Abstract
Background
Patients with COVID-19 pneumonia have an excess of inflammation and increased concentrations of cytokines including interleukin-1 (IL-1). We aimed to determine whether anakinra, a recombinant human IL-1 receptor antagonist, could improve outcomes in patients in hospital with mild-to-moderate COVID-19 pneumonia.
Methods
In this multicentre, open-label, Bayesian randomised clinical trial (CORIMUNO-ANA-1), nested within the CORIMUNO-19 cohort, we recruited patients from 16 University hospitals in France with mild-to-moderate COVID-19 pneumonia, severe acute respiratory syndrome coronavirus 2 infection confirmed by real-time RT-PCR, requiring at least 3 L/min of oxygen by mask or nasal cannula but without ventilation assistance, a score of 5 on the WHO Clinical Progression Scale (WHO-CPS), and a C-reactive protein serum concentration of more than 25 mg/L not requiring admission to the intensive care unit at admission to hospital. Eligible patients were randomly assigned (1:1) using a web-based secure centralised system, stratified by centre and blocked with varying block sizes (randomly of size two or four), to either usual care plus anakinra (200 mg twice a day on days 1–3, 100 mg twice on day 4, 100 mg once on day 5) or usual care alone. Usual care was provided at the discretion of the site clinicians. The two coprimary outcomes were the proportion of patients who had died or needed non-invasive or mechanical ventilation by day 4 (ie, a score of >5 on the WHO-CPS) and survival without need for mechanical or non-invasive ventilation (including high-flow oxygen) at day 14. All analyses were done on an intention-to-treat basis. The trial is registered with ClinicalTrials.gov, NCT04341584, and is now closed to accrual.
Findings
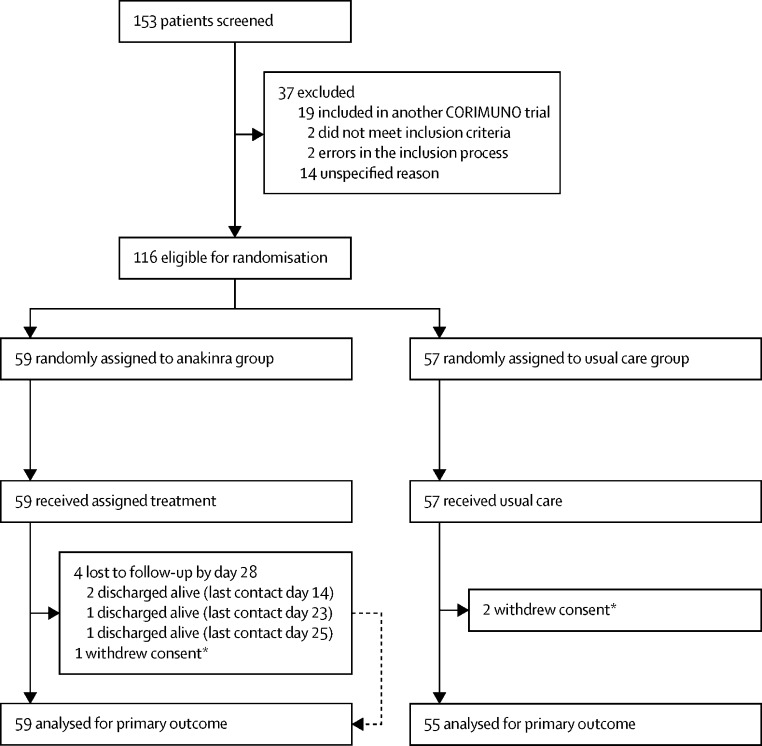
Between April 8 and April 26, 2020, we screened 153 patients. The study was stopped early following the recommendation of the data and safety monitoring board, after the recruitment of 116 patients: 59 were assigned to the anakinra group, and 57 were assigned to the usual care group. Two patients in the usual care group withdrew consent and were not analysed. In the analysable population, the median age was 66 years (IQR 59 to 76) and 80 (70%) participants were men. In the anakinra group, 21 (36%) of 59 patients had a WHO-CPS score of more than 5 at day 4 versus 21 (38%) of 55 in the usual care group (median posterior absolute risk difference [ARD] −2·5%, 90% credible interval [CrI] −17·1 to 12·0), with a posterior probability of ARD of less than 0 (ie, anakinra better than usual care) of 61·2%. At day 14, 28 (47%; 95% CI 33 to 59) patients in the anakinra group and 28 (51%; 95% CI 36 to 62) in the usual care group needed ventilation or died, with a posterior probability of any efficacy of anakinra (hazard ratio [HR] being less than 1) of 54·5% (median posterior HR 0·97; 90% CrI 0·62 to 1·52). At day 90, 16 (27%) patients in the anakinra group and 15 (27%) in the usual care group had died. Serious adverse events occurred in 27 (46%) patients in the anakinra group and 21 (38%) in the usual care group (p=0·45).
Interpretation
Anakinra did not improve outcomes in patients with mild-to-moderate COVID-19 pneumonia. Further studies are needed to assess the efficacy of anakinra in other selected groups of patients with more severe COVID-19.
Funding
The Ministry of Health, Programme Hospitalier de Recherche Clinique, Foundation for Medical Research, and AP-HP Foundation.
Introduction
COVID-19 is a respiratory disease, induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), that has already caused more than 1 million deaths over the word.1, 2, 3, 4 Most people with COVID-19 have only mild or uncomplicated symptoms, but approximately 10–15% of patients have moderate or severe disease that requires admission to hospital and oxygen support, and 3–5% require admission to an intensive care unit (ICU) mainly for ventilation assistance.4, 5 In severe cases, COVID-19 can be complicated by acute respiratory distress syndrome. Older age, male sex, and comorbid diseases are risk factors for death.1, 6, 7
At the beginning of the epidemic in France, when no standard of care was defined, we decided to set up the publicly supported CORIMUNO-19 (Cohort Multiple randomized controlled trials open-label of immune modulatory drugs and other treatments in COVID-19 patients) project, a cohort designed with the purpose of being able to carry out open-label, randomised clinical trials of immunomodulatory drugs in patients who had been admitted to hospital with mild-to-moderate or severe COVID-19. The overall objective of this initiative was to explore several immunomodulatory drugs to inform the design of larger trials to confirm the best drug in a defined population of patients with mild-to-moderate or severe COVID-19 pneumonia and eventually develop new standard of care.
Research in context.
Evidence before the study
Since the beginning of the COVID-19 pandemic, no definitive standard of care for mild-to-moderate COVID-19 pneumonia has clearly emerged. Patients with COVID-19 pneumonia have an excess of inflammation and increased concentrations of cytokines including interleukin-1 (IL-1). We searched PubMed for clinical trials published in English from database inception until March 30, 2020, assessing the effect of anakinra (a recombinant human IL-1 receptor antagonist) among patients with laboratory-confirmed COVID-19 using the search terms (“COVID-19”[All Fields] OR “2019-nCoV”[All Fields]) OR “SARS-CoV-2”[All Fields]) AND (“anakinra”[All Fields] (filters: Clinical Trial, Randomized Controlled Trial). We identified only cohort or observational studies and no randomised clinical trials that compared anakinra with usual care in patients with COVID-19.
Added value of this study
To our knowledge, this is the first randomised clinical trial to report the effects of anakinra in patients with mild-to-moderate COVID-19 pneumonia requiring at least 3 L/min of oxygen but not receiving non-invasive or invasive mechanical ventilation at randomisation. The study was stopped early for futility following the recommendation of the data and safety monitoring board. We found no difference between the anakinra group and the usual care group in terms of 4-day improvement, 14-day ventilation requirement or death, and 28-day and 90-day mortality.
