Abstract
Fecal shedding of SARS-CoV-2 from COVID-19 patients and presence of the viral RNA in wastewater have extensively been reported. Some wastewater treatment plant (WWTP) processes generate aerosols which have the potential to transmit pathogenic microorganisms and present a health risk for exposed individuals. We analyzed the presence of viral RNA of SARS-CoV-2 in raw wastewater and air samples of WWTPs. The risk that may arise from exposure to virus-contaminated aerosols of wastewater was estimated by developing a quantitative microbial risk analysis (QMRA) method. SARS-CoV-2 was detected in 9 of 24 (37.5%) wastewater samples with a concentration about 104 genomic copies L−1. The viral RNA was also detected in 40% (6/15) of air samples. QMRA analysis showed a relatively high risk of SARS-CoV-2 infection for wastewater workers via exposure to the viral aerosols. The estimated annual infection risk ranged from 1.1 × 10−2 to 2.3 × 10−2 per person per year (PPPY) for wastewater workers which was higher than the reference level recommended by WHO (10−3 pppy). However, due to the lack of data on survival of SARS-CoV-2 in wastewater and its fate in aerosolized state, more research is needed to determine the importance of wastewater in transmission of COVID-19.
Keywords: SARS-CoV-2, Wastewater workers, QMRA, Bioaerosol, Transmission
Graphical abstract
1. Introduction
Recently, a novel coronavirus, SARS-CoV-2, emerged as the etiological agent of a respiratory disease called COVID-19. COVID-19 was rapidly spread worldwide and the World Health Organization (WHO) declared the coronavirus outbreak a public health emergency of international concern (WHO, 2020a). Similar to severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), SARS-CoV-2 belongs to the Betacoronavirus genus. Betacoronaviruses are single-stranded, positive sense, enveloped RNA viruses which are responsible for respiratory infections (Yeo et al., 2020). The virus is primarily transmitted from person-to-person by exposure to respiratory droplets from infected patients (Kampf et al., 2020). However, some studies have been reported survival of human coronaviruses in the gastrointestinal tract and shedding in feces of infected patients (Kitajima et al., 2020). Information from researches on human coronaviruses especially SARS-CoV and MERS-CoV shows respiratory and gastrointestinal symptoms such as diarrhea after viral infection (Yeo et al., 2020). Intestinal infection in COVID-19 patients has also been reported and approximately 2–10% of cases with confirmed disease presented with diarrhea (Yeo et al., 2020). Some studies have reported detection of COVID-19 viral RNA in fecal samples of infected patients (Kitajima et al., 2020). During peak shedding, a high number of virus particles is released per gram of fecal matter of COVID-19 patients which could lead to widespread environmental dissemination of the virus through contaminated water and wastewater (Yeo et al., 2020). There are several reports of the molecular detection of SARS-CoV-2 in wastewater (Ahmed et al., 2020; Lodder and de Roda Husman, 2020; Medema et al., 2020; Nemudryi et al., 2020; Wurtzer et al., 2020) with concentrations of a maximum of over 106 copies per liter of untreated wastewater (Kitajima et al., 2020; Wurtzer et al., 2020). It has been proposed that the analysis of wastewater could be a powerful tool for COVID-19 surveillance. However, the documented presence of SARS-CoV-2 in wastewater raises the question regarding the potential transmission risk of this virus through wastewater, especially for those working with human waste and wastewater (Foladori et al., 2020; Yang et al., 2020; Gormley et al., 2020; El Baz and Imziln, 2020; Gwenzi, 2021). Aerosols from wastewater treatment processes have the ability to carry infectious agents, including respiratory viruses, and therefore, might contribute to transmission of viral infections to the wastewater workers through aerosols/droplets produced in wastewater treatment plants (WWTP) (Kitajima et al., 2020). Although, no evidence has emerged to support the role of wastewater in transmission of COVID-19, the ongoing global pandemic of this viral infection highlights the need for further studies to address any public concerns related to the wastewater (Gwenzi, 2021). Rimoldi et al. (2020) suggested a low risk of infection from river water, while in the case of wastewater indicated that the risk for public health should be estimated under a precautionary approach. Evidence from an outbreak of SARS-CoV in the Amoy Gardens apartment building in Hong Kong showed that aerosolized droplets of fecally contaminated water were responsible for the spread of the viral infection in large numbers of people (McKinney et al., 2006). Structural similarities between the SARS-CoV and SARS-CoV-2 (Corpuz et al., 2020), in combination with relatively high stability of SARS-CoV-2 in the aerosol state (Fears et al., 2020), calls for WWTPs to be considered as a potential source for transmission of COVID-19 through bioaerosols produced by wastewater treatment processes (Corpuz et al., 2020; Kitajima et al., 2020; Gwenzi, 2021). In other words, it is of considerable importance to understand the potential risk of SARS-CoV-2 transmission via wastewater for the sake of wastewater workers and public health. This is particularly important in developing countries, where the personal protective equipment has received limited attention and there is a lack of efficient barrier for controlling the aerosols emitted from wastewater processes (Gwenzi, 2021).
Accordingly, this study was designed to 1) investigate the presence of SARS-CoV-2 in wastewater and air samples of municipal wastewater treatment plants (WWTPs) during the COVID-19 epidemic in Isfahan, Iran. 2) estimate the risk of SARS-CoV-2 infection for WWTP workers which associated with the exposure to wastewater aerosols by a quantitative microbial risk analysis (QMRA) 3) develop a mathematical model that predicts the risk of SARS-CoV-2 for wastewater workers based on the cumulative number of COVID-19 cases at any one time.
