Abstract
A 70-year-old Japanese man contracted severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and required oxygen to maintain oxygen saturation (>90%), 5 weeks after onset of coronavirus disease 2019 (COVID-19) symptoms. Transbronchial lung cryobiopsy revealed pathological features of organizing pneumonia with alveolar epithelial injury, and prednisolone administration led to alleviation of respiratory symptoms and recovery of respiratory function. This case report is the first to demonstrate the use of corticosteroids to successfully treat post-COVID-19 respiratory failure in a patient with biopsy-proven organizing pneumonia. We propose that steroid treatment be considered for patients with persistent respiratory dysfunction as COVID-19 pneumonia sequelae.
Keywords: COVID-19, Cryobiopsy, Organizing pneumonia, Steroid
Abbreviations: COVID-19, novel coronavirus disease 2019; OP, organizing pneumonia; CT, computed tomography; CRP, C-reactive protein; PaO2, partial pressure of arterial oxygen; RT-PCR, real-time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SP-D, surfactant protein D; DAD, diffuse alveolar damage; BAL, bronchoalveolar lavage
1. Introduction
The novel coronavirus disease 2019 (COVID-19) can cause respiratory failure and respiratory symptoms even after patients recovery from acute illness. Mo et al. [1] reported that at least 30% of COVID-19 cases had abnormal pulmonary function with reduced carbon monoxide diffusion capacity at hospital discharge. Carfi et al. [2] reported that >40% of COVID-19 survivors had dyspnea. We encountered a patient with a similar case of respiratory failure that persisted 5 weeks after the onset of COVID-19. Transbronchial cryobiopsy revealed pathologically organizing pneumonia (OP) with alveolar epithelial injury. Respiratory failure improved with steroid therapy in our patient. This report may help guide treatment decisions in similar cases.
2. Case report
A 70-year-old Japanese man presented with symptoms of COVID-19 (fever, malaise, cough, and dyspnea) 11 days before admission. He had no history of smoking and no past medical history of lung disease. He had been taking medications for hypertension and dyslipidemia. On admission, he presented with a pulse rate of 92 bpm, temperature of 38.5 °C, blood pressure of 130/77 mmHg, and oxygen saturation of 91% under 6 L/min O2 administered via a mask. Laboratory data revealed elevated serum lactate dehydrogenase (LDH) 441 U/L, aspartate aminotransferase (AST) 58 U/L, C-reactive protein (CRP) 16.93 mg/dL, and ferritin 1428.0 ng/mL; lymphocytes had decreased to 318/μL. Partial pressure of arterial oxygen (PaO2) declined to 71.2 Torr under 6 L/min O2 administered via a mask. Real-time reverse transcription-polymerase chain reaction (RT-PCR) tests of nasopharyngeal swab specimens for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were positive. Chest X-ray revealed peripheral consolidation and volume reduction in the lower lung field, while computed tomography (CT) revealed peripheral non-segmental ground-glass opacities and consolidation along with broncho-vascular bundles with some traction bronchiectasis in both peripheral lung fields (Fig. 1 A and B). Due to his severe respiratory status, he required high-flow nasal cannula (fraction of inspiratory oxygen 0.65, oxygen flow rate 50 L/min) and was administered antiviral drug (favipiravir) from days 1–4, which was discontinued due to liver dysfunction. Methylprednisolone, 70 mg (1 mg/kg/day), was also administered for 5 days for its expected anti-inflammatory effect.
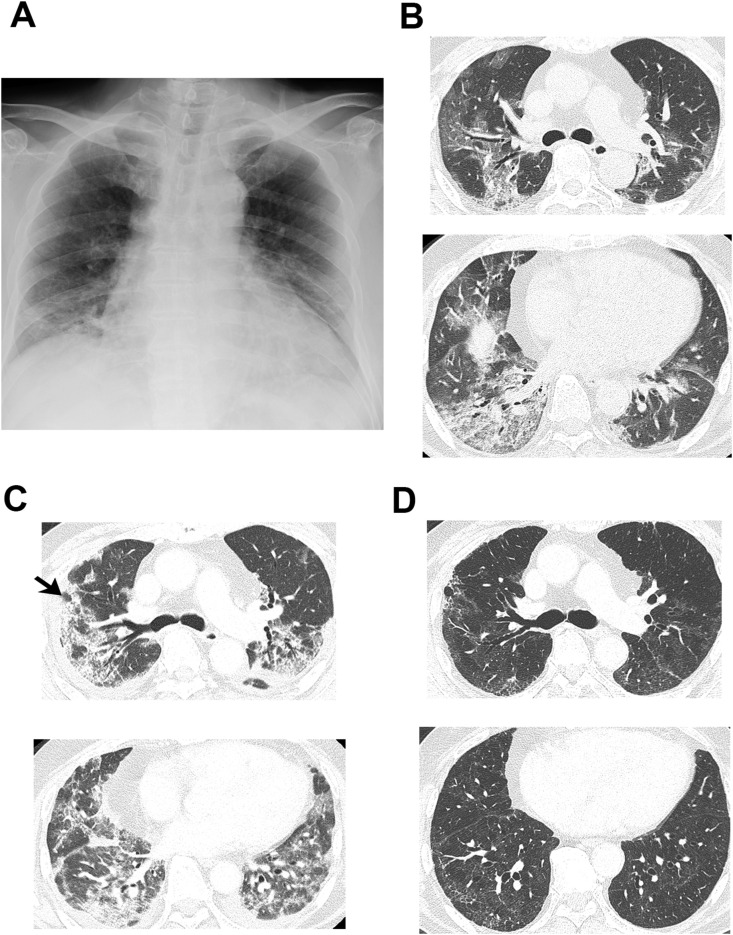
Fig. 1.