Implications of all the available evidence
Since the beginning of this study and based on a large randomised open trial done by the RECOVERY collaborative group, dexamethasone is now largely used in the treatment of mild-to-moderate, severe, or critical COVID-19. Our finding of no clinical benefit from anakinra treatment compared with usual care is not encouraging to set up a larger randomised controlled trial to test anakinra in the population of patients with mild-to-moderate COVID-19 pneumonia, but our findings do not preclude the possible efficacy of anakinra in more severe cases of COVID-19. Another trial within the CORIMUNO platform (CORIMUNO-ANA-2) that aims to assess the effect of anakinra in patients with more severe COVID-19 who are in intensive care units has now been completed and is being analysed.
Patients with COVID-19 pneumonia present with non-specific inflammatory responses, including oedema and inflammatory cell infiltration in the lungs. In addition to the specific pathogenic effect of SARS-CoV-2, this deleterious excessive and non-effective host immune response has an important role during the disease course. It is, at least in part, related to a hyperinflammatory status associated with production of a number of pro-inflammatory cytokines and chemokines, including interleukin 1 (IL-1).
Anakinra is a 17 kD recombinant human IL-1 receptor antagonist (therefore blocking both IL-1α and IL-1β), with a short half-life of about 3–4 h and good safety profile approved in the treatment of patients with rheumatoid arthritis, gouty arthritis, and some rare auto-inflammatory syndromes. Anakinra has also been used at higher dose with an intravenous regimen in the management of critically ill patients with haemophagocytic lymphohystiocytosis and in patients with septic shock.8, 9, 10, 11, 12, 13 Severe COVID-19 pneumonia and haemophagocytic lymphohistiocytosis share biological and clinical features,14, 15 and so the hypothesis that anakinra could be effective in COVID-19 has emerged.15, 16
Preliminary data from observational studies suggest the possible efficacy of anakinra for mild-to-moderate, severe, or critical COVID-19,15, 17, 18, 19 but until now no data were available from randomised clinical trials. Therefore, we aimed to assess the efficacy and safety of anakinra in patients in hospital with mild-to-moderate COVID-19 pneumonia in a randomised controlled trial setting.
Methods
Study design and participants
We enrolled patients with COVID-19 from University hospitals in France for a series of randomised controlled trials testing different therapeutic regimens (CORIMUNO-19 cohort). Patients with mild-to-moderate COVID-19 pneumonia and patients with severe and critical COVID-19 pneumonia were included in independent clinical trials. Here we report data from CORIMUNO-ANA-1, a CORIMUNO-19, multicentre, open-label, randomised controlled trial of patients with mild-to-moderate COVID-19 pneumonia.
Participants were recruited from 16 French University hospitals (appendix 2 p 2). The CORIMUNO cohort and all embedded trials (ie, trials using data collected in the CORIMUNO cohort) were approved by an ethics committee (Comité de Protection des Personnes Île-de-France VI) and relevant authorities. Legal issues and trial procedures are presented in detail in the appendix 2 (p 12). Written informed consent was obtained from all patients or from the patient's legal representative for entering the CORIMUNO cohort and longitudinal data (including clinical status, biological data, and outcomes) were recorded as part of their participation in the cohort. In this consent form, patients were made aware that a number of trials might occur inside the cohort, and that they would probably be offered participation in some of them. In practice, for logistical reasons, only one trial was done in each centre at a given time. Specific additional written consent was obtained from eligible patients who were randomly selected to be offered anakinra and agreed to receive this treatment. Eligible patients assigned to receive usual care were not notified about the trial but their data as part of the CORIMUNO cohort were available for analysis. Patients were eligible for inclusion in the CORIMUNO-19 cohort if they had confirmed SARS-CoV-2 infection (positive on real-time RT-PCR or chest CT scan typical of COVID-19 pneumonia, or both) with mild-to-moderate, severe, or critical pneumonia (ie, receiving oxygen at a flow of >3 L/min via mask or nasal cannula and a score of ≥5 points on the WHO Clinical Progression Scale [WHO-CPS] 10-point ordinal scale, which is described in the appendix 2 [pp 12–13]). Patients from the CORIMUNO-19 cohort were eligible for the CORIMUNO-ANA-1 trial if they had a C-reactive protein serum concentration of more than 25 mg/L not requiring admission to the hospital intensive care unit at the time of admission, and mild-to-moderate COVID-19 pneumonia with a WHO-CPS score of 5 points, receiving at least 3 L/min of oxygen but without ventilation assistance (eg, high-flow oxygen, non-invasive ventilation, or mechanical ventilation). Key exclusion criteria included known hypersensitivity to anakinra or any of its excipients, pregnancy, current documented bacterial infection, an absolute neutrophil count of 1·0 × 109 per L or less, a platelet concentration of less than 50 G/L, serum aspartate aminotransferase or serum alanine aminotransferase of more than five-times the upper limit of normal, or severe renal insufficiency defined by an estimated glomerular filtration rate of less than 30 mL/min. Full inclusion and exclusion criteria are listed in the appendix 2 (p 12).
Randomisation and masking
Participants were randomly assigned (1:1) using a web-based secure centralised system to either usual care plus anakinra or usual care alone. An independent statistician provided a computer-generated assignment randomisation list stratified by centre and blocked with varying block sizes (randomly of sizes two or four) unknown to the investigators. Patients and investigators were not masked to treatment assignment due to the nature of the intervention.
Procedures
Because of the emergency nature of the trial and feasibility issues, no placebo alternative for anakinra was prepared. Anakinra (Sobi, Puteaux, France) was given intravenously 200 mg twice a day (total 400 mg) on days 1–3 after randomisation, then at 100 mg twice a day (total 200 mg) on day 4, and 100 mg once on day 5. If no improvement was seen on the morning of day 4 (improvement was determined as a reduction in requirement of oxygen of more than 50%, but the decision was left to the treating physician), 3 supplementary days of treatment at 400 mg per day were done on days 4–6, followed by a decrease to 200 mg per day on day 7 and 100 mg per day on day 8, and no treatment thereafter. Usual care (antibiotic drugs, antiviral drugs, corticosteroids, vasopressor support, anticoagulants) was provided at the discretion of the site clinicians.
Outcomes
The two coprimary outcomes were the proportion of patients who had died or needed non-invasive or mechanical ventilation by day 4 (ie, a score of >5 points on the WHO-CPS); and survival with no need for mechanical or non-invasive ventilation (including high-flow oxygen) at day 14. Both outcomes were consistent with the core outcome set proposed by WHO for a minimal outcome measure for COVID-19 clinical outcomes.20
Prespecified secondary outcomes were clinical status assessed with the WHO-CPS at days 4, 7, and 14; overall survival at days 14, 28, and 90; time to discharge from hospital; time to oxygen supply independency; and time to negative viral excretion (not assessed due to paucity of data). We also measured biological factors (eg, C-reactive protein concentration) and adverse events. Time to discharge and time to oxygen supply independency was assessed at day 28 because this was the latest timepoint when data were complete for almost all patients.
Statistical analysis
We used a Bayesian monitoring and analytical approach based on the coprimary outcomes. The sample size was initially set at 120, with interim analyses presented weekly to the data and safety monitoring board (DSMB) and a provision to increase the sample size in case of promising but not conclusive results. We calculated that the trial would have frequentist power of 97·2% to detect a decrease in event rate from 0·50 to 0·20, and of 73·9% to detect a decrease in event rate from 0·50 to 0·30. For the day 4 outcome, we used a β prior distribution with parameters 1 and 1 for the proportion in each treatment group. For the day 14 outcome, we used a Gaussian prior distribution with a mean log hazard ratio (HR) of 0 and variance of 1 × 106 for the log HR. We then did sensitivity analyses using a range of prior distributions (appendix 2 pp 13–14, 19). The treatment effect was expressed in terms of absolute risk difference (ARD) for the day 4 outcome and HR for the day 14 outcome. We calculated posterior probabilities of ARD of less than 0 for the day 4 outcome and HR of less than 1 for the day 14 outcome, analytically for the ARD for the day 4 outcome and using Markov chain Monte Carlo methods for the HR for the day 14 outcome. According to the protocol, a posterior probability of more than 0·99 at the interim analysis or more than 0·95 at the final analysis indicated efficacy for both ARD and HR. We also calculated a posterior probability of ARD of less than −5·5% for the day 4 outcome and HR of less than 0·85 for the day 14 outcome (denoting moderate or greater effect). A futility boundary was set at a posterior probability of moderate or greater effect of less than 20% at the interim analysis. The treatment effect was summarised by the posterior median and equal-tail credible intervals (CrIs). We also did a prespecified subgroup analysis according to antiviral drug use at baseline; however, too few patients were on antivirals at baseline to enable this analysis. We did a post-hoc subgroup analysis according to use of corticosteroids, and particularly dexamethasone, in light of publications during the study period;21 however, too few patients were on dexamethasone at baseline to enable analysis of dexamethasone use. We did a post-hoc analysis according to C-reactive protein concentration. As a sensitivity analysis to the day 14 outcome, we repeated the analysis only considering mechanical ventilation and death (survival without need for mechanical ventilation). We analysed secondary outcomes using a frequentist framework, except for the analysis of the WHO-CPS scores as an ordinal variable. All efficacy analyses, except the posterior distribution of the ARD for the day 4 outcome, were adjusted for age and centre. The statistical analysis plan and details of the statistical analyses are in the appendix 2 (pp 12–14, 21–39).
