Abstract
Background
The COVID-19 pandemic has raised novel concerns for people living with MS regarding their safety.
Methods
Observational study of patients at a single comprehensive community MS center.
Results
48 patients with MS were suspected of developing COVID-19 March to May 2020; 2 died. Of the remainder, 17 were tested for COVID-19 antibodies as part of routine care. Average age of this subgroup was 49.8y ± 11.3 (age range 32-67), 76% female. 65% were treated with an anti-CD20 drug, 12% untreated, and 6% each received glatiramer acetate, interferon, natalizumab, or teriflunomide. 59% of patients were antibody negative.
Conclusions
The low incidence of SARS CoV2 antibodies following infection suggests that certain DMTs may alter SARS CoV2-Ab response or persistence.
Keywords: COVID-19, MS, Disease modifying treatment, Anti-B cell treatment, Observational study, COVID-19 antibodies
1. Introduction
As the COVID-19 viral pandemic ravaged the world, new concerns regarding safety of people living with multiple sclerosis (pwMS) emerged. While definitive answers will likely take many months or longer to establish, patients and clinicians are making daily decisions based on expert guidance from professional organizations and expert opinion (eg. CMSC, NMSS, and others). One immediate question has been – should treatment be altered – either by delaying treatment, or by changing the specific disease modifying treatment (DMT) as a direct result of the pandemic? Thus far, there has been reassuring evidence published regarding patients on all DMT classes, including ‘induction’ therapies, alemtuzumab (Carandini et al., 2020) and cladribine (Dersch et al., 2020), as well as continuous infusion, oral and injectable treatments (natalizumab (Borriello and Ianniello, 2020; Parrotta et al., 2020), anti-CD20 treatments (Parrotta et al., 2020; Thornton and Harel, 2020; Baker et al., 2020), dimethyl fumarate (Parrotta et al., 2020), fingolimod (Bollo et al., 2020)/siponimod (Parrotta et al., 2020), teriflunomide (Bollo et al., 2020), interferons (Parrotta et al., 2020) and glatiramer (Parrotta et al., 2020)) surviving infection. This mirrors our own center's experience as well. However, another significant question also warrants consideration – once an effective vaccine against COVID-19 is available, which is imminent as of this writing, how will DMT use affect its efficacy in pwMS? To begin to address this question, we evaluated our patients who were clinically diagnosed with COVID-19 for antibodies (Ab) against the virus.
2. Methods
This is an observational study of pwMS who are patients of the Holy Name MS Center and were either proven by PCR or highly suspected of active COVID-19 infection as of August 19, 2020. A portion of these patients had SARS CoV2 antibody testing with the Abbott Architect SARS CoV2 IgG test which has an estimated sensitivity of 100% and specificity of 99.6%, and one patient was tested at an outside institution with Siemens ADVIA Centaur SARS CoV2 IgM/IgG chemiluminescent immunoassay with an estimated sensitivity of 100% and specificity of 99.9% (EUA, 2020). Due to a shortage of tests at the start of the pandemic, it was not possible to test every symptomatic person, thus in many instances only the most severely ill were tested. We present descriptive statistics regarding their baseline demographics, and outcomes with respect to antibody status and DMT use. Patients provided research consent at the time of antibody testing.
3. Results
Of 48 patients suspected to be ill from COVID-19 infection in the March-May 2020 season (time of the first wave of the pandemic in New Jersey, USA), 79% (n=38) were confirmed positive by nasopharyngeal PCR; 6% (n=3) patients exhibited viral symptoms and had household contacts who tested positive for COVID-19 (thus it was felt unnecessary to test them given scarcity of testing supplies); another 6% (n=3) of patients had typical viral symptoms in March 2020 for which they were treated with antibiotics for possible COVID-19 but were not tested for unidentified reasons. Two patients (4%) with pneumonia presumed to be secondary to COVID-19 were treated at outside hospitals (one PCR negative, one unknown). Of the remaining patients, there was one (2%) each of the following: infectious symptoms of fever and cough in late March but COVID-19 PCR testing negative; one asymptomatic patient never tested for COVID-19 PCR but with positive Sars-CoV2-Ab.
We were informed of two patient deaths; one patient was a man, last known to our practice from 2 years ago and was prescribed dimethyl fumarate at the time, he was admitted to the hospital due to a thromboembolism (unspecified location) and died at age 55 (positive for COVID-19); another person was a woman who was treated with interferon beta-1a, and died following presumed COVID-19-associated pneumonia at age 76 (we do not have a record of her COVID-19 testing).