2. Materials and methods
2.1. Sample collection
2.1.1. Wastewater samples
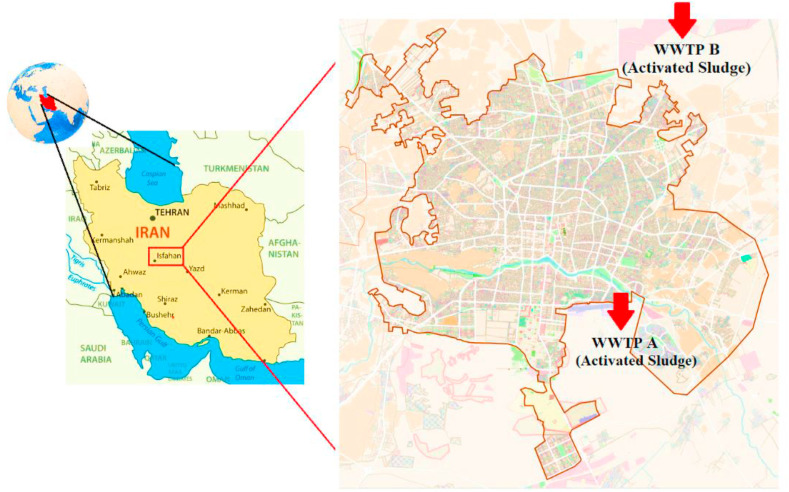
Wastewater samples were collected at 1-hourly intervals within a 12-h period from two wastewater treatment plants in Isfahan, Iran, from March 04 to March 17, 2020; during the early stages of the COVID-19 outbreak. The location and characteristics of WWTPs are presented in Fig. 1 and Table 1 , respectively. A total of 24 raw wastewater (after grit chamber) samples (12 samples from each WWTP), were collected in 250 mL sterile glasses and were transferred to the laboratory in an insulated box with cooling packs.
Fig. 1.
Maps of the study region and the location of WWTPs.
Table 1.
Characteristics of the wastewater treatment plants (WWTPs).
| WWTP | Served population | Capacity (m3 y−1) | Treatment process | Potential points of bioaerosol generation |
|---|---|---|---|---|
| South of Isfahan (WWTP A) | 790,000 | 45,990,000 | Activated Sludge | Pumping station, Aerated grit chamber, Aeration tank |
| North of Isfahan (WWTP B) | 980,000 | 83,950,000 | Activated Sludge | Pumping station, Aerated grit chamber, Aeration tank |
2.1.2. Air samples
For detection of SARS-CoV-2 in aerosols of WWTP, a total of 15 air samples were collected using all-glass impingers, containing phosphate buffer solution. Air sampling was performed at three sites in WWTP A, including pumping station and activated sludge plants at a height of 1.5 m above the ground level. Air samples (3500–4500 L) were collected using portable pumps at a flow rate of 7.5–8.5 L min−1 and then transferred to the laboratory in an insulated box with cooling packs.
2.2. SARS-CoV-2 detection
Before detection of SARS-CoV-2, 200 mL of wastewater samples were concentrated by aluminum hydroxide adsorption-precipitation method as described in Standard Methods for the examination of water and wastewater (APHA, 2012). However, for increase of detection sensitivity, some samples were more concentrated by application of polyethylene glycol (PEG) (Wu et al., 2020). Air samples were also concentrated by application of PEG (Wu et al., 2020). Viral RNA was extracted from concentrates using the RNeasy Mini Kit (QIAGEN, Germany) supplemented with β-mercaptoethanol and carrier RNA according to the manufacturer’s instructions. RNA was also extracted by application of TRIzol (Invitrogen).
Isolated RNA was used as a template for one-step reverse transcription quantitative polymerase chain reaction (RT-qPCR). Real-time PCR was performed using specific primers (F: 5′-TGTTAAACCAGGTGGAAC-3’; R: 5′-CTGTGTTGTAGATTGCG-3′) targeting RNA-dependent RNA polymerase (RdRp) gene of SARS-CoV to detect the presence of SARS-CoV-2. A 25 μL reaction contained 12.5 μL of 2 X reaction mix, 0.25 μL RT enzyme mix (QuantiTect RT-PCR, QIAGEN), 0.6 μM of each primer, 5 mM MgCl2, 0.25 mg mL−1 BSA and 8 μL template RNA. The cycling parameters were as RT at 50 °C for 30 min, followed by 95 °C for 15 min and then 45 cycles at 95 °C for 15 s, 57 °C for 30 s in a StepOne real-time PCR system (Applied Biosystems™, USA). Each run included a positive control (RNA from patients hospitalized with COVID-19) and negative control. A melting curve analysis was performed after the PCR run to differentiate between actual products and primer dimmers, and to eliminate the possibility of false-positive results. A novel coronavirus (2019-nCoV) detection kit was also used for detecting the ORF-1ab and N genes of SARS-CoV-2 (Sansure Biotech, China) according to the manufacturer’s instructions.
2.2.1. Quantification of SARS-CoV-2
SARS-CoV-2 RNA was quantified by plotting the quantification cycles (Cq) against of a 10-fold serial dilution of a quantified plasmid. RNA of SARS-CoV-2 was amplified by RT-PCR and then used for DNA cloning. Plasmids containing the SARS-CoV-2 insert were purified using High Pure Plasmid Isolation Kit (Roche, Germany), quantified and a ten-fold serial dilution was prepared. The limit of detection (LOD) resulted as 10 genomic copies per reaction.
2.3. Quantitative microbial risk assessment
Wastewater is containing pathogenic microorganisms such as viruses which could be aerosolized during wastewater treatment processes. In the study, a QMRA model was used to assess the risk of infection associated with inhalation of SARS-CoV-2 contaminated aerosols/droplets produced from wastewater treatment processes.
2.3.1. Hazard identification
Sewage workers may expose to pathogenic microorganisms from biological aerosols produced during wastewater treatment processes. Exposure to SARS-CoV-2 aerosols/droplets in WWTPs could pose a health risk for workers (Corpuz et al., 2020; Kitajima et al., 2020). Different stages of COVID-19 outbreak, in terms of percentage of infected population, can affect the concentration of viral particles in wastewater, aerosols emitted from WWTPs, and consequently estimated risk. Therefore, it may be better to use the human fecal shedding method for estimating SARS-CoV-2 concentrations in wastewater as recommended by Barker (2014); and Zaneti et al. (2021).
2.3.2. Exposure assessment
Aerosols are generated by some units at WWTPs such as pumping station and aeration tanks. High fractions of the aerosols have diameters ≤10 μ (88% of produced aerosols are less than 4 μ in diameter), which are considered respirable, could be deposited in the respiratory tract, and may reach the alveolar region of the lungs (USEPA, 2011).