We did all analyses on an intention-to-treat basis with no correction for multiplicity for prespecified secondary outcomes. Thus, these secondary outcomes are exploratory and reported as point estimates and 95% CrIs.
We analysed safety in all patients in the intention-to-treat population. We compared proportions of participants with at least one adverse event or at least one severe adverse event using Fisher's exact tests, and the total numbers of adverse events and severe adverse events using Poisson models.
We did all statistical analyses using SAS (version 9.4) or R (version 3.6.1). This study is registered with ClinicalTrials.gov, NCT04341584.
Data quality monitoring included both remote data monitoring and on-site monitoring done by dedicated staff who were independent of the site investigators, with source data verification done for all patients recruited at every site for all critical datapoints, inclusion and exclusion criteria, primary endpoints, survival until day 90 (appendix 2 p 13). On April 23, 2020, the DSMB met and recommended suspension of recruitment for futility on the basis of the interim analysis of the 102 first patients recruited, although the futility boundaries were not formally crossed. The sponsor decided to discontinue the study on April 26, 2020.
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between April 8 and April 26, 2020, 153 patients were screened and 116 were randomly assigned to the anakinra group (59) or the usual care group (57). Following an interim analysis, the study was closed to accrual on April 26, 2020 due to futility. Among the 57 patients assigned to receive usual care, two withdrew consent and were not analysed (figure 1 ). Among the 59 who received anakinra treatment, all received 2–15 injections of anakinra (median 11 [IQR 9–15]). Median dose of anakinra by perfusion was 180 mg (IQR 167–186), 55 (93%) patients received seven perfusions or more, and the median cumulative dose of anakinra was 1900 mg (1500–2700). Demographic and baseline clinical and biological characteristics of patients are shown in table 1 . The median age was 66 years (IQR 59–76) and 80 (70%) participants were men. The treatment groups were well balanced.
Figure 1.
Study profile
*According to French and European regulations, we were able to analyse data from patients who withdrew consent until the date of consent withdrawal, unless they asked for their personal data to be erased.
Table 1.
Baseline characteristics
| Anakinra group (n=59) | Usual care group (n=55) | ||
|---|---|---|---|
| Age, years | 67·0 (55·5–74·3; n=59) | 64·9 (59·5–78·3; n=55) | |
| Sex | |||
| Male | 43 (73%) | 37 (67%) | |
| Female | 16 (27%) | 18 (33%) | |
| Weight, kg | 78·0 (67·0–91·0; n=59) | 77·5 (70·0–95·0; n=46) | |
| BMI, kg/m2 | 27·4 (24·9 to 32·0; n=41) | 26·8 (24·7 to 31·5; n=42) | |
| ≥30 kg/m2* | 13/58 (22%) | 15/55 (27%) | |
| Temperature, °C | 37·8 (36·7–38·8; n=59) | 37·6 (37·0–38·5; n=55) | |
| Respiratory rate, breaths per min | 28·0 (24·0–32·0; n=55) | 28·0 (23·0–36·0; n=50) | |
| Oxygen flow, L/min | 5·0 (4·0–7·0; n=59) | 6·0 (4·0–9·0; n=55) | |
| SpO2, % | 95·0 (93·0–97·0; n=59) | 95·0 (93·0–97·0; n=55) | |
| Time from symptoms onset to randomisation, days | 10·0 (8·0–13·0; n=59) | 10·0 (7·0–13·0; n=54) | |
| Diagnosis of SARS CoV-2 infection† | |||
| Positive rRT-PCR | 54 (92%) | 48 (87%) | |
| Typical chest CT scan | 53 (90%) | 51 (93%) | |
| Coexisting conditions | |||
| Chronic cardiac disease | 22 (37%) | 14 (25%) | |
| Diabetes | 19 (32%) | 15 (27%) | |
| Chronic kidney disease (stage 1 to 3) or dialysis | 5 (8%) | 3 (5%) | |
| Asthma | 5 (8%) | 3 (5%) | |
| Chronic pulmonary disease (not asthma) | 6 (10%) | 3 (5%) | |
| Active malignant neoplasm | 5 (8%) | 6 (11%) | |
| Current or former smoker | 10/57 (17%) | 10/52 (18%) | |
| Laboratory values | |||
| C-reactive protein, mg/L | 121·0 (77·0–198·0; n=58) | 120·0 (87·0–191·5; n=52) | |
| D-dimer, μg/L | 991 (720–1499; n=50) | 1280 (750–2017; n=43) | |
| Ferritin, mg/L | 1479 (444–2334; n=38) | 1151 (847–2530; n=35) | |
| Neutrophil count, G/L | 5·4 (3·8–7·5; n=54) | 5·2 (3·4–7·1 n=50) | |
| Lymphocyte count, G/L | 0·8 (0·6–1·2; n=54) | 0·9 (0·7–1·3; n=50) | |
| Lymphocytes to neutrophils ratio | 0·2 (0·1–0·3; n=54) | 0·2 (0·1–0·4; n=50) | |
| Haemoglobin, g/dL | 12·3 (11·3–13·5; n=57) | 12·9 (11·7–13·8; n=55) | |
| Platelet count, g/L | 234 (166–327; n=57) | 263 (203–307; n=54) | |
| Alanine aminotransferase, IU/L | 44·0 (30·0–69·0; n=57) | 30·5 (20·0–48·5; n=52) | |
| Aspartate aminotransferase, IU/L | 56·0 (41·0–79·0; n=57) | 49·0 (35·0–68·0; n=50) | |
| Creatinine, μmol/L | 82·0 (69·0–101·0; n=57) | 70·0 (56·0–88·0; n=54) | |
| Lactate dehydrogenase, IU/L | 437 (346–614; n=44) | 475 (359–631; n=42) | |
| Treatments at baseline | |||
| Anticoagulants | 33 (59%) | 29 (53%) | |
| Azithromycin | 11 (19%) | 14 (25%) | |
| Hydroxychloroquine | 2 (3%) | 4 (7%) | |
| Lopinavir-ritonavir or lopinavir | 1 (2%) | 2 (4%) | |
| Other antivirals | 0 (0%) | 0 (0%) | |
| Dexamethasone | 1 (2%) | 0 (0%) | |
| Other glucocorticoids | 6 (10%) | 8 (15%) | |
Data are median (IQR; n) or n (%). BMI=body-mass index. rRT-PCR=real-time RT-PCR. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. SpO2=oxygen saturation.
This variable was also recorded as a binary condition at screening, hence the lower number of missing values.
All patients had either positive rRT-PCR for SARS-CoV-2 or chest CT scan typical of COVID-19 pneumonia.
