Abstract
Patients with COVID-19 report severe respiratory symptoms consistent with ARDS. The clinical presentation of ARDS in COVID-19 is often atypical, as patients with COVID-19 exhibit a disproportionate hypoxemia compared with relatively preserved lung mechanics. This pattern is more similar to neonatal respiratory distress syndrome secondary to surfactant deficiency, which has been shown to benefit from exogenous surfactant. We present our experience with exogenous surfactant treatment in a patient with COVID-19 experiencing COVID-19-related ARDS. The patient responded with improved oxygenation, and we believe surfactant was the catalyst for the successful extubation and clinical improvement of the patient.
Key Words: ARDS, COVID-19, surfactant
Patients hospitalized with COVID-19 experience severe respiratory symptoms consistent with ARDS. To date, despite various trials and medication regimens, no treatment has emerged as significantly efficacious in the treatment of patients with severe respiratory failure secondary to COVID-19, although some treatments have shown early promise.1 , 2 It is has been observed that COVID-19 patients with severe respiratory distress can present with an atypical form of ARDS, exhibiting significant discrepancy between their severe hypoxemia and their relatively preserved lung mechanics.3 This pattern is more similar to neonatal respiratory distress syndrome (RDS) secondary to surfactant deficiency, which has been shown to benefit from exogenous surfactant.4 We present our experience with exogenous surfactant treatment in a patient with COVID-19 experiencing respiratory failure.
Case Report
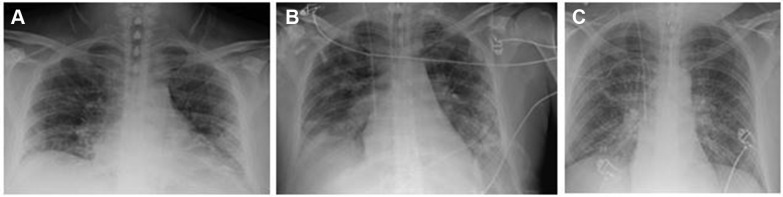
A 48-year-old male non-smoker, with a medical history of hyperlipidemia and prediabetes, was admitted to the hospital following 4 days of fever, dry cough, and exertional dyspnea. On presentation, the patient had speech dyspnea and was febrile (39.5°C) and tachycardic (109 beats/min). Oxygen saturation (Spo 2) at room air was 94%, with normal venous blood gas, mild hyponatremia (126 mEq/L), and lymphopenia (0.8 K/µL). Chest radiograph showed mild bibasilar opacities (Fig 1 A). Results of a COVID-19 test were positive.
Figure 1.
Chest radiograph of the study patient at admission (A) and following respiratory decline (B and C).
The patient was initially treated with azithromycin, hydroxychloroquine, and ceftriaxone, with azithromycin replaced by lopinavir/ritonavir because he did not improve. Repeat chest radiograph showed worsening bilateral opacities (Fig 1B); due to worsening respiratory status, the patient was admitted to the ICU, with a single dose of tocilizumab replacing lopinavir/ritonavir. He continued to desaturate despite noninvasive respiratory support and was intubated on day 3 of hospitalization and placed on the ARDS protocol, including extended proning. Despite full respiratory support, the patient’s ratio of Pao 2 (in millimeters of mercury) to Fio 2 continued to decline and on day 6 of hospitalization, he was placed on extracorporeal membrane oxygenation (ECMO).
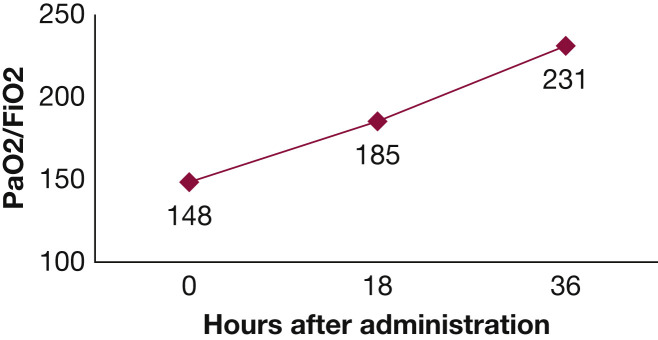
The patient remained stable but did not improve on ECMO for 5 days. On day 11 of hospitalization, he was administered five ampules of 6 mL surfactant (calfactant; 35 mg/mL phospholipid suspension), determined based on 20 mg phospholipids/kg of lean body weight, similar to dosing in a prior adult surfactant treatment trial that showed clinical improvment.5 The surfactant was administered via tracheobronchial suction catheter passed through the endotracheal tube with the distal suction tip positioned above the carina, and then dispersed directly into the lungs. The patient was sequentially turned right side down and then left side down immediately subsequent to administration. Prior to administration, oxygen saturation was 89% on Fio 2 100%, Pao 2/Fio 2 was 148, and compliance was measured at 31 mL/cm H2O. Subsequently, Pao 2/Fio 2 improved to 185 after 18 h and to 231 after 36 h (Fig 2 ), at which point the patient was weaned from ECMO. The patient was extubated the following day and was discharged 8 days later.