The daily dose (TCID50 d−1) of SARS-CoV-2 aerosols inhaled by the WWTP workers is given by (Barker, 2014):
| (1) |
where Cw is the concentration of SARS-CoV-2 in wastewater (TCID50 L−1); PCwa is the microbial water-to-air partitioning coefficient (L m−3); IR is the average inhalation rate (m3 h−1); texp is the daily exposure duration (h) which was considered 8 h for occupational exposure, and RR is the aerosol retention rate in the lungs which is calculated by equation (2) (Schoen and Ashbolt, 2011):
| (2) |
Here, fi 1 is the fraction of aerosols of size range i, and fi 2 is the fraction of the aerosols of size range i that are deposited in the lower respiratory tract (Schoen and Ashbolt, 2011). We assumed that WWTPs could generate inhalable-size aerosols (smaller than 10 μm) which may carry the virus.
For Cw, we used the best available data to estimate densities of infectious SARS-CoV-2 in the wastewater. The concentration of SARS-CoV-2 in wastewater is estimated by equation (3) (Barker, 2014).
| (3) |
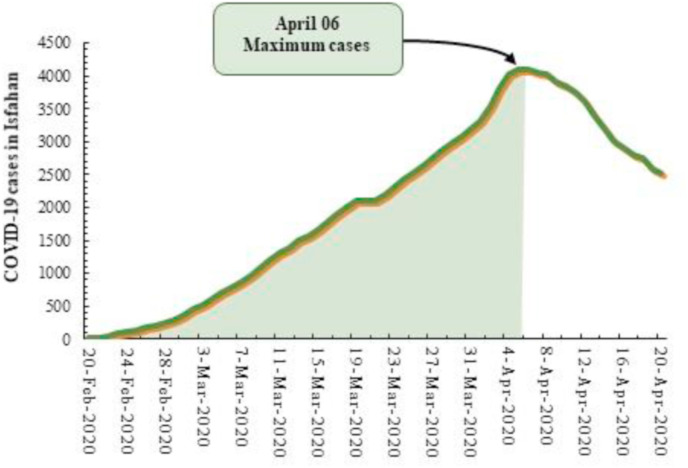
where Pi is the cumulative number of COVID-19 cases over a 46-day period (from February 20 to April 06, 2020). The first wave of infection in the study region was started to increase since the end of February 2020, reached a maximum on the 6th of April, 2020, and then has slowly decreased (Fig. 2 ).
Fig. 2.
Daily COVID-19 cases in the region of study from February 20-April 06, 2020.
Since gastrointestinal symptoms and subsequently virus excretion have been reported for some patients, PR is the percent of individuals with fecal shedding of SARS-CoV-2.
Shd and Shc are shedding duration (d) and shedding rate of SARS-CoV-2 (copy g−1) in feces of infected patients, respectively. Recent reports have revealed that some of COVID-19 patients have gastrointestinal symptoms for various durations of time from 1 to 39 days, and SARS-CoV-2 RNA load could be as high as 108 copies per gram of fecal sample (Kitajima et al., 2020). F is the daily fecal production by infected patients (g feces per person per day), Q is the flow rate of WWTP (m3 d−1) and CF is the conversion factor of genomic copy number to TCID50 and dt is 46 days.
No pathogen decay was considered during aerosolization or transport through air to a receptor.
2.3.3. Dose-response model and risk characterization
By the dose-response model, the probability of infection is estimated due to the inhaled dose. Because of the structural similarities of SARS-CoV-2 with SARS-CoV and human coronavirus and the lack of a dose-response model for SARS-CoV-2 we used exponential dose-response models which have been suggested by Watanabe et al. (2010) for human coronavirus (229E) (Eq. (4)) and SARS-CoV (Eq. (5)).
| (4) |
| (5) |
Pi(d) is the risk of infection per daily exposure of sewage workers to aerosols/droplets of SARS-CoV-2, d is the daily dose (TCID50 d−1); and k is the model parameter which has been considered as 5.39 × 10−2 and 2.46 × 10−3 with illness and death as endpoints of response for equations of 4 & 5, respectively (Watanabe et al., 2010).
The annual risk of SARS-CoV-2 infection per person (Pi(A)) is estimated by equation (6) (Moazeni et al., 2017).
| (6) |
where n is the frequency of exposure (the number of days per year on which a worker may be exposed to SARS-CoV-2 aerosols). We considered 20 working days per each month and an exposure period of 12 months (one year).
2.4. Model implementation
The values and statistical distributions of the variable parameters introduced in the study are presented in Table 2 . Monte-Carlo simulation technique with 10,000 random sampling from each distribution input was used to run the model to develop a distribution of likely risk and incorporate the uncertainty and variability around each parameter. All modeling and analysis were implemented using RStudio version 1.3.959. Sensitivity analysis was performed using Spearman’s rank order correlation to identify those variable input parameters contributing to the uncertainty of estimated infection risk and have the greatest influence on the estimated risk.
Table 2.
QMRA model input parameters.
| Parameter | Unit | Distribution | Reference |
|---|---|---|---|
| Cumulative cases of COVID-19 served by WWTP A (Pi) | Person | Beta (α1 = 0.59204, α2 = 0.75091) | This study |
| Cumulative cases of COVID-19 served by WWTP B (Pi) | Person | Beta (α1 = 0.59197, α2 = 0.85633) | This study |
| Shedding duration (Shd) | D | PERT (Min = 1, Mode = 8, Max39) | (Wu et al., 2020) |
| Shedding rate (Shc) | Copies per g feces | Uniform (Min = 6.3 × 105, Max = 1.3 × 108) | Kitajima et al. (2020) |
| Daily fecal production (F) | g person−1 d−1 | Normal (Mean = 243, SD = 130.2) | Rose et al. (2015) |
| Flow rate of WWTP A (Q) | m3 d−1 | Point (125,544) | This study |
| Flow rate of WWTP B (Q) | m3 d−1 | Point (229,785) | This study |
| Conversion factor (CF) | Copies TCID50−1 | Uniform (Min = 29, Max = 700) | (Kim et al., 2020; Mcbride et al., 2013) |
| Positive Rate (PR) | Percent | Beta (α1 = 2.1173, α2 = 1.8117) | Kitajima et al. (2020) |
| Microbial water-to-air partitioning coefficient (PCwa) | L m−3 | Uniform (Min = 10−4, Max = 10−5) | This study |
| Inhalation rate (IR) | m3 min−1 | Point (2.9 × 10−2) | (USEPA, 2011) |
| Exposure time (texp) | h | Point (8) | (USEPA, 2011) |
| Parameter for the exponential model; disease as endpoint response (k) death as endpoint response (k) |
Point (5.39 × 10−2) Point (2.46 × 10−3) |
Watanabe et al. (2010) |
Since the concentration of SARS-CoV-2 in wastewater and subsequently in aerosolized wastewater is affected by the extent of COVID-19 outbreak in a community, we presented a mathematical model (Eq. (7)) to predict the annual probability of SARS-CoV-2 infection for WWTP workers based on the number of COVID-19 cases in a community served by a WWTP.
| (7) |
All of other parameters in QMRA model were kept to their average value.
