Abstract
The increased prevalence of obesity, diabetes, and cardiovascular risk factors in people hospitalized with severe COVID-19 illness has engendered considerable interest in the metabolic aspects of SARS-CoV-2-induced pathophysiology. Here, I update concepts informing how metabolic disorders and their co-morbidities modify the susceptibility to, natural history, and potential treatment of SARS-CoV-2 infection, with a focus on human biology. New data informing genetic predisposition, epidemiology, immune responses, disease severity, and therapy of COVID-19 in people with obesity and diabetes are highlighted. The emerging relationships of metabolic disorders to viral-induced immune responses and viral persistence, and the putative importance of adipose and islet ACE2 expression, glycemic control, cholesterol metabolism, and glucose- and lipid-lowering drugs is reviewed, with attention to controversies and unresolved questions. Rapid progress in these areas informs our growing understanding of SARS-CoV-2 infection in people with diabetes and obesity, while refining the therapeutic strategies and research priorities in this vulnerable population.
Keywords: vaccination, immunity, glucose, adipose tissue, islet, virus
COVID-19 infection produces excess mortality in people with obesity or diabetes. Here we review the epidemiology, susceptibility to infection, pathophysiology, immunology, complications, potential therapeutic options, and response to vaccinations in people with metabolic disorders and SARS-CoV-2 infection.
Introduction
The world is fully engaged in all aspects of the COVID-19 pandemic, which has disrupted the health and well-being of people and nations on a global scale. The striking susceptibility of individuals with cardiovascular disease (CVD), type 2 diabetes (T2D), and obesity to severe cases of COVID-19, evident by increased rates of hospitalization and mortality, has focused the attention of the endocrine and metabolism community on the pandemic, both in the laboratory and in the clinic.
The increased prevalence of diabetes and obesity and greater rates of adverse outcomes in hospitalized subjects with COVID-19 raises important scientific questions with immediate clinical implications. These range from the extent, if any, of disproportionate immune dysregulation; the relative importance of mechanisms predisposing to enhanced disease severity; duration of viral shedding; the response to vaccines; the importance of optimizing metabolic control; and the safety, and potential benefits of commonly used medications, in people with T2D and obesity. Moreover, a flurry of reports has raised multiple competing hypotheses surrounding the pathophysiology of SARS-CoV-2 infection in people with diabetes and obesity, encompassing the gastrointestinal tract, liver, islets, and adipose tissue, raising uncertainty surrounding relevant biology and validated mechanisms.
Understandably, the majority of reports in this rapidly evolving field represent retrospective case series, often reporting associations, without randomized controls. Many of these reports have multiple scientific deficiencies. These include failing to fully account appropriately for multiple confounders; missing data; inappropriate statistical comparisons; reporting dynamic data often at a single, sometimes random time point; and selection of arbitrary endpoints for post hoc emphasis and analysis (Selvin and Juraschek, 2020). Moreover, many observational studies do not prospectively define primary and secondary outcomes, and sometimes fail to comprehensively report the pre-defined outcomes, focusing instead on other outcomes deemed to be of interest following retrospective analysis. Our understanding of how SARS-CoV-2 infection modifies the pathophysiology and clinical outcomes of people with metabolic disorders has advanced substantially during the first year of the COVID-19 pandemic. Nevertheless, the existing literature is replete with contrasting interpretations of similar data, frequently precluding definitive conclusions.
Here, I discuss key perspectives of general metabolic and translational interest, focusing on scientific data made available since the spring of 2020, with an emphasis, wherever possible, on research, relevant to people with obesity and diabetes. The available data are interpreted through a lens focusing on gaps and limitations, often precluding definitive conclusions. Readers are referred to earlier reviews that predominantly address clinical and therapeutic guidance (Bornstein et al., 2020; Drucker, 2020; Lim et al., 2021).
The epidemiology of COVID-19 in people with diabetes and obesity
People with diabetes or obesity do not exhibit increased susceptibility to SARS-CoV-2 infection. However, COVID-19 infection results in increased rates of hospitalization and greater severity of illness in people with type 1 diabetes (T1D), T2D, or obesity. A few illustrative reports highlight the extent of these findings, with relative proportions often differing across centers. On November 20, 2020, the International Severe Acute Respiratory and Emerging Infection Consortium (https://isaric.tghn.org/), representing dozens of countries and multiple continents, reported 95,966 clinical COVID-19 cases (93.4% with laboratory-confirmed SARS-CoV-2 infection) wherein prevalence of diabetes and obesity was 17.4% and 13.4%, respectively (Hall et al., 2020). In contrast, rates of diabetes and obesity reported in 5,700 COVID-19 cases in 12 New York City (NYC) hospitals from March 1 to April 4, 2020, were much higher, 33.8 and 41.7%, respectively (Richardson et al., 2020). Not surprisingly, consistent with lower population BMIs in Asia, the mean BMI in 7,337 subjects with COVID-19 in China was 24.7 and 23.4 in people with and without T2D, respectively (Zhu et al., 2020), further highlighting population differences in overweight and obesity.
Type 1 diabetes
People with COVID-19 and T1D do not invariably exhibit an increased risk for hospitalization or more severe illness (Vangoitsenhoven et al., 2020); however, older subjects with T1D (age > 65–75) exhibit higher rates of COVID-19-related mortality, as described in the Coronavirus-SARS-CoV-2 and Diabetes Outcomes (CORONADO) study in France (Wargny et al., 2020). Barron and colleagues reported that one-third of all COVID-19-related mortality (23,698 deaths) in England from March 1 to May 11, 2020, occurred in people with diabetes, 31.4% and 1.5% for T2D and T1D, respectively (Barron et al., 2020). After adjustments for age, sex, and geographical region, the odds ratios for adjusted in-hospital COVID-19-related death rates were 3.51 and 2.03 for T1D and T2D, respectively (Barron et al., 2020). Notably, no deaths were reported in the T1D cohort < 50 years of age and the association of diabetes (both T1D and T2D) with excess mortality was independent of age, sex, ethnicity, socioeconomic deprivation, and cardiovascular co-morbidities (Barron et al., 2020). Nevertheless, in the related National Health Service cohort of death certificate audits reported from February 16 to May 11, 2020, deaths were more common in people with COVID-19 and either T1D or T2D, older age, male sex, and a history of cardiovascular and renal disease (Holman et al., 2020). The risk of COVID-19-related mortality was increased in individuals with an elevated level of percent glycosylated hemoglobin (%HbA1c); only the greatest quartiles of %HbA1c elevation were associated with mortality in T1D, whereas the risk of mortality in people with T2D increased with progressive gradations in %HbA1c elevations, starting with a %HbA1c of 7.6 or greater (Holman et al., 2020). Both very low (<20 kg/m2) and high (>40 kg/m2) BMI was associated with increased mortality in people with T1D and T2D.
T2D, severity of illness, and associated complications
Increased rates of all-cause mortality for people with T2D and COVID-19 were reported in a retrospective analysis of patients admitted to a high-dependency unit (HDU) or intensive care unit (ICU) in England from March 1 to July 27, 2020, after correction for multiple confounding co-morbidities (Dennis et al., 2021). The authors scrutinized 19,256 admissions, 13,809 in an HDU (mean age 70 years) and 5,447 in the ICU (mean age 58), with data obtained from the COVID-19 Hospitalisation in England Surveillance System. People with T2D represented 18.3% of the admissions and obesity was present in 45% versus 31% of the T2D versus the non-T2D cohort. The unadjusted 30-day rates of mortality were 34.7% versus 25.5% for T2D versus no T2D, respectively. In contrast, after propensity matching, no increased risk of mortality was noted for people with T1D (n = 135). The relative risk of mortality for people with T2D decreased progressively with increasing age, highlighting the importance of T2D as a risk factor for severe COVID-19 illness, even in younger people 18–49 years old (Dennis et al., 2021). These findings were confirmed in an analysis of risk factors associated with mortality in hospitalized young adults ages 18–35 in NYC (Altonen et al., 2020). A diagnosis of T2D (14.5% of 5,307 people with COVID-19 in Korea) was similarly associated with greater severity of clinical illness and death in both male and female individuals, even after correction for multiple co-morbidities (Moon et al., 2020).
Obesity and COVID-19 outcomes
Obesity with or without T2D has been associated with higher rates of hospitalization and an increased severity of illness, in multiple retrospective observational analyses. Investigators in the French CORONADO trial observed that the risk of mechanical ventilation or death (the primary composite outcome) by day 7 of hospital admission in 1,965 people with SARS-CoV-2 infection and T2D rose progressively with increasing BMI, with odds ratios of 1.65, 1.93, and 1.98, for overweight, class I, and class II/III obesity, respectively (Smati et al., 2021). Nevertheless, increasing BMI alone was not a risk factor for mortality in the CORONADO study, and the relationship between BMI and the primary outcome was not evident in people >75 years of age (Smati et al., 2021).
Obesity (72.5%) was the most common co-morbidity identified in 6,760 health care personnel hospitalized in 14 US states from March 1 to May 31, 2020, reported by the COVID-19-Associated Hospitalization Surveillance Network (Kambhampati et al., 2020). Analysis of COVID-19-related mortality in participants from the United Kingdom (UK) BioBank linked higher BMI, waist circumference, waist-to-hip ratio, and waist to-height ratio in men and women with overweight and obesity with an increased risk of COVID-19-related mortality (Peters et al., 2021). Nevertheless, these analyses were not corrected for multiple confounding cardiometabolic risk factors in the same population. Hamer et al. examined 640 subjects with COVID-19 among 334,329 individuals in the UK from March 16 to April 26, 2020. The likelihood of hospitalization was greater with increasing BMI, with odds ratios of 1.39, 1.7, and 3.38 for BMI ranges of 25–29, 30–34.9, and >35, respectively (Hamer et al., 2020). The magnitude of these relationships was attenuated, but remained present, after correction for high-density lipoprotein (HDL) and %HbA1c. Similar relationships linking a progressive increase in BMI, obesity, and adverse outcomes were reported among 7,606 people enrolled in the American Heart Association’s COVID-19 Cardiovascular Disease Registry studied up to July 22, 2020. Notably, the linkage between class I–III obesity and increased mechanical ventilation and mortality was most pronounced in younger individuals <50 years of age (Hendren et al., 2021). These findings were corroborated by analysis of a large cohort of hospitalized individuals in Spain, where the association of increased BMI and COVID-19 severity in 10,862 hospitalizations was strongest for people <59 years old (Recalde et al., 2020).
Among critically ill subjects with acute hypoxemic respiratory failure admitted to two NY hospitals between March 1 and April 2, 2020, 46% were people with obesity, including 39 out of 55 individuals less than 50 years of age (Cummings et al., 2020). Similar findings were reported in 5,795 patients with COVID-19, ages 18–79, hospitalized in Paris from February 1 to April 20, 2020, with a mean BMI of 29.3 and 27.2 for women and men, respectively. The odds ratios for relative risk of mortality at 30 days for SARS-CoV-2-infected subjects with a BMI of 25–29.9, 30–35, 35–40, and >40 were 1.41, 1.89, 2.79, and 2.55, respectively, even after correction for age, sex, and co-morbidities (Czernichow et al., 2020). Notably, the relative risk of mortality for people with obesity increased progressively in older individuals. Increasing BMI was also linked to illness severity in 10,861 individuals with COVID-19 admitted to the Northwell Health hospitals in the NYC area from March 1 to April 27, 2020. A multivariate analysis of 20,133 hospitalized COVID-19 patients in England, Scotland, and Wales demonstrated that obesity, but not diabetes, was associated with excess in hospital mortality (hazard ratio 1.33) (Docherty et al., 2020).
In contrast, analysis of 10,131 US veterans testing positive for SARS-CoV-2 in the Veterans Affairs national health care system did not reveal difference in mortality, when comparing outcomes for individuals across a range of BMIs. Mortality was not different in those with a BMI > 35 (n = 1,968) versus people with a BMI from 18.5 to 24.9 (n = 1,889), after adjustment for age, socioeconomic characteristics, and co-morbidities (Ioannou et al., 2020). Within the same population, diabetes was associated with an increased risk of hospitalization and mechanical ventilation.
Mendelian randomization studies have analyzed potential causal associations linking 17 cardiometabolic risk factors, including BMI, with susceptibility to severe SARS-CoV-2 infection (Leong et al., 2020). Although genetic predisposition to an increased BMI was associated with increased susceptibility to infection and hospitalization, this relationship was mitigated by adjustment of the genetic risk for co-existing T2D. Whether the genetic predisposition to obesity might be associated with adverse clinical outcomes, including mortality, was not determined.
Summary and areas of uncertainty
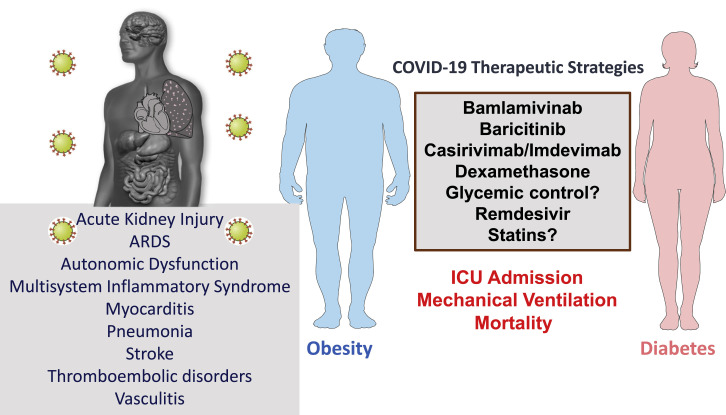
Substantial evidence supports a greater risk of more severe COVID-19 outcomes in people with T2D and obesity, two frequently co-existing conditions. Some of the risk attributed to obesity likely reflects coincident T2D and impaired cardiopulmonary fitness. The relative risk also appears greater in individuals with T2D, suboptimal health care, and in some, but not all, studies, socioeconomic deprivation (Ken-Dror et al., 2020; Sosa-Rubí et al., 2021), risk factors less frequently reported in most reports. The pathophysiology of T2D underlying enhanced SARS-CoV-2 pathogenicity remains uncertain, but may be associated with increased rates of coagulopathy underlying enhanced mortality, as described in severely ill, hospitalized people with COVID-19 in Wuhan (Chen et al., 2020a). Many older individuals with T2D and obesity also have pre-existing CVD. Shared pathophysiology underlying greater COVID-19 susceptibility in people with T2D or obesity may include heightened basal inflammatory tone, defective adaptive immune responses, endothelial dysfunction, and a greater propensity for development of coagulation-related complications (Figure 1 ). Collectively, the available data suggest that the relationship between increasing BMI, severity of SARS-CoV-2 infection, and outcomes is not always linear; more relevant in younger people (Burn et al., 2020), including children (Duarte-Salles et al., 2020; Fernandes et al., 2020; Tsankov et al., 2020); and frequently complicated in adults by co-existing cardiometabolic risk factors. A diagnosis of T2D is also associated with adverse COVID-19 outcomes in younger adults, even those between 20 and 39 years of age (Woolcott and Castilla-Bancayán, 2020).
