Abstract
Background
Atrial fibrillation (AF) is the most encountered arrhythmia and has been associated with worse in-hospital outcomes.
Objective
This study was to determine the incidence of AF in patients hospitalized with coronavirus disease 2019 (COVID-19) as well as its impact on in-hospital mortality.
Methods
Patients hospitalized with a positive COVID-19 polymerase chain reaction test between March 1 and April 27, 2020, were identified from the common medical record system of 13 Northwell Health hospitals. Natural language processing search algorithms were used to identify and classify AF. Patients were classified as having AF or not. AF was further classified as new-onset AF vs history of AF.
Results
AF occurred in 1687 of 9564 patients (17.6%). Of those, 1109 patients (65.7%) had new-onset AF. Propensity score matching of 1238 pairs of patients with AF and without AF showed higher in-hospital mortality in the AF group (54.3% vs 37.2%; P < .0001). Within the AF group, propensity score matching of 500 pairs showed higher in-hospital mortality in patients with new-onset AF as compared with those with a history of AF (55.2% vs 46.8%; P = .009). The risk ratio of in-hospital mortality for new-onset AF in patients with sinus rhythm was 1.56 (95% confidence interval 1.42–1.71; P < .0001). The presence of cardiac disease was not associated with a higher risk of in-hospital mortality in patients with AF (P = .1).
Conclusion
In patients hospitalized with COVID-19, 17.6% experienced AF. AF, particularly new-onset, was an independent predictor of in-hospital mortality.
Keywords: Atrial fibrillation, SARS-CoV-2, Pandemic, COVID-19, Mortality
Introduction
Atrial fibrillation (AF) is the most common arrhythmia and has been associated with worse outcomes in hospitalized patients.1 , 2 Its prevalence and clinical impact are even more prominent in patients with pulmonary disease, critical illness, and systemic inflammatory response syndrome.3, 4, 5
Coronavirus disease 2019 (COVID-19) in hospitalized patients is often characterized by respiratory involvement with hypoxia, systemic inflammatory response, and cardiac involvement, all of which are known predisposing factors for the development of AF. In addition, the onset of AF has deleterious hemodynamic effects6 that can further deteriorate the clinical presentation of a tenuous patient. Finally, the hypercoagulable state during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to influence thromboembolic events related with AF.7 The objectives of this study were to examine the incidence of AF in patients hospitalized with COVID-19 and to evaluate its impact on in-hospital mortality.
Methods
Study design and cohort definition
The study was conducted at 13 hospitals in the Northwell Health system. Patients admitted between March 1 and April 27, 2020, with a positive COVID-19 polymerase chain reaction test were included in the study. Patients were followed through May 31, 2020. Patients with multiple readmissions during the study period were evaluated as a single presentation. Patients with a negative or absent nasopharyngeal swab, patients without progress notes or documented basic laboratory values within the first 96 hours of initial registration, patients who were still in the hospital as of May 31, and those whose outcome could not be determined as of May 31 were excluded.
The Northwell Health Institutional Review Board approved this study. The research reported in this article adhered to Helsinki Declaration as revised in 2013.
Data collection
For data extraction, a detailed electronic medical record (EMR) search was performed, which included scanning of all medical notes, diagnoses, medications, orders, and electrocardiogram and telemetry interpretations. First available laboratory results within 96 hours from the initial presentation were used for the analyses. Natural language processing (NLP) techniques were used to detect AF diagnosis for each patient from a total of >24 million progress notes in the data set. To this end, a rule-based text classification algorithm was developed using the word “atrial fibrillation” and all possible variants (eg, “atrial flutter,” “rapid ventricular rate,” and “paroxysmal atrial fibrillation”) and abbreviations (eg, “AF,” “afib,” “RVR,” and “PAF”). The algorithm was designed to include misspellings and hyphenations in acronyms (eg, “a-fib”). It excluded irrelevant acronym matches and false partial matches (eg, “has a fibroid”). Furthermore, a set of explicit language rules were developed to handle negation (eg, “no evidence of AF”), uncertainty (eg, “at risk of AF”), a reference to family history, and other false-positive cases such as “afib[ ]” (where missing an “X” in square brackets indicates the absence). Python’s NLTK library was used to tokenize the text into sentences and regular expressions module was used to develop and implement inclusion/exclusion rules at the sentence level. To evaluate the accuracy of the algorithm in detecting the presence of an AF diagnosis in patients’ charts, a random set of 250 patients (∼2.6% of the population) were independently and blindly reviewed by 2 physicians. This revealed an overall accuracy of 99.6% (sensitivity 100%; specificity 98%) for AF detection. The rate of agreement between the 2 clinicians was 100%.
