Abstract
Ethnopharmacological relevance
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causes coronavirus disease 2019 (COVID-19), a highly pathogenic virus that has spread rapidly across the entire world. There is a critical need to develop safe and effective drugs, especially broad-spectrum antiviral and organ protection agents in order to treat and prevent this dangerous disease. It is possible that Chinese herbal medicine may play an essential role in the treatment of patients with SARS-CoV-2 infection.
Aim of the review: We aim to review the use of Chinese herbal medicine in the treatment of COVID-19 both in vitro and in clinical practice. Our goal was to provide a better understanding of the potential therapeutic effects of Chinese herbal medicine and to establish a “Chinese protocol” for the treatment of COVID-19.
Materials and methods
We systematically reviewed published research relating to traditional Chinese herbal medicines and the treatment of SARS-CoV-2 from inception to the 6th January 2021 by screening a range of digital databases (Web of Science, bioRxiv, medRxiv, China National Knowledge Infrastructure, X-MOL, Wanfang Data, Google Scholar, PubMed, Elsevier, and other resources) and public platforms relating to the management of clinical trials. We included the active ingredients of Chinese herbal medicines, monomer preparations, crude extracts, and formulas for the treatment of COVID-19.
Results
In mainland China, a range of Chinese herbal medicines have been recognized as very promising anti-SARS-CoV-2 agents, including active ingredients (quercetagetin, osajin, tetrandrine, proscillaridin A, and dihydromyricetin), monomer preparations (xiyanping injection, matrine-sodium chloride injection, diammonium glycyrrhizinate enteric-coated capsules, and sodium aescinate injection), crude extracts (Scutellariae Radix extract and garlic essential oil), and formulas (Qingfei Paidu decoction, Lianhuaqingwen capsules, and Pudilan Xiaoyan oral liquid). All these agents have potential activity against SARS-CoV-2 and have attracted significant attention due to their activities both in vitro and in clinical practice.
Conclusions
As a key component of the COVID-19 treatment regimen, Chinese herbal medicines have played an irreplaceable role in the treatment of SARS-CoV-2 infection. The “Chinese protocol” has already demonstrated clear clinical importance. The use of Chinese herbal medicines that are capable of inhibiting SARS-Cov-2 infection may help to address this immediate unmet clinical need and may be attractive to other countries that are also seeking new options for effective COVID-19 treatment. Our analyses suggest that countries outside of China should also consider protocols involving Chinese herbal medicines combat this fast-spreading viral infection.
Keywords: SARS-Cov-2, Chinese herbal medicine, Active ingredients, Broad-spectrum antiviral, Organ protection
Abbreviations: ACE2, angiotensin-converting enzyme 2; andro, andrographolide; CHIKV, Chikungunya virus; 3CLpro, 3C-Like protease; COVID-19, coronavirus disease 2019; EBOV, Ebola virus; EC50, half maximal effective concentration; FCV, feline calicivirus; GA, glycyrrhizic acid; HBV, Hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; GRP78, glucose regulating protein 78; H1N1, influenza A; HSV-1, herpes simplex virus type 1; IC50, half maximal inhibitory concentration; IL-10, interleukin-10; JHQG, Jinhua Qinggan granules; LHQW, Lianhuaqingwen capsules; MERS, Middle East respiratory syndrome coronavirus; MLAV, Mengla virus; Mpro, main protease; NHC PRC, National Health Commission of the People's Republic of China; nsp, nonstructural protein; PDL, Pudilan Xiaoyan oral liquid; PHEIC, public health emergency of international concern; PLpro, papain-like protease; RdRp, RNA-dependent RNA polymerase; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SI, selectivity index; TMPRSS2, transmembrane protease serine 2; TNF-α, tumor necrosis factor-α; WHO, World Health Organization; XYP, Xiyanping injection
Graphical abstract
1. Introduction
In late December 2019, a novel coronavirus disease (COVID-19) outbreak occurred in Wuhan, China (Jie et al., 2020). As the sixth public health emergency of international concern (PHEIC), this global pandemic still remains as a focus of concern due to the significant threat it poses to the lives of billions of individuals (WHO Emergency Committee, 2020). COVID-19 is causes by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and can cause multiple injuries to extrapulmonary organs and tissues, including the heart (Shi et al., 2020b), kidney (Puelles et al., 2020), liver (Zhang et al., 2020a), and brain (Rhea et al., 2020). However, the virus has also been shown to affect the ocular system (Wu et al., 2020), the gastrointestinal system (Redd et al., 2020), the musculoskeletal system (Cipollaro et al., 2020), the skin (Casas et al., 2020), and the cardiovascular system (Nishiga et al., 2020). There are numerous pathological mechanisms that are potentially involved in these forms of injury, including direct viral toxicity (Gupta et al., 2020), immune dysregulation (Acharya et al., 2020), endothelial derangement (Nizzoli et al., 2020), and an imbalance in the renin angiotensin aldosterone system (John et al., 2020). Given the complexity of the mechanisms involved, it is vital that we develop novel therapies against the SARS-CoV-2 virus that are novel, safe and effective.
Chinese herbal medicines are associated with a range of beneficial effects that had collectively led to this approach becoming an important option for fighting the epidemic in mainland China, including antiviral activity (Ma et al., 2020e), anti-inflammatory activity (Chen et al., 2019b), and anti-fibrotic activity (Yang et al., 2019b). On the 22nd January 2020, the National Health Commission of the People's Republic of China (NHC PRC) included traditional Chinese medicine as a recommended therapeutic option for the treatment of patients with COVID-19 The National Health Commission of the People's Republic of China (NHC PRC), 2020. As of the 23rd March 2020, a total of 74,187 patients infected with SARS-CoV-2 on the mainland of China (91.5% of the total number of confirmed cases at that time) were treated with traditional Chinese medicine; the overall efficacy of this approach exceeded 90% (Xinhua Net, 2020b; Yu, 2020b). As of the 6th January 2021, the COVID-19 epidemic has been well controlled in China; the current SARS-CoV-2 infection rate is 1279 cases (WHO, 2020). Chinese herbal medicine has played a critical role in the treatment of this novel viral infection by virtue of its proven activity against multiple SARS-CoV-2 pathways and targets (Huang et al., 2020). Globally, the epidemic is still ongoing, the World Health Organization (WHO) has reported that there are 84,780,171 confirmed cases across the world, including 23,707,908 cases of active SARS-CoV-2 infection (WHO, 2020). The rapid spread and highly infectious nature of this virus has created a health emergency on a global scale; none of the world's population is currently immune to this infection. It is vital that medical practitioners from across the world unite in a concerted effort to combat SARS-CoV-2 infection, including Chinese medicine practitioners.
The outstanding performance of Chinese herbal medicine, especially in mainland China, has led to the publication of several interesting reviews on COVID-19 from the perspectives of clinical experience (Lee et al., 2020; Shu et al., 2020; Zhuang et al., 2020), scientific foundation (Leung et al., 2020), efficacy and safety (Li et al., 2020f), pros and cons (Nugraha et al., 2020; López-Alcalde et al., 2020) and meta-analysis (Fan et al., 2020; Xiong et al., 2020b; Luo et al., 2020a). However, as yet, there has been no hierarchical (active ingredients, monomer preparations, crude extracts, and formulas) review covering the use of Chinese herbal medicine for the treatment of COVID-19 that covers both in vitro research and clinical practice.
The molecule (active ingredient) is responsible for the biological activity of Chinese herbal medicine. To better digest the potential therapeutic effects of Chinese herbal medicine, we attempted to focus on both representative and different categories of chemical components (involving high-quality anti-SARS-CoV-2 studies in vitro), rather than take a systematically driven approach. We also emphasize the unique advantages of Chinese herbal medicines in terms of organ protection and broad-spectrum activity against viruses.
This review presents a hierarchical overview of the current progress in potentially active anti-SARS-CoV-2 ingredients in Chinese herbal medicine, monomer preparations, crude extracts, and formulas. Our aim was to provide a ‘Chinese protocol’ for the treatment of COVID-19.
2. Promising active ingredients of Chinese herbal medicine that exhibit in vitro activity against SARS-Cov-2
The history of the modern pharmaceutical industry includes many anecdotes describing how traditional Chinese medicine inspired the discovery of several drugs; for example, artemisinin (Ma et al., 2020c) and arsenic trioxide (List et al., 2003). Chinese herbal medicine consists of a large group of secondary metabolites (e.g., flavonoids) that present wide structural diversity and includes a range of compounds (e.g., flavones, flavanols, flavanonols, and isoflavones) that mediate a wide range of valuable bioactivities, including anti-browning, anti-tuberculosis, anti-microbial, anti-cancer and anti-oxidant effects (Wang et al., 2013). As research continues, flavonoids have recently been recognized as promising antiviral agents against multiple viruses, including the influenza A (H1N1) virus (Roschek et al., 2009), Ebola virus (EBOV) (Fanunza et al., 2020), and severe acute respiratory syndrome coronavirus (SARS-CoV) (Jo et al., 2019).
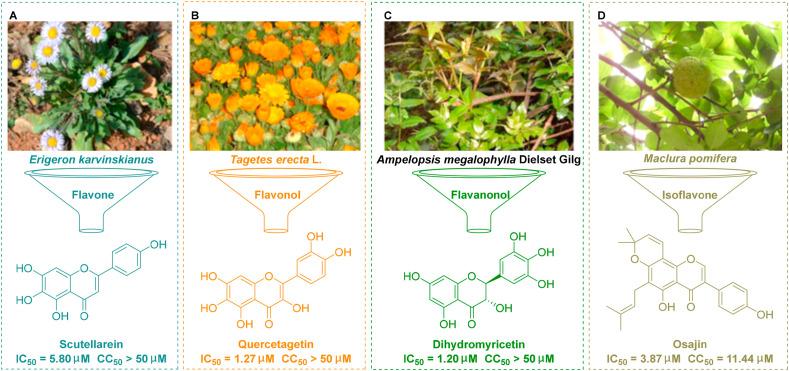
Scutellarein, a flavone monomer, is an important natural product that can be isolated from the plant Erigeron karvinskianus and used as a traditional herbal medicine (Fig. 1 A) (Miao et al., 2020). This agent has significant bioactivity against several diseases, including pulmonary fibrosis (Miao et al., 2020), but can also confer neuroprotective effects (Tang et al., 2014). Scutellarein has half maximal inhibitory concentration (IC50) values of 0.86 μM and 2.50 μM against SARS-CoV (Yu et al., 2012) and HIV (Sansei et al., 1997), respectively. Quercetagetin is a natural flavonol that is extracted from Tagetes erecta L. (marigold), a traditional form of herbal medicine (Fig. 1B) (Kang et al., 2013). Extensive studies have demonstrated that quercetagetin exerts a range of pharmacological activities, as an anti-inflammatory agent (Kang et al., 2013) and as an anti-viral agent (Ahmed-Belkacem et al., 2014). Furthermore, quercetagetin exhibits broad-spectrum activities against viruses, including feline calicivirus (FCV) at an IC50 of 2.8 μM (Fumian et al., 2018), hepatitis C virus (HCV) at a half maximal effective concentration (EC50) of 5.4 μM in Huh 7.5 cells, a stable HCV cell-line (Ahmed-Belkacem et al., 2014); and Chikungunya virus (CHIKV) at an IC50 of 43.52 μM (Lani et al., 2016). Dihydromyricetin, a flavanonol monomer, is a secondary metabolite isolated from the plant Ampelopsis megalophylla Dielset Gilg that has potential anti-oxidative effects (Fig. 1C) (Zhang et al., 2018a). Dihydromyricetin also been reported exert anti-inflammatory effects and was shown to reduce lung injury by inhibiting activation of the NLRP3 inflammasome in vascular endothelial cells (Wang et al., 2019).
Fig. 1.
Flavonoids that have been shown to exert activity against SARS-Cov-2 in vitro. (A) Scutellarein was isolated from the plant Erigeron karvinskianus. (B) Quercetagetin was isolated from the plant Tagetes erecta L. (C) Dihydromyricetin was isolated from the plant Ampelopsis megalophylla Dielset Gilg. (D) Osajin was isolated from the plant Maclura pomifera.
The main protease (Mpro) of SARS-CoV-2, also referred to as the 3C-like protease (3CLpro), plays an essential role in the maturation process of viral polyproteins and is therefore an attractive therapeutic target (Zhang et al., 2020f). Recent research by Liu et al. has shown that three natural flavonoids (scutellarein, quercetagetin, and dihydromyricetin) can effectively inhibit SARS-CoV-2 3CLpro activity in vitro and strongly inhibit the replication of SARS-CoV-2 in Vero cells at an IC50 of 5.80 μM, 1.27 μM, and 1.20 μM, respectively (Liu et al., 2020a).
Osajin, produced by the medicinal plant Maclura pomifera, is an antioxidative prenylated isoflavone (Fig. 1D) (Dilek et al., 2017). Osajin exhibits a range of significant pharmacological activities against prostate cancer, diabetes and bacterial infections (Dilek et al., 2017). Jeon et al. recently revealed that osajin could effectively inhibit the replication of SARS-CoV-2 in vitro with an EC50 at 3.87 μM with only mild levels of toxicity (selectivity index (SI) = 2.95) (Jeon et al., 2020). Based on molecular docking and binding stability, Kousar et al. further revealed that osajin could be considered as a potential inhibitor of the MTase (−8.2 kcal/mol) and helicase (−8.2 kcal/mol) in SARS-CoV-2 (Kousar et al., 2020). Collectively, this evidence has highlighted a new potential role for natural flavonoids for the inhibition of SARS-CoV-2 replication in vitro. Further research is now needed to investigate whether these agents also exhibit anti-viral activities in vivo.
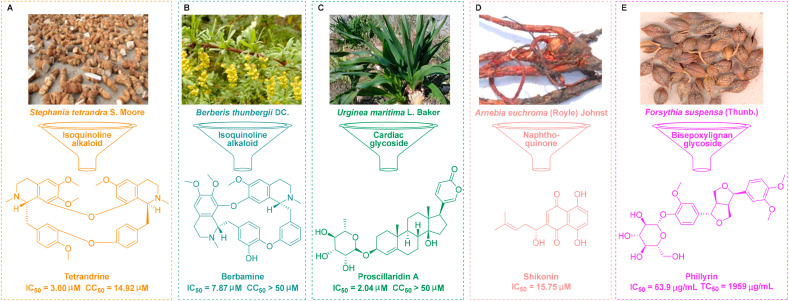
Bisbenzylisoquinoline alkaloids are structurally diverse natural products that contain approximately 500 compounds that are of medical importance (Weber and Opatz, 2019). Tetrandrine is a macrocyclic alkaloid and a natural product that can be extracted from the commonly used plant Stephania tetrandra S. Moore, a form of traditional Chinese medicine (Fig. 2 A) (Han et al., 2007). As a herbal alkaloid, tetrandrine has been widely used for its pharmacological activity against lung injury (Han et al., 2007). Furthermore, tetrandrine has been recognized as a promising broad-spectrum antiviral drug that exhibits in vitro activity against a range of viruses, including human coronavirus (HCoV) OC43 at an EC50 of 0.296 μM (Kim et al., 2019), EBOV at an IC50 of 0.055 μM (Sakurai et al., 2015), and human immunodeficiency virus (HIV)/Mengla virus (MLAV)-GP at an EC50 of 2.65 μM (Chen et al., 2019a). Very recently, Jeon et al. revealed that tetrandrine could effectively inhibit the replication of SARS-CoV-2 in Vero cells at an IC50 of 3.0 μM (Jeon et al., 2020), thus suggesting the clinical potential of tetrandrine for the treatment of SARS-CoV-2. Qian et al. also reported that tetrandrine could reduce the entry of SARS-CoV-2 by blocking the activity of the two-pore channel 2 which plays a critical role in endocytosis (Ou et al., 2020).
Fig. 2.
Other traditional Chinese medicines that exhibit bioactivity against SARS-Cov-2 in vitro. (A) Tetrandrine was isolated from the plant Stephania tetrandra S. Moore. (B) Berbamine was isolated from the plant Berberis thunbergii DC. (C) Proscillaridin A was isolated from the plant Urginea maritima L. Baker. (D) Shikonin was isolated from the plant Arnebia euchroma (Royle) Johnst. (E) Phillyrin was isolated from the plant Forsythia suspensa (Thunb.).
Berbamine, another typical bisbenzylisoquinoline alkaloid, can be extracted from Berberis thunbergii DC., a traditional form of Chinese medicine (Fig. 2B) (Zheng et al., 2017). Berbamine exhibits a range of promising pharmacological properties; for example, it can protect the heart from ischemia/reperfusion injury (Zheng et al., 2017) by maintaining cytosolic Ca(2+) homeostasis (Zhang et al., 2012). In addition, berbamine can exhibit efficacy for the treatment of influenza virus (Jin et al., 1986). Liu et al. recently revealed that berbamine can also effectively inhibit the replication of SARS-CoV-2 3CLpro in Vero cells at an IC50 of 7.87 μM with moderate levels of cytotoxicity (SI = 6.35) (Liu et al., 2020a).