3. Results and discussion
3.1. Detection of SARS-CoV-2 in raw wastewater
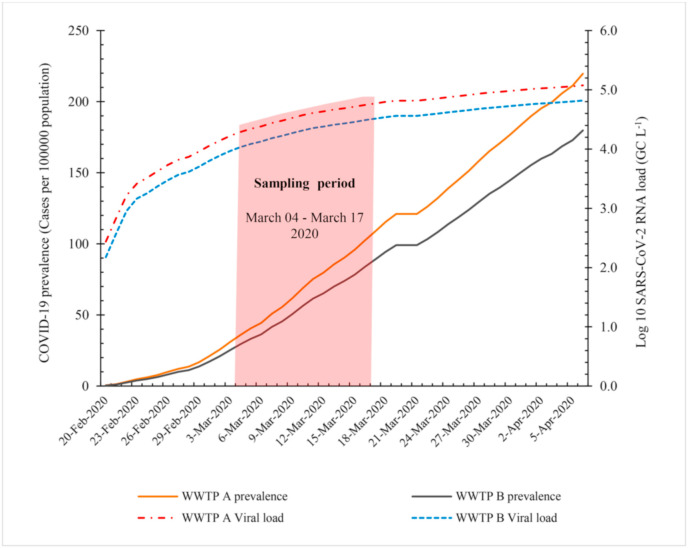
Monitoring of pathogenic microorganisms in wastewater is a promising approach to understand the prevalence and circulation of the microbial diseases in a community. In the study, we detected SARS-CoV-2 in 9 of 12 (37.5%) wastewater samples. Viral RNA was detected in 5 of 12 and 4 of 12 samples of raw wastewater from WWTP A and B, respectively (Table 3 ). SARS-CoV-2 was detected in wastewater samples of different countries have been facing COVID-19 epidemic such as Australia, Italy, Netherlands, America, France and India (Ahmed et al., 2020; La Rosa et al., 2020; Medema et al., 2020; Rimoldi et al., 2020; Wu et al., 2020; Wurtzer et al., 2020; Kumar et al., 2020). Results of an investigation in Milan and Rome, Italy showed positive results for SARS-CoV-2 RNA of 50% (6/12) raw wastewater samples (La Rosa et al., 2020). All samples (7 of 7) of pre-treated wastewater from the municipal wastewater treatment plant in Bozeman, Montana were positive for viral RNA of SARS-CoV-2 (Nemudryi et al., 2020). Among the nine untreated wastewater samples tested from urban catchments in Southeast Queensland, Australia, two (22.2%) samples were positive for viral RNA (Ahmed et al., 2020). Wastewater testing has been suggested as a sensitive tool to monitor the status and circulation of the COVID-19 infection in the community (Kitajima et al., 2020; Medema et al., 2020). Screening of SARS-CoV-2 targets in 24 h composite samples of incoming wastewater at different WWTPs in the Netherlands showed no positive sample three weeks before the first COVID-19 case, while 9 of 10 samples were positive 2.5 weeks after the outbreak (Medema et al., 2020). Therefore, the infection rate in a community could be an important parameter affects the detection frequency and concentration of the virus in wastewater samples. As shown in Fig. 3 , lower positive samples from WWTP B may in part be related to the lower prevalence of COVID-19 in the region served by WWTP B. Furthermore, despite the increasing prevalence of COVID-19 over the last days of sampling period (Fig. 3), SARS-CoV-2 was not detected in some samples. No detection of SARS-CoV-2 in the samples may be a consequence of the low recovery of aluminum hydroxide adsorption-precipitation method. In other words, SARS-CoV-2 was detected in samples which were more concentrated by PEG. Randazzo et al. (2020) reported a recovery of about 10% for influent samples by aluminum hydroxide adsorption-precipitation method which was tested by spiking of the samples with mengovirus (MgV).
Table 3.
Detected genes of SARS-CoV-2 in positive wastewater samples.
| Sampling site/Sample | Detected gene |
||
|---|---|---|---|
| RdRp | ORF-1ab | N | |
| WWTP A | |||
| 1 | + | – | – |
| 2 | + | – | – |
| 3 | + | – | – |
| 4 | + | – | – |
| 5 | – | + | + |
| WWTP B | |||
| 1 | + | – | – |
| 2 | + | – | + |
| 3 | + | – | – |
| 4 | + | – | – |
Fig. 3.
Cumulative prevalence of COVID-19 in regions served by WWTPs, and SARS-CoV-2 concentration in raw wastewater (Cw) as estimated by QMRA model during the study period.
We detected SARS-CoV-2 in a concentration of about 2–4 × 104 genomic copies (GC) per liter. In consistent with our results, Medema et al. (2020) detected SARS-CoV-2 in concentrations of 2.6–30 GC mL−1 in wastewater samples of Netherlands. Viral concentrations of 4–5 and more than 6 log10 GC L−1 have been reported in wastewaters of Massachusetts and France, respectively (Randazzo et al., 2020; Wu et al., 2020; Wurtzer et al., 2020). However, in Ahmed et al. (2020) study, among the nine wastewater samples tested, two (22.2%) samples were reported positive with a concentration of 12 and 1.9 copies per 100 mL of untreated wastewater. In consistent with previous studies, we observed discrepancy among different target genes for detection of SARS-CoV-2 (Table 3) in wastewater samples which may in part be related to the analytical sensitivity among the assays (Medema et al., 2020; Randazzo et al., 2020). As presented in Table 3, RdRp gene was the most frequently detected gene in our samples (8 of 9 positive samples). N and ORF-1ab genes were detected in 2 and 1 samples, respectively. In study of Rimoldi et al. (2020), ORF-1ab gene showed the highest frequency of positivity, while both the other two genes (N and E) failed to be amplified in two out of five positive samples of water and wastewater. Detection of SARS-CoV-2 in wastewater samples in Italy by RdRp gene showed a higher sensitivity compared to the assay targeting the spiked gene (La Rosa et al., 2020).
