Abstract
Background
There is limited data concerning the prevalence of arrhythmias, particularly atrial fibrillation (AF), which may develop as a consequence of direct myocardial injury and the inflammatory state existing in COVID-19.
Methods
This single-center study included data concerning 658 COVID-19 patients, who were hospitalized in our institute, between April 20th, 2020 and July 30th, 2020. Demographic data, findings of the imaging studies, and laboratory test results were retrieved from the institutional digital database.
Results
New onset AF (NOAF) was identified in 33 patients (5%). Patients who developed AF were older (72.42 ± 6.10 vs 53.78 ± 13.80, p < 0.001) and had higher frequencies of hypertension and heart failure compared to patients without NOAF (p < 0.001, for both). The CHA2DS2-VASc score was higher in patients, who developed NOAF, compared to those who did not during hospitalization for COVID-19 (p < 0.001). Subjects, who developed NOAF during hospitalization, had a higher leukocyte count, neutrophil / lymphocyte ratio (NLR), C-reactive protein, erythrocyte sedimentation rate, and procalcitonin levels compared to those without NOAF (p < 0.001 for all comparisons). Diffuse lung infiltration was also more frequent in COVID-19 patients, who developed NOAF, during hospitalization (p = 0.015). Multivariate logistic regression analysis demonstrated that age, CHA2DS2-VASc score, CRP, erythrocyte sedimentation rate, and presence of diffuse lung infiltration on thorax CT were predictive for NOAF.
Conclusion
The prevalence of NOAF in hospitalized COVID-19 patients is higher than the general population. Age, CHA2DS2-VASc score, C-reactive protein, erythrocyte sedimentation rate, and presence of diffuse lung infiltration on thorax CT may be used to identify patients at high risk for development of NOAF. Especially among these parameters, the presence of diffuse lung infiltration on thorax CT it was the most powerful independent predictor of NOAF development.
Keywords: COVID-19, Arrhythmia, Atrial fibrillation, Inflammation, CHA2DS2-VASc score
Introduction
The most common cardiac rhythm disorder in clinical practice, atrial fibrillation (AF), is a critical condition that is associated with hemodynamic disorders and thromboembolic events [1]. Although the frequency of AF increases with age, its prevalence in the general population is about 0.4% to 1% [2,3]. Contemporary data indicates that the risk of heart failure, ischemic stroke, and cardiovascular mortality are increased in patients with AF [1]. Even though the exact mechanisms leading to AF development are not fully understood, several risk factors, including age, hypertension, coronary artery disease, cerebrovascular disease, and diabetes have been suggested to be associated with the development of AF [4].
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is a new viral pneumonia that evolved into a pandemic within three months after the first confirmed cases. Although COVID-19 primarily affects the lungs, cardiovascular involvement has also been reported extensively [5]. Direct myocardial cell injury, myocardial oxygen supply/demand mismatch, acute plaque ruptures leading to acute coronary syndrome as a part of systemic inflammation and catecholamine surge, and increased thrombosis have been reported as cardiac manifestations. Some of these are directly caused by the disease while others are associated with the potential side effects of medications used for the treatment of COVID-19 [[6], [7], [8]].
Acute myocarditis, acute coronary syndrome, and increased likelihood of thromboembolic events have previously been reported in subjects with COVID-19 [[6], [7], [8]].
However, data concerning the prevalence of arrhythmias, particularly AF, which may develop as a consequence of direct myocardial injury and the inflammatory state existing in COVID-19 patients, is lacking.
The aim of this study was to describe the prevalence of new-onset AF (NOAF) and identify risk factors associated with the development of NOAF in subjects hospitalized for COVID-19.
Methods
This single-center, study, included data from patients admitted with COVID-19 and hospitalized at our institute between April 20th, 2020 and July 30th, 2020. Our center is one of the dedicated healthcare centers for the management of COVID-19 patients in Turkey and thus, the COVID-19 population demonstrated a high degree of variation in terms of characteristics. The study plan was approved by the institutional ethics committee and was conducted in accordance with the Helsinki declaration. Informed consent for inclusion into this study was obtained from all individual participants.
Patients, older than 18 years of age, who received a COVID-19 diagnosis via polymerase chain reaction (PCR) tests and had sinus rhythm at admission according to 12‑lead electrocardiogram (ECG), were included in this study. Patients were excluded from the study if any of the following criteria applied: preexisting permanent or persistent AF and patients with a previous history of AF attack, presence of malignancy, pregnancy, or breastfeeding. Patients with insufficient information in their hospital files and severe renal or liver disease were also excluded from the study.
