Abstract
Since the outbreak of coronavirus disease 2019 (COVID-19), a large number of COVID-19-related reports have been published in journals or submitted to preprint platforms. In this study, we search the COVID-19-related literature officially published and included in the Web of Science (WOS) database or submitted to four preprint platforms: bioRxiv, medRxiv, Preprints, and SSRN. Using data on the number of reports, author institution, country, and research category, we analyze global trends in COVID-19 research, including institution distribution and research hotspots. The results show that a large number of COVID-19-related reports have been produced; the United States has contributed the most published literature, followed by China. The United States has published the most reports included in the WOS in the categories of non-pharmaceutical interventions, treatment, and vaccine-related reports, while China has published the most literature in the categories of clinical features and complications, virology and immunology, epidemiology, and detection and diagnosis. Publication countries are concentrated in Asia, North America, and Europe, while South America and Africa have less literature. In conclusion, many scientific research issues related to COVID-19 need to be further clarified and COVID-19 research urgently needs global cooperation.
Keywords: COVID-19, Bibliometric, Web of Science, Preprint
1. Introduction
-
1.
Early in the 21st century, humans suffered severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), two epidemics caused by members of the coronavirus group. After each outbreak, a large number of related studies were carried out by countries greatly affected by the epidemic as well as those with a strong research tradition such as the United States.1 At the end of December 2019, a new coronavirus disease (COVID-19) outbreak and spread rapidly globally.2, 3 On March 11, 2020, the World Health Organization announced that COVID-19 had become a global pandemic.4 The outbreak of the COVID-19 pandemic poses a huge challenge to global public health and has a profound impact on the economic and social operations of countries.5 It has caused more than 100 million infections worldwide and more than 2.15 million people have been killed as of 28 January 2021.6 Since the outbreak of the pandemic, researchers globally have carried out relevant research from many aspects such as virology and immunology, disease transmission and clinical processes, disease diagnosis and management, experimental therapy, and vaccine development.7, 8, 9
Bibliometric analysis is an objective evaluation of scientific research, which can quantitatively present the research hotspots, development trends, and key research institutions of relevant scientific research activities; clarify ideas for scientific researchers; and provide a reference for research cooperation.10 The measurement of COVID-19-related literature is of great significance for understanding current research on COVID-19. This study comprehensively analyzes the literature published since the outbreak of COVID-19 based on the Web of Science (WOS) database and four preprint platforms: bioRxiv (https://www.biorxiv.org/), medRxiv (https://www.medRxiv.org/), Preprints (https://www.preprints.org/), and SSRN (https://www.ssrn.org/). In particular, we examine the number of reports, institution, country distribution, and research category to provide a reference for COVID-19-related researchers and decision-makers.
2. Methods
2.1. Data sources
A COVID-19-related literature search was conducted on October 14, 2020. The source of the literature was selected as the Science Citation Index Expanded database of the WOS and four preprint platforms: bioRxiv, medRxiv, Preprints, and SSRN. The WOS literature data were retrieved by setting the corresponding subject terms; the literature type was limited to “article,” the search field was the title, and the specific search formula was (TI = COVID-19 OR TI= “Coronavirus disease 2019” OR TI = COVID-2019 OR TI = 2019-nCoV OR TI = nCov-2019 OR TI = SARS-COV-2 OR TI = “Severe acute respiratory syndrome coronavirus 2” OR TI = “Novel Coronavirus”) AND “Article”[Publication Type]. The bioRxiv, medRxiv, Preprints, and SSRN platform literature data were obtained through COVID-19-related reports published on the respective websites.
In the literature in the WOS, 20 reports unrelated to the subject (mainly literature on the search term “Novel Coronavirus” before 2020) and 353 duplicated reports were excluded, finally yielding 12,021 reports; from the literature obtained from the bioRxiv platform, we excluded four reports that were irrelevant to the COVID-19, and finally 2040 reports were obtained; from the literature obtained from the medRxiv platform, we excluded six reports unrelated to the COVID-19, and 7555 reports were obtained; 1046 reports were obtained from the Preprints platform; and 2028 reports were obtained from the SSRN platform.
