Abstract
Background
While direct effects of occupational exposures on an individual’s respiratory health are evident, a new paradigm is emerging on the possible effects of pre-conception occupational exposure on respiratory health in offspring. We aimed to study the association between parental occupational exposure starting before conception and asthma in their offspring (at 0–15 years of age).
Methods
We studied 3985 offspring participating in the Respiratory Health in Northern Europe, Spain and Australia (RHINESSA) generation study. Their mothers or fathers (n = 2931) previously participated in the European Community Respiratory Health Survey (ECRHS). Information was obtained from questionnaires on parental job history pre- and post-conception which was linked to an asthma-specific job-exposure matrix (JEM). We assessed the association between parental occupational exposure and offspring asthma, applying logistic regression models, clustered by family and adjusted for study centre, offspring sex, parental characteristics (age, asthma onset, place of upbringing, smoking) and grandparents’ level of education.
Results
Parental occupational exposure to microorganisms, pesticides, allergens or reactive chemicals pre-conception or both pre- and post-conception was not related to offspring asthma; in general, subgroup analyses confirmed this result. However, maternal exposure both pre- and post-conception to allergens and reactive chemicals was associated with increased odds for early-onset asthma in offspring (0–3 years of age); odds ratio 1.70 (95% CI: 1.02–2.84) and 1.65 (95% CI: 0.98–2.77), respectively.
Conclusions
This study did not find evidence that parental occupational exposure, defined by an asthma JEM before conception only or during pre- and post-conception vs non-exposed, was associated with offspring asthma.
Keywords: Epidemiology, asthma, generation study, occupational exposure, air pollutants, occupation, job-exposure matrices
Key Messages
Parental occupational exposure to microorganisms, pesticides, allergens or reactive chemicals pre-conception or both pre- and post-conception does not seem to have a major impact on offspring asthma.
Maternal occupational exposure during both pre- and post-conception periods to allergens and reactive chemicals might be associated with an increased risk for early-onset offspring asthma.
Paternal occupational exposure pre-conception or during both pre- and post-conception periods did not seem to impact the development of asthma in offspring.
Introduction
In the early 1990s, associations were found between low birth weight or preterm birth and increased risk of ischaemic heart disease in adulthood.1,2 These findings led to the theory that exposures in utero could affect offspring health in adulthood,3,4 the Barker Hypothesis,1,2 and further developed into the Developmental Origins of Health and Diseases, including the role of early life environmental exposures such as chemicals in the aetiology of adult disease.5
During the last 30 years, the incidence of asthma has increased considerably,6,7 and asthma is now one of the most common global chronic diseases in children and adolescents.6–8 Occupational exposures to e.g. chemical and biological agents are known to affect respiratory health in adult workers,9–12 and a substantial number of agents have been linked to occupation-related asthma.13 It is estimated that ∼15% of adult asthma cases could be prevented if occupational exposures were avoided.14 A few studies15,16 have also suggested that maternal occupational exposures, for example to low molecular weight agents (from e.g. cleaning agents, hairdressing and dentistry chemicals, wood dust) during pregnancy can pose a risk for asthma in offspring. Furthermore, a recent study using data from the Respiratory Health in Northern Europe (RHINE) study17 found that paternal pre-conception exposure to occupational welding may influence the risk of asthma among their children born years after this exposure. These results suggest that exposures after the age of 15 years and before conception may constitute a ‘window of susceptibility’ among males to having future offspring with asthma.17 Evidence from mainly animal studies suggests that pre-conception environmental exposures in males may induce epigenetic alterations or damage sperm DNA, which may later be transferred to the offspring through the male germline.18,19
In this study, we aimed to investigate whether a range of parental occupational exposures influenced the risk of asthma in offspring, relative to the timing of exposure (pre-conception only vs both pre- and post-conception) and parental sex. We hypothesized that parental sex modifies the development of offspring asthma through pre-conception exposures in fathers and around pregnancy in mothers.
Methods
Study population
The study population was adult offspring ≥18 years of age from 10 centres who provided self-reported information collected in the RHINESSA generation study (n = 8204)—Respiratory Health in Northern Europe, Spain and Australia (www.rhinessa.net). Either the father or the mother (total n = 6026) of each offspring in the RHINESSA study had previously participated in the longitudinal European Community Respiratory Health Survey (ECRHS) initiated in 1989–1992 (www.ecrhs.org). The ECRHS was initially conducted to estimate variations in the prevalence of asthma and allergy in young adults in Europe and other parts of the world in a randomized sample of the general population aged 20–44 years. The ECRHS was performed in three stages, and we used the second stage (ECRHS II) which provides information on parents’ previous total job history. A detailed description of the RHINESSA generation study can be found elsewhere.20,21
In this two-generation, population-based cohort study, we used information from the 3985 eligible adult offspring (18–52 years—collected in RHINESSA, 2013–2016) with information on one of their parents, n = 2931 (26–54 years—collected in ECRHS II, 1999–2000). Information about the parents was obtained from parent’s self-reported data and the offspring’s information was obtained from the offspring’s self-reported data. We also obtained information about the educational level of the parents’ parents.
Data collection
For the seven Northern European ECRHS centres [Reykjavik (Iceland), Bergen (Norway), Umeå, Uppsala and Göteborg (Sweden), Aarhus (Denmark) and Tartu (Estonia)], parental information was available from a detailed self-administered questionnaire survey, RHINE II (the RHINE study, www.rhine.nu). Melbourne (Australia) and Huelva and Albacete (Spain) used interviewer-administered questionnaires from ECRHS II. Importantly, offspring information was obtained directly from the offspring themselves and available from the RHINESSA questionnaire. All questionnaires are available online from the study webpages listed above.