According to the definitions in the “COVID-19 Diagnosis and Treatment Guide” printed by the Turkish Ministry of Health, the clinical definition of the patients was as follows: Mild illness presents with features such as fever, muscle/joint pain, cough, sore throat, and nasal congestion, with or without mild pneumonia together with a respiratory rate < 30/min and an O2 saturation above 90% while breathing room air. Severe illness is defined with widespread findings of pneumonia in computed tomography (CT). Critical illness defines the requirement of the Intensive care unit (ICU). The routine criteria for ICU admission at our center were as follows (according to Ministry of Health guidelines): Signs conclusive for severe respiratory failure, including having an SpO2 of ≤92% in ambient air, need for ≥6 L O2/min, need for NIV (non-invasive ventilation) or IMV (invasive mechanical ventilation).
Immediately after the diagnosis of COVID-19, routine blood laboratory tests were performed at the time of hospitalization. Demographic data, findings of the imaging studies, and routine blood tests, including serum troponin I results were retrieved from the institutional digital database. Control troponin I levels measured in patients, who developed NOAF, were also found in the hospital registry system and were considered normal if they were below the upper reference limit of the 99th percentile.
The presence of pneumonia was confirmed by computerized tomography imaging (CTI) within 24 h of hospital admission for all patients. All COVID-19 patients in our study had signs of pneumonia on CTI.
Examination of the radiological findings showed that the lesions tended to be located more in the peripheral and lower lobes. The most common radiological findings of the patients were bilateral ground glass, diffuse infiltration, consolidation, and unilateral ground glass opacities.
The aim of the study was the finding of an occurrence of any episode of NOAF within the period of hospitalization as documented by medical records, ECGs, rhythm strips, and Holter monitors accordingly. All patients hospitalized in the intensive care unit (ICU) were followed up with a rhythm during their stay in the ICU. AF attacks lasting ≥30 s were recorded. The diagnosis of NOAF in other patients was determined according to ECG findings, evaluation of rhythm strips, and medical records.
The standard COVID-19 treatment protocol recommended by the Science Advisory Board of the Turkish Ministry of Health, including Oseltamivir phosphate 75 mg twice daily, hydroxychloroquine 200 mg twice daily, and azithromycin 250 mg once daily (following a 500 mg loading dose) were administered to all patients. All patients had a baseline ECG at the time of hospitalization. A daily ECG was taken 12 h after the first drug dose and on the following days in all patients. A QTc interval exceeding 500 ms was accepted as the warning limit (7).
The CHA2DS2-VASc score and CURB-65 (Confusion, Urea nitrogen, Respiratory rate, Blood pressure, and age > 65 years; Patients were given 1 point for each parameter) score was calculated for all subjects at the time of hospitalization. Depending on the CHA2DS2-VASC score, patients were given 1 score for congestive heart failure, hypertension, age 65–74 years, diabetes mellitus, vascular disease, female sex, and 2 points for age ≥ 75 years and prior stroke or transient ischemic attack.
Patients were divided into two groups as follows: Patients, who developed AF during hospitalization (Group 1) and patients, who did not develop AF during hospitalization (Group 2). The primary outcomes of this study were the differences between these two groups in terms of demographic characteristics, laboratory measurements, and imaging findings. The role of several risk factors on the development of NOAF was also evaluated via multivariate analysis.
Statistical analysis
Statistical analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) software for Windows. The distribution of quantitative variables was checked with the Shapiro Wilk test. Descriptive data was given as mean ± standard deviation and median (inter quartile range, IQR) depending on normality of distribution. The independent Sample t-test was used for the comparison of normally distributed quantitative variables and the Mann-Whitney U test was used for the comparison of non-normally distributed quantitative variables. The comparison of the distributions of categorical variables was performed with chi-square tests. Continuous variable distribution among the groups (patients with mild, severe, and critical illness) was done by One Way Anova. Variability between groups was performed by the LSD test. The effects of different variables on the development of NOAF were calculated with univariate analysis. Variables for which the unadjusted P value was <0.10 were included in the last multivariate model to identify NOAF predictors. P-values equal to or lower than 0.05 were considered to demonstrate statistical significance.
Results
A total of 726 patients hospitalized for COVID-19 were screened for inclusion. The initial ECG of 11 patients was AF, 6 patients had a previous history of AF, 51 patients were not included because of the other exclusion reasons, and the remaining 658 patients (92.5%) were included in the study (Fig. 1 ). The mean age of the patients was 54 ± 14, and 43.4% were female. The comorbidities with the highest frequency were as follows: hypertension (31.9%, n = 210), diabetes (18%, n = 119), and chronic respiratory diseases, such as asthma or COPD (16.8%, n = 111).
Fig. 1.