2.2. Statistical analysis
According to the search results, statistical analysis was performed in terms of the number of reports, author institution, country, and research category. The literatures of WOS and four preprint platforms were separate statistics, not matter its overlap. As the data on publications in October include only the period from October 1 to October 14, so some figures does not present data in October. The author’s institution and country were selected as the institution and country information of the first author (where the first author had multiple institutions, we selected the first). In the literature included in the WOS database, the Chinese literature contains the literature of Hong Kong and Macao, but excludes those of Taiwan of China, and the literature from the United Kingdom is divided into the literature of England, Scotland, and Wales. In the literature submitted on the preprint platforms, the UK literature includes the literature of England, Scotland, and Wales. Hence, in this study, for comparison purposes, the Chinese literature includes the literature of Hong Kong and Macao but excludes that of Taiwan of China, while the UK literature includes the literature of England, Scotland, and Wales.
The publication time of WOS reports was selected as the official publication time and the time of the reports on the preprint platforms was selected as the time when the preprint version was published. Journal impact factors were queried from the 2020 InCites Journal Citation Reports.
To understand the hotspots and trends of COVID-19 research, we referred to the classification from the WHO database of COVID-19 literature11 and divided the literature into the following 10 categories according to the research content: epidemiology (research on COVID-19 epidemiological characteristics and development of predictive models), non-pharmaceutical interventions (research on COVID-19 epidemic prevention and nosocomial infection control), treatment (research on COVID-19 drug development and clinical treatment plans), vaccines (COVID-19 research related to vaccine development), clinical characteristics and complications (research on COVID-19 clinical and imaging manifestations and complications), detection and diagnosis (COVID-19 detection markers and clinical diagnosis), virology and immunology (SARS-CoV-2 virology and immunology basic research and virus traceability research), transmission (research on COVID-19 transmission route), psychology (psychology-related research in the COVID-19 field), and other research (COVID-19 disease review, case reports, social impact, and social science research).
3. Results
3.1. Number of COVID-19 reports
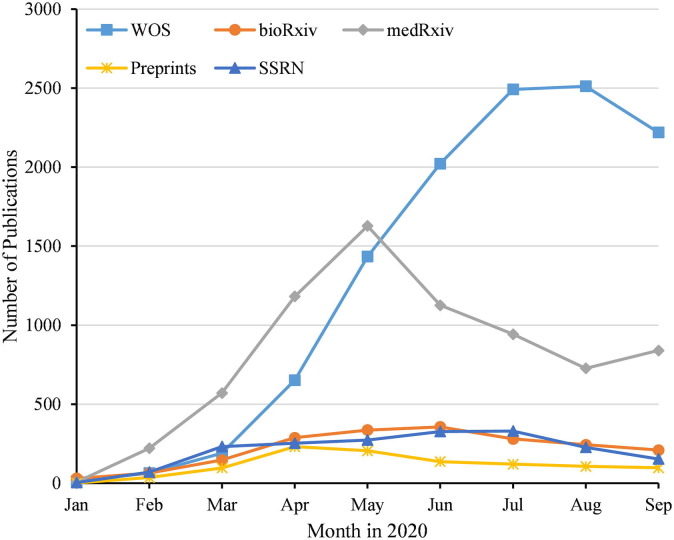
As of October 14, 2020, the WOS database included 12,021 reports related to COVID-19. For preprints, 2040 reports had been submitted to the bioRxiv platform, the first of which was submitted by Xiamen University on January 19, 202012; 7555 reports had been submitted to the medRxiv platform, the first of which was submitted by Lancaster University on January 23, 202013; 1046 reports had been submitted to the Preprints platform, the first of which was submitted by Shenzhen University on January 30, 202014; and 2028 reports had been submitted to the SSRN platform, the first of which was submitted by Boston Children’s Hospital on January 24, 2020.15 In January to May 2020, the number of reports submitted to the four preprint platforms monthly is more than the number of reports included in the WOS; from June 2020, the number of reports included in the WOS monthly continues to exceed the number submitted to the four preprint platforms. Currently, the growth rate of reports included in the WOS or submitted to these preprint platforms is flat (Fig. 1 ; Supplementary Table 1).