During the trial, including any time before or after randomisation, in the anakinra group, two (3%) of 59 patients were given antiviral drugs, 30 (51%) were given glucocorticoids, 52 (88%) were given antibiotics, and 53 (90%) were given anticoagulants. In the usual care group, four (7%) of 55 patients were given antiviral drugs, 29 (53%) were given glucocorticoids, 48 (87%) were given antibiotics, and 49 (89%) were given anticoagulants. Additional immunomodulators were given to one (2%) patient in the anakinra group (tocilizumab). Details of treatments received at the time of and after randomisation until day 14 are in the appendix 2 (p 15).
On day 4, 21 (36%) of 59 patients in the anakinra group had a WHO-CPS score of more than 5 versus 21 (38%) of 55 in the usual care group (median posterior ARD −2·5% [90% CrI −17·1 to 12·0]; table 2 ). The posterior probability of any efficacy of anakinra (ie, ARD of less than 0) was 61·2% (table 2). The median posterior adjusted odds ratio was 0·90 (90% CrI 0·47 to 1·73).
Table 2.
Primary and secondary efficacy outcomes
| Anakinra group (n=59) | Usual care group (n=55) | Treatment effect | ||
|---|---|---|---|---|
| Coprimary outcomes | ||||
| WHO-CPS score of >5 points at day 4 | 21 (36%) | 21 (38%) | −2·5% (90% CrI −17·1 to 12·0)* | |
| Posterior probability of any benefit | .. | .. | 61·2% | |
| Posterior probability of moderate or greater benefit | .. | .. | 36·9% | |
| Non-invasive ventilation, mechanical ventilation or death up to day 14 | 28 (47%) | 28 (51%) | 0·97 (90% CrI 0·62 to 1·52)† | |
| Posterior probability of any benefit | .. | .. | 54·5% | |
| Posterior probability of moderate or greater benefit | .. | .. | 31·7% | |
| Secondary outcomes | ||||
| Overall survival | ||||
| Mortality at day 14 | 9 (15%) | 13 (24%) | 0·56 (95% CI 0·23 to 1·39)‡ | |
| Mortality at day 28 | 13 (22%) | 13 (24%) | 0·77 (95% CI 0·33 to 1·77)‡ | |
| Mortality at day 90 | 16 (27%) | 15 (27%)§ | 0·97 (95% CI 0·46 to 2·04)‡ | |
| WHO-CPS score (10-point scale) | ||||
| Day 4 | 5 (5 to 6) | 5 (5 to 6) | 0·80 (95% CrI 0·38 to 1·68)¶ | |
| Day 7 | 5 (5 to 7)‖ | 5 (5 to 7)** | 0·69 (95% CrI 0·33 to 1·43)¶ | |
| Day 14 | 5 (2 to 8)†† | 5 (3 to 8)** | 0·70 (95% CrI 0·35 to 1·38)¶ | |
| Day 2 to 14 (longitudinal analysis) | .. | .. | 0·92 (95% CrI 0·32 to 2·65)¶ | |
| Time to discharge | ||||
| Discharged at day 28 | 34 (58%) | 34 (62%) | 0·91 (95% CI 0·56 to 1·48)‡ | |
| Time to oxygen supply independency | ||||
| Independent from oxygen at day 28 | 37 (63%) | 38 (69%) | 1·01 (95% CI 0·64 to 1·61)‡ | |
Data are n (%), median (IQR), or estimate with 90% or 95% CrI or 95% CI in parentheses. 95% CrIs are shown for Bayesian analyses, and 95% CIs for frequentist analyses. CrI=credible interval. WHO-CPS=WHO Clinical Progression Scale.
Median posterior absolute risk difference.
Median posterior hazard ratio adjusted for age and centre.
Hazard ratio, adjusted for age and centre.
One patient died on day 91 and is not counted here.
Median posterior odds ratio in a proportional odds model, adjusted for age and centre.
n=54 with available data.
n=53 with available data.
n=56 with available data.
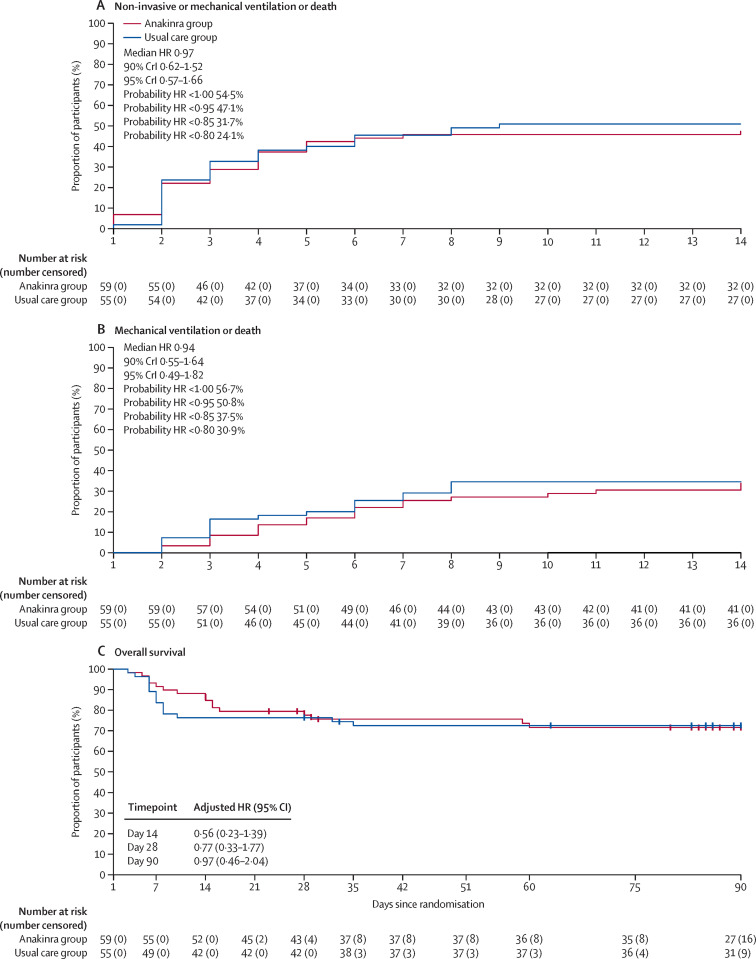
On day 14, at least one event of interest (non-invasive ventilation, high-flow oxygen, mechanical ventilation, or death) had occurred in 28 of 59 patients in the anakinra group (cumulative incidence of event 47%; 95% CI 33–59) and 28 of 55 patients in the usual care group (cumulative incidence 51% [95% CI 36–62]; figure 2 , table 2; appendix 2 p 15). The median posterior HR for survival with no need for mechanical or non-invasive ventilation was 0·97 (90% CrI 0·62 to 1·52), and the posterior probability of any efficacy of anakinra (ie, HR <1) was 54·5%, and of moderate or greater efficacy (HR <0·85) was 31·7%.
Figure 2.
Kaplan-Meier estimates of probability of mechanical or non-invasive ventilation or death (A), mechanical ventilation or death (B), and overall survival (C) during follow-up, for the anakinra group versus usual care group
In panel A, events occurring on day 1 occurred on the same day as but after randomisation. For the outcomes of death or ventilation support and death or mechanical ventilation, data are analysed in a Bayesian framework, and median posterior HRs and 90% CrIs are presented, together with posterior probabilities of achieving specified outcomes. Overall survival was analysed in a frequentist framework, and so posterior probabilities are not relevant and not calculated. In part C, HRs are adjusted for age and centre. CrI=credible interval. HR=hazard ratio.
In a sensitivity analysis of the day 14 outcome, the number of patients on mechanical ventilation or who had died at day 14 was 20 (34%) in the anakinra group and 19 (35%) in the usual care groups (figure 2).