Angiotensin-converting enzyme 2 (ACE2), a vital component of the angiotensin-regulating system, is a host cell entry receptor for SARS-CoV-2. Interaction between the glycosylated SARS-CoV-2 spike (S) protein and ACE2 is the first step of the SARS-CoV-2 infection (Clausen et al., 2020). SARS-CoV-2 can also bind to glucose regulating protein 78 (GRP78) (Elfiky, 2020) and transmembrane protease serine 2 (TMPRSS2) (Hoffmann et al., 2020). Balmeh et al. revealed that berbamine has high binding energy for ACE2 (−12.3kCal/mol), TMPRSS2 (−11.8kCal/mol) and GRP78 (−11kCal/mol) receptors; these are the most important receptors for SARS-Cov-2 (Balmeh et al., 2020).
Proscillaridin A, a Na+/K+ pump inhibitor, is an active cardiac glycoside that can be isolated from the traditional Chinese medicine Urginea maritima L. Baker; this is commonly used to treat heart failure (Fig. 2C) (Maryam et al., 2018). Studies have shown that Proscillaridin A can exert therapeutic effects against heart failure (Costa et al., 2019), apoptosis in human fibroblasts (Winnicka et al., 2010), and non-small-cell lung cancer (Li et al., 2018). More recently, Liu et al. carried out an in-depth study of proscillaridin A to identify its effect against COVID-19. The authors revealed that proscillaridin A could effectively inhibit the replication of SARS-CoV-2 3CLpro in Vero cells at an IC50 of 2.04 μM (Liu et al., 2020a). Furthermore, proscillaridin A exhibited a low binding energy (−80.06 Kcal/mol) and well interactions (six interactions) with SARS-CoV-2 Mpro; in addition, this study provided additional evidence relating to the potential molecular mechanisms of action (Aishwarya et al., 2020).
Shikonin is a natural naphthoquinone derived from the traditional Chinese medicine Arnebia euchroma (Royle) Johnst (Fig. 2D). Shikonin has already demonstrated a broad spectrum of pharmacological properties that show significant potential for therapeutic development (Zhang et al., 2018b). Specifically, shikonin exhibits significant pharmacological activities both in vitro and in vivo, including anti-inflammatory, anti-fungal, and anti-HIV effects (Wang et al., 2020c). Jin et al. recently reported that shikonin can effectively inhibit the replication of SARS-CoV-2 Mpro r in vitro with an EC50 at 15.75 μM (Jin et al., 2020).
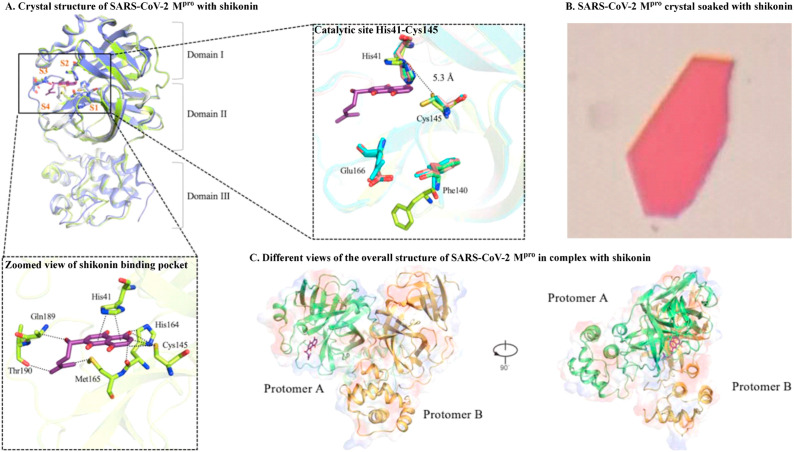
In order to support the precision design of a drug against SARS-Cov-2, Li et al. were the first to create a crystal structure of SARS-CoV-2 Mpro in complex with shikonin at 2.45 Å resolution (Fig. 3 A and B) and generated various different views of the overall structure of SARS-CoV-2 Mpro in complex with shikonin (Fig. 3C) (Li et al., 2020a). Li et al. demonstrated that the catalytic dyad His41 and Cys145 residues undergo huge conformational changes, creating a striking difference with other reported structures (Dai et al., 2020; Zhang et al., 2020e). Further analysis of the shikonin binding pocket revealed three novel interactions (π-π interactions with the His41 residue, hydrogen bond interactions with the Gln189 and Thr190 residues, and hydrogen bond interactions with the Met165, His164, and Cys145 residues) (Li et al., 2020a). This study demonstrated different binding patterns, thus suggesting significant diversity in terms of binding sites. However, Ma et al. recently revealed that shikonin might not be a target-specific SARS-CoV-2 Mpro inhibitor, due to the fact that its inhibitory ability is greatly reduced in the presence of 1,4-dithiothreitol (Ma et al., 2020a). Our growing understanding of different binding modes has offered an important strategy for designing and identifying effective anti-SARS-CoV-2 agents.
Fig. 3.
The crystal structure of SARS-CoV-2 Mpro with shikonin. (A) Comparison of SARS-CoV-2 Mpro structures, conformational difference in catalytic site, and a zoomed view of shikonin binding pocket. (B) SARS-CoV-2 Mpro crystal soaked with shikonin. (C) Different views of the overall structure of SARS-CoV-2 Mpro in complex with shikonin (image reproduced with permission from Li et al., 2020a).
Phillyrin, a form of bisepoxylignan, can be extracted from the traditional Chinese medicine Forsythia suspensa (Thunb.) (Fig. 2E) (Xia et al., 2010). Literature shows that phillyrin exhibits promising pharmacological properties against lung inflammation (Zhong et al., 2013), traumatic brain injury (Jiang et al., 2020) and acute kidney injury (Zhang et al., 2020b). Yang et al. recently revealed that phillyrin could effectively inhibit the replication of SARS-CoV-2 in Vero E6 cells at an IC50 of 63.90 μg/mL with a low toxicity profile (SI = 30.66) by inhibiting activation of the nuclear factor kappa B (NF-κB) signaling pathway (Ma et al., 2020d). Furthermore, network pharmacology and molecular docking analysis further revealed that phillyrin could block the binding of SARS-CoV-2 S-protein and Gln325 in ACE2 (Yu et al., 2020a).
Our growing understanding of the processes that can be used to identify potential anti-SARS-CoV-2 molecules has led to the use of active ingredients from Chinese herbal medicines being recognized as a potential strategy for treating COVID-19. Besides the small molecules mentioned above, several other active ingredients from Chinese herbal medicines have also been shown to exhibit potent anti-SARS-CoV-2 activities in vitro. Table 1 summarizes a range of studies investigating the in vitro effects of such agents against SARS-CoV-2. In future, we hope that the active ingredients from Chinese herbal medicines will prove to be effective for treating SARS-CoV-2 infection in animal models and in humans.
Table 1.
Summary of the active ingredients from Chinese herbal medicines that have been shown to exert activity against SARS-Cov-2 in vitro.
| No. | Compound | Plant | EC50 or IC50 (μM) | Reference |
|---|---|---|---|---|
| 1 | andrographolide | Herba andrographitis | 0.034 | Sa-ngiamsuntorn et al. (2020) |
| 2 | artemisinin | Artemisia annua | 64.45 | Cao et al. (2020) |
| 3 | baicalein | Scutellaria baicalensis | 0.39 | Liu et al. (2020a) |
| 4 | baicalin | Scutellaria baicalensis | 6.41 | Su et al. (2020) |
| 5 | berbamine | Berberis thunbergii | 7.87 | Liu et al. (2020a) |
| 6 | cannabidiol | Cannabis sativa | 7.91 | Raj et al. (2020) |
| 7 | cepharanthine | Stephania cephalantha | 0.35 | Ohashi et al. (2020) |
| 8 | chlorogenic acid | L. japonica | 39.5 | Su et al. (2020) |
| 9 | digitoxin | Digitalis purpurea | 0.23 | Jeon et al. (2020) |
| 10 | digoxin | Digitalis purpurea | 0.19 | Cho et al. (2020) |
| 11 | dihydromyricetin | Ampelopsis megalophylla | 1.20 | Liu et al. (2020a) |
| 12 | EGCG | Green tea | 0.017 | Jang et al. (2020) |
| 13 | emetine | Psychotria ipecacuanha | 0.46 | Choy et al. (2020) |
| 14 | forsythoside A | Forsythia suspensa | 3.18 | Su et al. (2020) |
| 15 | forsythoside B | Forsythia suspensa | 2.88 | Su et al. (2020) |
| 16 | glycyrrhizin | Glycyrrhiza uralensis | 0.53 | Sand et al. (2020) |
| 17 | homoharringtonine | Cephalotaxus harringtonii | 2.25 | Choy et al. (2020) |
| 18 | myricetin | Myrica rubra | 0.22 | Kuzikov et al. (2020) |
| 19 | osajin | Maclura pomifera | 3.87 | Jeon et al. (2020) |
| 20 | ouabain | Acocanthera ouabaio | 0.024 | Cho et al. (2020) |
| 21 | phillyrin | Forsythia suspensa | 1.13 | Ma et al. (2020d) |
| 22 | platycodin D | Platycodon grandiflorus | 1.19 | Kim et al. (2020) |
| 23 | proscillaridin A | Urginea maritima | 2.04 | Liu et al. (2020a) |
| 24 | pterostilbene | Pterocarpus santalinus | 19 | Ellen ter et al. (2020) |
| 25 | quercetagetin | Tagetes erecta | 1.27 | Liu et al. (2020a) |
| 26 | quercetin | Flos Sophorae Immaturus | 4.48 | Liu et al. (2020b) |
| 27 | resveratrol | Polygoni cuspidati rhizoma | 66 | Ellen ter et al. (2020) |
| 28 | scutellarein | Erigeron karvinskianus | 5.80 | Liu et al. (2020a) |
| 29 | shikonin | Arnebia euchroma | 15.75 | Jin et al. (2020) |
| 30 | Δ9-tetrahydrocannabinol | Cannabis sativa | 10.25 | Raj et al. (2020) |
| 31 | tetrandrine | Stephania tetrandra | 3.00 | Jeon et al. (2020) |
| 32 | theaflavin | Black tea | 0.015 | Jang et al. (2020) |
3. Promising Chinese herbal medicine monomer preparations for the treatment of SARS-CoV-2 infection in clinical practice
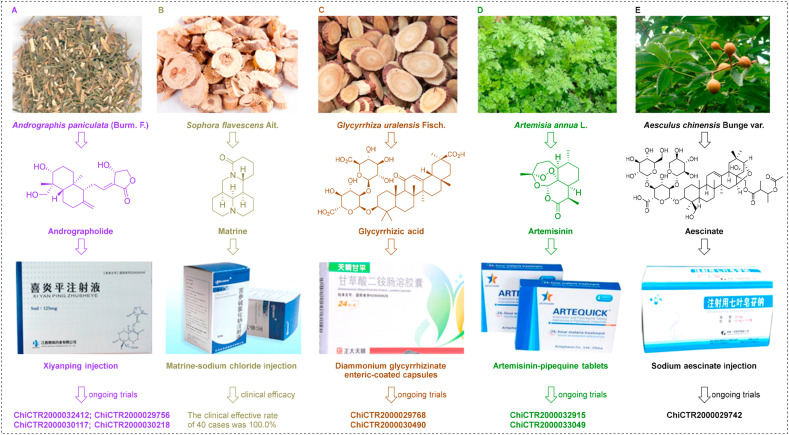
Herba andrographitis (Chinese name: Chuanxinlian), composed of the whole plant or leaves of Andrographis paniculata (Burm. F.) Nees, is a traditional herb that has been widely used over many centuries to treat a range of diseases in China (Fig. 4 A) (Kumar et al., 2019). Recent studies have reported numerous pharmacological properties of Herba andrographitis extracts, both in vitro and in vivo, against HIV (EC50 value of 5.49 μg/mL) (Feng et al., 2012), complement (EC50 value of 23.1–638.3 μg/mL) (Wen et al., 2020), and excito-repellency (96.7% escape at 0.5–5.0% w/v concentration) (Sukkanon et al., 2019). This agent has also been used to treat osteoarthritis in the knee; a 300 mg/day dose was effective and safe when applied to reduce pain (Hancke et al., 2019). Andrographolide (andro) is a major component of Herba andrographitis and belongs to the diterpenoid family. Consistent with the reported function of Herba andrographitis extracts, andro has demonstrated a broad spectrum of bioactivities, including anti-inflammatory, anti-cancer, and anti-bacterial effects (Kumar et al., 2019). Andro also exhibits broad-spectrum activities against several different types of viruses, including influenza virus (Ding et al., 2017), herpes simplex virus type 1 (HSV-1) (Seubsasana et al., 2011), Chikungunya virus (Gupta et al., 2017), and Hepatitis B virus (HBV) (Chen et al., 2014).
Fig. 4.
Monomer preparations for the treatment of SARS-CoV-2 infection in clinical practice. (A) Andrographolide, the major ingredient of Xiyanping injection, was isolated from the plant Herba andrographitis. (B) Matrine, the major ingredient of matrine injection, was isolated from the plant Sophora flavescens Aiton. (C) Glycyrrhizic acid, the major ingredient of diammonium glycyrrhizinate enteric-coated capsules, was isolated from the plant Glycyrrhizae uralensis Fisch. (D) Artemisinin, the major ingredient of artemisinin-pipequine tablets, was isolated from the plant Artemisia annua L. (E) Aescinate, the major ingredient of sodium aescinate injection, was isolated from the plant Aesculus chinensis Bunge var.
Very recently, andro has been identified as a potential inhibitor of SARS-COV-2 Mpro with excellent pharmacodynamic properties, as demonstrated by in silico and computational studies (Enmozhi et al., 2020). The bioavailability of andro is increased when it is administered intravenously. Andro sulfonate is the major ingredient of Xiyanping injection (XYP), an agent that has been approved to treat respiratory infectious diseases in China (Huang et al., 2019). Nie et al. (Nie et al., 2012) reported the effect of XYP on acute lung injury in a rat model via bronchoalveolar lavage; XYP was shown to inhibit excessive anti-inflammatory responses and reduce the production of several proinflammatory cytokines (interleukin (IL)-1β, IL-6, and IL-8). Recent studies have also shown that XYP can play a vital role in the treatment of viral pneumonia and is more effective than ribavirin (Nie et al., 2012). XYP has also proven to be effective for the treatment of acute upper respiratory diseases (Qi et al., 2018). Given its broad-spectrum of activities, XYP has become a key component of COVID-19 treatment. To further investigate anti-SARS-Cov-2 activity, clinical studies have been registered to evaluate the effect of XYP for the treatment of COVID-19 (www.chictr.org/cn/, China Clinical Trial Registry number: ChiCTR2000032412, ChiCTR2000029756, ChiCTR2000030117, and ChiCTR2000030218).
Matrine, a clinical drug in China, is an active quinolizidine alkaloid that can be isolated from Sophora flavescens Aiton, a traditional Chinese medicine (Fig. 4B) (Zhou et al., 2014). Matrine is known to exhibit a therapeutic effect on asthma, skin inflammation, Alzheimer's syndrome, viral hepatitis, and cancer (Zhang et al., 2020c). More recently, matrine has been reported to play an important role in reducing organ injury (Xu et al., 2016a). Li et al. (Li et al., 2012) showed that matrine was able to significantly reduce acute myocardial injury by reducing the expression levels of dimethylarginine dimethylaminohydrolase-2 and by attenuating the serum levels of asymmetric dimethylarginine. During the treatment of silicosis, the combination of a matrine injection with tetrandrine tablets was able to reduce lung markings very effectively (Miao et al., 2012). Interestingly, Xu et al. reported that matrine significantly improved renal function in rats with nephropathy (Xu et al., 2016b). Furthermore, a clinical study showed that matrine was able to protect liver function in patients with primary hepatic carcinoma (Lao, 2005). In another study, Huang et al. found that matrine was able to inhibit allergic airway inflammation in asthmatic mice by reducing the production of eotaxin and Th2 cytokine (Huang et al., 2014). Matrine sodium chloride injection, which has been approved for clinical application to treat cancers and cancer-related pain in China (Guo et al., 2015). With regards to the treatment of COVID-19 treatment, Sun et al. reported that the injection of matrine sodium chloride has anti-COVID-19 activity (lung index inhibition rate was 86.86% at a dose of 36.67 mL/kg/d) in a mouse model combining disease with the syndrome (Sun et al., 2020). More importantly, an in-depth clinical trial of matrine sodium chloride injection was launched to identify its efficacy against COVID-19; the effective clinical rate was 100.0% in 40 cases (Yang et al., 2020a). As with ACE2 and Mpro, RNA-dependent RNA polymerase (RdRp) is another primary target associated with SARS-CoV-2 infection (Gao et al., 2020b). Based on molecular docking experiments, Peng et al. revealed that matrine exhibits a high binding potential to RdRp (−6.3 kcal/mol), Mpro (−5.8 kcal/mol), and ACE2 (−6.1 kcal/mol) in SARS-CoV-2 (Peng et al., 2020). Furthermore, matrine could be used to treat COVID-19 by suppressing SARS-CoV-2 replication and by regulating inflammatory responses (Peng et al., 2020).