Fig. 1.
Monthly publications on COVID-19.
3.2. Country and institution distribution of COVID-19 literature
According to the first author information of the relevant literature, more than 5000 institutions in 173 countries or regions have invested in COVID-19 research. The leading countries in the literature included in the WOS are the United States (2561 reports, 21.3%), China (2483 reports, 20.7%), Italy (1138 reports, 9.5%), the United Kingdom (596 reports, 5.0%), and India (484 reports, 4.0%). The leading countries in the literature submitted to bioRxiv are the United States (732 reports, 35.9%), China (294 reports, 14.4%), India (141 reports, 6.9%), the United Kingdom (106 reports, 5.2%), and Germany (95 reports, 4.7%). The leading countries in the literature submitted to medRxiv are the United States (2007 reports, 26.6%), China (986 reports, 13.1%), the United Kingdom (862 reports, 11.4%), India (430 reports, 5.7%), and Germany (259 reports, 3.4%). The leading countries in the literature submitted to Preprints are the United States (156 reports, 14.9%), India (143 reports, 13.7%), China (89 reports, 8.5%), Italy (67 reports, 6.4%), and the United Kingdom (48 reports, 4.6%). The leading countries in the literature submitted to SSRN are China (649 reports, 32.0%), the United States (362 reports, 17.9%), India (148 reports, 7.3%), the United Kingdom (137 reports, 6.8%), and Italy (99 reports, 4.9%) (Table 1 ).
Table 1.
The top 10 countries of research on COVID-19.
| Ranking | Number of Publications |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WOS | Percent | bioRxiv | Percent | medRxiv | Percent | Preprints | Percent | SSRN | Percent | |
| 1 | United States (n = 2561) |
21.3% | United States (n = 732) |
35.9% | United States (n = 2007) |
26.6% | United States (n = 156) |
14.9% | China (n = 649) |
32.0% |
| 2 | China (n = 2483) |
20.7% | China (n = 294) |
14.4% | China (n = 986) |
13.1% | India (n = 143) |
13.7% | United States (n = 362) |
17.9% |
| 3 | Italy (n = 1138) |
9.5% | India (n = 141) |
6.9% | United Kingdom (n = 862) |
11.4% | China (n = 89) |
8.5% | India (n = 148) |
7.3% |
| 4 | United Kingdom (n = 596) |
5.0% | United Kingdom (n = 106) |
5.2% | India (n = 430) |
5.7% | Italy (n = 67) |
6.4% | United Kingdom (n = 137) |
6.8% |
| 5 | India (n = 484) |
4.0% | Germany (n = 95) |
4.7% | Germany (n = 259) |
3.4% | United Kingdom (n = 48) |
4.6% | Italy (n = 99) |
4.9% |
| 6 | Germany (n = 420) |
3.5% | France (n = 57) |
2.8% | Italy (n = 257) |
3.4% | Bangladesh (n = 41) |
3.9% | Germany (n = 50) |
2.5% |
| 7 | France (n = 399) |
3.3% | Canada (n = 56) |
2.7% | Brazil (n = 237) |
3.1% | Brazil (n = 40) |
3.8% | Spain (n = 45) |
2.2% |
| 8 | Spain (n = 369) |
3.1% | Italy (n = 55) |
2.7% | France (n = 226) |
3.0% | Iran (n = 24) |
2.3% | France (n = 44) |
2.2% |
| 9 | Canada (n = 250) |
2.1% | Japan (n = 42) |
2.1% | Spain (n = 204) |
2.7% | Spain (n = 22) |
2.1% | Canada (n = 39) |
1.9% |
| 10 | Brazil (n = 249) |
2.1% | Brazil (n = 38) |
1.9% | Canada (n = 159) |
2.1% | Germany (n = 21) |
2.0% | Brazil (n = 37) |
1.8% |
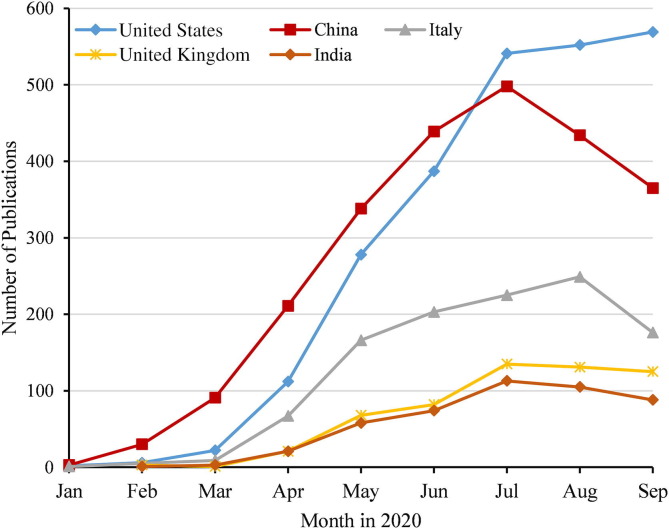
The country that has published the most reports included in the WOS monthly within the first six months of 2020 is China. Since July 2020, the US monthly literature included in the WOS has surpassed that of China. (Fig. 2 ; Supplementary Table 2).
Fig. 2.
Monthly publications on COVID-19 included in the WOS by the main countries.