We found relatively consistent result for the viral load in WWTPs as quantified by the real-time PCR assay with which was calculated by the QMRA model (Cw) (Fig. 3). However, some authors have reported much higher concentrations of the viral RNA in wastewater than the confirmed cases of COVID-19 in the community (Randazzo et al., 2020; Wu et al., 2020).
3.2. Detection of SARS-CoV-2 in wastewater aerosols
Aerosols produced by some operations or processes in WWTPs have the ability to carry pathogenic microorganisms (Kitajima et al., 2020). However, very few studies have investigated the presence and concentrations of airborne viruses in WWTPs (Kitajima et al., 2020; Corpuz et al., 2020). Our results revealed the detection of SARS-CoV-2 RNA in 40% (6/15) of air samples of WWTP A when prevalence of COVID-19 was very high in the region. SARS-CoV-2 RNA was detected in a range of 5–188 GC L−1 of air with the highest concentration at pumping station. Masclaux et al. (2014) reported a high frequency of detection of adenovirus in air samples (100% of summer samples and 97% of winter samples) of a WWTP. They reported that the highest airborne concentration of adenovirus was 2.27 × 106 genome equivalent m−3. However, they detected norovirus in only 3 of the 123 air samples. In another study, 56% (9/16) of air samples from a WWTP in Japan were positive for norovirus (NV) GII. Adenoviruses (4/16), NV GI (6/16), FRNA bacteriophages GIII (3/16), and enteroviruses (3/16) were also detected, but at lower frequencies (Matsubara and Katayama, 2019).
3.3. Risk of COVID-19 infection for wastewater workers
Presence of SARS-CoV-2 RNA in wastewater samples and wastewater aerosols could pose a health concern for WWTPs workers.
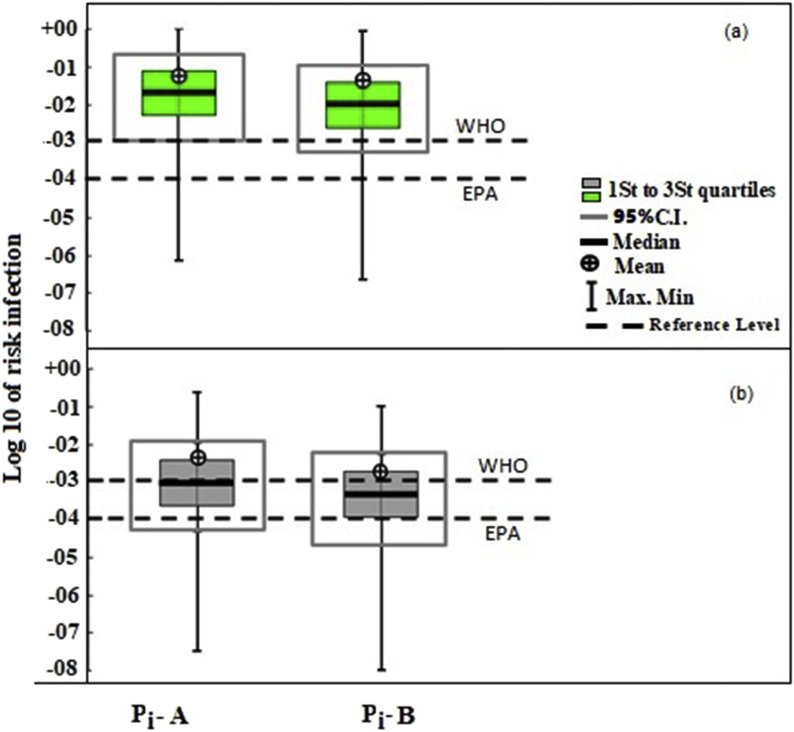
The results of this study showed a median infection risk (with illness as endpoint of response, Eq. (4)) of 2.3 × 10−2 (95% CI: 1.65 × 10− 3 – 4.9 × 10−1) and 1.1 × 10−2 (95% CI: 8 × 10− 4 - 2.9 × 10−1) per person per year (pppy) for workers of WWTP A and B, respectively. Fig. 4 compares the levels of estimated infection risk with the 10−4 and 10−3 pppy reference levels proposed by EPA (EPA, 2011) and WHO (Mara et al., 2007), respectively. As shown in Fig. 4, the estimated annual infection risk of SARS-CoV-2 for wastewater workers was about 1 log higher than the WHO guideline threshold of 10−3 pppy. The estimated risk was also higher than the tolerable infection risk of 5.5 × 10−4 pppy has been recommended by Zaneti et al. (2021) for SARS-CoV-2.
Fig. 4.
The box plots of estimated infection risk (pppy) for workers of WWTP A (Pi-A) and WWTP B (Pi-B) in comparison to 10−4 and 10−3 pppy reference levels, with considering (a) illness as endpoint response and (b) death as endpoint response.
The results showed a higher infection risk for workers of WWTP A which is related to the higher prevalence of COVID-19 in the region served by WWTP A (Fig. 3). Study of Zaneti et al. (2021) showed an infection risk of SARS-CoV-2 from 2.6 × 10−3 to 1.3 × 10−2 from accidental ingestion of sewage by WWTP workers while performing routine activities. They reported that the estimated risk was above the tolerable infection risk for SARS-CoV-2 of 5.5 × 10−4 pppy, thus reinforcing the concern about wastewater as a potential source of COVID-19 transmission. A QMRA analysis showed a high infection risk from adenoviruses for wastewater workers from exposure to bioaerosols from influent and biological oxidation tanks for >3 min exposure time (Carducci et al., 2018). Pepper and Gerba (2018) reported an infection risk of greater than 10−4 for exposure to spray irrigation of reclaimed water when the number of Legionella in the water exceeded 1000 colony forming units (CFU) per mL.