Glycyrrhizae Radix et Rhizoma (Chinese name: Gancao), the dried roots and rhizomes of Glycyrrhiza uralensis Fisch., is a traditional herb that is widely used to treat a variety of diseases, (Fig. 4C) (Gao et al., 2019). Glycyrrhizic acid (GA), an important active ingredient of Gancao, has therapeutic effects against cancer, bronchitis, and acquired immune deficiency syndrome (Sun et al., 2019). This triterpene glycoside has also played an important role not only in reducing myocardial injury (Yang et al., 2017), human coronary artery endothelial cell damage (Tang et al., 2020), lung injury (Yuan et al., 2020), and hepatotoxicity (Tian et al., 2020), but also in protecting the liver (Chen et al., 2016), and promoting neural repair (Cao et al., 2019). Furthermore, GA has been evaluated by many groups for its ability to inhibit infections to multiple viruses in vitro and in humans, including SARS-CoV (EC50 = 365 μM in Vero cells with an SI > 65) (Hoever et al., 2005), HIV (90% inhibition of HIV replication in 60% patients) (Sasaki et al., 2002), Epstein-Barr virus (Lin et al., 2008), and varicella-zoster virus (the 50% infectious dose was 710 μM with an SI = 30) (Baba et al., 1987). Multiple lines of evidence (Bailly et al., 2020; Sinha et al., 2020; Li et al., 2020d; Vardhan et al., 2020; Ray et al., 2020) now support the critical role of GA for the treatment of SARS-CoV-2 infection. Krawczyk et al. revealed that GA could effectively inhibit the replication of SARS-CoV-2 Mpro in Vero E6 cells at an EC50 of 0.44 mg/ml in vitro (Sand et al., 2020). GA is the major ingredient of diammonium glycyrrhizinate enteric-coated capsules; these are used as a hepatic protector in clinical practice in China. To further investigate anti-SARS-Cov-2 activity, clinical studies on diammonium glycyrrhizinate enteric-coated capsules for the treatment of COVID-19 have been carried out in open-label trials (www.chictr.org/cn/, China Clinical Trial Registry number: ChiCTR2000029768 and ChiCTR2000030490).
Artemisinin, a first-line antimalarial drug, is a sesquiterpene that was first isolated in 1972 from the traditional Chinese medicine Artemisia annua L. by Xia (Fig. 4D) (Xia et al., 2020a). Based on in-depth studies, accumulating evidence has shown that artemisinin also has therapeutic efficacy for respiratory diseases (Cheong et al., 2020), acute lung injury (Zhao et al., 2017), myocardial ischemia-reperfusion injury (Wang et al., 2020b), and acute kidney injury (Liu et al., 2019). Cao et al. (Cao et al., 2020) recently revealed that artemisinin was able to inhibit SARS-CoV-2 infection with an EC50 value of 64.45 μM in vitro. Molecular dynamics studies also confirmed that artemisinin exhibits potent binding to Lys353 and Lys31-binding hotspots in the SARS-CoV-2 S protein (Sehailia et al., 2020). Artemisinin is the major ingredient of artemisinin-pipequine tablets. Artemisinin is an approved drug with an excellent safety profile, highlighting a new potential role as an inhibitor of SARS-CoV-2 replication. To further investigate anti-SARS-Cov-2 activity, clinical studies have been registered to evaluate the effect of artemisinin-pipequine tablets against COVID-19 (www.chictr.org/cn/, China Clinical Trial Registry number: ChiCTR2000032915 and ChiCTR2000033049).
Sodium aescinate (SA), a prescription drug possessing organ-protective effects, is an important triterpene saponin product isolated from the traditional herbal medicine Aesculus chinensis Bunge var. (Fig. 4E), (Zhang et al., 2020e). SA exerts an important role in the neuroprotection of traumatic brain injury (via the nuclear factor erythroid 2-related factor 2/antioxidant-response element pathway) (Zhang et al., 2020d), protecting the liver (via decreasing aspartate aminotransferase and alanine aminotransferase activities) (Peng et al., 2016), promoting cardiopulmonary resuscitation (via increasing the expression of hypoxia-inducible factor-1α in the cerebral cortex) (Du et al., 2011), and protecting acute lung injury (via increasing myeloperoxidase activity and nitric oxide level in the lung (Tian et al., 2011), and decreasing malondialdehyde and matrix metalloproteinase gelatinase B levels (Du et al., 2012)). Importantly, no observable cytotoxicity or adverse events both in vivo and in vitro with SA treatment have been reported to date. SA is the major ingredient of sodium aescinate injection. To further investigate anti-SARS-Cov-2 activity, clinical studies on SA injection for the treatment of this novel viral infection has been registered to evaluate the treatment outcomes in the an open-label trial (i.e., www.chictr.org/cn/, China Clinical Trial Registry number: ChiCTR2000029742).
4. Promising crude extracts from Chinese herbal medicines for the treatment of SARS-CoV-2 infection
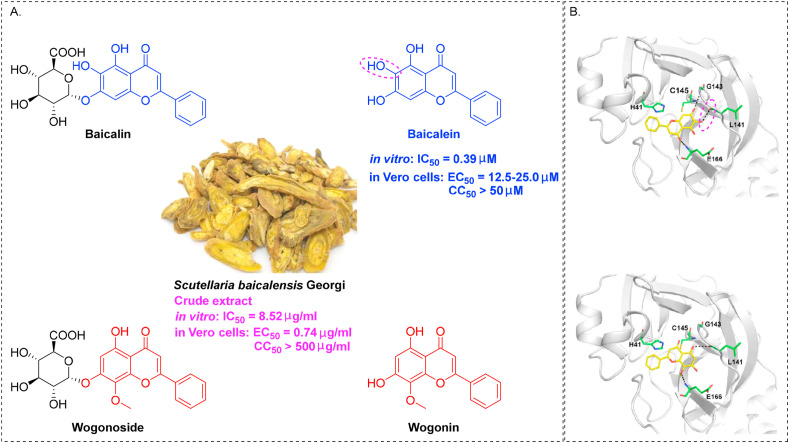
Scutellariae Radix (Chinese name: Huangqin), the dried roots of Scutellaria baicalensis Georgi, is an essential Chinese herbal medicine widely used over for 2000 years in China to treat a range of diseases including lung and liver complaints (Zhao et al., 2019). Due to a successful clinical trial, it is now listed officially in the 2020 Chinese Pharmacopoeia, 2020 British Pharmacopoeia, and European Pharmacopoeia v10.0. Huangqin's major chemical constituents are flavonoids, which contribute to its therapeutic effects, including anti-viral (Lin et al., 2016), lung injury (Yang et al., 2019a), hepatoprotective (Yang et al., 2019a), and neuroprotective activities (Li et al., 2019). A total of 100 flavonoids (56 free flavonoids and 44 flavonoid glycosides) have been isolated from Scutellaria baicalensis (Wang et al., 2018), among which baicalein, baicalin, wogonin, and wogonoside (Fig. 5 A) are the major components with broad-spectrum anti-viral effects active against H1N1 (Zhi et al., 2019), Dengue virus (Hassandarvish et al., 2016), HSV-1 (Luo et al., 2020b), and HBV (Guo et al., 2007).
Fig. 5.
Scutellaria baicalensis extracts for the treatment of SARS-CoV-2 infection. (A) Four major flavones derived from Scutellaria baicalensis. (B) Molecular docking patterns of baicalein and wogonin with SARS-CoV-2 3CLpro (image reproduced with permission from Liu et al., 2020a).
A new therapeutic strategy is currently being expanded. Lai et al. recently revealed that the crude extract of Scutellaria baicalensis could effectively inhibit the replication of SARS-CoV-2 in Vero E6 cells at an EC50 of 0.74 μg/mL with minimal toxicity (SI > 675.7) (Liu et al., 2020a). Additionally, baicalein is highly effective at inhibiting SARS-CoV-2, 3C-like protease (3CL pro) infection (IC50 = 0.39 μM) and has low toxicity (SI > 128.2), while wogonin only has 6.1% inhibition even at the concentration of 50 μM (Liu et al., 2020a). A molecular docking technique was used to better define the inhibitory activity of the flavones baicalein and wogonin. The docking model revealed that 6-OH in baicalein plays an essential role in inhibiting SARS-CoV-2 viral RNA replication (forming hydrogen bond interactions with the carbonyl group of L141), as shown in (Fig. 5B) (Liu et al., 2020a). Further study of the extract of Scutellaria baicalensis is needed to confirm its promising data. Scutellaria baicalensis is a widely used drug with a good safety profile, highlighting a new potential role for its extract in the inhibition of SARS-CoV-2 replication.
Very recently, organosulfur compounds have attracted significant attention due to their broad-spectrum anti-SARS-CoV-2 activities in vitro. For example, calpain inhibitor II could potently blocks SARS-Cov-2 Mpro infection at lower concentration (EC50 = 2.07 μM) with no observable cytotoxicity (SI > 48.3) (Ma et al., 2020b), S416 demonstrated significant inhibition of SARS-CoV-2 replication at an excellent EC50 of 17 nM with remarkable selectivity (SI > 5882) (Xiong et al., 2020a), auranofin could effectively inhibit the replication of SARS-Cov-2 in Huh7 cells at an EC50 of 1.40 μM (Rothan et al., 2020), and nelfinavir exhibits potent anti-COVID-19 activity (IC50 = 0.77 μM in VeroE6 cells) with a low toxicity profile (SI > 83.1) (Ohashi et al., 2020). As a valuable anti-virus source of organosulfur compounds, garlic has fueled significant attention and become a critical strategy in the treatment of SARS-Cov-2 infection.
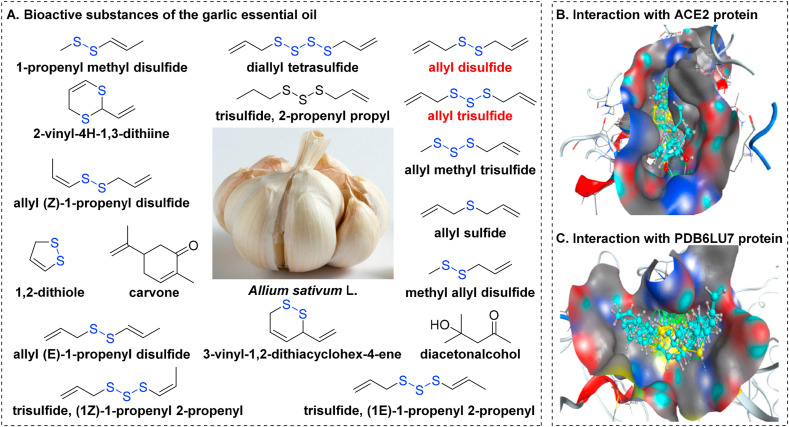
Garlic (Chinese name: Dasuan), the highly enlarged bulbs of Allium sativum L., is a well-known Chinese herbal medicine widely used for centuries in China to treat a range of ailments including arterial stiffness, asthma, common colds, leprosy, and influenza (Fig. 6 A) (Rose et al., 2018). Garlic's major chemical constituents are organosulfur compounds, which contribute to its pharmacological effects such as liver injury (Murugavel et al., 2007), cytoprotective (Pari et al., 2007), anti-oxidant (Suo et al., 2019), and neuroinflammation (Chung et al., 2006).
Fig. 6.
Garlic Essential Oil for the treatment of SARS-CoV-2 infection. (A) Bioactive compounds in essential garlic oil. (B) Interactions of organosulfur compounds with the ACE2 protein of SARS-CoV-2. (C) Interactions of organosulfur compounds with the PDB6LU7 protein of SARS-CoV-2 (image reproduced with permission from Thuy et al., 2020; https://pubs.acs.org/doi/10.1021/acsomega.0c00772#; Copyright © 2021, American Chemical Society).
Following extensive research of organosulfur compounds, multiple lines of evidence have implicated a critical role for garlic in viral infection. Thuy et al. (Thuy et al., 2020) showed that 99.4% (17/18) of the bioactive substances of garlic essential oil are organosulfur compounds (Fig. 6A)), which exhibit strong synergistic interactions with the ACE2 (host receptor for SARS-CoV-2, Fig. 6B) and the PDB6LU7 (main protease of SARS-CoV-2, Fig. 6C). The docking model revealed that allyl disulfide (docking score energy: −12.84 kcal mol−1 with the ACE2 Protein and −15.32 kcal mol−1 with the PDB6LU7 of SARS-CoV-2) and allyl trisulfide (docking score energy: −12.76 kcal mol−1 with the ACE2 protein and −15.02 kcal mol−1 with the PDB6LU7 protein of SARS-CoV-2) present the strongest anti-SARS-CoV-2 effects(Thuy et al., 2020). Numerous evidence has shown the potential usefulness of garlic essential oil as a treatment for viral infections, but further research is needed to explore whether it has anti-SARS-CoV-2 activity in vivo.
5. Promising Chinese herbal medicine formulas for the treatment of SARS-CoV-2 infection in clinical practice
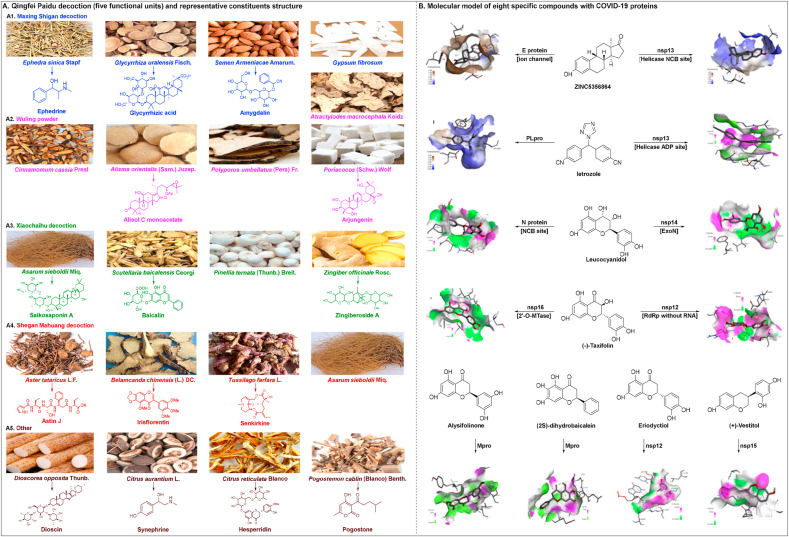
Qingfei Paidu decoction (QFPD) consists of 21 materials derived from four traditional Chinese medicine prescriptions: Maxing Shigan decoction, Wuling powder, Xiaochaihu decoction, and Shegan Mahuang decoction (Fig. 7 A) (Zhong et al., 2020). Maxing Shigan decoction, Wuling powder, and Xiaochaihu decoction were initially described in the Treatise on Febrile Diseases for the treatments of fever, influenza virus infections, asthma, and nephritic syndrome (Hsieh et al., 2012; Cheng et al., 2006; Yang et al., 2015). Shegan Mahuang decoction, derived from the Synopsis of Golden Chamber, was mainly used for the treatment of fever, asthma, inflammation and headache (Lin et al., 2020). QFPD has been widely used in treating SARS since 2002 in China (Chen et al., 2004a). Recently, QFPD has garnered considerable attention due to its ability to treat the mild, common, and severe types of COVID-19 (Wang et al., 2020a; Ren et al., 2020; Zhang et al., 2020h). Qingfeipaidu formula was shown to reach a total effective rate of 97.78% (n = 1202/1261) in COVID-19 patients without transfer from mild to severe cases (Xinhua Net, 2020a). According to the Diagnosis and Treatment Protocol for Coronavirus (2019-nCoV) Pneumonia (Trial Version 7), QFPD is effective (cure rate > 90%) for patients with SARS-CoV-2 infection at all stages (NHC PRC, 2020a). Based on a retrospective multicenter cohort study, Shi et al. revealed that QFPD can yield favorable clinical outcomes, including faster recovery times and a shorter duration of hospital stay (Shi et al., 2020a).
Fig. 7.
Qingfei Paidu decoction for the treatment of SARS-CoV-2 infection. (A) Raw materials of QFPD and its potential active compounds. (B) molecular model of eight specific compounds with COVID-19 proteins (image reproduced with permission from Chen et al., 2020a).
The mechanism of action involved in the inhibition of SARS-CoV-2 infection by QFPD is incredibly challenging given the 21 herbs and hundreds of chemical constituents within this product. Yang et al. (Yang et al., 2020b) used liquid chromatography quadrupole-time of flight mass spectrometry analysis to identify 129 compounds in QFPD (45% flavonoids,15% glycosides, 10% carboxylic acids, and 5% saponins); the representative molecules of QFPD are shown in Fig. 7A. Additionally, Yang et al. (Yang et al., 2020) found that the anti-inflammatory activity of Maxing Shigan decoction played an integral role in the treatment of COVID-19, as determined by a computational molecular networking model and an experimental rat model of pneumonia. Extensive research on the immunological mechanisms involved indicate that QFPD induces a significant elevation of proinflammatory cytokines (IL-1β, IL-18, tumor necrosis factor-α (TNF-α), and IL-8) (Kageyama et al., 2020).