Eligibility and exclusion criteria
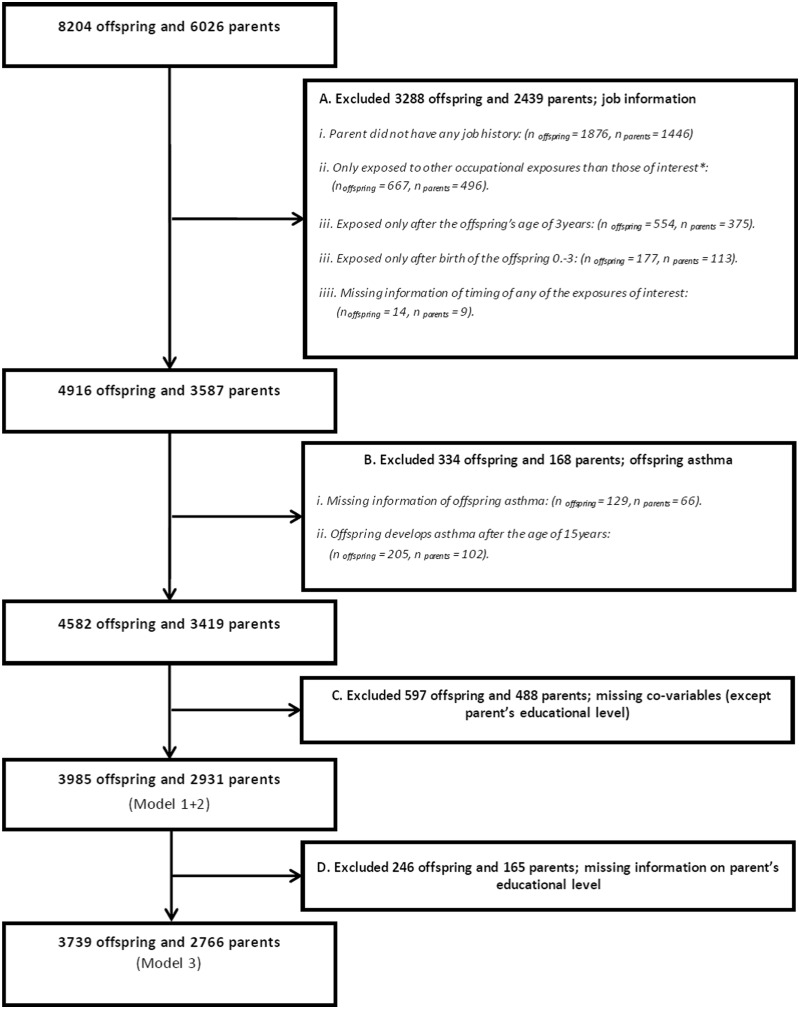
The eligibility criteria for inclusion were: offspring ≥18 years of age when they participated in the RHINESSA study, with a parent who had earlier participated in the ECRHS II/RHINE II. A flowchart of the study population is provided in Figure 1. We excluded 2439 parents and their 3288 offspring due to (i) missing information of job history, (ii) exposures to occupational exposures other than those of interest, (iii) no exposure in the relevant exposure windows, or (iv) missing information about the timing of exposures. Furthermore, 344 offspring and their 168 parents were excluded due to missing information about offspring asthma or the late onset of offspring asthma (>15years of age) to avoid contamination of data by occupational exposures of offspring. Information on parental educational level was not available for the centres in Melbourne, Huelva and Albacete. To keep these centres in our analysis, we applied two different adjustment models of co-variables, performing analysis both with and without adjustment for educational level. Due to missing co-variables (except parental educational level), we excluded 597 offspring and 488 parents; and due to missing information on parental educational level, we excluded 246 offspring and 165 parents in our fully adjusted model. A comparison of those included in the study vs those excluded is available in Supplementary Table S1, available as Supplementary data at IJE online. Those excluded had a lower educational level and a higher percentage were smokers compared with those included in the study.
Figure 1.
Flowchart of the exclusion process of the study population. Model 1 + 2—full information of study centre, offspring’s sex, parent’s characteristics (sex, age, asthma onset, place of upbringing and smoking) and grandmother’s and grandfather’s educational level. Model 3—full information of Model 1 + 2 and parent’s educational level. *Occupational exposures of interest: 1, microorganisms (moulds, endotoxin); 2, pesticides (herbicides, insecticides, fungicides); 3, allergens (animals, flour, house dust mites, storage mites, plant mites, enzymes, latex, fish/shellfish); 4, reactive chemicals (high level chemical disinfectants, isocyanates, acrylates, epoxy resins, persulfates/henna, aliphatic amines, bleach).
Parental occupational exposures
The question about parental job history was formulated as:
RHINE II—‘List your jobs including branch, your work tasks and the period of employment. Periods shorter than six months need not to be specified. Employment also includes work done by people with their own company.’
ECRHSII—‘If employed or self-employed or a full time house-person go to Q28: “Q 28. If you had more than one job in the same company, or if you were doing more than one job at the same time, we would like to talk about them separately. Please start with your current or last job.’
Parental job history was linked to an occupational asthma-specific job-exposure matrix (OAsJEM) to yield information about exposure to 30 specific agents.22 For each of these agents, experts have evaluated exposure for each job code in the International Standard Classification of Occupations, 1988 (ISCO-88). We focused on the 20 most specific agents grouped into four main exposure groups.
Microorganisms (molds, endotoxin).
Pesticides (herbicides, insecticides, fungicides).
Allergens (animals, flour, house dust mites, storage mites, plant mites, enzymes, latex, fish/shellfish).
Reactive chemicals (high-level chemical disinfectants, isocyanates, acrylates, epoxy resins, persulfates/henna, aliphatic amines, bleach).
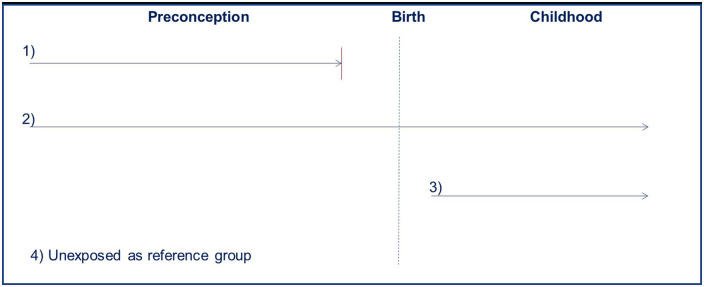
Each of the four groups of agents was dichotomized with respect to exposure (yes/no) for each of the exposure windows investigated: (i) pre-conception only; (ii) both pre- and post-conception; (iii) post-birth only; and (iv) unexposed (the reference group). However, due to the small sample size of exposed post-birth only (n = 145) even before stratification by each exposure category and parent's sex, post-birth only was not included. In contrast, the-pre conception window included 330 exposed and 51 asthma cases and during both the pre- and post-conception windows combined, 994 were exposed including a total of 147 asthma cases. Occupational exposure pre-conception only was chosen if the employment ended ≥2 years before the birth of a parent’s offspring. Occupational exposures during both pre- and post-conception periods were chosen if the parent was exposed both before and after the offspring’s year of birth (Figure 2).
Figure 2.
Exposure windows: 1) pre-conception only; 2) both pre- and post-conception; 3) post-birth only (not included due to power issues); 4) unexposed.
Asthma phenotypes in offspring
Adult offspring themselves provided information in the RHINESSA study on their ever asthma status through the following questions: ‘Do you have or have you ever had asthma?’, and if yes, information about age at asthma onset: ‘How old were you when you first experienced asthma symptoms?’ We included offspring asthma with onset between 0 and 15 years of age as the main outcome, which was further divided into asthma onset between 0 and 3 years (early-onset asthma) and between 4 and 15 years (late-onset asthma).23,24 An affirmative answer to the questions: ‘Do you have any nasal allergies including hay fever?’ or ‘Have you ever had eczema or any kind of skin allergy?’ was defined as offspring atopy.
Statistical procedures
Multivariable logistic regression models were applied to estimate the odds ratio (OR) of the association between parental occupational exposure and offspring asthma (onset at 0–15 years of age) using the PROC GENMOD function in the software package SAS. Each of the four different agent exposure groups was analysed separately. The reference group consisted of working parents not exposed to any of the 30 agents from the OAsJEM.
A priori, it was decided to separate all analyses by sex of the parent, adjust for study centre and include clustering by family to account for multiple offspring (siblings) from the same parent. We identified potential co-variables based on the literature and discussed direction and relationships of co-variables using directed acyclic graphs (DAGs) (Supplementary Figure S1, available as Supplementary data at IJE online). To be identified as a confounder in the DAGs, the covariate had to be a risk factor for both exposure and outcome. The following covariates were included as confounders from the DAGs: parent’s characteristics (age, early/late asthma onset, place of upbringing, educational level) and educational level of grandparents. Smoking and offspring sex were not considered as confounders according to the DAGs; however, smoking and offspring sex were included as co-variables in accordance with earlier studies.17,25–27 We applied the following three models.