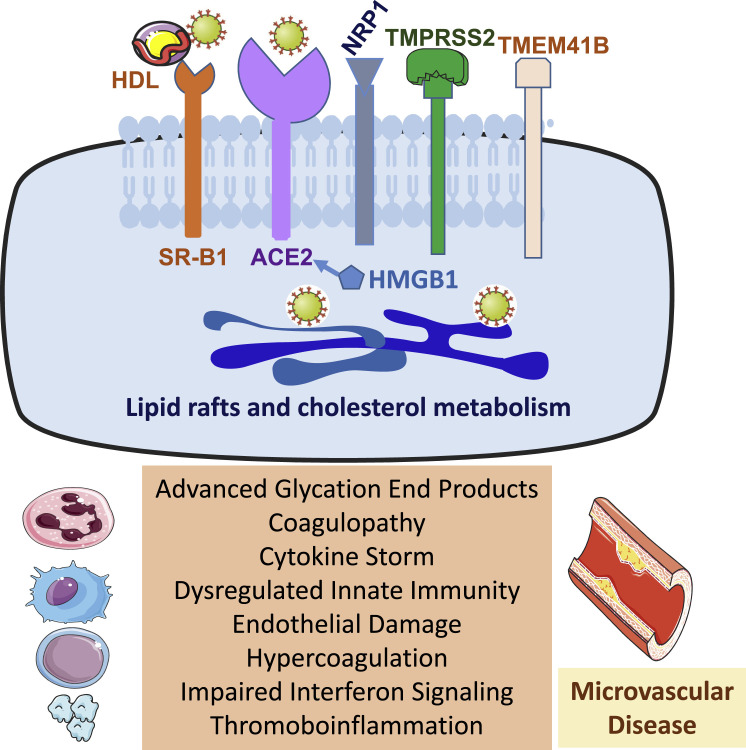
Figure 1.
Factors modifying viral entry, replication, and clinical secerity
Depiction of cell surface receptors and co-factors facilitating SARS-CoV-2 entry, the importance of cholesterol metabolism (top panel), and cellular, immune, and pathophysiological abnormalities predisposing people with obesity and diabetes to more severe COVID-19 outcomes (bottom panel). ACE2, angiotensin-converting enzyme 2; HMGB1, high-mobility group box 1 protein; NPR1, neuropilin 1; SR-B1, scavenger receptor class B type 1; TMPRSS2, transmembrane serine protease 2; TMEM41B, transmembrane protein 41B.
While it seems reasonable to support exercise and healthy eating behaviors to maximize immune and nutritional health and fitness in people with T2D and obesity during the pandemic, whether such regimens influence COVID-19 severity is uncertain. Moreover, restrictions on movement and associated stress have made it difficult for people with obesity to pursue a healthy lifestyle (Robinson et al., 2021). Importantly, in some large studies (McPadden et al., 2020), the diagnoses of T2D or obesity are not associated with adverse COVID-19 outcomes in hospitalized people, highlighting the importance of understanding the prevalent pathophysiology central to development of severe SARS-CoV-2 infection.
Susceptibility of people with diabetes and obesity to infection
T2D and obesity are metabolic disorders characterized by immune dysfunction, including increased accumulation of resident immune cells in multiple tissues, which in turn enables a heightened state of basal inflammation via enhanced cytokine and chemokine production, leading to impairment of β cell function and exacerbation of insulin resistance (Donath et al., 2019). These immune cell populations encompass macrophages, neutrophils, eosinophils, T cells, B cells, and dendritic cells, collectively impairing the control of insulin action and energy homeostasis (Figure 1). Moreover, the extent of systemic inflammation is further modified by adipokines, hepatokines, myokines, lipokines, branched-chain amino acids, and microbial metabolites, which may circulate at abnormal levels in some people with T2D or obesity. Collectively, these acquired abnormalities broadly impair cellular immune function, likely contributing to enhanced activation of the NLRP3 inflammasome, and a greater susceptibility to infection in vulnerable individuals (Rodrigues et al., 2021). There is considerable interest in whether the expression of viral entry factors essential for SARS-CoV-2 infectivity is enhanced in one or more tissues in people with T2D or obesity (Figure 1); however, the available data are inconclusive (Drucker, 2020). Considerable data support androgen upregulation of TMPRSS2 expression, providing a mechanistic hypothesis for greater illness severity in men, supporting investigation of agents that downregulate or block androgen-mediated TMPRSS2 activity.
Prior to the COVID-19 pandemic, diabetes was associated with increased risk for multiple infections, and infection-related morbidity and mortality often proportional to the degree of glycemic control (Critchley et al., 2018). In a primary care practice audit in the UK, 6% of infection-related hospitalizations and 12% of infection-related deaths were associated with a diagnosis of diabetes (Carey et al., 2018). The increased rate of complicated tuberculosis infections in people with T2D may be explained in part by defective T cell responses, including elevated frequencies of Th1 and Th17 cells, reduced numbers of regulatory T cells, and aberrant cytokine responses (Kumar et al., 2013).
Concerns surrounding the potential for people with obesity to exhibit a defective immune response to SARS-CoV-2 stem in part from experience with the influenza virus (Ledford, 2020). Obesity has been recognized as a risk factor for development of more severe clinical illness, with some, but not all, strains of influenza virus, most commonly influenza A, and suboptimal humoral and cellular immune responses to vaccination (for multiple different viruses) having been observed in people with increasing BMI (Painter et al., 2015; Sheridan et al., 2012). The response to influenza vaccination has been examined in a prospective study of 1,022 people (28% overweight, 44% with obesity) in North Carolina who received the trivalent inactivated influenza vaccine (IIV3) from 2013 to 2015. Obesity (but not diabetes) was associated with a 2-fold risk of developing influenza or an influenza-related illness. Despite greater clinical reports of illness, no differences were observed after vaccination in antibody seroconversion rates or seroprotective titers in the cohorts with normal weight versus obesity (Neidich et al., 2017). The differences in these studies may also reflect the timing of sampling, as some studies show a normal initial antibody titer response, but subsequently diminished antibody response in people with obesity when studied 12 months after vaccination (Sheridan et al., 2012). The enhanced susceptibility to viral infection in people with T2D and obesity, and defective immune responses in the context of influenza vaccination, has been recently discussed (Drucker, 2020). Defective immune responses to hepatitis B vaccines in people with obesity and a BMI > 35 have been reported in a small study of 68 individuals with NAFLD, with antibody titers measured after the third vaccine dose (Joshi et al., 2021). The number of individuals demonstrating HBsAg-stimulated proliferation of CD4+ T cells was also lower in people with a BMI > 35, as was the relative rate of T cell proliferation.
Immune responses in people with obesity, diabetes, and SARS-CoV-2 infection
Circulating leukocyte populations were characterized in 45 hospitalized subjects with SARS-CoV-2 infection, with (n = 30) and without (n = 15) T2D (Alzaid et al., 2020). BMI was increased (mean 28), %HbA1c was elevated (mean 7.8), and hypertension was more common in subjects with T2D. Lymphopenia was common in both groups; however, subjects with T2D had a 1.3-fold reduction in numbers of CD14+ monocytes, reflecting a 1.4-fold decrease in frequency of classical monocyte (CD14Hi, CD16−) populations. Moreover, monocyte CD14 expression was reduced and monocytes were larger in subjects with COVID-19 and T2D, reflecting the increased cell size of the CD14+ population. Furthermore, the expression of pro-inflammatory cytokines as well as IRF5 and IFNB1 was higher in peripheral blood mononuclear cells from people with T2D (Alzaid et al., 2020). Consistent with these findings, monocyte size was also greater in the T2D population requiring ICU admission. Whether the signature of relative monocytopenia, reduction of CD8+ cytotoxic lymphocytes, altered monocyte size, and an enhanced hyper-inflammatory type 1 interferon response in acutely ill subjects with T2D reflects the extent of dysglycemia and a greater severity of illness or other abnormalities intrinsic to T2D remains uncertain.
Hyperglycemia enhances SARS-CoV-2 replication in monocytes, increases surface ACE2 expression, and augments the monocyte pro-inflammatory cytokine response ex vivo (Codo et al., 2020). Additionally, monocytes isolated from individuals with T2D or obesity exhibited greater susceptibility to SARS-CoV-2 infection ex vivo (Codo et al., 2020). Moreover, the importance of glucose metabolism for monocyte infection was demonstrated using the glycolysis inhibitor 2-deoxyglucose, which blocked viral replication and attenuated induction of ACE2 and cytokine expression, findings mimicked by chemical inhibition of glycolysis. SARS-CoV-2 infection and induction of glycolysis-related genes in monocytes were attributed to upregulation of HIF-1α. Inhibition of HIF-1α blocked SARS-CoV-2 replication, attenuated induction of the glycolysis gene expression program, and diminished induction of cytokine expression (Codo et al., 2020). Intriguingly, conditioned medium from SARS-CoV-2-infected monocytes impaired T cell proliferation and decreased the viability of a human pulmonary epithelial cell line, extending concepts of how hyperglycemia may exacerbate the severity of SARS-CoV-2 infection (Codo et al., 2020).
Interpretation of humoral immune responses in people with COVID-19 is challenged by the lack of assay standardization, and substantial inter-assay differences in antigen recognition, sensitivity, and specificity. SARS-CoV-2 exposure was assessed from May 2 to May 9, 2020, in 10 cities in Brazil in 2,921 asymptomatic individuals analyzed for IgM responses. Of the 123 individuals with IgM positivity, 69 self-reported a diagnosis of hypertension and 23 a diagnosis of diabetes (Borges et al., 2020). A prospective seroprevalence survey in the US analyzing the presence of antibodies against multiple SARS-CoV-2 antigens in 4,469 people (24.1% with obesity) with a 7.2% rate of seropositivity did not detect any evidence for increased rates of SARS-CoV-2 infection in people with overweight or obesity. Furthermore, humoral immunity and helper T cell activity were not different in seropositive individuals with obesity (Nilles et al., 2020). Analysis of anti-IgG responses directed against the S1 domain in 2,112 persons with confirmed symptomatic SARS-CoV-2 infection at ~63 days after symptom onset revealed that people with overweight or obesity were less likely to be antibody-negative (Petersen et al., 2020). These findings were corroborated by analysis of non-hospitalized individuals with symptoms of COVID-19 and relatively greater IgG responses against the spike protein in people with obesity (Shields et al., 2020).
Antibody responses were examined in a consecutive cohort of 250 SARS-CoV-2-infected individuals in the context of a convalescent plasma screening program, with a median 61 days between SARS-CoV-2 positivity and antibody analysis (Boonyaratanakornkit et al., 2020). Diabetes was among the clinical correlates associated with higher (>1:80) neutralizing antibody titers (odds ratio 5.36 in a univariate analysis); however, the odds ratio was reduced (2.75) and no longer significant after multivariate analysis. Huang et al. examined IgM and IgG immune responses over time in 366 SARS-CoV-2-positive (as determined by PCR) individuals hospitalized in Wuhan, China. IgM antibody titers were not meaningfully different in people with (n = 65) or without (n = 301) diabetes (Huang et al., 2020a). In contrast a higher geometric mean reciprocal IgG antibody titer was detected in people with diabetes at the initial time point, ~5 days from symptom onset, whereas levels of IgG were not different in the two groups for the duration of the analysis out to 65 days. A more detailed characterization of the immune response to SARS-CoV-2 infection was provided in studies of hospitalized subjects from February 25 to April 19, 2020, in Milan. In total, 519 individuals with clinical COVID-19 infection were assessed, including 139 with established or newly diagnosed diabetes (Lampasona et al., 2020). Although hyperglycemia was associated with a less favorable outcome, IgG, IgA, and IgM antibody responses to the S1 receptor binding domain, spike protein, or nucleocapsid protein were generally similar in people with or without diabetes when evaluated over several weeks and were not impacted by the degree of glycemic control.
Viral shedding of SARS-CoV-2 in people with diabetes or obesity
Prolonged viral shedding has been described in people with obesity and influenza A H1N1 infections (Maier et al., 2018). Notwithstanding multiple reports of more severe COVID-19 illness in people with diabetes or obesity, limited data inform whether people with T2D or obesity may take longer to clear the virus, as assessed by detection of persistent SARS-CoV-2 positivity in respiratory secretions. Moriconi et al. reported the duration of viral shedding in 100 consecutive patients with COVID-19 (29 with obesity) admitted to the Cisanello Hospital in Pisa, Italy, from March 16 to April 15, 2020 (Moriconi et al., 2020). Individuals with obesity had higher levels of circulating ferritin, C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α) on admission, a prolonged hospital stay, and a longer time, relative to subjects without obesity, to negative testing for SARS-CoV-2 by PCR in nasal/oropharyngeal swabs (19 ± 8 versus 13 ± 7 days, respectively).
Viral shedding was also assessed using nasopharyngeal swabs and lower tracheal aspirates from 48 intubated patients with COVID-19 in Locarno, Switzerland. More prolonged persistence of SARS-CoV-2 (assessed by first negative PCR test) and a higher mortality rate were reported in the 13 individuals with T2D (hazard ratio for time to negativity of 0.23 after multivariate analysis) (Buetti et al., 2020a). Nevertheless, a second study from a subset of the same authors examined SARS-CoV-2 persistence in 267 lower respiratory tract samples from 90 patients (including 27 with T2D) hospitalized with acute respiratory distress syndrome in the ICU. Although viral load correlated with survival, diabetes, age, or cardiovascular co-morbidities were not associated with prolonged viral shedding (Buetti et al., 2020b).
In a cohort of 251 people with COVID-19 evaluated at the Mayo Clinic, no difference in time to cessation of viral shedding (two consecutive negative SARS-CoV-2 PCR tests in nasopharyngeal swabs) was observed in people with T2D (n = 34) despite older age, a greater severity of illness, and higher rates of hospitalization in subjects with T2D (Cano et al., 2021). Dicker and colleagues assessed time to SARS-CoV-2 negativity in nasopharyngeal swabs from 34 subjects recovering from COVID-19. Hospital stay was longer for people with overweight or obesity, and the time to negative PCR testing for the SARS-CoV-2 RNA-dependent RNA polymerase gene was marginally longer in people with an elevated BMI (Dicker et al., 2020).
Summary and areas of uncertainty
The available data do not support the hypothesis of broadly impaired humoral responses to SARS-CoV-2 infection in people with T2D or obesity. More information is needed to clarify the putative importance of defects in cellular immune responses in people with T2D and COVID-19. Insufficient data exist to inform the possibility of differences in viral clearance or shedding in people with obesity or diabetes. Very limited data inform our understanding of whether selective defects in innate immunity amplify the severity of cytokine storm and ARDS in people with T2D or obesity. Emerging data from SARS-CoV-2 vaccine trials reveal that the BNT162/PF-07302048 RNA vaccine was equally protective in people with obesity or T2D (Polack et al., 2020). In contrast, less information is available about initial responses to the ChAdOx1 nCoV-19 vaccine, where <3% of trial participants reported a diagnosis of T2D (Voysey et al., 2021). The mean BMI in the COVID-19 mRNA-1273 trial, which reported an overall vaccine efficacy of 94% (Moderna), was 28, with ~6% of subjects classified as people with severe obesity, and the baseline prevalence of T2D was 9.4%. The vaccine efficacy rate was 91% and 100% for people with severe obesity (BMI > 40) or T2D, respectively (https://www.fda.gov/media/144434/download).
Glucose control and COVID-19 outcomes
Glucose control preceding admission impacts illness severity and mortality in multiple retrospective reports of people with diabetes and COVID-19. The OpenSafely Collaborative describe results from 17,278,392 adults and 10,926 COVID-19-related deaths, where a %HbA1c > 7.5 within the previous 15 months was associated with an increased rate of mortality (Williamson et al., 2020). Zhu and colleagues reported a retrospective analysis of 7,337 people between the ages of 18 and 75, hospitalized with COVID-19 in Hubei province 952 with T2D (Zhu et al., 2020). Circulating biomarkers of inflammation (CRP and procalcitonin) and D-dimer levels were higher, whereas oxygen saturation on admission was lower in the group with T2D. Over a 28-day observation period, acute respiratory distress syndrome, acute kidney injury, septic shock, and disseminated intravascular coagulation were more frequent in the T2D group. Consistent with these findings, the in-hospital death rate was several-fold higher in the T2D cohort and remained elevated (1.49-fold higher) after adjusting for age, sex, and severity of illness (Zhu et al., 2020). Subjects with well-controlled glucose (5.2–7.5 mM, median 6.4 mM, %HbA1c 7.3) exhibited less severe illness, reduced neutrophil counts, and lower levels of CRP, interleukin-6 (IL-6), and procalcitonin, relative to the subgroup with higher (7.6–14.3 mM, median 10.9 mM, %HbA1c 8.1) glucose levels. Strikingly, the hazard ratio for reduction in mortality at day 28 was 0.14 for the well-controlled glucose group, even after propensity-matched adjustments for age, sex, co-morbidities, and the onset and severity of initial COVID-19 illness (Zhu et al., 2020).