Similarly, a rule-based NLP algorithm was developed to determine whether a patient has new-onset AF or a history of AF. SAS Rule Builder procedure was first used to automatically extract an initial rule set based on 500 sentences labeled by 2 clinicians. Rules were then fine-tuned by iteratively implementing and evaluating the results at the patient level. Satisfactory results were achieved after 11 iterations with a total of 46 inclusion/exclusion rules. The final validation of the algorithm in differentiating between new AF and known AF was performed by 2 clinicians who blindly and independently evaluated a random sample of 400 patients (∼4.1% of the population). They had disagreement rates of 4% on “history of AF” and 9% on “new-onset of AF.” Disagreements were settled with deliberation. The final labels showed an overall accuracy of 95.0% for “new-onset of AF” (sensitivity 98.6%; specificity 91.0%) and an overall accuracy of 98.8% for “history of AF” (sensitivity 98.3%; specificity 99.1%).
The AF group was defined as those patients who had AF during hospitalization irrespective of a history of AF. Those with AF were further classified into 2 subgroups: patients with new-onset AF and patients with a history of AF. Moreover, final diagnosis International Classification of Diseases codes (ICD-9 and ICD-10) were also reviewed in the subset of patients who had any prior hospitalization recorded in the EMR to identify preexisting conditions, including AF, not recorded during the patients’ COVID admission.
Comparison groups and outcome measures
The primary outcome of this study was in-hospital mortality. In-hospital mortality was compared between patients with AF and those without AF, between the 2 AF subgroups, and patients with new-onset AF and those without AF. Finally, to examine the effect of underlying cardiac disease (ie, coronary artery disease, congestive heart failure, and valvular heart disease) on hospital mortality in patients with AF, patients with a history of cardiac disease were compared with those without such a history.
Statistical analysis
Propensity score matching (PSM) was used to control for potential confounding variables. The efficiency of the PSM procedure in producing comparability of the 2 groups after matching was evaluated using standardized mean differences (SMDs). An absolute SMDs of <0.1 was deemed as adequate comparability.8 Descriptive statistics of the factors used to develop the propensity score in the entire cohort before and after matching are presented in Table 1 . Continuous factors are reported as mean ± SD or median (first quartile, third quartile) in their original units, and categorical factors are presented as frequency and proportion.
Table 1.
Comparability of the groups according to in-hospital AF status
|
Variable |
Before matching |
After matching |
||||
|---|---|---|---|---|---|---|
| AF (n = 1687) | No AF (n = 7877) | SMD | AF (n = 1238) | No AF (n = 1238) | SMD | |
| Age (y) | 74.6 ± 12.9 | 62.6 ± 16.2 | 0.816∗ | 73.1 ± 13.5 | 73.6 ± 13.3 | −0.034 |
| Sex: male | 1054 (62.5) | 4578 (58.1) | 0.089 | 777 (62.8) | 774 (62.5) | 0.005 |
| Smoking status | ||||||
| Current | 123 (7.3) | 411 (5.2) | 0.086 | 90 (7.3) | 89 (7.2) | 0.003 |
| Former | 298 (17.7) | 1062 (13.5) | 0.116∗ | 208 (16.8) | 217 (17.5) | −0.019 |
| Never | 1059 (62.8) | 5928 (75.3) | −0.272∗ | 783 (63.3) | 777 (62.8) | 0.010 |
