Abstract
Coronavirus Disease 2019 (COVID-19) is well documented as a cause of respiratory tract infection. Increasingly, multi-systemic effects, including COVID-19-related neurologic features, are being reported. Here we report, what we believe to be, the first reported case of acute haemorrhagic leukoencephalitis (AHLE) with presence of oligoclonal bands in the cerebrospinal fluid. AHLE is a rare fulminant demyelinating disease, associated with severe COVID-19 infection.
Keywords: COVID-19, Neurological features, Haemorrhagic leukoencephalitis, Oligoclonal bands
Highlights
-
•
Acute Haemorrhagic Leukoencephalitis (AHLE) could be a neurological complication of severe COVID-19 infection.
-
•
The presence of oligoclonal bands in the CSF could highlight the central inflammatory response.
-
•
Here a rare case of AHLE in a severe COVID-19 infection with positive oligoclonal bands in CSF is described.
1. Introduction
By January 2021, more than 89 million cases of Covid-19 have been reported worldwide resulting in more than 1 million death (D-19 Dashboard by the, 2020). Various clinical manifestations of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been reported including neurologic sequalae. Helms J. et al. reported several syndromes including agitation and confusion following withdrawal of sedation, diffuse corticospinal tract signs, and dysexecutive syndrome (Helms et al., 2020). Others have described COVID-19 related headache, dizziness, myalgia, anosmia, encephalopathy, encephalitis, necrotising haemorrhagic encephalopathy, stroke, epileptic seizures, rhabdomyolysis and Guillain-Barre syndrome (Carod-Artal, 2020).
This report describes a rare case of acute haemorrhagic leukoencephalitis (AHLE), also known as Hurst disease in a patient with severe COVID-19 requiring invasive ventilation. Oligoclonal bands were identified on cerebrospinal fluid analysis suggesting intra-thecal immune response.
2. Case report
A 56-year-old male presented to the emergency department with a 7-day history of flu-like symptoms. He was active and worked full time. His past medical history included hypertension, chronic kidney disease, hypercholesterolaemia, asthma, and pre-obesity (Body Mass Index (BMI): 29.72 kg/m2). He had no known neurological condition. The patient was admitted to critical care for mechanical ventilation within 24 h. A Polymerase Chain Reaction (PCR) test for 2019 SARS-CoV-2 Ribonucleic Acid (RNA) from the nasopharyngeal swab confirmed the diagnosis of COVID-19.
During the course of the admission, he developed an acute kidney injury (AKI), and required continuous veno-venous haemofiltration (CVVH) which was started on day 5 of his admission. On day 7 of the admission, D-dimer was >19.0 μg/mL (normal range in our centre: 0.27–0.5 μg/mL), fibrinogen of >10 g/L (normal range: 1.7–4.5 g/L), and platelets remained in normal limits during admission (ranging from 194 to 353 × 109/L). On day 10, in accordance with a change in local guidelines, he was commenced on therapeutic dose heparin infusion to reduce the risk of clot formation (he had received prophylactic dose prior to this). The APTT ratio remained within the target range of 1.5–2.5. No other anticoagulation or antiplatelet therapy medications were commenced during his ICU admission. No clinical trial medication was trialled in this case and his management mainly remained supportive. He also developed persistent hypertension on a background of known essential hypertension. This was controlled with multiple antihypertensive medications. His regular antihypertensive medications were gradually reintroduced when systolic blood pressure (SBP) ranged between 140 and 190 mmHg (these medicines had been held on admission since he required low dose vasopressors until day 10). His antihypertensive medications included doxazocin, amlodipine, bendroflumethiazide, and lisinopril. Hypertension peaked between days 20 and 23 when the SBP reached 220 mmHg and labetalol infusion was commenced. The high BP was controlled, and labetolol was converted to regular bisoprolol on day 28.
Initially, he was difficult to ventilate and oxygenate, but was unresponsive to prone positioning. Apart from initial hypoxia on admission that required urgent intubation and ventilation (89% O2 saturation on 60% oxygen via face mask), his oxygen saturation ranged between 91% and 100%. By the third week in critical care, his respiratory and renal function improved and CVVH was discontinued on day 21.
By day 15, sedative medications were reduced, and attempts were made to reduce ventilatory support. However, on stopping sedation, the patient’s Richmond Agitation-Sedation Scale (RASS) remained at −2. By day 24 all sedative infusions had stopped yet maximum Glasgow Coma Scale (GCS) was E4VTM1. No obvious focal neurological signs were noted on physical examination.
