Abstract
Background
To mitigate the effects of COVID-19, a vaccine is urgently needed. BBV152 is a whole-virion inactivated SARS-CoV-2 vaccine formulated with a toll-like receptor 7/8 agonist molecule adsorbed to alum (Algel-IMDG) or alum (Algel).
Methods
We did a double-blind, multicentre, randomised, controlled phase 1 trial to assess the safety and immunogenicity of BBV152 at 11 hospitals across India. Healthy adults aged 18–55 years who were deemed healthy by the investigator were eligible. Individuals with positive SARS-CoV-2 nucleic acid and/or serology tests were excluded. Participants were randomly assigned to receive either one of three vaccine formulations (3 μg with Algel-IMDG, 6 μg with Algel-IMDG, or 6 μg with Algel) or an Algel only control vaccine group. Block randomisation was done with a web response platform. Participants and investigators were masked to treatment group allocation. Two intramuscular doses of vaccines were administered on day 0 (the day of randomisation) and day 14. Primary outcomes were solicited local and systemic reactogenicity events at 2 h and 7 days after vaccination and throughout the full study duration, including serious adverse events. Secondary outcome was seroconversion (at least four-fold increase from baseline) based on wild-type virus neutralisation. Cell-mediated responses were evaluated by intracellular staining and ELISpot. The trial is registered at ClinicalTrials.gov (NCT04471519).
Findings
Between July 13 and 30, 2020, 827 participants were screened, of whom 375 were enrolled. Among the enrolled participants, 100 each were randomly assigned to the three vaccine groups, and 75 were randomly assigned to the control group (Algel only). After both doses, solicited local and systemic adverse reactions were reported by 17 (17%; 95% CI 10·5–26·1) participants in the 3 μg with Algel-IMDG group, 21 (21%; 13·8–30·5) in the 6 μg with Algel-IMDG group, 14 (14%; 8·1–22·7) in the 6 μg with Algel group, and ten (10%; 6·9–23·6) in the Algel-only group. The most common solicited adverse events were injection site pain (17 [5%] of 375 participants), headache (13 [3%]), fatigue (11 [3%]), fever (nine [2%]), and nausea or vomiting (seven [2%]). All solicited adverse events were mild (43 [69%] of 62) or moderate (19 [31%]) and were more frequent after the first dose. One serious adverse event of viral pneumonitis was reported in the 6 μg with Algel group, unrelated to the vaccine. Seroconversion rates (%) were 87·9, 91·9, and 82·8 in the 3 μg with Algel-IMDG, 6 μg with Algel-IMDG, and 6 μg with Algel groups, respectively. CD4+ and CD8+ T-cell responses were detected in a subset of 16 participants from both Algel-IMDG groups.
Interpretation
BBV152 led to tolerable safety outcomes and enhanced immune responses. Both Algel-IMDG formulations were selected for phase 2 immunogenicity trials. Further efficacy trials are warranted.
Funding
Bharat Biotech International.
Introduction
Spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections has led to a global COVID-19 pandemic. Vaccines from multiple manufacturers will be needed to address the global need for SARS-CoV-2 vaccines and thus far, 194 vaccine candidates are in development.1
A desirable characteristic for any COVID-19 vaccine candidate is the ability to induce T-helper-1 cell (Th1) responses.2 Whole-virion inactivated vaccines are usually formulated with Alum, which does not have the ability to induce cell-mediated responses.3, 4 An imidazoquinoline molecule, which is a toll-like receptor (TLR) 7/8 agonist, has been used to stimulate cell-mediated responses.5, 6 Algel-IMDG (an imidazoquinoline molecule chemisorbed on alum [Algel]) has been designed to traffic vaccine antigen directly to draining lymph nodes without diffusing into the systemic circulation. BBV152 is a whole-virion inactivated SARS-CoV-2 vaccine adjuvanted with Algel-IMDG.
Preclinical studies in mice, rats, and rabbits showed appropriate safety profiles and humoral and cell-mediated responses.7 Two live viral challenge protective efficacy studies in hamsters and non-human primates were done. In both studies, protection was evident by rapid clearance of virus in the lower and upper respiratory tract, and absence of lung pathology (after viral challenge).8, 9 Here, we report the interim findings from the randomised, controlled, double-blind phase 1 trial on the safety and immunogenicity of three different formulations of BBV152 and one control group containing Algel (without antigen). This phase 1 trial was done with the intention of selecting two formulations for progression to the phase 2 trial.
Research in context.
Evidence before this study
We searched PubMed on Jan 15, 2020, for published research articles using the search terms “SARS-CoV-2”, “COVID-19”, “vaccine”, and “clinical trial”, with no language or date restrictions. We found several publications on COVID-19 vaccine clinical trials from mRNA, adenovirus, protein subunit, and inactivated vaccines.
As of Jan 15, 2020, nine vaccines have received emergency use authorisation to be administered to prevent COVID-19. Inactivated vaccines have been approved for decades with well established safety profiles. Immune responses from two other inactivated vaccines have been reported; however, with few results on cell-mediated responses. Bharat Biotech has developed a vero cell-based whole-virion inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine (BBV152) formulated with alum and a TLR7/8 agonist producing a T-helper-1 cell skewed response. This vaccine candidate reported protection in two live viral non-human primate and hamster challenge models.
Added value of this study
We report the preliminary analyses for the safety and immunogenicity of the vaccine candidate BBV152 in 375 vaccinated adults. All vaccine groups had similar reactogenicity and serological outcomes to the control group. BBV152 led to enhanced immune responses; the 3-μg and 6-μg Algel-IMDG vaccines induced T-cell responses that were biased to T-helper-1 cells.
Implications of all the available evidence
Findings from other inactivated SARS-CoV-2 vaccine candidates are corroborating. However, to the best of our knowledge, ours is the only reported inactivated COVID-19 vaccine candidate inducing cell-mediated responses and humoral neutralising responses. Both Algel-IMDG formulations will be assessed in a phase 2 immunogenicity trial.
