Abstract
Background
Care homes have been disproportionately affected by the COVID-19 pandemic. We investigated the potential role of asymptomatic infection and silent transmission in London care homes that reported no cases of COVID-19 during the first wave of the pandemic.
Methods
Five care homes with no cases and two care homes reporting a single case of COVID-19 (non-outbreak homes) were investigated with nasal swabbing for SARS-CoV-2 RT-PCR and serology for SARS-CoV-2 antibodies five weeks later. Whole genome sequencing (WGS) was performed on RT-PCR positive samples. Serology results were compared with those of six care homes with recognised outbreaks.
Findings
Across seven non-outbreak homes, 718 (387 staff, 331 residents) individuals had a nasal swab and 651 (386 staff, 265 residents) had follow-up serology. Sixteen individuals (13 residents, 3 staff) in five care homes with no reported cases were RT-PCR positive (care home positivity rates, 0 to 7.6%) compared to 13 individuals (3.0 and 10.8% positivity) in two homes reporting a single case.
Seropositivity across these seven homes varied between 10.7-56.5%, with four exceeding community seroprevalence in London (14.8%). Seropositivity rates for staff and residents correlated significantly (rs 0.84, [95% CI 0.51-0.95] p <0.001) across the 13 homes. WGS identified multiple introductions into some homes and silent transmission of a single lineage between staff and residents in one home.
Interpretation
We found high rates of asymptomatic infection and transmission even in care homes with no COVID-19 cases. The higher seropositivity rates compared to RT-PCR positivity highlights the true extent of the silent outbreak.
Funding
PHE
Keywords: SARS-CoV-2 outbreak, Care home, Asymptomatic transmission
Research in context.
Evidence before this study
We searched PubMed with the terms “COVID-19” or “SARS-CoV-2” and “care home”, “nursing home”, “nursing facility” or “residential home” to identify publications relating to SARS-CoV-2 infections and COVID-19 outbreaks since January 2020, focusing particularly on enhanced outbreak investigations and antibody testing. Mass swabbing identified high rates of symptomatic and asymptomatic SARS-CoV-2 infections among residents and staff. There are limited investigations involving antibody testing for evidence of prior exposure to SARS-CoV-2 or to assess immunity against the virus in residents or staff members.
Added value of this study
We investigated the degree of exposure to SARS-CoV-2 in five care homes with no reported or suspected cases of COVID-19 and two care homes with single cases. Swabbing for SARS-CoV-2 RNA identified infection rates of 0 to 7.6% in care homes with no cases, half of whom remained asymptomatic, and 3.0-10.8% in homes reporting a single case. SARS-CoV-2 seropositivity ranged between 10.7-56.5% in non-outbreak care homes. There was a strong and significant correlation in seropositivity rates between staff and residents across all the care homes investigated (rs 0.84, p < 0.001).
Implications of all the available evidence
Given the high fatality rates associated with COVID-19 in care home residents, regular screening with rapid isolation of infected residents and staff may be the only effective option for preventing SARS-CoV-2 infection and transmission in these high-risk settings during the COVID-19 pandemic. Additionally, serological investigations provide a more accurate assessment of exposure in institutional settings and are critical for understanding of immune correlates of protection from reinfection and to inform future vaccination policy.
Alt-text: Unlabelled box
1. Introduction
Care homes have been particularly affected by COVID-19, with large outbreaks associated with high morbidity and mortality among residents [1], [2], [3], [4]. In England, the first imported cases of COVID-19 were confirmed at the end of January 2020. Cases started increasing rapidly from early March 2020 and the first wave plateaued in mid-April 2020 before declining [5] At the peak of the pandemic, the large number of COVID-19 outbreaks in care homes across London, one of the earliest affected areas in the UK, prompted outbreak investigations by Public Health England (PHE). Initially, six care homes reporting an outbreak to the PHE London Coronavirus Response Centre (LCRC) during 10-13 April 2020 were investigated with nasal swabbing of more than 500 residents and staff, with 45% of residents and 20% of staff found to be infected with SARS-CoV-2 (Phase 1) [4]. Around 80% of those infected were asymptomatic at the time of testing and half never developed any symptoms throughout their infection. Further outbreak investigations were initiated one week later in four care homes reporting only a single confirmed or suspected case of COVID-19 (Phase 2). In two of these care homes the virus had already spread extensively, affecting 32% and 61% of residents and staff, respectively [6]. In the other two homes, however, only 3% and 11% tested positive for SARS-CoV-2 infection. Infected residents and staff were isolated and stringent infection prevention and control (IPC) practices were reinforced in all four homes. Repeat swabbing a week later confirmed no further spread of the virus.