Model 1: Clustered by family and adjusted for centre.
Model 2: Model 1 and further adjustment for offspring sex, the parent’s characteristics (age, early/late asthma onset, place of upbringing and smoking) and educational level of grandparents.
Model 3: Model 2 and further adjustment for parent’s educational level.
In the description of results, we generally referred to Model 2. The significance level was set at a P-value of <0.05 (two-sided) and 95% confidence intervals (CIs) were calculated.
A priori, subgroup analyses were included to examine if offspring age at asthma onset, offspring atopy or offspring sex modified the association between parental occupational exposure and risk of offspring asthma. The impact of offspring age on asthma onset was investigated by multinomial logistic regression models (0–3 and 4–15 years of age) using the PROC GEE function in SAS (SAS Institute Inc., Cary, NC, USA). Due to statistical power issues, subgroup analyses were only performed for allergens and chemicals. All statistical analyses were performed using SAS version 14.1.
Results
The baseline characteristics of 3985 offspring, 2931 parents and grandparents are presented in Table 1. Offspring mean age was 30 years [standard deviation (SD) 7.5] when they responded to the questionnaire. A slightly higher proportion of offspring of exposed parents developed early-onset asthma (4.5%) compared with the offspring of non-exposed parents (3.6%). Parental mean age was 43 years (SD 6.5) when they responded to the questionnaire. More women than men participated, both among offspring (57.0% women) and parents (59.5% women). Exposed parents were more likely to have asthma, have lived on a farm and to have a lower educational level compared with unexposed parents.
Table 1.
Characteristics of offspring, parents and grandparents by parents exposed to either microorganisms, pesticides, allergens and/or chemicals or non-exposed
| Exposed | Non-exposed | Total | ||||
|---|---|---|---|---|---|---|
| Offspring, n | 1324 | 2661 | 3985 | |||
| Female, n (%) | 743 | (56.1) | 1528 | (57.4) | 2271 | (57.0) |
| Age at data collection mean, SD | 28.5 | (6.7) | 30.3 | (7.7) | 29.7 | (7.5) |
| Asthma 0–15 years, n (%) | 198 | (15.0) | 377 | (14.2) | 575 | (14.4) |
| Asthma onset 0–3 years, n (%) | 59 | (4.5) | 97 | (3.6) | 156 | (3.9) |
| Asthma onset 4–15 years, n (%) | 139 | (10.5) | 280 | (10.5) | 419 | (10.5) |
| Allergy, n (%) | 748 | (56.5) | 1535 | (57.7) | 2283 | (57.3) |
| Centre | ||||||
| Aarhus, n (%) | 80 | (6.0) | 239 | (9.0) | 319 | (8.0) |
| Albacete, n (%)a | <5 | (<0.4) | <25 | (<0.9) | <30 | (<0.8) |
| Huelva, n (%)a | <5 | (<0.4) | <15 | (<0.6) | <20 | (<0.5) |
| Reykjavik, n (%) | 134 | (10.1) | 399 | (15.0) | 533 | (13.4) |
| Bergen, n (%) | 206 | (15.6) | 581 | (21.8) | 787 | (19.7) |
| Gothenburg, n (%) | 83 | (6.3) | 204 | (7.7) | 287 | (7.2) |
| Umea, n (%) | 422 | (31.9) | 397 | (14.9) | 819 | (20.6) |
| Uppsala, n (%) | 327 | (24.7) | 529 | (19.9) | 856 | (21.5) |
| Melbourne, n (%) | 6 | (0.5) | 117 | (4.4) | 123 | (3.1) |
| Tartu, n (%) | 57 | (4.3) | 155 | (5.8) | 212 | (5.3) |
| Parents, n | 995 | 1936 | 2931 | |||
| Female | 572 | (57.5) | 1065 | (55.0) | 1637 | (55.9) |
| Age at data collection mean, SD | 42.4 | (6.6) | 43.8 | (6.4) | 43.3 | (6.5) |
| Asthma <10 years, n (%) | 40 | (4.0) | 68 | (3.5) | 108 | (3.7) |
| Asthma >10 years, n (%) | 81 | (8.1) | 149 | (7.7) | 230 | (7.8) |
| Place of upbringing, n (%) | ||||||
| Farm | 240 | (24.1) | 315 | (16.3) | 555 | (18.9) |
| Village, small town, suburb of city | 656 | (65.9) | 1317 | (68.0) | 1973 | (67.3) |
| Inner city | 99 | (9.9) | 304 | (15.7) | 403 | (13.7) |
| Educational levelb | ||||||
| Primary, n (%) | 112 | (11.6) | 173 | (9.6) | 285 | (10.3) |
| Secondary, n (%) | 411 | (42.5) | 637 | (35.4) | 1048 | (37.9) |
| University/College, n (%) | 445 | (46.0) | 988 | (54.9) | 1433 | (51.8) |
| Smokers, n (%) | 510 | (51.3) | 1015 | (52.4) | 1525 | (52.0) |
| Centre | ||||||
| Aarhus, n (%) | 66 | (6.6) | 192 | (9.9) | 258 | (8.8) |
| Albacete, n (%)a | <5 | (<0.5) | <20 | (<1.0) | <25 | (<0.9) |
| Huelva, n (%)a | <5 | (<0.5) | <10 | (<0.5) | <15 | (<0.5) |
| Reykjavik, n (%) | 112 | (11.3) | 306 | (15.8) | 418 | (14.3) |
| Bergen, n (%) | 149 | (15.0) | 405 | (20.9) | 554 | (18.9) |
| Gothenburg, n (%) | 66 | (6.6) | 153 | (7.9) | 219 | (7.5) |
| Umea, n (%) | 304 | (30.6) | 276 | (14.3) | 580 | (19.8) |
| Uppsala, n (%) | 236 | (23.7) | 380 | (19.6) | 616 | (21.0) |
| Melbourne, n (%) | 6 | (0.6) | 69 | (3.6) | 75 | (2.6) |
| Tartu, n (%) | 49 | (4.9) | 131 | (6.8) | 180 | (6.1) |
| Grandmothers, n | 995 | 1936 | 2931 | |||
| Educational level | ||||||
| Primary, n (%) | 692 | (69.5) | 1287 | (66.5) | 1979 | (67.5) |
| Secondary, n (%) | 216 | (21.7) | 470 | (24.3) | 686 | (23.4) |
| University/College, n (%) | 87 | (8.7) | 179 | (9.2) | 266 | (9.1) |
| Grandfathers, n | 995 | 1936 | 2931 | |||
| Educational level | ||||||
| Primary, n (%) | 584 | (58.7) | 1019 | (52.6) | 1603 | (54.7) |
| Secondary, n (%) | 271 | (27.2) | 579 | (29.9) | 850 | (29.0) |
| University/College, n (%) | 140 | (14.1) | 338 | (17.5) | 478 | (16.3) |
Due to data security, i.e. the European Data Protection Regulation, we are not allowed to show results based on microdata (<5 persons); data are available on request for authorized research units.