2.1. Investigations
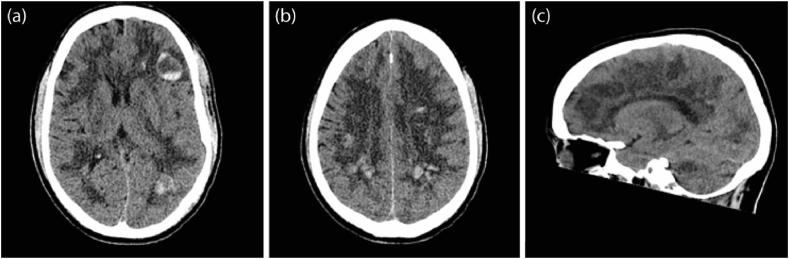
Computed tomography (CT) scan of the brain was performed on day 26 to investigate his persistently reduced GCS. This demonstrated probable Acute Haemorrhagic Leukoencephalitis (AHLE) (Fig. 1). Magnetic resonance imaging (MRI) brain (on day 27) and cerebrospinal fluid (CSF) analysis on day 30 were undertaken and neurosurgical and neurology opinions were sought.
Fig. 1.
First Computed Tomography (CT) scan of the head. (diffuse white matter hypodensity which possibly due to oedema or demyelination process, and multiple bilateral white matter haemorrhagic foci involving the corpus callosum with fluid blood level in some of them, with most of the blood is along the white matter venules).
2.2. Management
Due to slow weaning from respiratory support and a persistently reduced GCS, tracheostomy was performed on day 20. Anticoagulation was continued. There was no neurosurgical treatment available in this case and no specific treatments were trialled during management. Overall clinical management strategy remained supportive and the patient developed no new problems. By day 49, his GCS remained around 7 (E4VTM3), and he was discharged from ICU to a general respiratory ward. The tracheostomy remained in situ to aid with respiratory secretion management with no supplemental oxygen required.
2.3. Follow-up and outcome
A repeat unenhanced CT brain on day 47 of admission showed no evidence of acute infarction, or intra-axial or extra-axial haemorrhage. Mild generalised involutional changes and small vessel disease were present on the scan. A repeat MRI of his brain was performed on day 60 (5 weeks after the initial imaging scan) to assess for changes and improvement. The MRI findings were still compatible with haemorrhagic leukoencephalitis. The appearances were less marked in comparison with the initial MRI. It also illustrated evidence of leukoencephalitis involving the cerebellum and the pons, with no evidence of haemorrhage within them (Fig. 2). An electroencephalography (EEG) as follow-up on day 65 was performed. EEG was diffusely slow and poorly responsive in keeping with a diffuse encephalopathy or encephalitis with no epileptiform discharge.
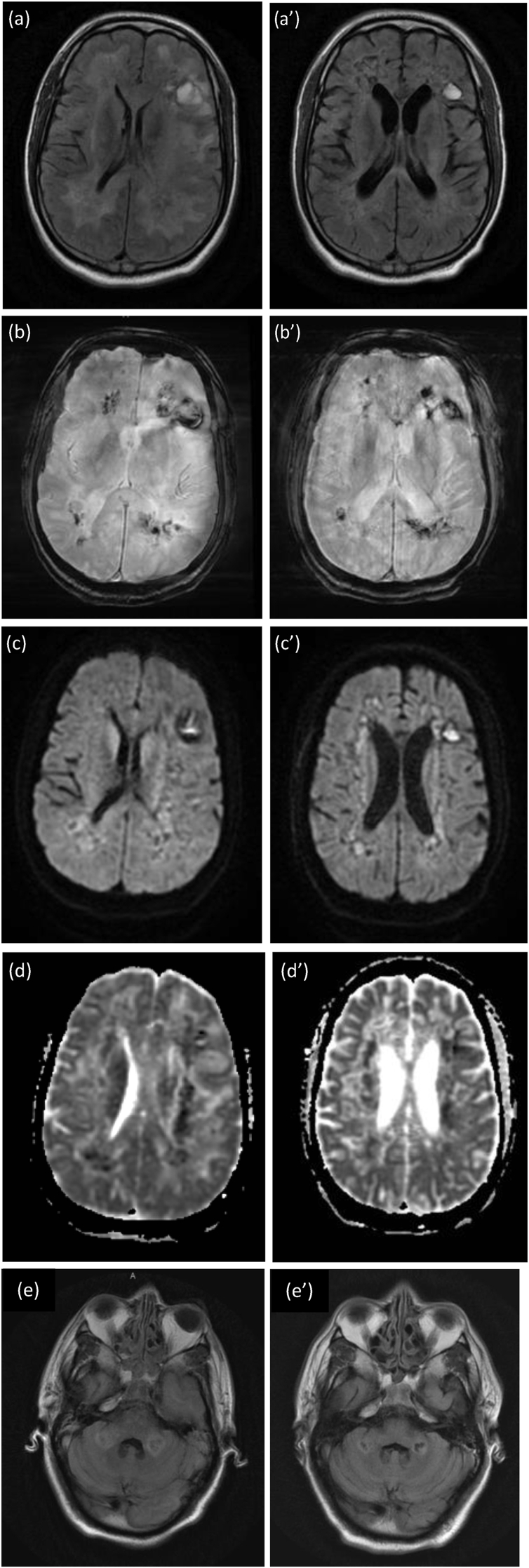
Fig. 2.