Figure 2.
Pao2/Fio2 of the study patient following administration of surfactant (tracked in hours).
Discussion
Although COVID-19 patients with severe respiratory symptoms can have ARDS consistent with the Berlin criteria,6 the clinical presentation of acute respiratory distress in COVID-19 is often atypical.7 Patients with COVID-19 exhibit disproportionate hypoxemia compared with a relatively preserved compliance and lung mechanics, a pattern more similar to RDS observed in premature infants who lack sufficient capacity to produce endogenous surfactant.3 SARS-CoV-2 has affinity for the angiotensin-converting enzyme receptor 2 (ACEr2) and predominantly damages cells expressing ACEr2, including type II alveolar cells, the source of lung surfactant.8 Damage to the type II alveolar cells markedly decreases the production of surfactant, which is necessary for effective gas exchange, leading to increased surface tension, alveolar flooding, and atelectasis.9
As such, a common pathophysiological feature of patients with COVID-19 and acute respiratory distress is dysfunction of the endogenous surfactant system, similar to the pathophysiology of RDS. Administration of exogenous pulmonary surfactant is an effective treatment of premature infants with RDS due to insufficient surfactant production.4 Although exogenous surfactant therapy has proven to be an effective treatment for RDS, no similar current effective therapy exists for patients with ARDS. Prior trials of surfactant administration to adults with ARDS have generally been disappointing,10 with the majority showing no benefit,11 although a minority have shown improvement in oxygenation and time to extubation.12
However, as noted, the pathophysiology of alveolar epithelial cell damage due to COVID-19 does not follow a classical pattern of ARDS; lung mechanics and compliance can be preserved, despite severe hypoxemia.13 Furthermore, other notable distinctions between classical ARDS and respiratory failure due to COVID-19 include the discrepancy between symptoms and radiologic presentation, delayed timing of respiratory failure from disease onset in COVID-19, and the direct involvement of ACERr2 in the infiltration of COVID-19.
The preferential targeting of COVID-19 for ACEr2, and consequently type II alveolar cells, implies a pivotal role of surfactant deficiency in the hypoxemic lung injury caused by COVID-19. Beyond the critical role surfactant plays in maintaining surface tension and preserving gas exchange, surfactant also exhibits antiinflammatory properties, reducing the expression of various cytokines,14 and resulting in decreased lung inflammation and damage.15 Accordingly, due to the similarities between neonatal RDS and COVID-19-induced respiratory distress, surfactant administration has been proposed as a potential treatment for COVID-19.3 , 16 , 17
More specifically, COVID-19 damages the type II alveolar cells, inhibiting production of surfactant. The reduction in natural surfactant in the lung leads to alveolar collapse and atelectasis, which as the process progresses, reduces the dynamic compliance of the lung; this scenario was observed in the current patient, who was administered surfactant at a relatively late stage of his infection. This is further exacerbated by an observed failure of the hypoxic pulmonary vasoconstriction mechanism in COVID-19, which, combined with alveolar collapse secondary to surfactant loss and lung edema, results in a substantial portion of the cardiac output perfusing nonaerated lung tissue, increasing intrapulmonary shunting.18 Restoration of surfactant would lower surface tension, reduce atelectasis and intrapulmonary shunting, and improve gas exchange, as well as counterbalance the proinflammatory effects of COVID-19.19
Our experience with administration of surfactant to a patient with COVID-19, while limited, is informative. The patient responded to surfactant with improved oxygenation, leading, we believe, to his clinical improvement and providing the catalyst that led to weaning from ECMO and extubation. Although it has been posited that surfactant administration would be more efficacious in ARDS if administered earlier in the course of the disease, prior to patients already having experienced substantial lung damage, similar benefits can be observed even during the refractory weaning phase of COVID-19 disease.3 , 16 Further studies can assist in determining the optimal timing and means of administering surfactant. For example, deposition of surfactant into the distal periphery of the lung under atelectatic conditions may be improved through aerosolizing the surfactant, potentially decreasing the particle size and allowing for more distal distribution to the periphery of the lung. In addition, sampling of surfactant levels in patients may provide further insight into the pathophysiology of COVID-19 ARDS and potentially identify patients who can benefit from surfactant administration.20 Future clinical trials, some of which are underway,21, 22, 23, 24, 25 will further elucidate the potential benefits of surfactant administration in COVID-19.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
