Abstract
Aim
Mortality is high in Coronavirus disease 2019 patients with pre-existing comorbidities and advanced age. Associated complications have added to the negative prognosis. Nevertheless, many have fully recovered, even among the most fragile. Factors associated with their survival was investigated.
Methods
Retrospective study of patients aged ≥90 years admitted for COVID-19 to the Internal Medicine wards of two hospitals in Lombardy, Italy.
Results
Among 34 patients with SARS-CoV-2 pneumonia, 33 (97.1%) had respiratory failure. Eighteen patients (52.9%) survived and 16 (47.1%) died during hospital stay. Survivors compared to deceased had a significantly longer hospitalization (19 vs. 10 days respectively; p = 0.02), a better PaO2:FiO2 ratio (241 vs. 171 respectively; p = 0.003), higher lymphocyte counts (p = 0.01) and lower serum LDH levels (p < 0.001) at admission. At multivariate analysis only higher PaO2:FiO2 was associated with survival (OR 1.06 [95%CI 1.0–1.03]; p = 0.02). Kaplan-Meier analysis showed a significant difference in event-free survival between patients treated or not with LMWH (p < 0.0001) and between those treated or not with beta-blockers (p = 0.008). Cox regression, performed in the subgroup of patients who received LMWH, did not show significant difference for sex (HR 2.7 [95% CI 0.53–14.3], p = 0.23), CCI (HR 0.7 [95% CI 0.37–1.45], p = 0.38), PaO2:FiO2 ratio (HR 0.98 [95% CI 0.97–1.0], p = 0.07), corticosteroid therapy (HR 0.99 [95% CI 0.22–4.5], p = 0.99) and beta-blocker therapy (HR 2.8 [95% CI 0.56–14,7], p = 0.21).
Conclusions
Despite higher mortality in elderly, treatment with LMWH and betablockers might be associated with better survival. Dedicated studies are required to confirm our result.
Keywords: COVID-19, Older patients, Anti-thrombotic therapy, Beta-blockers, Low molecular weight heparin
1. Introduction
The SARS-CoV2 infection has affected around 96 million people and has claimed more than 2 million lives worldwide so far (World Health Organization, 2021). Advanced age and the presence of comorbidities, including dementia, have been reported to contribute significantly to the worst outcome (Hariyanto, Putri, Arisa, Situmeang, & Kurniawan, 2020; Iaccarino, Grassi, & Borghi, 2020; Wu & McGoogan, 2020). The process of healing is further hindered by the limited capacity of elderly to deal with stressful events in forced isolation for the sake of public health (Chen, 2020). In Italy, the death rate in patients affected by COVID-19 was higher in the older people reaching about 33% in the 90-year-old age group (COVID-19 integrated surveillance: key national data as of 15 September 2020, 2020,). Shengmei et al. described the clinical characteristics of a small number of patients aged ≥80 years confirming the age-related high fatality rate (Niu, Tian, & Lou, 2020). Therefore, it seems worthwhile to identify the characteristics, and more specifically the modifiable factors, associated with favorable outcome for a better clinical management. Hence our study aimed at identifying factors associated with survival among older people, focusing on 90 years age group.
2. Methods
2.1. Study design and participants
We retrospectively studied consecutive patients ≥ 90 years of age with confirmed and/or probable COVID-19 infection admitted from 24/02/2020 to 23/04/2020 to the Internal Medicine Wards of two tertiary hospitals (“Spedali Civili di Brescia” and “Ospedale di Cremona”) located in Lombardy, Italy. SARS-CoV-2 infection was defined according to the laboratory and/or imaging diagnostic criteria of the European Centre for Disease Prevention and Control (Case definition for coronavirus disease 2019 (COVID-19), as of 29 May 2020, 2020). Patients aged < 90 years and without SARS-CoV-2 infection were excluded from this study.
In-hospital survival, defined by discharging the patient alive, was the outcome of interest. The difference in demographic and clinical characteristics such as age, gender, body mass index (BMI), comorbidities, symptoms, complications, laboratory findings, and in-hospital therapy was evaluated and compared between survivors and deceased patients.
2.2. Data collection
All data were collected from medical records. In most cases, family members of the patients were contacted by telephone to elucidate some unclear and missing data as far as the comorbidities and chronic treatment were concerned. Ethical clearance for epidemiological studies was obtained from local ethics committee. The collected data were handled as per hospital privacy regulations and informed consent was obtained for off label treatments.