Magnetic Resonance Imaging (MRI) of the brain.
Left Column, Initial brain MRI:(a) Axial fluid-attenuated inversion recovery (FLAIR), (b) Susceptible-weighted imaging (SWI), (c) Diffusion-weighted imaging (DWI), (d) Apparent Diffusion Coefficient (ADC) sequences, and (e) view of cerebellum involvement in Axial T2 fluid-attenuated inversion recovery (FLAIR) are demonstrated here.
Increased symmetrical FLAIR signal throughout the white matter. Diffuse haemosiderin staining throughout the white matter and the genu of the corpus callosum. There are also some cystic haemorrhagic areas containing a fluid-blood level within both cerebral hemispheres. There are some areas of restricted diffusion within the white matter, but this signal change is unreliable owing to the presence of acute haemorrhage.
Right Column, Repeat Follow-Up MRI on Day 60. (a’) Axial fluid-attenuated inversion recovery (FLAIR), (b’) Susceptible-weighted imaging (SWI), (c’) Diffusion-weighted imaging (DWI), (d’) Apparent Diffusion Coefficient (ADC) sequences, and (e’) view of cerebellum involvement in Axial T2 fluid-attenuated inversion recovery (FLAIR) are demonstrated here.
Extensive abnormal signal throughout the white matter bilaterally with haemorrhage compatible with haemorrhagic leukoencephalitis. the appearances are slightly less marked compared with the previous examination. There is evidence of leukoencephalitis involving the cerebellum and the pons, though there is no evidence of haemorrhage within the pons or cerebellum.
Following discharge from ICU, his neurological condition has remained stable with no obvious progression of AHLE. He was also decannulated prior to his discharge from the hospital and remained stable with no oxygen requirement. Despite his prognosis remaining uncertain, the patient was discharged to a neurorehabilitation centre.
3. Methods
3.1. Specimen collection
Nasopharyngeal swabs were collected according to the Public Health England (PHE) guideline. The samples were stored at 2–8 °C prior to transfer to the main laboratory for testing. Lumbar puncture (LP) was performed using full sterile technique in ICU, and local anaesthetic was used. The procedure was atraumatic and successful at the first attempt. The samples for culture, PCR, protein, and glucose were collected in sterile specimen containers. A paired serum glucose was also sent to the laboratory. The CSF sample was shipped to PHE for diagnostic testing.
3.2. Diagnostic testing
3.2.1. Nasopharyngeal testing
The platform was Brighton-m2000 Realtime System (Abbott). The COVID assay detects RdRp and N genes with an internal control running alongside to ensure amplification of the target genes occurs.
3.2.2. CSF testing
This specimen was tested in Viasure NCO2 assay to detect ORF1ab and N-gene, and also in parallel WHO E-gene assay.
3.2.3. MRI imaging
The patient was escorted by the anaesthetics team to the MRI scanner room. Following the imaging scan, the MRI scanner is decontaminated according to the trust guideline. The scans were reported by the trust radiologists.
4. Results
Findings at MRI brain were suggestive of underlying acute haemorrhagic leukoencephalitis (AHLE) (Fig. 2).
Lumbar puncture demonstrated opening pressure of >40 mmH2O (exceeding the capacity of the manometer), white blood cells of less than 1.0 per uL, red blood cells of 6 per uL, CSF protein of 0.71 g/L (normal limit of 0.15–0.45 g/L), and CSF glucose of 4.3 mmol/L with serum glucose of 8.6 mmol/L (normal limit of 3.0–6.0 mmol/L). No organisms were seen on gram stain of the CSF sample. CSF culture had no growth after two days. 2019 n-CoV SARS-CoV-2 RNA was not detected on the CSF sample. Herpes Simplex Virus-1 (HSV-1) Deoxyribonucleic Acid (DNA), HSV-2 DNA, Varicella zoster virus DNA, Enterovirus (group) RNA, and Parechovirus RNA were not detected on the CSF sample. West Nile virus serum IgM was negative.