Hyperglycemia on hospital admission in severely ill people with COVID-19 is very well recognized and is predictive of a poor outcome, even in subjects without pre-existing diabetes (Cai et al., 2020; Fadini et al., 2020; Huang et al., 2020b; Mamtani et al., 2020a). For example, dysregulated glucose control was associated with poor outcomes in 1,122 hospitalized people with COVID-19 analyzed in the Glytec database from March 1 to April 6, 2020 (Bode et al., 2020). In total, 194 people were identified with pre-existing diabetes defined as %HbA1c > 6.5 and a further 257 people were determined to have uncontrolled hyperglycemia, defined as two or more glucose readings >10 mM. Episodes of hyperglycemia and severe hypoglycemia were more common, duration of hospital stay was longer, and the mortality rate was higher in people with diabetes and poorly controlled glucose, relative to controls (28.8% versus 6.2%, respectively) (Bode et al., 2020). Both hyperglycemia and hypoglycemia during admission were associated with poor outcomes in a subsequent analysis of hospitalized individuals with COVID-19 in the Glytec database from March 1 to May 8, 2020 (Klonoff et al., 2021).
Analysis of the non-linear relationship between fasting blood glucose (FBG) determined within 24 h of admission, and adverse COVID-19 outcomes, was reported in a study of 417 hospitalized subjects (23% with a known previous diagnosis of diabetes) in Kuwait (Alahmad et al., 2020). Relatively greater rates of ICU admission occurred in people with glycemic increments in the 5–10 mM range whereas similar or greater numerical increases of glucose in the 10–20 mM range were not associated with the same fold-increase in risk of ICU admission. These findings revealed a steep slope of the odds ratio for ICU admission for glucose in the 5–10 mM range, which progressively flattened with further increases in fasting glycemia (Alahmad et al., 2020).
Glucose-lowering therapies and COVID-19 outcomes
Understanding whether the use of any specific class of glucose-lowering agents is uniquely beneficial or harmful in people at risk for or with active COVID-19 infection remains challenging in the absence of prospective randomized controlled trials (RCTs). Several glucose-lowering therapies, including metformin, thiazolidinediones, insulin, dipeptidyl peptidase-4 (DPP-4) inhibitors, and glucagon-like peptide-1 receptor (GLP-1R) agonists, exhibit anti-inflammatory actions in people with T2D (Drucker, 2020; Pollack et al., 2016), fostering hypotheses that one or more of these agents might be particularly useful in people with T2D and COVID-19. Analysis of 7- and 28-day COVID-19 outcomes for 1,317 people with diabetes (88.5% T2D) in French hospitals did not reveal any clear association between glucose-lowering agents and the severity of clinical illness, although metformin use prior to admission was lower and insulin use was higher in people who succumbed to their illness (Cariou et al., 2020b). Indeed, the CORONADO investigators observed that long-standing T2D, reflected by older age; presence of microvascular disease; and greater use of insulin and statins were associated with a higher risk of death. Nevertheless, these associations did not remain significant after multivariate adjustment for confounding risk factors. Analysis of COVID-19 outcomes stratified by prior use of glucose-lowering medicines in 1,762 people with T2D enrolled in the Spanish Society of Internal Medicine COVID-19 registry from March 1 to July 19, 2020, did not reveal any association between class of medication and a range of COVID-19 outcomes after analyses using multivariate logistic regression (Pérez-Belmonte et al., 2020).
Crouse and colleagues retrospectively analyzed outcomes using electronic health records at the University of Alabama from February 25 to June 22, 2020, identifying 604 individuals with COVID-19 (40% with T2D). While prior use of insulin did not impact clinical outcomes, antecedent metformin use (n = 76) prior to the diagnosis of COVID-19 was associated with a 50% reduction in mortality, relative to non-metformin-treated subjects, findings not explained by between-group differences in %HbA1c or BMI (Crouse et al., 2020). A putative benefit of antecedent metformin use in the US was also inferred from a retrospective analysis of health care claims data from January 1 to June 7, 2020, identifying 6,256 individuals hospitalized with COVID-19 (40.7% with T2D or obesity) in the United Health Group Clinical Discovery database (Bramante et al., 2021). Over 99% of subjects treated with metformin (for more than 90 days prior to hospital admission for COVID-19) had a diagnosis of T2D. After adjustment for covariates, metformin use was associated with a reduction in mortality in women, but not in men (Bramante et al., 2021).
Cheng and colleagues retrospectively analyzed outcomes in 678 metformin- versus 535 non-metformin-treated Chinese subjects, selected from among 2,563 people hospitalized with pre-existing T2D and COVID-19, after excluding 1,350 people from the analysis, for reasons including the exclusive use of insulin or no record of using glucose-lowering medications (Cheng et al., 2020). Although the analysis excluded individuals with contraindications for taking metformin, metabolic acidosis, including lactic acidosis, was more common in metformin-treated subjects, notably in people with clinically severe COVID-19 illness. Acidosis was more common in subjects with hypoxemia, higher metformin doses (2–3 g daily), and an estimated glomerular filtration rate (eGFR) < 60 (Cheng et al., 2020); however, all-cause mortality determined at day 28 of hospitalization was not different between groups.
Retrospective analyses have also described lower mortality in people with T2D treated with DPP-4 inhibitors, prior to or during hospitalization. Analysis of COVID-19-related health care claims and diagnostic codes in the Korean database of National Health Review and Assessment Services identified 5,080 people with COVID-19, including 832 people with diabetes. Among individuals with T2D prescribed DPP-4 inhibitors (31.6%), the odds ratio for ICU admission or death (after adjustment for age, sex, medication use, and co-morbidities) was 0.362 for users of DPP-4 inhibitors, suggesting that DPP-4-inhibitor use is associated with a better clinical outcome in COVID-19 patients (Rhee et al., 2020).
Solerte et al. reported improved outcomes, reduced need for critical care, and substantially lower mortality in hypoxic, sitagliptin-treated subjects with COVID-19 and T2D admitted with pneumonia, when compared to non-sitagliptin-treated individuals retrospectively matched for age and sex, after 30 days of observation (Solerte et al., 2020). However, the selected control group had higher levels of CRP, and procalcitonin and proinflammatory biomarkers trended higher. Moreover, prior insulin use and chronic kidney disease (CKD) were more common in the control group, whereas the sitagliptin-treated subjects had lower mean blood glucose levels in hospital. Notably, data collection for key parameters and endpoints was incomplete, and the primary and secondary outcomes reported deviated in part from those pre-defined on clinicaltrials.gov (NCT04382794). Similarly, in a retrospective analysis of 385 COVID-19 hospital admissions (90 with T2D), Mirani and colleagues concluded that use of DPP-4 inhibitors conferred a 7-fold lower risk of mortality relative to the 3-fold increased risk seen with insulin use, based on the outcomes of 11 people treated with DPP-4 inhibitors (Mirani et al., 2020). No one in the DPP-4 inhibitor group was treated with insulin; other risk factors and parameters of disease activity were imbalanced across the DPP-4 inhibitor versus insulin groups. In contrast, Dalan et al. carried out a retrospective analysis of 717 COVID-19 hospitalizations in Singapore, including 76 individuals with T2D, the majority (88%) treated with metformin. The cohort of 27 subjects treated with DPP-4 inhibitors exhibited a greater need for supplemental oxygen and had higher rates of ICU admission, associations that persisted after multivariate correction for other risk factors (Dalan et al., 2020). Furthermore, no association between clinical outcomes, including mortality, was observed in a retrospective analysis of hospitalized individuals with COVID-19 and T2D, including 142 people on DPP-4 inhibitors and 1,115 individuals on other glucose-lowering agents (Zhou et al., 2020). The putative benefit of DPP-4 inhibitors in some studies of COVID-19 potentially reflects the anti-inflammatory actions of these agents (Drucker, 2020). However, a study of 600 people with T2D, predominantly with overweight, obesity, and pre-existing CVD, did not detect any reduction of circulating biomarkers of inflammation in sitagliptin- versus placebo-treated subjects (Baggio et al., 2020) and biomarkers of inflammation were not reduced in people with COVID-19 treated with DPP-4 inhibitors (Zhou et al., 2020).
Although less information is available on users of SGLT-2 inhibitors who develop COVID-19, Sainsbury and colleagues compared reports of new COVID-19 infection in people with T2D using SGLT-2 inhibitors (SGLT-2i, n = 9,948) versus DPP-4 inhibitors (n = 14,917) followed from January 30 to July 27, 2020, in a UK primary care database. The incidence rates for a confirmed or clinically suspected new COVID-19 diagnosis were 19.7 versus 24.7/10,000 patient years for SGLT-2i versus DPP-4 inhibitors, respectively, when comparing propensity matched cohorts (7,676 matched subjects in each group) (Sainsbury et al., 2021). Insufficient information was available to infer differences in severity of illness or rates of mortality.
Insulin has been the glucose-lowering drug of choice for decades in the ICU for people with diabetes. In a retrospective analysis, Yu and colleagues concluded that use of insulin in 364 subjects (from a total of 689) studied in a single hospital in Wuhan was associated with enhanced systemic inflammation, organ injury, and adverse outcomes including increased mortality in people with T2D and COVID-19 (Yu et al., 2021). Notably, circulating levels of glucose, %HbA1c, multiple inflammatory biomarkers, D-dimer, and Nt-proBNP were higher and lymphocyte and platelet counts and albumin were lower at baseline in the insulin-treated group. Accordingly, propensity score matching was used to select a matched smaller cohort (n = 183 per group) more suitable for outcome comparisons; however, glucocorticoid use remained substantially greater in the insulin-treated cohort (Yu et al., 2021). Levels of several pro-inflammatory biomarkers increased further during hospitalization despite insulin administration. The precise timing of initiation of insulin therapy and the insulin doses required to achieve control of hyperglycemia were not reported. Moreover, %HbA1c was not measured or missing in the majority of study subjects on admission (Yu et al., 2021). The authors concluded that insulin use may be harmful and should be used with caution in people with severe COVID-19 infection.
Summary and areas of uncertainty
Hyperglycemia in hospitalized people with COVID-19, with or without pre-existing T2D, uniformly associates with greater illness severity, and hence may also be associated with and exacerbated by concomitant use of glucocorticoid therapy. Indeed, hyperglycemia associates with adverse outcomes in critically ill individuals without diabetes or COVID-19 (Mamtani et al., 2020b). Collectively, these observations reflect contributions of increased insulin resistance and deficient β cell functional reserve leading to stress-related hyperglycemia, supporting a hypothesis that early aggressive control of glucose levels in hospital might potentially modify COVID-19 outcomes. Nonetheless, this hypothesis would be difficult, if not ethically impossible, to test in an RCT, as outlined below.
The role of DPP-4 as a MERS-CoV receptor spurred considerable speculation as to whether DPP-4i might interfere with SARS-CoV-2 infectivity (Drucker, 2020). Nevertheless, experimental data do not support and actually refute the contention that human recombinant DPP-4 binds SARS-CoV-2 (Xi et al., 2020). Serum circulating levels of sDPP-4 were reduced by ~50% in 7 individuals hospitalized with COVID-19 (Schlicht et al., 2020); however, given the substantial intra- and inter-individual variability of sDPP-4 in humans, this preliminary observation must be confirmed in larger datasets (Baggio et al., 2020).
Inferring real associations between glucose-lowering drugs and COVID-19 outcomes from retrospective analyses can be perilous, as metformin is generally the first agent prescribed in people with new onset or easy to control T2D, and may be more common in people without long-standing T2D and its complications. In contrast, insulin use is frequently required and often the only therapy available for use in people with poorly controlled T2D, or in older individuals with a longer duration of T2D and established cardiovascular and kidney disease, risk factors known to be associated with suboptimal outcomes in people with severe COVID-19 (Drucker, 2020). Similarly, use of GLP-1 receptor agonists and insulin is more common in people with both T2D and obesity, a cohort at risk for cardiovascular complications and greater severity of COVID outcomes (Smati et al., 2021). Hence, it is not surprising that multiple retrospective studies of COVID-19 outcomes in T2D associate insulin use prior to hospitalization with greater COVID-19-related mortality (Agarwal et al., 2020; Chen et al., 2020b; Riahi et al., 2020).
Prospective RCTs of different glucose-lowering agents in hospitalized patients while achieving glycemic equipoise is likely to be extremely challenging. Commendably, the study of dapagliflozin in hospitalized people with COVID-19 will prospectively assess the safety and potential benefit of this SGLT-2i in the setting of severe SARS-CoV-2 infection. Many glucose-lowering agents have limitations on their use in critically ill people with impaired renal function (Drucker, 2020). Within the hospital, insulin may be the only therapy suitable for patients with the highest levels of blood glucose, often reflecting the presence of severe illness and marked insulin resistance. Hence, it may be difficult to resolve the risks or putative benefits of different glucose-lowering agents using RCTS in the context of COVID-19 infection (Figure 2 ). Moreover, it will not be ethically possible or feasible to randomize people with severe hyperglycemia and COVID-19 in the ICU to a treatment arm that withholds insulin, precluding definitive resolution of the “insulin is harmful” hypothesis.
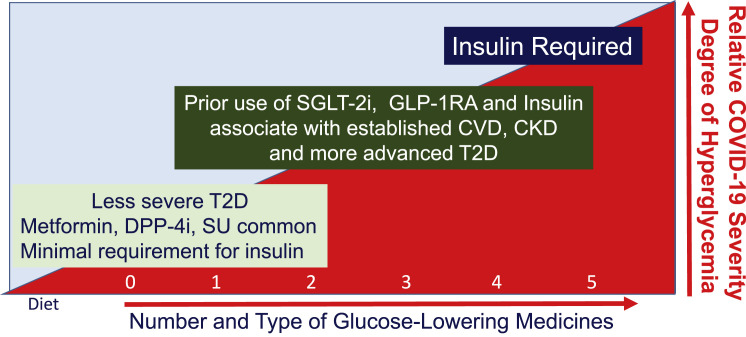
Figure 2.
The relationship between medication use, type 2 diabetes, and severity of COVID-19 infection
The progressive use of glucose-lowering medicines during the evolution of type 2 diabetes and severity of acute illness in people with COVID-19. More advanced stages of T2D necessitate use of multiple agents to achieve glucose control, and choice of agents is skewed toward insulin, SGLT-2 inhibitors, and GLP-1R agonists in people at risk of or with established cardiovascular and renal complications. CKD, chronic kidney disease; CVD, cardiovascular disease; DPP-4i, dipeptidyl peptidase-4 inhibitors; GLP-1-RA, glucagon-like peptide-1 receptor agonists; SGLT-2i, sodium glucose cotransporter-2 inhibitors; SU, sulfonylureas; T2D, type 2 diabetes.
Intracellular and extracellular cholesterol, lipids, and statins
Cholesterol is critical for viral entry and replication, raising interest in cholesterol metabolism as a potential target for modification of viral infectivity. Cholesterol-binding domains within the SARS-CoV-2 spike protein interact with HDL, indirectly facilitating viral entry through the SR-B1 cell surface receptor in an ACE2-dependent manner (Wei et al., 2020) (Figure 1). Cholesterol 25-hydroxylase (CH25H), a known interferon-stimulated gene, enables the synthesis of the oxysterol 25-hydroxy cholesterol (25HC) from cholesterol; notably, 25HC exhibits broad antiviral activity and inhibits intracellular entry of MERS-CoV and SARS-CoV-2 via selective depletion of plasma membrane, but not total cell cholesterol (Wang et al., 2020c). CH25H expression was upregulated in cells following SARS-CoV-2 infection ex vivo, and levels of CH25H were increased in macrophages and epithelial cells from bronchioalveloar lavage (BAL) fluid obtained from individuals hospitalized with SARS-CoV-2 infection (Wang et al., 2020c). Overexpression of CH25HC in cells ex vivo suppressed infection of a SARS-CoV-2 pseudovirus, findings mimicked by treatment of cells with 25HC. Viral entry was inhibited by 25HC in lung epithelial cells and organoids, via mechanisms requiring activation of acyl-CoA:cholesterol acyltransferase (ACAT), thereby promoting cholesterol transport from the cell membrane to the endoplasmic reticulum (Wang et al., 2020c). Consistent with these findings, Zang and colleagues used gain- and loss-of-function experiments to demonstrate key roles for interferon-stimulated CH25H and 25HC in the inhibition of viral membrane fusion and SARS-CoV-2 replication (Zang et al., 2020).