| Unknown | 207 (12.3) | 476 (6.0) | 0.217∗ | 157 (12.7) | 155 (12.5) | 0.005 |
| Race | ||||||
| Asian | 133 (7.9) | 676 (8.6) | −0.025 | 102 (8.2) | 117 (9.5) | −0.043 |
| Black | 299 (17.7) | 1766 (22.4) | −0.117∗ | 229 (18.5) | 227 (18.3) | 0.004 |
| White | 851 (50.4) | 2745 (34.9) | 0.319∗ | 585 (47.3) | 602 (48.6) | −0.027 |
| Other/multiracial | 355 (21.0) | 2321 (29.5) | −0.195∗ | 284 (22.9) | 247 (20.0) | 0.073 |
| Unknown | 49 (2.9) | 369 (4.7) | −0.093 | 38 (3.1) | 45 (3.6) | −0.031 |
| BMI | ||||||
| <18.5 kg/m2 | 41 (2.4) | 123 (1.6) | 0.062 | 29 (2.3) | 38 (3.1) | −0.045 |
| 18.5 to <25 kg/m2 | 371 (22.0) | 1370 (17.4) | 0.116∗ | 250 (20.2) | 266 (21.5) | −0.031 |
| 25 to <30 kg/m2 | 440 (26.1) | 2266 (28.8) | −0.060 | 333 (26.9) | 333 (26.9) | 0.000 |
| 30 to <40 kg/m2 | 364 (21.6) | 1981 (25.2) | −0.085 | 276 (22.3) | 260 (21.0) | 0.031 |
| ≥40 kg/m2 | 86 (5.1) | 479 (6.1) | −0.043 | 65 (5.3) | 65 (5.3) | 0.000 |
| Unknown | 385 (22.8) | 1658 (21.1) | 0.043 | 285 (23.0) | 276 (22.3) | 0.017 |
| Medical history | ||||||
| Chronic liver disease | 35 (2.1) | 210 (2.7) | −0.039 | 26 (2.1) | 23 (1.9) | 0.016 |
| Atrial fibrillation | 578 (34.3) | 109 (1.4) | 0.951∗ | 131 (10.6) | 109 (8.8) | 0.051 |
| Hypertension | 1318 (78.1) | 4691 (59.6) | 0.409∗ | 925 (74.7) | 940 (75.9) | −0.027 |
| Coronary artery disease | 853 (50.6) | 2543 (32.3) | 0.378∗ | 584 (47.2) | 593 (47.9) | −0.015 |
| Peripheral vascular disease | 97 (5.8) | 203 (2.6) | 0.159∗ | 61 (4.9) | 62 (5.0) | −0.004 |
| Chronic obstructive pulmonary disease | 229 (13.6) | 455 (5.8) | 0.266∗ | 149 (12.0) | 135 (10.9) | 0.039 |
| Asthma | 151 (9.0) | 742 (9.4) | −0.016 | 102 (8.2) | 98 (7.9) | 0.011 |
| End-stage renal disease | 165 (9.8) | 415 (5.3) | 0.172∗ | 101 (8.2) | 107 (8.6) | −0.018 |
| Chronic kidney disease | 254 (15.1) | 541 (6.9) | 0.264∗ | 151 (12.2) | 152 (12.3) | −0.003 |
| Diabetes | 771 (45.7) | 3009 (38.2) | 0.152∗ | 547 (44.2) | 548 (44.3) | −0.002 |
| Congestive heart failure | 451 (26.7) | 575 (7.3) | 0.535∗ | 244 (19.7) | 237 (19.1) | 0.016 |
| Cancer | 306 (18.1) | 825 (10.5) | 0.220∗ | 208 (16.8) | 210 (17.0) | −0.005 |
| Lactate | ||||||
| Normal, within range | 115 (6.8) | 637 (8.1) | −0.048 | 78 (6.3) | 79 (6.4) | −0.003 |
| Abnormal/high | 1064 (63.1) | 4858 (61.7) | 0.029 | 813 (65.7) | 807 (65.2) | 0.010 |
| Missing | 508 (30.1) | 2382 (30.2) | −0.003 | 347 (28.0) | 352 (28.4) | −0.009 |
| Magnesium | ||||||
| Abnormal/low | 60 (3.6) | 175 (2.2) | 0.080 | 43 (3.5) | 40 (3.2) | 0.013 |
| Normal, within range | 1201 (71.2) | 5202 (66.0) | 0.111∗ | 873 (70.5) | 880 (71.1) | −0.012 |
| Abnormal/high | 162 (9.6) | 621 (7.9) | 0.061 | 128 (10.3) | 124 (10.0) | 0.011 |
| Missing | 264 (15.7) | 1879 (23.9) | −0.21∗ | 194 (15.7) | 194 (15.7) | 0.000 |
| Procalcitonin | ||||||
| Normal, within range | 260 (15.4) | 1592 (20.2) | −0.126∗ | 196 (15.8) | 205 (16.6) | −0.020 |
| Abnormal/high | 1095 (64.9) | 4443 (56.4) | 0.175∗ | 810 (65.4) | 785 (63.4) | 0.042 |
| Missing | 332 (19.7) | 1842 (23.4) | −0.090 | 232 (18.7) | 248 (20.0) | −0.032 |
| D-dimer | ||||||
| Normal, within range | 184 (10.9) | 918 (11.7) | −0.024 | 118 (9.5) | 106 (8.6) | 0.034 |
| Abnormal/high | 1004 (59.5) | 4321 (54.9) | 0.094 | 769 (62.1) | 782 (63.2) | −0.022 |
| Missing | 499 (29.6) | 2638 (33.5) | −0.084 | 351 (28.4) | 350 (28.3) | −0.009 |
| Ferritin | ||||||
| Normal, within range | 320 (19.0) | 1540 (19.6) | −0.015 | 225 (18.2) | 201 (16.2) | 0.051 |
