Abstract
Background
Reports suggest that asymptomatic individuals (those with no symptoms at all throughout infection) with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are infectious, but the extent of transmission based on symptom status requires further study.
Purpose
This living review aims to critically appraise available data about secondary attack rates from people with asymptomatic, pre-symptomatic and symptomatic SARS-CoV-2 infection.
Data sources
Medline, EMBASE, China Academic Journals full-text database (CNKI), and pre-print servers were searched from 30 December 2019 to 3 July 2020 using relevant MESH terms.
Study selection
Studies that report on contact tracing of index cases with SARS-CoV-2 infection in either English or Chinese were included.
Data extraction
Two authors independently extracted data and assessed study quality and risk of bias. We calculated the secondary attack rate as the number of contacts with SARS-CoV-2, divided by the number of contacts tested.
Data synthesis
Of 927 studies identified, 80 were included. Summary secondary attack rate estimates were 1% (95% CI 0%–2%) with a prediction interval of 0%–10% for asymptomatic index cases in ten studies, 7% (95% CI 3%–11%) with a prediction interval of 1%–40% for pre-symptomatic cases in 11 studies and 6% (95% CI 5%–8%) with a prediction interval of 5%–38% for symptomatic index cases in 40 studies. The highest secondary attack rates were found in contacts who lived in the same household as the index case. Other activities associated with transmission were group activities such as sharing meals or playing board games with the index case, regardless of the disease status of the index case.
Limitations
We excluded some studies because the index case or number of contacts were unclear.
Conclusion
Asymptomatic patients can transmit SARS-CoV-2 to others, but our findings indicate that such individuals are responsible for fewer secondary infections than people with symptoms.
Systematic review registration
PROSPERO CRD42020188168.
Keywords: Asymptomatic, Coronavirus disease 2019, Secondary attack rate, Severe acute respiratory syndrome coronavirus 2, Transmission
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) demonstrates efficient transmission in populations without effective public health interventions; basic reproduction numbers (R0) range between 2 and 3 [1]. Although asymptomatic transmission has been described as the ‘Achilles’ heel’ of control efforts during this pandemic, the extent to which transmission of SARS-CoV-2 by people without symptoms drives this pandemic remains uncertain [2]. SARS-CoV-2 infection that is asymptomatic at the time of laboratory testing is widely reported [3]; however, studies that follow infected people over time suggest that many infections are not asymptomatic throughout the entire disease course, and a large proportion of these individuals ultimately develop a diverse range of symptoms [[4], [5], [6], [7]]. For instance, Sugano et al. reported a detailed cluster outbreak in music clubs in Japan, where the asymptomatic cases reported also included pre-symptomatic cases [8]. A living systematic review of studies published up to 10 June 2020 estimated that 20% (95% CI 17%–25%) of people who become infected with SARS-CoV-2 remain asymptomatic throughout infection [7].
One of the barriers to understanding the role of asymptomatic transmission is the lack of consistency in case definitions [9]. Although symptom severity exists on a spectrum, individuals infected with SARS-CoV-2 can be miscategorized as asymptomatic, when they have milder or atypical symptoms, leading to overestimation of the proportion without symptoms [3,10]. For instance, in a detailed study of SARS-CoV-2 infections in Iceland where individuals deemed at high risk for coronavirus disease 2019 (COVID-19), including those with a consistent syndrome, were screened in a targeted manner, and other individuals were tested via a population screening mechanism, more than one-third of people in the second group reported symptoms potentially consistent with COVID-19 [3]. However, it is increasingly becoming clear that some individuals experience more diverse symptoms, including taste and smell disturbance or myalgia, either for the entire course of the illness or preceding respiratory symptoms. These symptoms can be so mild and insidious that they do not limit patients' daily activities [4,11]. The situation is further complicated by subjective patient perception and differences between studies in the elicitation and reporting of symptoms.
There are reports describing asymptomatic individuals with SARS-CoV-2 who are infectious [12] and who have infected one or more contacts [13], but the extent and significance of asymptomatic transmission requires further understanding. The aim of this review is to summarize the available evidence about secondary attack rates (defined as the probability that an infected individual will transmit the disease to a susceptible individual) among the contacts of individuals with SARS-CoV-2 with different symptom status to provide information about how contagious they are, and their role in driving the pandemic.
Materials and methods
This systematic review was registered in PROSPERO on 8 June 2020 (CRD42020188168) and will be updated in 4–6 months according to the availability of new evidence as a living systematic review [14]. The larger review aims to answer transmission dynamics of SARS-CoV-2. The analysis in this report addresses one of the review questions; to identify secondary attack rate based on symptom status.
Definitions
We defined ‘asymptomatic’ as an individual with laboratory-confirmed SARS-CoV-2 infection but without symptoms throughout their entire course of infection, or after 14 days of follow up; ‘paucisymptomatic’ as an individual with laboratory-confirmed SARS-CoV-2 infection with mild symptoms, and ‘pre-symptomatic’ as an individual who reports no symptoms at the time of the initial positive test result, but who subsequently develops symptoms attributable to COVID-19. We used these definitions to categorize the index cases. Secondary attack rate was defined as the number of new SARS-CoV-2 infection cases among susceptible contacts of primary cases divided by the total number of susceptible contacts.
Search strategy
We retrieved articles about transmission of SARS-CoV-2 infection through systematic searches of eight databases: Medline, EMBASE, Europe PMC, Web of Science, SCOPUS, Chinese database (CNKI), and preprint servers (MedRxiv, BioRxiv) using relevant Medical Subject Headings (MeSH) terms (see Supplementary material, Appendix S1). The initial search was completed from 30 December 2019 to 21 May 2020, searches were repeated on 8 June 2020 and 3 July 2020, owing to the rapidly increasing number of studies.
Study selection
Studies were eligible if they met the inclusion criteria: (a) report on COVID-19 or SARS-CoV-2 infection and (b) report an outbreak investigation or contact tracing study. Exclusion criteria were: (a) review articles; (b) observational studies providing only the proportion of individuals infected; (c) studies that do not indicate the number of contacts or secondary infections; and (d) reports in media sources. We also manually screened the references of the included original studies and reviews to identify additional eligible studies.