2.3 Statistical methods
Descriptive data are summarized as mean, median, and interquartile range (IQR) for continuous variables. The frequency of categorical variables is expressed in percentages. Student's T-test with Fisher's exact correction and Pearson's Chi-squared test were used to calculate p-values for continuous and categorical variables, respectively. Odds ratio was calculated using crosstabs for categorical variables and binary logistic for continuous variables. Multivariate analysis was performed with logistic regression. The Kaplan-Meier method was used to analyze event-free survival in patients who received treatments with significant difference, and the groups were compared using the Mantel-Cox and Breslow tests. The relative importance of each prognostic factor, adjusted for the others, was assessed using the Cox proportional hazards model. All the statistical tests were 2 tailed. A value of p < 0.05 was considered statistically significant. All analyses were carried out with the SPSS statistical package (IBM SPSS Statistics 25). Kaplan Meier survival curves were drawn using GraphPad Prism 8.4.3.
3. Results
3.1 Study population
A total of 870 patients were admitted to the Internal Medicine Wards of two tertiary hospitals from 24/02/2020 to 23/04/2020. Among them, 34 patients were included in the study according to the eligibility criteria of age and diagnosis (26 from Cremona and 8 from Brescia). Out of 34 patients included, 29 (85.3%) were positive for RT-PCR for SARS-CoV-2 on throat swab, 4 (11.8%) had only the typical changes for a viral infection on chest X-ray and CT-scans, and 1 (2.8%) had a positive RT-PCR for SARS-CoV-2 identified from bronchoalveolar lavage. 18 patients (52.9%) discharged from the hospitals were defined as survivors while 16 patients (47.1%) who died during hospitalization were defined as non-survivors.
3.1.1 Demographic characteristics
The median age was 93 years in both groups of patients (survivors and non-survivors) (p = 0.98). The proportion of male and female was 50% in both groups and the mean BMI did not differ between the two study sub-populations (Table 1 ).
Table 1.
Demographic characteristics and clinical background.
| Survivors, n = 18 | Non-survivors, n = 16 | P-value | |
|---|---|---|---|
| Male (%) /Female (%) | 9 (50.0) / 9 (50.0) | 8 (50.0) / 8 (50.0) | |
| Age, years (range) | 93 (90–99) | 93 (90–101) | 0.98 |
| BMI, kg/m2 (IQR) | 23.2 (19.5–25.5) | 25.2 (22.4–27.6) | 0.22 |
| Comorbidities: | |||
| Hypertension (%) | 15 (83.3) | 13 (81.3) | 1 |
| Chronic heart failure (%) | 9 (50.0) | 5 (31.3) | 0.26 |
| Chronic kidney disease (%) | 9 (50.0) | 5 (31.3) | 0.26 |
| COPD (%) | 5 (27.8) | 1 (6.3) | 0.18 |
| Arrhythmias (%) | 5 (27.8) | 5 (31.3) | 0.82 |
| Dementia (%) | 5 (27.8) | 5 (31.3) | 0.82 |
| Venous thromboembolism (%) | 4 (22.2) | 0 (0.0) | 0.12 |
| Dyslipidemia (%) | 4 (22.2) | 2 (12.5) | 0.66 |
| Ischemic heart disease (%) | 2 (11.1) | 2 (12.5) | 1 |
| Diabetes mellitus (%) | 2 (11.1) | 3 (18.8) | 0.63 |
| Cerebrovascular event (%) | 2 (11.1) | 3 (18.8) | 0.63 |
| Charlson Comorbidity index (IQR) | 5 (4.7–6.0) | 5.5 (4.0–6.7) | 1 |
| Chronic home treatment: | |||
| Beta-blockers (%) | 10 (55.6) | 3 (18.8) | 0.03 |
| Anti-platelet agents (%) | 8 (44.4) | 11 (68.8) | 0.15 |
| Diuretics (%) | 7 (38.9) | 7 (43.8) | 0.77 |
| Calcium channel blockers (%) | 6 (33.3) | 4 (25.0) | 0.59 |
| ARBs (%) | 4 (22.2) | 2 (12.5) | 0.66 |
| Anticoagulant therapy (%) | 6 (33.3) | 1 (6.3) | 0.09 |
| ACE-inhibitors (%) | 2 (11.1) | 4 (25.0) | 0.38 |
COPD (chronic obstructive pulmonary disease), ARBs (angiotensin II receptor blockers), ACE (angiotensin converting enzyme), IQR (Inter-quartile range).
3.1.2 Pre-existing comorbidities
Most of the patients in both groups suffered from hypertension (83.3% survivors and 81.3% non-survivors, respectively); chronic heart failure, chronic kidney disease, and chronic obstructive pulmonary disease were more frequent in survivors than in non-survivors, despite the differences did not reach statistical significance.
No difference between the two groups was observed in the rate of arrhythmias (predominantly atrial fibrillation), coronary artery disease, diabetes mellitus, and dyslipidemia. Charlson Comorbidity Index median value was high in both groups, without any statistically significant difference (p = 0.5).