Educational level, missing: exposed n = 27, non-exposed n = 138, total n = 165.
Multivariable logistic regression analyses (Table 2) showed no clear associations between parent’s occupational exposure and offspring asthma at 0–15 years of age, neither for pre-conception exposures only nor for exposures both pre- and post-conception. These findings were consistent for both fathers and mothers in all four groups of exposure agents. Of note, no results are presented for maternal exposure to microorganisms and pesticides pre-conception only, due to a very low number of exposed individuals.
Table 2.
Multivariable logistic regression analyses clustering by family of paternal or maternal occupational exposure in relation to asthma in offspring (0–15 years)
| n | Asthma, % | ORModel1a | 95 % CI | ORModel2b | 95 % CI | ORModel3c | 95 % CI | |
|---|---|---|---|---|---|---|---|---|
| Microorganisms | ||||||||
| Fathers | ||||||||
| Unexposed | 1216 | 15.5 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 40 | 20.0 | 1.22 | (0.52; 2.86) | 1.19 | (0.49; 2.90) | 1.26 | (0.53; 3.01) |
| Exposed both pre- and post-conception | 109 | 11.0 | 0.57 | (0.28; 1.13) | 0.53 | (0.26; 1.06) | 0.61 | (0.30; 1.24) |
| Mothers | ||||||||
| Unexposed | 1445 | 13.0 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception onlyd | 18 | 0.0 | – | - | – | |||
| Exposed both pre- and post-conception | 31 | 12.9 | 0.96 | (0.36; 2.60) | 0.66 | (0.26; 1.64) | 0.56 | (0.21; 1.4) |
| Pesticides | ||||||||
| Fathers | ||||||||
| Unexposed | 1216 | 15.5 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 51 | 17.6 | 1.12 | (0.49; 2.55) | 1.09 | (0.46; 2.54) | 1.13 | (0.48; 2.65) |
| Exposed both pre- and post-conception | 126 | 13.5 | 0.72 | (0.39; 1.30) | 0.67 | (0.37; 1.22) | 0.74 | (0.40; 1.37) |
| Mothers | ||||||||
| Unexposed | 1445 | 13.0 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception onlyd | 16 | 0.0 | – | - | – | |||
| Exposed both pre- and post-conception | 32 | 9.4 | 0.77 | (0.24; 2.42) | 0.56 | (0.19; 1.69) | 0.46 | (0.14; 1.51) |
| Allergens | ||||||||
| Fathers | ||||||||
| Unexposed | 1216 | 15.5 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 76 | 10.5 | 0.70 | (0.31; 1.59) | 0.66 | (0.29; 1.51) | 0.67 | (0.29; 1.59) |
| Exposed both pre- and post-conception | 235 | 12.8 | 0.78 | (0.50; 1.23) | 0.77 | (0.48; 1.21) | 0.83 | (0.52; 1.31) |
| Mothers | ||||||||
| Unexposed | 1445 | 13.0 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 118 | 17.8 | 1.36 | (0.79; 2.31) | 1.04 | (0.59; 1.83) | 0.96 | (0.54; 1.70) |
| Exposed both pre- and post-conception | 523 | 16.3 | 1.26 | (0.95; 1.69) | 1.15 | (0.85; 1.54) | 1.16 | (0.85; 1.57) |
| Chemicals | ||||||||
| Fathers | ||||||||
| Unexposed | 1216 | 15.5 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 116 | 15.5 | 1.09 | (0.65; 1.85) | 0.98 | (0.58; 1.66) | 0.98 | (0.55; 1.66) |
| Exposed both pre- and post-conception | 300 | 14.0 | 0.88 | (0.60; 1.31) | 0.85 | (0.57; 1.26) | 0.86 | (0.57; 1.29) |
| Mothers | ||||||||
| Unexposed | 1445 | 13.0 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 112 | 18.8 | 1.49 | (0.88; 2.54) | 1.20 | (0.69; 2.11) | 1.11 | (0.63; 1.95) |
| Exposed both pre- and post-conception | 520 | 16.3 | 1.26 | (0.94; 1.68) | 1.17 | (0.87; 1.57) | 1.18 | (0.87; 1.61) |
ORModel1 cluster by family, adjusted for study center.
ORModel2 cluster by family, adjusted for study center, offspring's sex and parent's characteristic (age, asthma before the age of 10 years, asthma after the age of 10 years, place of upbringing, and smoking) as well as grandmother and grandfathers educational level.
ORModel3 cluster by family, same adjustment as ORModel2 + parent's educational level.
The category was included in the model, but due to very few exposed individuals, the result is not presented. Due to data security, i.e. the European Data Protection Regulation, we are not allowed to show results based on microdata (<5 persons). Data are available on request for authorized research units.
Subgroup analyses
Subgroup analyses of the age of asthma onset (Table 3) supported the main results (Table 2) with two exceptions. Maternal exposure to allergens during both pre- and post-conception periods was associated with increased odds for early-onset asthma, ORModel2 1.70 (1.02–2.84). Similarly, maternal exposure to chemicals both pre- and post-conception increased the odds for early-onset asthma [ORModel2 1.65 (0.98–2.77)], also seen in Models 1 [(OR 1.87 (1.12–3.13)] and 3 [OR 1.82 (1.06–3.12)]. However, no association was found for maternal allergen or chemical exposures and late-onset asthma, ORModel2 1.03 (0.73–1.45) and ORModel2 1.03 (0.73–1.45), respectively.
Table 3.