This novel virus can cause multiple extrapulmonary manifestations (Gupta et al., 2020). Chen et al. (Chen et al., 2020a) identified the protective effects of QFPD against COVID-19 injury mainly through antiviral, anti-inflammatory activity and metabolic programming. Following network topology analysis, the absorption, distribution, metabolism, elimination, and toxicity (ADMET) estimations, and drug-likeness test, Chen et al. (Chen et al., 2020a) showed that of the 8 chemical constituents of QFPD with the top molecular docking score, letrozole showed seven interactions with SARS-CoV-2-encoded nonstructural protein (nsp) 13 and five interactions with SARS-CoV-2 papain-like protease (PLpro), leucocyanidol showed eleven interactions with nucleocapsid (N) protein NCB site and five interactions with nsp14. The molecular modelling of the compounds associated with COVID-19 proteins is shown in Fig. 7B. Chen et al. (Chen et al., 2020a) demonstrated that the mechanism for the efficacy of QFPD might be involve the harmonious treatment of SARS-CoV-2 pathways and multiple related targets.
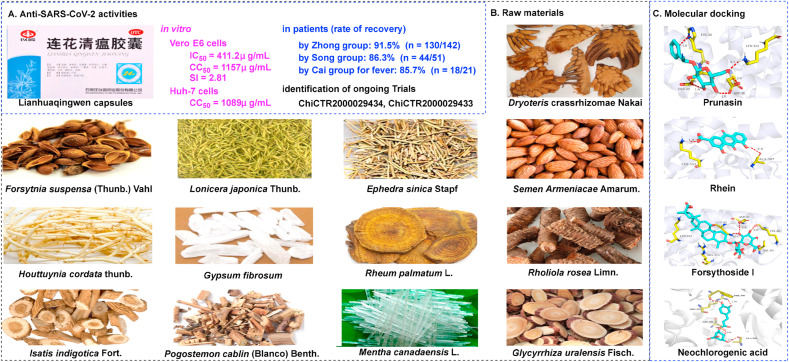
Lianhuaqingwen capsules (LHQW), a formulation developed by Shijiazhuang Yiling Pharmaceutical Co., Ltd, includes 13 types of traditional Chinese medicine that are derived from two classic prescriptions: Maxing Shigan decoction and Yinqiao powder (Fig. 8 B) (Zhang et al., 2020i). As one of the most common prescriptions, LHQW has already been approved as an effective agent to treat influenza and pneumonia in China (Zhuang et al., 2020). As of the 4th December 2020, LHQW has been approved in 16 other countries, including Brazil, Indonesia, Romania, Singapore, Russia, Philippines, and Ukraine, for diseases related to influenza and pneumonia. In addition, LHQW has been launched for registration in more than 30 other countries in the Middle East, Africa, and Latin America, due to its efficacy against COVID-19. Furthermore, in September 2020, Kuwait approved LHQW to boost the survival of patients with COVID-19 (Forbes China, 2020).
Fig. 8.
Lianhuaqingwen capsules for the treatment of SARS-CoV-2 infection. (A) Lianhuaqingwen capsules. (B) Raw materials of Lianhuaqingwen capsules. (C) Molecular docking of representative components (prunasin, rhein, forsythoside I, and neochlorogenic acid) with ACE2 (image reproduced with permission from Chen et al., 2020c).
Researchers have determined that LHQW demonstrates broad-spectrum activities against multiple viruses, including H1N1 (Gao et al., 2020a), H7N9 (NHFPC PRC, 2017), Middle East respiratory syndrome (MERS) coronavirus (NHFPC PRC, 2015), and SARS-CoV (CMACACM, 2004). Furthermore, LHQW has been shown to protect against acute lung injury in a rat model by downregulating the nuclear factor-kappa B (NF-κB) signaling pathway (Cui et al., 2016), thus reducing the infiltration of mononuclear macrophages (Li et al., 2020b). As a patented Chinese medicine, the potential of LHQW to treat SARS-CoV-2 has been documented in vitro and in a mouse model. Li et al. recently reported that LHQW could effectively inhibit the replication of SARS-CoV-2 in Vero E6 cells at an IC50 of 411.2 μg/mL and markedly reduce the mRNA expression of several proinflammatory cytokines (IL-6, TNF-α, and CCL-2/MCP-1) (Li et al., 2020c). Furthermore, Li et al. indicated that LHQW could alleviate lipopolysaccharide-induced endoplasmic reticulum stress and tumor necrosis factor-related apoptosis, thus inducing ligand expression in a co-culture model of inflammatory macrophages and alveolar epithelial cells and in a mouse model of acute lung injury (Li et al., 2020c). These data provided preliminary research evidence relating to the ability of LHQW to protect the lungs, implying its promising potential as a therapeutic for lung injury in patients with COVID-19.
Several clinical trials have attempted to evaluate the effects of LHQW on COVID-19. For example, Hu et al. conducted a clinical study (www.chictr.org/cn/, China Clinical Trial Registry number: ChiCTR2000029434) on 142 patients from 23 hospitals receiving LHQW therapy and reported that LHQW significantly improved the recovery rate (91.5%, n = 130/142), markedly shortened the recovery time (7 days), and improved the recovery of chest radiological abnormalities (83.8%), with no serious safety issues (Hu et al., 2020). Cheng et al. further showed that LHQW treatment led to a significant improvement in cardinal symptoms (86.3%, n = 44/51) (Cheng et al., 2020). Yao et al. also revealed that LHQW could significantly relieve cardinal symptoms, such as fever (85.7%, n = 18/21), cough (46.7%, n = 7/15), shortness of breath (77.8%, n = 7/9), and expectoration (64.3%, n = 9/14) (Yao et al., 2020). To explore appropriate treatments for patients with severe COVID-19, Li et al. conducted a clinical study (www.chictr.org/cn/, China Clinical Trial Registry number: ChiCTR2000030803) on the therapeutic effects of LHQW combined with other agents and determined that the combination of ribavirin, lopinavir/ritonavir, umifenovir, and LHQW, could exhibit a synergistic inhibitory effect in severe COVID-19 patients (improvement rate 84.9%, n = 28/33) (Li et al., 2020e). Other related clinical studies have also reported that LHQW can relieve the major symptoms of patients with COVID-19 (and suspected cases) with good levels of safety (Lv et al., 2020; Wang et al., 2020d; Cheng and Li, 2020).
From a mechanistic point-of-view, the combination of network pharmacology and molecular docking showed that the therapeutic effects of LHQW were closely associated with immune response, cell apoptosis, and virus infection (Xia et al., 2020b). In another study, Zhao et al. highlighted that Akt1 (a serine/threonine protein kinase) is a promising target for COVID-19 patients and could help to reduce lung injury and help eliminate viral infection (Zhao et al., 2020). Chen et al. further used a human exposure-based approach to identify the molecular mechanisms underlying the active components of LHQW. Results demonstrated that certain representative components exhibited high inhibitory effects on ACE2, including prunasin (affects the binding interface of ACE2 and S protein), rhein (the best binding interface of ACE2), forsythoside I (the binding interface of ACE2), and neochlorogenic acid (the binding interface of peptidase and S protein) (Fig. 8C) (Chen et al., 2020c). As a large formulation, LHQW may possess complex mechanisms of action; however, these are currently undefined and require additional research. Multiple lines of evidence have shown the value of LHQW as a treatment for SARS-CoV-2 infection, thus allowing this agent to be included in the Diagnosis and Treatment Protocol for Coronavirus (2019-nCoV) Pneumonia (Trial Version 8) (NHC PRC, 2020b).
Numerous traditional Chinese medicines have shown promising results and garnered considerable attention due to their ability to inhibit SARS-CoV-2 effectively. Pudilan Xiaoyan oral liquid (PDL), composed of four types of traditional Chinese medicine, has recently been recognized as a promising formulation with potent antiviral and antibacterial effects (Feng et al., 2018). Deng et al. (Deng et al., 2020) recently determined that PDL could effectively inhibit SARS-CoV-2 replication in Vero E6 cells in vitro at an EC50 of 1.078 mg/mL and with an excellent SI of 8.27. Furthermore, the effects of PDL were evaluated in SARS-CoV-2-infected hACE2 mice; results showed that PDL could relieve the symptoms of pneumonia, including asthma and chronic obstructive pulmonary disease (Deng et al., 2020). Liu et al. (Liu et al., 2020c) further showed that Jinhua Qinggan granules (JHQG) significantly reduced the recovery time by two days compared with a control group and was not associated with any adverse reactions. Kageyama et al. (Kageyama et al., 2020) showed that JHQG could down- and up-regulate the plasma levels of IL-6 and interferon-gamma (IFN-γ); these factors have been correlated with mortality in patients with SARS-CoV-2 infection (Blanco-Melo et al., 2020; Zhang et al., 2020g). Shuanghuanglian preparations, both oral liquid and injection, could effectively block SARS-CoV-2 replication in Vero E6 cells (Su et al., 2020). Chen et al. (Chen et al., 2020b) showed that 131 cases of COVID-19 patients were cured and discharged after receiving treatment with Ganlu Xiaodu decoction. Furthermore, Xuanfei Baidu decoction also improved the patient's clinical symptoms via its anti-inflammatory effect (Xiong et al., 2020c).
The safety profiles of traditional Chinese medicines have been evaluated in multiple clinical trials. Besides the above-mentioned prescriptions, several other clinical trial protocols have been registered to investigate the treatment outcomes of COVID-19 using traditional Chinese medicines (Table 2 ). It should be stressed that the small sample size of the control groups in these trials (often below 200 participants) is not large enough to detect statistically significant differences and support sufficiently scientific foundations for their clinical use in COVID-19 patients. Moreover, the majority of the registered Chinese herbal medicine formulas could not be completed due to the fact that the COVID-19 epidemic has been well controlled in China; this means that there were only small numbers of patients available for trials. However, as potential anti-SARS-CoV-2 agents, there is no doubt that Chinese herbal medicine formulas, including those have not yet completed clinical trials, could represent potential therapeutic options for the treatment of COVID-19.
Table 2.
Registered clinical trials relating to traditional Chinese medicine prescriptions for the treatment of patients with COVID-19 (Chinese Clinical Trial Registry, www.chictr.org/cn/, 2020/01/30–2020/09/08).
| Dosage form | Herbal formula | Ingredients (Latin name) | Registration number | Sample size of the control group |
|---|---|---|---|---|
| Pills | Liushen | Borneolum Syntheticum, Cinnabaris, Menthae Haplocalycis Herba, Bos taurus domesticus Gmelin, Moschus, Fel Ursi, Isatidis Radix, Realgar, Glycyrrhizae Radix et Rhizoma, Lonicerae Japonicae, Bufonis Venenum | ChiCTR2000030469 | 48 |
| Xiangsha Liujun pills | Aucklandiae Radix, Amomi Fructus, Codonopsis Radix, Atractylodes Macrocephala, Poria, Glycyrrhizae Radix et Rhizoma, Citri Reticulatae Pericarpium, Pinelliae Rhizoma, Zingiberis Rhizoma Recens, Jujubae Fructus | ChiCTR2000032237 | 100 | |
| Gushen Dingchuan pills | Radix Rehmanniae praepar ata, Radix Aconiti lateralis Preparata, Moutan Cortex, Radix Achyranthis Bidentatae, Psoraleae Fructus, Amomi Fructus, Plantaginis Semen, Poria, Fructus Alpinae Oxyphyllae, Cinnamomi Cortex, Dioscoreae Rhizoma, Alismatis Rhizoma, Rosae Laevigatae Frucyus | ChiCTR2000030937 | 72 | |
| Powder | Danggui Shaoyao powder | Paeoniae Radix Alba, Angelicae Sinensis Radix, Chuanxiong Rhizoma, Atractylodes Macrocephala, Poria, Alismatis Rhizoma | ChiCTR2000032098 | 300 |
| Shengjiang powder | Bombyx Batryticatus, CicadaePeriostracum, Curcumae Longae Rhizoma, Rhei Radix et Rhizoma | ChiCTR2000030314 | 40 | |
| Decoction | Qingfei Paidu decoction | Ephedrae Herba, Glycyrrhizae Radix et Rhizoma, Semen Armeniacae Amarum, Gypsum Fibrosum, Ramulus Cinnamomi, Alismatis Rhizoma, Polyporus, Atractylodes Macrocephala, Poria, Bupleuri Radix, Scutellariae Radix, Pinelliae Rhizoma, Zingiberis Rhizoma Recens, Radix Asteris, Flos Farfarae, Belamcandae Rhizoma, Herba Asari, Dioscoreae Rhizoma, Fructus Aurantii Immaturus, Citri Reticulatae Pericarpium, Pogostemonis Herba | ChiCTR2000029433 | 120 |
| ChiCTR2000030883 | 100 | |||
| ChiCTR2000032767 | 782 | |||
| Maxing Shigan decoction | Ephedrae Herba, Semen Armeniacae Amarum, Gypsum Fibrosum, Glycyrrhizae Radix et Rhizoma | ChiCTR2000030522 | 50 | |
| ChiCTR2000030314 | 40 | |||
| Yiqi Huashi Jiedu decoction | Radix pseudostellariae, Astragali Radix, Atractylodes Macrocephala, Alismatis Rhizoma, Polyporus, Herba Ephedrae, Herba Artemisiae scoparia, Rosae Laevigatae Frucyus, Semen Euryales, Herba Leonuri, Pheretima, Cinnamomi Cortex, Glycyrrhizae Radix et Rhizoma | ChiCTR2000030479 | 50 | |
| Xinguan I decoction | Scutellariae Radix, Saposhnikoviae Radix, Forsythiae Fructus, Lonicerae Japonicae, Radix Peucedani | ChiCTR2000029637 | 50 | |
| Xinguan II decoction | Ephedrae Herba, Semen Armeniacae Amarum, Gypsum Fibrosum, Semen Coicis, Atractylodis Rhizoma, Rhizoma Polygoni Cuspidati, Verbenae Herba, Pogostemonis Herba, Descurainiae Semen, Phragmitis Rhizoma, Exocarpium Citri Rubrum, Glycyrrhizae Radix et Rhizoma | ChiCTR2000029628 | 50 | |
| Xinguan III decoction | Astragali Radix, Radix Pseudostellariae, Radix Adenophorae, Poria, Atractylodes Macrocephala, Radix Ophiopogonis | ChiCTR2000030936 | 2130 | |
| Xuanfei Baidu decoction | Herba Ephedrae, Semen Armeniacae Amarum, Gypsum Fibrosum, Semen Coicis, Rhizoma Atractylodis, Herba Pogostemonis, Herba Artemisiae Annuae, Herba Artemisiae Annuae, Verbenaceae, Rhizoma Phragmitis, Semen Lepidii, Exocarpium Citri Grandis, Radix Glycyrrhizae | ChiCTR2000034795 | 22 | |
| Syrup | Kesuting syrup | Eriobotryae Folium, Ephedrae Herba, Papaveris Pericarpium, Platycodonis Radix, Mori Cortex, Reineckia carnea (Andr.) Kunth, Aletris pauciflora var. khasiana (Hook. f.) Wang et Tang, Rhizoma Polygoni Cuspidati, Polygonati Rhizoma | ChiCTR2000029991 | 24 |
| Capsules | Keqing capsules | Reineckia carnea (Andr.) Kunth, Papaveris Pericarpium, Physalis Calyx Seu Fructus, Rhizoma Polygoni Cuspidati, Eriobotryae Folium, Mori Cortex | ChiCTR2000029991 | 24 |
| Lianhuaqingwen capsules | Forsythiae Fructus, Lonicerae Japonicae, Ephedrae Herba, Semen Armeniacae Amarum, Gypsum Fibrosum, Isatidis Radix、Dryopteridis Crassirhizomatis Rhizoma, Herba Ephedrae, Pogostemonis Herba, Rhei Radix et Rhizoma, Rhodiola Rosae, menthol, Glycyrrhizae Radix et Rhizoma | ChiCTR2000029434 | 120 | |
| Tanreqing capsules | Scutellariae Radix, Fel Ursi, Corne Caprae Hirci, Lonicerae Japonicae, Forsythiae Fructus | ChiCTR2000029813 | 36 | |
| Bufei Huoxue capsules | Astragali Radix, Radix Padoniae Rubra, Psoraleae Fructus | ChiCTR2000032573 | 60 | |
| Xiaoyao capsules | Bupleuri Radix, Angelicae Sinensis Radix, Paeoniae Radix Alba, Atractylodes Macrocephala, Poria, Glycyrrhizae Radix et Rhizoma, Menthae Haplocalycis Herba | ChiCTR2000032399 | 100 | |
| Babaodan capsules | Bos taurus domesticus Gmelin, Agkistrodon Haly, Saiga tatarica Linnaeus, Margarita, Notoginseng Radix et Rhizoma, Moschus | ChiCTR2000029769 | 20 | |
| Injection | Xuebijing injection | Carthami Flos, Radix Padoniae Rubra, Chuanxiong Rhizoma, Salviae Miltiorrhizae Radix et Rhizoma, Angelicae Sinensis Radix | ChiCTR2000030388 | 30 |
| Tanreqing injection | Scutellariae Radix, Fel Ursi, Corne Caprae Hirci, Lonicerae Japonicae, Forsythiae Fructus | ChiCTR2000029432 | 72 | |
| Shenfu injection | Ginseng Radix Rubra, Aconiti Lateralis Radix Praeparata | ChiCTR2000030043 | 150 | |
| Shenqifuzheng injection | Codonopsis Radix, Astragali Radix | ChiCTR2000029780 | 80 | |
| Oral liquid | Kegan Liyan oral liquid | Lonicerae Japonicae, Scutellariae Radix, Schizonepetae Herba, Gardeniae Fructus, Forsythiae Fructus, Scrophulariae Radix, Bombyx Batryticatus, Rehmannia glutinosa, Belamcandae Rhizoma, Platycodonis Radix, Menthae Haplocalycis Herba, Cicadae Periostracum, Saposhnikoviae Radix, Glycyrrhizae Radix et Rhizoma | ChiCTR2000033720 | 240 |
| ChiCTR2000033745 | 240 | |||
| ChiCTR2000031982 | 240 | |||
| Shuanghuanglian oral liquid | Lonicerae Japonicae, Scutellariae Radix, Forsythiae Fructus | ChiCTR2000033133 | 30 | |
| ChiCTR2000029605 | 100 | |||
| Xiangxue antiviral oral liquid | Isatidis Radix, Gypsum Fibrosum, Phragmitis Rhizoma, Rehmannia glutinosa Libos, Curcumae Radix, Anemarrhenae Rhizoma, Acori Tatarinowii Rhizoma, Pogostemonis Herba, Forsythiae Fructus | ChiCTR2000030033 | 276 | |
| Granule | Sancai granule | Chebulae Fructus, Toosendan Fructus, Gardenia jasminoides Ellis | ChiCTR2000034794 | 30 |
| Huashi Baidu granule | Magnoliae Officinalis Cortex, Astragali Radix, Atractylodis Rhizoma, Pogostemonis Herba, Tsaoko Fructus, Glycyrrhizae Radix et Rhizoma, Pinelliae Rhizoma, Descurainiae Semen, Ephedrae Herba, Radix Padoniae Rubra, Semen Armeniacae Amarum, Rhei Radix et Rhizoma, Poria, Gypsum Fibrosum | ChiCTR2000030989 | 38 | |
| Xiaoer Huatan Zhike granule | Jiegeng Liujingao, Mori Cortex Liujingao, Emetine, Ephedrine Hydrochloride | ChiCTR2000030022 | 50 | |
| Toujie Quwen granule | Forsythiae Fructus, Pseudobulbus Cremastrae seu Pleiones, Lonicerae Japonicae, Scutellariae Radix, Isatidis Folium, Bupleuri Radix, Herba Artemisiae Annuae, Cicadae Periostracum, Radix Peucedani, Bulbus Fritillariae Cirrhosae, Bulbus Fritillariae Thunbergii, Fructus Mume, Scrophulariae Radix, Astragali Radix, Poria, Radix Pseudostellariae | ChiCTR2000031888 | 150 | |
| Jingyin granule | Schizonepetae Herba, Lonicerae Japonicae, Arctii Fructus, Isatidis Folium, Ilicis Chinensis Folium. | ChiCTR2000030255 | 200 |
6. Conclusion and future perspectives
At the time of writing, the COVID-19 epidemic has caused 1,853,525 deaths worldwide but continues to cause effect (WHO, 2020). Furthermore, mutation of the SARS-CoV-2 virus has strengthened increased the ability of this virus to infect and spread. In this scenario, safe and broad-spectrum anti-SARS-Cov-2 drugs are urgently needed. In mainland China, Chinese herbal medicines (active ingredients, monomer preparations, crude extracts, and formulas) have been recognized as very promising anti-SARS-CoV-2 agents due to their broad-spectrum antiviral activities against SARS-CoV (Chen et al., 2004b) and MERS-CoV (Antonelli et al., 2020) and their unique role in organ protection as antiviral, anti-inflammatory, and anti-fibrotic agents both in vitro and in clinical practice. As a key component of the COVID-19 treatment regimen, Chinese herbal medicines have played an irreplaceable role in the treatment of SARS-CoV-2 infection since January 2020. Due to this effective treatment regimen, as of March 2020, the COVID-19 epidemic has been well controlled and has reached a plateau in China.