The main institutions from which published COVID-19 literature has been indexed by the WOS include Huazhong University of Science and Technology (n = 300), Wuhan University (n = 170), Fudan University (n = 80), Columbia University (n = 66), and Zhejiang University (n = 66); the main institutions that have submitted literature to the bioRxiv platform include the University of Oxford (n = 20), Chinese Academy of Medical Sciences (n = 18), Washington University (n = 18), Stanford University (n = 63), and Fudan University (n = 16); the main institutions that have submitted literature to the medRxiv platform include the University of Oxford (n = 83), Imperial College London (n = 72), Huazhong University of Science and Technology (n = 67), Stanford University (n = 63), and University College London (n = 62); the main institutions that have submitted literature to the Preprints platform include the University of Dhaka (n = 9), the Bhawanipur Education Society College (n = 6), the University of Sao Paulo (n = 6), the University of Catania (n = 6), and the All India Institute of Medical Sciences (n = 5); and the main institutions that have submitted literature to the SSRN platform include Huazhong University of Science and Technology (n = 103), Wuhan University (n = 52), Shanghai Jiaotong University (n = 19), Fudan University (n = 18), and Fujian Medical University (n = 18) (Table 2 ).
Table 2.
The top 10 institutes publishing research on COVID-19.
| Ranking | Number of Publications |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WOS | Number | bioRxiv | Number | medRxiv | Number | Preprints | Number | SSRN | Number | |
| 1 | Huazhong Univ Sci & Technol | 300 | Univ Oxford | 20 | Univ Oxford | 83 | Univ Dhaka | 9 | Huazhong Univ Sci & Technol | 103 |
| 2 | Wuhan Univ | 170 | Chinese Acad Med Sci | 18 | Imperial Coll London | 72 | Bhawanipur Education Society College | 6 | Wuhan Univ | 52 |
| 3 | Fudan Univ | 80 | Washington Univ | 18 | Huazhong Univ Sci & Technol | 67 | Univ Sao Paulo | 6 | Shanghai Jiao Tong Univ | 19 |
| 4 | Columbia Univ | 66 | Stanford Univ | 17 | Stanford Univ | 63 | Univ Catania | 6 | Fudan Univ | 18 |
| 5 | Zhejiang Univ | 66 | Fudan Univ | 16 | Univ College London | 62 | All India Inst Med Sci | 5 | Fujian Med Univ | 18 |
| 6 | US CDC | 60 | Univ Calif San Diego | 16 | London Sch Hyg & Trop Med | 58 | Iran Univ Med Sci | 5 | Sun Yat-Sen Univ | 18 |
| 7 | Cent South Univ | 59 | Yale Univ | 16 | King’s College London | 56 | Univ Bologna | 5 | Zhejiang Univ | 15 |
| 8 | Icahn Sch Med Mt Sinai | 59 | Columbia Univ | 15 | Fudan Univ | 54 | Wuhan Univ | 5 | Harvard Univ | 14 |
| 9 | Shanghai Jiao Tong Univ | 57 | Chinese Acad Sci | 14 | Icahn Sch Med Mt Sinai | 51 | Amity Univ | 4 | Chinese Acad Med Sci | 13 |
| 10 | Univ Hong Kong | 57 | Massachusetts Institute of Technology | 14 | Harvard Univ | 50 | CSIR-Indian Institute of Chemical Biology | 4 | Massachusetts Institute of Technology | 13 |
3.3. The main journals of published COVID-19 literature
The 12,021 reports included in the WOS were published in 2076 journals. As shown in Table 3 , the main journals of published COVID-19 literature are the International Journal of Environmental Research and Public Health (n = 283), Journal of Medical Virology (n = 261), PLOS One (n = 182), International Journal of Infectious Diseases (n = 154), and Journal of Biomolecular Structure & Dynamics (n = 142).