CT findings during the clinical course. (A) Chest X-ray on day 0. Peripheral consolidation and volume reduction are noted in the lower lung field. (B) Computed tomography (CT) scan of the lungs on day 0. Peripheral non-segmental ground-glass opacities and consolidation are present along with broncho-vascular bundles with some traction bronchiectasis. (C) CT scan on day 23. The shadow had enlarged and changed to consolidation. The lower lobe of the right lung shrank, accompanying fibrosis, and pleural effusion emerged. Cryobiopsy was performed at the site indicated by the arrow. (D) CT scan on day 54. Most of the consolidation had improved compared with day 23.
Although his respiratory status improved, his need for supplemental oxygen persisted, with 4 L/min required to maintain oxygen saturation (>90%) (Fig. 2 ). Also, CRP level remained around 6 mg/dL without decreasing, suggesting persistent local lung inflammation. Moreover, serum sialylated carbohydrate antigen levels were elevated (Krebs von den Lungen-6 [KL-6]; 427 U/mL [upper limit: 401.2 U/mL]), and surfactant protein D (SP-D; 243.3 ng/mL [upper limit: 110.0 ng/mL]). Chest X-ray revealed increased lung consolidations and progressive volume reduction even on day 23, 5 weeks after the onset of COVID-19 symptoms. Common respiratory pathogens were not found in the laboratory tests. RT-PCR tests for SARS-CoV-2 became negative on day 16. There was no pulmonary embolism, and cardiac function was normal. On day 28, he underwent bronchoalveolar lavage (BAL) from the middle lobe and transbronchial lung cryobiopsy from the right lung upper lobe. RT-PCR tests for SARS-CoV-2 using the BAL fluid and lung tissue were negative. Other BAL cultures were unremarkable. In the BAL fluid, the cell count had increased to 4.47∗105/mL, with macrophages accounting for most (91%), and lymphocytes accounting for 6%; the CD4/8 ratio was 0.4.
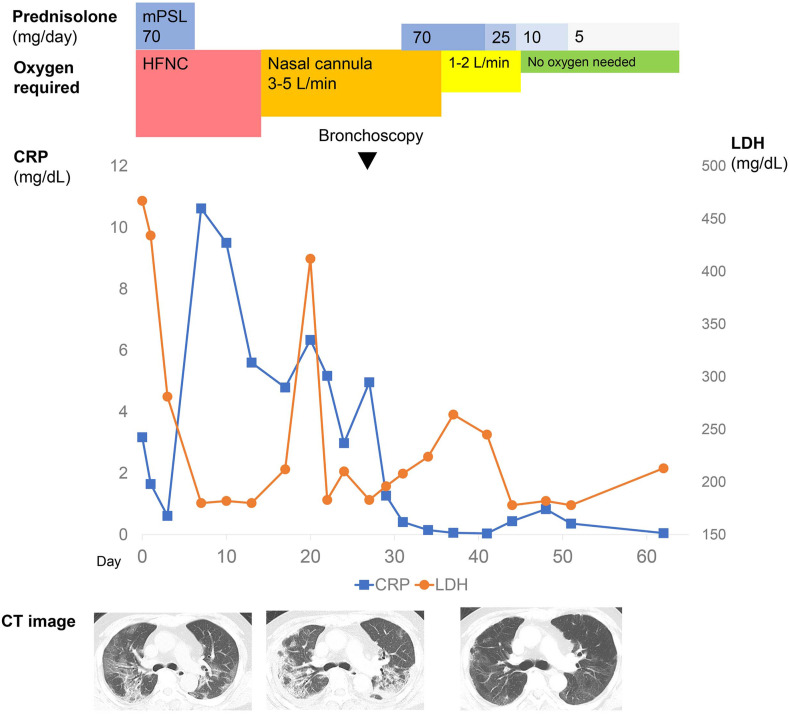
Fig. 2.
Clinical course of the case. Although the acute phase of infection of the novel coronavirus disease 2019 was completed, his need for supplemental oxygen persisted. Persistent lung consolidation and volume reduction were present on day 23. We administered oral prednisolone 70 mg (1 mg/kg/day) starting on day 31 and gradually tapered over 4 weeks. His need for supplemental oxygen at rest disappeared after 2 weeks of steroid therapy. Findings on chest computed tomography scans improved over time. mPSL, methylprednisolone; HFNC, high-flow nasal cannula; LDH, lactate dehydrogenase; CRP, C-reactive protein; CT, computed tomography.