Data extraction
Two authors (XQ and AIN) independently reviewed reports by title and abstract for relevance, with at least 20% of all reports being screened in duplicate to ensure consistency. Two authors then independently read the full-text report of all studies not excluded by title and abstract, to consider eligibility for inclusion. Any disagreements regarding study inclusion were resolved through discussion with a third author (MC). Data were extracted onto a standardized form. From each study, the following variables were extracted: the name of the first author, year of publication, country, sample size, details of index cases (categorized as asymptomatic, pre-symptomatic and symptomatic); event details such as environment, transmission details; number of contacts, number of secondary cases. If these data were not reported, we contacted authors to request them and checked with the authors about all symptoms that they sought.
Risk of bias in included studies
Two authors (XQ and AIN) independently assessed completeness of reporting and risks of bias, using an adapted version of the Joanna Briggs Institute Critical Appraisal Checklist for Case Series (see Supplementary material). Any disagreements were resolved through discussion with a third author (MC).
Data synthesis and statistical analysis
The studies are summarized in text and table form, descriptive statistics were completed for key outcome measures. Secondary attack rates were computed from raw data in each study, dividing the number of infected contacts of primary cases by the total number of susceptible exposed contacts. A pooled analysis was carried out to generate summary estimates for the secondary attack rate in each subgroup analysed (asymptomatic, pre-symptomatic and symptomatic index cases), in the framework of a random effect model. The Freeman–Tukey double-arcsine variance-stabilizing transformation was used to combine data, because of its advantage over log and logit transformations, which do not allow computation of the proportion in the presence of zero event counts [15]. Secondary attack rates are presented as a proportion along with 95% CIs in forest plots. Heterogeneity between study estimates was gauged by means of the Cochran's Q and I2 statistic: an I2 value above 75% indicates high heterogeneity [16]. Moreover, a 95% prediction interval is displayed in the forest plots, which is an index of dispersion, providing information on how widely the true effect size varies. It can also provide the range of values in which a future observation will fall [17]. Analyses were performed though the software MetaXL version 5.3 (Ersatz, EpiGear International, Sunrise Beach, QLD, Australia) [18].
Results
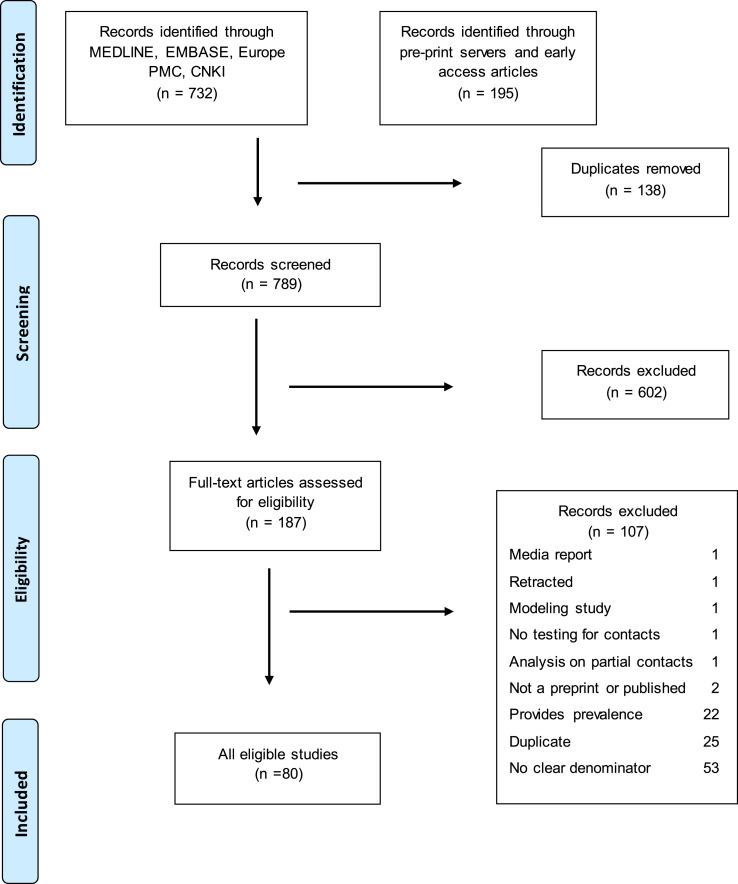
The systematic search identified 927 potentially relevant articles and 789 records were screened after removal of duplicates. Of 187 articles retrieved for full-text review and assessed for eligibility, 80 studies were included in the systematic review, and among those we identified 69 studies that indicated the symptom status of index case(s). In this analysis, we excluded 11 studies that reported asymptomatic and symptomatic index cases together or in which no symptom status of the index case was available. We re-classified three studies from asymptomatic to pre-symptomatic as the index cases developed symptoms later during the disease course after reviewing the details and contacting the authors [[19], [20], [21]]. The number of selected papers at each step of the screening and eligibility are reported in the flow diagram (Fig. 1 ).
Fig. 1.
Flowchart describing inclusion and exclusion of studies at each stage of the review.
Summary of secondary attack rates of asymptomatic index cases
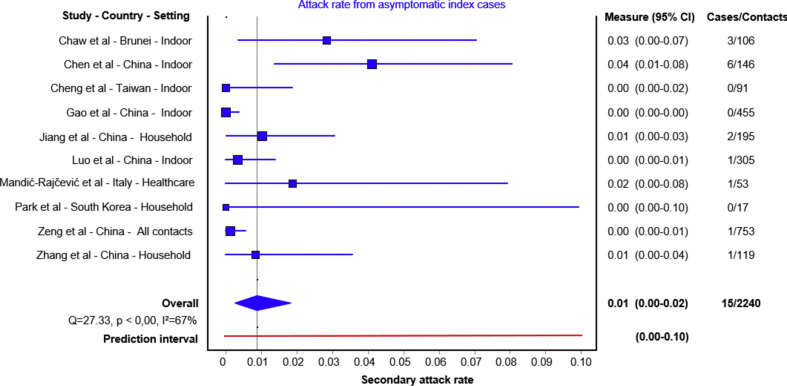
Ten studies were included in the quantitative analysis (Table 1 ) [6,13,[22], [23], [24], [25], [26], [27], [28], [29]]. Summary secondary attack rate estimate was 1% (95% CI 0%–2%) with a prediction interval range of 0%–10% (Fig. 2 ). All except one tested all close contacts for SARS-CoV-2, regardless of symptoms [26]. Cheng et al. only tested symptomatic cases, but they also tested high-risk populations regardless of symptoms including the household and hospital contacts [26]. Six studies reported on household contacts, two studies included hospital contacts and two studies included non-household close contacts.