Table 3.
The top 15 journals of publications on COVID-19.
| Ranking | Journal | Number | Journal Impact Factor (2019) |
|---|---|---|---|
| 1 | Int J Env Res Pub He | 283 | 2.8 |
| 2 | J Med Virol | 261 | 2.0 |
| 3 | PLOS One | 182 | 2.7 |
| 4 | Int J Infect Dis | 154 | 3.2 |
| 5 | J Biomol Struct Dyn | 142 | 3.3 |
| 6 | Sci Total Environ | 133 | 6.6 |
| 7 | J Chem Educ | 89 | 1.4 |
| 8 | Front Med-Lausanne | 85 | 3.9 |
| 9 | Mmwr-Morbid Mortal W | 82 | 13.6 |
| 10 | Head Neck-J Sci Spec | 79 | 2.5 |
| 11 | Front Public Health | 78 | 2.5 |
| 12 | Eurosurveillance | 74 | 6.5 |
| 13 | J Clin Virol | 73 | 2.8 |
| 14 | Sustainability | 72 | 2.6 |
| 15 | Epidemiol Infect | 69 | 2.2 |
A total of 170 articles were published in the Lancet, the New England Journal of Medicine, Nature, Science, and Cell, including 32 in the Lancet, 23 in the New England Journal of Medicine, 37 in Nature, 47 in Science, and 31 in Cell. The most published country is the United States with 63 articles, followed by China with 53 articles; other countries with articles in these journals include the United Kingdom (n = 14), Germany (n = 13), and France (n = 4). Institutions from the United States, China, the United Kingdom, Germany, and France published 241 articles, accounting for 86.4% (Table 4 ).
Table 4.
National distribution of publications on COVID-19 in the Lancet, the New England Journal of Medicine, Nature, Science, and Cell.
| Country | Number of Publications |
||||||
|---|---|---|---|---|---|---|---|
| Lancet | N Engl J Med | Nature | Science | Cell | Total | ||
| 1 | United States | 6 | 16 | 11 | 18 | 12 | 63 |
| 2 | China | 12 | 4 | 13 | 12 | 12 | 53 |
| 3 | United Kingdom | 4 | 3 | 7 | 14 | ||
| 4 | Germany | 1 | 1 | 4 | 4 | 3 | 13 |
| 5 | France | 1 | 2 | 1 | 4 | ||
| 6 | Italy | 2 | 1 | 1 | 4 | ||
| 7 | Switzerland | 2 | 2 | 4 | |||
| 8 | Netherlands | 3 | 3 | ||||
| 9 | Singapore | 1 | 1 | 2 | |||
| 10 | Spain | 2 | 2 | ||||
| Others | 2 | 1 | 1 | 1 | 3 | 8 | |
| Total | 32 | 23 | 37 | 47 | 31 | 170 | |
3.4. Literature research category
Literature based on clinical features and complications is the most common in the WOS (n = 1889); literature based on virology and immunology is the most submitted to bioRxiv (n = 1243); literature based on epidemiology is the most submitted to medRxiv (n = 1956); literature based on virology and immunology is the most submitted to Preprints (n = 209); and literature based on epidemiology is the most submitted to SSRN (n = 456) (Table 5 ).
