Abstract
Background
The indirect impact of the COVID-19 pandemic on cancer outcomes is of increasing concern. However, the extent to which key treatment modalities have been affected is unclear. We aimed to assess the impact of the pandemic on radiotherapy activity in England.
Methods
In this population-based study, data relating to all radiotherapy delivered for cancer in the English NHS, between Feb 4, 2019, and June 28, 2020, were extracted from the National Radiotherapy Dataset. Changes in mean weekly radiotherapy courses, attendances (reflecting fractions), and fractionation patterns following the start of the UK lockdown were compared with corresponding months in 2019 overall, for specific diagnoses, and across age groups. The significance of changes in radiotherapy activity during lockdown was examined using interrupted time-series (ITS) analysis.
Findings
In 2020, mean weekly radiotherapy courses fell by 19·9% in April, 6·2% in May, and 11·6% in June compared with corresponding months in 2019. A relatively greater fall was observed for attendances (29·1% in April, 31·4% in May, and 31·5% in June). These changes were significant on ITS analysis (p<0·0001). A greater reduction in treatment courses between 2019 and 2020 was seen for patients aged 70 years or older compared with those aged younger than 70 years (34·4% vs 7·3% in April). By diagnosis, the largest reduction from 2019 to 2020 in treatment courses was for prostate cancer (77·0% in April) and non-melanoma skin cancer (72·4% in April). Conversely, radiotherapy courses in April, 2020, compared with April, 2019, increased by 41·2% in oesophageal cancer, 64·2% in bladder cancer, and 36·3% in rectal cancer. Increased use of ultra-hypofractionated (26 Gy in five fractions) breast radiotherapy as a percentage of all courses (0·2% in April, 2019, to 60·6% in April, 2020; ITS p<0·0001) contributed to the substantial reduction in attendances.
Interpretation
Radiotherapy activity fell significantly, but use of hypofractionated regimens rapidly increased in the English NHS during the first peak of the COVID-19 pandemic. An increase in treatments for some cancers suggests that radiotherapy compensated for reduced surgical activity. These data will assist health-care providers in understanding the indirect consequences of the pandemic and the role of radiotherapy services in minimising these consequences.
Funding
None.
Introduction
The indirect consequences of the COVID-19 pandemic are of increasing concern. The UK was one of the most severely affected countries in Europe during the first wave of the pandemic. As cases escalated in March, 2020, the National Health Service (NHS) restructured in anticipation of large numbers of inpatients requiring respiratory support.1 Similar steps were taken by health-care providers globally.2
The impact of this pivot towards COVID-19, which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), for patients with cancer is of particular concern given their need for timely diagnosis, treatment, and symptom palliation. Alongside surgery and systemic anticancer therapy, radiotherapy plays a major part both as a curative treatment and in the palliation of localised symptoms from advanced disease. It is estimated that a third of all patients with cancer in the UK will receive radiotherapy during their disease course.3 At the outset of the pandemic, all three treatment modalities were affected by constraints on COVID-19 testing and staff shortages. Surgical services faced additional pressure as a consequence of the adaptation of theatre space for the care of acutely unwell patients requiring ventilation.
In response to these pressures, service providers, commissioners, and professional bodies within the UK and internationally issued revised guidance for cancer care. Drawing on evidence and expert consensus, these guidelines also addressed concerns about in-hospital transmission of SARS-CoV-2 and the potentially heightened risks of infection during cancer treatment.4, 5 Within these site-specific guidelines, suggestions included treatment omission or delay, the use of radiotherapy to replace or to bridge to surgery, and the wider use of short, high daily dose (hypofractionated) radiotherapy.5
Research in context.
Evidence before this study
The indirect consequences of the COVID-19 pandemic on the care of patients with cancer are of concern. However, the extent to which radiotherapy services were affected is unclear. To identify studies reporting on changes in radiotherapy activity during the COVID-19 pandemic, we searched PubMed for articles published in English between Jan 1 and Oct 1, 2020, using the search terms (“cancer” or “malignancy”) AND (“radiation therapy” OR “radiotherapy”) AND (“COVID-19” OR “coronavirus” OR “SARS-CoV-2”). So far, only analyses of radiotherapy activity across single or small numbers of centres, or larger survey-based studies assessing changes to radiotherapy practice have been undertaken. These studies are at risk of responder bias and are not sufficiently comprehensive to detail changes in radiotherapy activity or prescriptions for individual cancers, or to quantify how these have varied as the pandemic has progressed.
Added value of this study
To our knowledge, this is the first comprehensive, nationwide analysis of radiotherapy activity during the first wave of the COVID-19 pandemic, from national lockdown on March 23 to June 28, 2020. We show an overall decrease in radiotherapy activity in the English National Health Service over this period. This decline is predominantly attributable to a reduction in treatments for prostate and non-melanoma skin cancer—malignancies for which there is evidence for the safety of treatment delay. By contrast, treatments for oesophageal, bladder, and rectal cancers markedly increased. We also demonstrate an increase in the use of hypofractionated regimens. Radiotherapy activity remained suppressed up to June, 2020, which might reflect delays in cancer diagnostic pathways.
Implications of all the available evidence
Although radiotherapy activity decreased during the first wave of the pandemic, our data suggest that the overall impact of this decline is likely to be modest. In addition, radiotherapy appears to have mitigated against some of the indirect harms of the pandemic by maintaining curative treatment options despite the challenges facing surgical services. As COVID-19 cases again rise, these data are crucial for modelling indirect harms of the pandemic and establish a new baseline for radiotherapy treatments from which to plan for the ongoing delivery of care throughout subsequent pandemic waves and into the recovery beyond. They also reinforce the need to address any persisting delays in cancer diagnostic pathways.
As cases of COVID-19 have risen again, it is important to understand the indirect consequences of the first pandemic peak. However, currently, our understanding of changes to radiotherapy practice is limited to a small number of surveys of radiation oncology centres in Europe, the USA, and Latin America.6, 7, 8 These studies are at risk of responder bias, have limited information about individual cancers and regimen use, and are not able to quantify longitudinal changes during the pandemic. In the absence of this information, the indirect harms of the pandemic cannot be accurately modelled. Such data are also required by service providers, commissioners, and clinicians to mitigate against these indirect consequences. Mitigation measures include identifying cohorts of patients for whom treatment has been modified and who as a consequence might require tailored clinical follow-up, and establishing a new baseline for radiotherapy treatment patterns from which planning for future waves and for the longer-term recovery of cancer services can be developed.
In England, all NHS radiotherapy providers submit data directly from their treatment delivery systems to Public Health England (PHE) on a monthly basis to form the National Radiotherapy Dataset (RTDS).9 This dataset contains information on more than 135 000 courses of radiotherapy delivered across the English NHS each year. In this study, we used the RTDS to explore changes in radiotherapy activity during the first peak in COVID-19 cases.
Methods
Study design
In this population-based study, we analysed radiotherapy activity across all 52 English NHS radiotherapy providers during the year before the pandemic and during the first wave of COVID-19 cases, from the date of the beginning of the UK lockdown on March 23 to June 28, 2020. PHE routinely collects data on the diagnosis and treatment of patients with cancer within the NHS under section 251 of the Health and Social Care act (2006). There is limited radiotherapy capacity in England outside these NHS centres. Study-specific ethical approval was not sought for this work, which was considered to be operational research within PHE's core remit.