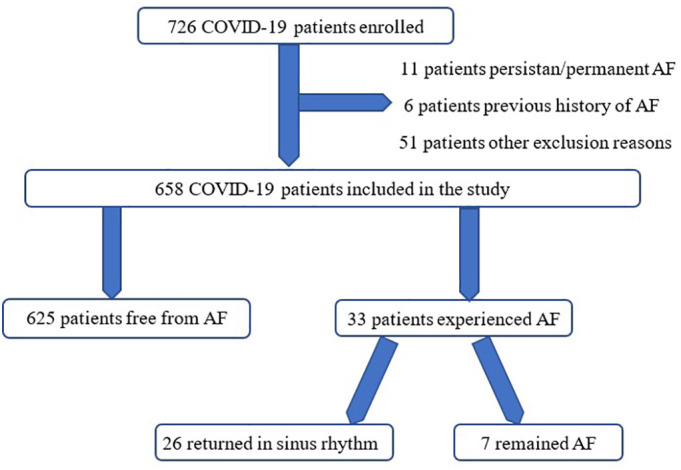
Patients' study flow schema.
NOAF was identified in 33 patients (5%). In 31 patients, NOAF developed within the first 72 h of hospitalization, and in the remaining 2 patients, NOAF developed within the first week of hospitalization. Among these 33 patients, 11 were followed-up in the ICU. All of them were taking hydroxychloroquine (HCQ), azithromycin, and favipiravir treatments. These patients had normal QTc (415 ms, average for all) intervals before treatment. After treatment, these QTc values still normal (460 ms, average for all). No patients needed to discontinue treatment due to QTc prolongation and thus, there were no treatments applied for this condition.
Baseline laboratory findings and demographic features of the study subjects are presented in Table 1 . Patients who developed AF were older (72.42 ± 6.10 years vs. 53.78 ± 13.80 years, p < 0.001) and had higher frequencies of hypertension and heart failure compared to patients without NOAF (66.6% vs. 30%, p < 0.001; 27.2% vs. 6.4%, p < 0.001, respectively). The CHA2DS2-VASc score was also significantly higher in patients, who developed NOAF, compared to COVID-19 patients without NOAF (3.42 ± 0.56 vs. 1.36 ± 1.27, p < 0.001).
Table 1.
Demographic and clinical characteristics, laboratory findings and in-hospital course of the general population with COVID-19 and in the two subgroups according to the occurrence of new onset atrial fibrillation (NOAF) during hospital stay.
| NOAF during hospitalization |
|||
|---|---|---|---|
| Yes | No | P value | |
| Number of patients | 33 (%5) | 625 (94.9) | – |
| Age, years | 72.42 ± 6.10 | 53.78 ± 13.80 | <0.001 |
| Gender, female, n | 17 (%51.5) | 269 (%43) | 0.338 |
| Diabetes Mellitus, n | 7 (%21.2) | 112 (%17.9) | 0.632 |
| Hypertension, n | 22 (%66.6) | 188 (%30) | <0.001 |
| Vascular disease, n | 6 (%18.1) | 91 (%14.5) | 0.567 |
| Heart Failure, n | 9 (%27.2) | 40 (%6.4) | <0.001 |
| Stroke/transient ischemic attack, n | 0 | 7 (%1) | 0.541 |
| Smoking, n | 6 (%18) | 74 (%12) | 0.277 |
| COPD/ Asthma, n | 7 (%21) | 104 (%16) | 0.497 |
| Family history of atrial fibrillation | 0 | 4 (%0.6) | 0.645 |
| Valvular heart diseases, | 2 (%6) | 17 (%2.7) | 0.264 |
| Previous episodes of CAP | 3 (%9) | 68 (%10.8) | 0.532 |
| CHA2DS2-VASc | 3.42 ± 0.56 | 1.36 ± 1.27 | <0.001 |
NOAF: new onset atrial fibrillation.
COPD: chronic obstructive pulmonary disease.
PAF: paroxysmal atrial fibrillation.
CAP: Community-acquired pneumonia.
CHA2DS2-VASc: Congestive heart failure/left ventricular dysfunction, Hypertension, Age (≥ 75 years 2 points), Diabetes, Stroke/transient ischemic attack (2 points), Vascular disease, Sex category.
The clinical parameters, laboratory, and radiographic findings of the patients are presented in Table 2 . Subjects, who developed NOAF during hospitalization, had higher C-reactive protein (CRP), erythrocyte sedimentation rate, procalcitonin, leukocyte count, and neutrophils/lymphocytes ratio (NLR) compared to those who did not develop NOAF (p < 0.001 for all comparisons).
Table 2.
Clinical parameters. Laboratory and radiographic findings of the study groups.