As shown in Fig. 4, risk assessment analysis by equation (5) with death as endpoint of response, showed lower risk of SARS-CoV-2 for workers from exposure to wastewater aerosols.
We assumed that the load of SARS-CoV-2 genomes in wastewater correlated with the number of symptomatic patients with a mean fecal shedding duration of 11.89 days and shedding rates from 6.3 × 105 to 1.3 × 108 RNA copies per gram (Table 2). Some studies have reported the detection of SARS-CoV-2 in fecal samples of non-symptomatic individuals as well as lower shedding rate about 102 -103 RNA copies per gram of fecal matter of symptomatic patients (Kitajima et al., 2020). It has been reported that viral RNA could be detected in the feces of 81.8% COVID-19 cases even with a negative throat swab result (Ling et al., 2020). In an investigation, shedding of SARS-CoV-2 in a cluster of 9 cases was 107 RNA copies g−1 feces one week after symptom onset which decreased to the 103 RNA copies g−1 three weeks after symptom onset (Medema et al., 2020). Therefore, due to the lack of sufficient information about this emerging pathogen, the estimated risk may be over- or underestimated.
3.4. Sensitivity analysis
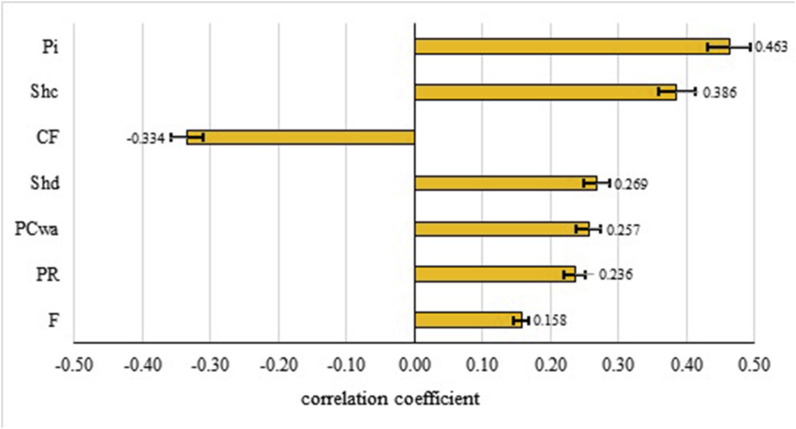
Sensitivity analysis was performed to determine the influence of variation in the input parameters on the estimated infection risk. As expected, the sensitivity analysis suggested that the infection risk of SARS-CoV-2 is greatly affected by the number of cases of COVID-19 in the population (Pi) (Fig. 5 ). However, the mathematical model developed in the study (Eq. (7)), can be readily used to predict the effect of variation of COVID-19 prevalence on the probability of infection risk. Our sensitivity analysis showed that conversion factor (CF) of genomic copies to TCID50 is also an important factor affects the infection risk (Fig. 5). Although it has been reported that coronaviruses may remain infectious in water and sewage for days (Qu et al., 2020), no accurate data is available for conversion of genomic copy numbers of SARS-CoV-2 to TCID50 in aquatic environments. Therefore, we used a wide range of CF from 29 (Kim et al., 2020) to 700 (Mcbride et al., 2013) based on the available data. Zaneti et al. (2021) considered a conversion factor of 1000 for estimation of risk of SARS-CoV-2 associated with the accidental ingestion of sewage by WWTPs workers. Shedding rate (Shc) and duration (Shd) of SARS-CoV-2 are other important influential factors which were positively correlated with the infection risk of SARS-CoV-2. These two parameters along with Pi affect the viral load or concentration of SARS-CoV-2 in wastewater. Other studies have also reported that the pathogen numbers in wastewater are among the most important variables correlated to the resultant risk from exposure to wastewater (Carducci et al., 2018; Farhadkhani et al., 2018; Moazeni et al., 2017). Legionella concentration was identified as the most important parameter affects the health risk from exposure to Legionella-contaminated aerosols in reclaimed water (Hamilton et al., 2018).
Fig. 5.
Tornado chart for the median estimates of the Spearman’s rank correlation between the input variables and the risk of infection, bounded by the 95% uncertainty.
The partitioning coefficient (PCwa), a function of the aerosolization efficiency, was identified as another important factor affects the infection risk (Fig. 5). In consistent with our results, Hamilton et al. (2018) reported that aerosol partitioning is an influential factor in health risk from exposure to Legionella-contaminated aerosols in reclaimed water (Hamilton et al., 2018). We assumed a PCwa of 10−4 - 10−5 L m−3 based on the analyzed concentrations of SARS-CoV-2 RNA in air samples of WWTP to the predicted concentration in wastewater. Estimated PCwa was one order of magnitude greater than values recommended for estimating bacterial transfer, in particular legionella, from air-to-water (Bauer et al., 2002; Chattopadhyay et al., 2017). However, the estimation of bioaerosol concentrations generated in WWTPs exhibits very high uncertainty for microbial pathogens (USEPA, 2011). Type and surface properties of pathogenic microorganisms and wastewater quality could affect the water-to-air partitioning coefficient of pathogens (Chattopadhyay et al., 2017). On the other hand, bioaerosolized viral particles may considerably dilute by air currents or inactivated by environmental factors. Pyankov et al. (2018) reported that the decay of airborne MERS-CoV virus is much higher for hot and dry air conditions, with only 4.7% survival over 60 min. However, we assumed no dilution or loss for viral particles which may overestimate the infection risk of COVID-19.
3.5. Limitations and further research needs
Although our findings suggest that wastewater may contribute to COVID-19 transmission, the estimated risk for wastewater workers still presents significant challenges due to the knowledge gaps about this emerging pathogen.
The lack of a dose-response model for SARS-CoV-2 is a critical limitation for conducting QMRA for this pathogen (Kitajima et al., 2020; Corpuz et al., 2020). In other words, the number of viral particles which is required to cause an infection differs among viral pathogens and it is not now clear for SARS-CoV-2 (Kitajima et al., 2020). In a QMRA study on adenovirus 40/41, a dose-response relationship for adenovirus type 4 was used and assumed that all adenoviruses display the same as adenovirus type 4 (Kundu et al., 2013). However, additional research is necessary to develop a dose-response relationship for SARS-CoV-2 based on the epidemiological studies.