Data sources
We extracted data for all external beam radiotherapy courses and attendances (which closely align to fractions) delivered to patients with cancer between Feb 4, 2019, and June 28, 2020. All ages and tumour sites were included.
Data items from RTDS used within this analysis were the diagnosis for which the treatment was delivered (defined using the 10th revision of the International Classification of Diseases [ICD-10]10) allocated to clinically appropriate groupings (eg, head and neck cancer), as detailed in the appendix (p 1); patient age and sex; treatment intent, defined by the treating clinician as curative (including both primary or radical and adjuvant), palliative, or other (including treatments for which intent was not submitted); planned dose in Gy; planned fractionation; date of course start; attendance dates; and provider organisation.
Data analysis
Radiotherapy courses were allocated to the week in which they started and attendances (fractions) to the week in which they occurred. We defined weeks using the International Organization for Standardization calendar and allocated them to the month in which they began. We defined radiotherapy activity by the mean weekly number of treatment courses and attendances per month. We calculated percentage change in activity for each month between February and June, 2020, compared with the equivalent month in 2019 (ordinarily, limited year-on-year variation in activity is anticipated). This approach ensured minor weekly fluctuations were smoothed across each month and that comparisons recognised seasonality and bank holiday periods. During the study period, between one and four providers per month had not submitted data at the time of data extraction (appendix p 1). To adjust for this missing data, we incorporated additional activity on the basis of the proportion of activity delivered by the missing centres in months for which complete data were available.
We examined the differences in courses and attendances compared with 2019 by provider, treatment intent, age, sex, diagnosis, and region. Given the known increase in risk of adverse COVID-19 outcomes with age,11 data were dichotomised at 70 years, in line with UK shielding advice.12
We assessed the significance of the change in activity following lockdown using interrupted time-series (ITS) analysis with multivariable generalised linear regression models.13 Lockdown and easing of lockdown were specified as binary variables, applied to all timepoints beyond March 23, 2020 (the date of the start of the lockdown) and June 1, 2020 (when some English schools partly reopened), respectively. These terms were interacted with time to parameterise the slope beyond the initial lockdown and lockdown easing. Adjustment was made for weeks that included a bank holiday, Christmas in 2019 (parameterised separately because this week includes two bank holidays), and seasonal variation (incorporated using Fourier terms; appendix p 2).13 We used Newey-West errors to recognise autoregressive errors and heteroscedasticity. Model predictions are presented graphically alongside the observed weekly course and attendance numbers. Separate models were fitted to both the adjusted (recognising missing data) and observed data (sensitivity analysis). Significance was defined as a p value of less than 0·05. The ITS model was used to predict the reduction in courses delivered between March 23 and June 28, 2020, following adjustment for missing data, with 95% CIs defined based on the linear model predictions.
We assessed change in treatment fractionation for patients aged 18 years or older for specified diagnoses (appendix p 1). Radical treatments were grouped into categories based on the prescribed dose per fraction (<2 Gy per fraction, standard fractionation [2·00–2·49 Gy per fraction], mild-to-moderate hypofractionation [2·5–4·9 Gy per fraction], and ultra-hypofractionation [≥5 Gy per fraction]). The proportion of activity delivered using each categorisation was assessed by diagnosis. Palliative treatments delivered to patients with these diagnoses were separately grouped as single fraction, two to five fractions, six to ten fractions, and greater than ten fractions, and then analysed similarly.
Based on the extensive changes observed in fractionation patterns delivered for breast cancer, we investigated these patterns further using ITS analysis. This analysis was done as detailed previously using a Poisson distribution to allow recognition of the small number of courses per week for some regimens.
All statistical analyses were carried out using StataIC 64, version 16. Data are presented graphically using Stata1C 64, Tableau, and Excel.
Role of the funding source
There was no funding source for this study.
Results