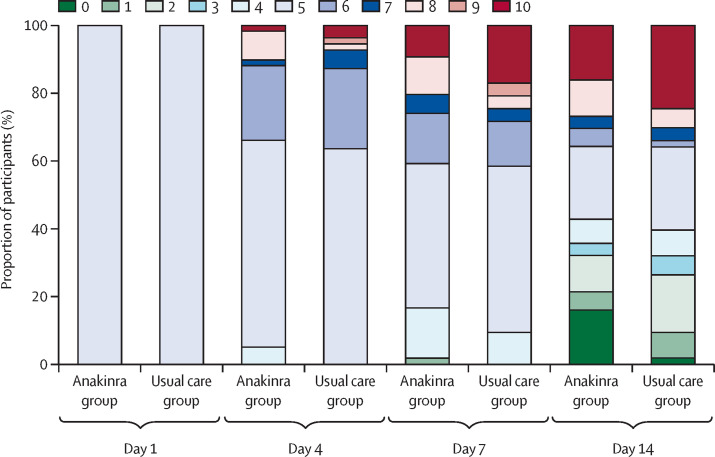
No difference was seen between groups in WHO-CPS score during 14-day follow up (table 2, figure 3 ; appendix 2 p 16). At day 90, 16 of 59 patients had died in the anakinra group (overall survival 72%, 95% CI 61–85) and 15 in the usual care group (overall survival 72%, 95% CI: 62–85; adjusted HR 0·97, 95% CI 0·46–2·04; figure 2, table 2; appendix 2 p 16).
Figure 3.
WHO Clinical Progression Scale score during 14-day follow up
The WHO Clinical Progression Scale is a 10-point scale, from 0 (uninfected and no symptoms) to 10 (death).
The cumulative incidence of patients who had been weaned from oxygen at day 28 was 63% (95% CI 49–74) in the anakinra group and 69% (55–80) in the usual care group (adjusted HR 1·01; 95% CI 0·64–1·61; table 2). The cumulative incidence of discharge from hospital by day 28 was 58% (95% CI 44–69) in the anakinra group and 62% (47–73) in the usual care group (adjusted HR 0·91 [95% CI 0·56 to 1·48; table 2).
Post-hoc analysis did not show any benefit of anakinra on the coprimary outcomes in subgroups of patients defined by C-reactive protein concentration of more than 150 mg/L and in patients with baseline corticosteroid use (appendix 2 p 18).
We assessed biological parameters over the course of 14 day follow up (appendix 2 p 20). Overall, we found no major difference between groups regarding the decrease of serum C-reactive protein level and the change in blood neutrophils and lymphocytes counts over time.
A total of 29 (49%) of 59 patients in the anakinra group and 23 (42%) of 55 in the usual care groups reported adverse events (table 3 ). 113 adverse events occurred in the anakinra group and 60 in the usual care group (p=0·0004 for the average number of events per patient). Serious adverse events occurred in 27 (46%) patients in the anakinra group and 21 (38%) in the usual care group (p=0·45). Bacterial and fungal sepsis occurred in 11 patients in the anakinra group (ten bacterial sepsis, one fungal sepsis) and in four patients in the usual care group (all bacterial sepsis; p=0·099).
Table 3.
Adverse events, serious adverse events, and causes of deaths
| Anakinra group (n=59) | Usual care group (n=55) | p value | ||
|---|---|---|---|---|
| Adverse events | ||||
| Patients with at least one adverse event | 29 (49%) | 23 (42%) | 0·46* | |
| Patients with multiple adverse events | 19 (32%) | 14 (25%) | .. | |
| Total number of adverse events | 113 | 60 | <0·0004† | |
| Serious adverse events | ||||
| Patients with at least one serious adverse event | 27 (46%) | 21 (38%) | 0·45* | |
| Patients with multiple serious adverse events | 8 (14%) | 5 (9%) | .. | |
| Total number of serious adverse events | 42 | 28 | 0·17† | |
| Events | ||||
| ARDS | 11 | 13 | .. | |
| Bacterial sepsis | 10 | 4 | .. | |
| Hepatic cytolysis | 7 | 0 | .. | |
| Multiple organ failure | 3 | 1 | .. | |
| Pulmonary embolism | 3 | 1 | .. | |
| Acute renal failure | 1 | 2 | .. | |
| Anaemia | 1 | 2 | .. | |
| Coronary syndrome | 1 | 0 | .. | |
| Cholestasis | 1 | 0 | .. | |
| Neutropenia | 1 | 0 | .. | |
| Fungal sepsis | 1 | 0 | .. | |
| Gastrointestinal bleeding | 1 | 0 | .. | |
| Myeloma | 1 | 0 | .. | |
| Sudden death | 0 | 1 | .. | |
| Arterial ischaemia | 0 | 1 | .. | |
| Thrombopenia | 0 | 1 | .. | |
| Complication of intubation | 0 | 1 | .. | |
| Metastatic progression | 0 | 1 | .. | |
| Causes of deaths | 16 (27%) | 16 (29%) | .. | |
| ARDS | 9 | 9 | .. | |
| Multiple organ failure | 3 | 0 | .. | |
| Bacterial sepsis | 2 | 2 | .. | |
| Pulmonary embolism and complication of intubation | 0 | 2 | .. | |
| Gastrointestinal bleeding | 1 | 0 | .. | |
| Bacterial sepsis and pulmonary embolism | 1 | 0 | .. | |
| Arterial ischaemia | 0 | 1 | .. | |
| Sudden death | 0 | 1 | .. | |
| Metastatic progression | 0 | 1 | .. | |
Data are n (%) or number of events. ARDS=acute respiratory distress syndrome.
Fisher's exact test.
Poisson model.
Discussion
We did not find any efficacy of anakinra (400 mg for 3 days, possibly repeated for 3 additional days) in patients with COVID-19 and mild-to-moderate pneumonia for decreasing the proportion of patients on non-invasive ventilation, high-flow oxygen, mechanical ventilation, or who died by day 14 of the proportion with a WHO-CPS score of more than 5 at day 4. Likewise, all the secondary outcomes, including survival up to 90 days, did not differ between the anakinra and the usual care groups.
Since the beginning of the COVID-19 pandemic, no definitive standard of care has emerged. The antiviral drug remdesivir reduced the length of recovery by 4 days but did not reduce the number of patients needing mechanical ventilation or the death rate.22 The RECOVERY collaborative group found that dexamethasone 6 mg per day for up to 10 days decreased 28-day mortality among patients receiving mechanical ventilation or oxygen.21 Therefore, dexamethasone is now largely used in most part of the world in the treatment of COVID-19.
Some observational studies have suggested the possible efficacy of anakinra for patients with mild-to-moderate, severe, or critical SARS-CoV-2 infection. The first retrospective monocentric cohort study was done in Italy and included patients with more severe illness than our study, all on non-invasive ventilation.18 This retrospective study only included patients with features of severe inflammatory status (serum C-reactive protein ≥100 mg/L or ferritin ≥900 ng/mL, or both). A low dose subcutaneous regimen of anakinra (100 mg twice a day) was ineffective, whereas a high dose (5 mg/kg twice a day intravenously) given to 29 patients led to a 77% decrease in mortality at 21 days compared with an historical control group of 16 patients with COVID-19 treated with usual care in the same hospital. However, in this study, mortality in patients with usual care was high, at around 50%.
The second prospective observational study was larger than the Italian study, and included patients from a single hospital in France who required oxygen support, and we included similar patients here in our study. Patients received subcutaneous anakinra at 100 mg twice a day for 3 days, then 100 mg daily for 7 days.19 Need for mechanical ventilation or death occurred in 13 (25%) of 52 patients in the anakinra group compared with 32 (73%) of 44 patients with COVID-19 treated with usual care in the same hospital. Day-28 mortality was also decreased by 50%. However, the day-28 mortality in the usual care group was again around 50%, whereas in our study mortality in the usual care group at day 28 was 24%.
In our trial, the chosen dose was approximately half that used in the Italian study, but we also chose an intravenous route that allows for better bioavailability of the drug. Although the typical dose of anakinra is 100–200 mg subcutaneous daily, we purposely used a dose and administration route that were around the same as those recently used successfully in haemophagocytic lymphohistiocytosis, in which hyper-inflammation is in the same range and even higher than in COVID-19.14, 15 However, we cannot exclude that anakinra might not inhibit lung inflammation because we used it at too low a dose. Alternatively, these negative findings might suggest that the hyperinflammatory status of most patients with mild-to-moderate COVID-19 pneumonia might not be due to excess of IL-1 signalling alone and might instead be a more subtle combination of proinflammatory cytokines.23
Our trial planned a statistical analysis of the coprimary outcomes after approximately 60 patients had reached day 14. After the second analysis, having included 114 patients up until this point, the DSMB decided to stop the trial on the ground of futility.