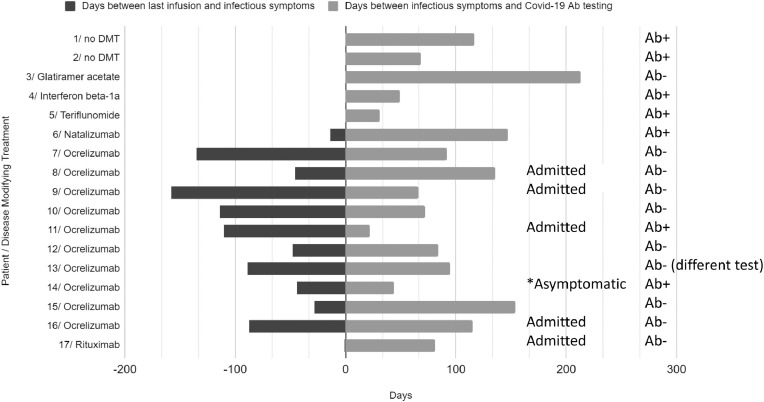
SARS CoV2 Ab testing became available at our hospital around May 6, 2020 and we started to routinely offer the testing to our patients as part of standard of care; 132 patients have been tested for antibodies as of August 19, 2020. Seventeen patients who were suspected of contracting COVID-19 have also had Sars-CoV2 IgG Ab testing. Average age of this subgroup was 49.8y ± 11.3 (age range 32-67), and they were 76% female. All were diagnosed with multiple sclerosis. 65% of patients (n=11) were treated with an anti-CD20 monoclonal Ab (10 ocrelizumab (OCR); 1 rituximab (RTX)), 12% (n=2) were untreated, and 6% each were on treatment with: glatiramer acetate, interferon, natalizumab, or teriflunomide. Only 41% of patients (n=7) were antibody positive. Fig. 1 demonstrates the timing of their preceding infusion dose (if applicable) relative to symptom onset and antibody testing (in days). Considering the patients based on viral disease severity; 29% (n=5) had a ‘more severe’ course, defined as requiring hospitalization. None of these patients required intubation; all survived. Four of the admitted patients (80%) were treated with ocrelizumab and one was treated with rituximab. Disease severity did not correlate with antibody status, as 80% of the admitted patients were eventually antibody negative
Fig. 1.
Patients presumed COVID-19 positive with subsequent SARS CoV2-Ab testing.
Legend:
• Each horizontal line indicates a single individual, numbered from 1 to 17, with their DMT at the time of infection listed. All but one patient were symptomatic.
• Patients who required hospitalization have the word “Admitted” to the right of the bar chart all others were managed as outpatients. One patient was never clearly symptomatic and she is detected with “*asymptomatic” to the right of the bar chart. Antibody status is annotated on the far right for each patient.
• In terms of infectious treatment Patients requiring admission for their severe COVID-19 related symptoms are annotated on the left. Patient #8, was treated with: azithromycin, zinc, hydroxychloroquine, high dose methylprednisolone and tocilizumab; Patient #9 required hydroxychloroquine, high dose methylprednisolone; Patient #11 admitted to an outside hospital for treatment of a blood clot due to non-adherence with anti-coagulation, treated with enoxaparin; Patient #16 was treated with hydroxychloroquine, zinc, azithromycin, high dose methylprednisolone and tocilizumab; Patient #17 was admitted to an outside hospital and reported treatment with intravenous steroids and antibiotics. None of the patients required intubation.
• Patients who were treated for their viral symptoms without admission to the hospital- Patient #1, received two antibiotics, budesonide, oxygen and via nasal cannula and nebulizer treatment; Patient #2 was treated with azithromycin, acetaminophen, prednisone and a cough suppressant; Patient #3, 4, 6, 7, 12 and 15 -treatment unknown; Patient #5 steroids; Patient #10 and #13 received azithromycin, Patient #14 was never treated given asymptomatic.
4. Discussion
In this case series, we present our center's experience with MS patients who were suspected of developing COVID-19 infection. Relative to the proportion of patients in our practice who are treated with anti-CD20 monoclonal antibodies (approximately 32%), this case series represents a disproportionate amount of people who were on these medications (OCR, RTX) and became ill, 40% (19/48). This is similar to the NYU Comprehensive MS Center experience, where about 33% of patients are treated with anti-CD20 therapy, and 47% of the COVID-19-infected MS patients were on this type of treatment (Parrotta et al., 2020). This is unsurprising however, as serious infections are a known complication of anti-CD20 treatments; in a study of over 6000 treated MS patients, RTX was associated with a 1.7 fold increased risk of serious infections relative to first line injectables (Luna et al., 2020).
We focused on patients who had antibody testing, and found that 59% of patients who were clinically suspected of having COVID-19 infection did not have antibodies upon subsequent testing. All patients were ill in March-May 2020, and testing for antibodies was performed May 6 – Aug 18, 2020, thus we would not suspect that timing of testing was a major limitation. The 41% Sars-Cov-2 Ab positivity rate in our patient population is significantly less than the 91% rate in recovered Icelandic people (general population) (Gudbjartsson et al., 2020).