The mean weekly number of radiotherapy courses delivered across the English NHS in 2019 was 2570 (SD 246). This number fell by 502 (−19·9%) in April, 2020, from 2526 (SD 178) in April, 2019 (table 1 ; appendix p 2). A fall of 151 (−6·2%) from 2425 (SD 172) was observed in May and 307 (−11·6%) from 2633 (60) in June, 2020. By comparison, greater reductions were observed for treatment attendances when compared with equivalent months in 2019: a fall of 10 290 (−29·1%) from 35 332 (SD 2544) in April, 10 573 (−31·4%) from 33 665 (2776) in May, and 11 380 (−31·5%) from 36 130 (233) in June, 2020.
Table 1.
Mean weekly courses and attendances in the months before and after the start of the first UK lockdown on March 23, 2020
|
Courses |
Attendances |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| February, 2020 | March, 2020 | April, 2020 | May, 2020 | June, 2020 | February, 2020 | March, 2020 | April, 2020 | May, 2020 | June, 2020 | |
| Total | ||||||||||
| Observed | 2631 | 2449 | 2024 | 2274 | 2130 | 36 121 | 34 716 | 25 042 | 23 092 | 22 631 |
| Adjusted | 2659 | 2449 | 2024 | 2274 | 2326 | 36 489 | 34 716 | 25 042 | 23 092 | 24 750 |
| SD | 107 | 239 | 226 | 246 | 59 | 514 | 2460 | 1017 | 1665 | 594 |
| Percentage change | −2·9% | −7·8% | −19·9% | −6·2% | −11·6% | −2·4% | −8·5% | −29·1% | −31·4% | −31·5% |
| Palliative | ||||||||||
| Observed | 847 | 827 | 653 | 789 | 788 | 3593 | 3380 | 2270 | 2623 | 2771 |
| Adjusted | 855 | 827 | 653 | 789 | 861 | 3629 | 3380 | 2270 | 2623 | 3030 |
| SD | 33 | 60 | 75 | 113 | 65 | 162 | 415 | 228 | 355 | 241 |
| Percentage change | 1·0% | −1·8% | −20·1% | −1·5% | −5·3% | 0·7% | −7·0% | −35·7% | −20·9% | −20·4% |
| Radical | ||||||||||
| Observed | 1774 | 1614 | 1362 | 1474 | 1332 | 32 426 | 31 227 | 22 684 | 20 376 | 19 792 |
| Adjusted | 1793 | 1614 | 1362 | 1474 | 1454 | 32 756 | 31 227 | 22 684 | 20 376 | 21 645 |
| SD | 83 | 206 | 152 | 137 | 16 | 570 | 2054 | 950 | 1355 | 364 |
| Percentage change | −3·1% | −9·3% | −19·4% | −8·7% | −15·2% | −2·2% | −8·2% | −28·3% | −32·6% | −32·9% |
| Other* | ||||||||||
| Observed | 11 | 9 | 9 | 10 | 10 | 103 | 108 | 88 | 93 | 68 |
| Adjusted | 11 | 9 | 9 | 10 | 11 | 104 | 108 | 88 | 93 | 75 |
| SD | 5 | 3 | 4 | 1 | 2 | 14 | 8 | 11 | 15 | 14 |
| Percentage change | −74·3% | −74·5% | −54·4% | 2·5% | 36·5% | −62·9% | −61·6% | −50·2% | −26·2% | −16·4% |
| Females aged younger than 70 years | ||||||||||
| Observed | 848 | 829 | 797 | 839 | 718 | 12 196 | 11 691 | 8974 | 8382 | 7459 |
| Adjusted | 857 | 829 | 797 | 839 | 784 | 12 320 | 11 691 | 8974 | 8382 | 8157 |
| SD | 47 | 50 | 99 | 101 | 19 | 143 | 516 | 400 | 743 | 229 |
| Percentage change | −2·6% | −2·8% | −2·2% | 5·3% | −8·2% | 3·0% | −7·3% | −23·4% | −25·7% | −33·6% |
| Females aged 70 years or older | ||||||||||
| Observed | 487 | 471 | 339 | 419 | 349 | 5464 | 5241 | 3526 | 3347 | 3100 |
| Adjusted | 492 | 471 | 339 | 419 | 381 | 5520 | 5241 | 3526 | 3347 | 3390 |
| SD | 31 | 48 | 21 | 67 | 5 | 107 | 443 | 233 | 362 | 136 |
| Percentage change | −2·3% | −1·8% | −25·8% | −2·1% | −18·9% | −2·9% | −6·9% | −31·9% | −32·6% | −36·1% |
| Males aged younger than 70 years | ||||||||||
| Observed | 584 | 518 | 459 | 508 | 488 | 9159 | 8905 | 6885 | 6560 | 6590 |
| Adjusted | 590 | 518 | 459 | 508 | 533 | 9252 | 8905 | 6885 | 6560 | 7207 |
| SD | 20 | 49 | 73 | 39 | 27 | 284 | 723 | 227 | 346 | 362 |
| Percentage change | 0·3% | −10·0% | −15·1% | −2·7% | −6·7% | −2·8% | −6·8% | −22·8% | −23·0% | −19·2% |
| Males aged 70 years or older | ||||||||||
| Observed | 713 | 631 | 429 | 508 | 575 | 9302 | 8878 | 5657 | 4803 | 5483 |
| Adjusted | 720 | 631 | 429 | 508 | 628 | 9397 | 8878 | 5657 | 4803 | 5996 |
| SD | 45 | 127 | 42 | 56 | 62 | 125 | 820 | 365 | 285 | 585 |
| Percentage change | −6·1% | −15·6% | −39·9% | −25·1% | −14·8% | −8·0% | −12·5% | −40·6% | −46·1% | −37·7% |
Observed weekly mean, weekly mean adjusted for missing data with SD, and percentage change compared with corresponding month of 2019.
Cancers for which treatment intent was not specified by the clinician; numbers in this group dropped steeply in 2019 with improvements to data collection, although, given their low frequency, this change is unlikely to have had a significant effect on the results more widely.
Substantial variation was seen across radiotherapy providers in both the direction and magnitude of change in mean weekly courses compared with the corresponding months in 2019, ranging from −53·5% to 13·3% in April, 2020, −45·7% to 15·4% in May, 2020, and −28·7% to 31·9% in June, 2020 (appendix p 8). All regions of the country saw a fall in courses in April with subsequent recovery, although the extent of this recovery varied (appendix p 9).
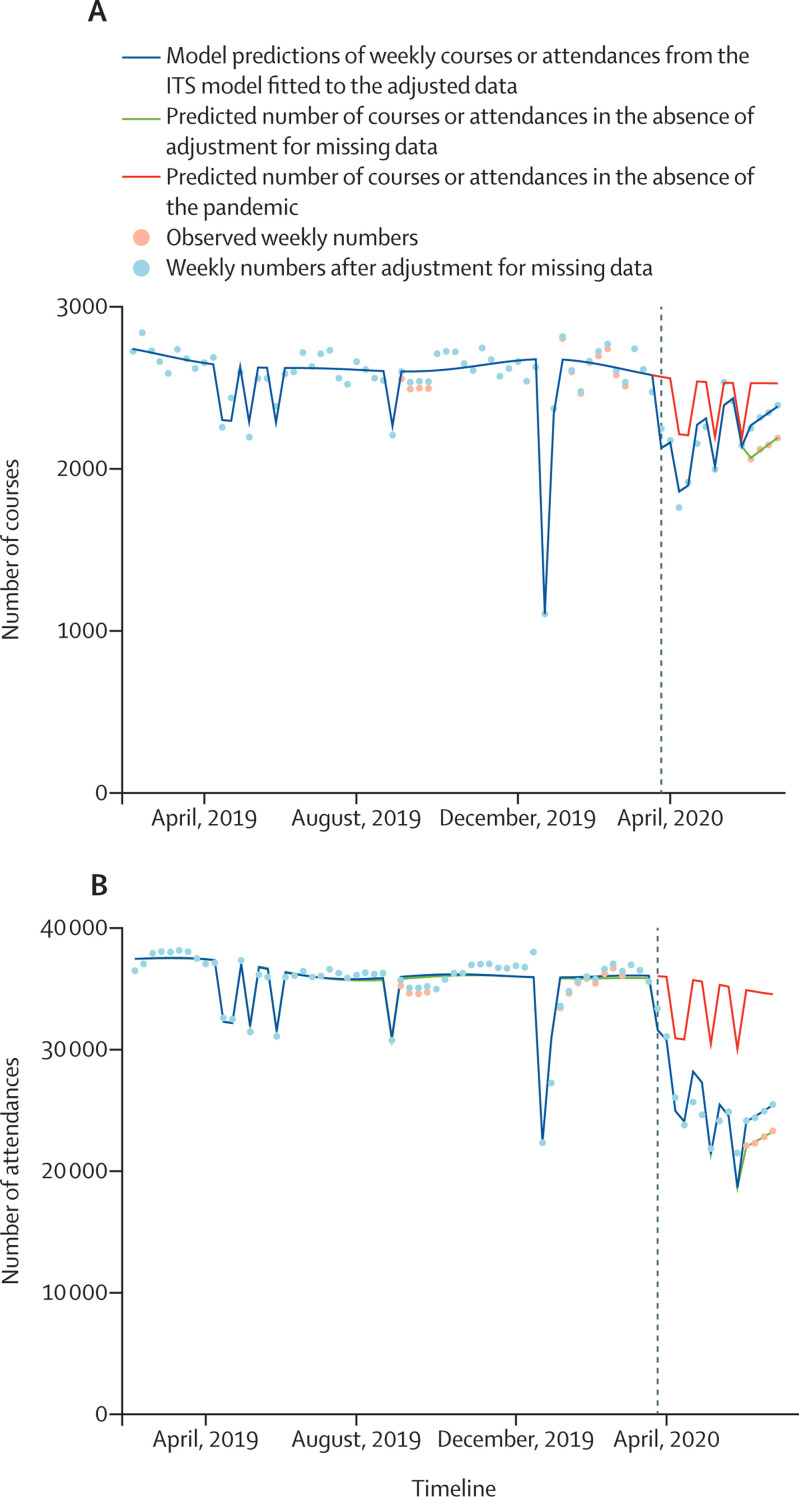
On ITS analyses, after adjustment for missing data, lockdown was associated with a significant reduction in courses and attendances (p<0·0001) for pandemic terms relating to both the change in activity at the point of lockdown and slope immediately afterwards. Model outputs are presented in figure 1 and the appendix (pp 4–5). Between March 23 and June 28, 2020, a predicted 3263 (95% CI 2936–3590) fewer treatment courses and 119 050 (112 632–125 470) fewer attendances were delivered in England than would have been expected had the pandemic not occurred.