| Abnormal/high | 1072 (63.5) | 4819 (61.2) | 0.049 | 818 (66.1) | 838 (67.7) | −0.034 |
| Missing | 295 (17.5) | 1518 (19.3) | −0.046 | 195 (15.8) | 199 (16.1) | −0.009 |
| CRP | ||||||
| Normal, within range | 22 (1.3) | 119 (1.5) | −0.018 | 18 (1.5) | 13 (1.1) | 0.036 |
| Abnormal/high | 1291 (76.5) | 5961 (75.7) | 0.020 | 957 (77.3) | 973 (78.6) | −0.031 |
| Missing | 374 (22.2) | 1797 (22.8) | −0.015 | 263 (21.2) | 252 (20.4) | 0.022 |
| Creatinine | ||||||
| Normal, within range | 835 (49.5) | 5527 (70.2) | −0.431∗ | 646 (52.2) | 604 (48.8) | 0.068 |
| Abnormal/high | 852 (50.5) | 2350 (29.8) | 0.431∗ | 592 (47.8) | 634 (51.2) | −0.068 |
| BUN | ||||||
| Normal, within range | 719 (42.6) | 5433 (69.0) | −0.55∗ | 561 (45.3) | 530 (42.8) | 0.050 |
| Abnormal/high | 968 (57.4) | 2444 (31.0) | 0.55∗ | 677 (54.7) | 708 (57.2) | −0.050 |
| Lymphocyte count | ||||||
| Abnormal/low | 1107 (65.6) | 4556 (57.8) | 0.161∗ | 814 (65.8) | 802 (64.8) | 0.020 |
| Normal, within range | 554 (32.8) | 3226 (41.0) | −0.169∗ | 405 (32.7) | 413 (33.4) | −0.014 |
| Abnormal/high | 26 (1.5) | 95 (1.2) | 0.029 | 19 (1.5) | 23 (1.9) | −0.025 |
| AST | ||||||
| Abnormal/low | 4 (0.2) | 25 (0.3) | −0.015 | 1 (0.1) | 1 (0.1) | 0.000 |
| Normal, within range | 665 (39.4) | 3241 (41.2) | −0.035 | 466 (37.6) | 492 (39.7) | −0.014 |
| Abnormal/high | 1018 (60.3) | 4611 (58.5) | 0.037 | 771 (62.3) | 745 (60.2) | 0.043 |
| ALT | ||||||
| Abnormal/low | 75 (4.5) | 210 (2.7) | 0.096 | 42 (3.4) | 42 (3.4) | 0.000 |
| Normal, within range | 1187 (70.4) | 4888 (62.1) | 0.176∗ | 860 (69.5) | 865 (69.9) | −0.009 |
| Abnormal/high | 425 (25.2) | 2779 (35.3) | −0.221∗ | 336 (27.1) | 331 (26.7) | 0.009 |
| ALK Phos | ||||||
| Abnormal/low | 50 (3.0) | 237 (3.0) | −0.003 | 33 (2.7) | 35 (2.8) | −0.010 |
| Normal, within range | 1371 (81.3) | 6482 (82.3) | −0.027 | 1017 (82.2) | 1019 (82.3) | −0.004 |
| Abnormal/high | 266 (15.8) | 1158 (14.7) | 0.030 | 188 (15.2) | 184 (14.9) | 0.009 |
| Serum glucose | ||||||
| Abnormal/low | 19 (1.1) | 80 (1.0) | 0.011 | 14 (1.1) | 9 (0.7) | 0.042 |
| Normal, within range | 225 (13.3) | 1076 (13.7) | −0.001 | 137 (11.1) | 150 (12.1) | −0.033 |
| Abnormal/high | 1443 (85.5) | 6721 (85.3) | 0.001 | 1087 (87.8) | 1079 (87.2) | 0.020 |
| Potassium | ||||||
| Abnormal/low | 172 (10.2) | 949 (12.1) | −0.059 | 131 (10.6) | 121 (9.8) | 0.027 |
| Normal, within range | 1361 (80.7) | 6465 (82.1) | −0.036 | 989 (79.9) | 1009 (81.5) | −0.041 |
| Abnormal/high | 154 (9.1) | 463 (5.9) | 0.124∗ | 118 (9.5) | 108 (8.7) | 0.028 |
| Sodium | ||||||
| Abnormal/low | 571 (33.9) | 2848 (36.2) | −0.048 | 430 (34.7) | 424 (34.3) | 0.010 |
| Normal, within range | 965 (57.2) | 4651 (59.1) | −0.037 | 706 (57.0) | 706 (57.0) | 0.000 |
| Abnormal/high | 151 (9.0) | 378 (4.8) | 0.165∗ | 102 (8.2) | 108 (8.7) | −0.017 |
| WBC | ||||||
| Abnormal/low | 119 (7.1) | 476 (6.0) | 0.041 | 86 (7.0) | 81 (6.5) | 0.016 |
| Normal, within range | 1160 (68.8) | 5682 (72.1) | −0.074 | 849 (68.6) | 871 (70.4) | −0.039 |
| Abnormal/high | 408 (24.2) | 1719 (21.8) | 0.056 | 303 (24.5) | 286 (23.1) | 0.032 |
AF = atrial fibrillation; ALK Phos = alkaline phosphatase; ALT = alanine transaminase; AST = asparate aminotransferase; BMI = body mass index; BUN = blood urea nitrogen; CRP = C-reactive protein; SMD = standardized mean difference; WBC = white blood count.