Here we report Phase 3 of the care home investigations which aimed to assess the presence of silent infection and transmission in five London care homes with no suspected or confirmed cases of COVID-19 at the peak of the pandemic. In addition to the initial swabbing of all residents and staff, blood samples for SARS-CoV-2 antibodies were obtained in the five care homes with no evidence of infection and two care homes with limited SARS-CoV-2 infection in the second phase of the investigations. Evidence of virus exposure by either nasal swab SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) positivity or SARS-CoV-2 antibody positivity were compared between these seven ‘non-outbreak’ homes and the initial six outbreak homes [4,7].
2. Methods
2.1. Recruitment
The PHE public health response to understand the effects of the COVID-19 pandemic on care homes involved enhanced surveillance of care homes reporting different COVID-19 case profiles in London. Six care homes, referred to as ‘outbreak’ homes, reporting at least two confirmed or suspected COVID-19 cases to the PHE London Health Protection Teams (HPTs) in early April 2020 were recruited into the first phase of the London care home investigations [4]. A week later, four care homes reporting a single case of suspected or confirmed COVID-19 were investigated in the second phase of the investigation;[6] two of these homes with low PCR positivity rates (<15%) were subsequently recruited to follow-up serological assessment. In the following week, care homes without any suspected or confirmed cases of COVID-19 were identified by cross-referencing the care home register held by the Care Quality Commission (CQC), the independent regulator of health and social care in England, with data held by local HPTs. The managers of these care homes were contacted to confirm that no suspected or confirmed cases of COVID-19 had been identified previously in residents or staff. Five care homes where the manager consented to participation on behalf of all their residents and staff (those with and without regular contact with residents) were recruited in phase 3 of the investigation to undertake nasal swab RT-PCR testing for SARS-CoV-2 and serum sampling. The care homes had experienced nursing staff and were provided with detailed written instructions for nasal swabbing. The staff swabbed themselves and the residents in their respective care homes. Symptom status during the 14 days before and at the time of swabbing was collected for all staff, who self-reported any symptoms, and residents, whose symptoms were recorded the care home staff. Daily interviews were undertaken with individual care homes to identify any newly symptomatic individuals. Typical COVID-19 symptoms at that time included fever ≥37.8⁰C, shortness of breath or cough, while atypical symptoms included, but were not restricted to, new confusion, reduced alertness, fatigue, lethargy, reduced mobility and diarrhoea. Serum samples were obtained a minimum of five weeks after the initial nose swabs and were taken by nursing staff working in the respective care homes. The five homes with no recognised cases and two homes with single cases and PCR positivity <15% recruited to serological follow-up are collectively referred to as ‘non-outbreak’ homes.
The investigation protocol was reviewed and approved by the PHE Research Ethics and Governance Group. PHE has legal permission, provided by Regulation 3 of the Health Service (Control of Patient Information) Regulation 2002, to process patient confidential information for national surveillance of communicable diseases. Verbal consent for testing was obtained by care home managers from staff members and residents or their next of kin as appropriate.
2.2. SARS-CoV-2 RT-PCR
Nucleic acid was extracted from samples and analysed by a previously described RT-PCR assay [8], targeting a conserved region of the open reading frame (ORF1ab) gene of SARS CoV-2, together with detection of an assay internal control to monitor the extraction and RT-PCR processes. This assay required 5μL RNA in a total RT-PCR reaction volume of 25μL. Reverse transcription and PCR amplification was performed on an Applied Biosystems 7500 FAST system.