Figure 1.
Courses (A) and attendances (B) of radiotherapy delivered within the English NHS over the year preceding and period following the first UK lockdown for the COVID-19 pandemic
The dashed line indicates the beginning of the lockdown on March 23, 2020. ITS=interrupted time series. NHS=National Health Service.
Changes in mean weekly curative treatment courses and attendances by diagnosis, are provided in table 2 and the appendix (pp 3, 10–11). The largest reduction in courses in 2020 was observed in prostate cancer (with a decrease of 266 [–77·0%] in April, 2020, from 346 [SD 43] in April, 2019) and non-melanoma skin cancer (a decrease of 58 [–72·4%] from 80 [SD 16], for the same period). Conversely, marked increases in the number of courses in 2020, compared with the equivalent months in 2019, were seen in other diagnoses: bladder cancer courses increased by 18 (64·2%) from 27 (SD 5) in April; oesophageal cancer courses increased by 14 (41·2%) from 32 (10) in April; and rectal cancer courses increased by 25 (36·3%) from 69 (11) in April. Attendances were similarly affected, although compared with courses relatively greater reductions were observed in breast cancer, rectal cancer, lymphoma, and palliative treatments (table 2; appendix pp 10–11).
Table 2.
Mean weekly number of radical episodes and attendances by month by diagnosis
|
Courses |
Attendances |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| February, 2020 | March, 2020 | April, 2020 | May, 2020 | June, 2020 | February, 2020 | March, 2020 | April, 2020 | May, 2020 | June, 2020 | |
| Anal cancer | ||||||||||
| Observed | 22 | 25 | 23 | 20 | 11 | 615 | 614 | 635 | 598 | 382 |
| Adjusted | 22 | 25 | 23 | 20 | 12 | 622 | 614 | 635 | 598 | 418 |
| SD | 6 | 5 | 7 | 4 | 1 | 45 | 25 | 27 | 49 | 63 |
| Percentage change | −4·4% | 21·5% | 9·7% | 2·6% | −48·9% | 11·2% | 8·7% | 16·7% | 12·5% | −27·2% |
| Bladder cancer | ||||||||||
| Observed | 20 | 28 | 45 | 55 | 26 | 370 | 521 | 730 | 992 | 674 |
| Adjusted | 20 | 28 | 45 | 55 | 28 | 373 | 521 | 730 | 992 | 737 |
| SD | 5 | 3 | 5 | 11 | 6 | 29 | 61 | 125 | 85 | 133 |
| Percentage change | −7·3% | −1·4% | 64·2% | 143·3% | 17·1% | −27·9% | 3·7% | 37·4% | 87·0% | 48·8% |
| Brain cancer | ||||||||||
| Observed | 58 | 51 | 43 | 42 | 41 | 1325 | 1145 | 948 | 845 | 817 |
| Adjusted | 58 | 51 | 43 | 42 | 45 | 1338 | 1145 | 948 | 845 | 893 |
| SD | 9 | 7 | 7 | 4 | 7 | 76 | 67 | 55 | 80 | 66 |
| Percentage change | −10·6% | −24·7% | −19·9% | −28·4% | −22·6% | 10·6% | −20·0% | −26·7% | −25·1% | −33·0% |
| Breast cancer | ||||||||||
| Observed | 634 | 597 | 570 | 618 | 493 | 9617 | 9289 | 6036 | 5400 | 4828 |
| Adjusted | 640 | 597 | 570 | 618 | 539 | 9715 | 9289 | 6036 | 5400 | 5279 |
| SD | 41 | 43 | 92 | 90 | 35 | 362 | 556 | 347 | 640 | 326 |
| Percentage change | 1·3% | −4·5% | −4·5% | 6·6% | −12·5% | 2·8% | −5·7% | −34·8% | −39·9% | −45·4% |
| Cervical cancer | ||||||||||
| Observed | 21 | 21 | 22 | 17 | 13 | 520 | 471 | 563 | 464 | 360 |
| Adjusted | 21 | 21 | 22 | 17 | 14 | 525 | 471 | 563 | 464 | 394 |
| SD | 4 | 7 | 5 | 5 | 4 | 48 | 30 | 15 | 49 | 18 |
| Percentage change | −10·9% | 6·3% | 0·7% | −37·7% | −32·7% | −9·9% | −16·6% | 12·2% | −19·3% | −37·6% |
| Head and neck cancer | ||||||||||
| Observed | 122 | 120 | 132 | 116 | 80 | 3249 | 3337 | 3415 | 3319 | 2467 |
| Adjusted | 124 | 120 | 132 | 116 | 87 | 3282 | 3337 | 3415 | 3319 | 2698 |
| SD | 14 | 16 | 23 | 23 | 3 | 98 | 62 | 147 | 205 | 249 |
| Percentage change | −0·2% | 4·2% | 9·7% | 4·0% | −25·6% | −6·6% | −2·9% | 5·1% | 3·4% | −18·8% |
| Lung cancer | ||||||||||
| Observed | 135 | 140 | 139 | 147 | 102 | 1955 | 2089 | 1893 | 1884 | 1350 |
| Adjusted | 136 | 140 | 139 | 147 | 111 | 1974 | 2089 | 1893 | 1884 | 1476 |
| SD | 7 | 13 | 13 | 22 | 19 | 77 | 72 | 80 | 155 | 158 |
| Percentage change | 6·8% | 8·5% | −1·9% | 10·8% | −11·5% | 8·2% | 9·0% | −5·8% | −0·1% | −21·5% |
| Lymphoma | ||||||||||
| Observed | 55 | 52 | 40 | 49 | 45 | 760 | 706 | 523 | 497 | 556 |
| Adjusted | 56 | 52 | 40 | 49 | 49 | 768 | 706 | 523 | 497 | 608 |
| SD | 5 | 9 | 7 | 9 | 4 | 31 | 56 | 48 | 84 | 5 |
| Percentage change | 5·9% | −5·5% | −19·2% | 23·1% | −1·7% | 12·2% | −10·8% | −25·1% | −14·2% | −7·9% |