References
- 1.RECOVERY Collaborative Group. Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koumbourlis A.C., Motoyama E.K. Lung mechanics in COVID-19 resemble RDS not ARDS: could surfactant be a treatment? Am J Respir Crit Care Med. 2020;202(4):624–626. doi: 10.1164/rccm.202004-1471LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sardesai S., Biniwale M., Wertheimer F., Garingo A., Ramanathan R. Evolution of surfactant therapy for respiratory distress syndrome: past, present, and future. Pediatr Res. 2017;81(1-2):240–248. doi: 10.1038/pr.2016.203. [DOI] [PubMed] [Google Scholar]
- 5.Amital A., Shitrit D., Raviv Y., et al. The use of surfactant in lung transplantation. Transplantation. 2008;86(11):1554–1559. doi: 10.1097/TP.0b013e31818a8418. [DOI] [PubMed] [Google Scholar]
- 6.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 7.Li X., Ma X. Acute respiratory failure in COVID-19: is it "typical" ARDS? Crit Care. 2020;24(1):198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason R.J. Thoughts on the alveolar phase of COVID-19. Am J Physiol Lung Cell Mol Physiol. 2020;319(1):L115–L120. doi: 10.1152/ajplung.00126.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willson D.F., Truwit J.D., Conaway M.R., Traul C.S., Egan E.E. The Adult Calfactant in Acute Respiratory Distress Syndrome trial. Chest. 2015;148(2):356–364. doi: 10.1378/chest.14-1139. [DOI] [PubMed] [Google Scholar]
- 11.Meng S.S., Chang W., Lu Z.H., et al. Effect of surfactant administration on outcomes of adult patients in acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. BMC Pulm Med. 2019;19(1):9. doi: 10.1186/s12890-018-0761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang C., Su M., Jiang T. Efficacy of pulmonary surfactant in the treatment of adult respiratory distress syndrome (ARDS) Biomedical Res. 2017;28(22):9801–9804. [Google Scholar]
- 13.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 does not lead to a "typical" acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spragg R.G., Lewis J.F., Walmrath H.D., et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004;351(9):884–892. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 15.Voelker D.R., Numata M. Phospholipid regulation of innate immunity and respiratory viral infection. J Biol Chem. 2019;294(12):4282–4289. doi: 10.1074/jbc.AW118.003229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busani S., Dall’Ara L., Tonelli R., et al. Surfactant replacement might help recovery of low-compliance lung in severe COVID-19 pneumonia. Ther Adv Respir Dis. 2020;14:1–6. doi: 10.1177/1753466620951043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Islam A.B., Khan M.A. Lung transcriptome of a COVID-19 patient and systems biology predictions suggest impaired surfactant production which may be druggable by surfactant therapy. Sci Rep. 2020;10(1):19395. doi: 10.1038/s41598-020-76404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhont S., Derom E., Van Braeckel E., et al. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir Res. 2020;21:198–207. doi: 10.1186/s12931-020-01462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirastschijski U., Dembinski R., Maedler K. Lung surfactant for pulmonary barrier restoration in patients with COVID-19 pneumonia. Front Med (Lausanne) 2020;7:254. doi: 10.3389/fmed.2020.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schousboe P., Wiese L., Heiring C., et al. Assessment of pulmonary surfactant in COVID-19 patients. Crit Care. 2020;24(1):552. doi: 10.1186/s13054-020-03268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institutes of Health Clinical Center. The safety and preliminary efficacy of lucinactant in adults with COVID-19. NCT04389671. ClinicalTrials.gov. National Institutes of Health; 2020. Updated October 8, 2020. https://clinicaltrials.gov/ct2/show/NCT04389671.
- 22.National Institutes of Health Clinical Center. Curosurf® in adult acute respiratory distress syndrome due to COVID-19 (Caards-1). NCT04384731. ClinicalTrials.gov. National Institutes of Health; 2020. Updated February 3, 2021. https://clinicaltrials.gov/ct2/show/NCT04384731.
- 23.National Institutes of Health Clinical Center. A clinical trial of nebulized surfactant for the treatment of moderate to severe COVID-19 (COVSurf). NCT04362059. ClinicalTrials.gov. National Institutes of Health; 2020. Updated September 9, 2020. https://clinicaltrials.gov/ct2/show/NCT04362059.
- 24.National Institutes of Health Clinical Center. London's exogenous surfactant study for COVID19 (LESSCOVID). NCT04375735. ClinicalTrials.gov. National Institutes of Health; 2020. Updated June 11, 2020. https://clinicaltrials.gov/ct2/show/NCT04375735.
- 25.National Institutes of Health. Poractant alfa - curosurf and SARS-COV-19 ARDS (Covid-19). NCT04502433. ClinicalTrials.gov. National Institutes of Health; 2020. Updated January 20, 2021. https://clinicaltrials.gov/ct2/show/NCT04502433.