Table 5.
Research categories of publications on COVID-19.
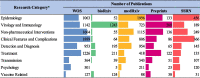 |
*In this table, other research (COVID-19 disease review, case reports, social impact, and social science research) is not presented.
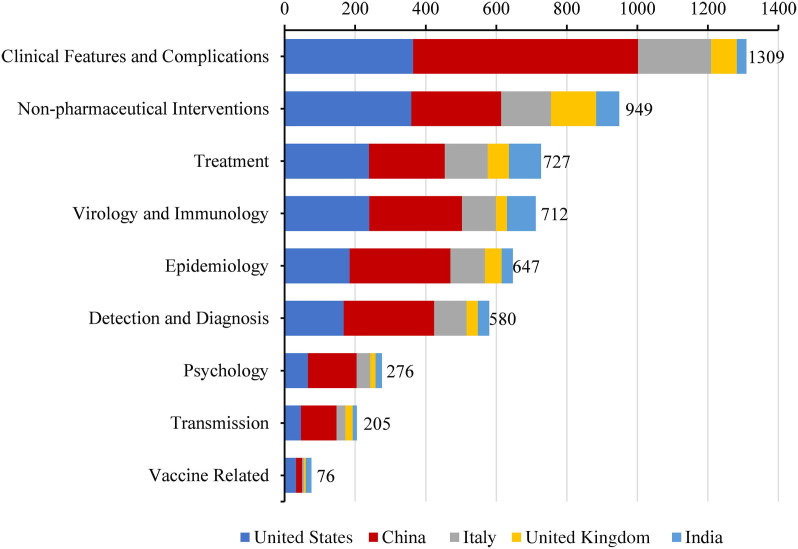
As for the national distribution of research categories in the WOS, US institutions publish the most in the categories of non-pharmaceutical interventions (n = 359), treatment (n = 239), and vaccine-related reports (n = 32), Chinese institutions publish the most in the categories of clinical features and complications (n = 638), virology and immunology (n = 263), epidemiology (n = 286), detection and diagnosis (n = 257), psychology (n = 138), and transmission (n = 101) (Fig. 3 , Supplementary Table 3).
Fig. 3.
Research categories of literature on COVID-19 included in the WOS. In this figure, other research (COVID-19 disease review, case reports, social impact, and social science research) is not presented.
4. Discussion
This study comprehensively analyzes the COVID-19-related literature based on the WOS database and four preprint platforms (bioRxiv, medRxiv, Preprints, and SSRN), which present global COVID-19 research in terms of the number of reports, distribution of countries, institutions, and research topics.
4.1. A large number of COVID-19-related reports have been produced, with US and Chinese institutions having the highest output
As of October 14, 2020, 12,021 COVID-19-related reports had been included in the WOS database and 12,669 articles had been submitted to the bioRxiv, medRxiv, Preprints, and SSRN platforms. In January 2020, the reports included in the WOS or submitted to the four preprint platforms monthly numbered in the dozens; the reports in February exceeded 100, and by March there were nearly 1000. After June, the number of reports monthly was nearly 4000.
Institutions that publish literature are concentrated in the United States, China, the United Kingdom, and Italy. Institutions from China and the United States publish the most reports. In the early months of the outbreak, Chinese institutions maintained the world’s highest monthly publication. As the country was the most affected by the initial outbreak of the pandemic, institutions from China contributed many reports to the COVID-19 research effort and played an important role in the epidemic response.16, 17 As of October 14, 2020, 20.7% of the reports submitted to the WOS had been from China. Moreover, Huazhong University of Science and Technology in China contributed the most reports included in the WOS. Since July 2020, the monthly literature in the WOS of US institutions has surpassed that of China. The proportion of reports included in the WOS from US institutions has reached 21.3% in October 2020; the proportion of US institutions published in leading journals is up to 37.1%.