| Oesophageal cancer | ||||||||||
| Observed | 24 | 30 | 46 | 43 | 25 | 519 | 621 | 735 | 1110 | 658 |
| Adjusted | 25 | 30 | 46 | 43 | 28 | 524 | 621 | 735 | 1110 | 720 |
| SD | 2 | 8 | 17 | 11 | 2 | 19 | 18 | 142 | 81 | 109 |
| Percentage change | −14·8% | 1·9% | 41·2% | 71·3% | −3·2% | −9·1% | −7·3% | 9·5% | 79·8% | 18·8% |
| Other cancer diagnosis | ||||||||||
| Observed | 148 | 124 | 107 | 113 | 115 | 2642 | 2284 | 1858 | 1837 | 1886 |
| Adjusted | 149 | 124 | 107 | 113 | 125 | 2668 | 2284 | 1858 | 1837 | 2062 |
| SD | 14 | 8 | 19 | 12 | 10 | 93 | 221 | 96 | 92 | 117 |
| Percentage change | 2·4% | −8·0% | −17·8% | 2·3% | −3·6% | 9·5% | −11·0% | −19·2% | −15·9% | −10·2% |
| Prostate cancer | ||||||||||
| Observed | 372 | 285 | 80 | 144 | 285 | 8471 | 7958 | 3706 | 2174 | 4595 |
| Adjusted | 375 | 285 | 80 | 144 | 311 | 8557 | 7958 | 3706 | 2174 | 5025 |
| SD | 30 | 115 | 11 | 54 | 44 | 249 | 976 | 852 | 224 | 1125 |
| Percentage change | −10·9% | −25·3% | −77·0% | −58·0% | −13·7% | −12·4% | −14·3% | −55·7% | −72·5% | −39·7% |
| Rectal cancer | ||||||||||
| Observed | 72 | 76 | 94 | 80 | 37 | 1435 | 1445 | 1252 | 907 | 602 |
| Adjusted | 73 | 76 | 94 | 80 | 41 | 1449 | 1445 | 1252 | 907 | 658 |
| SD | 7 | 9 | 24 | 14 | 9 | 30 | 68 | 82 | 171 | 41 |
| Percentage change | −5·2% | −5·7% | 36·3% | 22·3% | −43·7% | 9·5% | 0·2% | −8·6% | −29·3% | −55·8% |
| Non-melanoma skin cancer | ||||||||||
| Observed | 94 | 65 | 22 | 34 | 59 | 949 | 747 | 389 | 352 | 618 |
| Adjusted | 94 | 65 | 22 | 34 | 64 | 959 | 747 | 389 | 352 | 676 |
| SD | 18 | 24 | 3 | 8 | 6 | 84 | 133 | 56 | 59 | 104 |
| Percentage change | −12·7% | −29·2% | −72·4% | −57·8% | −28·4% | −8·5% | −24·7% | −52·5% | −56·7% | −28·8% |
Observed weekly mean, weekly mean adjusted for missing data with SD, and percentage change compared with the corresponding month in 2019 based on adjusted data are presented. Where diagnoses including small numbers of courses are considered, the adjustment for missing data must be interpreted with caution.
The mean weekly number of treatment courses delivered to patients aged 70 years or older in April, 2020, fell by 403 (−34·4%) to 768 (SD 63) from 1171 (94) in April, 2019. A smaller reduction was seen in patients younger than 70 years (a fall of 99 [–7·3%] to 1256 [SD 166] in April, 2020, from 1355 [92] in April, 2019). Of these patients, a weekly mean of less than 12 courses were delivered to patients aged younger than 18 years throughout the study period. Given these small numbers of courses, temporal changes over time were not assessed in patients younger than 18 years. The differential effect of age was most notable in patients with breast cancer (mean weekly courses falling by 59 [–32·5%] to a mean of 123 [SD 8] in April, 2020, from 182 [SD 25] in April, 2019, in those aged 70 years or older vs increasing by one [0·3%] from a mean of 513 [SD 42] in April, 2019, to 515 [86] in April, 2020, in those younger than 70 years), and non-melanoma skin cancer (falling by 57 [–71·0%] from a mean of 80 [14] in April, 2019, to 23 [4] in April, 2020, in those aged 70 years or older and six [–52·9%] from a mean of 12 [4] in April, 2019, to six [1] in April, 2020, in those younger than 70 years; appendix pp 12–14).
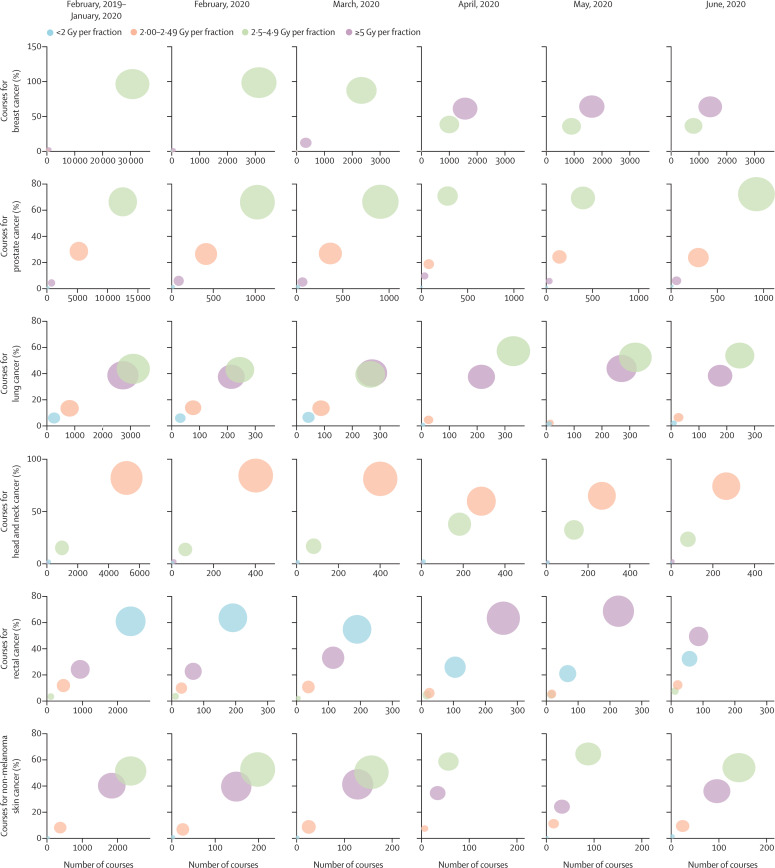
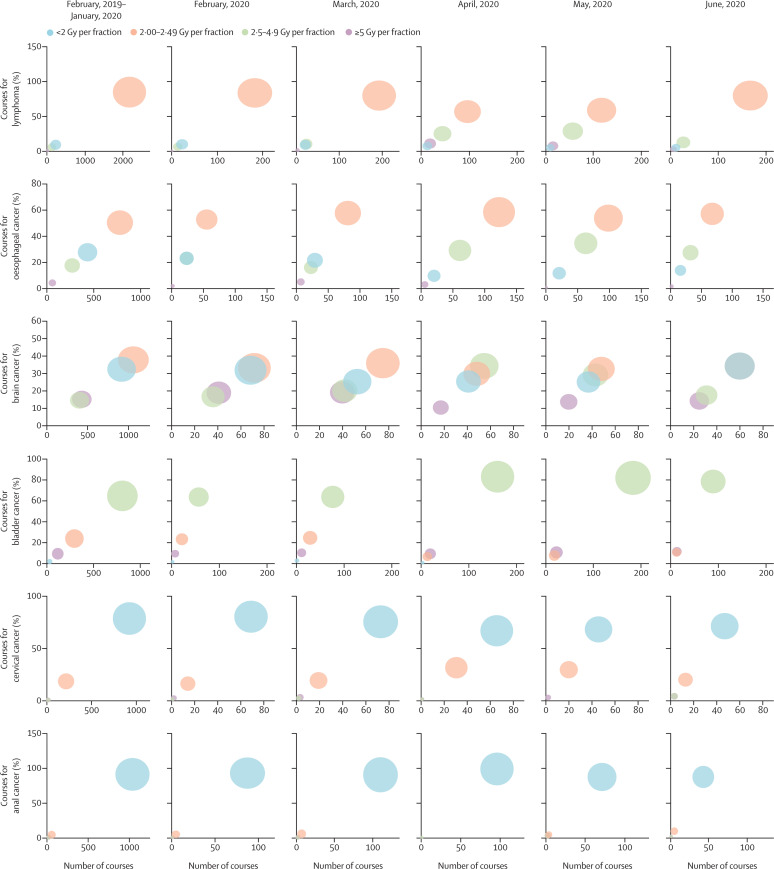
In April, 2019, a mean 949 (SD 78; 60·9%) of 1558 (121) weekly curative courses delivered to patients aged 18 years or older used a mild-to-moderately hypofractionated regimen (2·5–4·9 Gy per fraction). In 2020, these numbers fell to 486 (SD 32; 38·9%) of 1250 (140) mean weekly courses in April and remained low in May (543 [65; 39·9%] of 1360 [128]) and June (528 [17; 43·4%] of 1215 [19]). By contrast, ultra-hypofractionation (≥5 Gy per fraction) increased from 146 (SD 25; 9·4%) of 1558 (121) mean weekly courses in April, 2019, to 498 (113; 39·9%) of 1250 (140) mean weekly courses in April, 2020, 545 (62; 40·0%) of 1360 (128) mean weekly courses in May, 2020, and 414 (27; 34·0%) of 1215 (19) mean weekly courses in June, 2020. Figure 2 and the appendix (p 6) show these results for individual diagnoses.