Only 22.2% of the survivors had venous thromboembolism. None of the non-survivors had a pre-hospital diagnosis of pulmonary embolism and/or deep vein thrombosis (Table 1).
3.1.3 Chronic home therapy
About half of the survivors (55.6%) were on beta-blocker therapy while fewer non-survivors (18,8%) were treated with a beta-blocker (p = 0.03). Other pharmacological drugs’ use, including ACE-inhibitor or an Angiotensin II receptor blocker, was not different between the two groups (Table 1).
3.1.4 Symptoms at presentation
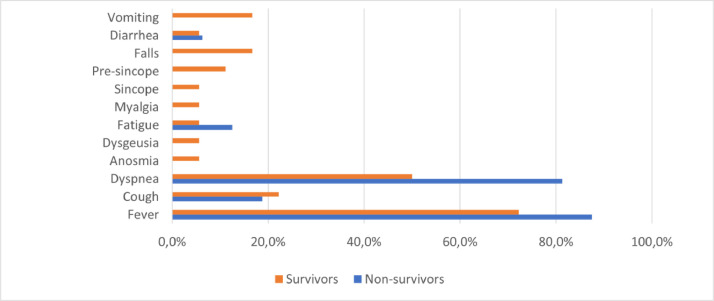
Most patients presented with fever (72.2% survivors vs 87.5% non-survivors, p = 0.41), dyspnea (50% survivors vs 81.3% non-survivors, p = 0.06) and cough (22.2% survivors vs 18.8% non-survivors, p = 1) respectively. In survivors, atypical symptoms like falls, pre-syncope, and syncope were reported in 16.7%, 11.1%, and 5.6% respectively, while none of such symptoms were referred by non-survivors at presentation. Vomiting was reported only by 3 survivors, and few patients complained of diarrhea in both groups. Anosmia and dysgeusia were also reported in a few survivors (Fig. 1 ).
Fig. 1.
Symptoms referred by patients at presentation to emergency department.
3.1.5 Clinical course and complications
The mean duration of hospital stay was 19 days (range 4–49) for survivors and 10 days (range 0–37) for non-survivors (p = 0.02).
Causes of death were respiratory failure (14/16, 87.5%) and acute coronary syndrome (2/16, 12.5%). A higher median PaO2:FiO2 ratio value was measured in patients who survived as compared to those who died (241 and 171 respectively, p = 0.003) indicating a more severe respiratory failure in the latter group. No difference was observed for the median baseline SOFA score or the maximum QTc interval (correction by Bazett formula) recorded during the hospital stay.
The clinical course was accompanied by several complications in both groups with no statistically significant difference. Acute kidney injury (AKI) according to KDIGO (Kidney Disease Improving Global Outcomes) criteria for AKI was the most common complication observed in about a third of patients in both groups. New-onset atrial fibrillation was almost similar in both survivors and deceased (5.6% and 6.3%, respectively). Acute coronary syndrome, indicated by ischemic changes on ECG and rise above 99th percentile upper reference limit in troponin I levels, complicated and resulted in a fatal outcome in 2 (12.5%) of the patients who died, while none of the survivors suffered by myocardial ischemia, but 2 (11.1%) of them were treated for acute heart failure. Only 1 (5.6%) patient among survivors had a pulmonary embolism and deep vein thrombosis, and 1 (6.3%) of non-survivor patients had deep vein thrombosis which was not further investigated for pulmonary embolism. A diagnosis of sepsis, based on a SOFA score ≥ 2 with or without positive blood cultures, was identified in 1 survivor and in 2 non-survivors (Table 2 ).
Table 2.
Clinical characteristics.