Nevertheless, cases of active SARS-CoV-2 infection have continuously advanced in other countries. It is clear that the “Chinese protocol” has shown important clinical value. Chinese herbal medicines that are capable of inhibiting SARS-Cov-2 infection may help to address this immediate unmet clinical need and may be attractive to other countries focusing on more effective COVID-19 treatment. Thus, countries outside China, should also pursue the use of Chinese herbal medicine protocols to combat this fast-spreading viral infection.
The safety and efficacy of traditional Chinese medicines, such as Lianhuaqingwen capsules, Xuanfei Baidu decoction, and Ganlu Xiaodu decoction, have been evaluated for patients with SARS-CoV-2 in several clinical trials. However, to respond effectively to concerns arising from the western medical community, it is crucial to investigate how Chinese herbal medicine exerts effect on SARS-Cov-2 infection (Cyranoski, 2020). The underlying molecular mechanisms involved remain elusive. To better understand the activity of Chinese herbal medicine, more validation studies, with high-quality evidence (both in vitro and in vivo), are now needed to systematically explore the underlying mechanisms. Furthermore, there is currently very little direct data associated with the protective effect of Chinese herbal medicines against extrapulmonary organ injuries during SARS-CoV-2 infection (Zhang et al., 2020d; He et al., 2020; Zhao et al., 2020). Nonetheless, Chinese herbal medicine has its own advantages. We sincerely hope that Chinese herbal medicine will prove to be a safe and effective therapy against SARS-Cov-2 worldwide.
Author contributions
Wang Zhong-lei and Yang Li-yan conceived the review. Wang Zhong-lei collected the literatures. Wang Zhong-lei and Yang Li-yan wrote and edited the manuscript.
Declarations of competing interest
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by the PhD research start-up funds of Qufu Normal University, China (Grant No. 614901, and 615201) and the project of introduction and cultivation for young innovation talents in the colleges and universities of Shandong Province (Grant No. 614202).
References
- Acharya D., Liu G.Q., Gack M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020;20:397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed-Belkacem A., Guichou J.F., Brillet R., Ahnou N., Hernandez E., Pallier C., Pawlotsky J.M. Inhibition of RNA binding to hepatitis C virus RNA-dependent RNA polymerase: a new mechanism for antiviral intervention. Nucleic Acids Res. 2014;42:9399–9409. doi: 10.1093/nar/gku632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aishwarya S., Gunasekaran K., Margret A.A. Computational gene expression profiling in the exploration of biomarkers, non-coding functional RNAs and drug perturbagens for COVID-19. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1850360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli M., Donelli D., Maggini V., Firenzuoli F. Phytotherapic compounds against coronaviruses: possible streams for future research. Phytother Res. 2020;34:1469–1470. doi: 10.1002/ptr.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M., Shigeta S. Antiviral activity of glycyrrhizin against varicella-zoster virus in vitro. Antivir. Res. 1987;7:99–107. doi: 10.1016/0166-3542(87)90025-8. [DOI] [PubMed] [Google Scholar]
- Bailly C., Vergoten G. Glycyrrhizin: an alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol. Therapeut. 2020;2020:107618. doi: 10.1016/j.pharmthera.2020.107618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmeh N., Mahmoudi S., Mohammadi N., Karabedianhajiabadi A. Predicted therapeutic targets for COVID-19 disease by inhibiting SARS-CoV-2 and its related receptors. Inform. Med. Unlocked. 2020;20:100407. doi: 10.1016/j.imu.2020.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R., Hu H., Li Y., Wang X., Xu M., Liu J., Zhang H., Yan Y., Zhao L., Li W., Zhang T., Xiao D., Guo X., Li Y., Yang J., Hu Z., Wang M., Zhong W. Anti-SARS-CoV-2 potential of artemisinins in vitro. ACS Infect. Dis. 2020;6:2524–2531. doi: 10.1021/acsinfecdis.0c00522. [DOI] [PubMed] [Google Scholar]
- Cao Y., Shi H., Sun Z., Wu J., Xia Y., Wang Y., Wu Y., Li X., Chen W., Wang A., Lu Y. Protective effects of magnesium glycyrrhizinate on methotrexate-induced hepatotoxicity and intestinal toxicity may be by reducing COX-2. Front. Pharmacol. 2019;10:119. doi: 10.3389/fphar.2019.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas C.G., Català A., Hernández G.C., Rodríguez-Jiménez P., Fernández-Nieto D., Lario A.R.V., Fernández I.N., Ruiz-Villaverde R., Falkenhain-López D., Velasco M.L., García-Gavín J., Baniandrés O., González-Cruz C., Morillas-Lahuerta V., Cubiró X., Nart I.F., Selda-Enriquez G., Romaní J., Fustà-Novell X., Melian-Olivera A., Roncero Riesco M., Burgos-Blasco P., Ortigosa J.S., Rodriguez M.F., García-Doval I. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020;183:71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Chan K.H., Jiang Y., Kao R.Y., Lu H.T., Fan K.W., Cheng V.C.C., Tsui W.H.W., Hung I.F.N., Lee T.S.W., Guan Y., Peiris J.S.M., Yuen K.Y. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Ma Y.B., Huang X.Y., Geng C.A., Zhao Y., Wang L.J., Guo R.H., Liang W.J., Zhang X.M., Chen J.J. Synthesis, structure–activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents. Bioorg. Med. Chem. Lett. 2014;24:2353–2359. doi: 10.1016/j.bmcl.2014.03.060. [DOI] [PubMed] [Google Scholar]
- Chen J., Wang Y.K., Gao Y., Hu L.S., Yang J.W., Wang J.R., Sun W.J., Liang Z.Q., Cao Y.M., Cao Y.B. Protection against COVID-19 injury by Qingfei Paidu decoction via anti-viral, anti-inflammatory activity and metabolic programming. Biomed. Pharmacother. 2020;129:110281. doi: 10.1016/j.biopha.2020.110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Zhang W., Zhang L., Zhang J., Chen X., Yang M., Chen T., Hong J. Glycyrrhetinic acid alleviates radiation-induced lung injury in mice. J. Radiat. Res. 2016;58:41–47. doi: 10.1093/jrr/rrw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Cheng Z.Q., Liu F., Xia Y., Chen Y.G. Analysis of 131 cases of COVID-19 treated with Ganlu Xiaodu decoction. China J. Chin. Mater. Med. 2020;45:2232–2238. doi: 10.19540/j.cnki.cjcmm.20200322.505. [DOI] [PubMed] [Google Scholar]
- Chen Q., Chen P.P., Xin Y.J., Tang K., Zhang X.Y., Guo Y. Establishment of a new genus of filovirus (Mengla virus) entry evaluating system and entry inhibitors discovery. Acta Pharm. Sin. 2019;54:1612–1619. [Google Scholar]
- Chen Q., Liu J., Wang W., Liu S., Yang X., Chen M., Cheng L., Lu J., Guo T., Huang F. Sini decoction ameliorates sepsis-induced acute lung injury via regulating ACE2-Ang (1-7)-Mas axis and inhibiting the MAPK signaling pathway. Biomed. Pharmacother. 2019;115:108971. doi: 10.1016/j.biopha.2019.108971. [DOI] [PubMed] [Google Scholar]
- Chen X., Wu Y., Chen C., Gu Y., Zhu C., Wang S., Chen J., Zhang L., Lv L., Zhang G., Yuan Y., Chai Y., Zhu M., Wu C. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Nakamura T. Statistical evidence for the usefulness of Chinese medicine in the treatment of SARS. Phytother Res. 2004;18:592–594. doi: 10.1002/ptr.1485. [DOI] [PubMed] [Google Scholar]
- Cheng D.Z., Li Y. Clinical effectiveness and case analysis in 54 NCP patients treated with Lanhuaqingwen granules. World Chin. Med. 2020;15:150–154. [Google Scholar]
- Cheng D.Z., Wang W.J., Li Y., Wu X.D., Zhou B., Song Q.Y. Analysis of 51 cases of novel coronavirus pneumonia treated with traditional Chinese medicine Lianhua Qingwen: a multicenter retrospective study. Tianjin J. Tradit. Chin. Med. 2020;37:509–516. [Google Scholar]
- Cheng P.W., Ng L.T., Lin C.C. Xiao chai hu tang inhibits CVB1 virus infection of CCFS-1 cells through the induction of Type I interferon expression. Int. Immunopharm. 2006;6:1003–1012. doi: 10.1016/j.intimp.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Cheong D.H.J., Tan D.W.S., Wong F.W.S., Tran T. Anti-malarial drug, artemisinin and its derivatives for the treatment of respiratory diseases. Pharmacol. Res. 2020;158:104901. doi: 10.1016/j.phrs.2020.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Medical Association and China Association of Chinese Medicine Guideline on diagnosis and treatment of SARS (2004 edition) Mod. Pract. Med. 2004;16:119–126. [Google Scholar]
- Cho J., Lee Y.J., Kim J.H., Kim S.I., Kim S.S., Choi B.S., Choi J.H. Antiviral activity of digoxin and ouabain against SARS-CoV-2 infection and its implication for COVID-19. Sci. Rep. 2020;10:16200. doi: 10.1038/s41598-020-72879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy K.T., Wong A.Y.L., Kaewpreedee P., Sia S.F., Chen D.D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.H., Huang X., Peiris M., Yen H.L. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung L.Y. The antioxidant properties of garlic compounds: allyl cysteine, alliin, allicin, and allyl disulfide. J. Med. Food. 2006;9:205. doi: 10.1089/jmf.2006.9.205. [DOI] [PubMed] [Google Scholar]
- Cipollaro L., Giordano L., Padulo J., Oliva F., Maffulli N. Musculoskeletal symptoms in SARS-CoV-2 (COVID-19) patients. J. Orthop. Surg. Res. 2020;15:178. doi: 10.1186/s13018-020-01702-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., Narayanan A., Majowicz S.A., Kwong E.M., McVicar R.N., Thacker B.E., Glass C.A., Yang Z., Torres J.L., Golden G.J., Bartels P.L., Porell R.N., Garretson A.F., Esko J.D. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183:1043–1057. doi: 10.1016/j.cell.2020.09.033. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E.M.D., Armaos G., McInnes G., Beaudry A., Moquin-Beaudry G., Bertrand-Lehouillier V., Caron M., Richer C., St-Onge P., Johnson J.R., Krogan N., Sai Y., Downey M., Rafei M., Boileau M., Eppert K., Flores-Díaz E., Haman A., Hoang T., Sinnett D., Beauséjour C., McGraw S., Raynal N.J.M. Heart failure drug proscillaridin A targets MYC overexpressing leukemia through global loss of lysine acetylation. J. Exp. Clin. Canc. Res. 2019;38:251. doi: 10.1186/s13046-019-1242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W.W., Jin X., Zhang Y.F., Chang L.P., Wang H.T. Effects of Lianhua Qingwen Capsules on IKK/IkB/NF-kB signal pathway in the mousewith LPS-induced acute lung injury. Chin. Tradit. Pat. Med. 2016;37:954. [Google Scholar]
- Cyranoski D. China is promoting coronavirus treatments based on unproven traditional medicines. Nature. 2020 doi: 10.1038/d41586-020-01284-x. [DOI] [PubMed] [Google Scholar]
- Dai W., Zhang B., Jiang X.M., Su H., Li J., Zhao Y., Xie X., Jin Z., Peng J., Liu F., Li C., Li Y., Bai F., Wang H., Cheng X., Cen X., Hu S., Yang X., Wang J., Liu X., Xiao G., Jiang H., Rao Z., Zhang L.K., Xu Y., Yang H., Liu H. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Xu Y., Kong Q., Xue J., Yu P., Liu J., Lv Q., Li F., Wei Q., Bao L. Therapeutic efficacy of Pudilan Xiaoyan oral liquid (PDL) for COVID-19 in vitro and in vivo. Signal Transduct. Tar. 2020;5:66. doi: 10.1038/s41392-020-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilek E., Erol H.S., Cakir A., Koc M., Halici M.B. Natural product inhibitors of carbonic anhydrase I and II isoenzymes: osajin and pomiferin. Arch. Physiol. Biochem. 2017;123:219–224. doi: 10.1080/13813455.2017.1303742. [DOI] [PubMed] [Google Scholar]
- Ding Y., Chen L., Wu W., Yang J., Yang Z., Liu S. Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-κB and JAKSTAT signaling pathway. Microb. Infect. 2017;19:605–615. doi: 10.1016/j.micinf.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Du Y., Wang T., Jiang N., Ren R.T., Li C., Li C.K., Fu F.H. Sodium aescinate ameliorates liver injury induced by methyl parathion in rats. Exp. Ther. Med. 2012;3:818–822. doi: 10.3892/etm.2012.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Wang T., Jiang N., Ren R.T., Zhao D.L., Li C., Fu F.H. Protective effect of sodium aescinate on lung injury induced by methyl parathion. Hum. Exp. Toxicol. 2011;30:1584–1591. doi: 10.1177/0960327110393764. [DOI] [PubMed] [Google Scholar]
- Elfiky A.A. SARS-CoV-2 spike-heat shock protein A5 (GRP78) recognition may be related to the immersed human coronaviruses. Front. Pharmacol. 2020;11:577467. doi: 10.3389/fphar.2020.577467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen ter B.M., Dinesh Kumar N., Bouma E.M., Troost B., Pol van de D.P.I., Ende van derMetselaar H.H., Apperloo L., Gosliga van D., Berge van den M., Nawijn M.C., Voort van der P.H.J., Moser J., Rodenhuis-Zybert I.A., Smit J.M. Resveratrol and pterostilbene potently inhibit SARS-COV-2 infection in vitro. bioRxiv. 2020 doi: 10.1101/2020.09.24.285940. [DOI] [Google Scholar]
- Enmozhi S.K., Raja K., Sebastine I., Joseph J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1760136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan A.Y., Gu S., Alemi S.F. Chinese herbal medicine for COVID-19: current evidence with systematic review and meta-analysis. J. Integr. Med. 2020;18:385–394. doi: 10.1016/j.joim.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanunza E., Iampietro M., Distinto S., Corona A., Quartu M., Maccioni E., Horvat B., Tramontano E. Quercetin blocks Ebola virus infection by counteracting the VP24 interferon-inhibitory function. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.00530-20. e00530-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Wang L., Ma Y.Y., Li M., Zhao G.Q. A potential in vitro and in vivo anti-HIV drug screening system for Chinese herbal medicines. Phytother Res. 2012;26:899–907. doi: 10.1002/ptr.3658. [DOI] [PubMed] [Google Scholar]
- Feng L., Yang N., Li C., Tian G., Wang J., Dong Z.B., Jia X.B., Di L.Q. Pudilan xiaoyan oral liquid alleviates LPS-induced respiratory injury through decreasing nitroxidative stress and blocking TLR4 activation along with NF-ΚB phosphorylation in mice. J. Ethnopharmacol. 2018;214:292–300. doi: 10.1016/j.jep.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Forbes China Lianhua Qingwen capsule was approved to land in 16 countries after the epidemic, and its revenue exceeded 2.8 billion yuan in the first three quarters. 2020. http://www.forbeschina.com/billionaires/52739