Strengths of this trial include the multicentre design, thorough monitoring to ensure data quality, and a homogeneous target population of patients with moderate pneumonia requiring at least 3 L/min of oxygen support. The groups were well balanced regarding baseline characteristics and additional treatments taken during the study.
Our study also had several limitations. The trial was not blinded because we aimed to collect data as quickly as possible in the pandemic setting, and so we did not have sufficient time to coordinate a double-blind academic study. Another limitation is that usual care could differ among centres and over time, especially regarding corticosteroid use. However, the short period of accrual and the stratification of randomisation might have restricted the effect of this absence of standardisation. The sample size was small, restricting the power of the study, and the CrIs and CIs were wide, but increasing the number of patients is unlikely to have affected our outcomes. Since arterial blood gas measurements were not done, we cannot provide an accurate measure of the ratio of partial pressure of oxygen to fractional concentration of oxygen in inspired air. Finally, in this trial we targeted a narrow segment of the COVID-19 patient population (patients with a WHO-CPS score of exactly 5 points and requiring at least 3 L/min of oxygen without any ventilatory support regardless of inflammatory status), and thus our results are not generalisable to the whole COVID-19 population. Another trial within the CORIMUNO platform (CORIMUNO-ANA-2) that aims to assess the effect of anakinra in patients with more severe COVID-19 who are in intensive care units (WHO-CPS score ≥6 points) has now been completed and is being analysed.
In summary, this randomised clinical trial suggests that anakinra was not effective in reducing the need for non-invasive or mechanical ventilation or death in patients with COVID-19 and mild-to-moderate pneumonia. These results are relevant for this patient population at the dose we used and cannot be extended to other populations with other doses. Further studies are needed to assess the efficacy of anakinra in other selected groups of patients with more severe COVID-19 and at other doses.
Correspondence to: Dr Xavier Mariette, Service D'Immuno-Rhumatologie Hôpital Bicêtre, Assistance Publique - Hôpitaux de Paris, 94270 Le Kremlin Bicêtre, France xavier.mariette@aphp.fr
Data sharing
The protocol will be available at ClinicalTrials.gov and the statistical analysis plan is available in the appendix 2 (pp 21–39). Consent forms, regulatory documents, and other relevant study materials were submitted to the journal with the manuscript. As described in the protocol, the trial steering committee will facilitate use of the study data and approval will not be unreasonably withheld. De-identified participant data collected during the CORIMUNO-ANA-1 trial (and the data dictionary) will be made available to bona fide researchers registered with an appropriate institution within 3 months of publication, and for 10 years thereafter. Proposals should be sent to Raphael Porcher (raphael.porcher@aphp.fr) and will be reviewed by the CORIMUNO scientific committee. The steering committee will need to be satisfied that any proposed publication is of high quality, honours the commitments made to the study participants in the consent documentation and ethical approvals, and is compliant with relevant legal and regulatory requirements (eg, relating to data protection and privacy). To gain access, data requesters will need to sign a data access agreement and confirm that data will only be used for the agreed purpose for which access was granted. The steering committee will have the right to review and comment on any draft manuscripts before publication.
Acknowledgments
Acknowledgments
This trial was publicly funded by the Ministry of Health, Programme Hospitalier de Recherche Clinique (PHRC COVID-19-20-0151, PHRC COVID-19-20-0029), Foundation for Medical Research, and AP-HP Foundation. Sobi (Puteaux, France) donated the anakinra and had no role in the study. An independent DSMB oversees all CORIMUNO trials (appendix 2 p 2). We thank all patients who participated in the CORIMUNO study, and their families. We also thank Maxime Dougados, who was in charge of the validation and opening of the centres, and the investigators who collaborated in this study (appendix 2 pp 2–11) and Universities of Paris, Paris-Saclay, Paris-Sorbonne, Paris-Nord Sorbonne, Paris-Est Créteil, Versailles-Saint Quentin and Strasbourg (Medical Students support), INSERM, and REACTing consortium for having provided medical students for help filling out electronic case report forms.
Contributors
XM, OH, PLT, MR-R, RP, and PR initially drafted the report and all members of the trial steering committee approved it before submission. MR-R organised collection of the data. RP and PR did the statistical analysis. All authors from the writing committee contributed to design of the trial and of study protocol, data interpretation and critical review and revision of the manuscript. The writing committee was responsible for the design, conduct, and reporting of the trial. RP and MR-R accessed and verified the raw data and vouch for the data and analyses, and for the fidelity of this report to the study protocol and statistical analysis plan. All members of the writing committee had full access to all of the data and the final responsibility to submit for publication.
The CORIMUNO-19 Collaborative group
Writing committee: France Xavier Mariette (Université Paris-Saclay, Assistance Publique-Hôpitaux de Paris [AP-HP], Hôpital Bicêtre, INSERM, Le Kremlin Bicêtre), Olivier Hermine (Université de Paris, AP-HP, Hôpital Necker, INSERM, Imagine Institute, Paris), Pierre Louis Tharaux (Paris Cardiovascular Centre, Université de Paris, INSERM, Paris), Matthieu Resche-Rigon (Centre of Research in Epidemiology and Statistics, Université de Paris, INSERM, Hôpital Saint Louis, Paris), Raphael Porcher, Philippe Ravaud (Centre of Research in Epidemiology and Statistics, Université de Paris, INSERM, INRAE, AP-HP, Hôpital Hôtel-Dieu, Paris). Steering Committee: France Philippe Ravaud (chair), Serge Bureau (AP-HP), Maxime Dougados (AP-HP), Olivier Hermine, Xavier Mariette, Matthieu Resche-Rigon, Pierre-Louis Tharaux, Annick Tibi (AP-HP).
Declaration of interests
The writing committee declares no competing interests.