| NOAF during hospitalization |
|||
|---|---|---|---|
| Yes | No | P value | |
| Number of patients | 33 (%5.0) | 625 (94.9) | – |
| Creatinine, mg/dl | 0.88 ± 0.14 | 1.10 ± 0.02 | 0.761 |
| AST, U/L | 22.37 ± 4.20 | 24.96 ± 3.50 | 0.531 |
| ALT, U/L | 18.15 ± 5.60 | 25.03 ± 7.35 | 0.158 |
| Hemoglobin, mg/dL | 12.95 ± 1.87 | 14.17 ± 2.12 | <0.001 |
| WBC, x103/ μL | 12.92 ± 2.12 | 8.49 ± 1.98 | <0.001 |
| Neutrophils/Lymphocytes ratio (NLR) | 10.03 ± 2.02 | 3.92 ± 1.94 | <0.001 |
| Platelets, x103/ μL | 234.9 ± 55.51 | 242.86 ± 42.29 | 0.550 |
| C-reactive protein, mg/L | 110.37 ± 23.3 | 37.32 ± 11.83 | <0.001 |
| ESR | 49.21 ± 30.17 | 27.17 ± 23.25 | <0.001 |
| Procalcitonin, ng/mL | 2.32 ± 0.47 | 0.32 ± 0.05 | <0.001 |
| Troponin I | 0.01 | 0.01 | 1 |
| Pneumonia Severity and Scores | |||
| CURB-65 | 1.72 ± 0.28 | 0.69 ± 0.09 | <0.001 |
| Bilateral Ground Glass, n | 22 (%66) | 375 (%60) | 0.445 |
| Diffuse lung infiltration, n | 11 (%33) | 105 (%16) | 0.015 |
| Course of Hospitalization and Outcome | |||
| Hospital Stay, days | 10.63 ± 1.67 | 8.97 ± 2.94 | <0.001 |
| ICU Admission, days | 11 (%32) | 48 (%8) | <0.001 |
| In-Hospital Mortality, n | 2 (%6) | 12 (%1.9) | 0.108 |
| Severety of Ilness | |||
| Mild | – | 66 (%10.5) | <0.001 |
| Severe | 22 (%66.6) | 511 (% 81.7) | <0.001 |
| Critical | 11 (%33.3) | 48 (% 7.6) | <0.001 |
ESR: Erythrocyte sedimentation rate.
NOAF: New onset atrial fibrillation.
WBC: White Blood Cell.
CURB-65: Confusion. Urea. Respiratory rate. Blood pressure. Age > 65 years.
ICU: intensive care unit.
ICU admission: Number and percentage of patients who need intensive care and are followed up in intensive care unit.
The CURB-65 score, which was used to stratify the severity of the pneumonia in patients, was significantly higher in those who developed NOAF compared to those without NOAF (1.72 ± 0.28 vs. 0.69 ± 0.09, p < 0.001). The frequency of diffuse lung infiltration was also higher in those with NOAF compared to those without (33% vs. 16%, p = 0.015). Length of hospital and ICU stay were also significantly longer in patients, who developed NOAF.
Baseline characteristics and the ECG findings of the patients with mild, severe, and critical illness are shown in Table 4. Accordingly, critically ill patients were older. In addition, it has been observed that critically ill patients have more comorbidities, such as hypertension (HT), heart failure, have higher CHADS2VASc and CURB-65 scores, and stay in the hospital longer. Serum CRP and WBC levels were also higher (P < 0.001, for all). As expected, the heart rates of critically ill patients were higher (p < 0.001).
Table 4.
Baseline characteristics and the ECG findings of the patients with mild, severe and critical illness.
| Variables | MİLD (n = 66) | SEVERE (n = 533) | CRİTİCAL (n = 59) | P Value |
|---|---|---|---|---|
| Age, years | 53.5 ± 14.2 | 56.1 ± 13.7 | 63.6 ± 10 | <0.001 |
| Gender, female, n | 33 (50%) | 223 (41%) | 30 (50%) | 0.152 |
| Diabetes Mellitus, n | 12 (16%) | 91 (17%) | 16 (27%) | 0.155 |
| Hypertension, n | 14 (21%) | 163 (30%) | 33 (55%) | <0.001 |
| Vascular disease, n | 2 (3%) | 83 (15%) | 12 (20%) | 0.029 |
| Heart Failure, n | 1 (1%) | 30 (5%) | 18 (30%) | <0.001 |
| Valvuler Heart Disease, n | 0 | 15 (2.8%) | 4 (%4) | 0.076 |
| COPD /Asthma, n | 2 (3%) | 95 (17%) | 14 (23%) | 0.003 |
| CHA2DS2-VASc | 1.34 ± 0.7 | 1.57 ± 0.96 | 2.42 ± 1.03 | <0.001 |
| CURB-65 | 0.64 ± 0.41 | 0.78 ± 0.38 | 1.27 ± 0.61 | <0.001 |
| Hospital Stay, days | 5.1 ± 1.3 | 8.9 ± 2.1 | 14.5 ± 1.2 | <0.001 |
| Laboratory findings | ||||
| CRP, mg/L | 29 ± 13.4 | 40.9 ± 11.8 | 57.2 ± 12.3 | <0.001 |
| WBC, x103/ μL | 8.45 ± 4.2 | 8.66 ± 4.21 | 9.48 ± 4.56 | <0.001 |
| ESR | 21.9 ± 3.4 | 28.3 ± 1.3 | 33.2 ± 5.6 | 0.189 |
| Radiographic Findings | ||||
| Bilateral Ground Glass, n | 4 (6%) | 388 (72.7) | 5 (8.4%) | <0.001 |
| Diffuse lung infiltration, n | 0 | 62 (11.6%) | 54 (91.5%) | <0.001 |
| ECG Finding | ||||
| Heart rate (beat/min) | 76.6 ± 13 | 77.6 ± 16.4 | 104.4 ± 12.7 | <0.001 |
| PR, ms | 148.2 ± 5.14 | 157.4 ± 18.6 | 154.3 ± 7.42 | 0.314 |
| QTc interval (ms) | 410.6 ± 18.3 | 415.1 ± 22.9 | 419.8 ± 22.5 | 0.091 |
| QRS duration (ms) | 85.2 ± 9.6 | 86.27 ± 12.4 | 87.08 ± 16.01 | 0.722 |
CURB-65: Confusion, Urea, Respiratory rate, Blood pressure, age > 65 years.