| Survivors n = 18 | Non-survivors n = 16 | P-value | |
|---|---|---|---|
| Total hospital stay, days (IQR) | 19 (4–49) | 10 (0–37) | 0.02 |
| Baseline SOFA score, (IQR) | 3 (2–5) | 4 (3–6) | 0.16 |
| Max QTc on ECG, msec (IQR) | 452 (433–493) | 468 (434–494) | 0.8 |
| Laboratory findings: | |||
| Leukocytes, x109/L (IQR) [3.9 - 10.6] | 11.1 (9.5–12.8) | 11.5 (8–17.7) | 0.52 |
| Hemoglobin, g/L (IQR) [137–175] | 129 (112–142) | 114 (102–133) | 0.18 |
| Platelets, x109/L (IQR) [150 - 400] | 187 (148–224) | 151 (133–194) | 0,31 |
| Lymphocytes, x109/L (IQR) [0.7 - 5.0] | 0.95 (0.66–1.23) | 0.62 (0.54–0.91) | 0.01 |
| *Troponine I, ng/L [<15.6] | 54 (2.7–447.6) | 51.5 (10–2625.2) | 0.86 |
| Creatinine, mmol/L (IQR) [0.05–0.09] | 0.12 (0.07–0.18) | 0.13 (0.09–0.17) | 0.97 |
| AST, μmol/s•L (IQR) [0.17 - 0.7] | 0.51 (0.34–0.78) | 0.64 (0.53–0.81) | 0.68 |
| ALT, μmol/s•L (IQR) [0.0 - 0.68] | 0.52 (0.26–0.86) | 0.38 (0.23–0.51) | 0.24 |
| C-reactive protein, nmol/L (IQR) [0.0 - 46.7] | 840 (477–1682) | 1417 (766–2236) | 0.27 |
| *LDH, μmol/s•L (IQR) [<4.1] | 4.5 (3.1–4.9) | 7 (5.3–9.3) | 0.00 |
| *D-Dimer, mg/L (IQR) [<0.5] | 1.63 (0.63–5.6) | 2.72 (1.6–5.6) | 0.46 |
| *Ferritin, nmol/L (IQR) [0.01 - 0.45] | 1.0 (0.4–2.36) | 1.6 (0.7–5.3) | 0.23 |
| PaO2:FiO2 (IQR) | 241 (222–268) | 171 (83–218) | 0.003 |
| In-hospital treatment | |||
| Hydroxychloroquine (%) | 15 (83.3) | 13 (81.3) | 1 |
| Corticosteroids (%) | 9 (50.0) | 7 (43.8) | 0.71 |
| Canakinumab (%) | 0 (0.0) | 1 (6.3) | 0.47 |
| Antibiotic therapy (%) | 17 (94.4) | 15 (93.8) | 1 |
| Antiviral therapy (%) | 7 (38.9) | 10 (62.5) | 0.17 |
| LMWH (%) | 18 (100.0) | 11 (68.8) | 0.02 |
| Beta-blockers (%) | 10 (55.6) | 2 (12.5) | 0.01 |
| In-hospital complications | |||
| Acute kidney failure (%) | 6 (33.3) | 5 (31.3) | 0.89 |
| Acute heart failure (%) | 2 (11.1) | 0 (0.0) | 0.49 |
| Atrial fibrillation (%) | 1 (5.6) | 1 (6.3) | 1 |
| Deep vein thrombosis (%) | 1 (5.6) | 1 (6.3) | 1 |
| Pulmonary embolism (%) | 1 (5.6) | 0 (0.0) | 1 |
| Bleeding (%) | 1 (5.6) | 0 (0.0) | 1 |
| Sepsis (%) | 1 (5.6) | 2 (12.5) | 0.59 |
| Acute coronary syndrome (%) | 0 (0.0) | 2 (12.5) | 0.21 |
Laboratory findings with missing data, LMWH (Low molecular weight heparin), SOFA (Sequential Organ Failure Assessment), Max (maximum), QTc (corrected QT interval), ECG (Electrocardiogram), [normal laboratory values].
3.1.6 Laboratory findings
Lymphocyte absolute count was significantly higher, and serum LDH level was significantly lower in the group of patients who survived, while no other significant differences were observed between the 2 groups (Table 2).
3.1.7 In hospital treatment
Most of the patients were treated with a combination of intravenous glucocorticoids (47%), hydroxychloroquine (82.4%), antivirals (50%), and antibiotic (94%) therapy. All survivors (n = 18) were treated with Low Molecular Weight Heparin (LMWH) at prophylactic (n = 13) or therapeutic dose (n = 5) as compared to 11 (68.8%) in the group of non-survivors (p = 0.02). Among the non-survivors five patients who did not receive anti-thrombotic prophylaxis were either on a palliative care regimen or had been judged to be at high bleeding risk by attending physician. The number of patients treated with a beta-blocker during the hospitalization was significantly higher among survivors than non-survivors (55.6% and 12.5%, respectively, p = 0.01). Oxygen therapy was delivered by rebreather mask, Venturi mask, or nasal cannula. Only two patients who died (12.5%) were treated with non-invasive ventilation, while one patient in the survivor group (5.6%) did not require oxygen therapy (Table 2).
3.2 Survival analysis
At univariate analysis odds of survival was significantly associated with longer hospital stay, higher PaO2:FiO2 ratio, higher lymphocyte counts, lower LDH levels, use of beta-blockers at home and during the hospital stay. Multivariate analysis performed with logistic regression confirmed better survival only for higher PaO2:FiO2 ratio. Lymphocyte counts and LDH levels were excluded from the model because of the missing data issue (Table 3 ).
Table 3.