- Fumian T.M., Tuipulotu D.E., Netzler N.E., Lun J.H., Russo A.G., Yan G.J.H., White P.A. Potential therapeutic agents for feline calicivirus infection. Viruses. 2018;10:433. doi: 10.3390/v10080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Niu M., Wei S.Z., Zhang C.E., Zhou Y.F., Yang Z.W., Li L., Wang J.B., Zhang H.Z., Zhang L., Xiao X.H. Identification of a pharmacological biomarker for the bioassay-based quality control of a thirteen-component TCM formula (Lianhua Qingwen) used in treating influenza A virus (H1N1) infection. Front. Pharmacol. 2020;11:746. doi: 10.3389/fphar.2020.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Li X., He L., Yang J., Ye X., Xiao F., Wei H. Diammonium glycyrrhizinate mitigates liver injury via inhibiting proliferation of NKT cells and promoting proliferation of tregs. Drug Des. Dev. Ther. 2019;13:3579–3589. doi: 10.2147/DDDT.S220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L., Ge J., Zheng L., Zhang Y., Wang H., Zhu Y., Zhu C., Hu T., Hua T., Zhang B., Yang X., Li J., Yang H., Liu Z., Xu W., Guddat L.W., Wang Q., Lou Z., Rao Z. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q., Zhao L., You Q., Yang Y., Gu H., Song G., Lu N., Xin J. Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antivir. Res. 2007;74:16–24. doi: 10.1016/j.antiviral.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Guo Y.M., Huang Y.X., Shen H.H., Sang X.X., Ma X., Zhao Y.L., Xiao X.H. Efficacy of compound kushen injection in relieving cancer-related pain: a systematic review and meta-analysis. Evid. Based. Complement Alternat. Med. 2015;2015:840742. doi: 10.1155/2015/840742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., Wan E.Y., Freedberg D.E., Kirtane A.J., Parikh S.A., Maurer M.S., Nordvig A.S., Accili D., Bathon J.M., Mohan S., Bauer K.A., Leon M.B., Krumholz H.M., Uriel N., Mehra M.R., Elkind M.S.V., Stone G.W., Schwartz A., Ho D.D., Bilezikian J.P., Landr D.W. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Mishra K.P., Ganju L. Broad-spectrum antiviral properties of andrographolide. Arch. Virol. 2017;162:611–623. doi: 10.1007/s00705-016-3166-3. [DOI] [PubMed] [Google Scholar]
- Han K.Y., Lu F.Z., Li J.N. Effects of tetrandrine on pulmonary capillary permeability in rats with acute lung injury induced by lipopolysaccharide. Chin. J. Integr. Tradit. West. Med. 2007;27:1016–1019. [PubMed] [Google Scholar]
- Hancke J.L., Srivastav S., Cáceres D.D., Burgos R.A. A double-blind, randomized, placebo-controlled study to assess the efficacy of Andrographis paniculata standardized extract (ParActin®) on pain reduction in subjects with knee osteoarthritis. Phytother Res. 2019;33:1469–1479. doi: 10.1002/ptr.6339. [DOI] [PubMed] [Google Scholar]
- Hassandarvish P., Rothan H.A., Rezaei S., Yusof R., Abubakar S., Zandi K. In silico study on baicalein and baicalin as inhibitors of dengue virus replication. RSC Adv. 2016;6:31235–31247. [Google Scholar]
- He T., Qu R., Qin C., Wang Z., Zhang Y., Shao X., Lu T. Potential mechanisms of Chinese Herbal Medicine that implicated in the treatment of COVID-19 related renal injury. Saudi Pharmaceut. J. 2020;28:1138–1148. doi: 10.1016/j.jsps.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L., Gat H.W.D., Cinatl J. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J. Med. Chem. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.F., Lo C.W., Liu C.H., Lin S., Yen H.R., Lin T.Y., Horng J.T. Mechanism by which ma-xing-shi-gan-tang inhibits the entry of influenza virus. J. Ethnopharmacol. 2012;143:57–67. doi: 10.1016/j.jep.2012.05.061. [DOI] [PubMed] [Google Scholar]
- Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z.H., Zhong N.S. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.C., Chan C.C., Wu S.J., Chen L.C., Shen J.J., Kuo M.L., Chen M.C., Liou C.J. Matrine attenuates allergic airway inflammation and eosinophil infiltration by suppressing eotaxin and Th2 cytokine production in asthmatic mice. J. Ethnopharmacol. 2014;151:470–477. doi: 10.1016/j.jep.2013.10.065. [DOI] [PubMed] [Google Scholar]
- Huang X., Duan X., Zhu Y., Wang K., Wu J., Tian X. Comparative efficacy of Chinese herbal injections for the treatment of community-acquired pneumonia: a Bayesian network meta-analysis of randomized controlled trials. Phytomedicine. 2019;63:153009. doi: 10.1016/j.phymed.2019.153009. [DOI] [PubMed] [Google Scholar]
- Huang Y.F., Bai C., He F., Xie Y., Zhou H. Review on the potential action mechanisms of Chinese medicines in treating coronavirus disease 2019 (COVID-19) Pharmacol. Res. 2020;158:104939. doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S., Ko M., Lee J., Choi I., Byun S.Y., Park S., Shum D., Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Chemother. 2020;64:e00819–e00820. doi: 10.1128/AAC.00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M., Park Y.I., Cha Y.E., Park R., Namkoong S., Lee J.I., Park J. Tea polyphenols EGCG and theaflavin inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Evid-Based. Compl. Alt. Med. 2020;2020:5630838. doi: 10.1155/2020/5630838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Chen J., Long X., Yao X., Zou X., Yang Y., Huang G., Zhang H. Phillyrin protects mice from traumatic brain injury by inhibiting the inflammation of microglia via PPARγ signaling pathway. Int. Immunopharm. 2020;79:106083. doi: 10.1016/j.intimp.2019.106083. [DOI] [PubMed] [Google Scholar]
- Jie G.W., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Sui W.Z. Influence of berbamine on immune function in mice infected with influenza virus. Acta Pharmacol. Sin. 1986;7:475–479. [PubMed] [Google Scholar]
- Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- Jo S., Kim S., Shin D.H., Kim M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzym. Inhib. Med. Chem. 2019;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John T.J., John K. Renin–angiotensin–aldosterone system dysregulation and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Eur. Heart J. 2020;41:2126–2127. doi: 10.1093/eurheartj/ehaa423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y., Aida K., Kawauchi K., Morimoto M., Ebisui T., Akiyama T., Nakamura T. Jinhua Qinggan granule, a Chinese herbal medicine against COVID-19, induces rapid changes in the plasma levels of IL-6 and IFN-γ. medRxiv. 2020 doi: 10.1101/2020.06.08.20124453. [DOI] [Google Scholar]
- Kang G.J., Han S.C., Ock J.W., Kang H.K., Yoo E.S. Anti-inflammatory effect of quercetagetin, an active component of immature citrus unshiu, in HaCaT human keratinocytes. Biomol. Ther. 2013;21:138–145. doi: 10.4062/biomolther.2013.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.E., Min J.S., Jang M.S., Lee J.Y., Shin Y.S., Song J.H., Kim H.R., Kim S., Jin Y.H., Kwon S. Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules. 2019;9:696. doi: 10.3390/biom9110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.Y., Jeon S., Jang Y., Gotina L., Won J., Ju Y.H., Kim S., Jang M.W., Won W., Park M.G., Pae A.N., Han S., Kim S., Lee C.J. Platycodin D prevents both lysosome- and TMPRSS2-driven SARS-CoV-2 infection in vitro by hindering membrane fusion. bioRxiv. 2020 doi: 10.1101/2020.12.22.423909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousar K., Majeed A., Yasmin F., Hussain W., Rasool N. Phytochemicals from selective plants have promising potential against SARS-CoV-2: investigation and corroboration through molecular docking, MD simulations, and quantum computations. BioMed Res. Int. 2020;2020:6237160. doi: 10.1155/2020/6237160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G., Singh D., Tali J.A., Dheer D., Shankar R. Andrographolide: chemical modification and its effect on biological activities. Bioorg. Chem. 2019;95:103511. doi: 10.1016/j.bioorg.2019.103511. [DOI] [PubMed] [Google Scholar]
- Kuzikov M., Costanzi E., Reinshagen J., Esposito F., Vangeel L., Wolf M., Ellinger B., Claussen C., Geisslinger G., Corona A., Iaconis D., Talarico C., Manelfi C., Cannalire R., Rossetti G., Gossen J., Albani S., Musiani F., Herzog K., Ye Y., Giabbai B., Demitri N., Jochmans D., Jonghe S.D., Rymenants J., Summa V., Tramontano E., Beccari A.R., Leyssen P., Storici P., Neyts J., Gribbon P., Zaliani A. Identification of inhibitors of SARS-CoV-2 3CL-Pro enzymatic activity using a small molecule in-vitro repurposing screen. bioRxiv. 2020 doi: 10.1101/2020.12.16.422677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lani R., Hassandarvish P., Shu M.H., Phoon W.H., Chu J.J.H., Higgs S., Vanlandingham D., Bakar S.A., Zandi K. Antiviral activity of selected flavonoids against Chikungunya virus. Antivir. Res. 2016;133:50–61. doi: 10.1016/j.antiviral.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Lao Y. Clinical study on effect of matrine injection to protect the liver function for patients with primary hepatic carcinoma after trans-artery chemo-embolization (TAE) J. Chin. Med. Mater. 2005;28:637–638. [PubMed] [Google Scholar]
- Lee D.Y.W., Li Q.Y., Liu J., Efferth T. Traditional Chinese herbal medicine at the forefront battle against COVID-19: clinical experience and scientific basis. Phytomedicine. 2020;80:153337. doi: 10.1016/j.phymed.2020.153337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung E.L.H., Pan H.D., Huang Y.F., Fan X.X., Wang W.Y., He F., Cai J., Zhou H., Liu L. The scientific foundation of Chinese herbal medicine against COVID-19. Engineering. 2020;6:1099–1107. doi: 10.1016/j.eng.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhou X., Zhang Y., Zhong F., Lin C., McCormick P.J., Jiang F., Luo J., Zhou H., Wang Q., Fu Y., Duan J., Zhang J. Crystal structure of SARS-CoV-2 main protease in complex with the natural product inhibitor shikonin illuminates a unique binding mode. Sci. Bull. 2020 doi: 10.1016/j.scib.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Li Q.Q., Jia J.N., Sun Q.Y., Zhou H.H., Jin W.L., Mao X.Y. Baicalein exerts neuroprotective effects in FeCl3-induced posttraumatic epileptic seizures via suppressing ferroptosis. Front. Pharmacol. 2019;10:638. doi: 10.3389/fphar.2019.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Ran Q., Sun L., Yin J., Luo T., Liu L., Zhao Z., Yang Q., Li Y., Chen Y., Weng X., Wang Y., Cai W., Zhu X. Lian Hua Qing Wen Capsules, a potent epithelial protector in acute lung injury model, block proapoptotic communication between macrophages, and alveolar epithelial cells. Front. Pharmacol. 2020;11:522729. doi: 10.3389/fphar.2020.522729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Hou Y., Huang J., Pan W., Ma Q., Shi Y., Li C., Zhao J., Jia Z., Jiang H., Zheng K., Huang S., Dai J., Li X., Hou X., Wang L., Zhong N., Yang Z. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Wu K., Li Y., Liang X., Lai K.P., Chen J. Integrative pharmacological mechanism of vitamin C combined with glycyrrhizic acid against COVID-19: findings of bioinformatics analyses. Briefings Bioinf. 2020 doi: 10.1093/bib/bbaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.Z., Fan X.X., Duan F.G., Jiang Z.B., Pan H.D., Luo L.X., Zhou Y.L., Li Y., Yao Y.J., Yao X.J., Leung E.L.H., Liu L. Proscillaridin A induces apoptosis and suppresses non-small-cell lung cancer tumor growth via calcium-induced DR4 upregulation. Cell Death Dis. 2018;9:696. doi: 10.1038/s41419-018-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang X., Guo Y., Deng N., Zheng P., Xu Q., Wu Y., Dai G. Regulation of endothelial nitric oxide synthase and asymmetric dimethylarginine by matrine attenuates isoproterenol-induced acute myocardial injury in rats. J. Pharm. Pharmacol. 2012;64:1107–1108. doi: 10.1111/j.2042-7158.2012.01502.x. [DOI] [PubMed] [Google Scholar]
- Li X., Yang Y., Liu L., Yang X., Zhao X., Li Y., Ge Y., Shi Y., Lv P., Zhang J., Bai T., Zhou H., Luo P., Huang S. Effect of combination antiviral therapy on hematological profiles in 151 adults hospitalized with severe coronavirus disease 2019. Pharmacol. Res. 2020;160:105036. doi: 10.1016/j.phrs.2020.105036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xu H., Lang H., Li J., Bi L., Li Y., Dong L., Zhang L., Liang X., Zhu H. The efficacy and safety of Chinese traditional medicine injections on patients with coronavirus disease 2019: a protocol for systematic review and meta analysis. Medicine. 2020;99:31. doi: 10.1097/MD.0000000000021024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.C., Wang Y.Y., Chen S.M., Liu Y.T., Li J.Q., Li F., Dai J.C., Zhang T., Qiu F., Liu H., Dai Z., Zhang Z.D. Shegan-Mahuang decoction ameliorates asthmatic airway hyperresponsiveness by downregulating Th2/Th17 cells but upregulating CD4+FoxP3+ Tregs. J. Ethnopharmacol. 2020;253:112656. doi: 10.1016/j.jep.2020.112656. [DOI] [PubMed] [Google Scholar]
- Lin H., Zhou J., Lin K., Wang H., Liang Z., Ren X., Huang L., Xia C. Efficacy of Scutellaria baicalensis for the treatment of hand, foot, and mouth disease associated with encephalitis in patients infected with EV71: a multicenter, retrospective analysis. BioMed Res. Int. 2016;2016:5697571. doi: 10.1155/2016/5697571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.C., Cherng J.M., Hung M.S., Baltina L.A., Baltina L., Kondratenko R. Inhibitory effects of some derivatives of glycyrrhizic acid against Epstein-Barr virus infection: structure-activity relationships. Antivir. Res. 2008;79:6–11. doi: 10.1016/j.antiviral.2008.01.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List A., Beran M., DiPersio J., Slack J., Vey N., Rosenfeld C.S., Greenberg P. Opportunities for Trisenox (arsenic trioxide) in the treatment of myelodysplastic syndromes. Leukemia. 2003;17:1499–1507. doi: 10.1038/sj.leu.2403021. [DOI] [PubMed] [Google Scholar]
- Liu H., Ye F., Sun Q., Liang H., Li C., Lu R., Huang B., Tan W., Lai L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. bioRxiv. 2020 doi: 10.1101/2020.04.10.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lu J., Liao Y., Liu S., Chen Y., He R., Men L., Lu C., Chen Z., Li S., Xiong G., Yang S. Dihydroartemisinin attenuates lipopolysaccharide-induced acute kidney injury by inhibiting inflammation and oxidative stress. Biomed. Pharmacother. 2019;117:109070. doi: 10.1016/j.biopha.2019.109070. [DOI] [PubMed] [Google Scholar]
- Liu X., Raghuvanshi R., Ceylan F.D., Bolling B.W. Quercetin and its metabolites inhibit recombinant human angiotensin-converting enzyme 2 (ACE2) activity. J. Agric. Food Chem. 2020. 2020;68:13982–13989. doi: 10.1021/acs.jafc.0c05064. [DOI] [PubMed] [Google Scholar]
- Liu Z., Li X., Gou C., Li L., Luo X., Zhang C., Zhang Yin, Zhang J., Jin A., Li H., Zeng Y., Li T., Wang X. Effect of Jinhua Qinggan granules on novel coronavirus pneumonia in patients. J. Tradit. Chin. Med. 2020;40:467–472. doi: 10.19852/j.cnki.jtcm.2020.03.016. [DOI] [PubMed] [Google Scholar]
- López-Alcalde J., Yan Y., Witt C.M., Barth J. Current state of research about Chinese herbal medicines (CHM) for the treatment of coronavirus disease 2019 (COVID-19): a scoping review. J. Alternative Compl. Med. 2020;26:557–570. doi: 10.1089/acm.2020.0189. [DOI] [PubMed] [Google Scholar]