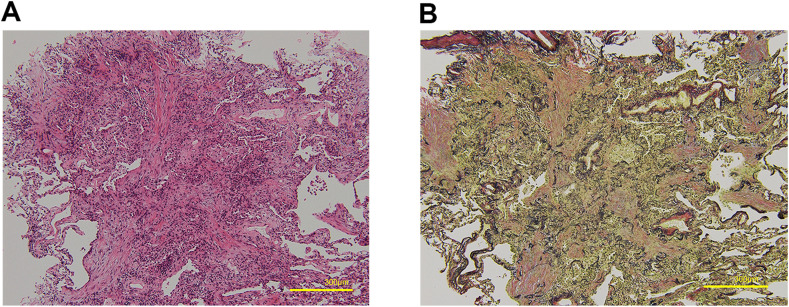
Microscopically, OP with alveolar epithelial injury was noted. Fibrosis was focally embedded in a myxoid matrix, predominantly involving the alveolar spaces and alveolar ducts. In the central part of the fibrosis, the elastic fibers in the alveoli were destroyed. Lymphoplasmacytic infiltration and scattered alveolar macrophages were observed within the airspaces. Neither vasculitis nor capillaritis was observed (Fig. 3 A and B). Immunostaining of the specimen did not detect SARS-CoV-2 nucleoprotein.
Fig. 3.
Pathological findings. (A) Pathological findings in the upper lobe of the right lung (hematoxylin-eosin stain, × 100). (B) Pathological findings in the upper lobe of the right lung (Verhoeff–Van Gieson stain, × 100). Fibrosis with myxoid stroma filled the alveolar spaces and alveolar ducts. Fragments of elastic fibers were found in the dense fibrosis.
We administered oral prednisolone 70 mg (1 mg/kg/day) starting on day 31 and gradually tapered over 4 weeks (Fig. 2). His need for supplemental oxygen at rest, previously 4 L/min, disappeared after 2 weeks of steroid therapy. Three weeks later, he did not need supplemental oxygen, even on exertion. Findings on chest CT scans improved over time. Some ground-glass opacities remained around previous consolidation, but almost all volume reduction improved, and extensive infiltrative shadows disappeared (Fig. 1D). Serum KL-6 normalized, and SP-D decreased to 121.3 ng/mL. He was discharged home on day 56, and respiratory dysfunction has not relapsed as of this writing 3 months later.
3. Discussion
To our knowledge, this is the first report of a case in which steroid therapy was used to successfully treat respiratory failure after COVID-19 in a patient with biopsy-proven OP. Several reports exists on pathological features of patients with COVID-19. Pogatchinik et al. [3] reported a correlation between lung consolidation and OP in the acute phase. Copin et al. [4] reported organizing diffuse alveolar damage (DAD) patterns in the lungs of autopsy cases.
The pathology in our case showed OP with alveolar epithelial injury, suggesting severe local inflammation. This finding may be related to the fact that SARS-CoV-2 targets angiotensin-converting enzyme 2 receptor, which is expressed on the surface of type II alveolar epithelial cells [5]. We did not find viral nucleoprotein in the lung tissue, which suggests that the inflammation was caused not by persistent infection but by an ongoing immune response. Hence, steroid treatment may have been useful.
The optimal timing of restarting steroid therapy in the late phase of COVID-19 remains a challenge. We currently believe that it is better to reintroduce steroid therapy if improvement of respiratory status is not sufficient or imaging findings worsen, for example, showing enlargement of shadows or shrinkage of the lung volume even after initial steroid therapy. The number of patients with COVID-19 continues to increase, and there is now a growing problem that many survivors may have persistent dyspnea and respiratory failure as sequelae [2]. These patients who complain of dyspnea are often treated with only respiratory rehabilitation and home oxygen therapy. This case report suggests that consolidation with volume reduction in the lungs of patients with persistent respiratory symptoms after recovery from COVID-19 is consistent with OP and that steroid therapy could be useful for treating the sequelae. We propose that steroid treatment be considered for patients with post-COVID-19 pneumonia who have persistent respiratory dysfunction.
Informed consent
We obtained informed consent from the patient to publish.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Prior abstract publication/presentation
None.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
We are grateful to Drs. Noriko Nakajima and Tadaki Suzuki for helpful discussions of immunostaining of SARS-CoV-2 nucleoprotein. We thank Dr. Tamiko Takemura for very thoughtful advice and comments on histological appearance.
References
- 1.Mo X., Jian W., Su Z., Chen M., Peng H., Peng P., et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carfì A., Bernabei R., Landi F. For the gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. J Am Med Assoc. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pogatchnik B.P., Swenson K.E., Sharifi H., Bedi H., Berry G.J., Guo H.H. Radiology–pathology correlation demonstrating organizing pneumonia in a patient who recovered from COVID-19. Am J Respir Crit Care Med. 2020;202:598–599. doi: 10.1164/rccm.202004-1278IM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Copin M., Parmentier E., Duburcq T., Poissy J., Mathieu D., Caplan M., et al. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020;46:1124–1126. doi: 10.1007/s00134-020-06057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]