The CSF sample tested positive for Oligoclonal Bands. These were found in both the CSF and serum with additional bands in the CSF. Serum Galactomannan (Aspergillus Antigen), and Beta Glucan antigen were also negative. He was also screened for Cytomegalovirus (CMV), Epstein-Barr virus (EBV), Hepatitis A, hepatitis B, hepatitis C, and Human Immunodeficiency Viruses (HIV) which were all negative.
5. Discussion
Disturbance of consciousness has been reported in around 15% of severe COVID-19 cases according to Mao et al. (Mao et al., 2020). However, direct infection of the brain is an unusual feature of Covid-19 5. Possible aetiologies for the neurological manifestations of COVID-19 infection include a direct neurotropic element of the disease or the secondary consequence of cytokine storm (Kandemirli et al., 2020). In our case, AHLE is presumed to be the direct complication of severe COVID-19 infection. AHLE is a rare demyelinating condition with significant associated mortality (Fontoura et al., 2002). It has previously been reported as a complication of other viral illnesses (Solis et al., 2017). AHLE is thought to be the most fulminant variant of acute disseminated encephalomyelitis (ADEM) with rapid progression of white matter inflammation (Rawal et al., 2019). In our case, the distribution of the white matter disease on imaging scan matches the ADEM distribution, as it usually involves the subcortical and deep white matter. In severe cases, the brainstem and cerebellum could be involved. Although the haemorrhagic component of AHLE is usually small, we believe in our case, this was severe enough to be depicted by CT due to possible aggravation effects of anticoagulation and hypertension. The pathophysiology of AHLE is not known but cross reactivation of host myelin and viral antigens is thought to be one possible mechanism (Fontoura et al., 2002; Solis et al., 2017; Duggal et al., 2014). Moreover, reported cases of COVID-19 related diffuse leukoencephalopathy and microhaemorrhages have been thought to be likely in association with hypoxaemia (Radmanesh et al., 2020a). Although, there has been a report of presumed diagnosis of delayed post-hypoxic leukoencephalopathy (DPHL) (Radmanesh et al., 2020b) following acute respiratory failure in a COVID-19 patient, after the initial period, our patient’s oxygenation remained stable during his ICU admission and his MRI findings were in keeping with AHLE.
Intracranial haemorrhage (ICH) has been observed in COVID-19 infection, especially in patients with risk factors (such as hypertension) (Cheruiyot et al., 2020). However, ICH incidence in COVID-19 is lower than ischemic stroke, with only 0.7% incidence (Cheruiyot et al., 2020). It has been shown that ICH in COVID-19 could be linked with anticoagulation treatment. More than half of these patients were on anticoagulation due to significantly high D-dimer levels, and a larger number of ICH patients were on heparin (treatment dose) (Melmed et al., 2020). It is speculated that could be rather a sign of severity of the illness than the increased risk of ICH while on heparin infusion (Melmed et al., 2020).
In addition, a reduced 28-day mortality in severe COVID-19 patients (with a significant D-dimer elevation) who were on anticoagulation therapy has been illustrated (Tang et al., 2020). It is postulated that anticoagulation treatment of severe COVID-19 patients with significant rise in D-dimer could be beneficial (Tang et al., 2020). The use of anticoagulation therapy in severe COVID-19 patients remains controversial. Further studies are required, and individual risk-benefit assessment should be considered. In our case, in view of considerable raised D-dimer and fibrinogen (maximum of 19.23 μg/mL in D-dimer prior to the start of heparin infusion), treatment dose anticoagulation was started as per local guideline. ICH could be thought as a differential diagnosis for such patient population with risk factors (including hypertension, and anticoagulation). However, there were signs of altered mental status in this case, since day 15 (during sedation withdrawal). This was before significant hypertensive episodes occurred. Within 72 h of the first imaging scan, the D-dimer and fibrinogen were still high (D-dimer mean: 6.23 μg/mL, Fibrinogen mean: 6.02 g/L), with APTT ratio in target range between 1.5 and 2.5, and normal platelet level during his admission.