Methods
Study design and participants
This is a randomised, double-blind, multicentre, phase 1 trial to assess the safety, reactogenicity, tolerability, and immunogenicity of the whole-virion inactivated SARS-CoV-2 vaccine (BBV152) in healthy adult volunteers, at 11 hospitals across nine states of India (appendix pp 5, 13). Participants were aged 18–55 years and deemed healthy by the investigator at the time of enrolment. At the screening visit, participants were tested with both SARS-CoV-2 nucleic acid (TRUPCR SARS-CoV-2 RT-PCR; 3B BlackBio Biotech, Bhopal, India) and serology (chemiluminescence immunoassay; LIAISON SARS-CoV-2 S1/S2 IgG; DiaSorin, Saluggia, Italy) tests (conducted at Dr Dangs Lab [New Delhi, India] using commercially available assays; appendix p 3). If found positive for any one test, they were excluded from the trial. The median time between the screening visit and vaccination visit was 4 days (range 3–6). Other key exclusion criteria were an axillary temperature of more than 37·0°C and known allergy to any vaccine component. Participants were screened for eligibility on the basis of their health status, including their medical history, laboratory findings (haematology, biochemistry, and urine tests), vital signs, and physical examination results, and were enrolled after providing signed and dated informed consent forms. Full inclusion and exclusion criteria are in the protocol.
The trial was approved by the National Regulatory Authority (India) and the respective ethics committees and was conducted in compliance with all International Council for Harmonization Good Clinical Practice guidelines.
Randomisation and masking
The master randomisation list was uploaded on the interactive web response system, which contained the randomisation number and intended allocation. The depot manager uploaded the kit code list and assigned the kits to the sites that had the kit codes and the allocation groups. At the site level, the system would set the randomisation number and the allotment of the kit without displaying the true group allocation, and the system would allocate the same treatment group for the second visit. For the first 50 participants, a block size of five with ten blocks was generated for the 3 μg with Algel-IMDG and control groups at a ratio of 4:1. In the remaining participants, the number of blocks was 20. For the first 15 blocks, a block size of 16 was used to randomly assign participants (3:5:5:3) to 3 μg with Algel-IMDG, 6 μg with Algel-IMDG, 6 μg with Algel, or Algel-only control. The next five blocks were size 17, and used to randomly assign participants (3:5:5:4) to 3 μg with Algel-IMDG, 6 μg with Algel-IMDG, 6 μg with Algel, or Algel-only control. An unmasked contract research organisation, Sclin Soft Technologies, generated the randomisation list for the study.
Participants, investigators, study coordinators, study-related personnel, and the funder were masked to treatment group allocation (excluding an unmasked member of the contract research organisation, who was tasked with the dispatch and labelling of vaccine vials and the generation of the master randomisation code). Participants were assigned a computer-generated randomisation code that maintained masking. The masked study nurse was responsible for vaccine preparation and administration. Each vial contained a unique code that ensured appropriate masking. The appearance, colour, and viscosity were identical across all vaccine and control formulations.
Procedures
The virus strain (NIV-2020-770) containing the Asp614Gly mutation, isolated from a COVID-19 patient and sequenced at the Indian Council of Medical Research National Institute of Virology, was provided to Bharat Biotech.10 Biosafety level 3 manufacturing facilities and a well established Vero cell manufacturing platform (with proven safety in other licensed live and inactivated vaccines) were used for the rapid development of BBV152.11, 12, 13, 14, 15, 16
BBV152 (manufactured by Bharat Biotech) is a whole-virion β-propiolactone-inactivated SARS-CoV-2 vaccine. The NIV-2020-770 strain contains the Asp614Gly mutation, which is characterised by aspartic acid to glycine shift at the amino acid position 614 of the spike protein.10
The candidates were formulated with two adjuvants: Algel (alum) and Algel-IMDG, an imidazoquinoline class molecule (TLR7 and TLR8 agonist) adsorbed onto Algel. After their eligibility was established, participants were assigned to the four groups. The control group contained only a sterile phosphate-buffered solution and Algel. Both the vaccine and control were stored at 2–8°C.
The vaccine (BBV152) and the control were provided as a sterile liquid that was injected intramuscularly (deltoid muscle) at a volume of 0·5 mL/dose in a two-dose regimen on day 0 (day of randomisation) and day 14. This accelerated schedule was chosen given the context of the ongoing pandemic. No onsite dose preparation was required. Each glass vial contained a single dose of either vaccine or control formulation that required no additional dilution steps. No prophylactic medication (ibuprofen or acetaminophen) was prescribed either before or after vaccination.
The follow-up visits were scheduled on days 7, 28, 42, 104, and 194 after vaccination. The study was done in a dose-escalation manner after completing vaccination in the first 50 participants with 3 μg with Algel-IMDG (the lowest antigen concentration) and the control; these participants were monitored for 7 days for safety. The independent data safety monitoring board reviewed masked safety data and decided whether the trial was allowed to continue with enrolment of the remaining participants into all groups.
Outcomes
The primary outcome was the number and proportion of participants with solicited local and systemic reactogenicity events at 2 h and 7 days after vaccination and throughout the full study duration, including serious adverse events. The secondary outcomes was immunogenicity, in terms of geometric mean titres (GMTs) and four-fold seroconversion rate of neutralising antibodies, from baseline to days 14, 28, 42, 104, and 194.
Safety assessments
The unsolicited adverse events were recorded for 28 days after vaccination. Laboratory values (serum chemistry, haematology, and urine) were compared before vaccination (day 0) and after vaccination (day 28).
Participants were observed for 2 h after vaccination to assess reactogenicity. They were instructed to record local and systemic reactions within 7 days (days 0–7 and days 14–21) after vaccination using a diary card. The diary card contained fields for symptom onset, severity, time to resolution, concomitant medication, and was collected during the next visit to the site. Routine telephone calls were scheduled after the first 7 days after each vaccination.
Solicited local adverse events were pain at the injection site and swelling, and systemic adverse events, including fever, fatigue or malaise, myalgia, body aches, headaches, nausea or vomiting, anorexia, chills, generalised rash, and diarrhoea. All unsolicited adverse events were reported by participants throughout the study. Adverse events were graded according to the severity score (mild, moderate, or severe) and whether they were related or not related to the investigational vaccine, as detailed in the protocol (appendix p 6).