Of the 10 patients who tested negative for antibodies, 90% were on anti-CD20 therapy. Examined from the perspective of the 11 patients who were on treatment with OCR/RTX, only 2 (18%) developed antibodies. These findings are in line with another case series of two MS patients treated with OCR who developed COVID-19 and remained SARS CoV2-Ab negative (Thornton and Harel, 2020). As well, it is consistent with the VELOCE study which challenged patients receiving OCR or controls (including no DMT or interferon beta treated patients) to inoculation with non-live vaccines and found that the humoral response to vaccines is attenuated by approximately 20% in the OCR treated patients (Bar-Or et al., 2020). Thus, we postulate that the OCR/RTX patients’ response to a future COVID-19 vaccine will likely be quite attenuated as well. These results may have an implication for policies surrounding business/societal “re-opening” plans particularly if establishments require proof of antibodies to return to work, as certain patients may be unable to mount an antibody response. Although we have only single digit experience with MS patients on other DMTs developing COVID-19, our results indicate that patients treated with teriflunomide, interferon and natalizumab are probably more likely to form post-infectious antibodies, similarly to the experience of others regarding fingolimod and teriflunomide (Bollo et al., 2020).
This study has several limitations. It was an observational study without a control group. It was a single center study with a modest number of subjects, thus we are unable to draw firm conclusions about the severity of the infection with respect to a given DMT, however, we maintain that it remains suspicious that 100% of our hospitalized cohort were on anti-CD20 therapy. Patients obtained antibody testing at the time of their standard of care visits, per the decision of their treating neurologist, therefore timing of testing with respect to infection onset was not standardized, however was generally more than a month following onset of infectious symptoms (except in the singular case of the individual who tested positive for antibodies without prior concern for contracting the illness). Nevertheless, in light of our present day pandemic, given the popularity of anti-CD20 therapy in the treatment of MS, our study suggests that pwMS may be at a disadvantage with respect to antibody formation, which may have public health ramifications.
Disclosures
AIW: Has received fellowship funding from the NMSS and consulting fees from Biogen and Genentech. These funding sources were unrelated to the present work.
MAP: Has received speaker and consulting fees from Biogen, Genentech, EMD Serono, Sanofi-Genzyme, Viela Bio, BMS. These funding sources were unrelated to the present work.
Declaration of Competing Interest
None.
Acknowledgment
There was no other targeted funding for this study. The authors would like to thank Mr. Matthew Schiebel with assistance in data collection.
References
- Baker D., Roberts C.A.K., Pryce G., et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin. Exp. Immunol. 2020 doi: 10.1111/cei.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Calkwood J.C., Chognot C., et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020 doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollo L., Guerra T., Bavaro D.F., et al. Seroconversion and indolent course of COVID-19 in patients with multiple sclerosis treated with fingolimod and teriflunomide. J. Neurol. Sci. 2020;416 doi: 10.1016/j.jns.2020.117011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriello G., Ianniello A. COVID-19 occurring during Natalizumab treatment: a case report in a patient with extended interval dosing approach. Mult. Scler. Relat. Disord. 2020;41 doi: 10.1016/j.msard.2020.102165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini T., Pietroboni A.M., Sacchi L., et al. Alemtuzumab in multiple sclerosis during the COVID-19 pandemic: a mild uncomplicated infection despite intense immunosuppression. Mult. Scler. 2020 doi: 10.1177/1352458520926459. [DOI] [PubMed] [Google Scholar]
- Dersch R., Wehrum T., Fahndrich S., Engelhardt M., Rauer S., Berger B. COVID-19 pneumonia in a multiple sclerosis patient with severe lymphopenia due to recent cladribine treatment. Mult. Scler. 2020 doi: 10.1177/1352458520943783. [DOI] [PubMed] [Google Scholar]
- EUA Authorized serology test performance. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance. Accessed Nov 30, 2020.
- Gudbjartsson D.F., Norddahl G.L., Melsted P., et al. Humoral immune response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna G., Alping P., Burman J., et al. Infection risks among patients with multiple sclerosis treated with Fingolimod, Natalizumab, Rituximab, and injectable therapies. JAMA Neurol. 2020;77(2):184–191. doi: 10.1001/jamaneurol.2019.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrotta E., Kister I., Charvet L., et al. COVID-19 outcomes in MS: observational study of early experience from NYU multiple sclerosis comprehensive care center. Neurol. Neuroimmunol. Neuroinflamm. 2020;7(5) doi: 10.1212/NXI.0000000000000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J.R., Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in two MS patients treated with ocrelizumab. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102341. [DOI] [PMC free article] [PubMed] [Google Scholar]