2.3. SARS-CoV-2 antibody testing
Serum samples were tested for IgG to the SARS-CoV-2 nucleocapsid (N) protein using a commercial antibody test (Abbott®) following the manufacturer's instructions and were also tested using an in-house recombinant SARS-CoV-2 IgG spike (S) protein receptor binding domain (RBD) indirect ELISA (PHE). Briefly, 96 well microtiter plates (Nunc, Cat-439454) coated with recombinant SARS-CoV-2 RBD (Sino Biological Inc, Cat-40592-V05H) were blocked with phosphate buffered saline solution with 5% milk, 1% bovine serum albumin and 0.05% Tween, and incubated with serum at 1:100 dilution. RBD specific IgG antibodies present in serum bound by the plate coated antigen were detected using a polyclonal goat anti-human Fab secondary antibody horseradish peroxidase conjugate (Sigma, Cat-A0293). The sera were analysed in duplicate on each plate. Each plate also contained at least one positive control human serum in duplicate, and a negative human serum (true negative, collected prior to the pandemic), analysed in four wells. For analysis, mean optical density (OD) values were calculated for each study serum, the controls and negative sera. In-house RBD IgG results are presented as ratios of the OD of the sample to the OD of the true negative (TN), analysed on the same plate. OD/TN ratios of greater than or equal to 5.0 were considered positive. This cut-off value was determined through analysis of ROC curves determined for a mixture of positive and negative samples (N positive 325, N negative 1431, N total 1756). Positive samples were collected from individuals with PCR-confirmed SARS-CoV-2 infection, while negatives were collected before January 2020. The cut-off resulted in a specificity of 98.1% (95% CI 97.3-98.8) and sensitivity of 89.8% (95% CI 86.0-92.9). Qualitative results and index values were used in analyses.
2.4. Whole genome sequencing
Whole genome sequencing (WGS) was performed on all RT-PCR positive samples [9]. Viral amplicons were sequenced using Illumina library preparation kits (Nextera) and sequenced on Illumina short-read sequencing machines (Nextseq or Hiseq). The bioinformatics protocol to generate consensus sequences utilised Trimmomatic, BWA (mapping), and an in-house variant caller (quasibam) to align against a SARS-CoV-2 reference genome (NC_045512.2). Consensus sequences were generated using a depth cut-off of 20 reads and ambiguities called where a minority variant detected at ≥20%, these were aligned using MAFFT (Multiple Alignment using Fast Fourier Transform, version 7.310), manually curated and maximum likelihood phylogenetic trees derived using IQtree (version 2.04). Genomes were included in analysis where the coverage of the reference genomes was ≥80%. Completed viral genomes were deposited in GISAID (Supplementary Table 1) [10].
2.5. Statistical analysis
Descriptive analyses were performed. Continuous data that did not follow a normal distribution were described as medians with interquartile ranges and differences compared using the Mann-Whitney U test. Seropositivity rates between outbreak and non-outbreak care homes were also compared using Mann-Whitney U test. Concordance of antibody positivity to N and S (RBD) protein was assessed using McNemar's test. Abbott SARS-CoV-2 IgG results are presented as index values with medians and 95% confidence intervals (95% CI). Antibody index values were compared using Kruskal-Wallis test. Data were analysed using GraphPad Prism. Seropositivity rates were compared to concurrent London community seroprevalence estimates (adjusted prevalence, 14.8%) [11].
2.6. Role of the funding source
This study was funded by PHE. The authors had sole responsibility for the study design, data collection, data analysis, data interpretation, and writing of the report. The authors are all employed by PHE, the study funder, which is a public body — an executive agency of the Department of Health and Social Care.