Intriguingly, genetic screens for determinants of SARS-CoV-2 infectivity in cells ex vivo, as well as in related gene knockdown experiments, revealed that inactivation of genes in the cholesterol pathway, such as SCAP, MBTPS1, MBTPS2, and NPC1, conferred resistance to infection (Hoffmann et al., 2020; Wang et al., 2020b). These findings were mimicked by experiments using chemical inhibitors of intracellular cholesterol metabolism (Figure 1). Novel genes such as HMGB1 identified in CRISPR screens modulate SARS-CoV-2 viral entry indirectly through controlling levels of ACE2 expression, and depletion of HMGB1 confers protection against viral infectivity (Wei et al., 2021). Intriguingly, hyperglycemia upregulates HMGB1 expression in endothelial cells (Liu et al., 2018), raising the hypothesis that poorly controlled diabetes may indirectly contribute to enhanced ACE2 expression and viral entry. Indeed, ACE2 expression is upregulated in the lungs and kidney of mice with experimental diabetes (Batchu et al., 2020). Similarly, Neuropilin 1 is known to bind furin cleavage products and was shown to potentiate SARS-CoV-2 cell infectivity when co-expressed with ACE2 and TMPRSS2 (Cantuti-Castelvetri et al., 2020; Daly et al., 2020) (Figure 1).
Complementary, yet distinct, results were obtained in a CRISPR screen to identify genes important for SARS-CoV-2 infection. Pathways regulating cholesterol biosynthesis were associated with suppression of viral entry in studies using human A549 pulmonary epithelial carcinoma cells engineered to overexpress ACE2. Genetic inactivation of RAB7A, PIK3C3, NPC1, CCDC22, ATP6V1A, and ATP6AP1 resulted in increased cellular cholesterol biosynthesis and was associated with resistance to viral entry (Daniloski et al., 2021). Whether loss of these genes was associated with changes in cholesterol abundance in specific cellular compartments, at the cell membrane, for example, resulting in compensatory upregulation of cholesterol biosynthesis, was not determined.
Multiple observational analyses have examined relationships between circulating lipids, statin use, and outcomes, often with conflicting conclusions. A retrospective analysis of outcomes in 654 hospitalized individuals with COVID-19 revealed that mortality was associated with lower total and low-density lipoprotein (LDL) cholesterol and lymphopenia throughout the hospitalization, which correlated inversely with circulating biomarkers of inflammation (Aparisi et al., 2020). Lipid values, with the exception of triglycerides, were generally lower on admission, relative to baseline values, when available from pre-admission records. Age, lymphopenia, LDL cholesterol ≤ 69 mg/dL, and CRP > 88 mg/dL on admission were independent predictors of mortality.
LDL binds bacterial toxins, including lipopolysaccharide, and the results of preclinical studies manipulating levels of LDL together with some, but not all, correlational studies in humans infer that LDL may be associated with protection against sepsis-related morbidity and mortality (Feng et al., 2019). Moreover, an inverse correlation between measured levels of HDL and LDL cholesterol and hospitalizations for infectious disease was detected in 407,558 British individuals in the UK Biobank, whereas genetically determined levels of HDL were also inversely associated with hospitalizations for infections and rates of sepsis-related mortality (Trinder et al., 2020). In agreement with these findings, higher levels of HDL were associated with a reduced risk of COVID-19 infection in the UK Biobank population (Scalsky et al., 2020), whereas low levels of HDL on admission have been associated with greater severity of COVID-19 illness in retrospective observational analyses (Wang et al., 2020a).
Analysis of the Clalit Health Care System data warehouse records identified 8,681 adults with SARS-CoV-2 infection and hospitalization in Israel. Simultaneous analysis of linked outpatient pharmacy records identified a reduced likelihood for hospitalization in individuals taking medications known to interact with the cholesterol synthesis pathway, specifically ubiquinone (OR 0.25), ezetimibe (OR 0.51), and rosuvastatin (OR 0.75) in the 30 days prior to diagnosis (Israel et al., 2020).
Divergent results surround the potential benefit or harm of statin use prior to or during hospitalization, in people with COVID-19 and T2D. Several retrospective observational studies have inferred lower mortality in SARS-CoV-2 hospitalized patients known to be taking 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-co-reductase) inhibitors (namely, statins). Statin use in hospital was identified in 1,219 subjects from a total cohort of 13,981 individuals hospitalized with SARS-CoV-2 infection in Hubei province, China (Zhang et al., 2020). Statin use was more common in older subjects, and in those with a history of heart, kidney, and cerebrovascular disease, who also exhibited increased circulating biomarkers of inflammation and higher levels of total and LDL cholesterol. Analysis of propensity score-matched cohorts demonstrated that in hospital statin use was associated with a lower risk of all-cause mortality over 28 days, whether or not individuals were also taking ACE inhibitors or angiotensin II receptor blockers for hypertension (Zhang et al., 2020). These findings were associated with lower in-hospital trajectories for neutrophil counts, circulating levels of CRP, and IL-6 in statin users.
Analysis of electronic medical records for antecedent statin use prior to hospitalization in 2,626 individuals with COVID-19 revealed 951 statin users, who were more likely to report a history of T2D, kidney disease, hypertension, coronary artery disease, and heart failure (Gupta et al., 2020). In hospital, statin use was documented for 77% of subjects with a history of prior statin use versus 8.6% of control subjects without a history of antecedent prescriptions for statin therapy. Consistent with statin history, levels of total and LDL cholesterol were lower in individuals with antecedent statin use. Comparison of outcomes in propensity-matched cohort subgroups revealed that antecedent statin use was associated with reduced levels of CRP on admission and decreased in-hospital mortality (the primary study endpoint) as well as a composite secondary outcome of reduced need for mechanical ventilation or mortality within 30 days of hospitalization (Gupta et al., 2020).
Statin use, but not levels of total cholesterol, was also associated with a lower risk of mortality in people with T2D (but not T1D) in the National Diabetes Audit population in England (Holman et al., 2020). Gupta et al. analyzed the electronic medical records of 2,626 patients with COVID-19 hospitalized in several NY hospitals between February 1 and May 12, 2020, with 36.2% of subjects on statins prior to admission (Gupta et al., 2020). Statin-treated subjects were older, had lower levels of total and LDL cholesterol, and were more likely to have previous hypertension, coronary artery disease, heart failure, and CKD. Analysis of propensity-matched cohorts demonstrated that statin users had lower levels of CRP on admission and reduced 30-day mortality (14.8% versus 26.5% for statin versus non-statin users, respectively) (Gupta et al., 2020). Antecedent statin use was also associated with a reduced odds ratio for the secondary composite endpoint of in-hospital mortality or invasive mechanical ventilation within 30 days.
Saeed et al. reported the impact of statin use on in-hospital mortality in 4,252 patients (53% with diabetes, 72% with hypertension, 26% with atherosclerotic heart disease) admitted to a single NY hospital between March 1 and May 2, 2020 (Saeed et al., 2020). Statin-treated subjects (1,355) were older and had a higher Charlson co-morbidity index, yet the statin-treated cohort exhibited a lower in-hospital mortality (23% versus 37% for statin versus non-statin use, respectively). The mean BMI was 28 versus 29 in statin versus non-statin users. The survival benefit was magnified in people with T2D (n = 2,266, mean %HbA1c of 7.8 within 3 months of admission), as mortality was 24% versus 39% for statin versus no statin use in T2D, respectively (49% reduction in mortality). Use of statins was not associated with a survival benefit in people without T2D (Saeed et al., 2020). It was not possible to ascertain the proportion of subjects who were on statins prior to hospitalization, or the duration of prior statin therapy.
In direct contrast to the “statins may be beneficial” hypothesis, analysis of the CORONADO dataset of hospitalized people with T2D and COVID-19 demonstrated that antecedent statin use prior to hospitalization (48.7% of study subjects) was associated with greater mortality at 7 and 28 days (Cariou et al., 2020a). Information on statin use in the hospital was not available. Notably, statin users had greater rates of CVD, pulmonary disease and obstructive sleep apnea, hypertension, dyslipidemia, microvascular disease, a lower eGFR, a longer duration of pre-existing T2D, and more use of insulin and GLP-1R agonists. Nevertheless, after propensity matching and correction of cohort imbalances using logistic regression and inverse probability of treatment weighting (IPTW), the investigators concluded that a history of statin use appears harmful in this population of people with long-standing T2D (Cariou et al., 2020a). Whether these divergent conclusions reflect the greater burden of illness in the statin users, and/or limitations of the IPTW methodology in correcting for differences between groups, is challenging to resolve. Further illustrating the lack of agreement among these types of studies, antecedent statin use had no impact on COVID-19 outcomes in 4,842 SARS-CoV-2-infected Danish subjects (17.4% had received a prior prescription for statins in the 6 months prior to a diagnosis COVID-19) (Butt et al., 2020).
Summary and areas of uncertainty
The putative benefit of statins in the context of COVID-19 is inconsistent across multiple studies and requires verification in prospective RCTs. Conversely, the preponderance of evidence suggests that statins are not likely harmful and do not need to be discontinued in the hospital (Masana et al., 2020). Many of the reports do not describe the presence or absence of atherosclerotic vascular disease in statin users with COVID-19. Beyond reduction of LDL, statins may exert beneficial effects on blood vessels, plaque stabilization, reduction of inflammation, and theoretical modulation of SARS-CoV-2 replication. Although considerable observational data link statin use to a reduction in sepsis-related mortality, an RCT of rosuvastatin in hospitalized subjects with sepsis-related acute respiratory distress syndrome demonstrated no clinical benefit or reduction in mortality in statin-treated subjects (Truwit et al., 2014). Statin use is common in individuals with increasing age, hypertension, T2D, obesity, and CVD, risk factors that increase the risk and severity of COVID-19-associated hospitalization. Several RCTS are underway that are designed to examine the impact of statin use on the outcome of SARS-CoV-2 infection.
Ketonemia, ketoacidosis, and insulin deficiency
As viral infections have been associated with increased reports of T1D, the putative relationship between SARS-CoV-2 infection and the incidence rates of T1D continues to receive scrutiny. Nevertheless, reports from several centers do not support an increased incidence of new-onset T1D comparing historical rates with those from the recent pandemic period (Lawrence et al., 2021; Tittel et al., 2020). Analysis of incident diabetes rates reported by 216 German pediatric diabetes centers from March 13 to May 13 each year from 2011 to 2020 in children and adolescents <18 years of age revealed no relative increase in cases reported in 2020 (Tittel et al., 2020). Moreover, retrospective analysis demonstrates that rates of hospitalization for 2,336 Belgian subjects with T1D do not appear to be increased from February 1 to April 30, 2020, relative to hospitalization numbers for individuals without T1D (Vangoitsenhoven et al., 2020). Similarly, there does not appear to be any relationship between SARS-CoV-2 positivity and development of T1D in children, including individuals at greater risk due to islet autoantibodies (Hippich et al., 2020). Consistent with reduced rates of clinical infection and the generally mild course of COVID-19 in children, the available data do not support T1D as a risk factor for severe COVID-19 in children or adolescents (Cardona-Hernandez et al., 2020).
Several small case series and reports have described ketoacidosis or increased ketonemia in individuals with COVID-19, most commonly in subjects with a history of T2D or T1D, with or without prior insulin therapy (Armeni et al., 2020; Chamorro-Pareja et al., 2020; Li et al., 2020). Severe insulinopenia underlies some of these cases, leading to the suggestion that SARS-CoV-2 infection directly or indirectly compromises β cell function, manifesting in insulin deficiency, hyperglycemia, and ketonemia in susceptible individuals (Armeni et al., 2020). Case reports have described new-onset T1D during the pandemic in some individuals with recent COVID-19 infection, with (Marchand et al., 2020) or without islet antibodies (Hollstein et al., 2020), raising the question of whether SARS-CoV-2 infection may promote insulitis or destruction of islet β cells, possibly via dysregulation of the immune system or direct viral infection of the endocrine pancreas. Indeed, the development of more classical T1D has been temporally associated with exposure to viral infection (Vehik et al., 2019); hence, it seems reasonable to query mechanisms whereby SARS-CoV-2 might trigger insulin deficiency and T1D.
Moreover, ketosis on admission has been observed in relatively young individuals (mean age 47 versus 58 for those with and without ketosis in a single hospital in China) and is associated with a longer duration of hospitalization and a poor prognosis in individuals with COVID-19 with or without a prior history of diabetes (Li et al., 2020). Similar observations, namely a high (50%) mortality rate, were reported in 50 individuals with diabetic ketoacidosis (DKA), predominantly people with a diagnosis of T2D (88%), over a 7-week period at a single US hospital (Chamorro-Pareja et al., 2020). Increased rates of ketoacidosis have also been reported in the pediatric population, including in children with new-onset T1D and SARS-CoV-2 infection (Unsworth et al., 2020).
Summary and areas of uncertainty
Despite concern about Sars-CoV-2 triggering T1D, there does not yet appear to be compelling epidemiological evidence supporting a population-based increase in the incidence of T1D in individuals with COVID-19. Although new cases of T1D draw understandable attention during the pandemic, the worldwide annual incidence of T1D ranges from 10 to 30/100,000 (Diaz-Valencia et al., 2015); hence, tracking potential changes in COVID-19-associated incidence rates will require careful ascertainment and may be challenging.
Localization of ACE2 and accessory proteins mediating susceptibility to SARS-CoV-2 infection
Whether the expression of ACE2 and its cofactors facilitating SARS-CoV-2 infectivity (Figure 1) is dysregulated in cells and tissues of some people with diabetes or obesity, contributing to the pathophysiology of SARS-CoV-2 infection, remains highly debated, yet uncertain. Analysis of ACE2 expression in human islet cells has yielded conflicting results, perhaps reflecting the choice of experimental model, conditions for obtaining islets and islet RNA, and the sensitivity and specificity of the techniques used for detection and localization of ACE2 expression. Hikmet and colleagues analyzed human ACE2 expression by immunohistochemistry (IHC) using 2 different antibodies in more than 150 cell types (Hikmet et al., 2020). Within the pancreas, ACE2+ cells were localized to capillaries, principally endothelial cells and pericytes as well as interlobular ducts; ACE2 expression was not observed within the endocrine pancreas of 6 female or 4 male adult donors (Hikmet et al., 2020).
Yang et al. assessed ACE2 expression and susceptibility to infection with a SARS-CoV-2 pseudovirus in human pluripotent stem cell (hPSC)-derived islet-like cells, as well as in a limited number of human islets (Yang et al., 2020). ACE2 protein expression was detected in hPSC-derived α and β cells, but not δ cells, by IHC using a monoclonal ACE2 antibody, findings corroborated by analysis of ACE2 and TMPRSS2 expression in human islet cells, as well as in human pancreatic ductal, acinar, and endothelial cells by single-cell RNA sequencing (scRNA-seq) (Yang et al., 2020). Consistent with these findings, SARS-CoV-2 pseudovirus infection was observed, as detected by luciferase reporter gene expression, within insulin (INS)+ and glucagon (GCG)+ hPSC-derived islet cells, and human islets. Moreover, hPSC-derived islet xenografts transplanted under the kidney capsule of severe combined immunodeficiency-beige mice were susceptible to pseudovirus infection, with viral-derived luciferase detected in INS+ and GCG+ cells within the xenograft.