The small vessel white matter disease is usually evident in hypertensive cerebrovascular disease imaging, and if the haemorrhage occurs, it is most frequently seen in basal ganglia followed by thalamus, brain stem and cerebellum (all are spared in our case). 10% of hypertensive haemorrhage can be lobar, but this incidence is controversial, because older hypertension patients may also have amyloid angiopathy. Hypertension and anticoagulation in our case, are thought to have aggravated the bleeding rather than caused it. Furthermore, hypertensive micro-bleeds are encountered in the deep grey matter and posterior fossa more than subcortically. Other differential diagnosis could be posterior reversible encephalopathy syndrome, which usually involves the cortex and subcortical white matter with no preservation of the subcortical U-fibres.
In our case, the radiological findings are suggestive of the diagnosis of AHLE with diffuse white matter involvement, foci of haemorrhages, surrounding oedema, subcortical U-fibres, and cortical sparing. In contrast, in one study looking at haemorrhagic stroke in Covid-19, the punctuate haemorrhages of parenchymal bleeding were usually found to involve the cortices (Dogra et al., 2020). Imaging scans play a crucial role in the early identification of AHLE. MRI could also differentiate AHLE from ADEM through demonstrating the presence of petechial haemorrhages and extensive white matter lesions in AHLE (Grzonka et al., 2020). Another differential diagnosis of AHLE could be infectious encephalitis. In HSV-1, brain involvement is often in temporal and limbic areas (Grzonka et al., 2020). Infectious encephalitis was excluded with negative CSF PCR and serology results.
Poyiadji et al. reported a case of acute haemorrhagic necrotizing encephalopathy (ANE) linked with SARS-COV-218 where the patient presented with altered conscious level and no abnormal findings on CSF analysis (Poyiadji et al., 2020). In our case, no focal neurological features were noted at presentation but high opening pressure at lumbar puncture, high CSF protein, and serum and CSF oligoclonal bands with additional bands in CSF were identified. Furthermore, a fatal case of ANE in COVID-19 has been reported. However, no oligoclonal bands were identified in their CSF analysis. The CT head showed brain oedema, whereas our case’s CT head showed multiple bilateral white matter demyelination, and haemorrhagic foci involving the corpus callosum as well as oedema (Elkady and Rabinstein, 2020)..(Needham et al., 2020)
Although the AHLE diagnosis is usually confirmed at autopsy and biopsy (Solis et al., 2017), MRI characteristics are useful in early detection of the condition. MRI findings show multifocal lesions (Solis et al., 2017; Poyiadji et al., 2020). Susceptibility weighted sequences demonstrate haemorrhage. There is white matter signal alteration with sparing of the grey matter and subcortical U-fibers, in a predominantly frontoparietal distribution. The Basal ganglia, brain stem and spinal cord may also be affected (Solis et al., 2017). Some have suggested a potential role for corticosteroids or intravenous immunoglobulin as treatment (Poyiadji et al., 2020; Bridwell et al., 2020; Pilotto et al., 2020). These were not considered viable options in this case due to the risks of ongoing infection.
Oligoclonal bands which are common to both CSF and serum imply a systemic inflammatory response, whilst bands which are restricted to the CSF suggest an additional specific central nervous system (CNS) response. Drouet et al. reported a case of borrelia burgdorferi infection related leukoencephalopathy with oligoclonal Immunoglobulin (Ig) appearance (Drouet et al., 2003). The SARS-CoV-2 RNA was not detected in the CSF. Due to temporary distribution of SARS-CoV-2 virus in CSF, isolating the virus is challenging (Ye et al., 2020). It is postulated that significant immune response could cause damage in the blood brain barrier (BBB) and this may result in CNS inflammation (Ye et al., 2020; De Felice et al., 2020). Oligoclonal IgG in CSF could present in the subacute phase of an infective encephalitis (Sindic et al., 2003). In our case, although the SARS-CoV-2 PCR was negative in CSF, the presence of additional oligoclonal bands in the CSF may be an indication of an immunologic response to the infection.
Repeat MRI head performed at day 60 suggested possible slight improvement of AHLE appearances. This raises the question of whether AHLE in COVID-19 could be self-limiting in the subacute phase.
6. Conclusion
To the best of our knowledge this is the first reported case of AHLE with positive oligoclonal bands associated with SARS-CoV-2 infection to date with follow-up MRI scan and EEG. Further data is essential to establish the causative mechanism, optimal management, and to elucidate the prognosis of this condition associated with COVID-19.