Absolute SMD values ≥ 0.1 indicate the presence of an imbalance between groups.
Exact binomial methods were used to compute the proportion of patients with AF along with the corresponding 95% confidence intervals (CIs).
The McNemar test for matched pairs was used to assess whether the risk of in-hospital mortality differed according to the status of AF and relevant subgroups during the patients’ hospital stay.9 Risk ratios for the matched-pair analyses were computed and corresponding 95% CIs were estimated using 1000 bootstrap resamples with replacement.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
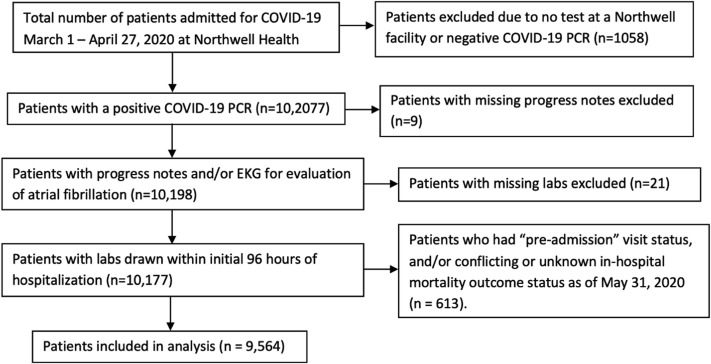
A total of 10,207 patients with COVID-19 were admitted between March 1 and April 27, 2020. Of those patients, 9564 patients met inclusion criteria. Eight thousand fifty-eight patients (84.3%) had 1 hospital admission and 1506 (15.7%) had more than 1 hospital admission. Figure 1 shows the flow diagram of the cohort selection. Out of the total, 4938 patients (51.6%) did not have a prior hospitalization within the Northwell Health system. This was a sick population, with 1881 (19.7%) receiving mechanical ventilation.
Figure 1.
Flow diagram of the cohort selection. COVID-19 = coronavirus disease 2019; EKG = electrocardiogram; PCR = polymerase chain reaction.
Of the total 9564 patients, 1687 (17.6%; 95% CI 16.9%–18.4%) experienced AF during the course of hospitalization. Of the 1687 patients with AF, 1109 patients (65.7%; 95% CI 63.4%–68.0%) had new-onset AF and 578 (34.3%; 95% CI 32.0%–36.6%) had a medical history of AF, of whom 518 were identified using NLP only and 60 patients were additionally identified using ICD-9/ICD-10 codes from prior hospitalization records in the EMR. There were 109 patients with a history of AF but no documented AF during hospitalization (1.4%).
AF status
Before matching, the mean age of the cohort was 64.8 ± 16.4 years and 5632 (58.9%) were men. Comorbidities that were more frequent in patients with AF included a history of hypertension, coronary artery disease, congestive heart failure, peripheral vascular disease, renal disease, chronic obstructive pulmonary disease, cancer, and diabetes (Table 1). As measured by the SMDs, Blood Urea Nitrogen and creatinine values were higher in patients with AF than in patients with sinus rhythm. The lymphocyte count was lower in the AF group. There were no differences in inflammatory biomarkers, namely, C-reactive protein (CRP), ferritin, and D-dimer. Patients with in-hospital AF were more likely to receive mechanical ventilation than patients who never experienced AF during their hospitalization (37.5% vs 15.9%; P < .0001).