Taneera et al. examined ACE2 and TMPRSS2 expression in human islets by microarray and RNA-seq. Levels of ACE2 and TMPRSS2 were very low in islets from non-diabetic donors, when assessed by microarray, and ACE2, but not TMPRSS2, expression was upregulated (~1.4-fold) in islets from donors with T2D (Taneera et al., 2020). In contrast, relative TMPRSS2 expression was higher in islets from donors with obesity. Similarly, ACE2 expression was reported at the lower limit of detection, whereas TMPRSS2 mRNA transcripts were more readily detectable by RNA-seq in RNA from fluorescence-activated cell sorting (FACS)-sorted human α, β, and exocrine cells (Bramswig et al., 2013). ACE2 localization was assessed by IHC using multiple antibodies in human pancreatic tissue obtained from 7 non-diabetic donors, demonstrating ACE2 immunopositivity, in ductal, endothelial, and endocrine cells, preferentially in ~57% of INS+ β cells (Fignani et al., 2020). A short form of ACE2 protein was preferentially localized to β cells. In contrast, ACE2 expression was very low or absent in the majority (90%) of α cells and considerable inter-islet heterogeneity in the extent of ACE2 protein expression was observed. ACE2 mRNA transcripts were detected by qPCR in RNA from collagenase-isolated human islet preps from 4 different non-diabetic donors, as well as in islet RNA isolated by laser capture microdissection from 5 different non-diabetic donors. Exposure of human EndoC-βH1 cells or human islets to a pro-inflammatory cytokine cocktail markedly upregulated ACE2 RNA and protein expression, whereas relative ACE2 expression was not different in human islets exposed to palmitate, or in islet RNA isolated from diabetic donors (Fignani et al., 2020).
In contrast, Coate and colleagues evaluated ACE2 expression in human islet bulk and scRNA-seq datasets and found extremely low or undetectable levels of ACE2 and TMPRSS2 expression, with less than 1% of interrogated β cells containing ACE2 or TMPRSS2 mRNA transcripts (Coate et al., 2020). ACE2 expression was detectable in <1% of ductal or acinar cells, whereas TMPRSS2 mRNA was detected in ~35% of these two pancreatic cell types. The proportion of ACE2 and TMPRSS2 co-expression in ductal and acinar cells (15% and 5%, respectively) was somewhat higher in the human islet RNA database described by Segerstolpe et al. (2016). Remarkably, using multiple antisera to examine islet ACE2, Coate and colleagues did not detect ACE2 protein expression in the adult or juvenile human endocrine pancreas. Notably, ACE2 expression was evaluated in pancreatic sections from normal non-diabetic donors (n = 14), and donors with T2D (n = 12) or T1D (n = 11) (Coate et al., 2020). The majority of ACE2 protein expression in the human pancreata was localized to microvascular structures, specifically CD31+ capillaries and pericytes, whereas TMPRSS2 protein expression was not detected within islets, but was visualized within the exocrine pancreas, predominantly within ducts (Figure 3 ). Most cytokeratin-19+ TMPRSS2+ ductal cells did not contain ACE2 immunopositivity (Coate et al., 2020). Examination of pancreatic tissue from 7 COVID-19 subjects (3 with T2D) did not reveal histological evidence of insulitis or pancreatic inflammation.
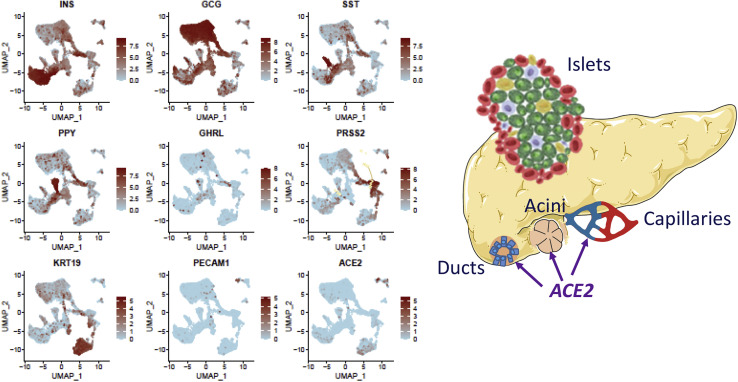
Figure 3.
ACE2 is expressed in the non-endocrine compartment within human islets
Expression of ACE2 in 9,940 human pancreatic cells, including mRNA transcripts for different cell lineages in islet endocrine cells (insulin [INS], glucagon [GCG], somatostatin [SST], and pancreatic polypeptide [PPY] for β, α, δ, and PP cells, respectively), acinar cells (PRSS2), ductal cells (KRT19), and endothelial cells (PECAM1). Data were retrieved and aggregated from Segerstolpe et al. (2016), Enge et al. (2017), and Camunas-Soler et al. (2020).
Related findings were reported independently by Kusmartseva et al. (2020) by employing IHC, western blotting, in situ hybridization, and RNA-seq databases. Using single-molecule fluorescence in situ hybridization, pancreatic ACE2 and TMPRSS2 expression localized to acinar cells, ducts, and CD34+ endothelial cells, but not in the majority of islet endocrine cells from normal non-diabetic donors. A similar pattern of ACE2 protein expression was obtained using 4 independently validated ACE2 antisera; ACE2 was predominantly localized to the ductal epithelium and pancreatic blood vessels in several dozen pancreata obtained from neonates, children, adolescents, middle age, and older human subjects. Moreover, ACE2 expression localized to the ductal epithelium and vascular endothelium, and SARS-CoV-2 nucleocapsid protein was detected in ducts, but not islets, in pancreata (two with pre-existing T2D) obtained from COVID-19+ individuals (Kusmartseva et al., 2020).
The regulation and importance of ACE2 in the setting of T2D and obesity have also been examined in extrapancreatic tissues. Kasela et al. examined ACE2 expression in bronchial airway epithelial cells from individuals in several phenotype cohorts in the absence of SARS-CoV-2 infection. ACE2 expression was increased in bronchial washings from individuals with obesity, as well in people with a history of smoking or hypertension, in several cohorts (Kasela et al., 2020). Hepatic ACE2 expression was assessed using surgically obtained human biopsies from non-diseased livers of 165 subjects of European ancestry, including 25 with T2D. ACE2 (and at lower levels, TMPRSS2) expression was upregulated in the human liver in people with T2D, and correlated with hepatic fat, but not with BMI (Soldo et al., 2020). Neither fasting glucose nor insulin sensitivity correlated with hepatic ACE2 mRNA. Whether SARS-CoV-2-associated liver inflammation is more common in people with T2D or obesity is not clear.
SARS-CoV-2 entry factors and adipose tissue
Adipose tissue plays a central role in energy storage, and dysregulated adipose tissue inflammation may theoretically contribute to the pathophysiology of SARS-CoV-2 infection. Despite the contention that ACE2 expression may be upregulated in white adipose tissue (WAT), enabling WAT to serve as a functional reservoir for SARS-CoV-2 in obese individuals (Ledford, 2020), data supporting this hypothesis are limited (Figure 4 ). Gene expression profiles from 1,471 male and female individuals (ages 35–85) across three human datasets demonstrated lower ACE2 expression in subcutaneous WAT from individuals with obesity or T2D relative to control subjects (El-Sayed Moustafa et al., 2020). Notably, expression of ACE2 and TMPRSS2 was relatively low in subcutaneous WAT and ACE2 expression was higher with increasing age. Genetic estimation of cell proportion in bulk RNA-seq analyses suggested that WAT ACE2 expression correlated with the proportion of microvascular endothelial cells within WAT, whereas lower ACE2 expression correlated with the abundance of macrophages (Ledford, 2020). Notably, ACE2 expression was not associated with the relative proportion of adipocytes in subcutaneous WAT.
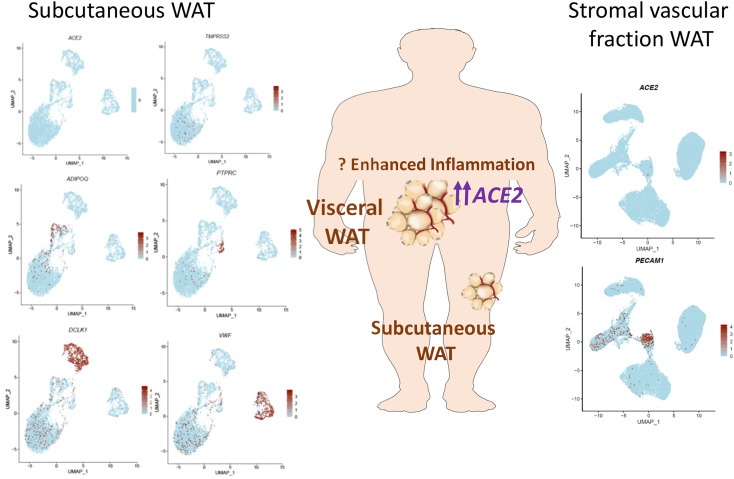
Figure 4.
ACE2 expression in human adipose tissue
ACE2 mRNA transcripts are expressed at low levels in human subcutaneous adipose tissue and at higher levels in visceral adipose tissue. The majority of adipose tissue ACE2 expression is in non-adipocyte cell types. ACE2 and TMPRSS2 expression in 5,041 cells from human neck subcutaneous WAT is shown in the left panel. VWF (endothelial cells), DCLK1 (preadipocytes), ADIPOQ (adipocytes), and PTPRC (CD45+ immune cell). Expression of ACE2 in 26,961 cells in human adipose stromal vascular fraction is depicted in the right panel. PECAM1 marks the endothelial cell population. Data were retrieved from Sun et al. (2020) and Vijay et al. (2020).
Gene expression profiles within human visceral adipose tissue (VAT) were examined in biopsies from normal weight (n = 19), obese (n = 21), and malnourished (n = 14) male and female human subjects at the time of surgery (Pinheiro et al., 2017). Adipocyte area was higher and lower in VAT from individuals with obesity versus malnutrition, respectively, relative to normal weight controls. Although levels of ACE, IL6, and TNFa mRNA transcripts were increased in VAT from subjects with obesity or malnutrition, ACE2 mRNA transcripts were not different across the 3 groups.
The available data on ACE2 expression in human WAT and adipocytes predominantly reflect information from subcutaneous tissue, from individuals without active COVID-19 infection. Hence, definitive conclusions on whether one or more WAT depots, such as VAT that expresses higher levels of ACE2, might serve as targets for direct SARS-CoV-2 infection await more substantive experimental interrogation of these questions (Figure 4). Moreover, much less information is available on ACE2 protein expression in human WAT, relative to the considerable information available from analyses of ACE2 protein localization in human pancreatic tissue.
Summary and areas of uncertainty
The discrepant localization of gene and protein expression between studies, as evident in studies of ACE2 localization in human islets, is common in biology, and frequently reflects the use of different reagents and techniques for analysis of sensitivity and specificity (Drucker, 2016). Interpretation of gene expression data using isolation of RNA from whole islets or single islet cells may be confounded by contamination from non-islet cell types. It seems less likely that inter-individual heterogeneity in islet donor characteristics accounts for these often fundamentally different observations. Despite hypotheses that adipose tissue may be a reservoir for SARS-CoV-2, adipocyte ACE2 expression appears low (Figure 4), and data interrogating viral infection in different adipose tissue depots have not been forthcoming. Collectively, the available data suggest that direct SARS-CoV-2 infection of islet β cells is likely a rare event, but cannot be excluded.
Long-COVID syndrome and indirect consequences of COVID-19
Symptoms of illness persisting after resolution of acute SARS-CoV-2 infection are common, yet incompletely described. A prospective observational cohort of 4,182 people positive for SARS-CoV-2 in Great Britain, the US, and Sweden revealed persistent symptoms for more than 28 days (long COVID LC28) in 13.3% of study volunteers (Sudre et al., 2020). The proportion of people with obesity was higher in the LC28 cohort relative to the cohort reporting shorter duration of symptoms. The number of symptoms reported in the first week of illness, older age, and female sex were the strongest predictors of long-COVID syndrome. Moreover, persistent symptoms beyond 30 days of COVID-19 clinical diagnosis are more common in those with severe illness, yet 14.3% of survey respondents in a US general adult population with mild COVID-19 illness report persistent symptoms more than 30 days later (Cirulli et al., 2020). Preliminary interrogation of individuals more than 90 days after hospitalization with a COVID-19 diagnosis including a substantial proportion of subjects with diabetes and obesity revealed high levels of persistent fatigue, anxiety, and neurological symptoms (Savarraj et al., 2020). Among individuals followed in home health care after discharge from the hospital after COVID-19 in NY, the risk of readmission and subsequent death was higher in people with complications associated with T2D. It seems certain that ongoing studies of people recovering from COVID-19 infection will have further direct implications for the subset of people with T2D or obesity recovering from COVID-19. Follow-up of 1,733 individuals with COVID-19 discharged from a single hospital in Wuhan over 6 months (12% with diabetes on admission) revealed 53 people with a new diagnosis of diabetes, but no other disproportionate outcomes were reported in the cohort with diabetes (Huang et al., 2021).
The pivoting of global health care systems to acute COVID-19 care, coupled with stay-at-home edicts, has predictably delayed the diagnosis and treatment of many complex medical diseases, including T2D. While telemedicine has extensively supported the management of people with T2D, it does not fully replace traditional primary care medicine. Analysis of 1,709 UK general practices in an electronic database revealed reduced rates of diabetes-related testing (%HbA1c), substantial reductions in new T2D diagnoses, and increased T2D-associated mortality from March 1 to July 10, 2020 (Carr et al., 2020).
Limitations of the data and future perspectives
There is currently limited information on the extent of tissue inflammation and dysregulation of gene expression in metabolically important tissues in the context of active SARS-CoV-2 infection. Post-mortem analyses have focused on inflammatory responses in lung, brain, heart, kidney, and vascular pathology (Schurink et al., 2020), whereas analyses of the pancreas, endocrine organs, or adipose tissue are much less frequently reported. Furthermore, there is scant molecular information to date from analysis of tissue biopsies from individuals with diabetes, obesity, and SARS-CoV-2 infection. Substantial inferences regarding the biology of ACE and ACE2, and the properties of glucose-lowering drugs, including actions impacting inflammation, are derived from preclinical studies, or people without COVID-19 (Drucker, 2020), which may not be relevant for understanding human SARS-CoV-2 infection.
Hundreds of clinical trials are now underway, testing interventions ranging from vaccines to immunotherapy (Figure 5 ), and the safety of specific glucose-lowering drugs in the context of acute SARS-CoV-2 infection. Given the high rates of hospitalization for people with T2D and obesity and clinical illness with COVID-19, we are likely to learn a great deal about which interventions work equally well, better, or are suboptimally effective in people with cardiometabolic disorders. The available data from retrospective observations are consistent with the hypothesis that safe optimization of glycemic control might reduce the risk of severe COVID-19 (Figure 5); however, it seems unlikely that this hypothesis will be prospectively tested in a clinical trial. Beyond insulin, it would be useful to understand the safety profile of glucose-lowering agents (Figure 2), including GLP-1R agonists and SGLT-2 inhibitors, in people with clinical symptoms of COVID-19. Moreover, GLP-1R agonists are increasingly prescribed for obesity, and there is little information informing whether this class of drugs is safe, poses any unique risk, or confers any benefit in people with obesity and SARS-CoV-2 infection. Preclinical studies demonstrate reduced virus-associated lung inflammation in mice treated with GLP-1R agonists (Bai et al., 2020; Bloodworth et al., 2018; Sato et al., 2020), yet data for the actions of GLP-1R agonists in experimental or clinical SARS-CoV-2 infection are not yet available.
Figure 5.
Key considerations for SARS-CoV-2 infection in people with obesity and diabetes
Major morbidities arising in people with severe SARS-CoV-2 infection (left panel) and emerging therapeutic interventions with potential benefit in people with diabetes and COVID-19 (right panel).