Multivariable logistic regression analyses clustering by family of paternal or maternal occupational exposure in relation to asthma in offspring; sensitivity analyses of age at asthma onset (0–3 years and 4–15 years)
| n | Asthma, % | ORModel1a | 95 % CI | ORModel2b | 95 % CI | ORModel3c | 95 % CI | |
|---|---|---|---|---|---|---|---|---|
| Allergens—fathers | ||||||||
| Asthma 0–3 years | ||||||||
| Unexposed | 1216 | 4 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception onlyd | 76 | <7 | – | - | – | |||
| Exposed both pre- and post-conception | 235 | 3.8 | 0.93 | (0.43; 2.01) | 0.77 | (0.34; 1.78) | 0.88 | 0.39; 1.99 |
| Asthma 4–15 years | ||||||||
| Unexposed | 1216 | 11.1 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 76 | 6.6 | 0.60 | (0.21; 1.78) | 0.59 | (0.20; 1.76) | 0.61 | 0.21; 1.81 |
| Exposed both pre- and post-conception | 235 | 8.9 | 0.73 | (0.43; 1.24) | 0.74 | (0.44; 1.26) | 0.79 | 0.46; 1.36 |
| Allergens—mothers | ||||||||
| Asthma 0-3 years | ||||||||
| Unexposed | 1445 | 3.0 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception onlyd | 118 | <5 | – | - | – | |||
| Exposed both pre- and post-conception | 523 | 5.4 | 1.99 | (1.20; 3.30) | 1.70 | (1.02; 2.84) | 1.85 | 1.08; 3.15 |
| Asthma 4–15 years | ||||||||
| Unexposed | 1445 | 10.0 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 118 | 16.1 | 1.55 | (0.89; 2.69) | 1.21 | (0.69; 2.14) | 1.07 | 0.60; 1.90 |
| Exposed both pre- and post-conception | 523 | 10.9 | 1.07 | (0.76; 1.49) | 0.99 | (0.70; 1.40) | 0.98 | 0.69; 1.40 |
| Chemicals—fathers | ||||||||
| Asthma 0–3 years | ||||||||
| Unexposed | 1216 | 4.4 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 116 | 6.9 | 1.90 | (0.85; 4.26) | 1.37 | (0.57; 3.28) | 1.35 | 0.54; 3.36 |
| Exposed both pre- and post-conception | 300 | 3.7 | 0.94 | (0.46; 1.89) | 0.77 | (0.35; 1.68) | 0.65 | 0.29; 1.47 |
| Asthma 4–15 years | ||||||||
| Unexposed | 1216 | 11.1 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 116 | 8.6 | 0.81 | (0.41; 1.61) | 0.80 | (0.41; 1.55) | 0.75 | 0.37; 1.52 |
| Exposed both pre- and post-conception | 300 | 10.3 | 0.87 | (0.55; 1.37) | 0.86 | (0.55; 1.37) | 0.92 | 0.58; 1.47 |
| Chemicals—mothers | ||||||||
| Asthma 0–3 years | ||||||||
| Unexposed | 1445 | 3.0 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception onlyd | 112 | <5 | – | - | – | |||
| Exposed both pre- and post-conception | 520 | 5.2 | 1.87 | (1.12; 3.13) | 1.65 | (0.98; 2.77) | 1.82 | 1.06; 3.12 |
| Asthma 4–15 years | ||||||||
| Unexposed | 1445 | 10.0 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 112 | 16.1 | 1.62 | (0.93; 2.84) | 1.34 | (0.76; 2.36) | 1.18 | 0.67; 2.09 |
| Exposed both pre- and post-conception | 520 | 11.2 | 1.09 | (0.78; 1.52) | 1.03 | (0.73; 1.45) | 1.03 | 0.72; 1.46 |
ORModel1 cluster by family, adjusted for study center.
ORModel2 cluster by family, adjusted for study center, and parent's characteristic (age, asthma before the age of 10 years, asthma after the age of 10 years, place of upbringing, and smoking) as well as grandmother and grandfathers educational level.
ORModel3 cluster by family, same adjustment as ORModel2 + parent's educational level.
The category was included in the model, but due to very few exposed individuals, the result is not presented. Due to data security, i.e. the European Data Protection Regulation, we are not allowed to show results based on microdata (<5 persons). Data are available on request for authorized research units.
The subgroup analyses stratified by offspring allergic status (Table 4) and by offspring sex (Table 5) showed similar results for allergic and non-allergic asthma as well as offspring sex. No results are available for parental exposure to allergens pre-conception only and early-onset asthma due to very few exposed individuals. Similarly, no results are presented for maternal exposure to chemicals pre- and post-conception and early-onset asthma.
Table 4.
Multivariable logistic regression analyses clustering by family of paternal or maternal occupational exposure in relation to asthma in their offspring (0–15years); subgroup analysis by allergic and non-allergic offspring
| n | Asthma, % | ORModel1a | 95 % CI | ORModel2b | 95 % CI | ORModel3c | 95 % CI | |
|---|---|---|---|---|---|---|---|---|
| Allergens—fathers | ||||||||
| Asthma 0–15 years atopic | ||||||||
| Unexposed | 684 | 20.5 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 51 | 13.7 | 0.72 | (0.30; 1.74) | 0.72 | (0.29; 1.78) | 0.74 | (0.30; 1.80) |
| Exposed both pre- and post-conception | 129 | 16.3 | 0.70 | (0.40; 1.22) | 0.74 | (0.42; 1.29) | 0.83 | (0.48; 1.45) |
| Asthma 0–15 years non-atopic | ||||||||
| Unexposed | 532 | 9.2 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception onlyd | - | 4.0 | – | - | – | |||
| Exposed both pre- and post-conception | 106 | 8.5 | 0.89 | (0.39; 1.99) | 0.79 | (0.34; 1.81) | 0.81 | (0.36; 1.82) |
| Allergens—mothers | ||||||||
| Asthma 0–15 years atopic | ||||||||
| Unexposed | 851 | 16.8 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 66 | 24.2 | 1.48 | (0.79; 2.77) | 1.28 | (0.69; 2.38) | 1.15 | (0.62; 2.15) |
| Exposed both pre- and post-conception | 292 | 21.6 | 1.32 | (0.93; 1.87) | 1.18 | (0.82; 1.71) | 1.19 | (0.82; 1.75) |
| Asthma 0–15 years non-atopic | ||||||||
| Unexposed | 594 | 7.6 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 52 | 9.6 | 1.29 | (0.48; 3.45) | 0.79 | (0.27; 2.32) | 0.82 | (0.28; 2.40) |
| Exposed both pre- and post-conception | 231 | 9.5 | 1.34 | (0.77; 2.35) | 1.21 | (0.69; 2.13) | 1.25 | (0.70; 2.20) |
| Chemicals—fathers | ||||||||
| Asthma 0–15 years atopic | ||||||||
| Unexposed | 684 | 20.5 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 77 | 18.2 | 1.04 | (0.58; 1.87) | 1.01 | (0.55; 1.85) | 0.98 | (0.52; 1.81) |
| Exposed both pre- and post-conception | 175 | 17.7 | 0.82 | (0.51; 1.30) | 0.81 | (0.50; 1.29) | 0.82 | (0.51; 1.33) |
| Asthma 0–15 years non-atopic | ||||||||
| Unexposed | 532 | 9.2 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception onlyd | - | 10.3 | – | - | – | |||
| Exposed both pre- and post-conception | 125 | 8.8 | 0.91 | (0.44; 1.92) | 0.80 | (0.37; 1.71) | 0.79 | (0.36; 1.75) |
| Chemicals—mothers | ||||||||
| Asthma 0–15 years atopic | ||||||||
| Unexposed | 851 | 16.8 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 63 | 25.4 | 1.58 | (0.85; 2.93) | 1.45 | (0.79; 2.67) | 1.31 | (0.71; 2.42) |
| Exposed both pre- and post-conception | 291 | 22.0 | 1.35 | (0.95; 1.92) | 1.24 | (0.86; 1.79) | 1.25 | (0.85; 1.83) |
| Asthma 0–15 years non-atopic | ||||||||
| Unexposed | 549 | 8.2 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 44 | 11.4 | 1.45 | (0.54; 3.86) | 0.95 | (0.33; 2.79) | 0.94 | (0.32; 2.74) |
| Exposed both pre- and post-conception | 208 | 10.1 | 1.24 | (0.71; 2.19) | 1.14 | (0.65; 2.01) | 1.18 | (0.66; 2.09) |
ORModel1 cluster by family, adjusted for study centre.
ORModel2 cluster by family, adjusted for study center, and parent's characteristic (age, asthma before the age of 10 years, asthma after the age of 10 years, place of upbringing and smoking) as well as grandmother and grandfathers educational level.
ORModel3 cluster by family, same adjustment as ORModel2 + parent's educational level.
The category was included in the model, but due to very few exposed individuals, the result is not presented. Due to data security, i.e. the European Data Protection Regulation, we are not allowed to show results based on microdata (<5 persons); data are available on request for authorized research units.