Immunogenicity assessments
IgG responses against the spike (S1) glycoprotein, receptor-binding domain, and nucleocapsid protein of SARS-CoV-2 were assessed by an in-house-developed ELISA and are expressed as GMTs. Neutralising antibody titres were assessed by wild-type virus neutralisation assays: a microneutralisation assay (MNT50) and a plaque-reduction neutralisation test (PRNT50), at Bharat Biotech. These assays were based on the Asp614Gly strain (appendix p 4). To establish interlaboratory comparability, a subset of randomly selected serum samples (n=50) was analysed by MNT50 at the National Institute of Virology. Additionally, three laboratory strains were used in vitro for PRNT50 at the National Institute of Virology: the BBV152 strain NIV-2020-770 homologous, and two heterologous strains from the O clade (nCoV-Q111 and nCoV-Q100). Genomic analyses of strains were reported by Potdar and colleagues.17 Only the NIV-2020-770 strain contained the Asp614Gly mutation.10
To compare vaccine-induced responses to natural SARS-CoV-2 infections, 41 convalescent serum samples (collected within 1–3 months after nucleic acid test-based diagnosis) were tested by MNT50. These serum samples were collected from both self-reported symptomatic (n=25) and asymptomatic (n=16) patients with COVID-19 at Nizam's Institute of Medical Sciences (NIMS; Hyderabad, India). The age of these participants was 23–62 years. For symptomatic patients, ascertainment of severity grading and requirement of supplemental oxygen was not obtainable. A participant who achieved seroconversion was defined as having a post-vaccination titre at least four-fold greater than their respective pre-vaccination titre. Serum samples were analysed in a masked manner at Bharat Biotech and the National Institute of Virology.
Cell-mediated responses were assessed in a subset of participants at one site (NIMS). The contract research organisation generated a random code containing randomisation numbers, which was provided to the staff to identify participants. Blood (3–5 mL) was collected from those participants who consented to the additional volume on days 0 and 28. Peripheral blood mononuclear cells were collected to assess IFN-γ by ELISpot (13 in vaccinated groups and six in the control group). Intracellular cytokine staining was used to assess T-cell responses in the remaining samples that contained an adequate number of cells. To ensure equal distribution, eight samples in each vaccine group were selected. These assays were done at Indoor Biotechnologies (Bangalore, India) and Bharat Biotech. All samples were analysed in a masked manner. The details of all assay methods are in the appendix (p 5).
Statistical analysis
Using a two-sided 5% significance level, power was calculated for several levels of the absolute difference between seroconversion rates for vaccine formulations, and we decided on the power to find a statistically significant difference between rates if the true underlying absolute difference was at least 20%. The allocation ratio was 1:1:1 for three vaccine formulations and 4:1 for the vaccine (all formulations combined) to placebo. The placebo group was not included in the sample size calculations. For a sample size of 90 for each formulation, the power to find a statistically significant absolute difference for a true underlying difference of 20% was at least 80% if the lower seroconversion rate for two formulations was at least 52%, which is lower than the seroconversion rate we expected for an effective vaccine. The sample size chosen was 100 per vaccine formulation, to allow for loss of data because of withdrawals or loss to follow-up. We did not incorporate an adjustment for multiple comparisons, because this phase 1 study was not a pivotal study for licensure, and we planned to choose two vaccine formulations from the phase 1 study for further assessment. Sample size estimation was done using PASS 13 software, version 13.0.17.
Safety endpoints are described as frequencies (%). GMTs with 95% CI are used for immunological endpoints. For continuous variables (<20 observations), medians and IQRs are reported. The exact binomial calculation was used for the CI estimation of proportions. The Wilson's test was used to test differences in proportions. CI estimation for the GMT was based on the log10 (titre) and the assumption that the log10 (titre) was normally distributed. A comparison of GMTs was done with t tests on the means of the log10 (titre). Significance was set at p<0·05 (two-sided). This preliminary report contains results regarding immunogenicity (days 0–28) and safety outcomes (days 0–42). Descriptive and inferential statistics were assessed using SAS, version 9.2. The trial was registered at ClinicalTrials.gov (NCT04471519).
Role of the funding source
The funder of the study had no role in data collection, data analysis, data interpretation, or writing of the statistical report, but was involved in study design. Data cleaning and analysis was conducted by a third party contract research organisation (Sclin Soft Technologies). Masked laboratory assessments were done at the respective laboratories and masked data sheets were sent to the contract research organisation for decoding and analysis. The unmasked randomisation list was not shared with the sponsor. All authors had full access to masked data in the study and had final responsibility for the decision to submit for publication.
Results
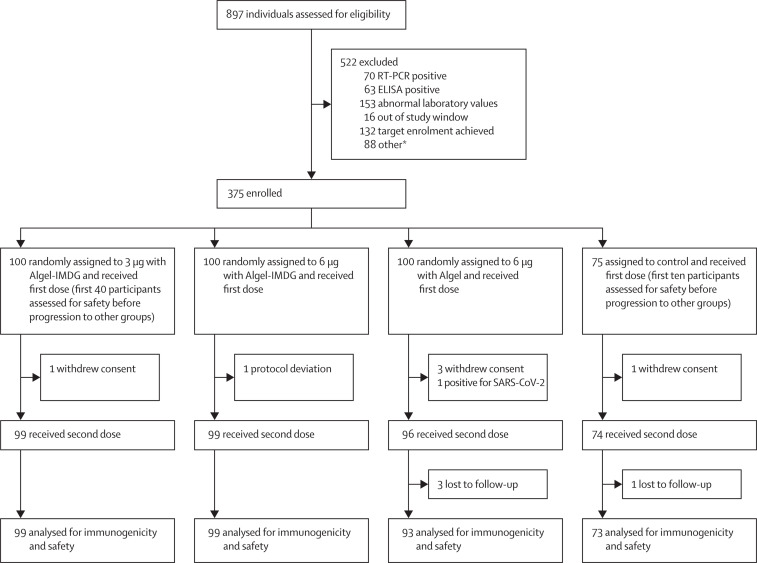
Between July 13 and 30, 2020, 897 individuals were screened and 375 were enrolled. Of the 522 initially screened individuals who were excluded, 133 participants were excluded because they were positive for SARS-CoV-2 by nucleic acid test or serology and 153 were excluded because of abnormal laboratory values (figure 1 ). The first 50 participants enrolled were monitored for 7 days after vaccination, and on the basis of the independent data safety monitoring board review of masked safety data, the trial was allowed to continue with enrolment of the remaining participants into all groups. Among the enrolled participants, 100 each were randomly assigned to the three vaccine groups, and 75 were randomly assigned to the control group (Algel only). Demographic characteristics of the participants were similar across groups (table 1 ).
Figure 1.
Trial profile
Due to a sudden increase in interest from potential participants, parallel enrolment at several sites, and an oversight in the trial software, 27 of the 132 people who reached target enrolment after the planned size of 375 people was reached received a first dose and 26 of them a second dose. They were not included in further safety or immunogenicity trial data, but were monitored for safety with 3 people reporting injection site pain and 1 person a tingling sensation after the first dose, and 1 person drowsiness, 2 people headache, and 1 person injection site pain after the second dose. No serious adverse events occurred. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. *Unable to contact the participant for vaccination or withdrawal of consent.
Table 1.