The adverse outcomes, including increased mortality, observed in hospitalized people with T2D and obesity are not currently amenable to disease-specific therapeutic interventions, in part due to a lack of understanding of mechanistic causality. It remains unclear whether enhanced SARS-CoV-2 infectivity, ARDS, cellular or humoral immune dysfunction, disorders of coagulation, cardiopulmonary injury, or vasculitis contribute preferentially to the clinical presentation of severe COVID-19 illness in people with metabolic disorders. Hence, elucidating the contributions of these potential mechanisms represents an important research priority to enable therapies that might be preferentially effective in people with T2D or obesity.
Beyond social distancing and use of masks, few options are available to people with obesity who wish to mitigate their risk of SARS-CoV-2 infection. Whether medically induced weight loss might reduce the risk of severe COVID-19 infection has not been formally studied. A prior history of bariatric surgery was associated with reduced need for mechanical ventilation and decreased mortality, even after correcting for BMI, in a retrospective analysis of hospitalized people with obesity and COVID-19 in France (Iannelli et al., 2020). Individuals with obesity are at particularly high risk for thromboembolic disease (Figure 5) when immobile in the hospital; hence, it will be extremely important to determine whether the forthcoming results of various anti-coagulant strategies to prevent SARS-CoV-2-related coagulopathy can be extrapolated to individuals across a broad range of BMIs.
A retrospective analysis reported that an elevated neutrophil:lymphocyte ratio greater than 6.11 on admission to hospital was associated with a poor prognosis yet a better therapeutic response to steroid therapy (Cai et al., 2021). Nevertheless, corticosteroid-treated individuals with T2D did not exhibit a reduction in mortality, while exhibiting a greater rate of hyperglycemia and infection requiring antibiotics. In contrast to these retrospective analyses, different conclusions ensued from the analysis of outcomes for people with diabetes in the prospective RECOVERY trial (25% of study subjects). The RECOVERY trial results demonstrated the benefit of dexamethasone in severely ill hospitalized patients; however, the proportion of study volunteers with overweight or obesity was not reported (Horby et al., 2020). Although not reported in the initial publication, analysis of outcomes in people with or without T2D in the RECOVERY trial by the European Medicines Agency revealed that people with diabetes exhibited a similar reduction in mortality, whether or not they required mechanical ventilation (https://www.ema.europa.eu/en/documents/other/dexamethasone-covid19-article-53-procedure-assessment-report_en.pdf).
Similarly, substantial proportions (30%–40%) of trial volunteers reported antecedent T2D and obesity (40%) in trials with remdesivir alone (Beigel et al., 2020; Spinner et al., 2020) or in combination with baricitinib. However, whether these regimens are meaningfully helpful in people with T2D or obesity is not yet clear from limited subgroup analyses. Although rapid strides have been made in the deployment of telemedicine for the management of diabetes, the report of a pandemic-related 10-fold increase in amputations in a single Ohio hospital (Casciato et al., 2020) is a sobering reminder of the current barriers to optimal diabetes care.
In summary, the rapid pace of often incomplete COVID-19-related science has reminded us to respect uncertainty, acknowledge conflicting views and lines of evidence, and be willing to consider multiple lines of evidence. Notwithstanding the torrent of daily new reports, it remains important to thoughtfully probe the scientific validity of reported findings in formulating mechanistic concepts of how SARS-CoV-2 infection impacts human biology. Ongoing rigorous scrutiny of unanswered scientific questions in the laboratory, and at the bedside, will progressively resolve key conundrums, generate new testable hypotheses, and improve the outcomes of people with diabetes, obesity, and COVID-19.
Acknowledgments
I thank Drs. A.C. Gingras and L.L. Baggio for critical review of the paper and Dr. C.K. Wong (all from Mt. Sinai Hospital) for analysis of the RNA-seq data shown in Figures 3 and 4. D.J.D. is supported by CIHR grant 154321. Investigator-initiated studies of peptide hormone action in the Drucker lab are sponsored by Novo Nordisk, Takeda Inc., a Banting and Best Diabetes Centre-Novo Nordisk Chair in Incretin Biology, and the Novo Nordisk Foundation-Mt. Sinai Hospital fund for the study of gut peptides.
Declaration of interests
There are no relevant interests related to COVID-19 and the subject matter of this review article. My other disclosed interests, related to gut hormone actions, but not related to this article, are as follows: D.J.D. has served as an advisor or consultant or speaker within the past 12 months to Forkhead Biotherapeutics, Intarcia Therapeutics, Kallyope, Merck Research Laboratories, Novo Nordisk Inc., and Pfizer Inc. Neither Dr. Drucker nor his family members hold stock directly or indirectly in any of these companies. GLP-2 is the subject of a patent license agreement between Shire Inc. and the University of Toronto, Toronto General Hospital (UHN), and D.J.D.
References
- Agarwal S., Schechter C., Southern W., Crandall J.P., Tomer Y. Preadmission diabetes-specific risk factors for mortality in hospitalized patients with diabetes and coronavirus disease 2019. Diabetes Care. 2020;43:2339–2344. doi: 10.2337/dc20-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmad B., Al-Shammari A.A., Bennakhi A., Al-Mulla F., Ali H. Fasting blood glucose and COVID-19 severity: nonlinearity matters. Diabetes Care. 2020;43:3113–3116. doi: 10.2337/dc20-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altonen B.L., Arreglado T.M., Leroux O., Murray-Ramcharan M., Engdahl R. Characteristics, comorbidities and survival analysis of young adults hospitalized with COVID-19 in New York City. PLoS ONE. 2020;15:e0243343. doi: 10.1371/journal.pone.0243343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzaid F., Julla J.B., Diedisheim M., Potier C., Potier L., Velho G., Gaborit B., Manivet P., Germain S., Vidal-Trecan T., et al. Monocytopenia, monocyte morphological anomalies and hyperinflammation characterise severe COVID-19 in type 2 diabetes. EMBO Mol. Med. 2020;12:e13038. doi: 10.15252/emmm.202013038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparisi A., Iglesias-Echeverria C., Ybarra-Falcon C., Cusacovich I., Uribarri A., Garcia-Gomez M., Ladron R., Fuertes R., Candela J., Hinojosa W., et al. Low-density lipoprotein cholesterol levels are associated with poor clinical outcomes in COVID-19. medRxiv. 2020 doi: 10.1101/2020.10.06.20207092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armeni E., Aziz U., Qamar S., Nasir S., Nethaji C., Negus R., Murch N., Beynon H.C., Bouloux P., Rosenthal M., et al. Protracted ketonaemia in hyperglycaemic emergencies in COVID-19: a retrospective case series. Lancet Diabetes Endocrinol. 2020;8:660–663. doi: 10.1016/S2213-8587(20)30221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio L.L., Varin E.M., Koehler J.A., Cao X., Lokhnygina Y., Stevens S.R., Holman R.R., Drucker D.J. Plasma levels of DPP4 activity and sDPP4 are dissociated from inflammation in mice and humans. Nat. Commun. 2020;11:3766. doi: 10.1038/s41467-020-17556-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Lian P., Li J., Zhang Z., Qiao J. The active GLP-1 analogue liraglutide alleviates H9N2 influenza virus-induced acute lung injury in mice. Microb. Pathog. 2020;150:104645. doi: 10.1016/j.micpath.2020.104645. [DOI] [PubMed] [Google Scholar]
- Barron E., Bakhai C., Kar P., Weaver A., Bradley D., Ismail H., Knighton P., Holman N., Khunti K., Sattar N., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchu S.N., Kaur H., Yerra V.G., Advani S.L., Kabir M.G., Liu Y., Klein T., Advani A. Lung and kidney ACE2 and TMPRSS2 in renin angiotensin system blocker treated comorbid diabetic mice mimicking host factors that have been linked to severe COVID-19. Diabetes. 2020 doi: 10.2337/db20-0765. [DOI] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., et al. ACTT-1 Study Group Members Remdesivir for the treatment of Covid-19 - final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodworth M.H., Rusznak M., Pfister C.C., Zhang J., Bastarache L., Calvillo S.A., Chappell J.D., Boyd K.L., Toki S., Newcomb D.C., et al. Glucagon-like peptide 1 receptor signaling attenuates respiratory syncytial virus-induced type 2 responses and immunopathology. J. Allergy Clin. Immunol. 2018;142:683–687.e12. doi: 10.1016/j.jaci.2018.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode B., Garrett V., Messler J., McFarland R., Crowe J., Booth R., Klonoff D.C. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J. Diabetes Sci. Technol. 2020;14:813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit J., Morishima C., Selke S., Zamora D., McGuffin S.A., Shapiro A.E., Campbell V.L., McClurkan C.L., Jing L., Gross R., et al. Clinical, laboratory, and temporal predictors of neutralizing antibodies to SARS-CoV-2 among COVID-19 convalescent plasma donor candidates. J. Clin. Invest. 2020 doi: 10.1172/JCI144930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges L.P., Martins A.F., de Melo M.S., de Oliveira M.G.B., Neto J.M.R., Dósea M.B., Cabral B.C.M., Menezes R.F., Santos A.A., Matos I.L.S., et al. Seroprevalence of SARS-CoV-2 IgM and IgG antibodies in an asymptomatic population in Sergipe, Brazil. Rev. Panam. Salud Publica. 2020;44:e108. doi: 10.26633/RPSP.2020.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., Boehm B., Amiel S., Holt R.I., Skyler J.S., et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramante C.T., Ingraham N.E., Murray T.A., Marmor S., Hovertsen S., Gronski J., McNeil C., Feng R., Guzman G., Abdelwahab N., et al. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. The Lancet Healthy Longevity. 2021;2:e34–e41. doi: 10.1016/S2666-7568(20)30033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramswig N.C., Everett L.J., Schug J., Dorrell C., Liu C., Luo Y., Streeter P.R., Naji A., Grompe M., Kaestner K.H. Epigenomic plasticity enables human pancreatic α to β cell reprogramming. J. Clin. Invest. 2013;123:1275–1284. doi: 10.1172/JCI66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetti N., Trimboli P., Mazzuchelli T., Lo Priore E., Balmelli C., Trkola A., Conti M., Martinetti G., Elzi L., Ceschi A., et al. Diabetes mellitus is a risk factor for prolonged SARS-CoV-2 viral shedding in lower respiratory tract samples of critically ill patients. Endocrine. 2020;70:454–460. doi: 10.1007/s12020-020-02465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetti N., Wicky P.H., Le Hingrat Q., Ruckly S., Mazzuchelli T., Loiodice A., Trimboli P., Forni Ogna V., de Montmollin E., Bernasconi E., et al. SARS-CoV-2 detection in the lower respiratory tract of invasively ventilated ARDS patients. Crit. Care. 2020;24:610. doi: 10.1186/s13054-020-03323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn E., Sena A.G., Prats-Uribe A., Spotnitz M., DuVall S., Lynch K.E., Matheny M.E., Nyberg F., Ahmed W.U., Alser O., et al. Use of dialysis, tracheostomy, and extracorporeal membrane oxygenation among 240,392 patients hospitalized with COVID-19 in the United States. medRxiv. 2020 doi: 10.1101/2020.11.25.20229088. [DOI] [Google Scholar]
- Butt J.H., Gerds T.A., Schou M., Kragholm K., Phelps M., Havers-Borgersen E., Yafasova A., Gislason G.H., Torp-Pedersen C., Køber L., Fosbøl E.L. Association between statin use and outcomes in patients with coronavirus disease 2019 (COVID-19): a nationwide cohort study. BMJ Open. 2020;10:e044421. doi: 10.1136/bmjopen-2020-044421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Shi S., Yang F., Yi B., Chen X., Li J., Wen Z. Fasting blood glucose level is a predictor of mortality in patients with COVID-19 independent of diabetes history. Diabetes Res. Clin. Pract. 2020;169:108437. doi: 10.1016/j.diabres.2020.108437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Li H., Zhang C., Chen Z., Liu H., Lei F., Qin J.-J., Liu Y.-M., Zhou F., Song X., et al. The neutrophil-to-lymphocyte ratio determines clinical efficacy of corticosteroid therapy in patients with COVID-19. Cell Metab. 2021 doi: 10.1016/j.cmet.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camunas-Soler J., Dai X.Q., Hang Y., Bautista A., Lyon J., Suzuki K., Kim S.K., Quake S.R., MacDonald P.E. Patch-seq links single-cell transcriptomes to human islet dysfunction in diabetes. Cell Metab. 2020;31:1017–1031.e4. doi: 10.1016/j.cmet.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano E., Corsini Campioli C., O’Horo J.C. Nasopharyngeal SARS-CoV-2 viral RNA shedding in patients with diabetes mellitus. Endocrine. 2021;71:26–27. doi: 10.1007/s12020-020-02516-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona-Hernandez R., Cherubini V., Iafusco D., Schiaffini R., Luo X., Maahs D.M. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr. Diabetes. 2020 doi: 10.1111/pedi.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey I.M., Critchley J.A., DeWilde S., Harris T., Hosking F.J., Cook D.G. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018;41:513–521. doi: 10.2337/dc17-2131. [DOI] [PubMed] [Google Scholar]
- Cariou B., Goronflot T., Rimbert A., Boullu S., Le May C., Moulin P., Pichelin M., Potier L., Smati S., Sultan A., et al. CORONADO investigators Routine use of statins and increased mortality related to COVID-19 in inpatients with type 2 diabetes: results from the CORONADO study. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., Amadou C., Arnault G., Baudoux F., Bauduceau B., et al. CORONADO investigators Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M.J., Wright A.K., Leelarathna L., Thabit H., Milne N., Kanumilli N., Ashcroft D.M., Rutter M.K. Impact of COVID-19 on the diagnoses, HbA1c monitoring and mortality in people with type 2 diabetes: a UK-wide cohort study involving 13 million people in primary care. medRxiv. 2020 doi: 10.1101/2020.10.25.20200675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciato D.J., Yancovitz S., Thompson J., Anderson S., Bischoff A., Ayres S., Barron I. Diabetes-related major and minor amputation risk increased during the COVID-19 pandemic. J. Am. Podiatr. Med. Assoc. 2020 doi: 10.7547/20-224. [DOI] [PubMed] [Google Scholar]
- Chamorro-Pareja N., Parthasarathy S., Annam J., Hoffman J., Coyle C., Kishore P. Letter to the editor: unexpected high mortality in COVID-19 and diabetic ketoacidosis. Metabolism. 2020;110:154301. doi: 10.1016/j.metabol.2020.154301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Chen Y., Wu C., Wei M., Xu J., Chao Y.C., Song J., Hou D., Zhang Y., Du C., et al. Coagulopathy is a major extrapulmonary risk factor for mortality in hospitalized patients with COVID-19 with type 2 diabetes. BMJ Open Diabetes Res. Care. 2020;8:e001851. doi: 10.1136/bmjdrc-2020-001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C., Liu C., Xiong M., Deng A., Zhang Y., et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43:1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- Cheng X., Liu Y.M., Li H., Zhang X., Lei F., Qin J.J., Chen Z., Deng K.Q., Lin L., Chen M.M., et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;32:537–547.e3. doi: 10.1016/j.cmet.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli E.T., Barrett K.M.S., Riffle S., Bolze A., Neveux I., Dabe S., Grzymski J.J., Lu J.T., Washington N.L. Long-term COVID-19 symptoms in a large unselected population. medRxiv. 2020 doi: 10.1101/2020.10.07.20208702. [DOI] [Google Scholar]