CHA2DS2-VASc: Congestive heart failure/left ventricular dysfunction, Hypertension, Age (≥ 75 years 2 points), Diabetes. Stroke/transient ischemic attack (2 points), Vascular disease, Sex category.
CRP: Creactive protein, WBC: White Blood Cell count, ESR: Eritrocyte Sedimentation Rate.
After including the variables that were found significant in the univariate analysis, age, HT, heart failure, CRP, WBC, ESR, Procalcitonin, the presence of diffuse lung infiltration on thorax CT, and CHA2DS2-VASc and CURB-65 scores emained for multivariate logistic regression analysis.
Multivariate logistic regression analysis demonstrated that age (OR: 1.135, 95% CI: 1.0197–1.263, p = 0.020), CHA2DS2-VASc score (OR: 2.511, 95% CI: 1.182–5.331, p = 0.017), CRP (OR: 1.017, 95% CI: 1.005–1.029, p = 0.004), erythrocyte sedimentation rate (OR: 1.031, 95% CI: 1.003–1.059, p = 0.026), and presence of diffuse lung infiltration on thorax CT (OR: 24.443, 95% CI: 3.905–152.994, p = 0.001) were predictive for the development of NOAF in hospitalized COVID-19 patients (Table 3 ). Especially, among these parameters, the presence of diffuse lung infiltration on thorax CT was the most powerful independent predictor of NOAF development.
Table 3.
Univariate and multivariate predictors of new onset atrial fibrillation (NOAF) in hospitalized patients with COVID-19.
| Unadjusted OR | 95% CI | p value | Adjusted OR | 95% CI | p value | |
|---|---|---|---|---|---|---|
| Age, years | 1.136 | 0.999–1.291 | 0.051 | 1.135 | 1.019–1.263 | 0.020 |
| Hypertension | 1.560 | 0.247–9.835 | 0.636 | – | – | – |
| Heart Failure | 0.728 | 0.111–4.776 | 0.741 | – | – | – |
| CURB-65 | 0.805 | 0.176–3.681 | 0.780 | – | – | – |
| Diffuse lung infiltration | 12.417 | 0.928–166.005 | 0.057 | 24.443 | 3.905–152.994 | 0.001 |
| CHA2DS2-VASc | 2.674 | 1.089–6.561 | 0.032 | 2.511 | 1.182–5.331 | 0.017 |
| Procalcitonin | 1.012 | 0.888–1.154 | 0.848 | – | – | – |
| ESR | 1.035 | 1.002–1.069 | 0.037 | 1.031 | 1.003–1.059 | 0.026 |
| WBC | 1.056 | 0.903–1.236 | 0.490 | – | – | – |
| CRP | 1.013 | 0.999–1.028 | 0.064 | 1.017 | 1.005–1.029 | 0.004 |
CURB-65: Confusion, Urea, Respiratory rate, Blood pressure, age > 65 years.
CHA2DS2-VASc: Congestive heart failure/left ventricular dysfunction, Hypertension, Age (≥ 75 years 2 points), Diabetes. Stroke/transient ischemic attack (2 points), Vascular disease, Sex category.
ESR: Eritrocyte Sedimentation Rate.
WBC: White Blood Cell count.
CRP: C reactive protein.
On the discharge documents of the patients, it was seen that 26 patients with NOAF returned to sinus rhythm, while 7 did not. These patients were treated according to the AF treatment guideline of European Society of Cardiology (ESC). As known, if the CHA2DS2VASC score is 1 in males, and 2 in women, anticoagulant treatment is recommended according to the ESC guideline. The CHA2DS2VASc score of patients, who developed NOAF in our study group was calculated separately for each patient, anticoagulant treatment was given to these patients as recommended in the ESC guideline. At the same time, since NOAF was detected in patients, rhythm control was performed in eligible patients. Rate control treatment was applied to patients who could not be successful with rhythm control [9].