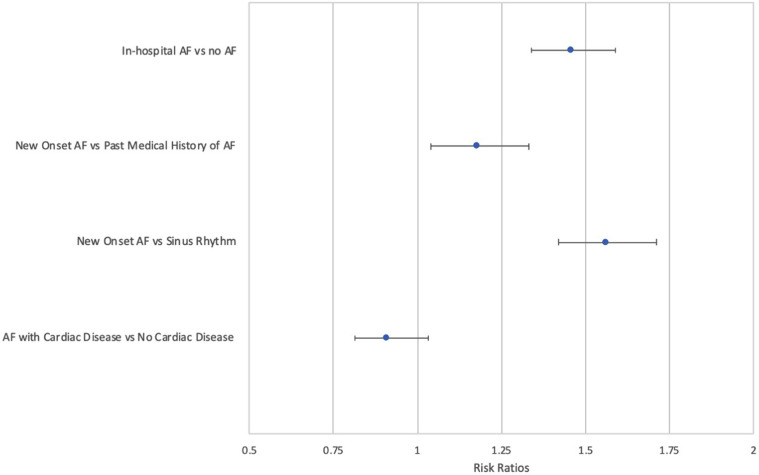
After controlling for possible confounding factors using PSM, 1238 patients with AF were successfully matched with patients without AF for a total of 2476 patients. In-hospital mortality was observed in 672 patients with AF as compared with 460 patients without AF (54.3% vs 37.2%; P < .0001). The relative risk (RR) of in-hospital mortality in those with AF compared with those without AF was 1.46 (95% CI 1.34–1.59).
New-onset AF vs a history of AF
To examine the incremental effect of new-onset AF within the AF group, 500 patients with new-onset AF were again matched with 500 patients with a history of AF. Among those patients, 276 with new-onset AF died in the hospital compared with 234 with a history of AF (55.2% vs 46.8%; P = .009) for a RR of in-hospital mortality of 1.18 (95% CI 1.04–1.33).
New-onset AF vs no AF
The in-hospital mortality in the new-onset AF group was also compared with that in the no AF group. Of 1109 patients with new-onset AF, 1107 patients were matched and compared with patients without AF. In-hospital mortality risk was significantly higher in the new-onset AF group (56.1% vs 36.0%), yielding a RR of 1.56 (95% CI 1.42–1.71; P < .0001).
AF with cardiac disease vs AF with no cardiac disease
The relationship between AF and in-hospital mortality was also examined in patients with cardiac disease. PSM analysis of 568 matched pairs did not suggest a difference in risk of in-hospital mortality between patients with AF with cardiac disease and patients without cardiac disease. Finally, 48.1% of those with a history of cardiac disease and AF died in the hospital compared with 52.6% without cardiac disease history and AF (RR 0.91; 95% CI 0.81–1.03; P = .12). The RR of in-hospital mortality for each analysis is shown in Figure 2 .
Figure 2.
Relative risk ratios of in-hospital mortality. The plot provides risk ratios along with corresponding 95% confidence intervals for 4 separate analyses. Each risk ratio should be interpreted independently and not directly compared across all 4. AF = atrial fibrillation.
Discussion
The main findings of this study in a sick cohort of patients hospitalized with COVID-19 were as follows: (1) 17.6% experienced AF, and 12.5% of patients without a history of AF were diagnosed with new-onset AF; (2) the risk of in-hospital mortality was significantly higher in patients who experienced AF (54% vs 37%); and (3) AF, particularly new-onset AF, was independently associated with in-hospital mortality.
It is estimated that 33.5 million people carry a diagnosis of AF worldwide,1 , 2 with AF more prevalent in the elderly, men, and patients with hypertension, heart failure, coronary artery disease, valvular disease, obesity, diabetes, and renal disease.3, 4, 5 Patients with the above comorbidities are also more likely to develop a more aggressive course of COVID-19 and be hospitalized.10 The baseline characteristics of the entire unmatched cohort showed that patients who developed AF had a higher prevalence of the above comorbidities, were more likely to receive mechanical ventilation, and had a significantly higher in-hospital mortality.
Incidence of AF
The prevalence and incidence of AF during hospitalization for COVID-19 is unclear; however, one should expect similarities with other systemic inflammatory response syndromes and sepsis. In the largest series of 60,209 Medicare beneficiaries with sepsis and a mean age of 80.2 years, AF was present in 25.5% of patients. In the present study, 17.6% of patients experienced AF, which is particularly high, considering that this cohort was much younger and included patients treated in a non–intensive care unit (ICU) setting.11 In a different series of 49,082 patients (mean age 69 years; 48% women) with severe sepsis, AF was newly diagnosed in 5.9% of patients.12 Bhatla et al13 reported a 3.5% incidence of AF in 700 patients (mean age 50 ± 18 years) hospitalized with COVID-19, of whom 79 (11.2%) were in an ICU setting. This is in contrast to the much higher 11.6% of new-onset AF in this study. In addition to the much greater size of the present cohort, differences are most likely due to the stricter admission criteria mandated by the much larger magnitude of the epidemic surge in New York compared with other regions, leading to hospitalization of patients only with advanced or aggressive presentation of COVID-19. The high proportion of patients requiring mechanical ventilation, the readmission rate of 15.7%, and the high mortality rate are indicative of the effort to preserve hospital beds for the sickest population.