- Coate K.C., Cha J., Shrestha S., Wang W., Gonçalves L.M., Almaça J., Kapp M.E., Fasolino M., Morgan A., Dai C., et al. HPAP Consortium SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β cells. Cell Metab. 2020;32:1028–1040.e4. doi: 10.1016/j.cmet.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codo A.C., Davanzo G.G., Monteiro L.B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., Prodonoff J.S., Carregari V.C., de Biagi Junior C.A.O., Crunfli F., et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32:437–446.e5. doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley J.A., Carey I.M., Harris T., DeWilde S., Hosking F.J., Cook D.G. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care. 2018;41:2127–2135. doi: 10.2337/dc18-0287. [DOI] [PubMed] [Google Scholar]
- Crouse A., Grimes T., Li P., Might M., Ovalle F., Shalev A. Metformin use is associated with reduced mortality in a diverse population with Covid-19 and diabetes. medRxiv. 2020 doi: 10.1101/2020.07.29.20164020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., Aaron J.G., Claassen J., Rabbani L.E., Hastie J., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernichow S., Beeker N., Rives-Lange C., Guerot E., Diehl J.L., Katsahian S., Hulot J.S., Poghosyan T., Carette C., Jannot A.S., AP-HP / Universities / INSERM COVID-19 research collaboration and AP-HP COVID CDR Initiative Obesity doubles mortality in patients hospitalized for severe acute respiratory syndrome coronavirus 2 in Paris hospitals, France: a cohort study on 5,795 patients. Obesity (Silver Spring) 2020;28:2282–2289. doi: 10.1002/oby.23014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalan R., Ang L.W., Tan W.Y.T., Fong S.W., Tay W.C., Chan Y.H., Renia L., Ng L.F.P., Lye D.C., Chew D.E.K., Young B.E. The association of hypertension and diabetes pharmacotherapy with COVID-19 severity and immune signatures: an observational study. Eur. Heart J. Cardiovasc. Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J.L., Simonetti B., Klein K., Chen K.E., Williamson M.K., Antón-Plágaro C., Shoemark D.K., Simón-Gracia L., Bauer M., Hollandi R., et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniloski Z., Jordan T.X., Wessels H.H., Hoagland D.A., Kasela S., Legut M., Maniatis S., Mimitou E.P., Lu L., Geller E., et al. Identification of required host factors for SARS-CoV-2 infection in human cells. Cell. 2021;184:92–105.e16. doi: 10.1016/j.cell.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis J.M., Mateen B.A., Sonabend R., Thomas N.J., Patel K.A., Hattersley A.T., Denaxas S., McGovern A.P., Vollmer S.J. Type 2 diabetes and COVID-19-related mortality in the critical care setting: a national cohort study in England, March-July 2020. Diabetes Care. 2021;44:50–57. doi: 10.2337/dc20-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Valencia P.A., Bougnères P., Valleron A.J. Global epidemiology of type 1 diabetes in young adults and adults: a systematic review. BMC Public Health. 2015;15:255. doi: 10.1186/s12889-015-1591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker D., Lev S., Gottesman T., Kournos T., Dotan M., Ashorov N., Marcoviciu D., Golan R. A time frame for testing negative for SARS-COV2 in people with obesity. Obes. Facts. 2020;13:528–533. doi: 10.1159/000511738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., et al. ISARIC4C investigators Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath M.Y., Dinarello C.A., Mandrup-Poulsen T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat. Rev. Immunol. 2019;19:734–746. doi: 10.1038/s41577-019-0213-9. [DOI] [PubMed] [Google Scholar]
- Drucker D.J. Never waste a good crisis: confronting reproducibility in translational research. Cell Metab. 2016;24:348–360. doi: 10.1016/j.cmet.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Drucker D.J. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr. Rev. 2020;41:457–470. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Salles T., Vizcaya D., Pistillo A., Casajust P., Sena A.G., Lai L.Y.H., Prats-Uribe A., Ahmed W.U., Alshammari T.M., Alghoul H., et al. Baseline characteristics, management, and outcomes of 55,270 children and adolescents diagnosed with COVID-19 and 1,952,693 with influenza in France, Germany, Spain, South Korea and the United States: an international network cohort study. medRxiv. 2020 doi: 10.1101/2020.10.29.20222083. [DOI] [Google Scholar]
- El-Sayed Moustafa J.S., Jackson A.U., Brotman S.M., Guan L., Villicana S., Roberts A.L., Zito A., Bonnycastle L., Erdos M.R., Narisu N., et al. ACE2 expression in adipose tissue is associated with COVID-19 cardio-metabolic risk factors and cell type composition. medRxiv. 2020 doi: 10.1101/2020.08.11.20171108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge M., Arda H.E., Mignardi M., Beausang J., Bottino R., Kim S.K., Quake S.R. Single-cell analysis of human pancreas reveals transcriptional signatures of aging and somatic mutation patterns. Cell. 2017;171:321–330.e14. doi: 10.1016/j.cell.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini G.P., Morieri M.L., Boscari F., Fioretto P., Maran A., Busetto L., Bonora B.M., Selmin E., Arcidiacono G., Pinelli S., et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res. Clin. Pract. 2020;168:108374. doi: 10.1016/j.diabres.2020.108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q., Wei W.Q., Chaugai S., Leon B.G.C., Mosley J.D., Leon D.A.C., Jiang L., Ihegword A., Shaffer C.M., Linton M.F., et al. Association between low-density lipoprotein cholesterol levels and risk for sepsis among patients admitted to the hospital with infection. JAMA Netw. Open. 2019;2:e187223. doi: 10.1001/jamanetworkopen.2018.7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes D.M., Oliveira C.R., Guerguis S., Eisenberg R., Choi J., Kim M., Abdelhemid A., Agha R., Agarwal S., Aschner J.L., et al. Tri-State Pediatric COVID-19 Research Consortium Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J. Pediatr. 2020 doi: 10.1016/j.jpeds.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fignani D., Licata G., Brusco N., Nigi L., Grieco G.E., Marselli L., Overbergh L., Gysemans C., Colli M.L., Marchetti P., et al. SARS-CoV-2 receptor angiotensin I-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front. Endocrinol. (Lausanne) 2020;11:596898. doi: 10.3389/fendo.2020.596898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Madhavan M.V., Poterucha T.J., DeFilippis E.M., Hennessey J.A., Redfors B., Eckhardt C., Bikdeli B., Platt J., Nalbandian A., et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Res Sq. 2020 doi: 10.21203/rs.3.rs-56210/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M., Pritchard M., Dankwa E.A., Baillie J.K., Carson G., Citarella B.W., Docherty A., Donnelly C.A., Dunning J., Fraser C., et al. ISARIC clinical data report 20 November 2020. medRxiv. 2020 doi: 10.1101/2020.07.17.20155218. [DOI] [Google Scholar]
- Hamer M., Gale C.R., Kivimäki M., Batty G.D. Overweight, obesity, and risk of hospitalization for COVID-19: a community-based cohort study of adults in the United Kingdom. Proc. Natl. Acad. Sci. USA. 2020;117:21011–21013. doi: 10.1073/pnas.2011086117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendren N.S., de Lemos J.A., Ayers C., Das S.R., Rao A., Carter S., Rosenblatt A., Walchok J., Omar W., Khera R., et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: results from the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143:135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
- Hikmet F., Méar L., Edvinsson Å., Micke P., Uhlén M., Lindskog C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020;16:e9610. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippich M., Holthaus L., Assfalg R., Zapardiel Gonzalo J.M., Kapfelsperger H., Heigermoser M., Haupt F., Ewald D.A., Welzhofer T.C., Marcus B.A., et al. Public health antibody screening indicates a six-fold higher SARS-CoV-2 exposure rate than reported cases in children. Med (N Y) 2020 doi: 10.1016/j.medj.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H.H., Sánchez-Rivera F.J., Schneider W.M., Luna J.M., Soto-Feliciano Y.M., Ashbrook A.W., Le Pen J., Leal A.A., Ricardo-Lax I., Michailidis E., et al. Functional interrogation of a SARS-CoV-2 host protein interactome identifies unique and shared coronavirus host factors. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein T., Schulte D.M., Schulz J., Glück A., Ziegler A.G., Bonifacio E., Wendorff M., Franke A., Schreiber S., Bornstein S.R., Laudes M. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: a case report. Nat. Metab. 2020;2:1021–1024. doi: 10.1038/s42255-020-00281-8. [DOI] [PubMed] [Google Scholar]
- Holman N., Knighton P., Kar P., O’Keefe J., Curley M., Weaver A., Barron E., Bakhai C., Khunti K., Wareham N.J., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., et al. RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Lu Q.B., Zhao H., Zhang Y., Sui Z., Fang L., Liu D., Sun X., Peng K., Liu W., Guan W. Temporal antibody responses to SARS-CoV-2 in patients of coronavirus disease 2019. Cell Discov. 2020;6:64. doi: 10.1038/s41421-020-00209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Guo H., Zhou Y., Guo J., Wang T., Zhao X., Li H., Sun Y., Bian X., Fang C. The associations between fasting plasma glucose levels and mortality of COVID-19 in patients without diabetes. Diabetes Res. Clin. Pract. 2020;169:108448. doi: 10.1016/j.diabres.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannelli A., Bouam S., Schneck A.S., Frey S., Zarca K., Gugenheim J., Alifano M. The impact of previous history of bariatric surgery on outcome of COVID-19. A nationwide medico-administrative French study. Obes. Surg. 2020 doi: 10.1007/s11695-020-05120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou G.N., Locke E., Green P., Berry K., O’Hare A.M., Shah J.A., Crothers K., Eastment M.C., Dominitz J.A., Fan V.S. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw. Open. 2020;3:e2022310. doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel A., Schäffer A.A., Cicurel A., Feldhamer I., Tal A., Cheng K., Sinha S., Schiff E., Lavie G., Ruppin E. Large population study identifies drugs associated with reduced COVID-19 severity. medRxiv. 2020 doi: 10.1101/2020.10.13.20211953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S.S., Davis R.P., Ma M.M., Tam E., Cooper C.L., Ramji A., Kelly E.M., Jayakumar S., Swain M.G., Jenne C.N., Coffin C.S. Reduced immune responses to hepatitis B primary vaccination in obese individuals with nonalcoholic fatty liver disease (NAFLD) NPJ Vaccines. 2021;6:9. doi: 10.1038/s41541-020-00266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambhampati A.K., O’Halloran A.C., Whitaker M., Magill S.S., Chea N., Chai S.J., Daily Kirley P., Herlihy R.K., Kawasaki B., Meek J., et al. COVID-NET Surveillance Team COVID-19-associated hospitalizations among health care personnel - COVID-NET, 13 states, March 1-May 31, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1576–1583. doi: 10.15585/mmwr.mm6943e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasela S., Ortega V.E., Martorella M., Garudadri S., Nguyen J., Ampleford E., Pasanen A., Nerella S., Buschur K.L., Barjaktarevic I.Z., et al. Genetic and non-genetic factors affecting the expression of COVID-19 relevant genes in the large airway epithelium. medRxiv. 2020 doi: 10.1101/2020.10.01.20202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ken-Dror G., Wade C., Sharma S., Law J., Russo C., Sharma A., Joy E., John J., Robin J., John S., et al. COVID-19 outcomes in UK centre within highest health and wealth band: a prospective cohort study. BMJ Open. 2020;10:e042090. doi: 10.1136/bmjopen-2020-042090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonoff D.C., Messler J.C., Umpierrez G.E., Peng L., Booth R., Crowe J., Garrett V., McFarland R., Pasquel F.J. Association between achieving inpatient glycemic control and clinical outcomes in hospitalized patients with COVID-19: a multicenter, retrospective hospital-based analysis. Diabetes Care. 2021;44:578–585. doi: 10.2337/dc20-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N.P., Sridhar R., Banurekha V.V., Jawahar M.S., Nutman T.B., Babu S. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J. Infect. Dis. 2013;208:739–748. doi: 10.1093/infdis/jit241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartseva I., Wu W., Syed F., Van Der Heide V., Jorgensen M., Joseph P., Tang X., Candelario-Jalil E., Yang C., Nick H., et al. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 2020;32:1041–1051.e6. doi: 10.1016/j.cmet.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampasona V., Secchi M., Scavini M., Bazzigaluppi E., Brigatti C., Marzinotto I., Davalli A., Caretto A., Laurenzi A., Martinenghi S., et al. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia. 2020;63:2548–2558. doi: 10.1007/s00125-020-05284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C., Seckold R., Smart C., King B.R., Howley P., Feltrin R., Smith T.A., Roy R., Lopez P. Increased paediatric presentations of severe diabetic ketoacidosis in an Australian tertiary centre during the COVID-19 pandemic. Diabet. Med. 2021;38:e14417. doi: 10.1111/dme.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford H. How obesity could create problems for a COVID vaccine. Nature. 2020;586:488–489. doi: 10.1038/d41586-020-02946-6. [DOI] [PubMed] [Google Scholar]
- Leong A., Cole J., Brenner L.N., Meigs J.B., Florez J.C., Mercader J.M. Cardiometabolic risk factors for COVID-19 susceptibility and severity: a Mendelian randomization analysis. medRxiv. 2020 doi: 10.1101/2020.08.26.20182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes. Metab. 2020;22:1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S., Bae J.H., Kwon H.S., Nauck M.A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Luo Q., You W., Jin M. MicroRNA-106 attenuates hyperglycemia-induced vascular endothelial cell dysfunction by targeting HMGB1. Gene. 2018;677:142–148. doi: 10.1016/j.gene.2018.07.063. [DOI] [PubMed] [Google Scholar]
- Maier H.E., Lopez R., Sanchez N., Ng S., Gresh L., Ojeda S., Burger-Calderon R., Kuan G., Harris E., Balmaseda A., Gordon A. Obesity increases the duration of influenza A virus shedding in adults. J. Infect. Dis. 2018;218:1378–1382. doi: 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamtani M., Athavale A.M., Abraham M., Vernik J., Amarah A.R., Ruiz J.P., Joshi A.J., Itteera M., Zhukovski S.D., Madaiah R.P., et al. Association of hyperglycemia with hospital mortality in nondiabetic COVID-19 patients: a cohort study. medRxiv. 2020 doi: 10.1101/2020.08.31.20185157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamtani M., Kulkarni H., Bihari S., Prakash S., Chavan S., Huckson S., Pilcher D. Degree of hyperglycemia independently associates with hospital mortality and length of stay in critically ill, nondiabetic patients: results from the ANZICS CORE binational registry. J. Crit. Care. 2020;55:149–156. doi: 10.1016/j.jcrc.2019.11.003. [DOI] [PubMed] [Google Scholar]
- Marchand L., Pecquet M., Luyton C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol. 2020;57:1265–1266. doi: 10.1007/s00592-020-01570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masana L., Correig E., Rodriguez-Borjabad C., Anoro E., Arroyo J.A., Jerico C., Pedragosa A., Miret M., Naf S., Pardo A., et al. Effect of statin therapy on SARS-COV-2 infection-related mortality in hospitalized patients. Eur. Heart J. Cardiovasc. Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPadden J., Warner F., Young H.P., Hurley N.C., Pulk R.A., Singh A., Durant T.J., Gong G., Desai N., Haimovich A., et al. Clinical characteristics and outcomes for 7,995 patients with SARS-CoV-2 infection. medRxiv. 2020 doi: 10.1101/2020.07.19.20157305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirani M., Favacchio G., Carrone F., Betella N., Biamonte E., Morenghi E., Mazziotti G., Lania A.G. Impact of comorbidities and glycemia at admission and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes with COVID-19: a case series from an academic hospital in Lombardy, Italy. Diabetes Care. 2020;43:3042–3049. doi: 10.2337/dc20-1340. [DOI] [PubMed] [Google Scholar]