Although in a recent study it was reported that SARS-CoV-2 aerosols could maintain their infectivity for up to 16 h (Fears et al., 2020), detection of SARS-CoV-2 RNA in wastewater and wastewater aerosols dose not necessarily indicate viability and infectivity of the viral particles (Foladori et al., 2020). In other words, the lack of data on the environmental stability of SARS-CoV-2 and its viability and fate in wastewater is a major uncertainty affects the potential risk of exposure to wastewater aerosols. Most researches are based on the molecular detection of SARS-CoV-2 which do not indicate the presence of infectious virus (Corpuz et al., 2020). Therefore, more researches are needed to perform molecular detection of SARS-CoV-2 along with cell culture isolation to validate infectivity of the virus and role of wastewater in transmission of COVID-19.
Furthermore, our bioaerosol information for driving water-to-air partitioning coefficient (PCwa) of viral particles is very limited. This highlights the importance of further research to develop more accurate data for estimating viral emission from wastewater to air.
Further improvements in information about the fecal shedding rate and duration of SARS-CoV-2 in infected patients are also necessary to reduce uncertainties associated with the risk outcome.
3.6. Risk management strategies and recommendations
As the COVID-19 pandemic continues, preventive measures must be taken everywhere there is a potential risk for transmission of the viral particles. To reduce the potential of COVID-19 infection incidence among WWTP workers, processes which produce bioaerosols should be covered, and workers should be encouraged to use protective wears. As outlined by WHO, workers should wear appropriate personal protective equipment (PPE), which includes protective outerwear, gloves, goggles or a face shield and a mask. It is recommended that wastewater workers wear N95 respirator which is very efficient for filtration of airborne particles such as bioaerosols. They should perform hand hygiene frequently; and they should avoid touching eyes, nose, and mouth with unwashed hands (WHO, 2020b). On the other hand, a reduction in working hours and consequently exposure time decreases the infection risk of SARS-CoV-2 for WWTPs workers.
4. Conclusion
Our results showed the detection of SARS-CoV-2 in wastewater samples as a result of COVID-19 outbreak. Detection of SARS-CoV-2 in air samples of WWTP demonstrated that wastewater aerosols may contribute to the transmission of COVID-19. QMRA analysis showed a relatively high risk of SARS-CoV-2 infection for wastewater workers through exposure to bioaerosols from WWTP. The risk assessment model developed in this study can be a useful tool for health services to predict the likely risk of SARS-CoV-2 from aquatic environments. However, the finding of this study provides the first data of risk analysis of SARS-CoV-2 associated with the exposure to bioaerosols and its future development could be very useful in risk management of SARS-CoV-2. In other words, the ongoing COVID-19 outbreak and its potential transmission through environments underscore the need to obtain more reliable information on the survival and fate of SARS-CoV-2 in wastewater. Epidemiological evidence is also required to validate the COVID-19 transmission through wastewater aerosols.
Author statement
Sahar Gholipour: Methodology, Investigation; Farzaneh Mohammadi: Software, Formal analysis; Mahnaz nikaeen: Conceptualization, Supervision, Writing – review & editing Zahra shamsizadeh: Inestigation; Atefeh Khazeni: Resources. Zohreh Sahbaei: Resources; Seyed Mohammad Mousavi: Resources; Mojtaba Ghobadian: Resources; Hossein Mirhendi: Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research was supported by the Vice Chancellery for Research at the Isfahan University of Medical Sciences (Grant No. 198237). The authors would like to thank managers of WWTPs for kindly providing wastewater samples.
Handling Editor: Dr. R Ebinghaus
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APHA . American Public Health Association; 2012. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- Barker S.F. Risk of norovirus gastroenteritis from consumption of vegetables irrigated with highly treated municipal wastewater-evaluation of methods to estimate sewage quality. Risk Anal. 2014;34:803–817. doi: 10.1111/risa.12138. [DOI] [PubMed] [Google Scholar]
- Bauer H., Fuerhacker M., Zibuschka F., Schmid H., Puxbaum H. Bacteria and fungi in aerosols generated by two different types of wastewater treatment plants. Water Res. 2002;36:3965–3970. doi: 10.1016/s0043-1354(02)00121-5. [DOI] [PubMed] [Google Scholar]
- Carducci A., Donzelli G., Cioni L., Federigi I., Lombardi R., Verani M. Quantitative microbial risk assessment for workers exposed to bioaerosol in wastewater treatment plants aimed at the choice and setup of safety measures. Int. J. Environ. Res. Publ. Health. 2018;15 doi: 10.3390/ijerph15071490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S., Perkins S.D., Shaw M., Nichols T.L. Evaluation of exposure of brevundimonas diminuta and Pseudomonas aeruginosa during showering. J. Aerosol Sci. 2017;114:77–93. doi: 10.1016/j.jaerosci.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpuz M.V.A., Buonerba A., Vigliotta G., Zarra T., Ballesteros F., Campiglia P., Belgiorno V., Korshin G., Naddeo V. Viruses in wastewater: occurrence, abundance and detection methods. Sci. Total Environ. 2020;745:140910. doi: 10.1016/j.scitotenv.2020.140910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Baz S., Imziln B. Can aerosols and wastewater be considered as potential transmissional sources of COVID-19 to humans? Eur. J. Environ. Public Heal. 2020;4 [Google Scholar]
- Farhadkhani M., Nikaeen M., Yadegarfar G., Hatamzadeh M., Pourmohammadbagher H., Sahbaei Z., Rahmani H.R. Effects of irrigation with secondary treated wastewater on physicochemical and microbial properties of soil and produce safety in a semi-arid area. Water Res. 2018;144:356–364. doi: 10.1016/j.watres.2018.07.047. [DOI] [PubMed] [Google Scholar]