Discussion
In our group of hospitalized patients with COVID-19, NOAF was observed in 5%. Patients, who developed NOAF during hospitalization for COVID-19, were more likely to have hypertension, heart failure, older age, and higher CHA2DS2-VASc score compared to those who did not develop NOAF. CRP erythrocyte sedimentation rate, procalcitonin level, leukocyte count, and (NLR) were significantly higher in patients with NOAF than those without NOAF during hospital stay. Moreover, diffuse lung infiltration was also more frequent in COVID-19 patients, who developed NOAF. Older age, higher CHA2DS2-VASc score, higher CRP, higher erythrocyte sedimentation rate, and presence of diffuse lung infiltration on thorax CT were identified as predictors for the development of AF in hospitalized COVID-19 patients.
The prevalence of AF in the general population is estimated to be 0.4% to 1.0% (2). AF also constitutes a common rhythm disorder for hospitalized patients, particularly for those with severe medical illness. Furthermore, AF has been reported to occur in 25%–30% of patients undergoing cardiac surgery and in about 3% of patients undergoing non-cardiac surgery. The risk of NOAF in patients hospitalized for pneumonia has been investigated in a number of studies. In a recent prospective study conducted by Pieralli et al., the authors reported that 10.3% of the study population, who were admitted for community-acquired pneumonia (CAP), experienced NOAF during hospitalization. Their findings were also remarkable for the role of CHA2DS2-VASc in predicting the development of NOAF in this patient population [10]. In a national cohort study, which included 32.689 patients hospitalized with pneumonia and 12% of the study population, were reported to have a new diagnosis of cardiac arrhythmia within 90 days of admission [11]. These findings show that patients hospitalized for pneumonia may be at higher risk for AF development. It has also been reported that increased serum inflammatory cytokines and presence of acute metabolic disorders (such as electrolyte abnormalities, hypo/hyperthermia, and hypoxemia) were indicated as triggers for AF during hospitalization for pneumonia [12,13]. Among these factors, inflammation has been shown to play a critical role in the development of NOAF, regardless of the presence of traditional risk factors (hypertension and coronary artery disease) [[14], [15], [16]]. Additionally, inflammatory mediators, including CRP, interleukin-6 and tumor necrosis factor-alpha (TNF-alpha), have been shown to induce AF development in patients with coronary artery disease or chronic kidney disease [[17], [18], [19]].
COVID-19 is novel coronavirus infection, which predominantly affects the lungs and leads to rapidly progressing respiratory failure in a substantial amount of subjects. In Covid-19, after the respiratory system, the system that is most affected is the cardiovascular system. Many processes that affect the cardiovascular system directly and indirectly work together.
Pre-existing cardiovascular disease (CVD), hypoxia, adult respiratory distress syndrome (ARDS), cytokine storm, hypotension, shock, microvascular thromboses, arrhythmias, stress-induced cardiomyopathy, cardiogenic shock, viral myocardial invasion, or inflammation altogether lead to cardiac injury. For this reason, some authors have suggested the term “Acute Covid-19 Cardiovascular Syndrome”. Since acute cardiac damage, shock, and arrhythmia have been seen in substantial numbers in the course of the disease, this term, which sums up the situation well, is increasingly used [20,21].
Extensive data reveals that inflammatory state and cytokine storm accompanies pneumonia in a subset of patients with COVID-19 [22]. Circulating TNF-alpha, IL-6, and IL-1β have been shown to increase in patients with COVID-19 infection [23]. However, despite the link between the inflammation and AF, data concerning the prevalence of NOAF, which may worsen clinical course in patients with COVID-19, is lacking. To the best of our knowledge, this is the first study to have evaluated the prevalence of NOAF and associated risk factors in hospitalized COVID-19 patients.
The prevalence of NOAF in our study population was found to be 5%. Although this rate is significantly higher than the prevalence values reported for the general population, it was significantly lower than the rates found in patients hospitalized for CAP. The reason for this can be explained by the fact that the patients in our study group had fewer comorbid diseases and their pneumonia severity was lower than the aforementioned studies. In particular, the fact that the average age of the patients in our study was much lower than in the mentioned studies may have decreased the incidence of NOAF. İn addition, the fact that the patients in the mentioned studies were also selected from patients with more critical and likelihood of intensive care needs may have increased the incidence of AF.
Consistent with the previous evidence in patients with pneumonia, we found that inflammatory markers (CRP, procalcitonin, erythrocyte sedimentation rate, and NLR) were significantly increased among patients with NOAF compared to those without NOAF. In addition, hypertension and heart failure were more frequent, and age, CURB-65 score, and CHA2DS2-VASc score were significantly higher in patients with NOAF compared to those without NOAF. Moreover, our findings show that markers of inflammatory state, such as CRP and erythrocyte sedimentation rate, may predict the development of NOAF in COVID-19 patients. These findings confirm the results of previous studies, which underline the role of inflammation in the pathogenesis of AF.