Funding
No specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgement
We are thankful to our patient’s family for giving us permission to report this rare manifestation of the COVID-19. We thank Dr Stephanie Jane Smith for providing the testing details. We thank Dr Jeffery Kimber for his expert neurology opinion. We would like to acknowledge the immense hard work and dedication of our ICU team who were key to make an enormous difference in our patients’ disease journey.
References
- Bridwell R., Long B., Gottlieb M. Neurologic complications of COVID-19. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carod-Artal F.J. Neurological complications of coronavirus and COVID-19. Rev. Neurol. 2020;70(9):311–322. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- Cheruiyot I., Sehmi P., Ominde B. Intracranial hemorrhage in coronavirus disease 2019 (COVID-19) patients. Neurol. Sci. 2020 doi: 10.1007/s10072-020-04870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins university. 2020. https://coronavirus.jhu.edu/map.html
- De Felice F.G., Tovar-Moll F., Moll J., Munoz D.P., Ferreira S.T. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the central nervous system. Trends Neurosci. April. 2020;21 doi: 10.1016/j.tins.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra S., Jain R., Cao M. Hemorrhagic stroke and anticoagulation in COVID-19. J. Stroke Cerebrovasc. Dis. 2020;29(8) doi: 10.1016/j.jstrokecerebrovasdis.2020.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouet A., Meyer X., Guilloton L. Acute severe leukoencephalitis with posterior lesions due to Borrelia burgdorferi infection. Presse Med. 2003;32(34):1607–1609. [PubMed] [Google Scholar]
- Duggal Ne, Ahmed I., Ni Duggal. Cute hemorrhagic leukoencephalitis associated with autoimmune myopathy. J. Vasc. Interv. Neurol. 2014;7(4):19–22. [PMC free article] [PubMed] [Google Scholar]
- Elkady A., Rabinstein A.A. Acute necrotizing encephalopathy and myocarditis in a young patient with COVID-19. Neurol. Neuroimmunol. Neuroinflamm. Sep. 2020;7(5) doi: 10.1212/NXI.0000000000000801. [DOI] [Google Scholar]
- Fontoura P., Mendes A., Correia M., Melo-Pires M. Weston Hurst acute haemorrhagic leukoencephalitis. Neuropathological study of one case. Abstract. Rev. Neurol. 2002;35(4):328–331. [PubMed] [Google Scholar]
- Grzonka P., Scholz M.C., De Marchis G.M. Acute hemorrhagic leukoencephalitis: a case and systematic review of the literature. Front. Neurol. 2020;11:899. doi: 10.3389/fneur.2020.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandemirli S.G., Dogan L., Sarikaya Z.T. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiol. May. 2020;8 doi: 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Wang M., Chen S. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed K.R., Cao M., Dogra S. Risk factors for intracerebral hemorrhage in patients with COVID-19. J. Thromb. Thrombolysis. 2020 doi: 10.1007/s11239-020-02288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham E.J., Chou S.H., Coles A.J., Menon D.K. Neurological implications of COVID-19 infections. Neurocritical Care. 2020;32:667–671. doi: 10.1007/s12028-020-00978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Odolini S., Stefano Masciocchi S. Steroid-responsive encephalitis in covid-19 disease. Ann. Neurol. May. 2020;17 doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiol. March. 2020;31 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmanesh A., Derman A., Lui Y.W. COVID-19 -associated diffuse leukoencephalopathy and microhemorrhages. Radiol. May. 2020;21 doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmanesh A., Derman A., Ishida K. COVID-19-associated delayed posthypoxic necrotizing leukoencephalopathy. J. Neurol. Sci. May 2020 doi: 10.1016/j.jns.2020.116945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acute hemorrhagic encephalomyelitis (AHEM) mimicking abscess (case of the Week) Rawal S., Machnowska M., Pauranik A., section, editors. Am. J. Neuroradiol. July. 2019;4 http://www.ajnr.org/content/cow/07042019/tab-diagnosis [Google Scholar]
- Sindic C.J., Van Antwerpen M.P., Goffette S. Clinical relevance of polymerase chain reaction (PCR) assays and antigen-driven immunoblots for the diagnosis of neurological infectious diseases. Abstract. Brain Res. Bull. 2003;61(3):299–308. doi: 10.1016/s0361-9230(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Solis W.G., Waller S.E., Harris A.K., Sugo E., Hansen M.A., Lechner-Scott J. Favourable outcome in a 33-year-old female with acute haemorrhagic leukoencephalitis. Case Rep. Neurol. 2017;9:106–113. doi: 10.1159/000472706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemostasis. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav. Immun. April. 2020;10 doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]