- Fears A.C., Klimstra W.B., Duprex P., Hartman A., Weaver S.C., Plante K.S., Mirchandani D., Plante J.A., Aguilar P.V., Fernández D., Nalca A., Totura A., Dyer D., Kearney B., Lackemeyer M., Bohannon J.K., Johnson R., Garry R.F., Reed D.S., Roy C.J. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg. Infect. Dis. 2020;26:2168–2171. doi: 10.3201/eid2609.201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;743:140444. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A. COVID-19: mitigating transmission via wastewater plumbing systems. Lancet Glob. Heal. 2020;8:e643. doi: 10.1016/S2214-109X(20)30112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwenzi W. Leaving no stone unturned in light of the COVID-19 faecal-oral hypothesis? A water, sanitation and hygiene (WASH) perspective targeting low-income countries. Sci. Total Environ. 2021;753:141751. doi: 10.1016/j.scitotenv.2020.141751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K.A., Hamilton M.T., Johnson W., Jjemba P., Bukhari Z., Lechevallier M., Haas C.N. Health risks from exposure to Legionella in reclaimed water aerosols : toilet fl ushing , spray irrigation , and cooling towers. Water Res. 2018;134:261–279. doi: 10.1016/j.watres.2017.12.022. [DOI] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-I., Kim S.-G., Kim S.-M., Kim E.-H., Park S.-J., Yu K.-M., Chang J.-H., Kim E.J., Lee S., Casel M.A.B., Um J., Song M.-S., Jeong H.W., Lai V.D., Kim Y., Chin B.S., Park J.-S., Chung K.-H., Foo S.-S., Poo H., Mo I.-P., Lee O.-J., Webby R.J., Jung J.U., Choi Y.K. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27:704–709. doi: 10.1016/j.chom.2020.03.023. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu A., McBride G., Wuertz S. Adenovirus-associated health risks for recreational activities in a multi-use coastal watershed based on site-specific quantitative microbial risk assessment. Water Res. 2013;47:6309–6325. doi: 10.1016/j.watres.2013.08.002. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Xu S.-B., Lin Y.-X., Tian D., Zhu Z.-Q., Dai F.-H., Wu F., Song Z.-G., Huang W., Chen J., Hu B.-J., Wang S., Mao E.-Q., Zhu L., Zhang W.-H., Lu H.-Z. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J. (Engl). 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020;5:533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mara D.D., Sleigh P.A., Blumenthal U.J., Carr R.M. Health risks in wastewater irrigation : comparing estimates from quantitative microbial risk analyses and epidemiological studies. J. Water Health. 2007:39–50. doi: 10.2166/wh.2006.055. [DOI] [PubMed] [Google Scholar]
- Masclaux F.G., Hotz P., Gashi D., Savova-Bianchi D., Oppliger A. Assessment of airborne virus contamination in wastewater treatment plants. Environ. Res. 2014;133:260–265. doi: 10.1016/j.envres.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Matsubara K., Katayama H. Development of a portable detection method for enteric viruses from ambient air and its application to a wastewater treatment plant. Pathogens. 2019;8 doi: 10.3390/pathogens8030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcbride G.B., Stott R., Miller W., Bambic D. Discharge-based QMRA for estimation of public health risks from exposure to stormwater- borne pathogens in recreational waters in the United States. Water Res. 2013;47:5282–5297. doi: 10.1016/j.watres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- McKinney K.R., Gong Y.Y., Lewis T.G. Environmental transmission of SARS at Amoy Gardens. J. Environ. Health. 2006;68:22–26. [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Moazeni M., Nikaeen M., Hadi M., Moghim S., Mouhebat L., Hatamzadeh M., Hassanzadeh A. Estimation of health risks caused by exposure to enteroviruses from agricultural application of wastewater effluents. Water Res. 2017;125:104–113. doi: 10.1016/j.watres.2017.08.028. [DOI] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Reports Med. 2020;1:100098. doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper I.L., Gerba C.P. Risk of infection from Legionella associated with spray irrigation of reclaimed water. Water Res. 2018;139:101–107. doi: 10.1016/j.watres.2018.04.001. [DOI] [PubMed] [Google Scholar]
- Pyankov O.V., Bodnev S.A., Pyankova O.G., Agranovski I.E. Survival of aerosolized coronavirus in the ambient air. J. Aerosol Sci. 2018;115:158–163. doi: 10.1016/j.jaerosci.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G., Li X., Hu L., Jiang G. An imperative need for research on the role of environmental factors in transmission of novel coronavirus (COVID-19) Environ. Sci. Technol. 2020;54:3730–3732. doi: 10.1021/acs.est.0c01102. [DOI] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose C., Parker A., Jefferson B., Cartmell E. The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 2015;45:1827–1879. doi: 10.1080/10643389.2014.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen M.E., Ashbolt N.J. An in-premise model for Legionella exposure during showering events. Water Res. 2011;45:5826–5836. doi: 10.1016/j.watres.2011.08.031. [DOI] [PubMed] [Google Scholar]
- USEPA . Final Report; Washington, DC: 2011. Exposure Factors Handbook 2011 Edition. [Google Scholar]
- Watanabe T., Bartrand T.A., Weir M.H., Omura T., Haas C.N. Development of a dose-response model for SARS coronavirus. Risk Anal. 2010;30:1129–1138. doi: 10.1111/j.1539-6924.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV) (Geneva, Switzerland) [Google Scholar]
- WHO . 2020. Surface Sampling of Coronavirus Disease (COVID-19): A Practical “How to” Protocol for Health Care and Public Health Professionals; pp. 1–26. COVID-19 WHO Surveilllance, case Investig. Epidemiol. Protoc. [Google Scholar]
- Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Moniz K., Erickson T., Chai P., Thompson J., Alm E. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. Msystems. 2020;5 doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. 2020. Evaluation of Lockdown Effect on SARS-CoV-2 Dynamics through Viral Genome Quantification in Waste Water. Greater Paris, France, 5 March to 23 April 2020. Eurosurveillance 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Cai C., Dai X. The potential exposure and transmission risk of SARS-CoV-2 through sludge treatment and disposal. Resour. Conserv. Recycl. 2020;162:105043. doi: 10.1016/j.resconrec.2020.105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaneti R.N., Girardi V., Spilki F.R., Mena K., Westphalen A.P.C., da Costa Colares E.R., Pozzebon A.G., Etchepare R.G. Quantitative microbial risk assessment of SARS-CoV-2 for workers in wastewater treatment plants. Sci. Total Environ. 2021;754:142163. doi: 10.1016/j.scitotenv.2020.142163. [DOI] [PMC free article] [PubMed] [Google Scholar]