Demographic characteristics of the participants in the intention-to-treat population
| BBV152 3 μg with Algel-IMDG(n=100) | BBV152 6 μg with Algel-IMDG (n=100) | BBV152 6 μg with Algel(n=100) | Algel only (n=75) | ||
|---|---|---|---|---|---|
| Age, years | |||||
| Median (IQR) | 32·5 (25·0–40·0) | 35·0 (25·0–40·0) | 32·0 (25·0–40·0) | 29·0 (24·0–38·0) | |
| ≥18 to ≤25 | 29 (29%) | 28 (28%) | 31 (31%) | 22 (29%) | |
| ≥26 to ≤40 | 47 (47%) | 47 (47%) | 45 (45%) | 37 (49%) | |
| >40 to ≤55 | 24 (24%) | 25 (25%) | 24 (24%) | 16 (21%) | |
| Sex | |||||
| Men | 78 (78%) | 82 (82%) | 76 (76%) | 61 (81%) | |
| Women | 22 (22%) | 18 (18%) | 24 (24%) | 14 (19%) | |
| Body-mass index*, kg/m2 | 24·8 (3·5) | 25·8 (4·2) | 24·9 (3·7) | 24·6 (3·5) | |
| Vital signs | |||||
| Systolic blood pressure, mm Hg | 122·9 (8·5) | 123·5 (7·9) | 121·6 (8·3) | 123·6 (8·5) | |
| Diastolic blood pressure, mm Hg | 79·4 (5·9) | 79·3 (6·5) | 79·2 (5·3) | 79·4 (6·4) | |
| Pulse rate, beats per min | 77·4 (7·3) | 78·1 (8·2) | 78·0 (5·9) | 78·3 (7·6) | |
| Respiratory rate, breaths per min | 16·9 (2·3) | 16·7 (2·6) | 17·1 (2·6) | 16·9 (2·2) | |
| Temperature, °C | 36·6 (0·4) | 36·5 (0·6) | 36·5 (0·4) | 36·6 (0·4) | |
| Sites | |||||
| All India Institute of Medical Sciences, New Delhi | 3 (3%) | 6 (6%) | 3 (3%) | 4 (5%) | |
| All India Institute of Medical Sciences, Patna | 25 (25%) | 9 (9%) | 6 (6%) | 7 (9%) | |
| Gillukar Multispeciality Hospital | 10 (10%) | 14 (14%) | 19 (19%) | 12 (16%) | |
| Institute of Medical Sciences and SUM Hospital | 4 (4%) | 5 (5%) | 9 (9%) | 5 (7%) | |
| Jeevan Rekha Hospital | 1 (1%) | 1 (1%) | 2 (2%) | 0 | |
| Nizam's Institute of Medical Sciences | 11 (11%) | 14 (14%) | 15 (15%) | 7 (9%) | |
| Pandit Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences | 22 (22%) | 10 (10%) | 15 (15%) | 16 (21%) | |
| Prakhar Hospital | 8 (8%) | 10 (10%) | 11 (11%) | 10 (13%) | |
| Rana Hospital and Trauma Centre | 1 (1%) | 3 (3%) | 2 (2%) | 2 (3%) | |
| Redkar Hospital | 7 (7%) | 14 (14%) | 13 (13%) | 9 (12%) | |
| SRM Hospital and Research Center | 8 (8%) | 14 (14%) | 5 (5%) | 3 (4%) | |
Data are n (%) or mean (SD) unless otherwise stated. The intention-to-treat population included all participants who received at least one dose.
Calculation was based on the bodyweight and height measured at the time of screening. No data on race were collected; all participants were south Asian.
After dose 1, solicited local adverse reactions were reported by five (5%; 95% CI 1·9–11·8) participants in the 3 μg with Algel-IMDG group, five (5%; 1·9–11·8) in the 6 μg with Algel-IMDG group, one (1%; 0·05–6·2) in the 6 μg with Algel group, and three (3%; 1·04–12·03), in the Algel-only control group. Solicited systemic adverse reactions were reported by five (5%; 1·9–11·8) participants in the 3 μg with Algel-IMDG group, 14 (14%; 8·1–22·7) in the 6 μg with Algel-IMDG group, eight (8%; 3·8–15·6) in the 6 μg with Algel group, and seven (7%; 4·2–18·9) in the Algel-only group (table 2 ; appendix p 14). The most common solicited adverse events were injection site pain (17 [5%] of 375 participants), headache (13 [3%]), fatigue (11 [3%]), fever (nine [2%]), and nausea or vomiting (seven [2%]). All adverse events were mild or moderate in severity and resolved within 24 h of onset. After both doses, solicited local and systemic adverse reactions were reported by 17 (17%; 95% CI 10·5–26·1) participants in the 3 μg with Algel-IMDG group, 21 (21%; 13·8–30·5) in the 6 μg with Algel-IMDG group, 14 (14%; 8·1–22·7) in the 6 μg with Algel group, and ten (10%; 6·9–23·6) in the Algel-only group. All adverse events were mild (43 [69%] of 62) or moderate (19 [31%]) and were more frequent after the first dose than the second. No significant differences were observed between the vaccinated and control groups.
Table 2.