3. Results
3.1. Demographics
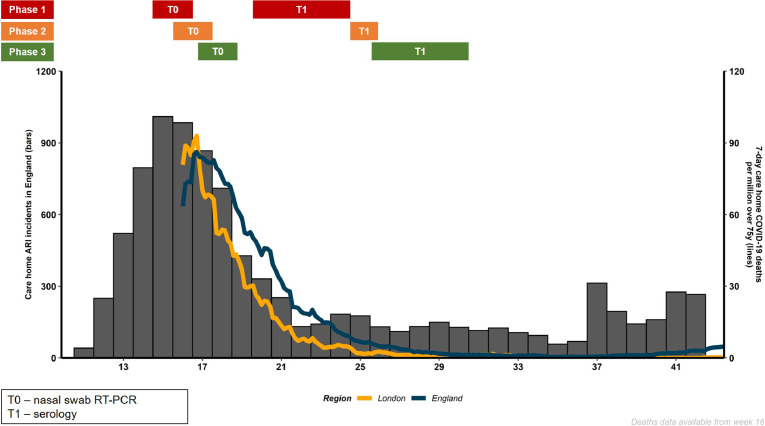
The timing of the investigations in these London care homes in relation to reported outbreaks of acute respiratory illnesses and COVID-19 related deaths in care homes across England is depicted in Fig. 1. The seven non-outbreak care homes included five phase 3 homes with no reported or suspected cases of COVID-19 (A-E), and two phase 2 homes with a single case (F and G). In total, 718 (387 staff, 331 residents) were investigated with a nasal swab for SARS-CoV-2 RT-PCR at the first visit (timepoint T0) and 651 (386 staff, 265 residents) consented to SARS-CoV-2 antibody testing at follow-up (T1), including 570 (87.6%) individuals (318 staff, 252 residents) who also had the initial T0 nasal swab. One asymptomatic PCR positive resident from the care homes with no reported cases died in the week following the positive test; this individual had been receiving end of life care in the preceding weeks. Two PCR positive residents from the care homes with single cases died, one on the day of the positive test and the other 11 days later. Both experienced COVID-19 symptoms prior to the test and the deaths were attributed to COVID-19.
Fig. 1.
Schematic of care home acute respiratory outbreaks (ARI; blue bars) and total COVID-19 related deaths in care homes in England (pink line) and London (yellow line) reported by ISO week of 2020. The enhanced outbreak testing periods for nasal swabbing SARS-CoV-2 RT-PCR - time-point 0 (T0) - and serology for SARS-CoV-2 antibodies – time-point 1 (T1) - are indicated for the care homes with outbreaks (phase 1 - red), single cases (phase 2 - amber) and those with no reported cases (phase 3 - green).
From the six phase 1 outbreak care homes (H-M), 586 (370 staff, 216 residents) consented to SARS-CoV-2 antibody testing at T1, 405 (69.1%) of whom (209 staff, 196 residents) had undergone nasal swabbing for SARS-CoV-2 RT-PCR at T0. Across these six homes, 23 residents died during the five-week interval between RT-PCR and serological testing, including 19 who had been SARS-CoV-2 RT-PCR positive at a median of 4 (IQR 2 to 8) days prior to death.
3.2. Care home characteristics
Between the non-outbreak (N=7) and outbreak (N=6) homes, there were no differences in the number of beds, percentage occupancy or number of staff. All six outbreak care homes were nursing care homes providing a range of specialty support, compared to four of the seven non-outbreak care homes (Table 1). Using criteria applied by regulators of care homes, there were no differences in the characteristics of the care homes or case mix (Table 1).
Table 1.
Care home characteristics.