Another important finding of this study was that CHA2DS2-VASc score was identified as an independent predictor for the development of NOAF in COVID-19 patients. The CHA2DS2-VASc score is a simple and practical scoring scheme, which was initially used for stroke risk discrimination among patients with AF. The CHA2DS2-VASc score has been shown to predict NOAF in patients with type-2 diabetes, dyslipidemia, community-acquired pneumonia, or -cancer. It is also predictive for postoperative AF in patients undergoing cardiac surgery [10,22,[24], [25], [26]]. This is the first study to suggest CHA2DS2-VASc as a parameter to identify patients at high risk for developing NOAF during COVID-19.
Of note, it is also remarkable that the severity of the pneumonia (as measured by CURB-65 score) was identified to be effective in the development of NOAF. Furthermore, the presence of diffuse lung infiltration on thorax CT was determined as an independent predictor for the development of NOAF in patients with COVID-19. It is accepted that the disease enters the cell through angiotensin converting enzyme (ACE-2) receptors in the upper respiratory tract mucosa epithelium and lower respiratory tract, and replication continues. Thus, pneumonia findings became prominent and determinant in the clinical course of the disease. However, respiratory system findings have been seen in different clinical severity during the course of the disease. It ranges from uncomplicated mild illness to severe pneumonia and ARDS [27]. Since diffuse lung infiltration is an indicator for more severe pneumonia, it may also be associated with a more prominent inflammatory state during the course of COVID-19. The pathophysiological mechanism may be a cytokine-mediated vasoconstriction that causes ischemia at the pulmonary venous atrial interface, where AF mostly occurs [28]. In addition to the increased inflammatory state, the further increase in endogenous catecholamine release and hemodynamic instability may also trigger AF. With the development of pulmonary inflammation, disruption in gas exchange, i.e. ventilation / perfusion imbalance occurs, which leads to hypoxemia. As a result, diffuse lung infiltration may precipitate hypoxemia, which could be another explanation for the development of NOAF in patients with COVID-19. In the present study, the presence of diffuse lung infiltration on thorax CT was found to be the strongest independent parameter affecting NOAF formation.
With these results and previous studies in mind, we showed that the risk of NOAF is higher in hospitalized COVID-19 patients. Moreover, age, CHA2DS2-VASc score, C-reactive protein, erythrocyte sedimentation rate, and presence of diffuse lung infiltration on thorax CT may be used to identify COVID-19 patients at high risk for development of NOAF. In-hospital mortality rate was similar for those with and without NOAF during the hospital course. However, this study was not designed to assess mortality and it is evident that a larger sample size would be required to elucidate the role of NOAF on mortality in COVID-19 patients.
Limitations.
This study has some limitations to be mentioned. The retrospective design is the main drawback of this study. Second, echocardiography data was not available for all patients and therefore could not be used in data analysis. Third, the relatively small number of patients and the fact that it was a single center study. Fourth, because the drug treatment algorithm recommended by the Ministry of Health was applied to all hospitalized subjects and almost all subjects received the same agents for medical treatment, the specific role of drugs could not be compared in patients with and without NOAF. Lack of Holter monitoring or long-term ECG monitoring for all patients is also one of the main limitations of the study and, it is likely that silent AF may have been undetected. Hence, we may have underestimated the real incidence of AF in COVİD-19. Larrger and multicenter studies should be performed to better analyze all the possible predictors of AF. Nevertheless, we believe that our findings add valuable information to the current knowledge on the prevalence of NOAF and associated risk factors for the development of NOAF in COVID-19 patients.
Conclusions
The prevalence of NOAF in hospitalized COVID-19 patients is higher than the general population. Age, CHA2DS2-VASc score, C-reactive protein, erythrocyte sedimentation rate, and presence of diffuse lung infiltration on thorax CT may be used to identify patients at high risk for the development of AF in patients hospitalized for COVID-19. Among these parameters, we found that the presence of diffuse lung infiltration on thorax CT was the most powerful independent predictor of NOAF development.
Funding source
This research did not receive any grants from funding agencies in the public, commercial, or not-for-profit sectors.
Autor statement
Conceptualization, Methodology: SK, DE, YY, EO Software: SK, DE,YY, ZBD, MG Data curation, Writing- Original draft preparation: SK, AUK, SD,ZS, BC Visualization, Investigation: SK,DE Supervision: DE Validation: SK, YY, EO, MG Writing- Reviewing and Editing: SK,DE.
Declaration of Competing Interest
No potential conflict of interest was reported by the authors.