Solicited adverse events in the safety set
|
Dose 1 |
Dose 2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 μg with Algel-IMDG (n=100) | 6 μg with Algel-IMDG (n=100) | 6 μg with Algel (n=100) | Algel only (n=75) | 3 μg with Algel-IMDG (n=100) | 6 μg with Algel-IMDG (n=100) | 6 μg with Algel (n=100) | Algel only (n=75) | ||
| Local reactions | |||||||||
| Pain at injection site | |||||||||
| Mild | 4 (4%; 1·1– 9·9) | 4 (4%; 1·1–9·9) | 1 (1%; 0·0–5·5) | 2 (3%; 0·3–9·3) | 2 (2%; 0·2–7·0) | 1 (1%; 0·03–5·5) | 1 (1%; 0·0–5·5) | 0 | |
| Moderate | 1 (1%; 0·0–5·5) | 1 (1%; 0·0–5·5) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Swelling | |||||||||
| Mild | 0 | 0 | 0 | 1 (1%; 0·0–7·2) | 0 | 0 | 0 | 0 | |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Systemic reactions | |||||||||
| Fever | |||||||||
| Mild | 0 | 1 (1%; 0·0–5·5) | 1 (1%; 0·0–5·5) | 0 | 2 (2%; 0·2–7·0) | 1 (1%; 0·0–5·5) | 1 (1%; 0·0–5·5) | 0 | |
| Moderate | 0 | 1 (1%; 0·0–5·5) | 2 (2%; 0·2–7·0) | 0 | 0 | 0 | 0 | 0 | |
| Body ache | |||||||||
| Mild | 0 | 1 (1%; 0·03–5·5) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Moderate | 0 | 1 (1%; 0·0–5·5) | 1 (1%; 0·0–5·5) | 0 | 1 (1%; 0·0–5·5) | 0 | 0 | 0 | |
| Fatigue | |||||||||
| Mild | 1 (1%; 0·0–5·4) | 0 | 0 | 0 | 1 (1%; 0·03–5·4) | 0 | 3 (3%; 0·6–8·5) | 0 | |
| Moderate | 2 (2%; 0·2–7·0) | 3 (3%; 0·6–8·5) | 0 | 0 | 1 (1%; 0·0–5·5) | 0 | 0 | 0 | |
| Headache | |||||||||
| Mild | 1 (1%; 0·03–5·5) | 2 (2%; 0·2–7·0) | 0 | 5 (7%; 2·2–14·9) | 0 | 0 | 0 | 0 | |
| Moderate | 0 | 3 (3%; 0·6–8·5) | 2 (2%; 0·2–7·0) | 0 | 0 | 0 | 0 | 0 | |
| Nausea or vomiting | |||||||||
| Mild | 1 (1%; 0·03–5·5) | 2 (2%; 0·2–7·0) | 2 (2%; 0·2–7·0) | 2 (3%; 0·3–9·3) | 0 | 0 | 0 | 0 | |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Data are n (%; 95% CI). The safety set includes all participants who received one dose of the vaccine (n=375). Dose 1 events are from days 0–7 and dose 2 events are days 14–21. The grading scale for most adverse events was based on the US Food and Drug Administration (FDA) guidance document for toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. For adverse events where grading was not mentioned in the FDA guidance document, we have used the common terminology criteria for adverse events grading. There were no severe adverse events.
44 unsolicited adverse events were reported by 24 (6%) of 375 participants (appendix p 6). Biochemical, haematological, and urine parameters outside of the normal ranges had no corroborating clinical manifestations (appendix pp 7–9).
One serious adverse event was reported in the 6 μg with Algel group. The participant was screened on July 25 and vaccinated on July 30. 5 days later, the participant reported fever and headache (initially reported as a solicited adverse event), and on Aug 8 tested positive for SARS-CoV-2 (by a nucleic acid test). The symptoms were initially mild in nature, with the onset of relapsing fever requiring admission to hospital on Aug 15. The participant had stable vital signs (except body temperature) during their hospital stay and did not require supplemental oxygen. The participant was discharged on Aug 22 after a negative nucleic acid test result. The event was not causally associated with the vaccine. No other symptomatic SARS-CoV-2 infections were reported between days 0 and 75. However, follow-up of routine SARS-CoV-2 nucleic acid testing was not done on any scheduled or illness visit.
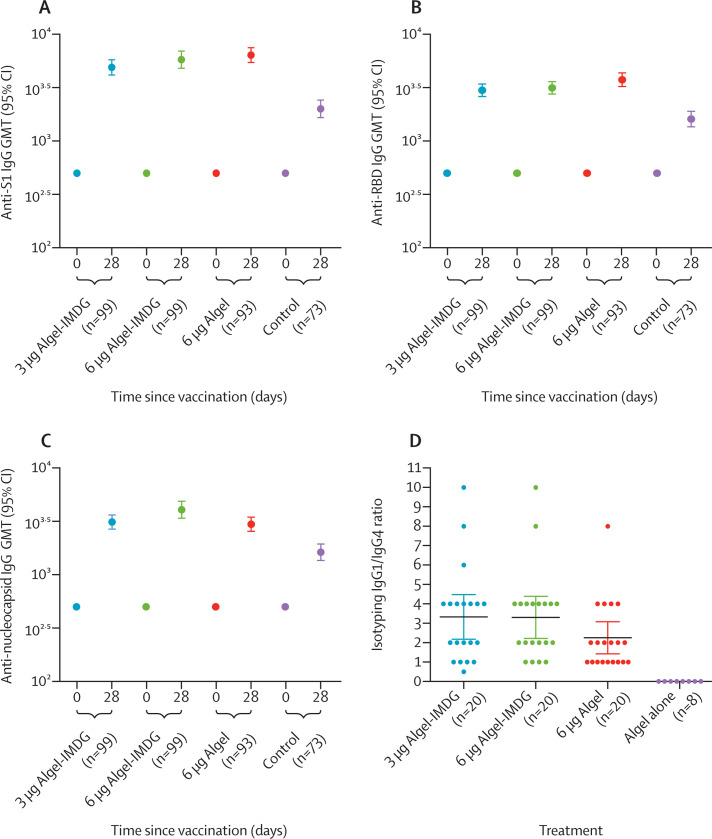
IgG titres (GMTs) to all epitopes (spike protein, receptor-binding domain, and nucleocapsid protein) increased rapidly after the administration of both doses (figure 2A–C ; appendix pp 3–4). Both 3 μg and 6 μg with Algel-IMDG groups reported similar anti-spike, anti-receptor binding, and anti-nucleoprotein IgG titres (GMTs), adding to the dose-sparing effect of the adjuvant. Binding antibody titres to the whole-virion inactivated antigen are shown in the appendix (p 15). The mean isotyping ratios (IgG1/IgG4) were greater than 1 for all vaccinated groups, which was indicative of a Th1 bias (figure 2D).
Figure 2.