- Moon S.J., Rhee E.J., Jung J.H., Han K.D., Kim S.R., Lee W.Y., Yoon K.H. Independent impact of diabetes on the severity of coronavirus disease 2019 in 5,307 patients in South Korea: a nationwide cohort study. Diabetes Metab. J. 2020;44:737–746. doi: 10.4093/dmj.2020.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriconi D., Masi S., Rebelos E., Virdis A., Manca M.L., De Marco S., Taddei S., Nannipieri M. Obesity prolongs the hospital stay in patients affected by COVID-19, and may impact on SARS-COV-2 shedding. Obes. Res. Clin. Pract. 2020;14:205–209. doi: 10.1016/j.orcp.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidich S.D., Green W.D., Rebeles J., Karlsson E.A., Schultz-Cherry S., Noah T.L., Chakladar S., Hudgens M.G., Weir S.S., Beck M.A. Increased risk of influenza among vaccinated adults who are obese. Int. J. Obes. 2017;41:1324–1330. doi: 10.1038/ijo.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilles E.J., Siddiqui S.M., Fischinger S., Bartsch Y.C., de Saint Aubin M., Zhou G., Gluck M., Berger S., Rhee J., Petersen E., et al. Epidemiological and immunological features of obesity and SARS-CoV-2. medRxiv. 2020 doi: 10.1101/2020.11.11.20229724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter S.D., Ovsyannikova I.G., Poland G.A. The weight of obesity on the human immune response to vaccination. Vaccine. 2015;33:4422–4429. doi: 10.1016/j.vaccine.2015.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Belmonte L.M., Torres-Peña J.D., López-Carmona M.D., Ayala-Gutiérrez M.M., Fuentes-Jiménez F., Huerta L.J., Muñoz J.A., Rubio-Rivas M., Madrazo M., Garcia M.G., et al. SEMI-COVID-19 Network Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: a nationwide cohort study. BMC Med. 2020;18:359. doi: 10.1186/s12916-020-01832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S.A.E., MacMahon S., Woodward M. Obesity as a risk factor for COVID-19 mortality in women and men in the UK biobank: comparisons with influenza/pneumonia and coronary heart disease. Diabetes Obes. Metab. 2021;23:258–262. doi: 10.1111/dom.14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L.R., Sami S., Vuong N., Pathela P., Weiss D., Morgenthau B.M., Henseler R.A., Daskalakis D.C., Atas J., Patel A., et al. Lack of antibodies to SARS-CoV-2 in a large cohort of previously infected persons. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro T.A., Barcala-Jorge A.S., Andrade J.M.O., Pinheiro T.A., Ferreira E.C.N., Crespo T.S., Batista-Jorge G.C., Vieira C.A., Lelis D.F., Paraíso A.F., et al. Obesity and malnutrition similarly alter the renin-angiotensin system and inflammation in mice and human adipose. J. Nutr. Biochem. 2017;48:74–82. doi: 10.1016/j.jnutbio.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R.M., Donath M.Y., LeRoith D., Leibowitz G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care. 2016;39(Suppl 2):S244–S252. doi: 10.2337/dcS15-3015. [DOI] [PubMed] [Google Scholar]
- Recalde M., Pistillo A., Fernandez-Bertolin S., Roel E., Aragon M., Freisling H., Prieto-Alhambra D., Burn E., Duarte-Salles T. Body mass index and risk of COVID-19 diagnosis, hospitalisation, and death: a population-based multi-state cohort analysis including 2,524,926 people in Catalonia, Spain. medRxiv. 2020 doi: 10.1101/2020.11.25.20237776. [DOI] [Google Scholar]
- Rhee S.Y., Lee J., Nam H., Kyoung D.-S., Kim D.J. Effects of a DPP-4 inhibitor and RAS blockade on clinical outcomes of patients with diabetes and COVID-19. medRxiv. 2020 doi: 10.1101/2020.05.20.20108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riahi S., Sombra L.R.S., Lo K.B., Chacko S.R., Neto A.G.M., Azmaiparashvili Z., Patarroyo-Aponte G., Rangaswami J., Anastasopoulou C. Insulin use, diabetes control, and outcomes in patients with COVID-19. Endocr. Res. 2020 doi: 10.1080/07435800.2020.1856865. [DOI] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., et al. the Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E., Boyland E., Chisholm A., Harrold J., Maloney N.G., Marty L., Mead B.R., Noonan R., Hardman C.A. Obesity, eating behavior and physical activity during COVID-19 lockdown: a study of UK adults. Appetite. 2021;156:104853. doi: 10.1016/j.appet.2020.104853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues T.S., de Sá K.S.G., Ishimoto A.Y., Becerra A., Oliveira S., Almeida L., Gonçalves A.V., Perucello D.B., Andrade W.A., Castro R., et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021;218:e20201707. doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed O., Castagna F., Agalliu I., Xue X., Patel S.R., Rochlani Y., Kataria R., Vukelic S., Sims D.B., Alvarez C., et al. Statin use and in-hospital mortality in diabetics with COVID-19. J. Am. Heart Assoc. 2020;9:e018475. doi: 10.1161/JAHA.120.018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury C., Wang J., Gokhale K., Acosta-Mena D., Dhalla S., Byne N., Chandan J.S., Anand A., Cooper J., Okoth K., et al. Sodium-glucose co-transporter-2 inhibitors and susceptibility to COVID-19: a population-based retrospective cohort study. Diabetes Obes. Metab. 2021;23:263–269. doi: 10.1111/dom.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Shimizu T., Fujita H., Imai Y., Drucker D.J., Seino Y., Yamada Y. GLP-1 receptor signaling differentially modifies the outcomes of sterile vs viral pulmonary inflammation in male mice. Endocrinology. 2020;161:bqaa201. doi: 10.1210/endocr/bqaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarraj J.P., Burkett A.B., Hinds S.N., Paz A.S., Assing A., Juneja S., Colpo G.D., Torres L.F., Gusdon A., McCullough L., et al. Three-month outcomes in hospitalized COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.10.16.20211029. [DOI] [Google Scholar]
- Scalsky R.J., Desai K., Chen Y.J., O’Connell J.R., Perry J.A., Hong C.C. Baseline cardiometabolic profiles and SARS-CoV-2 risk in the UK biobank. medRxiv. 2020 doi: 10.1101/2020.07.25.20161091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht K., Rohmann N., Geisler C., Hollstein T., Knappe C., Hartmann K., Schwarz J., Tran F., Schunk D., Junker R., et al. Circulating levels of soluble dipeptidylpeptidase-4 are reduced in human subjects hospitalized for severe COVID-19 infections. Int. J. Obes. 2020;44:2335–2338. doi: 10.1038/s41366-020-00689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurink B., Roos E., Radonic T., Barbe E., Bouman C.S.C., de Boer H.H., de Bree G.J., Bulle E.B., Aronica E.M., Florquin S., et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstolpe Å., Palasantza A., Eliasson P., Andersson E.M., Andréasson A.C., Sun X., Picelli S., Sabirsh A., Clausen M., Bjursell M.K., et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 2016;24:593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin E., Juraschek S.P. Diabetes epidemiology in the COVID-19 pandemic. Diabetes Care. 2020;43:1690–1694. doi: 10.2337/dc20-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan P.A., Paich H.A., Handy J., Karlsson E.A., Hudgens M.G., Sammon A.B., Holland L.A., Weir S., Noah T.L., Beck M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012;36:1072–1077. doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A.M., Faustini S.E., Perez-Toledo M., Jossi S., Allen J.D., Al-Taei S., Backhouse C., Dunbar L., Ebanks D., Emmanuel B., et al. Serological responses to SARS-CoV-2 following non-hospitalised infection: clinical and ethnodemographic features associated with the magnitude of the antibody response. medRxiv. 2020 doi: 10.1101/2020.11.12.20230763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smati S., Tramunt B., Wargny M., Caussy C., Gaborit B., Vatier C., Vergès B., Ancelle D., Amadou C., Bachir L.A., et al. CORONADO investigators Relationship between obesity and severe COVID-19 outcomes in patients with type 2 diabetes: results from the CORONADO study. Diabetes Obes. Metab. 2021;23:391–403. doi: 10.1111/dom.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldo J., Heni M., Königsrainer A., Häring H.U., Birkenfeld A.L., Peter A. Increased hepatic ACE2 expression in NAFL and diabetes-a risk for COVID-19 patients? Diabetes Care. 2020;43:e134–e136. doi: 10.2337/dc20-1458. [DOI] [PubMed] [Google Scholar]
- Solerte S.B., D’Addio F., Trevisan R., Lovati E., Rossi A., Pastore I., Dell’Acqua M., Ippolito E., Scaranna C., Bellante R., et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diabetes Care. 2020;43:2999–3006. doi: 10.2337/dc20-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa-Rubí S.G., Seiglie J.A., Chivardi C., Manne-Goehler J., Meigs J.B., Wexler D.J., Wirtz V.J., Gómez-Dantés O., Serván-Mori E. Incremental risk of developing severe COVID-19 among Mexican patients with diabetes attributed to social and health care access disadvantages. Diabetes Care. 2021;44:373–380. doi: 10.2337/dc20-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner C.D., Gottlieb R.L., Criner G.J., Arribas López J.R., Cattelan A.M., Soriano Viladomiu A., Ogbuagu O., Malhotra P., Mullane K.M., Castagna A., et al. GS-US-540-5774 Investigators Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C.E., Capdevila Pujol J., Klaser K., Antonelli M., Canas L.S., et al. Attributes and predictors of Long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv. 2020 doi: 10.1101/2020.10.19.20214494. [DOI] [Google Scholar]
- Sun W., Dong H., Balaz M., Slyper M., Drokhlyansky E., Colleluori G., Giordano A., Kovanicova Z., Stefanicka P., Balazova L., et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature. 2020;587:98–102. doi: 10.1038/s41586-020-2856-x. [DOI] [PubMed] [Google Scholar]
- Taneera J., El-Huneidi W., Hamad M., Mohammed A.K., Elaraby E., Hachim M.Y. Expression profile of SARS-CoV-2 host receptors in human pancreatic islets revealed upregulation of ACE2 in diabetic donors. Biology (Basel) 2020;9:215. doi: 10.3390/biology9080215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittel S.R., Rosenbauer J., Kamrath C., Ziegler J., Reschke F., Hammersen J., Mönkemöller K., Pappa A., Kapellen T., Holl R.W., DPV Initiative Did the COVID-19 lockdown affect the incidence of pediatric type 1 diabetes in Germany? Diabetes Care. 2020;43:e172–e173. doi: 10.2337/dc20-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinder M., Walley K.R., Boyd J.H., Brunham L.R. Causal inference for genetically determined levels of high-density lipoprotein cholesterol and risk of infectious disease. Arterioscler. Thromb. Vasc. Biol. 2020;40:267–278. doi: 10.1161/ATVBAHA.119.313381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truwit J.D., Bernard G.R., Steingrub J., Matthay M.A., Liu K.D., Albertson T.E., Brower R.G., Shanholtz C., Rock P., Douglas I.S., et al. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N. Engl. J. Med. 2014;370:2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankov B.K., Allaire J.M., Irvine M.A., Lopez A.A., Sauvé L.J., Vallance B.A., Jacobson K. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;103:246–256. doi: 10.1016/j.ijid.2020.11.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth R., Wallace S., Oliver N.S., Yeung S., Kshirsagar A., Naidu H., Kwong R.M.W., Kumar P., Logan K.M. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care. 2020;43:e170–e171. doi: 10.2337/dc20-1551. [DOI] [PubMed] [Google Scholar]
- Vangoitsenhoven R., Martens P.J., van Nes F., Moyson C., Nobels F., Van Crombrugge P., Wierckx K., van Pottelbergh I., Van Huffel L., Gillard P., Mathieu C. No evidence of increased hospitalization rate for COVID-19 in community-dwelling patients with type 1 diabetes. Diabetes Care. 2020;43:e118–e119. doi: 10.2337/dc20-1246. [DOI] [PubMed] [Google Scholar]
- Vehik K., Lynch K.F., Wong M.C., Tian X., Ross M.C., Gibbs R.A., Ajami N.J., Petrosino J.F., Rewers M., Toppari J., et al. TEDDY Study Group (2019). Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat. Med. 2019;25:1865–1872. doi: 10.1038/s41591-019-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay J., Gauthier M.F., Biswell R.L., Louiselle D.A., Johnston J.J., Cheung W.A., Belden B., Pramatarova A., Biertho L., Gibson M., et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab. 2020;2:97–109. doi: 10.1038/s42255-019-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Oxford COVID Vaccine Trial Group Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Zhang Q., Zhao X., Dong H., Wu C., Wu F., Yu B., Lv J., Zhang S., Wu G., et al. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: an observational study. Lipids Health Dis. 2020;19:204. doi: 10.1186/s12944-020-01382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Simoneau C.R., Kulsuptrakul J., Bouhaddou M., Travisano K., Hayashi J.M., Carlson-Stevermer J., Oki J., Holden K., Krogan N.J., et al. Functional genomic screens identify human host factors for SARS-CoV-2 and common cold coronaviruses. bioRxiv. 2020 doi: 10.1101/2020.09.24.312298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Li W., Hui H., Tiwari S.K., Zhang Q., Croker B.A., Rawlings S., Smith D., Carlin A.F., Rana T.M. Cholesterol 25-hydroxylase inhibits SARS-CoV-2 and other coronaviruses by depleting membrane cholesterol. EMBO J. 2020;39:e106057. doi: 10.15252/embj.2020106057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargny M., Gourdy P., Ludwig L., Seret-Bégué D., Bourron O., Darmon P., Amadou C., Pichelin M., Potier L., Thivolet C., et al. CORONADO investigators Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO Study. Diabetes Care. 2020;43:e174–e177. doi: 10.2337/dc20-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Wan L., Yan Q., Wang X., Zhang J., Yang X., Zhang Y., Fan C., Li D., Deng Y., et al. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat Metab. 2020;2:1391–1400. doi: 10.1038/s42255-020-00324-0. [DOI] [PubMed] [Google Scholar]
- Wei J., Alfajaro M.M., DeWeirdt P.C., Hanna R.E., Lu-Culligan W.J., Cai W.L., Strine M.S., Zhang S.M., Graziano V.R., Schmitz C.O., et al. Genome-wide CRISPR screens reveal host factors critical for SARS-CoV-2 infection. Cell. 2021;184:76–91.e13. doi: 10.1016/j.cell.2020.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolcott O.O., Castilla-Bancayán J.P. Diabetes and mortality among 1.6 million adult patients screened for SARS-CoV-2 in Mexico. medRxiv. 2020 doi: 10.1101/2020.11.25.20238345. [DOI] [Google Scholar]
- Xi C.R., Di Fazio A., Nadvi N.A., Patel K., Xiang M.S.W., Zhang H.E., Deshpande C., Low J.K.K., Wang X.T., Chen Y., et al. A novel purification procedure for active recombinant human DPP4 and the inability of DPP4 to bind SARS-CoV-2. Molecules. 2020;25:5392. doi: 10.3390/molecules25225392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Han Y., Nilsson-Payant B.E., Gupta V., Wang P., Duan X., Tang X., Zhu J., Zhao Z., Jaffré F., et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–136.e7. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Li C., Sun Y., Wang D.W. Insulin treatment is associated with increased mortality in patients with COVID-19 and type 2 diabetes. Cell Metab. 2021;33:65–77.e2. doi: 10.1016/j.cmet.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang R., Case J.B., Yutuc E., Ma X., Shen S., Gomez Castro M.F., Liu Z., Zeng Q., Zhao H., Son J., et al. Cholesterol 25-hydroxylase suppresses SARS-CoV-2 replication by blocking membrane fusion. Proc. Natl. Acad. Sci. USA. 2020;117:32105–32113. doi: 10.1073/pnas.2012197117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.J., Qin J.J., Cheng X., Shen L., Zhao Y.C., Yuan Y., Lei F., Chen M.M., Yang H., Bai L., et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32:176–187.e4. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.H., Wu B., Wang W.X., Lei F., Cheng X., Qin J.J., Cai J.J., Zhang X.J., Zhou F., Liu Y.M., et al. No significant association between dipeptidyl peptidase-4 inhibitors and adverse outcomes of COVID-19. World J. Clin. Cases. 2020;8:5576–5588. doi: 10.12998/wjcc.v8.i22.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., Lei F., Wang H., Xie J., Wang W., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]