- Luo X., Ni X., Lin J., Zhang Y., Wu L., Huang D., Liu Y., Guo J., Wen W., Cai Y., Chen Y., Lin L. The add-on effect of Chinese herbal medicine on COVID-19: a systematic review and meta-analysis. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., Kuang X., Zhou Q., Yan C., Li W., Gong H., Kurihara H., Li W., Li Y., He R. Inhibitory effects of baicalein against herpes simplex virus type 1. Acta Pharm. Sin. B. 2020;10:2323–2338. doi: 10.1016/j.apsb.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv R.B., Wang W.J., Li X. Combined with western medicine conventional therapy in the treatment of 63 suspected cases of Coronavirus Disease 2019. J. Tradit. Chin. Med. 2020;61:655–659. [Google Scholar]
- Ma C., Hu Y., Townsend J.A., Lagarias P.I., Marty M.T., Kolocouris A., Wang J. Ebselen, disulfiram, carmofur, PX-12, tideglusib, and shikonin are nonspecific promiscuous SARS-CoV-2 main protease inhibitors. ACS Pharmacol. Transl. Sci. 2020;3:1265. doi: 10.1021/acsptsci.0c00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Sacco M.D., Hurst B., Townsend J.A., Hu Y., Szeto T., Zhang X., Tarbet B., Marty M.T., Chen Y., Wang J. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020;30:678–692. doi: 10.1038/s41422-020-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Zhang Z., Liao F., Jiang T., Tu Y. The birth of artemisinin. Pharmacol. Therapeut. 2020;2020:107658. doi: 10.1016/j.pharmthera.2020.107658. [DOI] [PubMed] [Google Scholar]
- Ma Q., Li R., Pan W., Huang W., Liu B., Xie Y., Wang Z., Li C., Jiang H., Huang J., Shi Y., Dai J., Zheng K., Li X., Hui M., Fu L., Yang Z. Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-κB) signaling pathway. Phytomedicine. 2020;78:153296. doi: 10.1016/j.phymed.2020.153296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Pan W., Li R., Liu B., Li C., Xie Y., Wang Z., Zhao J., Jiang H., Huang J., Shi Y., Dai J., Zheng K., Li X., Yang Z. Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-κB signaling pathway. Pharmacol. Res. 2020;158:104850. doi: 10.1016/j.phrs.2020.104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryam A., Mehmood T., Yan Q., Li Y., Khan M., Ma T. Proscillaridin A promotes oxidative stress and ER stress, inhibits STAT3 activation, and induces apoptosis in A549 lung adenocarcinoma cells. Oxid. Med. Cell Longev. 2018. 2018:3853409. doi: 10.1155/2018/3853409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao K., Pan T., Mou Y., Zhang L., Xiong W., Xu Y., Yu J., Wang Y. Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis. Ther. Adv. Chronic. Dis. 2020 doi: 10.1177/2040622320940185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R.M., Fang Z.H., Yao Y.M. Therapeutic efficacy of tetrandrine tablets combined with matrine injection in treatment of silicosis. Chin. J. Ind. Hyg. Occup. Dis. 2012;30:778–780. [PubMed] [Google Scholar]
- Murugavel P., Pari L. Effects of diallyl tetrasulfide on cadmium-induced oxidative damage in the liver of rats. Hum. Exp. Toxicol. 2007;26:527–534. doi: 10.1177/0960327107073810. [DOI] [PubMed] [Google Scholar]
- National Health and Family Planning Commission of People's Republic of China Guideline on diagnosis and treatment of human infection with avian influenza A (H7N9) virus (2017 version) Chin. J. Viral Dis. 2017;7:1–4. [Google Scholar]
- National Health and Family Planning Commission of People’s Republic of China Guideline on diagnosis and treatment of Middle East respiratory syndrome (2015 version) Chin. J. Viral. Dis. 2015;5:352–354. [Google Scholar]
- Nhc Prc. National Health Commission of the People’s Republic of China Notice on the issunance of guidelines of diagnosis and treatment for 2019-nCoV infected pneumonia (version 7) 2020. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml Available from:
- Nhc Prc. National Health Commission of the People’s Republic of China Notice on the issunance of guidelines of diagnosis and treatment for 2019-nCoV infected pneumonia. 2020. http://www.nhc.gov.cn/yzygj/s7653p/202008/0a7bdf12bd4b46e5bd28ca7f9a7f5e5a.shtml (trial version 8). Available from:
- Nie Y.L., Fan B., Yan H., Tang C.S., Peng J., Guo N., Zhao X.L., Cui H.F., Feng S.Y., Yang X.L., Li Z.Y., Yu Y.H. Effects of cytokine content of Xiyanping injection on acute lung injury induced by LPS in rat bronchoalveolar lavage. Chin. J. Basic Med. Tradit. Chin. Med. 2012;18:976–978. [Google Scholar]
- Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizzoli M.E., Merati G., Tenore A., Picone C., Consensi E., Perotti L., Ferretti V.V., Sambo M., Sabatino A.D., Iotti G.A., Arcaini L., Bruno R., Belliato M. Circulating endothelial cells in COVID-19. Am. J. Hematol. 2020;95:E187–E188. doi: 10.1002/ajh.25881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugraha R.V., Ridwansyah H., Ghozali M., Khairani A.F., Atik N. Traditional herbal medicine candidates as complementary treatments for COVID-19: a review of their mechanisms, pros and cons. Evid. base Compl. Alternative Med. 2020;2020:2560645. doi: 10.1155/2020/2560645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi H., Watashi K., Saso W., Shionoya K., Iwanami S., Hirokawa T., Shirai T., Kanaya S., Ito Y., Kim K.S., Nishioka K., Ando S., Ejima K., Koizumi Y., Tanaka T., Aoki S., Kuramochi K., Suzuki T., Maenaka K., Matano T., Muramatsu M., Saijo M., Aihara K., Iwami S., Takeda M., McKeating J.A., Wakita T. Multidrug treatment with nelfinavir and cepharanthine against COVID-19. bioRxiv. 2020 doi: 10.1101/2020.04.14.039925. [DOI] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pari L., Murugavel P. Diallyl tetrasulfide improves cadmium induced alterations of acetylcholinesterase, ATPases and oxidative stress in brain of rats. Toxicology. 2007;234:44–50. doi: 10.1016/j.tox.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Peng C., Fang K., Gong J. Aescin reduces oxidative stress and provides neuroprotection in experimental traumatic spinal cord injury. Free Radic. Biol. Med. 2016;99:405–417. doi: 10.1016/j.freeradbiomed.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Peng W., Xu Y., Han D., Feng F., Wang Z., Gu C., Zhou X., Wu Q. Potential mechanism underlying the effect of matrine on COVID-19 patients revealed through network pharmacological approaches and molecular docking analysis. Arch. Physiol. Biochem. 2020 doi: 10.1080/13813455.2020.1817944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., Braun F., Lu S., Pfefferle S., Schröder A.S., Edler C., Gross O., Glatzel M., Wichmann D., Wiech T., Kluge S., Pueschel K., Aepfelbacher M., Huber T.B. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi R.H., Fang S.N., Li D.M., Zhang H.C. System evaluation and meta-analysis of Xiyanping injection in the treatment of adult viral pneumonia. Mod. Chin. Clin. Med. 2018;25:29–33. [Google Scholar]
- Raj V., Park J.G., Cho K.H., Choi P., Kim T., Ham J., Lee J. Assessment of antiviral potencies of cannabinoids against SARSCoV-2 using computational and in vitro approaches. Int. J. Biol. Macromol. 2020 doi: 10.1016/j.ijbiomac.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray M., Sarkar S., Rath S.N. Druggability for COVID19-in silico discovery of potential drug compounds against nucleocapsid (N) protein of SARS-CoV-2. chemRxiv. 2020 doi: 10.26434/chemrxiv.12387290.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd W.D., Zhou J.C., Hathorn K.E., McCarty T.R., Bazarbashi A.N., Thompson C.C., Shen L., Chan W.W. Prevalence and characteristics of gastrointestinal symptoms in patients with SARS-CoV-2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020;159:765. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020;155:104743. doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea E.M., Logsdon A.F., Hansen K.M., Williams L., Reed M., Baumann K., Holden S., Raber J., Banks W.A., Erickson M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier: kinetics, distribution, mechanisms, and influence of ApoE genotype, sex, and inflammation. bioRxiv. 2020 doi: 10.1101/2020.07.15.205229. [DOI] [Google Scholar]
- Roschek B., Fink R.C., McMichael M.D., Li D., Alberte R.S. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry. 2009;70:1255–1261. doi: 10.1016/j.phytochem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Rose P., Moore P.K., Zhu Y.Z. Garlic and gaseous mediators. Trends Pharmacol. Sci. 2018;39:624–634. doi: 10.1016/j.tips.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Rothan H.A., Stone S., Natekar J., Kumari P., Arora K., Kumar M. The FDA-approved gold drug auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Virology. 2020;547:7–11. doi: 10.1016/j.virol.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand, L.V.D., Bormann, M., Alt, M., Schipper, L., Heilingloh, C.S., Todt, D., Dittmer, U., Elsner, C., Witzke, O., Krawczyk, A., Glycyrrhizin effectively neutralizes SARS-CoV-2 in vitro by inhibiting the viral main protease. bioRxiv doi: 10.1101/2020.12.18.423104. [DOI] [PMC free article] [PubMed]
- Sakurai Y., Kolokoltsov A.A., Chen C.C., Tidwell M.W., Bauta W.E., Klugbauer N., Grimm C., Wahl-Schott C., Biel M., Davey R.A. Ebola virus. two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347:995–998. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Takei M., Kobayashi M., Pollard R.B., Suzuki F. Effect of glycyrrhizin, an active component of licorice roots, on hiv replication in cultures of peripheral blood mononuclear cells from HIV-seropositive patients. Pathobiology. 2002;70:229–236. doi: 10.1159/000069334. [DOI] [PubMed] [Google Scholar]
- Sansei N., Katsuhiko O., Hideo N., Tomoko K., Yukio N., Toshihiro T. Studies on constituents of plantaginis herba inhibitory effects of flavonoids from plantago herb on HIV-reverse transcriptase activity. Nat. Med. 1997;51:547–549. [Google Scholar]
- Sa-ngiamsuntorn K., Suksatu A., Pewkliang Y., Thongsri P., Kanjanasirirat P., Manopwisedjaroen S., Charoensutthivarakul S., Wongtrakoongate P., Pitiporn S., Khemawoot P., Chutipongtanate S., Borwornpinyo S., Thitithanyanont A., Hongeng S. Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component Andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. bioRxiv. 2020 doi: 10.1101/2020.12.08.415836. [DOI] [PubMed] [Google Scholar]
- Sehailia M., Chemat S. Antimalarial-agent artemisinin and derivatives portray more potent binding to Lys353 and Lys31-binding hotspots of SARS-CoV-2 spike protein than hydroxychloroquine: potential repurposing of artenimol for COVID-19. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1796809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubsasana S., Pientong C., Ekalaksananan T., Thongchai S., Aromdee C. A potential andrographolide analogue against the replication of herpes simplex virus type 1 in vero cells. Med. Chem. 2011;7:237–244. doi: 10.2174/157340611795564268. [DOI] [PubMed] [Google Scholar]
- Shi N., Liu B., Liang N., Ma Y., Ge Y., Yi H., Wo H., Gu H., Kuang Y., Tang S., Zhng Y., Tong L., Liu S., Zhao C., Chen R., Bai W., Fan Y., Shi Z., Li L., Liu J., Gu H., Zhi Y., Wang Z., Li Y., Li H., Wang J., Jiao L., Tian Y., Xiong Y., Huo R., Zhang X., Bai J., Chen H., Chen L., Feng Q., Guo T., Hou Y., Hu G., Hu X., Hu Y., Huang J., Huang Q., Huang S., Ji L., Jin H., Lei X., Li C., Wu G., Li J., Li M., Li Q., Li X., Liu H., Liu J., Liu Z., Ma Y., Mao Y., Mo L., Na H., Wang J., Song F., Sun S., Wang D., Wang M., Wang X., Wang Y., Wang Y., Wu W., Wu L., Xiao Y., Xie H., Xu H., Xu S., Xue R., Yang C., Yang K., Yang P., Yuan S., Zhang G., Zhang J., Zhang L., Zhao S., Zhao W., Zheng K., Zhou Y., Zhu J., Zhu T., Wang G., Li W., Zhang H., Wang Y., Wang Y. Association between early treatment with Qingfei Paidu decoction and favorable clinical outcomes in patients with COVID-19: a retrospective multicenter cohort study. Pharmacol. Res. 2020;161:105290. doi: 10.1016/j.phrs.2020.105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Z., Zhou Y., Chang K., Liu J., Min X., Zhang Q., Sun J., Xiong Y., Zou Q., Zheng Q., Ji J., Poon J., Liu B., Zhou X., Li X. Clinical features and the traditional Chinese medicine therapeutic characteristics of 293 COVID-19 inpatient cases. Front. Med. 2020 doi: 10.1007/s11684-020-0803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S.K., Prasad S.K., Islam M.A., Gurav S.S., Patil R.B., AlFaris N.A., Aldayel T.S., AlKehayez N.M., Wabaidur S.M., Shakya A. Identification of bioactive compounds from Glycyrrhiza glabra as possible inhibitor of SARS-CoV-2 spike glycoprotein and non-structural protein-15: a pharmacoinformatics study. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1779132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H.X., Yao S., Zhao W.F., Li M.J., Liu J., Shang W.J., Xie H., Ke C.Q., Hu H.C., Gao M.N., Yu K.Q., Liu H., Shen J.S., Tang W., Zhang L.K., Xiao G.F., Ni L., Wang D.W., Zuo J.P., Jiang H.L., Bai F., Wu Y., Ye Y., Xu Y.C. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol. Sin. 2020;41:1167–1177. doi: 10.1038/s41401-020-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukkanon C., Karpkird T., Saeung M., Leepasert T., Panthawong A., Suwonkerd W., Bangs M.J., Chareonviriyaphap T. Excito-repellency activity of andrographis paniculata (lamiales: acanthaceae) against colonized mosquitoes. J. Med. Entomol. 2019;57:192–203. doi: 10.1093/jme/tjz139. [DOI] [PubMed] [Google Scholar]
- Sun J., Zhao R.H., Guo S.S., Shi Y.J., Bao Lei, Geng Z.H., Gao Y.J., Liu J., Li Q., Cui X.L. Effect of matrine sodium chloride injection on a mouse model combining disease with syndrome of human coronavirus pneumonia with cold-dampness pestilence attacking the lung. Acta Pharm. Sin. 2020;55:366–373. [Google Scholar]
- Sun Z.G., Zhao T.T., Lu N., Yang Y.A., Zhu H.L. Research progress of glycyrrhizic acid on antiviral activity. Mini Rev. Med. Chem. 2019;19:826–832. doi: 10.2174/1389557519666190119111125. [DOI] [PubMed] [Google Scholar]
- Suo J., Zhang C., Wang P., Hou L., Wang Q., Zhao X. Allyl sulfide counteracts 1-bromopropane-induced neurotoxicity by inhibiting neuroinflammation and oxidative stress. Toxicol. Sci. 2019;167:397–407. doi: 10.1093/toxsci/kfy240. [DOI] [PubMed] [Google Scholar]
- Tang H., Tang Y., Li N., Shi Q., Guo J., Shang E., Duan J.A. Neuroprotective effects of scutellarin and scutellarein on repeatedly cerebral ischemia-reperfusion in rats. Pharmacol. Biochem. Behav. 2014;118:51–59. doi: 10.1016/j.pbb.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Tang Q., Cao Y., Xiong W., Ke X., Zhang J., Xia Y., Liu D. Glycyrrhizic acid exerts protective effects against hypoxia/reoxygenation-induced human coronary artery endothelial cell damage by regulating mitochondria. Exp. Ther. Med. 2020;20:335–342. doi: 10.3892/etm.2020.8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Health Commission of the People's Republic of China (NHC PRC) NHC PRC; 2020. Notice on the issuance of the new 3rd version of the COVID-19 Diagnosis and Treatment Guideline.http://www.nhc.gov.cn/yzygj/s7653p/202001/f492c9153ea9437bb587ce2ffcbee1fa/files/39e7578d85964dbe81117736dd789d8f.pdf [Google Scholar]
