyarchuk E.A., Manuylov V.A., et al. Safety and immunogenicity of the GamTBvac, the recombinant subunit tuberculosis vaccine candidate: A Phase II, multi-center, double-blind, randomized, placebo-controlled study. Vaccines. 2020;8:652. doi: 10.3390/vaccines8040652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Whitlow E., Mustafa A.S., Hanif S.N.M. An Overview of the Development of New Vaccines for Tuberculosis. Vaccines. 2020;8:586. doi: 10.3390/vaccines8040586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Safar H.A., Mustafa A.S., McHugh T.D. COVID-19 vaccine development: What lessons can we learn from TB? Ann. Clin. Microbiol. Antimicrob. 2020;19:56. doi: 10.1186/s12941-020-00402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Van Dissel J.T., Arend S.M., Prins C., Bang P., Tingskov P.N., Lingnau K., Nouta J., Klein M.R., Rosenkrands I., Ottenhoff T.H., et al. Ag85B-ESAT-6 adjuvanted with IC31 promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naïve human volunteers. Vaccine. 2010;28:3571–35781. doi: 10.1016/j.vaccine.2010.02.094. [DOI] [PubMed] [Google Scholar]
- 193.Rodo M.J., Rozot V., Nemes E., Dintwe O., Hatherill M., Little F., Scriba T.J. A comparison of antigen-specific T cell responses induced by six novel tuberculosis vaccine candidates. PLoS Pathog. 2019;15:e1007643. doi: 10.1371/journal.ppat.1007643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mustafa A.S., Shaban F.A., Abal A.T., Al-Attiyah R., Wiker H.G., Lundin K.E., Oftung F., Huygen K. Identification and HLA restriction of naturally derived Th1-cell epitopes from the secreted Mycobacterium tuberculosis antigen 85B recognized by antigen-specific human CD4(+) T-cell lines. Infect. Immun. 2000;68:3933–3940. doi: 10.1128/IAI.68.7.3933-3940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mustafa A.S., Abal A.T., Shaban F., El-Shamy A.M., Amoudy H.A. HLA-DR binding prediction and experimental evaluation of T-cell epitopes of mycolyl transferase 85B (Ag85B), a major secreted antigen of Mycobacterium tuberculosis. Med. Princ. Pract. 2005;14:140–146. doi: 10.1159/000084629. [DOI] [PubMed] [Google Scholar]
- 196.He H., Yang H., Deng Y. Mycobacterium tuberculosis dormancy-associated antigen of Rv2660c induces stronger immune response in latent Mycobacterium tuberculosis infection than that in active tuberculosis in a Chinese population. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:1103–1109. doi: 10.1007/s10096-015-2335-8. [DOI] [PubMed] [Google Scholar]
- 197.Lin P.L., Dietrich J., Tan E., Abalos R.M., Burgos J., Bigbee C., Bigbee M., Milk L., Gideon H.P., Rodgers M., et al. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J. Clin. Investig. 2012;122:303–314. doi: 10.1172/JCI46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Billeskov R., Tan E.V., Cang M., Abalos R.M., Burgos J., Pedersen B.V., Christensen D., Agger E.M., Andersen P. Testing the H56 vaccine delivered in 4 different adjuvants as a BCG-booster in a non-human primate model of tuberculosis. PLoS ONE. 2016;11:e0161217. doi: 10.1371/journal.pone.0161217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Reither K., Katsoulis L., Beattie T., Gardiner N., Lenz N., Said K., Mfinanga E., Pohl C., Fielding K.L., Jeffer Y.H., et al. Safety and immunogenicity of H1/IC31®, an adjuvanted TB subunit vaccine, in HIV-infected adults with CD4+ lymphocyte counts greater than 350 cells/mm3: A phase II, multi-centre, double-blind, randomized, placebo-controlled trial. PLoS ONE. 2014;9:e114602. doi: 10.1371/journal.pone.0114602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lenz N., Schindler T., Kagina B.M., Zhang J.D., Lukindo T., Mpina M., Bang P., Kromann I., Hoff S.T., Andersen P., et al. Antiviral innate immune activation in HIV-infected adults negatively affects H1/IC31-induced vaccine-specific memory CD4+ T Cells. Clin. Vaccine Immunol. 