Figure 2.
Bubble plot showing the change in fractionation patterns over time for courses delivered with curative intent for a range of diagnoses
Diagnoses are presented in descending order of total number of courses. The size of the bubble reflects the number of treatments delivered using the specified fractionation category.
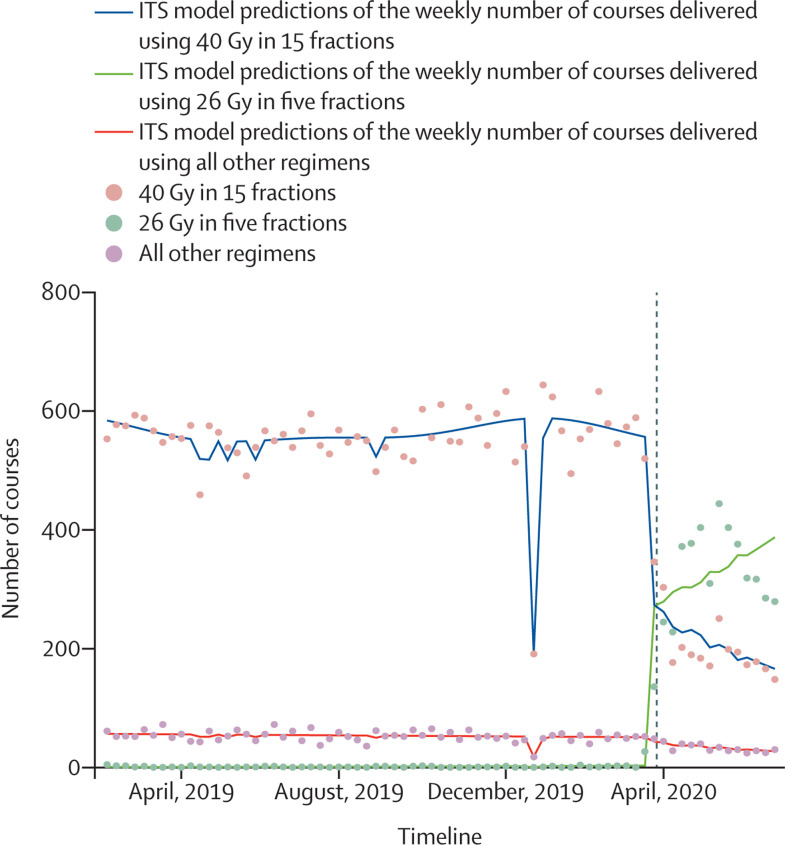
A major contributor to the increase in ultra-hypofractionation was the increased use of 26 Gy in five fractions for adjuvant breast cancer treatment. Whereas in April, 2019, one (SD 1; 0·2%) of 597 (54) mean weekly courses were delivered using this regimen, this number increased to 345 (79; 60·6%) of 570 (92) mean weekly courses in April, 2020. Conversely, the use of 40 Gy in 15 fractions fell from 546 (SD 49; 91·5%) of 597 (54) mean weekly courses to 188 (11; 33·0%) of 570 (92) mean weekly courses on the same comparison. ITS regression confirmed the significance of these changes (p<0·0001 for the change in use of 26 Gy in five fractions during lockdown; figure 3 ; appendix p 7). A marked increase in the use of ultra-hypofractionation in the neoadjuvant treatment of rectal cancer was also observed, with a reduction in the use of less than 2 Gy per fraction regimens.
Figure 3.
Change in fractionation patterns delivered for breast cancer across the English NHS before and after the first UK lockdown
Model predictions (lines) of the use of differing regimens for the adjuvant treatment of breast cancer with observed weekly courses (dots). The dashed line indicates the beginning of the lockdown on March 23, 2020. ITS=interrupted time series. NHS=National Health Service.
The proportion of palliative treatment courses delivered using a single fraction rose from a weekly mean of 223 (39·3%) of 568 (SD 37) in April, 2019, to 233 (50%) of 463 (62) in April, 2020, with a corresponding fall in treatments delivered using more than five fractions over the same period (125 [22·0%] of 568 [37] to 74 [16·0%] of 463 [62]).
Discussion
We have shown that the number of patients receiving radiotherapy in the English NHS fell significantly during the first wave of the COVID-19 pandemic. When compared with a year previously, a 20% reduction in radiotherapy courses was seen in April, 2020, immediately after the beginning of the UK national lockdown. Recovery was not complete by June, 2020 (12% reduction), despite the easing of lockdown and decrease in number of NHS inpatients with COVID-19. We project that compared with the same period a year previously (March 23 to June 28, 2019), 3263 fewer treatment courses were delivered with 119 050 fewer treatment attendances across the English NHS. The disproportionately greater fall in treatment attendances largely reflects a rapid increase in the use of ultra- hypofractionated treatment regimens across several tumour sites.
These analyses are based on a comprehensive national dataset. However, the reduction in activity that we report compares favourably with the more limited surveys of radiation oncology departments undertaken following the first pandemic peak in both the USA and Europe.7, 8 An approximate 25% reduction in patient volume was, for example, reported for centres in Europe, including the UK, with patient volume in the USA predicted to be a third lower. By contrast, at 8%, the reported median reduction in patient volume in Latin America is far more modest than observed in the UK.6
Beyond overall changes in radiotherapy activity, we also highlight how these changes varied by age group and diagnosis. At the onset of the pandemic, several professional bodies issued guidance for safely maintaining radiotherapy services.4, 5 A key concern at the time related to the potential for hospitals to act as a reservoir for SARS-CoV-2,14 and for a potentially heightened risk from COVID-19 for patients with cancer, particularly in those aged 70 years or older.11 Reflecting this concern, many guidelines advocated the deferral of treatment if it was safe to do so, or if the potential risks of treatment outweighed the benefits.