References
- 1.January C.T., Wann L.S., Alpert J.S., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Go A.S., Hylek E.M., Phillips K.A., et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and risk factors in atrial fibrillation (ATRIA) study. Jama. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Feinberg W.M., Blackshear J.L., Laupacis A., et al. Prevalence, age distribution, and gender of patients with atrial fibrillation: analysis and implications. Arch. Intern. Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 4.Miller J.D., Aronis K.N., Chrispin J., et al. Obesity, exercise, obstructive sleep apnea, and modifiable atherosclerotic cardiovascular disease risk factors in atrial fibrillation. J. Am. Coll. Cardiol. 2015;66:2899–2906. doi: 10.1016/j.jacc.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 5.Yang W., Cao Q., Qin L., et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang. China J Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong T.-Y., Redwood S., Prendergast B., et al. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020;41(19):1798–1800. doi: 10.1093/eurheartj/ehaa231. PMID: 32186331; PMCID: PMC7454513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haseeb S., Gul E.E., Çinier G., et al. Value of electrocardiography in coronavirus disease 2019 (COVID-19) J. Electrocardiol. 2020 Aug 6;62:39–45. doi: 10.1016/j.jelectrocard.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T. Description and proposed Management of the Acute COVID-19 cardiovascular syndrome. Circulation. 2020;141(23):1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirchhof P., Benussi S., Kotecha D., et al. ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016 Oct 7;37(38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 10.Pieralli F., Biondo B., Vannucchi V., et al. Performance of the CHA(2)DS(2)-VASc score in predicting new onset atrial fibrillation during hospitalization for community-acquired pneumonia. Eur J Intern Med. 2019;62:24–28. doi: 10.1016/j.ejim.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Soto-Gomez N., Anzueto A., Waterer G.W., et al. Pneumonia: an arrhythmogenic disease? Am. J. Med. 2013;126:43–48. doi: 10.1016/j.amjmed.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Restrepo M.I., Reyes L.F. Pneumonia as a cardiovascular disease. Respirology. 2018;23:250–259. doi: 10.1111/resp.13233. [DOI] [PubMed] [Google Scholar]
- 13.Corrales-Medina V.F., Musher D.M., Shachkina S., et al. Acute pneumonia and the cardiovascular system. Lancet. 2013;381:496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 14.Boos C.J., Anderson R.A., Lip G.Y. Is atrial fibrillation an inflammatory disorder? Eur. Heart J. 2006;27:136–149. doi: 10.1093/eurheartj/ehi645. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y., Lip G.Y., Apostolakis S. Inflammation in atrial fibrillation. J. Am. Coll. Cardiol. 2012;60:2263–2270. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 16.Issac T.T., Dokainish H., Lakkis N.M. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J. Am. Coll. Cardiol. 2007;50:2021–2028. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 17.Marcus G.M., Whooley M.A., Glidden D.V., et al. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the heart and soul study. Am. Heart J. 2008;155:303–309. doi: 10.1016/j.ahj.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amdur R.L., Mukherjee M., Go A., et al. Interleukin-6 is a risk factor for atrial fibrillation in chronic kidney disease: findings from the CRIC study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren M., Li X., Hao L., et al. Role of tumor necrosis factor alpha in the pathogenesis of atrial fibrillation: a novel potential therapeutic target? Ann. Med. 2015;47:316–324. doi: 10.3109/07853890.2015.1042030. [DOI] [PubMed] [Google Scholar]
- 20.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T. Description and proposed Management of the Acute COVID-19 cardiovascular syndrome. Circulation. 2020;141(23):1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yenerçağ Mustafa, Arslan Uğur. Mustafa Doğduş et all. Evaluation of electrocardiographic ventricular repolarization variables in patients with newly diagnosed COVID-19. J. Electrocardiol. 2020 Jul 21;62:5–9. doi: 10.1016/j.jelectrocard.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson L.A., Canna S.W., Schulert G.S., et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheum. 2020;72:1059–1063. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madjid M., Safavi-Naeini P., Solomon S.D., et al. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. PMID: 32219363. [DOI] [PubMed] [Google Scholar]
- 24.Hu W.S., Lin C.L. Comparison of CHA(2)DS(2)-VASc, CHADS(2) and HATCH scores for the prediction of new-onset atrial fibrillation in cancer patients: a nationwide cohort study of 760,339 study participants with competing risk analysis. Atherosclerosis. 2017;266:205–211. doi: 10.1016/j.atherosclerosis.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y.L., Zeng M., Liu Y., et al. CHA(2)DS(2)-VASc score for identifying patients at high risk of postoperative atrial fibrillation after cardiac surgery: a meta-analysis. Ann. Thorac. Surg. 2020;109:1210–1216. doi: 10.1016/j.athoracsur.2019.07.084. [DOI] [PubMed] [Google Scholar]
- 26.Barkas F., Elisaf M., Korantzopoulos P., et al. The CHADS(2) and CHA(2)DS(2)-VASc scores predict atrial fibrillation in dyslipidemic individuals: role of incorporating low high-density lipoprotein cholesterol levels. Int. J. Cardiol. 2017;241:194–199. doi: 10.1016/j.ijcard.2017.04.062. [DOI] [PubMed] [Google Scholar]
- 27.ZheXu LeiShi, Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols Larry. Pneumonia as a trigger for atrial fibrillation. J Rural Med. 2017;12(2):146–148. doi: 10.2185/jrm.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]