Table 1.
Transmission from truly asymptomatic index cases
| Index cases | Environment | Number of contacts | Number of secondary cases | Asymptomatic SAR (95% CI) | Symptomatic SAR (95% CI) | |
|---|---|---|---|---|---|---|
| Chaw et al.a [13] | 3 | Household Non-household |
106 | 3 | 2.8% (0.06–8.0) | 14.4% (8.8–19.9) 0.7% (0.01–1.3) |
| Chen Y et al. [29] | 30 | Household Non-household |
146 | 6 | 4.1% (1.7–9.1) | 6.3% (5.3–7.5) |
| Cheng et al. [26] | 9 | Non-household | 91 | 0 | 0% (0.0–4.1) | Mild 3.76 (1.1–12.8) Severe 3.99 (1.0–15.8) |
| Gao et al. [25] | 1 | Household and healthcare | 455 | 0 | 0% (0.0–0.08) | |
| Jiang et al. [22] | 3 | Household | 195 | 2 | 1% (0.1–3.7) | |
| Luo et al. [28] | 8 | Household and non-household | 305 | 1 | 0.33% (0.0–1.8) OR (0.29 (0.04–2.2)) |
Mild 3.3% (OR 0.48; 0.28–0.82) Mod 5.6% (OR 1.0) Sev 6.2% (OR 1.19; 0.7–2.1) |
| Mandić-Rajčević et al. [23] | 1 | Healthcare | 53 | 1 | 1.9% (0.0,10.0) | |
| Park et al. [24] | 4 | Household | 17 | 0 | 0% (0.0–19.5) | 16.2 % (11.6–22.0) |
| Zeng et al. [27] | All contacts | 753 | 1 | 0.13% (0.0–0.7) | 2.02% (1.8–2.3) | |
| Zhang et al. [6] | 12 | Household | 119 | 1 | 0.8% (0.0–4.6) | Mild 3.5% (1.5–8.0) Mod 5.7% (2.5–12.8) Severe 4.5% (0.8–21.8) |
Abbreviations: SAR, secondary attack rate; sev, severe.
Authors contacted for more details.
Fig. 2.
Secondary attack rates from asymptomatic index cases to their contacts. For each study the secondary attack rate is reported with its 95% CI. A prediction interval at the bottom of the forest plot is depicted.
Three studies identified no secondary cases after following up 17, 91 and 455 close contacts of asymptomatic index cases (asymptomatic secondary attack rate of 0%) [[24], [25], [26]]. Of those, two studies demonstrated higher symptomatic secondary attack rates: Cheng et al. demonstrated that mild cases had a secondary attack rate of 3.8% (95% CI 1.1%–12.8%) and severe cases had a 4% (95% CI 1.0%–15.8%) secondary attack rate [26], while Park et al. reported a household symptomatic secondary attack rate of 16.2% (95% CI 11.6%–22.0%) [24]. In another study, 305 contacts of eight asymptomatic cases were followed up, identifying one secondary case (secondary attack rate 0.3%, 95% CI 0.0%–1.8%) [28]. In the same study, attack rates from index cases with mild, moderate and severe diseases were 3.3%, 5.6% and 6.2%, respectively. Zhang et al. followed up 119 close contacts of 12 asymptomatic index cases and identified one secondary case, an asymptomatic secondary attack rate of 0.8% (95% CI 0.0%–4.6%). In the same study, the secondary attack rate was 3.5% (95% CI 1.5%–8.0%) for those with mild, 5.7% (95% CI 2.5%–12.8%) for those with moderate and 4.5% (95% CI 0.8%–21.8%) for those with severe symptoms [6]. In this study, close contacts that lived with an index case had 12 times the risk of infection as those who did not live with the index case (relative risk 12.5, 95% CI 1.6–100.8) and those who had frequent contact with an index case patient, and those who had more than five contacts had 29 times the risk of infection as those with fewer contacts (relative risk 29.0, 95% CI 3.6–232.3). Two studies indicated an asymptomatic secondary attack rate of 1% and 1.9% [22,23]. Chaw et al. reported asymptomatic and pre-symptomatic contacts together. The authors clarified that three asymptomatic index cases and their 106 close contacts were followed up, leading to three secondary cases, a secondary attack rate of 2.8% (95% CI 0.06%–8.0%). In this study, the overall secondary attack rate was 10.6% in the household setting, which was higher for symptomatic cases (14.4%, 95% CI 8.8%–19.9%) than that of asymptomatic cases and for non-household contacts 0.7 (95% CI 0.1%–1.3) [13]. Zeng et al. conducted the largest contact tracing study, following up 753 close contacts of asymptomatic index cases and identified one secondary case, an asymptomatic secondary attack rate of 0.13% (95% CI 0.0%–0.7%) [27].