Contributor Information
The CORIMUNO-19 Collaborative group:
Pierre-Louis Tharaux, Gilles Pialoux, Arthur Pavot, Xavier Mariette, Olivier Hermine, Matthieu Resche-Rigon, Raphael Porcher, Philippe Ravaud, Serge Bureau, Maxime Dougados, Annick Tibi, Elie Azoulay, Jacques Cadranel, Joseph Emmerich, Muriel Fartoukh, Bertrand Guidet, Marc Humbert, Karine Lacombe, Matthieu Mahevas, Frédéric Pene, Valérie Pourchet-Martinez, Frédéric Schlemmer, Yazdan Yazdanpanah, Gabriel Baron, Elodie Perrodeau, Damien Vanhoye, Cécile Kedzia, Lauren Demerville, Anne Gysembergh-Houal, Alexandre Bourgoin, Sarah Dalibey, Nabil Raked, Lakhdar Mameri, Stéphanie Alary, Samir Hamiria, Thinhinane Bariz, Hala Semri, Dhiaa Meriem Hai, Moustafa Benafla, Mohamed Belloul, Pernelle Vauboin, Saskia Flamand, Claire Pacheco, Anouk Walter-Petrich, Emilia Stan, Souad Benarab, Corine Nyanou, Claire Montlahuc, Lucie Biard, Robin Charreteur, Celine Dupré, Kévin Cardet, Blandine Lehmann, Kamyl Baghli, Claire Madelaine, Eric D'Ortenzio, Oriane Puéchal, Caroline Semaille, Laurent Savale, Anatole Harrois, Samy Figueiredo, Jacques Duranteau, Nadia Anguel, Xavier Monnet, Christian Richard, Jean-Louis Teboul, Philippe Durand, Pierre Tissieres, Mitja Jevnikar, David Montani, Sophie Bulifon, Xavier Jaïs, Olivier Sitbon, Stéphan Pavy, Nicolas Noel, Olivier Lambotte, Lelia Escaut, Stéphane Jauréguiberry, Elodie Baudry, Christiane Verny, Mathilde Noaillon, Edouard Lefèvre, Mohamad Zaidan, Clotilde Le Tiec Le Tiec, Céline Verstuyft Verstuyft, Anne-Marie Roques, Lamiae Grimaldi, Domitille Molinari, Gaël Leprun, Alain Fourreau, Laurent Cylly, Myriam Virlouvet, Ramdane Meftali, Solène Fabre, Marion Licois, Asmaa Mamoune, Yacine Boudali, Sophie Georgin-Lavialle, Patricia Senet, Angèle Soria, Antoine Parrot, Hélène François, Nathalie Rozensztajn, Emmanuelle Blin, Pascaline Choinier, Juliette Camuset, Jean-Simon Rech, Antony Canellas, Camille Rolland-Debord, Nadège Lemarié, Nicolas Belaube, Marine Nadal, Martin Siguier, Camille Petit-Hoang, Julie Chas, Elodie Drouet, Matthieu Lemoine, Audrey Phibel, Lucie Aunay, Eliane Bertrand, Sylviane Ravato, Marie Vayssettes, Anne Adda, Celine Wilpotte, Pélagie Thibaut, Julie Fillon, Isabelle Debrix, Soraya Fellahi, Jean-Philippe Bastard, Guillaume Lefèvre, Vincent Fallet, Jacques-Eric Gottenberg, Yves Hansmann, Emmanuel Andres, Sophie Bayer, Guillaume Becker, Frédéric Blanc, Stéphane Brin, Vincent Castelain, Emmanuel Chatelus, Eva Chatron, Olivier Collange, François Danion, Frédéric De Blay, Eric Demonsant, Pierre Diemunsch, Sophie Diemunsch, Renaud Felten, Bernard Goichot, Valentin Greigert, Aurélien Guffroy, Bob Heger, Anne Hutt, Charlotte Kaeuffer, Loic Kassegne, Anne Sophie Korganow, Pierrick Le Borgne, Nicolas Lefebvre, Tristan Martin, Paul Michel Mertes, Catherine Metzger, Nicolas Meyer, Gabriel Nisand, Eric Noll, Mathieu Oberlin, Sophie Ohlmann-Caillard, Vincent Poindron, Julien Pottecher, Yvon Ruch, Cédric Sublon, Hakim Tayebi, François Weill, Arsène Mekinian, Dorothée Chopin, Olivier Fain, Marc Garnier, Jessica Krause le Garrec, Marjolaine Morgand, Jerome Pacanowski, Tomas Urbina, Chloe McAvoy, Maria Pereira, Gladys Aratus, Laurence Berard, Tabassome Simon, Anne Daguenel-Nguyen, Marie Antignac, Céline Leplay, Jean-Benoit Arlet, Jean-Luc Diehl, Florence Bellenfant, Anne Blanchard, Alexandre Buffet, Bernard Cholley, Antoine Fayol, Edouard Flamarion, Anne Godier, Thomas Gorget, Sophie-Rym Hamada, Caroline Hauw-Berlemont, Jean-Sébastien Hulot, David Lebeaux, Marine Livrozet, Adrien Michon, Arthur Neuschwander, Marie-Aude Penet, Benjamin Planquette, Brigitte Ranque, Olivier Sanchez, Geoffroy Volle, Sandrine Briois, Mathias Cornic, Virginie Elisee, Denis, Jesuthasan, Juliette Djadi-Prat, Pauline Jouany, Ramon Junquera, Mickael Henriques, Amina Kebir, Isabelle Lehir, Jeanne Meunier, Florence Patin, Valérie Paquet, Anne Tréhan, Véronique Vigna, Brigitte Sabatier, Damien Bergerot, Charléne Jouve, Camille Knosp, Olivia Lenoir, Nassim Mahtal, Léa Resmini, F-Xavier Lescure, Jade Ghosn, Antoine BACHELARD, Timothee BIRONNE, Raphael BORIE, Agathe BOUNHIOL, Catherine BOUSSARD, Jeanne CHAUFFiER, Solaya CHALAL, Lynda CHALAL, Malikhone CHANSOMBAT, Paul CRESPIN, Bruno CRESTANI, Olivia DACONCEICAO, Laurene DECONINCK, Philippe DIEUDE, Antoine DOSSIER, Marie DUBERT, Greggory DUCROCQ, Axelle FUENTES, Anne GERVAIS, Marie GILBERT, Valentina ISERNIA, Sophie ISMAEL, Veronique JOLY, Zelie JULIA, Sylvie LARIVEN, Sylvie LE GAC, Diane LE PLUART, Francoise LOUNI, Awa NDIAYE, Thomas PAPO, Marion PARISEY, Bao PHUNG, Annabelle POURBAIX, Anne RACHLINE, Christophe RIOUX, Aurelie SAUTEREAU, Gabriel STEG, Hassan TARHINI, Simon VALAYER, Dorothee VALLOIS, Paul VERMES, Thomas VOLPE, Yann Nguyen, Vasco Honsel, Emmanuel Weiss, Anaïs Codorniu, Virginie Zarrouk, Victoire De Lastours, Matthieu Uzzan, Olivier Olivier, Geoffrey Rossi, Naura Gamany, Roza Rahli, Zeina Louis, David Boutboul, Lionel Galicier, Yaël Amara, Gabrielle Archer, Amira Benattia, Anne Bergeron, Louise Bondeelle, Nathalie De Castro, Melissa Clément, Michaël Darmont, Blandine Denis, Clairelyne Dupin, Elsa Feredj, Delphine Feyeux, Adrien Joseph, Etienne Lengliné, Pierre Le Guen, Geoffroy Liégeon, Gwenaël Lorillon, Asmaa Mabrouki, Eric Mariotte, Grégoire Martin de Frémont, Adrien Mirouse, Jean-Michel Molina, Régis Peffault de Latour, Eric Oksenhendler, Julien Saussereau, Abdellatif Tazi, Jean-Jacques Tudesq, Lara Zafrani, Isabelle Brindele, Emmanuelle Bugnet, Karine Celli Lebras, Julien Chabert, Lalia Djaghout, Catherine Fauvaux, Anne Lise Jegu, Ewa Kozaliewicz, Martine Meunier, Marie-Thérèse Tremorin, Claire Davoine, Isabelle Madeleine, Sophie Caillat-Zucman, Constance Delaugerre, Florence Morin, Damien SENE, Ruxandra BURLACU, Benjamin CHOUSTERMAN, Bruno MEGARBANE, Pascal RICHETTE, Jean-Pierre RIVELINE, Aline FRAZIER, Eric VICAUT, Laure BERTON, Tassadit HADJAM, Miguel Alejandro VASQUEZ-IBARRA, Clément JOURDAINE, Aude JACOB, Julie SMATI, Stéphane RENAUD, Philippe MANIVET, Claire PERNIN, Lydia SUAREZ, Luca Semerano, Sebastien ABAD, Ruben Benainous, Coralie Bloch Queyrat, Nicolas Bonnet, Sabrina Brahmi, johann Cailhol, Yves Cohen, Celine Comparon, Hugues Cordel, Robin Dhote, Nathalie Dournon, Boris Duchemann, Nathan Ebstein, Benedicte Giroux-Leprieur, Jeanne Goupil de Bouille, Anne Jacolot, Hilario Nunes, Johanna Oziel, Vanessa Rathouin, Marthe Rigal, Dominique Roulot, Claire Tantet, Yurdagul Uzunhan, Nathalie COSTEDOAT-CHALUMEAU, Zakaria Ait Hamou, Sarah Benghanem, Philippe BLANCHE, Etienne CANOUI, Nicolas CARLIER, Benjamin CHAIGNE, Adrien CONTEJEAN, Bertrand DUNOGUE, Pierre DUPLAND, Aurélie DUREL - MAURISSE, Remy GAUZIT, Paul JAUBERT, Hassan Joumaa, Mathieu Jozwiak, Solen KERNEIS, Marie LACHATRE, Hélène Lafoeste, Paul LEGENDRE, Liem Binh LUONG NGUYEN, Jonathan MAREY, Caroline MORBIEU, Luc MOUTHON, Lee NGUYEN, Lola-Jade Palmieri, Alexis REGENT, Tali-Anne SZWEBEL, Benjamin TERRIER, Corinne GUERIN, Jérémie ZERBIT, Kahina CHEREF, Kamil