Table 5.
Multivariable logistic regression analyses clustering by family of paternal or maternal occupational exposure in relation to asthma in their offspring (0–15 years); subgroup analysis by offspring sex
| n | Asthma, % | ORModel1a | 95 % CI | ORModel2b | 95 % CI | ORModel3c | 95 % CI | |
|---|---|---|---|---|---|---|---|---|
| Allergens—fathers | ||||||||
| Asthma 0–15 years—boys | ||||||||
| Unexposed | 519 | 15.4 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 39 | 12.8 | 0.90 | (0.34; 2.37) | 0.74 | (0.28; 1.96) | 0.80 | (0.31; 2.04) |
| Exposed both pre- and post-conception | 102 | 14.7 | 0.94 | (0.48; 1.82) | 0.82 | (0.42; 1.59) | 0.95 | (0.49; 1.84) |
| Asthma 0–15 years—girls | ||||||||
| Unexposed | 697 | 15.6 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception onlyd | - | 8.1 | – | - | – | |||
| Exposed both pre- and post-conception | 133 | 11.3 | 0.66 | (0.35; 1.23) | 0.67 | (0.35; 1.27) | 0.71 | (0.37; 1.35) |
| Allergens—mothers | ||||||||
| Asthma 0–15 years—boys | ||||||||
| Unexposed | 614 | 14.0 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 51 | 17.6 | 1.36 | (0.63; 2.96) | 1.05 | (0.46; 2.37) | 0.98 | (0.43; 2.22) |
| Exposed both pre- and post-conception | 224 | 15.2 | 1.15 | (0.75; 1.77) | 1.03 | (0.66; 1.61) | 0.98 | (0.62; 1.56) |
| Asthma 0–15 years—girls | ||||||||
| Unexposed | 831 | 12.3 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 67 | 17.9 | 1.37 | (0.64; 2.94) | 1.05 | (0.48; 2.31) | 0.94 | (0.42; 2.11) |
| Exposed both pre- and post-conception | 299 | 17.1 | 1.38 | (0.92; 2.06) | 1.23 | (0.81; 1.86) | 1.29 | (0.84; 1.97) |
| Chemicals—fathers | ||||||||
| Asthma 0–15 years—boys | ||||||||
| Unexposed | 519 | 15.4 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 57 | 14.0 | 0.97 | (0.44; 2.11) | 0.81 | (0.36; 1.78) | 0.83 | (0.37; 1.87) |
| Exposed both pre- and post-conception | 135 | 13.3 | 0.82 | (0.46; 1.48) | 0.74 | (0.41; 1.33) | 0.76 | (0.42; 1.40) |
| Asthma 0–15 years—girls | ||||||||
| Unexposed | 697 | 15.6 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 59 | 16.9 | 1.17 | (0.56; 2.43) | 1.11 | (0.54; 2.28) | 1.05 | (0.48; 2.27) |
| Exposed both pre- and post-conception | 165 | 14.5 | 0.96 | (0.57; 1.61) | 0.94 | (0.55; 1.59) | 0.94 | (0.55; 1.62) |
| Chemicals—mothers | ||||||||
| Asthma 0–15 years—boys | ||||||||
| Unexposed | 614 | 14.0 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 50 | 20.0 | 1.61 | (0.77; 3.36) | 1.27 | (0.59; 2.73) | 1.24 | (0.58; 2.68) |
| Exposed both pre- and post-conception | 231 | 16.5 | 1.25 | (0.82; 1.90) | 1.15 | (0.74; 1.79) | 1.12 | (0.71; 1.76) |
| Asthma 0–15 years—girls | ||||||||
| Unexposed | 831 | 12.3 | 1.00 | 1.00 | 1.00 | |||
| Exposed pre-conception only | 62 | 17.7 | 1.38 | (0.63; 3.06) | 1.11 | (0.49; 2.54) | 0.97 | (0.42; 2.22) |
| Exposed both pre- and post-conception | 289 | 16.3 | 1.28 | (0.86; 1.93) | 1.16 | (0.76; 1.76) | 1.20 | (0.78; 1.87) |
ORModel1 cluster by family, adjusted for study center.
ORModel2 cluster by family, adjusted for study center, and parent's characteristic (age, asthma before the age of 10 years, asthma after the age of 10 years, place of upbringing and smoking) as well as grandmother and grandfathers educational level.
ORModel3 cluster by family, same adjustment as ORModel2 + parent's educational level.
The category was included in the model, but due to very few exposed individuals, the result is not presented. Due to data security, i.e. the European Data Protection Regulation, we are not allowed to show results based on microdata (<5 persons). Data are available on request for authorized research units.
Discussion
In this study, parental occupational exposure to microorganisms, pesticides, allergens or reactive chemicals pre-conception only as well as both pre- and post-conception did not show clear associations with offspring development of childhood asthma. In general, subgroup analyses by age of asthma onset, offspring atopic status and offspring sex confirmed the negative finding except for an increased odds for early-onset offspring asthma in the case of maternal pre- and post-conception exposure to allergens and chemicals.
Comparison with other studies
Maternal occupational exposure and offspring asthma
We did not identify any other studies investigating pre-conception occupational exposure in mothers, but maternal exposure to occupational factors during pregnancy has been reported to be related to offspring asthma in previous studies. Christensen et al. found that combined intrauterine and postnatal occupational exposure to low molecular weight agents, but not high molecular weight agents, might be related to asthma in 7-year-old children.15 We did find associations between mothers exposed to allergens both pre- and post-conception, though only for early-onset asthma (0–3 years). Christensen et al. did not distinguish between age at asthma onset and included only children up to the age of 7 years.15
Tagiyeva et al. found that maternal antenatal occupational exposure to latex and biocides/fungicides was associated with an increased likelihood of asthma in 7-year-old children.28 However, no effect was seen after adjustment for postnatal exposures, indicating that the main effect could be due to the ‘carry-home’ effect. We wanted to include an exposure window for those only exposed after the birth of the offspring to compare with the window of those exposed both pre- and post-conception to examine possible carry-home effects. Unfortunately, we did not have sufficient statistical power to do this in the post-birth exposure window as we a priori used a cut-off point at age 3 years of the offspring to minimize the risk of offspring developing asthma before the parent was occupationally exposed. We had too few cases to produce firm results for the exposure window pre-conception only for pesticides and microorganisms; the results for allergens and chemicals did not reveal any clear results for offspring asthma. Due to data security, i.e. the European Data Protection Regulation, we are not allowed to show results based on microdata (<5 persons). Data are available on request for authorized research units. Magnusson et al. found that some maternal jobs (e.g. engineering technicians, social workers and librarians) during pregnancy were associated with an elevated risk of asthma in their children (14–18 years old).16
In agreement with other studies,29,30 Svanes et al. reported that among parents of 24 168 offspring from the RHINE study,17 there was an increased early onset of offspring asthma if mothers smoked around pregnancy. This is in line with our findings for maternal occupational exposure to reactive chemicals during both pre- and post-conception periods related to early-onset asthma in offspring. The parents in our study constitute a subpopulation of the larger study population used by Svanes et al. However, we obtained information directly from the offspring of the parents themselves in the RHINESSA study. Similar to Svanes et al.,17 we did not find a clear association for mothers only exposed pre-conception in the main analysis. These findings should be interpreted with caution as we had too few cases to produce firm results for the exposure window pre-conception only in relation to early-onset asthma.