We demonstrate that treatment courses fell by a much a greater degree in patients aged 70 years or older than in patients younger than 70 years. This might partly be a consequence of decisions made by clinicians and patients to defer treatment in this higher-risk group. It might also in part reflect the age profile of patients with prostate cancer and non-melanoma skin cancer, for whom falls greater than anticipated from European surveys were observed. In prostate cancer, randomised evidence supports a delay in delivery of radiotherapy of up to 6 months between diagnosis and treatment if patients are commenced on androgen deprivation therapy, or even active surveillance in patients with low-risk disease.15, 16 The extent to which evidence supports treatment delays in non-melanoma skin cancer is less well defined, although for small basal cell carcinomas delay is unlikely to change the likelihood of cure.17 Additionally, a differential effect of age on treatment delivery was seen in breast cancer, potentially reflecting altered clinical decision making based on an assessment of risk and informed by the results of the PRIME-II trial.18 Similarly, specific concerns for adverse COVID-19 outcomes in patients with lung cancer might have contributed to the reduction in 2 Gy per fraction treatments (often delivered with concurrent chemotherapy) in favour of mild-to-moderate hypofractionation.
By contrast, a rise in curative courses was observed for rectal, bladder, and oesophageal cancer during April and May, 2020; cancers in which disease biology precludes substantial treatment delays. The increase in courses observed in this study might reflect the use of radiotherapy as an alternative definitive treatment approach to surgery. Several modelling studies have estimated large numbers of excess deaths due to limitations to surgical services.19, 20 However, these studies have not taken into account the use of radiotherapy in place of surgery, as shown here. Equally, although equipoise exists between radiotherapy and surgery for the treatment of bladder cancer and oesophageal squamous cell carcinoma, surgery is superior in oesophageal adenocarcinoma.21, 22 For rectal cancer, radiotherapy offers a mechanism to support delayed surgery with the potential for a substantial minority to avoid resection entirely. One immediate consequence of this shift in treatment patterns should be an urgent review of post-treatment surveillance protocols to ensure that patients who received an alternative treatment approach, and for whom cancer recurs can, where appropriate, be swiftly identified and referred for salvage resection. In the long-term, analysis of the outcomes of patients who have undergone radiotherapy in place of surgery, for reasons unrelated to their individual baseline condition, could provide valuable comparative data in settings in which randomisation between surgery and radiotherapy has historically been challenging.21
In line with guidance advocating reductions in hospital visits, treatment attendances fell significantly post-lockdown as a consequence of the wider use of hypofractionated radiotherapy. Most strikingly, the results of the FAST-Forward trial were incorporated into national guidelines supporting rapid and widespread adoption of a 26 Gy in five fractions regimen in place of the previous 40 Gy in 15 fractions standard for adjuvant breast cancer radiotherapy.23, 24 In this context, the decision in March, 2020, to move away from a per-attendance tariff for national radiotherapy commissioning is likely to have supported providers in rapidly adopting this new evidence base.1 In addition, as a UK-wide study, the experience of delivering these quality-assured hypofractionated regimens within a trial setting will likely have aided its rapid implementation.23 These changes show that, at least for some indications, the pandemic has beneficially catalysed the adoption of a new evidence base.
An increase in hypofractionation was also seen for palliative treatments, with half of these delivered as a single fraction in April, 2020. This change is appropriate and in keeping with evidence for most palliative indications. However, the concomitant reduction in the number of palliative treatment courses is concerning given the role of these treatments in improving quality of life for patients with localised symptoms due to advanced incurable cancer.25
Finally, in keeping with centres catching up on deferred treatments, prostate and non-melanoma skin cancer treatments were returning to baseline in June, 2020. Across a range of other diagnoses, despite smaller declines during lockdown, a reduction in activity was observed in June, 2020, compared with June, 2019. For some diagnoses (eg, cervical cancer), the temporary reduction or cessation of screening programmes might have played a part. However, NHS waiting time data demonstrate that in June, 2020, referrals for possible symptomatic cancer remained 21% below those in June, 2019. New diagnoses were suppressed by 26%, which is probably a key contributor to the ongoing suppression in radiotherapy activity up to June, 2020.26 Consistent with this finding, there was limited change in compliance with the 31-day treatment targets in radiotherapy, which remained above 95% throughout the study period.27 The time between diagnosis and commencing treatment might have limited the effect of the pandemic in May (compared with April and June), as previously diagnosed patients began their treatment. These results reinforce concerns about the effect of the COVID-19 pandemic on cancer diagnostic pathways and, in turn, outcomes.28, 29 This will require examination in the future, once complete cancer registration data are available.
To our knowledge, this is the first comprehensive national analysis of changes in radiotherapy provision during the first wave of the COVID-19 pandemic. Nevertheless, this study does have limitations. Data were only available for England and a lag in data collection and availability (of approximately 2–3 months) means more contemporaneous data are not available, so that longer-term changes in radiotherapy activity beyond the first wave of the pandemic cannot yet be seen. In addition, data were missing from four centres, which had not completed their activity submission in June, 2020. However, having adjusted for these missing data within our analyses, it is unlikely to substantially affect the conclusions reached. A small number of private providers deliver radiotherapy in England. Data from these providers are not routinely collected, so we cannot comment on the role of the private sector. Due to the limitations of and longitudinal changes in COVID-19 testing in England, it is extremely challenging to interpret the association between regional COVID-19 prevalence and radiotherapy delivery, and as such this was not attempted. Finally, although the RTDS provides robust data on the changes in courses and attendances for radiotherapy, it does not provide data on why these changes were made. Although assumptions can be made for the population as a whole, the data cannot provide definitive information on an individual patient level. Data relating to individual patient treatment decisions made during the COVID-19 pandemic will be collated by the UK National Cancer Research Institute Clinical and Translational Radiotherapy Research Working Group COVID radiotherapy initiative, and will be linked with other national datasets to determine the effect on patient outcomes.30
Radiotherapy activity in the English NHS fell significantly during the first wave of the COVID-19 pandemic. This decrease occurred predominantly in cancers for which treatment can be safely delayed and through the use of hypofractionation. By contrast, increased activity in specific diagnoses suggests that radiotherapy was used to compensate for reduced surgical activity. Overall, the effect on cancer outcomes of changes in radiotherapy activity during the first pandemic peak is likely to be modest, and an increase in radiotherapy use might have helped to mitigate against the loss of surgical capacity. However, the continued suppression in radiotherapy activity up to June, 2020, supports an urgent need to restore diagnostic pathways.
This online publication has been corrected. The corrected version first appeared at thelancet.com/oncology on June 1, 2021
Data sharing
The aggregate data and meta-data used in this analysis along with further information is available online at http://www.ncin.org.uk/cancer_type_and_topic_specific_work/topic_specific_work/covid19. Access to patient-level data can be arranged via https://www.gov.uk/government/publications/accessing-public-health-england-data/about-the-phe-odr-and-accessing-data. The data presented here are available to NHS clinicians and service managers via PHE's Cancerstats2 dashboard, which will be updated regularly. We look forward to working with clinicians to use these data in order to inform the process of recovery, restoration, and, where necessary, reconfiguration over the coming months.