AF as a marker of in-hospital mortality
AF is previously described as a risk factor for increased all-cause mortality.14, 15, 16, 17 In 3100 patients hospitalized with sepsis, AF was associated with a 1.45 RR increase in mortality.18 A retrospective study of 3,240,083 patients showed that patients with severe sepsis who experienced AF were more likely to die (odds ratio 1.19; 95% CI 1.14–1.24).19 In particular, new-onset AF is an independent risk factor for mortality in patients admitted to the ICU with severe sepsis or septic shock.20 For cardiac patients, AF is an independent mortality predictor for patients presenting with myocardial infarction21 or heart failure exacerbation.17 The present cohort supports the notion that AF is a marker of severe systemic illness similarly to the sepsis literature. Nevertheless, the results of the strict PSM that controlled for all previously described demographic, clinical, and laboratory confounders support an independent association between COVID-19 and AF that has not been described previously. The strong association between AF and COVID-19 was most likely driven by the new AF cases, with >50% of patients with newly diagnosed AF dying, again suggesting the strong association between the development of AF and in-hospital mortality.
This study highlights the importance of using AF as a clinical and noninvasive marker of in-hospital mortality in patients hospitalized with COVID-19. To date, laboratory markers on admission, such as CRP and D-dimer, have been used clinically to identify patients with COVID-19 with a poorer prognosis. In a recent report, higher levels of CRP and D-dimer level > 2.0 mg/L have an RR of 11.97 and 10.17, respectively, for mortality.22, 23, 24 In the present study, both CRP and D-dimer did not differ between the AF and no AF groups. In addition, both biomarkers were controlled for in all PSM analyses. The RR of 1.56 for new-onset AF provides an incremental risk to the 2 inflammatory biomarkers that are currently used as mortality predictors, suggesting that the association between COVID-19 and AF might be due to mechanisms other than systemic inflammatory response.
Potential pathogenesis of AF in COVID-19
Recently, it has been suggested that the SARS-CoV-2 virus may directly contribute to the pathogenesis of AF through attaching to pericytes, cells responsible for microvascular integrity of cardiac tissue. This results in the release of a number of growth factors, causing cardiac tissue inflammation and altering atrial cellular electrophysiology.25, 26, 27 Similarly, dysregulation of cellular angiotensin-converting enzyme 2 receptors by the SARS-CoV-2 virus results in the release of angiotensin II, further contributing to AF.28, 29, 30
Understanding how AF contributes to the increased risk of in-hospital mortality is important. The present cohort is reflective of a sick population with COVID-19 admitted to the hospital at the height of the New York pandemic. The health system was flooded with the sickest patients with COVID-19, overwhelming care and rapidly depleting resources. Most patients were treated with a rate-controlled strategy. Transesophageal echocardiograms and cardioversions were discouraged because of exposure risk. The use of anticoagulation across the health system evolved rapidly during the study period. Although most patients admitted during the study period were likely to have received some form of anticoagulation for COVID-19, the true prevalence and type of anticoagulation in this cohort, especially related to the timing of the development of AF, was not in the scope of this study.
Limitations
The study is subject to the same limitations as other retrospective studies. Because of the nature of collecting the data from the EMR, and the decreased rigor in documentation during the pandemic, the true incidence of AF may be higher because of reporting bias. However, the NLP algorithm was created after rigorous review of a random sample of medical records by electrophysiologists and enabled a review of >24 million notes. The results are not generalizable to those who may develop AF in the outpatient setting.
Conclusion
AF occurred in 17.6% of patients hospitalized with COVID-19. In 12.5% of patients, there was no history of AF (new-onset AF). The occurrence of any AF, and particularly new-onset AF, was independently associated with a significantly higher in-hospital mortality.
Footnotes
Funding sources: The authors have no funding sources to disclose.