Paternal occupational exposure and offspring asthma
We did not find any associations between paternal occupational exposure and offspring asthma. In comparison, Svanes et al.17 suggested that paternal occupational exposure to welding as well as smoking increased the risk of asthma in offspring if exposed to welding pre-conception. The association was found for only non-allergic asthma with an onset before the age of 10 years, suggesting effects on children’s lung function rather than allergy-related outcomes.17 Similarly, Accordini et al. in a study of 1964 fathers participating in the ECRHS study reported that paternal smoking during early puberty was associated with a higher risk of non-allergic offspring asthma.25
Males are suggested to have three possible vulnerability windows in sperm development: in utero, before completion of puberty and post-puberty (reproductive cycle), where the pre-puberty window is suggested to be the most vulnerable.17–19 Even though we included parent job history throughout their working life, it is most likely that they entered the labour market after their pre-puberty years. In contrast, the germ cells in women are believed to be more vulnerable during the period around pregnancy.17 This could explain why we find an effect of pre- and post-conceptional exposure and early-onset offspring asthma in mothers but not for fathers. Epigenetic research on exposure effects across generations is novel and sparse. Environmental exposures could potentially affect the development of germ cells in a fetus, which could directly affect one or two generations, referred to as intergenerational inheritance.31 The intergenerational inheritance of epigenetic information is, though, far from understood.
Strengths and limitations
To our knowledge, this is the first study to investigate the association between multiple parental occupational exposures pre-conception and offspring risk of childhood asthma. An important strength of this study is that information about parental occupational exposure was collected independently and before the offsprings’ report of their asthma status.
Several limitations also need to be considered. Parental job information of the parent who did not participate in the study is lacking. Even if there was a tendency towards couples being more likely to have the same type of job, the associations found in mothers are unlikely to be explained by their partner’s exposure, as we did not observe associations for paternal exposure; however, the true contrast between the sexes of the parents could be larger than observed.
Even though the study design was prospective, questionnaire data were collected retrospectively and recall bias could have been introduced. Parents’ total employment history could thus be incomplete but it is unlikely that this would be different between exposed and unexposed parents. The use of the OAsJEM will leave out the risk of recall bias concerning exposure to specific agents at their jobs, as these are based on expert evaluations. However, the OAsJEM introduces non-differential misclassifications, as the job titles only constitute a proxy for the exposure to specific agent exposures. It is, therefore, uncertain whether the individual parents were exposed to the agents for which they were chosen. Hence, parents could have been exposed to agents other than those included in the OAsJEM. Even though we excluded all individuals exposed to any of the 30 other occupational asthma-specific agents from the reference group, other (non-occupational) exposures could have constituted a risk factor for the development of asthma, which may have contaminated the reference group data. However, it is unlikely that the findings are due to exposure misclassification of the JEMs. JEM-based exposure assessment is mainly affected by the Berkson error, which results in nearly unbiased effect estimates at the expense of loss of statistical power.32 We did not include measurements of air pollution, which is suspected to be a risk factor for childhood asthma33 and this might have influenced the associations. However, adjustment for various possible confounders including place of upbringing, parental smoking as well as parental and grandparents’ highest educational level did not substantially alter the associations. However, we cannot rule out residual confounding. We used complete case analysis to deal with missing data, which potentially could have under- or over-estimated the effect.34
Asthma is a heterogenetic disorder with multiple phenotypes where age at asthma onset may define some of the heterogeneity. We included offspring with asthma onset at 0–15 years of age, which was further divided into onset between 0–3 years (early-onset asthma) and 4–15 years (late-onset asthma) to account for possible different underlying mechanisms for different phenotypes.35 As asthma and age at asthma onset were assessed based on adult offsprings' self-report, recall bias could have led to misclassification. Svanes et al.17 found smoking and welding pre-conception to be associated with an increased risk of only non-allergic early-onset asthma. Due to the few available cases, it was not feasible to stratify the early-onset groups of asthma into atopic status or sex of the offspring. We cannot rule out that having allergic offspring in the analysis would have biased the association towards the null. However, no clear associations were seen in the main analyses of childhood asthma between 0 and 15 years of age, and similar results were seen in the main analysis among atopic and non-atopic children. As an isolated finding, maternal occupational exposure to allergens and reactive chemicals during the pre- and post-conception period was associated with increased odds of early-onset offspring asthma. Although this finding is not supported by the other analyses and could be a result of multiple comparisons, it holds some biological plausibility and could be considered as hypothesis-generating to be further investigated in animal or observational studies. Current results should be interpreted and generalized with caution until further studies are conducted.
Conclusion
In conclusion, we did not find evidence to support the impact of only pre-conception or both pre-and post-conception parental occupational exposure measured by JEMs on childhood asthma in offspring in this study.
Ethics approval and consent to participate
The study was approved by regional committees of medical research ethics in each study centre according to national legislation. Furthermore, informed consent was obtained from each participant in all study centres. For a list of ethics committees and numbers for each centre, please see www.rhinessa.net.
Availability of data and material
The dataset is held and managed by the RHINESSA coordinating centre at the Department of Occupational Medicine, Haukeland University Hospital, Bergen, Norway. Data cannot be made freely available as they are subject to Norwegian Data Protection regulations, but de-identified data can be made available to researchers upon request. Requests for data can be sent to the principal investigator of RHINESSA: C.S., cecilie.svanes@med.uib.no.
Supplementary data
Supplementary data are available at IJE online
Funding
This work was supported by funding from The Faculty of Health, Aarhus University, Denmark [Project No. 240008], The Wood Dust Foundation [Project No. 444508795], The Danish Lung Association, the Swedish Heart and Lung Foundation, the Swedish Association Against Asthma and Allergy, the Swedish Association against Heart and Lung Disease, the Swedish Council for Working Life and Social Research, The Bror Hjerpstedt Foundation, The Vårdal Foundation for Health Care and Allergic Research, The Norwegian Research Council [project 135773/33], The Norwegian Asthma and Allergy Association, HelseVest Norway [Grant no. 911 631], NFR [Grant no. 214123, 230827/F20, 228174], The University of Iceland Research Fund, The Icelandic GP's Research Fund, The Estonian Science Foundation [Grant No. 4350], The Estonian Research Council [Grant no. PUT562], the Australian National Health Medical Research Council, Melbourne University, Sociedad Española de Neumologia y Cirugía Toracica, SEPAR Spain and Horizon2020 PHC1 [Grant no. 633212]. For more information, please see www.rhinessa.net. V.S. and C.S. are members of the COST BM1201 network. K.P. received a PhD scholarship from Aarhus University and the Danish Working Environment Research Fund, Denmark [Grant no. 17–2015–09/20150067134]. X.L. is supported by the Danish Council for Independent Research [Project No. DFF-5053-00156B].
Author contributions
K.P.: principal author, conception and design of the work, statistical analysis, interpretation of data, drafting the manuscript. All other authors: conception and design of the work, acquisition and interpretation of data and revision of the manuscript for important intellectual content. All authors approved the final version.
Conflict of interest
None declared.