Since the outbreak of the COVID-19 epidemic, researchers worldwide have responded quickly and published many reports in a short period.18 So far, COVID-19-related research has involved non-pharmaceutical interventions, epidemiology, clinical characteristics, treatment, detection and diagnosis, virology and immunology, disease transmission, vaccines, and other categories. Additionally, psychological studies have examined the psychological status of the public and medical staff during the epidemic.19 In terms of the number of articles, the leading categories are non-pharmaceutical interventions, treatment, and clinical features and complications. There are relatively few vaccine-related reports. Only 127 of the reports included in the WOS are vaccine-related, accounting for only 1%, which may be related to the relatively long time required for vaccine development. Taking the reports included in the WOS as an example, the United States has published more reports in the non-pharmaceutical interventions, treatment, and vaccine-related report categories, whereas the categories most frequently published in by Chinese institutions are clinical features and complications, virology and immunology, epidemiology, detection and diagnosis.
4.2. Preprint platforms have played an important role in COVID-19-related science research
In terms of monthly publications, much of the literature has been submitted to the preprint platform. Within the first five months, the total number of reports submitted to the four preprint platforms each month was greater than the literature included in the WOS. In response to the Ebola and Zika outbreaks, fewer than 5% of articles were submitted to preprint platforms.20 In recent years, preprint platforms have attracted increasing attention from researchers because of their fast and free open-source release. Since the establishment of the first physics preprint platform arXiv in 1991, there have been dozens of preprint platforms involved in various fields, including the medRxiv and bioRxiv platforms in the biomedical field and the Chemrxiv platform in the chemical field. In 2017, Science ranked the preprint platform among the top 10 scientific and technological advances in that year. As it matures, the preprint platform is considered to have great potential for accelerating the spread of scientific discoveries, disseminating information in emergencies, and supporting infectious disease outbreak response20, 21; the preprint platform is also considered to be helpful for scientific and technological exchanges.22 However, recent studies have pointed out that in the COVID-19 epidemic response, articles submitted to preprint platforms have not been peer-reviewed by experts, and paper quality is worrying.23 For example, a paper by an Indian research team submitted to bioRxiv in February suggested that the new coronavirus may contain HIV inserts. The research team subsequently admitted it was incorrect and retracted the article. Therefore, the prospects for preprints and how to effectively use preprint literature in scientific research and emergency response to infectious diseases are matters worth discussing.
4.3. Many COVID-19-related scientific research issues remain unclear, so the fight against the pandemic urgently requires the cooperation of scientific researchers globally
From a global perspective, COVID-19 brought unprecedented challenges to public health systems. Although the epidemic in China, Japan, and South Korea has tended to be flattened by their effective prevention and control measures, the situations in Europe, North America, and other countries are still grim and those in Africa and South America are not yet optimized. Many related scientific research issues such as the natural origin, capacity and means of transmission, vaccine protection time and effective treatment of COVID-19 remain not fully clarified.9 There are also large differences in economic and social conditions, medical resources, and the ability to respond to public health security incidents in various countries.24, 25 The direction in which the COVID-19 epidemic will eventually go is unknown. Kissler et al. state that the epidemic may not end in one or two years, without effective treatment and vaccines, a strategy based on close contact tracking and effective isolation can reduce the incidence of SARS-COV-2, but the long-term development of the epidemic would have a huge impact on the medical systems and economies of various countries.26 To a certain extent, the publication of literature represents the corresponding scientific and technological level at the global and regional scale. The United States, China, Italy, and the United Kingdom contribute nearly 60% of the reports included in the WOS and nearly 80% of those published in the top journals. There is relatively more literature from Asia, Europe, and North America than from Africa and South America. And, there is an urgent need for the cooperation of governments and scientific researchers globally to jointly fight the epidemic.