Summary of pre-symptomatic secondary attack rates
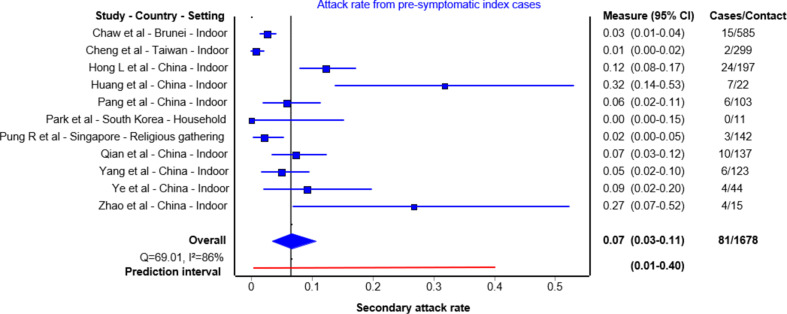
Sixteen papers reported either outbreak investigations or contact tracing studies reporting transmission from an index case during the pre-symptomatic period [13,19,21,26,[30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]] (Table 2 ). Of those, 11 studies were included in the quantitative analysis. The summary secondary attack rate estimate was 7% (95% CI 3%–11%) with a prediction interval of 1%–40% (Fig. 3 ). These studies followed up 22 to 585 close contacts whose initial exposure occurred before symptom onset of the index case. Even in studies that followed up large numbers of people, including community contacts, the majority of secondary cases identified were from the same household or among gatherings of friends. In these studies, having meals together, or playing cards with the index case were exposure activities associated with transmission. The remaining one study reported an outbreak in a restaurant [40] and four studies exclusively reported family cluster outbreaks [30,32,33,39]; these investigations did not test contacts outside the household, and it is challenging to truly differentiate transmission during the pre-symptomatic period from symptomatic transmission in the household setting (see Supplementary material, Fig. S1).
Table 2.
Transmission during pre-symptomatic period
| Index cases | Environment | Number of contacts | Number of secondary cases | Pre-symptomatic SAR (95% CI) | Secondary cases | |
|---|---|---|---|---|---|---|
| Contract tracing | ||||||
| Chaw et al.a [13] | 7 | Household and non-household | 585 | 15 | 2.56% (1.4–4.2) | |
| Cheng et al. [26] | NR | Household and non-household | 299 | 2 | 0.7% (0.1–2.4) | |
| Hong L et al. [31] | 41 | Household and non-household | 197 | 24 | 12.2% (8.0–17.6) | Friends, family, card-playing partners |
| Huang et al. [19] | 1 | Friends | 22 | 7 | 31.8% (13.0–54.9) | Shared meal with index |
| Pang et al. [34] | 1 | Household and non-household | 103 | 6 | 5.8% (2.2–12.2) | Living together or sharing meal |
| Park et al. [24] | 4 | Household | 11 | 0 | 0% (0.0–2.8) | |
| Pung R et al. [38] | 2 | Religious gathering | 142 | 3 | 2.1% (0.5–6.5) | |
| Qian et al. [35] | 1 | Household and non-household | 137 | 10 | 7.3% (3.6–13.0) | Living together or sharing meal |
| Yang et al. [36] | 2 | Household and non-household | 123 | 6 | 4.9% (1.8–10.3) | All secondary cases lived together |
| Ye et al. [21] | 1 | Family | 44 | 4 | 9.1% (2.5–21.4) | Extended family |
| Zhao et al. [37] | 1 | Friends | 15 | 4 | 26.7% (7.8–55.1) | Meal and Mahjong game gathering |
| Outbreak investigation | ||||||
| Chen M et al. [30] | 1 | Household | 3 | 2 | 66.7% (9.4–99.2) | Family cluster outbreak |
| Li P et al. [32] | 1 | Household | 5 | 4 | 80% (28.4–99.5) | Family cluster outbreak |
| Lu J et al. [40] | 1 | Restaurant | 82 | 9 | 11% (5.9–19.6) | |
| Jiang Y et al. [39] | 1 | Household | 7 | 3 | 42.9% (11.8–79.8) | Family cluster outbreak |
| Qian G et al. [33] | 2 | Household | 4 | 3 | 75% (10.4–99.4) | Family cluster outbreak |
Abbreviation: SAR, secondary attack rate.
Authors contacted for more details.
Fig. 3.
Secondary attack rates from pre-symptomatic index cases to their contacts. For each study the secondary attack rate is reported with its 95% CI. A prediction interval at the bottom of the forest plot is depicted.
Summary of symptomatic secondary attack rates
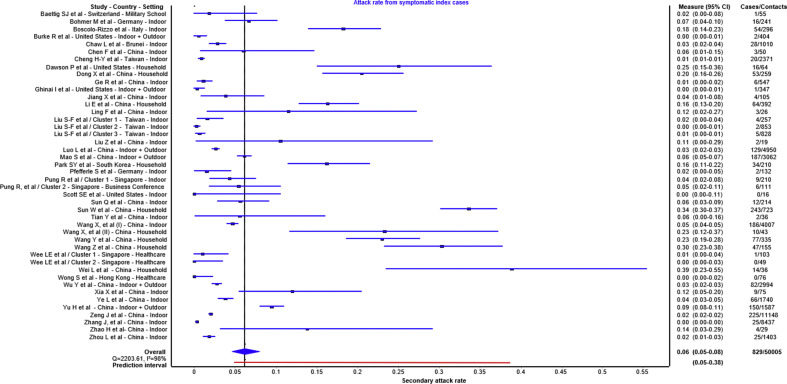
Forty-six papers reported either outbreak investigations or contact tracing studies reporting transmission from symptomatic index case(s). Of those, 40 contact tracing studies reported secondary attack rates ranging from 0% to 38.89% [13,24,27,28,38,[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75]] and six reported outbreak investigation [[76], [77], [78], [79], [80]] (see Supplementary material, Table S1). Forty contact tracing studies with 44 observations were included in the quantitative analysis (Fig. 4 ). The summary estimate of secondary attack rate from symptomatic index subjects was 6% (95% CI 5%–8%) with a prediction interval of 5%–38%. Of those, nine studies reported less than 1% secondary attack rates, two of these were in a health-care setting, two included outdoor interaction and four included non-household contacts. Higher frequency of contacts and household contacts were reported to be higher risk than non-household contacts.
Fig. 4.
Secondary attack rates from symptomatic index cases to their contacts. For each study the secondary attack rate is reported with its 95% CI. A prediction interval at the bottom of the forest plot is depicted.
Quality assessment
All papers included a clear definition of symptomatic and asymptomatic cases, number of secondary cases and number of contacts. The majority of studies identified index cases with a clear diagnosis, had an acceptable case definition and sufficiently followed up close contacts (for a minimum of 14 days). However, in some studies the definition of close contact and setting of transmission was not provided. In addition, it was unclear in four reports whether all potential close contacts were included; therefore, the direction of bias was uncertain. The quality assessment is summarized in the Supplementary material (Table S2).