| Care home | Type of care (CQC) | Beds‡ | Occupancy (%) | No. staff | Registered Care Categories (CQC)† | Min age | CQC rating |
|---|---|---|---|---|---|---|---|
| A | Residential | 50 | 92.3 | 30 | Dementia | 50 | Good |
| B | Nursing | 60 | 88.3 | 78* | Dementia, old age | 65 | Good |
| C | Residential | 50 | 86.7 | 58 | Dementia, old age | 65 | Good |
| D | Nursing | 110 | 40.4 | 45 | Dementia, old age, sensory impairment | 65 | Requires improvement |
| E | Nursing | 60 | 79.7 | 69 | Dementia, eating disorders, learning disability, mental health condition, old age, physical disability, sensory impairment, substance misuse | 40 | Good |
| F | Residential | 50 | 87.8 | 34 | Dementia, learning disability, old age | 65 | Good |
| G | Nursing | 60 | 85.0 | 106 | Old age, physical disability | 65 | Good |
| H | Nursing | 100 | 90.0 | 135 | Dementia, mental health condition, old age, physical disability | 60 | Good |
| I | Nursing | 80 | 76.9 | 85 | Dementia, old age | 60 | Requires improvement |
| J | Nursing | 90 | 84.7 | 110 | Dementia, mental health condition, old age, physical disability, sensory impairment | 65 | Good |
| K | Nursing | 40 | 90.7 | 14 | Old age | NS | Good |
| L | Nursing | 60 | 90.6 | 70 | Dementia, old age | 65 | Good |
| M | Nursing | 50 | 57.1 | 65 | Old age | 65 | Requires improvement |
minimum number of staff; NS = not specified. Colour coding: red = outbreak reported; amber – single case reported; green = no cases reported at time of investigation. CQC: Care Quality Commission.
Care homes in the UK are regulated by the CQC. Registration with the CQC includes a description of the care categories offered by the home.
Rounded to the nearest 10, analysis has been performed on exact numbers.
3.3. RT-PCR positivity in care homes without reported cases of COVID-19
Across the five care homes (A-E) with no reported COVID-19 cases, nasal swabbing identified 16 SARS-CoV-2 RT-PCR positive individuals (13 residents, 3 staff). Eight (1 staff, 7 residents) reported symptoms during the follow-up period, having been asymptomatic at the time of testing. RT-PCR positive individuals had cycle threshold values ranging between 21 and 37 (median, 35). SARS-CoV-2 RT-PCR positivity rates ranged between 0% and 7.6% across these five care homes (Table 2). Care home B had no SARS-CoV-2 RT-PCR positive individuals, while two care homes (A and C) had SARS-CoV-2 RT-PCR positive residents, but no staff positive. Repeat nasal swabs taken a week later from 14 of the RT-PCR positive individuals (range 3-10 days) found that four (28.6%) remained positive. None were positive two weeks later (15-19 days after the first swab).
Table 2.
Relationship of SARS-CoV-2 PCR positivity on nasal swab at T0 to convalescent IgG positivity to nucleocapsid (N) protein (Abbott®) at T1.
| PCR positive (%) | PCR positive seropositive N(%) | PCR negative seropositive N(%) | Total seropositive N (% [95% CI]) | |
|---|---|---|---|---|
| A | 4/91 (4.4) | 2/3 (66.7) | 16/57 (28.1) | 20/66 (30.3 [20.6 to 42.2]) |
| B | 0/124 (0.0) | NA | 12/112 (10.7) | 14/129 (10.9 [6.6 to 17.4]) |
| C | 1/90 (1.1) | 1/1 (100) | 22/66 (33.3) | 23/70 (32.9 [23.0 to 44.5]) |
| D | 7/92 (7.6) | 3/4 (75) | 30/52 (57.7) | 39/69 (56.5 [44.8 to 67.6]) |
| E | 4/104 (3.8) | 4/4 (100) | 42/84 (50.0) | 53/107 (49.5 [40.2 to 58.9]) |
| F | 9/83 (10.8) | 5/5 (100) | 6/67 (9.0) | 14/79 (17.7 [10.9 to 27.6]) |
| G | 4/134 (3.0) | 1/1 (100) | 12/114 (10.5) | 14/131 (10.7 [6.5 to 17.1]) |
| H | 35/94 (37.2) | 13/15 (86.7) | 21/36 (58.3) | 59/90 (65.9 [55.3 to 74.6]) |
| I | 14/72 (19.4) | 9/11 (81.8) | 31/45 (69.9) | 58/84 (68.3 [57.6 to 77.4]) |
| J | 23/98 (23.5) | 15/17 (88.2) | 61/73 (83.6) | 124/153 (81.0 [74.1 to 86.5]) |
| K | 47/74 (63.5) | 36/39 (92.3) | 15/22 (68.1) | 63/75 (84.0 [74.1 to 90.6]) |
| L | 18/97 (18.6) | 13/14 (92.9) | 24/71 (33.8) | 49/117 (41.9 [33.3 to 50.9]) |
| M | 21/83 (25.3) | 14/16 (87.5) | 39/46 (84.8) | 56/67 (83.6 [72.9 to 90.6]) |
Total seropositivity includes individuals who did not have PCR at T0. The previously reported SARS-CoV-2 RT-PCR positivity for care homes F and G is shown for comparison.6 A subset of results for care homes H-M were included in a previous publication.4 NA: not applicable.