Univariate and multivariate analysis for statistically significant variables.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Hospital stay duration | 1.003 | 1.0–1.1 | 0.035 | 1.004 | 0.99–1.1 | 0.08 |
| PaO2:FiO2 | 1.02 | 1.0–1.03 | 0.02 | 1.06 | 1.0–1.03 | 0.02 |
| Lymphocyte count | 91 | 1.84–4563.7 | 0.023 | |||
| LDH levels | 0.98 | 0.97–0.99 | 0.02 | |||
| B-blockers at home | 5.4 | 1.15–25.8 | 0.034 | 0.24 | 0.06–3.8 | 0.3 |
| B-blockers in hospital | 8.7 | 1.5–50.3 | 0.015 | 0.22 | 0.013–3.6 | 0.3 |
OR (odds ratio), CI (confidence interval).
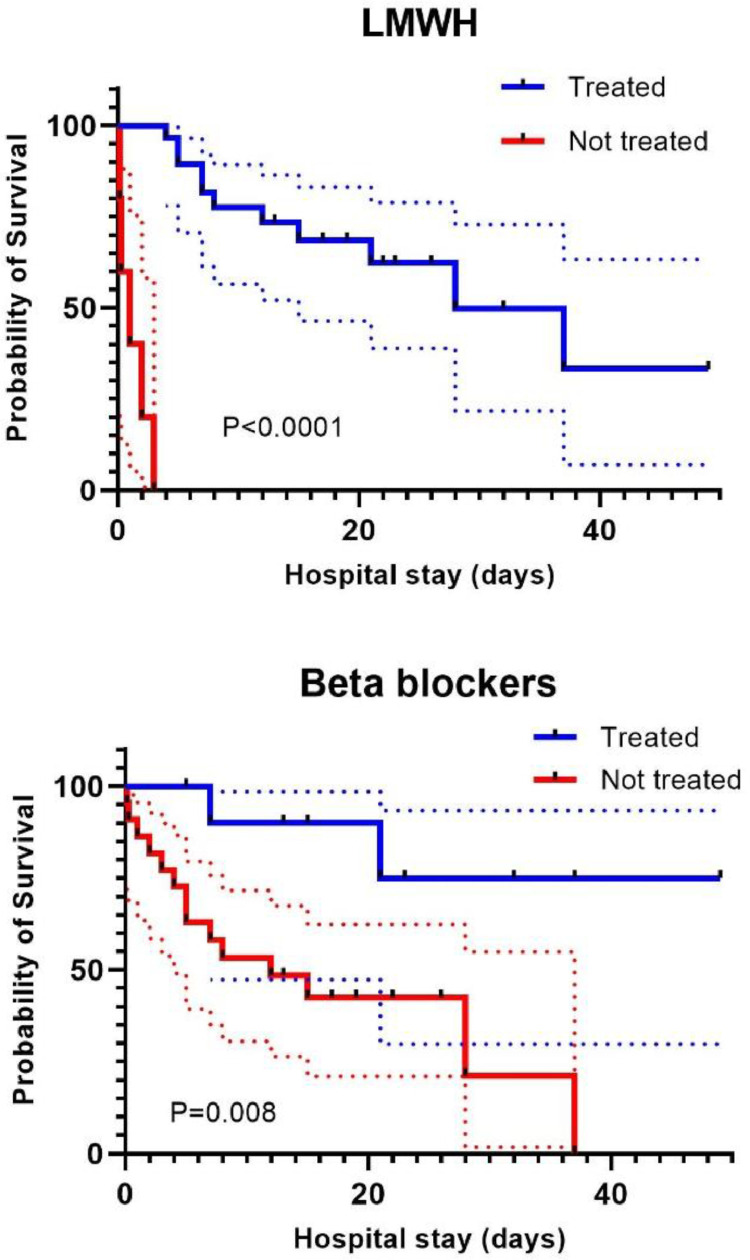
Univariate analysis did not yield results for LMWH most probably because of a complete separation issue (all patients in the survivors group received LMWH). To investigate the effect of LMWH we performed survival analysis by Kaplan Meier which showed a significant difference in event-free survival between patients treated or not with LMWH (p < 0.0001 by the Mantel-Cox test, p < 0.0001 by the Breslow test). The effect of in hospital beta-blockers alone was also evaluated with Kaplan Meier which showed a significant difference between those treated and those who did not receive it (p = 0.008 by the Mantel-Cox test, p = 0.015 by the Breslow test) however with notable overlap of confidence intervals (Fig. 2 ).
Fig. 2.
Survival curves by Kaplan Meier for low molecular weight heparin (LMWH) (above) and beta-blocker (below) treatment during the hospital stay, dotted lines show the confidence intervals.