Discussion
This systematic review provides comprehensive data on secondary attack rates based on symptom status of the index case(s). Although asymptomatic patients can transmit the virus to others [81], the findings from ten studies in this review found summary secondary attack rates of 1% with a prediction interval of 0%–10% for asymptomatic index cases compared with secondary attack rates of 6% with a prediction interval of 5%–38% in symptomatic cases and 7% with a prediction interval of 1%–40% in pre-symptomatic cases. These findings suggest that individuals who are asymptomatic throughout the disease course are responsible for fewer secondary infections than symptomatic and pre-symptomatic cases. Most transmission events were associated with living with the index case or group activities such as sharing meals and playing board games.
Given the importance of transmission heterogeneity in propagating the pandemic, it is important that we learn about the various factors that contribute to transmission. According to modelling and contact tracing studies, around 80% of secondary infections can be linked to 20% of cases, which distinguishes SARS-CoV-2 from seasonal influenza, although a similar pattern was also observed in SARS-CoV and Middle East respiratory syndrome-CoV [[82], [83], [84]]. There are multiple factors (environmental factors, contact patterns and socio-economic inequalities) that contribute to this heterogeneity, but some evidence is starting to emerge about the influence of individual's infectiousness on transmission dynamics. In this systematic review, we found that index cases with symptoms had a higher secondary attack rate compared with truly asymptomatic index cases. Although there is a need to better understand this difference, it may be due to shorter duration of infectiousness. In a living systematic review including studies published up to 6 June 2020, we found that asymptomatic people had a shorter duration of RNA shedding than symptomatic individuals [85]. Asymptomatic patients may therefore be contagious but for a shorter duration than symptomatic people; this might contribute to lower transmission to their contacts. However, we do not yet know the relative importance of behavioral factors by the host versus environmental factors in determining transmission risk. It is not known whether the size of the cluster of secondary infections would be different according to index case symptom status in a high-risk environment with no mitigation measures in place.
Modelling studies suggest that it is not possible to have widespread infection without substantial pre-symptomatic transmission. Viral load dynamics of SARS-CoV-2 derived from confirmed cases suggest that peak viral loads are detected at the start of symptom onset up to day 5 of illness, indicating that the highest infectiousness occurs just before or within the first few days after symptom onset [85]. So far, several contact tracing studies emphasize that the highest risk of transmission occurs during the prodromal phase or early in the disease course [64,86]. For instance, in a prospective contact tracing study of 100 confirmed cases of COVID-19 and 2761 close contacts, no secondary cases were identified when the exposure occurred more than 5 days after symptom onset [26]. Our findings therefore have important implications from a public health perspective. In settings such as nursing homes, homeless shelters, prisons, cruise ships and meat-packing plants in which many people spend prolonged periods of time together in the same environment including sleeping, dining and sharing common facilities, and where several outbreaks have been documented, pre-symptomatic transmission may contribute substantially to transmission [87,88]. In these settings, when infection develops, most patients are already inside the facility with high viral loads that increase the risk of onward transmission. This highlights the importance of mitigation measures and surveillance in these settings to identify those patients early in the disease course to prevent onward transmission inside the facility.
This systematic review has several strengths. First, this is a living systematic review examining the transmission of SARS-CoV-2 through contact tracing and outbreak investigation studies. Second, we only included studies with clear case definitions, which indicated the number of contacts and secondary cases. We excluded studies in which the index case was unclear, or the numbers of contacts were not provided. The estimates from individual studies are also subject to limitations, such as imprecision resulting from small study size, and multiple sources of bias in the estimation of the true secondary attack rate, which are detailed in this paper [89]. Moreover, while the number of index cases could influence the confidence interval estimation for secondary attack rate due to heterogeneity among index cases, we have constructed a prediction interval to yield conservative confidence interval estimates.
We identified two other systematic reviews that investigated asymptomatic transmission, with different research questions, which results in different search terms and studies retrieved. One living systematic review, which included studies published up to 10 June 2020, identified five studies that directly compared secondary attack rates between asymptomatic and symptomatic index cases; all were included in our review [7]. This study only included studies that provided data to allow relative risks to be estimated. The summary risk ratios for asymptomatic versus symptomatic (0.35, 95% CI 0.10–1.27) and pre-symptomatic versus symptomatic (0.63, 95% CI 0.18–2.26) are consistent with our findings. The second review estimated only household secondary attack rates and included studies published up to 29 July 2020 [90]. Of three studies that included asymptomatic index cases, two were included in our review. We excluded one of the studies because the number of contacts of asymptomatic index cases was not specified; we have not yet received details of the study after contacting the authors. Advantages of our review over these two studies are inclusion of studies published in Chinese, search terms that aimed to capture studies specifically estimating secondary attack rates.
In summary, although asymptomatic transmission is a major concern for SARS-CoV-2 community spread, secondary attack rates from those who remain asymptomatic throughout their course of infection are low, suggesting limited infectiousness. Although it is difficult to estimate the proportion of pre-symptomatic transmission, these patients are likely to be highly infectious just before and around the time of symptom onset and appear to transmit efficiently, particularly within households. Given these results, in the context of limited resources, approaches should be targeted predominantly at identifying and immediately isolating patients with prodromal or mild symptoms and their contacts, which may avert a significant number of community transmission clusters [91]. Future clinical studies should incorporate clear definitions and assess a broad range of symptoms associated with COVID-19, include longitudinal follow up of patients, and calculate secondary attack rates for a wider range of settings and populations [9].
Transparency declaration
The authors declare that there are no conflicts of interest.
Contribution statement
XQ contributed to investigation, data curation and writing the original draft; AN contributed to investigation, data curation and review and editing the article; AEM contributed to methodology, formal analysis and writing the original draft; IB and NL contributed to interpretation and to reviewing and editing the article; M. Cevik contributed to conceptualization, methodology, investigation, writing the original draft and supervision.
Funding
No funding was received.