- Thuy B.T.P., My T.T.A., Hai N.T.T., Hieu L.T., Hoa T.T., Loan H.T.P., Triet N.T., Anh T.T.V., Quy P.T., Tat P.V., Hue N.V., Quang D.T., Trung N.T., Tung V.T., Huynh L.K., Nhung N.T.A. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega. 2020;5:8312–8320. doi: 10.1021/acsomega.0c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Li X., Zhao L., Shen P., Wang Z., Zhu L., Li C., Su C., Zhang Y. Glycyrrhizic acid promotes neural repair by directly driving functional remyelination. Food Funct. 2020;11:992–1005. doi: 10.1039/c9fo01459d. [DOI] [PubMed] [Google Scholar]
- Tian W., Wang T., Sun W.P., Deng X.H., Xue Q., Li T.S., Chen Z.F., Jin H.F., Ni L., Zhao B., Du J.B., Ge B.M. The impact of sodium aescinate on acute lung injury induced by oleic acid in rats. Exp. Lung Res. 2011;37:585–599. doi: 10.3109/01902148.2011.622426. [DOI] [PubMed] [Google Scholar]
- Vardhan S., Sahoo S.K. In silico ADMET and molecular docking study on searching potential inhibitors from limonoids and triterpenoids for COVID-19. Comput. Biol. Med. 2020;124:103936. doi: 10.1016/j.compbiomed.2020.103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E.C., Tang L., Xu K., Feng Q.s. Efficacy evaluation of Qingfeipaidu decoction in the treatment of 75 cases of mild and common type of COVID-19 with enzymatic index. Pharm. Clin. Chin. Mat. Med. 2020;11:3–5. [Google Scholar]
- Wang F., Gao Q., Yang J., Wang C., Cao J., Sun J., Fan Z., Fu L. Artemisinin suppresses myocardial ischemia–reperfusion injury via NLRP3 inflammasome mechanism. Mol. Cell. Biochem. 2020;474:171–180. doi: 10.1007/s11010-020-03842-3. [DOI] [PubMed] [Google Scholar]
- Wang F., Pozo F.M., Tian D., Geng X., Yao X., Zhang Y., Tang J. Shikonin inhibits cancer through P21 upregulation and apoptosis induction. Front. Pharmacol. 2020;11:861. doi: 10.3389/fphar.2020.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.C., Shen B.X., He C.Y., Zhao W.C., Nie S.L. Clinical efficacy and mechanism of Lianhua Qingwen granule on COVID-19 based on network pharmacology research. Pharmacol. Clin. Chin. Mater. Med. 2020;36:93–101. [Google Scholar]
- Wang Y.C., Liu Q.X., Zheng Q., Liu T., Xu X.E., Liu X.H., Gao W., Bai X.J., Li Z.F. Dihydromyricetin alleviates sepsis-induced acute lung injury through inhibiting NLRP3 inflammasome-dependent pyroptosis in mice model. Inflammation. 2019;42:1301–1310. doi: 10.1007/s10753-019-00990-7. [DOI] [PubMed] [Google Scholar]
- Wang Z., Yang L., Yang X., Zhang X. Advances in the first total synthesis of natural flavonoids. Synth. Commun. 2013;43:3093–3114. [Google Scholar]
- Wang Z.L., Wang S., Kuang Y., Hu Z.M., Qiao X., Ye M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018;56:465–484. doi: 10.1080/13880209.2018.1492620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C., Opatz T. Bisbenzylisoquinoline alkaloids. Alkaloids - Chem. Biol. 2019;81:1–114. doi: 10.1016/bs.alkal.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Wen Q., Jin X., Lu Y., Chen D.F. Anticomplement ent-labdane diterpenoids from the aerial parts of Andrographis paniculata. Fitoterapia. 2020;142:104528. doi: 10.1016/j.fitote.2020.104528. [DOI] [PubMed] [Google Scholar]
- Who Coronavirus disease (COVID-2019) situation reports. 2020. https://covid19.who.int/
- WHO Emergency Committee . 2020. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) [Google Scholar]
- Winnicka K., Bielawski K., Bielawska A., Miltyk W. Dual effects of ouabain, digoxin and proscillaridin A on the regulation of apoptosis in human fibroblasts. Nat. Prod. Res. 2010;24:274–285. doi: 10.1080/14786410902991878. [DOI] [PubMed] [Google Scholar]
- Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L., Wu K. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia E.Q., Ai X.X., Zang S.Y., Guan T.T., Xu X.R., Li H.B. Ultrasound-assisted extraction of phillyrin from Forsythia suspensa. Ultrason. Sonochem. 2011;18:549–552. doi: 10.1016/j.ultsonch.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Xia M., Liu D., Liu Y., Liu H. The therapeutic effect of artemisinin and its derivatives in kidney disease. Front. Pharmacol. 2020;11:380. doi: 10.3389/fphar.2020.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q.D., Xun Y., Lu J.L., Lu Y.C., Yang Y.Y., Zhou P., Hu J., Li C., Wang S.G. Network pharmacology and molecular docking analyses on Lianhua Qingwen capsule indicate Akt1 is a potential target to treat and prevent COVID‐19. Cell Prolif. 2020;53 doi: 10.1111/cpr.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xinhua Net Academician Xiaolin Tong: the total effective rate of Qingfeipaidu formula was 97%, none transfer from mild to severe cases. 2020. http://www.kunlunce.com/ssjj/fl1/2020-03-18/141570.html Available from.
- Xinhua Net TCM treatment effective on over 90 pct of COVID-19 patients on China's mainland: official. 2020. http://www.xinhuanet.com/english/2020-03/23/c_138908334.htm
- Xiong R., Zhang L.K., Li S.L., Sun Y., Ding M.Y., Wang Y., Zhao Y.L., Wu Y., Shang W.J., Jiang X.M., Shan J.W., Shen Z.H., Tong Y., Xu L.X., Yu C., Liu Y.L., Zou G., Lavillete D., Zhao Z.J., Wang R., Zhu L.L., Xiao G.F., Lan K., Li H.L., Xu K. Novel and potent inhibitors targeting DHODH, a rate-limiting enzyme in de novo pyrimidine biosynthesis, are broad-spectrum antiviral against RNA viruses including newly emerged coronavirus SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.03.11.983056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Wang P., Su K., Cho W.C., Xing Y. Chinese herbal medicine for coronavirus disease 2019: a systematic review and meta-analysis. Pharmacol. Res. 2020;160:105056. doi: 10.1016/j.phrs.2020.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W.Z., Wang G., Du J., Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19: a pilot randomized clinical trial. Integr. Med. Res. 2020;2020:100489. doi: 10.1016/j.imr.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Wang K.Q., Xu W.H., Li Y.H., Qi Y., Wu H.Y., Li J.Z., He Z.G., Hu H.G., Wang Y., Zhang J.P. The matrine derivate MASM prolongs survival, attenuates inflammation, and reduces organ injury in murine established lethal sepsis. J. Infect. Dis. 2016;214:1762–1772. doi: 10.1093/infdis/jiw445. [DOI] [PubMed] [Google Scholar]
- Xu Y., Lin H., Zheng W., Ye X., Yu L., Zhuang J., Yang Q., Wang D. Matrine ameliorates adriamycin-induced nephropathy in rats by enhancing renal function and modulating Th17/Treg balance. Eur. J. Pharmacol. 2016;791:491–501. doi: 10.1016/j.ejphar.2016.09.022. [DOI] [PubMed] [Google Scholar]
- Yang M.W., Chen F., Zhu D.J., Li J.Z., Zhu J.L., Zeng W., Qu S.L., Zhang Y. Clinical efficacy of matrine and sodium chloride injection in treatment of 40 cases of COVID-19. China J. Chin. Mater. Med. 2020;45:2221–2231. doi: 10.19540/j.cnki.cjcmm.20200323.501. [DOI] [PubMed] [Google Scholar]
- Yang J., Shi Y., Chen H., Wang X., Chen Y., Yang B. Glycyrrhizic acid attenuates myocardial injury: involvement of RIP140/NF-kB pathway. Biomed. Pharmacother. 2017;95:62–67. doi: 10.1016/j.biopha.2017.08.031. [DOI] [PubMed] [Google Scholar]
- Yang J., Xiang D., Xiang D., He W., Liu Y., Lan L., Li G., Jiang C., Ren X., Liu D., Zhang C. Baicalin protects against 17α-ethinylestradiol-induced cholestasis via the sirtuin 1/hepatic nuclear receptor-1α/farnesoid X receptor pathway. Front. Pharmacol. 2019;10:1685. doi: 10.3389/fphar.2019.01685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Liu H., Bai C., Wang Y., Zhang X., Guo R., Wu S., Wang J., Leung E., Chang H., Li P., Liu T., Wang Y. Chemical composition and pharmacological mechanism of Qingfei Paidu decoction and Ma Xing Shi Gan decoction against coronavirus disease 2019 (COVID-19): in silico and experimental study. Pharmacol. Res. 2020;157:104820. doi: 10.1016/j.phrs.2020.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Chen Y.Y., Liu J.R., Zhao H., Vaziri N.D., Guo Y., Zhao Y.Y. Natural products against renin-angiotensin system for antifibrosis therapy. Eur. J. Med. Chem. 2019;179:623–633. doi: 10.1016/j.ejmech.2019.06.091. [DOI] [PubMed] [Google Scholar]
- Yang Y., Zhang D.M., Liu J.H., Hu L.S., Xue Q.C., Ding X.Q., Kong L.D. Wuling San protects kidney dysfunction by inhibiting renal TLR4/MyD88 signaling and NLRP3 inflammasome activation in high fructose-induced hyperuricemic mice. J. Ethnopharmacol. 2015;169:49–59. doi: 10.1016/j.jep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Yao K.T., Liu M.Y., Li X., Huang J.H., Cai H.B. Retrospective clinical analysis on treatment of novel coronavirusinfected pneumonia with traditional Chinese medicine Lianhua Qingwen. Chin. J. Exp. Trad. Med. Formulae. 2020;26:8–12. [Google Scholar]
- Yu J.W., Wang L., Bao L.D. Exploring the active compounds of traditional Mongolian medicine in intervention of novel coronavirus (COVID-19) based on molecular docking method. J. Funct. Foods. 2020;71:104016. doi: 10.1016/j.jff.2020.104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.Y. People’s Daily Overseas Edition; 2020. The Total Effective Rate of Traditional Chinese Medicine for the Treatment of COVID-19 Exceeds 90% 2020-03-24(002. [Google Scholar]
- Yu M.S., Lee J., Lee J.M., Kim Y., Chin Y.W., Jee J.G., Keum Y.S., Jeong Y.J. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg. Med. Chem. Lett. 2012;22:4049–4054. doi: 10.1016/j.bmcl.2012.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T., Wang J., Chen L., Shan J.J., Di L. Glycyrrhizic acid improving the liver protective effect by restoring the composition of Lactobacillus. J. Funct. Foods. 2019;52:219–227. [Google Scholar]
- Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.M., Gao L., Zheng Y.J., Yang H.T. Berbamine protects the heart from ischemia/reperfusion injury by maintaining cytosolic Ca(2+) homeostasis and preventing calpain activation. Circ. J. 2012;76:1993–2002. doi: 10.1253/circj.cj-11-1431. [DOI] [PubMed] [Google Scholar]
- Zhang D., Qi B., Li D., Feng J., Huang X., Ma X., Huang L., Wang X., Liu X. Phillyrin relieves lipopolysaccharide-induced AKI by protecting against glycocalyx damage and inhibiting inflammatory responses. Inflammation. 2020;43:540. doi: 10.1007/s10753-019-01136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chen L., Sun X., Yang Q., Wan L., Guo C. Matrine: a promising natural product with various pharmacological activities. Front. Pharmacol. 2020;11:588. doi: 10.3389/fphar.2020.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Chen Y., Luo H., Sun L., Xu M., Yu J., Zhou Q., Meng G., Yang S. Recent update on the pharmacological effects and mechanisms of dihydromyricetin. Front. Pharmacol. 2018;9:1204. doi: 10.3389/fphar.2018.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.L., Li W.X., Li Y., Wong M.S., Wang Y.J., Zhang Y. Therapeutic options of TCM for organ injuries associated with COVID-19 and the underlying mechanism. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Fei M., Wang H., Zhu Y. Sodium aescinate provides neuroprotection in experimental traumatic brain injury via the Nrf2-ARE pathway. Brain Res. Bull. 2020;157:26–36. doi: 10.1016/j.brainresbull.2020.01.019. [DOI] [PubMed] [Google Scholar]
- Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Cui J.H., Meng Q.Q., Li S.S., Zhou W., Xiao S. Advance in anti-tumor mechanisms of shikonin, alkannin and their derivatives. Mini Rev. Med. Chem. 2018;18:164–172. doi: 10.2174/1389557517666170228114809. [DOI] [PubMed] [Google Scholar]
- Zhang X., Tan Y., Ling Y., Lu G., Liu F., Yi Z., Jia X., Wu M., Shi B., Xu S., Chen J., Wang W., Chen B., Jiang L., Yu S., Lu J., Wang J., Xu M., Yuan Z., Zhang Q., Zhang X., Zhao G., Wang S., Chen S., Lu H. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583:437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Geng L., Chen J., Ma Y., Zheng Q., Guo D. Qingfeipaidu decoction combined with Western medicine in the treatment of 1 case of severe type of COVID-19. Tianjin J. Tradit. Chin. Med. 2020;37:861–865. [Google Scholar]
- Zhang Z.J., Morris‐Natschke S.L., Cheng Y.Yi, Lee K.H., Li R.T. Development of anti‐influenza agents from natural products. Med. Res. Rev. 2020;40:2290–2338. doi: 10.1002/med.21707. [DOI] [PubMed] [Google Scholar]
- Zhao D., Zhang J., Xu G., Wang Q. Artesunate protects LPS-induced acute lung injury by inhibiting TLR4 expression and inducing Nrf2 activation. Inflammation. 2017;40:798–805. doi: 10.1007/s10753-017-0524-6. [DOI] [PubMed] [Google Scholar]
- Zhao J., Tian S., Lu D., Yang J., Zeng H., Zhang F., Tu D., Ge G., Zheng Y., Shi T., Xu X., Zhao S., Yang Y., Zhang W. Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Yang J., Cui M.Y., Liu J., Fang Y., Yan M., Qiu W., Shang H., Xu Z., Yidiresi R., Weng J.K., Pluskal T., Vigouroux M., Steuernagel B., Wei Y., Yang L., Hu Y., Chen X.Y., Martin C. The reference genome sequence of Scutellaria baicalensis provides insights into the evolution of wogonin biosynthesis. Mol. Plant. 2019;12:935–950. doi: 10.1016/j.molp.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Gu S., Li X., Tan J., Liu S., Jiang Y., Zhang C., Gao L., Yang H.T. Berbamine postconditioning protects the heart from ischemia/reperfusion injury through modulation of autophagy. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi H.J., Zhu H.Y., Zhang Y.Y., Lu Y., Li H., Chen D.F. In vivo effect of quantified flavonoids-enriched extract of Scutellaria baicalensis root on acute lung injury induced by influenza A virus. Phytomedicine. 2019;57:105–116. doi: 10.1016/j.phymed.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Zhong J.L.L.D., Lam W.C., Yang W., Chan K.W., Sze S.C.W., Miao J., Yung K.K.L., Bian Z., Wong V.T. Potential targets for treatment of coronavirus disease 2019 (COVID-19): a review of Qing-Fei-Pai-Du-Tang and its major herbs. Am. J. Chin. Med. 2020;48:1051–1071. doi: 10.1142/S0192415X20500512. [DOI] [PubMed] [Google Scholar]
- Zhong W.T., Wu Y.C., Xie X.X., Zhou X., Wei M.M., Soromou L.W., Ci X.X., Wang D.C. Phillyrin attenuates LPS-induced pulmonary inflammation via suppression of MAPK and NF-κB activation in acute lung injury mice. Fitoterapia. 2013;90:132–139. doi: 10.1016/j.fitote.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Zhou H., Xu M., Gao Y., Deng Z., Cao H., Zhang W., Wang Q., Zhang B., Song G., Zhan Y., Hu T. Matrine induces caspase-independent program cell death in hepatocellular carcinoma through bid-mediated nuclear translocation of apoptosis inducing factor. Mol. Canc. 2014;13:59. doi: 10.1186/1476-4598-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang W., Fan Z., Chu Y., Wang H., Yang Y., Wu L., Sun N., Sun G., Shen Y., Lin X., Guo G., Xi S. Chinese patent medicines in the treatment of coronavirus disease 2019 (COVID-19) in China. Front. Pharmacol. 2020;11:1066. doi: 10.3389/fphar.2020.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]