SARS-CoV-2 IgG titres against anti-spike protein (A), receptor-binding domain (B), and nucleocapsid IgG (C) and anti-spike protein IgG1/IgG4 ratio (D)
ELISA results at baseline (day 0) and 2 weeks after the second vaccination (day 28). In A–C, error bars show 95% CIs. The cutoff for detectable antibodies was 1/500. Some samples were positive for SARS-CoV-2 in the control group, as evident by the antibody titres on day 28. Endpoint titre dilution for day 28 sera samples was established with baseline (day 0), interpolated from the absorbance of the corresponding day 0 sample. Cutoff (mean ± 3 SD) for day 0 was calculated considering the absorbance of all sera dilutions (1/500 to 1/32000) tested, except the lowest dilution (1/500). ELISA titres (endpoint titres) on day 14 were not analysed. In D, the isotyping ratio was calculated (in a randomly selected subset) as IgG1/IgG4; dots show the individual datapoints and horizontal bars show means with error bars for 95% CIs. Endpoint titre=the highest sera dilution at which the absorbance was above the cutoff. GMT=geometric mean titre. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Seroconversion rates (after the second dose), based on MNT50 were 87·9% (95% CI 79·8–94·3) in the 3 μg with Algel-IMDG group, 91·9% (84·6–96·0) in the 6 μg with Algel-IMDG group, and 82·8% (73·7–89·2) in the 6 μg with Algel group (figure 3A ). Seroconversion (at day 28) in the control group was reported in six (8% [3·6–17·2]) of 75 participants, suggestive of asymptomatic infection. The post-second-dose GMTs (MNT50) were 61·7 (49·5–76·9) in the 3 μg with Algel-IMDG group, 66·4 (53·4–82·4) in the 6 μg with Algel-IMDG group, and 48·0 (37·7–61·1) in the 6 μg with Algel group. Responses in the Algel-IMDG groups were not significantly different to the response in the 6 μg with Algel group. The vaccine-induced responses were similar to those observed in the convalescent serum collected from 41 patients who had recovered from COVID-19 (figure 3B). On these 41 patients, the median titre of symptomatic patients (n=25; median 142·2 [IQR 56·6–350]) was significantly higher than that of the asymptomatic patients (n=16; 22·6 [9·0–56·5]; appendix p 16). Seroconversion rates analysed by PRNT50 (after the second dose) were 93·4% (95% CI 83·7–97·8) in the 3 μg with Algel-IMDG group, 86·4% (75·1–93·2) in the 6 μg with Algel-IMDG group, and 86·6% (74·3–93·6) in the 6 μg with Algel group (figure 3C).
Figure 3.
SARS-CoV-2 wild-type MNT50 seroconversion rates (A) and GMT (B) and PRNT50 seroconversion rates (C) and medians (D)
Results at baseline (day 0), 2 weeks after the first vaccination (day 14), and 2 weeks after the second vaccination in the immunogenicity cohort. Seroconversion rates were defined by the proportion of titres achieving at least four-fold greater than baseline. In A–C, error bars show 95% CIs. In B, the human convalescent serum panel included specimens from participants with PCR-confirmed symptomatic or asymptomatic COVID-19, obtained at least 30 days after diagnosis (41 samples for MNT50). In D, randomly selected serum samples from day 28 were analysed by PRNT50 at the National Institute of Virology for homologous (NIV-2020-770) and heterologous (nCoV-Q11 and nCoV-Q100) assessments; dots show individual datapoints and horizontal bars show medians with error bars for IQRs. GMT=geometric mean titre. MNT50=microneutralisation assay. PRNT50=plaque-reduction neutralisation test. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
MNT50 wild-type neutralising antibody responses for a subset of paired serum samples (n=50) were analysed at the National Institute of Virology and Bharat Biotech (on day 28, 2 weeks after the second vaccination in all groups). Additionally, neutralising antibodies were analysed by PRNT50 at Bharat Biotech and the National Institute of Virology. Similar results were obtained for MNT50 and PRNT50 assays at both laboratories (appendix p 17). Randomly selected serum samples from day 28 were analysed by PRNT50 at the National Institute of Virology with homologous and heterologous strain assessments. Neutralisation responses, regardless of the challenge strain, were observed (figure 3D).
In a subset of randomly selected blood samples at one site, IFN-γ ELISpot responses against SARS-CoV-2 peptides peaked at about 100–120 spot-forming cells per million peripheral blood mononuclear cells in all vaccinated groups on day 28. Both the Algel-IMDG groups elicited CD3+, CD4+, and CD8+ T-cell responses that were reflected in the IFN-γ production, albeit in a small number of samples. However, there was a minimal detection of less than 0·5% of CD3+, CD4+, and CD8+ T-cell responses in the 6 μg with Algel group and the Algel only group (appendix p 16).
Discussion
We report the interim findings from the phase 1 clinical trial of BBV152, a whole-virion inactivated SARS-CoV-2 vaccine. The vaccine was well tolerated in all dose groups with no vaccine-related serious adverse events. Both humoral and cell-mediated responses were observed in the recipients of the Algel-IMDG-based vaccines.
The most common adverse event was pain at the injection site, followed by headache, fatigue, and fever. The overall incidence of solicited local and systemic adverse events in this study was 14–21% in all vaccine-treated groups, which is noticeably lower than the rates for other SARS-CoV-2 vaccine platform candidates18, 19, 20, 21, 22, 23 and similar to the rates for other inactivated SARS-CoV-2 vaccine candidates24, 25 One serious adverse event (positive for SARS-CoV-2 by a nucleic acid test) in an individual in the 6 μg with Algel group was not related to vaccination. Because the event occurred in the 5 days after vaccination, the development of a protective immune response was not likely.
BBV152 induced binding and neutralising antibody responses that were similar to those induced by other SARS-CoV-2 inactivated vaccine candidates.24, 25 Titres from the Anti-spike IgG ELISA assay correlated positively with live virus microneutralisation assay titres (R2=0·51). We assessed an accelerated schedule (vaccination 2 weeks apart) and did not include a routine schedule (vaccination 4 weeks apart). It has been reported that a routine schedule for another SARS-CoV-2 vaccine candidate offers better immune responses, as is to be expected.26 The 4-week schedule for BBV152 3 μg and 6 μg with Algel-IMDG is being assessed in a phase 2 trial in 380 volunteers (NCT04471519). Here, we showed that all vaccine formulations were Th1 skewed with IgG1/IgG4 ratios greater than 1. Furthermore, the Algel-IMDG formulations were associated with an increase in the frequency of CD4+ INF-γ+ T cells compared with the 6 μg with Algel formulation, which is indicative of a Th1 bias. Additionally, cell-mediated responses from other SARS-CoV-2 inactivated vaccine candidates have not been reported thus far.