2015;22:688–696. doi: 10.1128/CVI.00092-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hussein J., Zewdie M., Yamuah L., Bedru A., Abebe M., Dagnew A.F., Chanyalew M., Yohannes A.G., Ahmed J., Engers H., et al. A phase I, open-label trial on the safety and immunogenicity of the adjuvanted tuberculosis subunit vaccine H1/IC31® in people living in a TB-endemic area. Trials. 2018;19:24. doi: 10.1186/s13063-017-2354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mearns H., Geldenhuys H.D., Kagina B.M., Musvosvi M., Little F., Ratangee F., Mahomed H., Hanekom W.A., Hoff S.T., Ruhwald M., et al. THYB04 study group. H1:IC31 vaccination is safe and induces long-lived TNF-α+IL-2+CD4 T cell responses in M. tuberculosis infected and uninfected adolescents: A randomized trial. Vaccine. 2017;35:132–141. doi: 10.1016/j.vaccine.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 203.Suliman S., Luabeya A.K.K., Geldenhuys H., Tameris M., Hoff S.T., Shi Z., Tait D., Kromann I., Ruhwald M., Rutkowski K.T., et al. H56-035 trial group. Dose optimization of H56:IC31 vaccine for tuberculosis-endemic populations. A double-blind, placebo-controlled, dose-selection trial. Am. J. Respir. Crit. Care Med. 2019;199:220–231. doi: 10.1164/rccm.201802-0366OC. [DOI] [PubMed] [Google Scholar]
- 204.Bekker L.G., Dintwe O., Fiore-Gartland A., Middelkoop K., Hutter J., Williams A., Randhawa A.K., Ruhwald M., Kromann I., Andersen P.L., et al. HVTN 602/Aeras A-042 Protocol Team. A phase 1b randomized study of the safety and immunological responses to vaccination with H4:IC31, H56:IC31, and BCG revaccination in Mycobacterium tuberculosis-uninfected adolescents in Cape Town, South Africa. EClinicalMedicine. 2020;21:100313. doi: 10.1016/j.eclinm.2020.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mustafa A.S. Progress towards the development of new anti-tuberculosis vaccines. In: Smithe L.T., editor. Focus on Tuberculosis Research. Nova Science Publishers, Inc.; New York, NY, USA: 2005. pp. 47–76. [Google Scholar]
- 206.Jones G.J., Gordon S.V., Hewinson R.G., Vordermeier H.M. Screening of predicted secreted antigens from Mycobacterium bovis reveals the immunodominance of the ESAT-6 protein family. Infect. Immun. 2010;78:1326–1332. doi: 10.1128/IAI.01246-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jones G.J., Hewinson R.G., Vordermeier H.M. Screening of predicted secreted antigens from Mycobacterium bovis identifies potential novel differential diagnostic reagents. Clin. Vaccine Immunol. 2010;17:1344–1348. doi: 10.1128/CVI.00261-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lewinsohn D.M., Swarbrick G.M., Cansler M.E., Null M.D., Rajaraman V., Frieder M.M., Sherman D.R., McWeeney S., Lewinsohn D.A. Human Mycobacterium tuberculosis CD8 T cell antigens/epitopes identified by a proteomic peptide library. PLoS ONE. 2013;8:e67016. doi: 10.1371/journal.pone.0067016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Safar H.A., Mustafa A.S., Amoudy H.A., El-Hashim A. The effect of adjuvants and delivery systems on Th1, Th2, Th17 and Treg cytokine responses in mice immunized with Mycobacterium tuberculosis-specific proteins. PLoS ONE. 2020;15:e0228381. doi: 10.1371/journal.pone.0228381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mahmood A., Srivastava S., Tripathi S., Ansari M.A., Owais M., Arora A. Molecular characterization of secretory proteins Rv3619c and Rv3620c from Mycobacterium tuberculosis H37Rv. FEBS J. 2011;278:341–353. doi: 10.1111/j.1742-4658.2010.07958.x. [DOI] [PubMed] [Google Scholar]