The relative importance on survival of other prognostic factors, including sex, Charlson Comorbidity Index, PaO2:FiO2 as a parameter of respiratory failure severity, treatment with corticosteroids and beta-blockers was evaluated in the subgroup of patients treated with LMWH. The association with death was assessed by the Cox proportional hazard model which did not show significant difference for sex (HR 2.7 [95% CI 0.53–14.3], p = 0.23), CCI (HR 0.7 [95% CI 0.37–1.45], p = 0.38), PaO2:FiO2 ratio (HR 0.98 [95% CI 0.97–1.0], p = 0.07), corticosteroid therapy (HR 0.99 [95% CI 0.22–4.5], p = 0.99) and beta-blocker therapy (HR 2.8 [95% CI 0.56–14,7], p = 0.21). Patients who did not receive LMWH were excluded from the multivariate analysis as for the noticeably short duration of hospital stay, they could not be compared with all the others.
4. Discussion
In this study, we examined the characteristics of a group of patients at an exceedingly high risk of death for their age and comorbidities, managed within an intermediate intensity ward of Internal Medicine. This decision was made because of extremely limited resources respecting the ethical guidance for Intensive Care Unit (ICU) admission proposed by the Italian Society of Anesthesia, Analgesia, Resuscitation, and Intensive Care (Ethical guidance for ICU admission & suspension in conditions of exceptional imbalance between resources needed & those available. Italian Society of Anesthesia, Analgesia, Resuscitation & Intensive Care, 2020). Appropriate palliative care was also set up whenever it was necessary (Ethical guidance for ICU admission & suspension in conditions of exceptional imbalance between resources needed & those available. Italian Society of Anesthesia, Analgesia, Resuscitation & Intensive Care, 2020). Alongside advanced age, chronic diseases are identified as predictors of death among COVID-19 patients (Li, Huang, & Wang, 2020). In a report published by Italy's National Statistics Institute, death charts of 4942 confirmed COVID-19 cases, which represented 15.6% of all deaths notified as of 25 May 2020, showed that 71.8% had at least one comorbidity. Hypertensive heart disease (~19%), diabetes mellitus (~14%), and ischemic heart disease (~13%) were the most common conditions associated with fatal outcome in advanced age (Impact of COVID-19 epidemic on mortality: causes of death in COVID-19 laboratory confirmed cases. Istat, 2020). In our study, we could not identify any statistically significant difference in comorbidities, including traditional CVD risk conditions like diabetes, cerebrovascular disease, and hyperlipidemia, between survivors and non-survivors. Hypertension was the most common comorbidity among both survivors and non-survivors. The prevalence of chronic heart failure was also higher in patients who survived, and most of them were receiving optimal medical therapy; however, we cannot exclude the presence of undiagnosed chronic heart disease among patients who died, taking into consideration their longstanding history for hypertension. Impaired immunity secondary to aging and to associated conditions like hyperlipidemia and diabetes may influence susceptibility to severe forms of COVID-19 (Andersen, Murphy, & Fernandez, 2016; Liu, van der Zeijst, Boog, & Soethout, 2011). A balanced presence of these factors in both groups raises the question if the severity of respiratory failure might have depended on different mutated forms of SARS-CoV-2 (Becerra-Flores & Cardozo, 2020). In fact, patients who survived presented a milder respiratory failure indicated by higher PaO2:FiO2 values.
Except for atypical presentations like falls, presyncope, and syncope among survivors, other predominant symptoms like fever, cough, and dyspnea were already known in hospitalized patients (Huang, Wang, & Li, 2020; International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC) COVID-19 Report as of 08 April 2020, 2020). On the opposite, olfactory and taste dysfunctions, referred by few patients alive, despite they have been reported as very frequent manifestations of mild to moderate disease (Lechien, Chiesa-Estomba, & Place, 2020). Atypical presentations require a thorough clinical assessment as they might, during the pandemic, conceal highly deleterious conditions for individual and public health.
Most of the patients in our study were tested for routine blood tests and only a few had been tested for specific parameters. Hence, we were not able to assess coagulopathy and the hyperinflammatory syndrome described in COVID-19 (Tang, Li, Wang, & Sun, 2020; Toniati, Piva, & Cattalini, 2020). However, lower lymphocyte count and higher serum LDH, some of the factors identified as predictors of severe outcome (Lippi & Plebani, 2020), were normal or only slightly altered among survivors as compared to those who died. Coagulation dysfunction most probably indicates thrombotic complications requiring further investigation and appropriate anticoagulation (Klok, Kruip, & van der Meer, 2020). In our study, the incidence of venous thromboembolism was very low, probably due to the prompt prescription of thromboprophylaxis with LMWH at admission in most of the patients. Anti-thrombotic therapy in our study population, prescribed at weight adjusted prophylactic dose or therapeutic dose (100 UI/kg b.i.d.) as per internal protocol, was associated with a better survival. The positive effect of anticoagulant therapy on in-hospital survival was also observed in another study with a larger population (Paranjpe, Fuster, & Lala, 2020). Anti-thrombotic dosage modifications have been proposed in COVID-19 patients with several risk factors for thrombosis such as obesity, previous deep vein thrombosis and active cancer, but more studies addressing this approach with high quality evidence in the light of bleeding risk are still lacking (Marietta, Ageno, & Artoni, 2020; McBane, Torres Roldan, & Niven, 2020; Testa, Paoletti, Giorgi-Pierfranceschi, & Pan, 2020).