A few animal studies of SARS-CoV and Middle East respiratory syndrome-CoV inactivated or vectored vaccines adjuvanted with alum have shown Th2 responses resulting in eosinophilic infiltration in the lungs.27, 28, 29 Adverse events might be associated with the induction of weakly neutralising or non-neutralising antibodies that lead to antibody-dependent enhancement or enhanced respiratory disease, thus prompting the attempt to develop SARS-CoV-2 vaccines that induce a CD4+ Th1 response with a minimal Th2 response.2, 30, 31, 32 Whole-virion inactivated vaccines are mostly developed with Algel (alum) as the adjuvant. The response generated by alum is primarily Th2 biased, with the induction of strong humoral responses by neutralising antibodies.33 To circumvent this concern of antibody-dependent enhancement, we have assessed this vaccine with Algel and a TLR7/8 agonist that results in immune responses that are biased to Th1. Previous studies have shown that the toll-like receptors play an integral role in bridging the innate and adaptive immune responses, leading to the differentiation of CD4+ T cells into Th1 cells, which produce IFN-γ.34 Geeraedts and colleagues35 reported that the stimulation of TLR7 by an influenza whole-virion inactivated vaccine was a significant determinant of a greater immune response and Th1 polarisation. Thus, it is imperative to develop such whole-virion inactivated vaccines with adjuvants that can synergistically contribute to the full potential. Algel-IMDG contains an imidaquizoquinoline class TLR7/8 agonist adsorbed to Algel. Preclinical studies on BBV152 adjuvanted with this molecule reported a Th1-biased response in mice.7 Furthermore, in a non-human primate and hamster live viral challenge studies, Algel-IMDG formulations led to higher neutralising antibodies, which might have resulted in improved upper and lower airway viral clearance (after challenge).8, 9
This study was done at a time of rapidly increasing daily diagnoses of COVID-19. Among all 897 individuals screened for this trial, 70 (8%) had positive SARS-CoV-2 nucleic acid test results and 63 (7%) had positive SARS-CoV-2 serology results. Seroconversion (at day 28) in the control group was reported in six (8%) of 75 participants from five separate study sites. Because substantial SARS-CoV-2 was observed at enrolment and some of the control group recipients seroconverted, post-vaccination titres from the vaccinated recipients might be slightly inflated, in the event of natural exposure to SARS-CoV-2. No symptomatic COVID-19 cases were reported in the control group.
Because this is an interim report, we are not reporting any data on the persistence of vaccine-induced antibody responses or long-term safety outcomes. The results reported here do not permit efficacy assessments. The analysis of safety outcomes requires more extensive phase 2 and 3 clinical trials. Pre-vaccination laboratory values were similar to values after vaccination. However, transient laboratory abnormalities might have been resolved by day 28. The analysis of T-cell responses by Th2 cytokines was not done and is planned for phase 2. We were unable to assess other immune responses of convalescent serum because of insufficient number of samples. The proportion of samples collected from asymptomatic individuals was high (39%), and no additional data on the severity of disease from symptomatic individuals was obtained. This study population did not have ethnic diversity and most of the participants were men, further underscoring the importance of assessing BBV152 in other populations.
However, this study has several strengths. To ensure generalisability, this study was conducted with participants from diverse geographic locations within India (appendix p 13), enrolling 375 participants across 11 hospitals. The first 50 participants were enrolled into the 3 μg with Algel-IMDG and control groups. Before granting the recommendation to proceed with the enrolment of other cohorts, masked safety data was reviewed by the data safety monitoring board. As a result, no operational bias was introduced. Despite enrolment occurring during a national lockdown, which led to several operational challenges, the overall participant retention rate was 97%. The sample size was intentionally large to enable the inference of meaningful conclusions regarding neutralising responses. With several reports questioning the efficacy of SARS-CoV-2 vaccines against antigenically divergent strains, we report neutralising responses to homologous and heterologous strains. The BBV152 vaccine strain, based on the Asp614Gly mutation, has been reported to have differential sensitivity to neutralisation by vaccine-elicited antibodies or by antibodies produced by natural infection.36, 37 The increase in Asp614Gly infectivity results in the virus being more susceptible to neutralising antibodies,38 which is corroborated by marginal reductions in neutralising titres in the PRNT50 assays with heterologous strains, which are devoid of the Asp614Gly mutation.
BBV152 induced binding and neutralising antibody responses and with the inclusion of the Algel-IMDG adjuvant, this is the first inactivated SARS-CoV-2 vaccine that has been reported to induce a Th1-biased response. BBV152 is stored at 2–8°C, which is compatible with immunisation cold-chain requirements. Both Algel-IMDG formulations were selected for the phase 2 immunogenicity trials. Further efficacy trials are warranted.
This online publication has been corrected twice. The corrected versions first appeared at thelancet.com/infection on February 23, 2021, and January 18, 2023
Data sharing
Deidentified individual participant data will be made available when the trial is complete, upon requests directed to the corresponding author; after approval of a proposal, data can be shared through a secure online platform.
Acknowledgments
Acknowledgments
Our sincere thanks to the principal and co-principal investigators, study coordinators, and health-care workers that were involved in this study. We express our gratitude to Dr Sivasankar Baalasubramaniam from Indoor Biotechnologies (Bangalore), who assisted with cell-mediated response analyses and Dr Dipankar Das from Bharat Biotech (Hyderabad, India), for binding antibody estimation. We thank Dr Rakeshchandra Meka, Dr Ramulu Chintala, and Ms Spandana Sure for cell-mediated assessments. A special thanks to Dr Arjun Dang and Dr Leena Chatterjee of Dr Dangs Lab (New Delhi, India), which was the central laboratory for clinical laboratory testing. We appreciate the guidance from Dr William Blackwelder for sample size estimation and statistical analysis planning. Dr Shashi Kanth Muni, Dr Sapan Kumar Behera, Dr Jagadish Kumar, Dr Vinay Aileni, Vamshi Sarangi, and Akhila Naidu of Bharat Biotech participated in protocol design and clinical trial monitoring. We thank the data safety monitoring board members (Dr Kiran Kumar, Dr Kiran Kishore, Dr Srinivasa Rao, Dr Sudha Madhuri, and Naradamuni Naidu) for their continued support and guidance on this ongoing clinical study. Development of this vaccine candidate (COVAXIN) would not have been possible without the efforts of Bharat Biotech's manufacturing team, quality control team, and the discovery team and the supply of Algel-IMDG adjuvant by Dr Sunil David (Virovax, Lawrence, KS, USA). All authors would like to express their gratitude for all front-line health-care workers during this pandemic. This work was supported and funded by Bharat Biotech International.
Contributors
RE and KMV accessed and verified the data. HJ, BG, PY, and GS led the immunogenicity experiments. KMV, SPr, VS, and RE contributed to the analysis and manuscript preparation. SR was the study coordinator and helped immensely with the protocol design and interim report generation. PA, SPr, NG, and BB contributed various neutralising antibody assays and participated in the writing of this manuscript. SPa reviewed the manuscript. PR, SV, SKR, CS, SVR, CSG, JSK, SM, VR, and RG were involved with the scientific review of this manuscript. KE was responsible for overall supervision of the project and review of the final paper.
Declaration of interests
RE, HJ, BG, KMV, SPr, VS, KE, and SR are employees of Bharat Biotech, with no stock options or incentives. KE is the Chairman and Managing Director of Bharat Biotech. PY, GS, PA, NG, SPa, and BB are employees of The Indian Council of Medical Research. All other authors declare no competing interests.