Supplementary Material
References
- 1. Barker DJ. The fetal and infant origins of adult disease. BMJ 1990;301:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barker DJP, Osmond C, Winter PD, Margetts B, Simmonds SJ.. Weight in infancy and death from ischaemic heart disease. Lancet 1989;334:577–80. [DOI] [PubMed] [Google Scholar]
- 3. Kyle UG, Pichard C.. The Dutch Famine of 1944-1945: a pathophysiological model of long-term consequences of wasting disease. Curr Opin Clin Nutr Metab Care 2006;9:388–94. [DOI] [PubMed] [Google Scholar]
- 4. Painter RC, Roseboom TJ, Bleker OP.. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol 2005;20:345–52. [DOI] [PubMed] [Google Scholar]
- 5. Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ.. Developmental origins of non-communicable disease: implications for research and public health. Environ Health 2012;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asher I, Pearce N.. Global burden of asthma among children. Int J Tuberc Lung Dis 2014;18:1269–278. [DOI] [PubMed] [Google Scholar]
- 7. Masoli M, Fabian D, Holt S, Beasley R.. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy 2004;59:469–78. [DOI] [PubMed] [Google Scholar]
- 8.Global Initiative for Asthma. (*NEW) 2017 GINA Report: Global Strategy for Asthma Management and Prevention [Internet]. 2017. http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/ (5 February 2018, date last accessed).
- 9. Alif SM, Dharmage SC, Bowatte G. et al. Occupational exposure and risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Expert Rev Respir Med 2016;10:861–72. [DOI] [PubMed] [Google Scholar]
- 10. Alif SM, Dharmage SC, Benke G. et al. Occupational exposure to pesticides are associated with fixed airflow obstruction in middle-age. Thorax 2017;72:990–97. [DOI] [PubMed] [Google Scholar]
- 11. Lau A, Tarlo SM.. Update on the management of occupational asthma and work-exacerbated asthma. Allergy Asthma Immunol Res 2018;11:188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sadhra S, Kurmi OP, Sadhra SS, Lam KBH, Ayres JG.. Occupational COPD and job exposure matrices: a systematic review and meta-analysis. COPD 2017; 12:725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baur X, Bakehe P.. Allergens causing occupational asthma: an evidence-based evaluation of the literature. Int Arch Occup Environ Health 2014;87:339–63. [DOI] [PubMed] [Google Scholar]
- 14. Bardana EJ. Occupational asthma. J Allergy Clin Immunol 2008;121(2, Supplement 2):S408–411. [DOI] [PubMed] [Google Scholar]
- 15. Christensen BH, Thulstrup AM, Hougaard KS. et al. Maternal occupational exposure to asthmogens during pregnancy and risk of asthma in 7-year-old children: a cohort study. BMJ Open 2013;3:e002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Magnusson LL, Wennborg H, Bonde JP, Olsen J.. Wheezing, asthma, hay fever, and atopic eczema in relation to maternal occupations in pregnancy. Occup Environ Med 2006. Sep;63:640–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Svanes C, Koplin J, Skulstad SM. et al. Father’s environment before conception and asthma risk in his children: a multi-generation analysis of the Respiratory Health in Northern Europe study. Int J Epidemiol 2017;46:235–45. [DOI] [PubMed] [Google Scholar]
- 18. Mima M, Greenwald D, Ohlander S.. Environmental Toxins and Male Fertility. Curr Urol Rep 2018;19:50. [DOI] [PubMed] [Google Scholar]
- 19. Soubry A, Hoyo C, Jirtle RL, Murphy SK.. A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. Bioessays 2014;36:359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuiper IN, Svanes C, Benediktsdottir B. et al. Agreement in reporting of asthma by parents or offspring—the RHINESSA generation study. BMC Pulm Med 2018;18:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pape K, Svanes C, Malinovschi A. et al. Agreement of offspring-reported parental smoking status: the RHINESSA generation study. BMC Public Health 2019;19:1–9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6341700/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moual NL, Zock J-P, Dumas O. et al. Update of an occupational asthma-specific job exposure matrix to assess exposure to 30 specific agents. Occup Environ Med 2018;75:507–14. [DOI] [PubMed] [Google Scholar]
- 23. Liu X, Olsen J, Agerbo E, Yuan W, Sigsgaard T, Li J.. Prenatal stress and childhood asthma in the offspring: role of age at onset. Eur J Public Health 2015;25:1042–046. [DOI] [PubMed] [Google Scholar]
- 24. Liu X, Madsen KP, Sejbaek CS. et al. Risk of childhood asthma following prenatal exposure to negative life events and job stressors: a nationwide register-based study in Denmark. Scand J Work Environ Health 2019;45:174–82. [DOI] [PubMed] [Google Scholar]
- 25. Accordini S, Calciano L, Johannessen A. et al. A three-generation study on the association of tobacco smoking with asthma. Int J Epidemiol 2018;47:1106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bråbäck L, Lodge CJ, Lowe AJ, Dharmage SC, Olsson D, Forsberg B.. Childhood asthma and smoking exposures before conception: a three-generational cohort study. Pediatr Allergy Immunol 2018;29:361–68. [DOI] [PubMed] [Google Scholar]
- 27. Lee A, Mathilda Chiu Y-H, Rosa MJ. et al. Prenatal and postnatal stress and asthma in children: temporal- and sex-specific associations. J Allergy Clin Immunol 2016;138:740–47.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tagiyeva N, Devereux G, Semple S. et al. Parental occupation is a risk factor for childhood wheeze and asthma. Eur Respir J 2010;35:987–93. [DOI] [PubMed] [Google Scholar]
- 29. Svanes C, Omenaas E, Jarvis D, Chinn S, Gulsvik A, Burney P.. Parental smoking in childhood and adult obstructive lung disease: results from the European Community Respiratory Health Survey. Thorax 2004;59:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burke H, Leonardi-Bee J, Hashim A. et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics 2012;129:735–44. [DOI] [PubMed] [Google Scholar]
- 31. Knudsen TM, Rezwan FI, Jiang Y, Karmaus W, Svanes C, Holloway JW.. Trans- and inter-generational epigenetic inheritance in allergic diseases. J Allergy Clin Immunol 2018;142:765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Armstrong BG. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med 1998;55:651–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khreis H, Cirach M, Mueller N. et al. Outdoor air pollution and the burden of childhood asthma across Europe. Eur Respir J 2019;54:1802194. [DOI] [PubMed] [Google Scholar]
- 34. Knol MJ, Janssen KJM, Donders ART. et al. Unpredictable bias when using the missing indicator method or complete case analysis for missing confounder values: an empirical example. J Clin Epidemiol 2010;63:728–36. [DOI] [PubMed] [Google Scholar]
- 35. Sbihi H, Koehoorn M, Tamburic L, Brauer M.. Asthma trajectories in a population-based birth cohort. Impacts of Air pollution and greenness. Am J Respir Crit Care Med 2017;195:607–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset is held and managed by the RHINESSA coordinating centre at the Department of Occupational Medicine, Haukeland University Hospital, Bergen, Norway. Data cannot be made freely available as they are subject to Norwegian Data Protection regulations, but de-identified data can be made available to researchers upon request. Requests for data can be sent to the principal investigator of RHINESSA: C.S., cecilie.svanes@med.uib.no.