Acknowledgments
Acknowledgments
No study-specific funding was provided for this analysis. Data for this analysis are based on patient-level information collected by the NHS, as part of the care and support of patients with cancer. The data are collated, maintained, and quality assured by the National Cancer Registration and Analysis Service, which is part of PHE. KS is funded through a University of Leeds University Academic Fellowship. CMJ is supported by a Wellcome Trust Clinical Research Fellowship (203914/Z/16/Z). EM's contribution was supported by Cancer Research UK (C23434/A23706), Health Data Research UK, and the National Institute for Health Research Oxford Biomedical Centre.
Contributors
KS led study design, analysed data, developed figures, interpreted data, and developed the initial manuscript draft. CMJ contributed to study design and figure development, interpreted data, and contributed to the writing of the manuscript. RG, CR, MS, and SL contributed to study design; data extraction, verification, and interpretation; and writing of the manuscript. RS contributed to study design and data analysis, interpreted the data, and reviewed the manuscript. LM contributed to the study design, data extraction, and figure development, and reviewed the final manuscript. PL, ME, DS-M, and TR contributed to the interpretation of the results and development of the manuscript. EM contributed to the study design, data analysis and interpretation, and development of the manuscript. All authors had full access to all of the data extracted from PHE, and the corresponding author had final responsibility for the decision to submit for publication.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Stevens S, Pritchard A. Next steps on NHS response to COVID-19. March 17, 2020. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/20200317-NHS-COVID-letter-FINAL.pdf
- 2.WHO In WHO global pulse survey, 90% of countries report disruptions to essential health services since COVID-19 pandemic. Aug 31, 2020. https://www.who.int/news/item/31-08-2020-in-who-global-pulse-survey-90-of-countries-report-disruptions-to-essential-health-services-since-covid-19-pandemic
- 3.Borras JM, Lievens Y, Dunscombe P, et al. The optimal utilization proportion of external beam radiotherapy in European countries: an ESTRO-HERO analysis. Radiother Oncol. 2015;116:38–44. doi: 10.1016/j.radonc.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence COVID-19 rapid guideline: delivery of radiotherapy. March 28, 2020. https://www.nice.org.uk/guidance/NG162 [PubMed]
- 5.Royal College of Radiologists Coronavirus (COVID-19): cancer treatment documents. https://www.rcr.ac.uk/college/coronavirus-covid-19-what-rcr-doing/clinical-information/coronavirus-covid-19-cancer
- 6.Martinez D, Sarria GJ, Wakefield D, et al. COVID's impact on radiation oncology: a Latin American survey study. Int J Radiat Oncol Biol Phys. 2020;108:374–378. doi: 10.1016/j.ijrobp.2020.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Society for Radiation Oncology COVID-19's impact on radiation oncology. Initial results of a nationwide physician survey, 5/20/20. May 20, 2020. https://www.astro.org/ASTRO/media/ASTRO/News%20and%20Publications/PDFs/ASTROCOVID19Survey1-ExecSummary.pdf
- 8.Slotman BJ, Lievens Y, Poortmans P, et al. Effect of COVID-19 pandemic on practice in European radiation oncology centers. Radiother Oncol. 2020;150:40–42. doi: 10.1016/j.radonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Registration and Analysis Service National Radiotherapy Dataset (RTDS) http://www.ncin.org.uk/collecting_and_using_data/rtds
- 10.WHO International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) version for 2010, chapter II. Neoplasms (C00–D48) http://apps.who.int/classifications/icd10/browse/2010/en#/II
- 11.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Health Service Who's at higher risk from coronavirus (COVID-19) https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/whos-at-higher-risk-from-coronavirus/
- 13.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–355. doi: 10.1093/ije/dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaorsky NG, Yu JB, McBride SM, et al. Prostate cancer radiation therapy recommendations in response to COVID-19. Adv Radiat Oncol. 2020;5:659–665. doi: 10.1016/j.adro.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisansky TM, Hunt D, Gomella LG, et al. Duration of androgen suppression before radiotherapy for localized prostate cancer: radiation therapy oncology group randomized clinical trial 9910. J Clin Oncol. 2015;33:332–339. doi: 10.1200/JCO.2014.58.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwan W, Wilson D, Moravan V. Radiotherapy for locally advanced basal cell and squamous cell carcinomas of the skin. Int J Radiat Oncol Biol Phys. 2004;60:406–411. doi: 10.1016/j.ijrobp.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Kunkler IH, Williams LJ, Jack WJL, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16:266–273. doi: 10.1016/S1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 19.Sud A, Jones ME, Broggio J, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020;31:1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai AG, Pasea L, Banerjee A, et al. Estimating excess mortality in people with cancer and multimorbidity in the COVID-19 emergency. medRxiv. 2020 doi: 10.1101/2020.05.27.20083287. published online June 1. (preprint). [DOI] [Google Scholar]
- 21.Huddart RA, Birtle A, Maynard L, et al. Clinical and patient-reported outcomes of SPARE—a randomised feasibility study of selective bladder preservation versus radical cystectomy. BJU Int. 2017;120:639–650. doi: 10.1111/bju.13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crosby T, Hurt CN, Falk S, et al. Long-term results and recurrence patterns from SCOPE-1: a phase II/III randomised trial of definitive chemoradiotherapy +/- cetuximab in oesophageal cancer. Br J Cancer. 2017;116:709–716. doi: 10.1038/bjc.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunt AM, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395:1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coles C, Aristei C, Bliss J, et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol. 2020;32:279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer K, Parrish R, Barton R, Henry A. Palliative radiotherapy. BMJ. 2018;360:k821. doi: 10.1136/bmj.k821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NHS Providers Government updates. https://nhsproviders.org/topics/covid-19/coronavirus-member-support/national-guidance/government-updates/daily-updates
- 27.NHS England Cancer waiting times. https://www.england.nhs.uk/statistics/statistical-work-areas/cancer-waiting-times/
- 28.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035–1044. doi: 10.1016/S1470-2045(20)30392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis PJ, Morris EJA, Chan CSK, Darley K, Sebag-Montefiore D, Evans M. COVID RT—assessing the impact of COVID-19 on radiotherapy in the UK. A National Cancer Research Institute Clinical and Translational Radiotherapy Research Working Group Initiative in Partnership with the Royal College of Radiologists, the Society of Radiographers and the Institute of Physics and Engineering in Medicine. Clin Oncol (R Coll Radiol) 2020 doi: 10.1016/j.clon.2020.08.008. published online Sept 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The aggregate data and meta-data used in this analysis along with further information is available online at http://www.ncin.org.uk/cancer_type_and_topic_specific_work/topic_specific_work/covid19. Access to patient-level data can be arranged via https://www.gov.uk/government/publications/accessing-public-health-england-data/about-the-phe-odr-and-accessing-data. The data presented here are available to NHS clinicians and service managers via PHE's Cancerstats2 dashboard, which will be updated regularly. We look forward to working with clinicians to use these data in order to inform the process of recovery, restoration, and, where necessary, reconfiguration over the coming months.