Abstract
Cell culture medium, nasopharyngeal and sera samples spiked with SARS-CoV-2 were subjected to heat inactivation for various periods of time, ranging from 30 s to 60 min. Our results showed that SARS-CoV-2 could be inactivated in less than 30 min, 15 min, and 3 min at 56 °C, 65 °C, and 95 °C, respectively. These data could help laboratory workers to improve their protocols by handling the virus in biosafety conditions.
Keywords: Heat, Inactivation, SARS-CoV-2, Covid-19
In December 2019, a new coronavirus named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) emerged in Wuhan City, Hubei Province in China and quickly became pandemic, spreading to almost all countries in about 2 months. This highly contagious virus has already caused a great number of infections and deaths, and the scientific community is facing new challenges to combat the ongoing outbreak. As no specific therapeutics and vaccines are available for disease control, lockdowns, social distancing, and quick detection of cases are currently the main weapons against the virus. Diagnostic and serological tools help to detect SARS-CoV-2 carriers and immunized recovered patients as soon as possible, and are therefore part of this fighting strategy and necessary to consider a return to normal life. In this context, viral inactivation procedures are urgently needed to allow safe experimental laboratory conditions. Amplification of viral RNA by quantitative RT-PCR is currently the gold standard procedure for diagnosis recommended by the World Health Organization.1 Viral RNA extraction kits sometimes require an initial lysis step at 70 °C or more for 5 min. Heat inactivation of the virus is also needed for serum treatment before ELISA and serological assays.
Although 56 °C is commonly used for inactivation of enveloped viruses,2, 3 higher temperatures can be used in some cases.4, 5, 6 This temperature is also used to eliminate serum complement. In this study, we exposed SARS-CoV-2 to three different inactivation temperatures (56 °C, 65 °C, and 95 °C) for various periods of time and tested its infectivity using the TCID50 method.
A human strain of SARS-CoV-2, isolated from a French patient hospitalized in February 2020 (GISAID accession number: EPI_ISL_437689), was grown on Vero E6 cells for three passages. The cells were maintained in Dulbecco's modified Eagle's medium (DMEM 1X, GIBCO) supplemented with 5% fetal calf serum (FCS) and antibiotics (0.1 units penicillin, 0.1 mg/mL streptomycin, GIBCO) at 37 °C in a humidified 5% CO2 incubator. The SARS-CoV-2 virus was titrated by the TCID50 method, as described previously,7 except that Vero E6 cells were used and examination for cytopathic effect was performed after 5 days. The viral titer was adjusted from 6.5 log10 TCID50/mL to a final titer of 6 log10 TCID50/mL in three different kinds of media, that is, cell culture medium (DMEM 1X), pooled nasopharyngeal samples of several patients tested negative for SARS-CoV-2, and pooled sera from donors collected before the 1st of January 2020 at a time when the virus was not circulating in France.
Diluted samples (500 µL) were submitted in triplicate to various temperatures for different periods of time in a calibrated and verified dry water bath, cooled on ice, and tested for infectivity by the TCID50 method as described above. All experiments were conducted under strict BSL3 conditions.
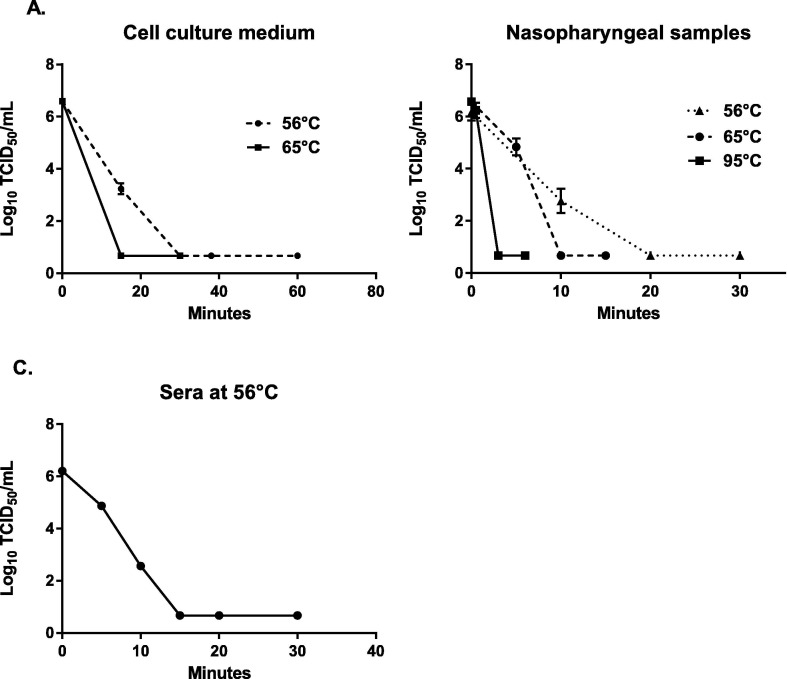
The mean viral titers obtained for each condition are presented in Table 1, Table 2, Table 3 . Viral inactivation over time is shown in Fig. 1 .
Table 1.
Log10 TCID50 per mL titers obtained after inactivation of cell culture medium containing 6 log10 TCID50/mL of SARS-CoV-2. ND: not detected (below the limit of virus detection which corresponded to 0.67 log10 TCID50 per ml).
| 56 °C |
65 °C |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | 0 | 15 | 30 | 38 | 60 | 0 | 15 | 30 | 60 | |
| Cell culture medium | 6.6 | 3.23 | ND | ND | ND | 6.6 | ND | ND | ND | |
Table 2.