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Chugh S.S., Havmoeller R., Narayanan K. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colilla S., Crow A., Petkun W., Singer D.E., Simon T., Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 3.Zoni-Berisso M., Lercari F., Carazza T., Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–220. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McManus D.D., Rienstra M., Benjamin E.J. An update on the prognosis of patients with atrial fibrillation. Circulation. 2012;126:e143–e146. doi: 10.1161/CIRCULATIONAHA.112.129759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang C.E., Naditch-Brule L., Murin J. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real- life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol. 2012;5:632–639. doi: 10.1161/CIRCEP.112.970749. [DOI] [PubMed] [Google Scholar]
- 6.Clarke D.M., Plumb V.J., Epstein A.E. Haemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol. 1997;30:1039–1045. doi: 10.1016/s0735-1097(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 7.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austin P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 9.Austin P.C. Comparing paired vs non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Stat Med. 2011;30:1292–1301. doi: 10.1002/sim.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanyaolu A., Okorie C., Marinkovic A. Comorbidity and its impact on patients with COVID-19 [published online ahead of print June 25, 2020]. SN Compr Clin Med. https://doi.org/10.1007/s42399-020-00363-4 [DOI] [PMC free article] [PubMed]
- 11.Walkey A.J., Greiner M.A., Heckbert S.R. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165:949–955.e3. doi: 10.1016/j.ahj.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walkey A.J., Wiener R.S., Ghobrial J.M., Curtis L.H., Benjamin E.J. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–2254. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatla A., Mayer M.M., Adusumalli S. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin E.J., Wolf P.A., D’Agostino R.B., Silbershatz H., Kannel W.B., Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 15.Stewart S., Hart C.L., Hole D.J., McMurray J.J. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 16.Andersson T., Magnuson A., Bryngelsson I.L. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish nationwide long-term case- control study. Eur Heart J. 2013;34:1061–1067. doi: 10.1093/eurheartj/ehs469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mountantonakis S.E., Grau-Sepulveda M.V., Bhatt D.L., Hernandez A., Peterson E., Fonarow G. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of Get With The Guidelines-Heart Failure. Circ Heart Fail. 2012;5:191–201. doi: 10.1161/CIRCHEARTFAILURE.111.965681. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi S., Litt D., Narula N. New-onset atrial fibrillation in sepsis is associated with increased morbidity and mortality. Neth Heart J. 2015;23:82–88. doi: 10.1007/s12471-014-0641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar G.D.A., Taneja A., Kumar N. Atrial fibrillation is more frequent in patients with severe sepsis and septic shock predicts a worse outcome. Chest. 2010;138:899. [Google Scholar]
- 20.Wells G.L., Morris P.E. Incidence and prognosis of atrial fibrillation in patients with sepsis. Cardiol Res. 2011;2:293–297. doi: 10.4021/cr108w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau D.H., Huynh L.T., Chew D.P., Astley C.M., Soman A., Sanders P. Prognostic impact of types of atrial fibrillation in acute coronary syndromes. Am J Cardiol. 2009;104:1317–1323. doi: 10.1016/j.amjcard.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 22.Sahu B.R., Kampa R.K., Padhi A., Panda A.K. C-reactive protein: a promising biomarker for poor prognosis in COVID-19 infection. Clin Chim Acta. 2020;509:91–94. doi: 10.1016/j.cca.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada T., Wakabayashi M., Yamaji T. Value of leukocytosis and elevated C-reactive protein in predicting severe coronavirus 2019 (COVID-19): a systematic review and meta-analysis. Clin Chim Acta. 2020;509:235–243. doi: 10.1016/j.cca.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao Y., Cao J., Wang Q. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. 2020;8:49. doi: 10.1186/s40560-020-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweeney M., Foldes G. It takes two: endothelial-perivascular cell cross-talk in vascular development and disease. Front Cardiovasc Med. 2018;5:154. doi: 10.3389/fcvm.2018.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stark K., Eckart A., Haidari S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy-Lydon T. Immune functions and properties of resident cells in the heart and cardiovascular system: pericytes. Adv Exp Med Biol. 2017;1003:93–103. doi: 10.1007/978-3-319-57613-8_5. [DOI] [PubMed] [Google Scholar]
- 28.Granger D.N., Rodrigues S.F., Yildirim A., Senchenkova E.Y. Microvascular responses to cardiovascular risk factors. Microcirculation. 2010;17:192–205. doi: 10.1111/j.1549-8719.2009.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKinney C.A., Fattah C., Loughrey C.M., Milligan G., Nicklin S.A. Angiotensin-(1-7) and angiotensin-(1-9): function in cardiac and vascular remodelling. Clin Sci (Lond) 2014;126:815–827. doi: 10.1042/CS20130436. [DOI] [PubMed] [Google Scholar]
- 30.Jansen H.J., Mackasey M., Moghtadaei M. Distinct patterns of atrial electrical and structural remodeling in angiotensin II mediated atrial fibrillation. J Mol Cell Cardiol. 2018;124:12–25. doi: 10.1016/j.yjmcc.2018.09.011. [DOI] [PubMed] [Google Scholar]