Supportive care should aim at preventing the known complications associated to COVID-19. In addition to anti-thrombotic therapy, beta-blockers could also play a protective role in COVID-19 patients same as they have been proved to reduce all-cause mortality and in-hospital mortality in patients with BPCO (Yang et al., 2020). In our study, beta-blocker therapy, as part of chronic treatment, was not withdrawn or was introduced de novo to control heart rate in patients with atrial fibrillation and was associated with better survival. Acute myocardial injury mediated directly by angiotensin-converting enzyme 2 (ACE2) receptors or caused indirectly by hypoxemia and systemic inflammation can provoke cardiac arrhythmias like, events commonly encountered in COVID-19 (Bhatla, Mayer, & Adusumalli, 2020; Driggin, Madhavan, & Bikdeli, 2020; Guo, Fan, & Chen, 2020; Hudson, Kurt, Petty, & Genton, 1973; Kwenandar, Japar, & Damay, 2020). Beta-blocker therapy could exert its protective role by minimizing the risk of tachyarrhythmias and guideline directed medical therapy including beta-blockers is recommended in COVID-19 patients with chronic heart disease (ESC Guidance for the Diagnosis & Management of CV Disease during the COVID-19 Pandemic. European Society of Cardiology, 2020; Priori, Blomström-Lundqvist, & Mazzanti, 2015), however studies focusing on the effect of beta-blockers in COVID-19 patients are still lacking. In the absence of continuous heart rhythm monitoring in our study population we could not exclude cardiac arrhythmias other than atrial fibrillation.
4.1 Limitations
The study represents a real-life description of results obtained in a peculiar subpopulation of very frail patients, and it should be read in the light of several limitations, including the small sample size, the retrospective analysis, missing laboratory data and lack of information on exact timing of onset of symptoms before hospitalization.
4.2 Conclusions
These new data in the elderly can give insight to do further study with larger sample size focusing on this population. The severity of respiratory failure and the use of anti-thrombotic and beta-blockers seemed to affect the outcome of the older patients. Treatment with LMWH and beta-blockers prevent the thrombotic and arrhythmic complications described in Covid-19 patients. Since the risks of bleeding and bradyarrhythmia might not be negligible in the elderly, the role of anti-thrombotic and beta-blockers should be further investigated in randomized studies.
Author contribution
All authors contributed equally to the preparation of this manuscript.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors have declared no conflicts of interest for this article.
Acknowledgments
All authors contributed equally to development of this study from data collection to statistical analysis and in writing this manuscript.
References
- Andersen C.J., Murphy K.E., Fernandez M.L. Impact of obesity and metabolic syndrome on immunity. Advances in nutrition (Bethesda, Md.) 2016;7(1):66–75. doi: 10.3945/an.115.010207. Published 2016 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra-Flores M., Cardozo T. SARS-CoV-2 viral spike G614 mutation exhibits higher case fatality rate [published online ahead of print, 2020 May 6] The International Journal of Clinical Practice. 2020:e13525. doi: 10.1111/ijcp.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatla A., Mayer M.M., Adusumalli S., Hyman MC., Eric Oh., Tierney A., Deo R., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm : The Official Journal of the Heart Rhythm Society. 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case definition for coronavirus disease 2019 (COVID-19), as of 29 May 2020. European center for disease control (2020). Available At: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition Accessed September 27, 2020.
- Chen L.K. Older adults and COVID-19 pandemic: Resilience matters. Archives of Gerontology and Geriatrics. 2020;89 doi: 10.1016/j.archger.2020.104124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 integrated surveillance: Key national data as of 15 September 2020. Available at: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_15-settembre-2020.pdf Accessed September 27, (2020).
- Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G., Parikh SA., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. Journal of the American College of Cardiology. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. European Society of Cardiology (2020). Available at: https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance Accessed October 7, 2020.
- Ethical guidance for ICU admission and suspension in conditions of exceptional imbalance between resources needed and those available. Italian Society of Anesthesia, Analgesia, Resuscitation, and Intensive Care (2020). Available At: http://siaarti.it Accessed September 27, 2020.
- Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Lu Z., et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19) JAMA Cardiology. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Putri C., Arisa J., Situmeang R.F.V., Kurniawan A. Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: A systematic review and meta-analysis [published online ahead of print, 2020 Nov 19] Archives of Gerontology and Geriatrics. 2020;93 doi: 10.1016/j.archger.2020.104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Cao B., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30] Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L.D., Kurt T.L., Petty T.L., Genton E. Arrhythmias associated with acute respiratory failure in patients with chronic airway obstruction. Chest. 1973;63(5):661–665. doi: 10.1378/chest.63.5.661. [DOI] [PubMed] [Google Scholar]
- Iaccarino G., Grassi G., Borghi C., Ferri C., Salvetti M., Volpe M. Age and multimorbidity predict death among COVID-19 patients: Results of the SARS-RAS study of the Italian Society of Hypertension. Hypertension. 2020;76(2):366–372. doi: 10.1161/HYPERTENSIONAHA.120.15324. [DOI] [PubMed] [Google Scholar]
- Impact of COVID-19 epidemic on mortality: Causes of death in COVID-19 laboratory confirmed cases. Istat (2020). Available at: http://www.istat.it Accessed September 27, 2020.
- International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC) COVID-19 Report as of 08 April 2020. Summary. ISARIC (2020). Available at: https://media.tghn.org/medialibrary/2020/04/ISARIC_Data_Platform_COVID-19_Report_8APR20.pdf Accessed September 27, 2020.
- Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous MS., Gommers DAMPJ., Kant KM., Endeman H., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwenandar F., Japar K.V., Damay V., Hariyanto TI., Tanaka M., Lugito NPH, Kurniawan A. Coronavirus disease 2019 and cardiovascular system: A narrative review. International Journal of Cardiology. Heart & Vasculature. 2020;29 doi: 10.1016/j.ijcha.2020.100557. Published 2020 Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q., Saussez S., et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. Journal of Internal Medicine. 2020;288(3):335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.Q., Huang T., Wang Y.Q., Wang ZP., Liang Y., Huang TB., Wang Y. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. Journal of Medical Virology. 2020;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clinical Chemistry and Laboratory Medicine. 2020;58(7):1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- Liu W.M., van der Zeijst B.A., Boog C.J., Soethout E.C. Aging and impaired immunity to influenza viruses: Implications for vaccine development. Human Vaccines. 2011;7(Suppl:94–98) doi: 10.4161/hv.7.0.14568. [DOI] [PubMed] [Google Scholar]
- Marietta M., Ageno W., Artoni A., De Candia E., Gresele P., Marchetti M., Tripodi A., et al. COVID-19 and haemostasis: A position paper from Italian Society on Thrombosis and Haemostasis (SISET) Blood Transfusion = Trasfusione del sangue. 2020;18(3):167–169. doi: 10.2450/2020.0083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBane R.D., 2.nd, Torres Roldan V.D., Niven A.S., Pruthi R.K., Franco P.M., Linderbaum J.A., Murad M.H., et al. Anticoagulation in COVID-19: A systematic review, meta-analysis, and rapid guidance from Mayo Clinic. Mayo Clinic Proceedings. 2020;95(11):2467–2486. doi: 10.1016/j.mayocp.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S., Tian S., Lou J., Kang X., Zhang L., Lian H., Zhang J. Clinical characteristics of older patients infected with COVID-19: A descriptive study. Archives of Gerontology and Geriatrics. 2020;89 doi: 10.1016/j.archger.2020.104058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe I., Fuster V., Lala A., Russak AJ., Glicksberg BS., Levin MA., Nadkarni GN., et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. Journal of the American College of Cardiology. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori S.G., Blomström-Lundqvist C., Mazzanti A., Blom N., Borggrefe M., Camm J., Van Veldhuisen DJ., et al. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) European Heart Journal. 2015;36(41):2793–2867. doi: 10.1093/eurheartj/ehv316. 2015. [DOI] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa S., Paoletti O., Giorgi-Pierfranceschi M., Pan A. Switch from oral anticoagulants to parenteral heparin in SARS-CoV-2 hospitalized patients. Internal and Emergency Medicine. 2020;15(5):751–753. doi: 10.1007/s11739-020-02331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., Latronico N., et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmunity Reviews. 2020;19(7) doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Coronavirus Disease (COVID-19) Dashboard. World Health Organization (2021). Available at: https://covid19.who.int/ Accessed January 22, 2021.
- Wu Z., McGoogan J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yang Y.L., Xiang Z.J., Yang J.H., Wang W.J., Xu Z.C., Xiang R.L. Association of β-blocker use with survival and pulmonary function in patients with chronic obstructive pulmonary and cardiovascular disease: A systematic review and meta-analysis. European Heart Journal. 2020;41(46):4415–4422. doi: 10.1093/eurheartj/ehaa793. [DOI] [PMC free article] [PubMed] [Google Scholar]