Supplementary Material
References
- 1.WHO Draft landscape of COVID-19 candidate vaccines. Dec 8, 2020. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 2.Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368:945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 3.Johnson TR, Graham BS. Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during primary respiratory syncytial virus infection. Pediatr Infect Dis J. 2004;23(suppl):S46–S57. doi: 10.1097/01.inf.0000108192.94692.d2. [DOI] [PubMed] [Google Scholar]
- 4.Delgado MF, Coviello S, Monsalvo AC, et al. Lack of antibody affinity maturation due to poor toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Philbin VJ, Dowling DJ, Gallington LC, et al. Imidazoquinoline toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol. 2012;130:195–204.e9. doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shukla NM, Salunke DB, Balakrishna R, Mutz CA, Malladi SS, David SA. Potent adjuvanticity of a pure TLR7-agonistic imidazoquinoline dendrimer. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganneru B, Jogdand H, Dharam VK, et al. Evaluation of safety and immunogenicity of an adjuvanted, TH-1 skewed, whole virion inactivated SARS-CoV-2 vaccine—BBV152. bioRxiv. 2020 doi: 10.1101/2020.09.09.285445. published online Sept 9. (preprint) [DOI] [Google Scholar]
- 8.Yadav P, Ella R, Kumar S, et al. Remarkable immunogenicity and protective efficacy of BBV152, an inactivated SARS-CoV-2 vaccine in rhesus macaques. Res Square. 2020 doi: 10.21203/rs.3.rs-65715/v1. published online Sept 10, 2020. (preprint) [DOI] [Google Scholar]
- 9.Mohandas S, Yadav PD, Shete A, et al. Immunogenicity and protective efficacy of BBV152: a whole virion inactivated SARS CoV-2 vaccine in the Syrian hamster model. Res Square. 2020 doi: 10.21203/rs.3.rs-76768/v1. published online Sept 16. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkale P, Patil S, Yadav PD, et al. First isolation of SARS-CoV-2 from clinical samples in India. Indian J Med Res. 2020;151:244–250. doi: 10.4103/ijmr.IJMR_1029_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Weekly epidemiological record. Jan 24, 2020. https://extranet.who.int/iris/restricted/bitstream/handle/10665/330607/WER9504-eng-fre.pdf?ua=1
- 12.Sampath G, Madhusudana SN, Sudarshan MK, et al. Immunogenicity and safety study of indirab: a vero cell based chromatographically purified human rabies vaccine. Vaccine. 2010;28:4086–4090. doi: 10.1016/j.vaccine.2010.03.064. [DOI] [PubMed] [Google Scholar]
- 13.Vadrevu KM, Potula V, Khalatkar V, Mahantshetty NS, Shah A, Ella R. Persistence of immune responses with an inactivated Japanese encephalitis single-dose vaccine, JENVAC and interchangeability with a live-attenuated vaccine. J Infect Dis. 2020;222:1478–1487. doi: 10.1093/infdis/jiz672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhandari N, Rongsen-Chandola T, Bavdekar A, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian children in the second year of life. Vaccine. 2014;32(suppl 1):A110–A116. doi: 10.1016/j.vaccine.2014.04.079. [DOI] [PubMed] [Google Scholar]
- 15.Ella R, Babji S, Ciarlet M, Blackwelder WC, Vadrevu KM. A randomized, open-labelled, non-inferiority phase 4 clinical trial to evaluate the immunogenicity and safety of the live, attenuated, oral rotavirus vaccine, ROTAVAC in comparison with a licensed rotavirus vaccine in healthy infants. Vaccine. 2019;37:4407–4413. doi: 10.1016/j.vaccine.2019.05.069. [DOI] [PubMed] [Google Scholar]
- 16.Ella R, Bobba R, Muralidhar S, Babji S, Vadrevu KM, Bhan MK. A phase 4, multicentre, randomized, single-blind clinical trial to evaluate the immunogenicity of the live, attenuated, oral rotavirus vaccine (116E), ROTAVAC, administered simultaneously with or without the buffering agent in healthy infants in India. Hum Vaccin Immunother. 2018;14:1791–1799. doi: 10.1080/21645515.2018.1450709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potdar V, Cherian SS, Deshpande GR, et al. Genomic analysis of SARS-CoV-2 strains among Indians returning from Italy, Iran & China, & Italian tourists in India. Indian J Med Res. 2020;151:255–260. doi: 10.4103/ijmr.IJMR_1058_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 19.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu F-C, Li Y-H, Guan X-H, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu F-C, Guan X-H, Li Y-H, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020 doi: 10.1056/NEJMoa2027906. published online Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y-J, Zeng G, Pan H-X, et al. Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: report of the randomized, double-blind, and placebo-controlled phase 2 clinical trial. medRxiv. 2020 doi: 10.1101/2020.07.31.20161216. published online Aug 10. (preprint) [DOI] [Google Scholar]
- 25.Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2020;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graepel KW, Kochhar S, Clayton EW, Edwards KE. Balancing Expediency and scientific rigor in severe acute respiratory syndrome coronavirus 2 vaccine development. J Infect Dis. 2020;222:180–182. doi: 10.1093/infdis/jiaa234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotez PJ, Corry DB, Bottazzi ME. COVID-19 vaccine design: the Janus face of immune enhancement. Nat Rev Immunol. 2020;20:347–348. doi: 10.1038/s41577-020-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diamond MS, Pierson TC. The challenges of vaccine development against a new virus during a pandemic. Cell Host Microbe. 2020;27:699–703. doi: 10.1016/j.chom.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiskopf D, Schmitz KS, Raadsen MP, et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489. doi: 10.1016/j.cell.2020.05.015. 501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arvin AM, Fink K, Schmid MA, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584:353–363. doi: 10.1038/s41586-020-2538-8. [DOI] [PubMed] [Google Scholar]
- 33.He P, Zou Y, Hu Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccin Immunother. 2015;11:477–488. doi: 10.1080/21645515.2014.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 35.Geeraedts F, Goutagny N, Hornung V, et al. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by toll-like receptor signalling. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812. doi: 10.1016/j.cell.2020.06.043. 27.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020 doi: 10.1056/NEJMoa2028436. published online Sept 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weissman D, Alameh M-G, de Silva T, et al. D614G spike mutation increases SARS CoV-2 susceptibility to neutralization. medRxiv. 2020 doi: 10.1101/2020.07.22.20159905. published online Sept 12. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified individual participant data will be made available when the trial is complete, upon requests directed to the corresponding author; after approval of a proposal, data can be shared through a secure online platform.