Acknowledgements
We would like to thank the authors of Chau et al. (Dr Tan Le Van), Mandić-Rajčević et al. (Dr Stefan Mandić-Rajčević) and Chaw et al. (Dr Liling Chaw) for providing further details about asymptomatic cases in their reports, also Prof Stephen Gillespie for his comments on the first draft of this manuscript. We would like to acknowledge Dr Shuang Jin for searching and downloading the Chinese database for this review.
Editor: M. Paul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.01.011.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Ruan L., Wen M., Zeng Q. New measures for the coronavirus disease 2019 response: a lesson from the Wenzhou experience. Clin Infect Dis. 2020;71:866–869. doi: 10.1093/cid/ciaa386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N Engl J Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gudbjartsson D.F., Helgason A., Jonsson H. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382:2302–2315. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cevik M., Bamford C.G.G., Ho A. COVID-19 pandemic-a focused review for clinicians. Clin Microbiol Infect. 2020;26:842–847. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Liu Y., Liu L., Wang X., Luo N., Li L. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China. J Infect Dis. 2020;221:1770–1774. doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W., Cheng W., Luo L. Secondary transmission of coronavirus disease from presymptomatic persons, China. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2608.201142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buitrago-Garcia D., Egli-Gany D., Counotte M.J. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugano N., Ando W., Fukushima W. Cluster of severe acute respiratory syndrome coronavirus 2 infections linked to music clubs in Osaka, Japan. J Infect Dis. 2020;222:1635–1640. doi: 10.1093/infdis/jiaa542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyerowitz E.A., Richterman A., Bogoch, Low N., Cevik M. Towards an accurate and systematic characterisation of persistently asymptomatic infection with SARS-CoV-2. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30837-9. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavezzo E., Franchin E., Ciavarella C. Suppression of COVID-19 outbreak in the municipality of Vo, Italy. medRxiv. 2020 2020.04.17.20053157. [Google Scholar]
- 11.Kim E.S., Chin B.S., Kang C.K. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean Cohort Study on COVID-19. J Korean Med Sci. 2020;35:e142. doi: 10.3346/jkms.2020.35.e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arons M.M., Hatfield K.M., Reddy S.C. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaw L., Koh W., Jamaludin S., Naing L., Alikhan M., Wong J. 2020. Analysis of SARS-CoV-2 transmission in different settings, Brunei. Emerg Infect Dis. 26:2598–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott Jh, Synnot A, Turner T Living systematic review: 1. Introduction—the why, what, when, and how. J Clin Epidemiol. 2017;91:23–30. doi: 10.1016/j.jclinepi.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Lin L., Xu C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Sci Rep. 2020;3:e178. doi: 10.1002/hsr2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sedgwick P. Meta-analyses: what is heterogeneity? BMJ. 2015;350:h1435. doi: 10.1136/bmj.h1435. [DOI] [PubMed] [Google Scholar]
- 17.IntHout J., Ioannidis J.P.A., Rovers M.M., Goeman J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barendregt J.J., Doi S.A., Lee Y.Y., Norman R.E., Vos T. Meta-analysis of prevalence. J Epidemiol Commun Health. 2013;67:974. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 19.Huang L., Zhang X., Zhang X. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16-23 years outside Wuhan and characteristics of young patients with COVID-19: a prospective contact-tracing study. J Infect. 2020;80:e1–e13. doi: 10.1016/j.jinf.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C., Ji F., Wang L. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, Xuzhou, China. Emerg Infect Dis. 2020;26:31. doi: 10.3201/eid2607.200718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye F., Xu S., Rong Z. Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster. Int J Infect Dis. 2020;94:133–138. doi: 10.1016/j.ijid.2020.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X.L., Zhang X.L., Zhao X.N. Transmission potential of asymptomatic and paucisymptomatic SARS-CoV-2 infections: a three-family cluster study in China. J Infect Dis. 2020;22:22. doi: 10.1093/infdis/jiaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandic-Rajcevic S., Masci F., Crespi E. 2020. Contact tracing and isolation of asymptomatic spreaders to successfully control the COVID-19 epidemic among healthcare workers in Milan (Italy) [DOI] [Google Scholar]
- 24.Park S.Y., Kim Y.M., Yi S. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26:23. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao M., Yang L., Chen X. A study on infectivity of asymptomatic SARS-CoV-2 carriers. Respir Med. 2020:106026. doi: 10.1016/j.rmed.2020.106026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng H.-Y., Jian S.-W., Liu D.-P. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.曾 晶, 邱乐 平, 邹 晏. 四川省新型冠状病毒肺炎密切接触者分析. 中国公共卫生. 2020;36:503–506. [Google Scholar]
- 28.Luo L., Liu D., Liao X. Contact settings and risk for transmission in 3410 close contacts of patients with COVID-19 in Guangzhou, China. Ann Intern Med. 2020;173:879–887. doi: 10.7326/M20-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y., Wang A.H., Yi B. Epidemiological characteristics of infection in COVID-19 close contacts in Ningbo city. Chung Hua Liu Hsing Ping Hsueh Tsa Chih. 2020;41:667–671. doi: 10.3760/cma.j.cn112338-20200304-00251. [DOI] [PubMed] [Google Scholar]