- 211.Yang E., Lu Y., Xu Y., Liang Q., Wang C., Wang H., Shen H. Recombinant BCG coexpressing Ag85B, ESAT-6 and Rv3620c elicits specific Th1 immune responses in C57BL/6 mice. Microb. Pathog. 2014;69–70:53–59. doi: 10.1016/j.micpath.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 212.Ansari M.A., Zubair S., Mahmood A., Gupta P., Khan A.A., Gupta U.D., Arora A., Owais M. RD antigen based nanovaccine imparts long term protection by inducing memory response against experimental murine tuberculosis. PLoS ONE. 2011;6:e22889. doi: 10.1371/journal.pone.0022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Safar H.A., El-Hashim A.Z., Amoudy H., Mustafa A.S. Mycobacterium tuberculosis-specific antigen Rv3619c effectively alleviates allergic asthma in mice. Front. Pharmacol. 2020;11:532199. doi: 10.3389/fphar.2020.532199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Duthie M.S., Coler R.N., Laurance J.D., Sampaio L.H., Oliveira R.M., Sousa A.L., Stefani M.M., Maeda Y., Matsuoka M., Makino M., et al. Protection against Mycobacterium leprae infection by the ID83/GLA-SE and ID93/GLA-SE vaccines developed for tuberculosis. Infect. Immun. 2014;82:3979–3985. doi: 10.1128/IAI.02145-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Baldwin S.L., Reese V., Granger B., Orr M.T., Ireton G.C., Coler R.N., Reed S.G. The ID93 tuberculosis vaccine candidate does not induce sensitivity to purified protein derivative. Clin. Vaccine Immunol. 2014;21:1309–1313. doi: 10.1128/CVI.00372-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Baldwin S.L., Reese V.A., Huang P.W.D., Beebe E.A., Podell B.K., Reed S.G., Coler R.N. Protection and long-lived immunity induced by the ID93/GLA-se vaccine candidate against a clinical Mycobacterium tuberculosis isolate. Clin. Vaccine Immunol. 2016;23:137–147. doi: 10.1128/CVI.00458-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cha S.B., Kim W.S., Kim J.S., Kim H., Kwon K.W., Han S.J., Cho S.N., Coler R.N., Reed S.G., Shin S.J. Pulmonary immunity and durable protection induced by the ID93/GLA-SE vaccine candidate against the hyper-virulent Korean Beijing Mycobacterium tuberculosis strain K. Vaccine. 2016;34:2179–2187. doi: 10.1016/j.vaccine.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 218.Larsen S.E., Baldwin S.L., Orr M.T., Reese V.A., Pecor T., Granger B., Dubois Cauwelaert N., Podell B.K., Coler R.N. Enhanced anti-Mycobacterium tuberculosis immunity over time with combined drug and immunotherapy treatment. Vaccines. 2018;6:30. doi: 10.3390/vaccines6020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kwon K.W., Lee A., Larsen S.E., Baldwin S.L., Coler R.N., Reed S.G., Cho S.N., Ha S.J., Shin S.J. Long-term protective efficacy with a BCG-prime ID93/GLA-SE boost regimen against the hyper-virulent Mycobacterium tuberculosis strain K in a mouse model. Sci. Rep. 2019;9:15560. doi: 10.1038/s41598-019-52146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Penn-Nicholson A., Tameris M., Smit E., Day T.A., Musvosvi M., Jayashankar L., Vergara J., Mabwe S., Bilek N., Geldenhuys H., et al. TBVPX-114 study team. Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: A randomised, double-blind, placebo-controlled phase 1 trial. Lancet Respir. Med. 2018;6:287–298. doi: 10.1016/S2213-2600(18)30077-8. [DOI] [PubMed] [Google Scholar]
- 221.Coler R.N., Day T.A., Ellis R., Piazza F.M., Beckmann A.M., Vergara J., Rolf T., Lu L., Alter G., Hokey D., et al. TBVPX-113 Study Team. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: First-in-human trial. NPJ Vaccines. 2018;3:34. doi: 10.1038/s41541-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]