Log10 TCID50 per mL titers obtained after inactivation of nasopharyngeal samples spiked with 6 log10 TCID50/mL of SARS-CoV-2. ND: not detected (below the limit of virus detection which corresponded to 0.67 log10 TCID50 per ml).
| 56 °C |
65 °C |
95 °C |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | 0 | 10 | 20 | 30 | 0 | 5 | 10 | 15 | 0 | 0.5 | 3 | 6 | ||
| Nasopharyngeal samples | 6.17 | 2.77 | ND | ND | 6.57 | 4.83 | ND | ND | 6.57 | 6.23 | ND | ND | ||
Table 3.
Log10 TCID50 per mL titers obtained after inactivation of human sera spiked with 6 log10 TCID50/mL of SARS-CoV-2. ND: not detected (below the limit of virus detection which corresponded to 0.67 log10 TCID50 per ml).
| 56 °C |
||||||
|---|---|---|---|---|---|---|
| Time (min) | 0 | 5 | 10 | 15 | 20 | 30 |
| Sera | 6.2 | 4.87 | 2.57 | ND | ND | ND |
Fig. 1.
Viral titers in log10 TCID50/mL obtained after heat inactivation of infected cell culture medium (A), nasopharyngeal samples spiked with SARS-CoV-2 (B), and sera samples from negative donors spiked with SARS-CoV-2 (C). Each condition was performed in triplicate. Each dot represents the mean viral titer and vertical lines represent the standard deviation.
At 56 °C, no infectious virus was detected within 30 min in cell culture medium (Fig. 1A) and within 20 min in nasopharyngeal samples and sera (Fig. 1B and 1C). As expected, increasing the temperature had a negative effect on viral infectivity as no infectious virus was detected within 15 min at 65 °C (Fig. 1A and 1B). At 95 °C, 3 min was sufficient to inactivate the virus in nasopharyngeal samples (Fig. 1B). The high quantity of infectious virus detected after 30 s (6.23 log10 TCID50/mL) was probably due to the time necessary for the media and the tubes to reach 95 °C inside.
To complement our results, viral RNA from each sample, except from nasopharyngeal samples treated at 56 °C, was extracted using a Nucleospin RNA Virus kit (Macherey-Nagel) for quantitative RT-PCR. Primers and probes targeting two regions of the RdRP gene developed by the French National Reference Center for Respiratory Infections Viruses and a LightCycler 480 II instrument were used as previously described.8 The E gene was used as a tertiary target for confirmation, following the Charité protocol.9 The results showed that the mean genomic RNA copy number ranged between 9.9 × 107 copy genome/5 µL and 5.3 × 109 copy genome/5 µL (data not shown). For each condition, the quantity of viral RNA was very stable over time, suggesting that viral RNA remained intact in virus particles. At 95 °C, the results showed a slight decrease in viral RNA quantity over time with approximately a 2.2-fold increase in the cq value, which could potentially prevent SARS-CoV-2 detection in samples with low viral loads.10, 11
These results are similar to our previous results obtained with the Middle East Respiratory Syndrome coronavirus (MERS-CoV),5 which belongs to the same genus of viruses. In conclusion, SARS-CoV-2 is relatively sensitive to heat inactivation under our laboratory conditions. These data can help laboratory workers to elaborate and improve their protocols for SARS-CoV-2 experiments, and reinforce our current knowledge on coronavirus survival.12, 13
CRediT authorship contribution statement
Christophe Batéjat: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing - review & editing. Quentin Grassin: Investigation. Jean-Claude Manuguerra: Resources, Writing - review & editing. India Leclercq: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.WHO-COVID-19-lab_testing-2020.1-eng.pdf. Accessed April 28, 2020. https://apps.who.int/iris/bitstream/handle/10665/331509/WHO-COVID-19-lab_testing-2020.1-eng.pdf
- 2.Cutts T., Grolla A., Jones S., Cook B.W.M., Qiu X., Theriault S.S. Inactivation of Zaire ebolavirus variant Makona in human serum samples analyzed by enzyme-linked immunosorbent assay. J Infect Dis. 2016;214(suppl 3):S218–S221. doi: 10.1093/infdis/jiw289. [DOI] [PubMed] [Google Scholar]
- 3.Park S.L., Huang Y.-J.S., Hsu W.-W., Hettenbach S.M., Higgs S., Vanlandingham D.L. Virus-specific thermostability and heat inactivation profiles of alphaviruses. J Virol Methods. 2016;234:152–155. doi: 10.1016/j.jviromet.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Edwards S. Survival and inactivation of classical swine fever virus. Vet Microbiol. 2000;73(2):175–181. doi: 10.1016/S0378-1135(00)00143-7. [DOI] [PubMed] [Google Scholar]
- 5.Leclercq I., Batéjat C., Burguière A.M., Manuguerra J.-C. Heat inactivation of the Middle East respiratory syndrome coronavirus. Influenza Other Respir Viruses. 2014;8(5):585–586. doi: 10.1111/irv.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saluzzo J.F., Leguenno B., Van der Groen G. Use of heat inactivated viral haemorrhagic fever antigens in serological assays. J Virol Methods. 1988;22(2-3):165–172. doi: 10.1016/0166-0934(88)90099-7. [DOI] [PubMed] [Google Scholar]
- 7.Dublineau A, Batéjat C, Pinon A, Burguière AM, Leclercq I, Manuguerra J-C. Persistence of the 2009 pandemic influenza A (H1N1) virus in water and on non-porous surface. PloS One 2011;6(11):e28043. doi:10.1371/journal.pone.0028043 [DOI] [PMC free article] [PubMed]
- 8.real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf. Accessed April 29, 2020. https://www.who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf?sfvrsn=3662fcb6_2
- 9.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3). doi:10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed]
- 10.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wölfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020:1–5. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 12.Chin A.W.H., Chu J.T.S., Perera M.R.A. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020;0(0) doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]