- 30.Chen M., Fan P., Liu Z. A SARS-CoV-2 familial cluster infection reveals asymptomatic transmission to children. J Infect Public Health. 2020;13:883–886. doi: 10.1016/j.jiph.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong L.X., Lin A., He Z.B. Mask wearing in pre-symptomatic patients prevents SARS-CoV-2 transmission: an epidemiological analysis. Travel Med Infect Dis. 2020;36:101803. doi: 10.1016/j.tmaid.2020.101803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P., Fu J.B., Li K.F. Transmission of COVID-19 in the terminal stages of the incubation period: a familial cluster. Int J Infect Dis. 2020;96:452–453. doi: 10.1016/j.ijid.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian G., Yang N., Ma A.H.Y. A COVID-19 transmission within a family cluster by presymptomatic infectors in China. Clin Infect Dis. 2020;71:861–862. doi: 10.1093/cid/ciaa316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.庞 秋艳, 李 朋, 李 天忠, 李 化荣. 起高速服务区新型冠状病毒肺炎聚集性疫情调查. 安徽预防医学杂志. 2020;26:130–132. [Google Scholar]
- 35.钱 丽珍, 郑 志强, 洪 万胜, 孙 芳红, 吴 方楠, 金 瑞盈. 瑞安市一起新型冠状病毒肺炎聚集性疫情调查. 预防医学. 2020;32:486–488+91. [Google Scholar]
- 36.阳 雅兰, 李 林洪, 李 长凤, 肖 玉春, 彭 君. 重庆市一起新型冠状病毒肺炎家庭聚集性疫情调查分析. 中国公共卫生. 2020;36:285–288. [Google Scholar]
- 37.Zhao H., Li B.S., Xia Y. Investigation of transmission chain of a cluster COVID-19 cases. Chung Hua Liu Hsing Ping Hsueh Tsa Chih. 2020;41:E064. doi: 10.3760/cma.j.cn112338-20200227-00198. [DOI] [PubMed] [Google Scholar]
- 38.Pung R., Chiew C.J., Young B.E. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395:1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Y., Niu W., Wang Q., Zhao H., Meng L., Zhang C. Characteristics of a family cluster of severe acute respiratory syndrome coronavirus 2 in Henan, China. J Infect. 2020;23:23. doi: 10.1016/j.jinf.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu J., Gu J., Li K. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26:2. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baettig S.J., Parini A., Cardona I., Morand G.B. Case series of coronavirus (SARS-CoV-2) in a military recruit school: clinical, sanitary and logistical implications. BMJ Mil Health. 2020;16:16. doi: 10.1136/bmjmilitary-2020-001482. [DOI] [PubMed] [Google Scholar]
- 42.Bohmer M.M., Buchholz U., Corman V.M. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis. 2020;15:15. doi: 10.1016/S1473-3099(20)30314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boscolo-Rizzo P., Borsetto D., Spinato G. New onset of loss of smell or taste in household contacts of home-isolated SARS-CoV-2-positive subjects. Eur Arch Oto-Rhino-Laryngol. 2020;277:2637–2640. doi: 10.1007/s00405-020-06066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke R.M., Midgley C.M., Dratch A. Active monitoring of persons exposed to patients with confirmed COVID-19 – United States, January-February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:245–246. doi: 10.15585/mmwr.mm6909e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng H.Y., Jian S.W., Liu D.P., Ng T.C., Huang W.T., Lin H.H. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180:1156–1163. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dawson P., Rabold E.M., Laws R.L. Loss of taste and smell as distinguishing symptoms of coronavirus disease 2019. Clin Infect Dis. 2021;72:682–685. doi: 10.1093/cid/ciaa799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ge R., Tian M., Gu Q. The role of close contacts tracking management in COVID-19 prevention: a cluster investigation in Jiaxing, China. J Infect. 2020;81:e71–e74. doi: 10.1016/j.jinf.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghinai I., McPherson T.D., Hunter J.C. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang X.L., Zhang X.L., Zhao X.N. Transmission potential of asymptomatic and paucisymptomatic severe acute respiratory syndrome coronavirus 2 infections: a 3-family cluster study in China. J Infect Dis. 2020;221:1948–1952. doi: 10.1093/infdis/jiaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu S.F., Kuo N.Y., Kuo H.C. Three Taiwan's domestic family cluster infections of coronavirus disease 2019. J Med Virol. 2020;28:28. doi: 10.1002/jmv.25949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao S., Huang T., Yuan H. Research Square; 2020. Epidemiological analysis of 67 local COVID-19 clusters in Sichuan Province, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfefferle S., Guenther T., Kobbe R. Low and high infection dose transmission of SARS-CoV-2 in the first COVID-19 clusters in Northern Germany. medRxiv. 2020 doi: 10.1101/2020.06.11.20127332. [DOI] [Google Scholar]
- 53.Scott S.E., Zabel K., Collins J. First mildly ill, non-hospitalized case of coronavirus disease 2019 (COVID-19) without viral transmission in the United States – Maricopa County, Arizona, 2020. Clin Infect Dis. 2020;71:807–812. doi: 10.1093/cid/ciaa374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun W.W., Ling F., Pan J.R. Epidemiological characteristics of 2019 novel coronavirus family clustering in Zhejiang Province. Chung Hua Yu Fang I Hsueh Tsa Chih. 2020;54:E027. doi: 10.3760/cma.j.cn112150-20200227-00199. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z., Ma W., Zheng X., Wu G., Zhang R. Household transmission of SARS-CoV-2. J Infect. 2020;81:179–182. doi: 10.1016/j.jinf.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wee L.E., Sim J.X.Y., Conceicao E.P. Containment of COVID-19 cases amongst healthcare workers: the role of surveillance, early detection and outbreak management. Infect Control Hosp Epidemiol. 2020:1–21. doi: 10.1017/ice.2020.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei L., Lv Q., Wen Y. Household transmission of COVID-19, Shenzhen, January-February 2020. medRxiv. 2020 doi: 10.1101/2020.05.11.20092692. [DOI] [Google Scholar]
- 58.Wong S.C.Y., Kwong R.T., Wu T.C. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. 2020;105:119–127. doi: 10.1016/j.jhin.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia X.Y., Wu J., Liu H.L., Xia H., Jia B., Huang W.X. Epidemiological and initial clinical characteristics of patients with family aggregation of COVID-19. J Clin Virol. 2020;127:104360. doi: 10.1016/j.jcv.2020.104360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye L.X., Wang H.B., Lu H.C. Investigation of a cluster epidemic of COVID-19 in Ningbo. Chung Hua Liu Hsing Ping Hsueh Tsa Chih. 2020;41:E065. doi: 10.3760/cma.j.cn112338-20200316-00362. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J.Z., Zhou P., Han D.B. Investigation on a cluster epidemic of COVID-19 in a supermarket in Liaocheng, Shandong province. Chung Hua Liu Hsing Ping Hsueh Tsa Chih. 2020;41:E055. doi: 10.3760/cma.j.cn112338-20200228-00206. [DOI] [PubMed] [Google Scholar]