CHITOUR, Mamadou Salif CISSE, Ada CLARKE, Gaelle CLAVERE, Isabelle DUSANTER, Caroline GAUDEFROY, Moez JALLOULI, Sami KOLTA, Catherine LE BOURLOUT, Nathalie MARIN, Nathalie MENAGE, Alexandre MOORES, Isabelle PEIGNEY, Cédric PIERRON, Samira SALEH-MGHIR, Mathilde VALLET, Marc MICHEL, Giovanna MELICA, Jean-Daniel LELIEVRE, Elena FOIS, Pascal LIM, Marie MATIGNON, Constance GUILLAUD, Alaki THIEMELE, David SCHMITZ, Marion BOUHRIS, Syllia BELAZOUZ, Laetitia LANGUILLE, Armand MEKONTSO-DESSAPS, Thiziri SADAOUI, Julien Mayaux, Patrice Cacoub, Jean-Christophe Corvol, Céline Louapre, Sara Sambin, Louise-Laure Mariani, Carine Karachi, Florence Tubach, Candice Estellat, Linda Gimeno, Karine Martin, Aïcha Bah, Vixra Keo, Sabrine Ouamri, Yasmine Messaoudi, Nessima Yelles, Pierre Faye, Sébastien Cavelot, Cecile Larcheveque, Laurence Annonay, Jaouad Benhida, Aida Zahrate-Ghoul, Soumeya Hammal, Ridha Belilita, Marie Lecronier, Alexandra Beurton, Luc Haudebourg, Robin Deleris, Julien Le Marec, Sara Virolle, Safaa Nemlaghi, Côme Bureau, Pierre Mora, Martin De Sarcus, Olivier Clovet, Baptiste Duceau, Paul Henri Grisot, Marie hélène Pari, Jérémy Arzoine, Ulrich Clarac, Morgane Faure, Julie Delemazure, Maxence Decavele, Elise Morawiec, Alexandre Demoule, Martin Dres, Mathieu Vautier, Yves Allenbach, Olivier Benveniste, Gaelle Leroux, Aude Rigolet, Perrine Guillaume-Jugnot, Fanny Domont, Anne Claire Desbois, Cloé Comarmond, Nicolas Champtiaux, Segolene Toquet, Amine Ghembaza, Matheus Vieira, Georgina Maalouf, Gonçalo Boleto, Yasmina Ferfar, Fanny Charbonnier, Claire AGUILAR, Fanny ALBY-LAURENT, Marie-Alexandra ALYANAKIAN, Prissile BAKOUBOULA, Christine BROISSAND, Carole BURGER, Clara CAMPOS-VEGA, Nathalie CHAVAROT, Laure CHOUPEAUX, Benjamin FOURNIER, Sophie GRANVILLE, Elodie ISSORAT, Claire ROUZAUD, Damien VIMPERE, Guillaume Geri, Nawal Derridj, Naima Sguiouar, Hakim Meddah, Mourad Djadel, Helene Chambrin-Lauvray, Jean-Charles Duclos-Vallée, Faouzi Saliba, Sophie-Caroline Sacleux, Ilias Koumis, Jean-Marie Michot, Annabelle Stoclin, Emeline Colomba, Fanny Pommeret, Chistophe Willekens, Madona Sakkal, Rosa Da Silva, Valérie Dejean, Yasmina Mekid, Ines Ben-Mabrouk, Caroline Pradon, Laurence Drouard, Valérie Camara-Clayette, Alexandre Morel, Gilles Garcia, Abolfazl Mohebbi, Férial Berbour, Mélanie Dehais, Anne-Lise Pouliquen, Alison Klasen, Loren Soyez-Herkert, Jonathan London, Younes Keroumi, Emmanuelle Guillot, Guillaume Grailles, Younes El Amine, Fanny Defrancq, Hanane Fodil, Chaouki Bouras, Dominique Dautel, Nicolas Gambier, Thierno Dieye, Anaïs Razurel, Boris Bienvenu, Victor Lancon, Laurence Lecomte, Kristina Beziriganyan, Belkacem Asselate, Laure Allanic, Elena Kiouris, Marie-Hélène Legros, Christine Lemagner, Pascal Martel, Vincent Provitolo, Félix Ackermann, Mathilde Le Marchand, Aurélie Clan Hew Wai, Dimitri Fremont, Elisabeth Coupez, Mireille Adda, Frédéric Duée, Lise Bernard, Antoine Gros, Estelle Henry, Claire Courtin, Anne Pattyn, Pierre-Grégoire Guinot, Marc Bardou, Agnes Maurer, Julie Jambon, Amélie Cransac, Corinne Pernot, Bruno Mourvillier, Amélie Servettaz, Gaetan Deslée, Alain Wynckel, Philippe Benoit, Eric Marquis, Damien Roux, Coralie Gernez, Cécile Yelnik, Julien Poissy, Mandy Nizard, Fanette Denies, Hélène Gros, Jean-Jacques Mourad, Emmanuelle Sacco, and Sophie Renet
Supplementary Materials
References
- 1.Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020;71:706–712. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children*. Pediatr Crit Care Med. 2014;15:401–408. doi: 10.1097/PCC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 10.Ocon AJ, Bhatt BD, Miller C, Peredo RA. Safe usage of anakinra and dexamethasone to treat refractory hemophagocytic lymphohistiocytosis secondary to acute disseminated histoplasmosis in a patient with HIV/AIDS. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-221264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Néel A, Wahbi A, Tessoulin B, et al. Diagnostic and management of life-threatening adult-onset still disease: a French nationwide multicenter study and systematic literature review. Crit Care. 2018;22:88. doi: 10.1186/s13054-018-2012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lind-Holst M, Hartling UB, Christensen AE. High-dose anakinra as treatment for macrophage activation syndrome caused by refractory Kawasaki disease in an infant. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2019-229708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wohlfarth P, Agis H, Gualdoni GA, et al. Interleukin 1 receptor antagonist anakinra, intravenous immunoglobulin, and corticosteroids in the management of critically ill adult patients with hemophagocytic lymphohistiocytosis. J Intensive Care Med. 2019;34:723–731. doi: 10.1177/0885066617711386. [DOI] [PubMed] [Google Scholar]
- 14.Eloseily EM, Weiser P, Crayne CB, et al. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2020;72:326–334. doi: 10.1002/art.41103. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos G, de Mast Q, Markou N, et al. Favorable anakinra responses in severe COVID-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020;28:117–123. doi: 10.1016/j.chom.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson LA, Canna SW, Schulert GS, et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020;72:1059–1063. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aouba A, Baldolli A, Geffray L, et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020;79:1381–1382. doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- 18.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The protocol will be available at ClinicalTrials.gov and the statistical analysis plan is available in the appendix 2 (pp 21–39). Consent forms, regulatory documents, and other relevant study materials were submitted to the journal with the manuscript. As described in the protocol, the trial steering committee will facilitate use of the study data and approval will not be unreasonably withheld. De-identified participant data collected during the CORIMUNO-ANA-1 trial (and the data dictionary) will be made available to bona fide researchers registered with an appropriate institution within 3 months of publication, and for 10 years thereafter. Proposals should be sent to Raphael Porcher (raphael.porcher@aphp.fr) and will be reviewed by the CORIMUNO scientific committee. The steering committee will need to be satisfied that any proposed publication is of high quality, honours the commitments made to the study participants in the consent documentation and ethical approvals, and is compliant with relevant legal and regulatory requirements (eg, relating to data protection and privacy). To gain access, data requesters will need to sign a data access agreement and confirm that data will only be used for the agreed purpose for which access was granted. The steering committee will have the right to review and comment on any draft manuscripts before publication.