CRediT authorship contribution statement
Panpan Wang: Data curation, Formal analysis, Writing - original draft Deqiao Tian: Conceptualization, Methodology, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bonilla-Aldana D.K., Quintero-Rada K., Montoya-Posada J.P. SARS-CoV, MERS-CoV and now the 2019-novel CoV: Have we investigated enough about coronaviruses? – a bibliometric analysis. Travel Med Infect Dis. 2020;33:101566. doi: 10.1016/j.tmaid.2020.101566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. The Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Available from: <https://www.who.int/dg/speeches/detail/who-director-general-s-opening -remarks-at-the-media-briefing-on-covid-19---11-march-2020>; 2020.
- 5.Nicola M., Alsafi Z., Sohrabi C. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surgery. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Coronavirus disease (COVID-19) outbreak situation. Available from: <https://www.who.int/emergencies/diseases/novel-coronavirus-2019>; (Jan 28, 2021).
- 7.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 8.Oberfeld B., Achanta A., Carpenter K. SnapShot: COVID-19. Cell. 2020;181(4):954-e1. doi: 10.1016/j.cell.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2020;(Oct 6):1–14. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durieux V., Gevenois P.A. Bibliometric indicators: quality measurements of scientific publication. Radiology. 2010;255(2):342–351. doi: 10.1148/radiol.09090626. [DOI] [PubMed] [Google Scholar]
- 11.WHO. COVID-19 Global literature on coronavirus disease. Available from: <https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/>; 2020.
- 12.Chen T, Rui J, Wang Q, Zhao Z, Cui J-A, Yin L. A mathematical model for simulating the transmission of Wuhan novel Coronavirus; 2020:2020.01.19.911669.
- 13.Read JM, Bridgen JR, Cummings DA, Ho A, Jewell CP. Novel coronavirus 2019-nCoV: early estimation of epidemiological parameters and epidemic predictions; 2020:2020.01.23.20018549. [DOI] [PMC free article] [PubMed]
- 14.Cheng Z.J., Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48(2):155–163. doi: 10.1007/s15010-020-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majumder M, Mandl KD. Early Transmissibility assessment of a novel coronavirus in Wuhan, China. SSRN[Preprint] 2020;Jan 24:3524675. [Google Scholar]
- 16.Azman A.S., Luquero F.J. From China: hope and lessons for COVID-19 control. Lancet Infect Dis. 2020;20(7):756–757. doi: 10.1016/S1473-3099(20)30264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiang Y.-T., Li W., Zhang Q. Timely research papers about COVID-19 in China. The Lancet. 2020;395(10225):684–685. doi: 10.1016/S0140-6736(20)30375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emma Stoye. China coronavirus: how many papers have been published? Available from: <https://www.nature.com/articles/d41586-020-00253-8>; (Jan 30, 2020). [DOI] [PubMed]
- 19.Pfefferbaum B., North C.S. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510–512. doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]
- 20.Johansson M.A., Reich N.G., Meyers L.A., Lipsitch M. Preprints: an underutilized mechanism to accelerate outbreak science. PLoS Med. 2018;15(4) doi: 10.1371/journal.pmed.1002549. e1002549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majumder M.S., Mandl K.D. Early in the epidemic: impact of preprints on global discourse about COVID-19 transmissibility. The Lancet Global Health. 2020;8(5):e627–e630. doi: 10.1016/S2214-109X(20)30113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarabipour S. Preprints are good for science and good for the public. Nature. 2018;560(7720):553. doi: 10.1038/d41586-018-06054-4. [DOI] [PubMed] [Google Scholar]
- 23.Kwon D. How swamped preprint servers are blocking bad coronavirus research. Nature. 2020;581(7807):130–131. doi: 10.1038/d41586-020-01394-6. [DOI] [PubMed] [Google Scholar]
- 24.Kelley M., Ferrand R.A., Muraya K., Chigudu S., Molyneux S., Pai M., Barasa E. An appeal for practical social justice in the COVID-19 global response in low-income and middle-income countries. The Lancet Global Health. 2020;8(7):e888–e889. doi: 10.1016/S2214-109X(20)30249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandel N., Chungong S., Omaar A., Xing J. Health security capacities in the context of COVID-19 outbreak: an analysis of International Health Regulations annual report data from 182 countries. The Lancet. 2020;395(10229):1047–1053. doi: 10.1016/S0140-6736(20)30553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]