- 62.Zhao H., Li B.S., Xia Y. Investigation of transmission chain of a cluster COVID-19 cases. Chung Hua Liu Hsing Ping Hsueh Tsa Chih. 2020;41:E064. doi: 10.3760/cma.j.cn112338-20200227-00198. [DOI] [PubMed] [Google Scholar]
- 63.Wang X., Zhou Q., He Y. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J. 2020;55:2000544. doi: 10.1183/13993003.00544-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y., Tian H., Zhang L. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y., Song S., Kao Q., Kong Q., Sun Z., Wang B. Risk of SARS-CoV-2 infection among contacts of individuals with COVID-19 in Hangzhou, China. Public Health. 2020;185:57–59. doi: 10.1016/j.puhe.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu H-j, Hu Y-f, Liu X-x. Household infection: the predominant risk factor for close contacts of patients with COVID-19. Travel Med Infect Dis. 2020;36:101809. doi: 10.1016/j.tmaid.2020.101809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong X.C., Li J.M., Bai J.Y. Epidemiological characteristics of confirmed COVID-19 cases in Tianjin. Chung Hua Liu Hsing Ping Hsueh Tsa Chih. 2020;41:638–642. doi: 10.3760/cma.j.cn112338-20200221-00146. [DOI] [PubMed] [Google Scholar]
- 68.凌 锋, 刘 社兰, 倪 朝荣. 浙江省首例新型冠状病毒肺炎报告病例流行病学调查. 预防医学. 2020;32:109–112. [Google Scholar]
- 69.刘 仲, 赵 梦娇, 杨 国樑. 1例不明原因新型冠状病毒肺炎及其密切接触者调查分析 山东大学学报(医学版) 2020;58:49–53. [Google Scholar]
- 70.周 林, 刘 晓雪, 李 战, 耿 兴义, 刘 庆皆. 新型冠状病毒肺炎病例1403例密切接触者发病分析. 山东大学学报(医学版) 2020;58:58–61. [Google Scholar]
- 71.孙 倩莱, 李 作超, 谭 夏林. 起新型冠状病毒肺炎聚集性疫情调查. 实用预防医学. 2020;27:389–392. [Google Scholar]
- 72.田元睿, 吴志明, 韩小亮, 王月萍, 刘泓, 扬州市首起输入性新型冠状病毒肺炎家庭聚集性疫情的流行病学调查 上海预防医学: 1–7.
- 73.陈 凤阳, 李 少雄. 江西某地一起新型冠状病毒肺炎聚集性疫情调查. 江西医药. 2020;55:346–348. [Google Scholar]
- 74.Li W., Zhang B., Lu J. The characteristics of household transmission of COVID-19. Clin Infect Dis. 2020;71:1943–1946. doi: 10.1093/cid/ciaa450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X., Pan Y., Zhang D. Basic epidemiological parameter values from data of real-world in mega-cities: the characteristics of COVID-19 in Beijing, China. BMC Infect Dis 20. 2020;526 doi: 10.1186/s12879-020-05251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang R., Xia J., Chen Y., Shan C., Wu C. A family cluster of SARS-CoV-2 infection involving 11 patients in Nanjing, China. Lancet Infect Dis. 2020;20:534–535. doi: 10.1016/S1473-3099(20)30147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li C.X., Wu B., Luo F., Zhang N. Clinical study and CT findings of a familial cluster of pneumonia with coronavirus disease 2019 (COVID-19) Sichuan Da Xue Xue Bao Yi Xue Ban. 2020;51:155–158. doi: 10.12182/20200360107. [DOI] [PubMed] [Google Scholar]
- 78.Song R., Han B., Song M. Clinical and epidemiological features of COVID-19 family clusters in Beijing, China. J Infect. 2020;23:23. doi: 10.1016/j.jinf.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tong Z.D., Tang A., Li K.F. Potential presymptomatic transmission of SARS-CoV-2, Zhejiang province, China, 2020. Emerg Infect Dis. 2020;26:1052–1054. doi: 10.3201/eid2605.200198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen D., Li Y., Deng X. Four cases from a family cluster were diagnosed as COVID-19 after 14-day of quarantine period. J Med Virol. 2020 doi: 10.1002/jmv.25849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chau N.V.V., Thanh Lam V., Thanh Dung N. The natural history and transmission potential of asymptomatic SARS-CoV-2 infection. Clin Infect Dis. 2020;71:2679–2687. doi: 10.1093/cid/ciaa711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bi Q., Wu Y., Mei S. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20:911–919. doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Althouse B.M., Wenger E.A., Miller J.C. Superspreading events in the transmission dynamics of SARS-CoV-2: opportunities for interventions and control. PLOS Biol. 2020;18 doi: 10.1371/journal.pbio.3000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adam D.C., Wu P., Wong J.Y. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat Med. 2020;26:1714–1719. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- 85.Cevik M., Tate M., Lloyd O., Maraolo A.E., Schafers J., Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microb. 2021;2:e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Böhmer M.M., Buchholz U., Corman V.M. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis. 2020;20:920–928. doi: 10.1016/S1473-3099(20)30314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adam D.C., Wu P., Wong J.Y. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat Med. 2020;26:1714–1719. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- 88.Leclerc Q.J.F.N., Knight L.E. What settings have been linked to SARS-CoV-2 transmission clusters? [version 1; peer review: 1 approved with reservations] Wellcome Open Res. 2020;5:83. doi: 10.12688/wellcomeopenres.15889.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Accorsi E., Qiu Xueting, Rumpler Eva. Harvard Library; 2020. How to detect and reduce potential sources of biases in epidemiologic studies of SARS-CoV-2.https://nrs.harvard.edu/URN-3:HUL.INSTREPOS:37366192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Madewell Z.J., Yang Y., Longini I.M., Jr., Halloran M.E., Dean N.E. Household Transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.31756. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peak C.M., Kahn R., Grad Y.H. Individual quarantine versus active monitoring of contacts for the mitigation of COVID-19: a modelling study. Lancet Infect Dis. 2020;20:1025–1033. doi: 10.1016/S1473-3099(20)30361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.