3.4. SARS-CoV-2 seropositivity in care homes independent of outbreak profile
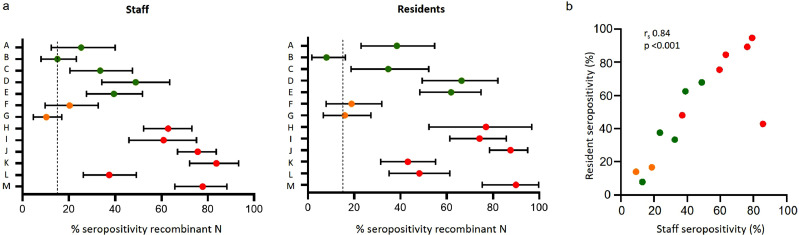
Seropositivity rates were significantly lower in the non-outbreak care homes (A-G) compared to the outbreak homes (H-M) (median seropositivity 30.3% vs. 74.7%; Mann-Whitney U test p=0.0047). Between individual care homes, however, seropositivity rates were highly variable, with seropositivity ranging from 10.7% to 56.5% in care homes A-G and from 41.9% to 84.0% in care homes H-M (Table 2). Four of the non-outbreak care homes (A, C, D, E) and all the outbreak care homes had seropositivity rates exceeding the community estimated seroprevalence in London at the time of the investigation (14.8%). In contrast, care homes B, F and G had seropositivity rates around or below the community seroprevalence, but with seropositivity in both staff and residents within the care homes (Fig. 2).
Fig. 2.
a) Summary data showing percentage IgG seropositivity against SARS-CoV-2 nucleocapsid (N) protein (Abbott®) and 95% confidence intervals for care home staff (left panel) and residents (right panel) for each care home (A-M). Dashed line indicates coincident estimated community seroprevalence in London [11]. b) Correlation of resident and staff seropositivity for each of the 13 care homes (Spearman rank correlation coefficient 0.84, p < 0.001). Colour coding: green = no cases reported at time of investigation; amber = single case reported; red = outbreak reported. Red defined as ‘outbreak’ homes; Amber and green homes defined as ‘non-outbreak’ homes.
3.5. Seroconversion in SARS-CoV-2 RT-PCR positive staff and residents
Of the 187 SARS-CoV-2 RT-PCR positive individuals (60 staff, 127 residents) across the 13 London care homes, 130 (48 staff, 82 residents) had follow-up serology, including 126 (96.9%) individuals (47 staff, 79 residents) who seroconverted a median 36 days (IQR 35 to 38) after their positive SARS-CoV-2 RT-PCR nasal swab. Within this cohort, 116 (92.1%; 45 staff, 71 residents) had detectable SARS-CoV-2 antibodies to the N protein and 123 (97.6%; 47 staff, 76 residents) to S protein in the RBD assay (McNemar's test of difference between N and S (RBD) test outcomes, p=0.096) (Table 2; Supplement Figure 1).
3.6. Seropositivity rates in staff and residents
Seropositivity rates for staff and residents in non-outbreak care homes (amber and green) were significantly lower than those of outbreak care homes (red); the Mann-Whitney U test for staff seropositivity between outbreak and non-outbreak homes was p=0.0047 and for residents was p=0.014) (Fig. 2a). Within the 13 homes, there was a strong and significant correlation in seropositivity rates for staff and resident (rs 0.84, [95% CI 0.51-0.95] p<0.001) (Fig. 2b).
3.7. Seropositivity by age
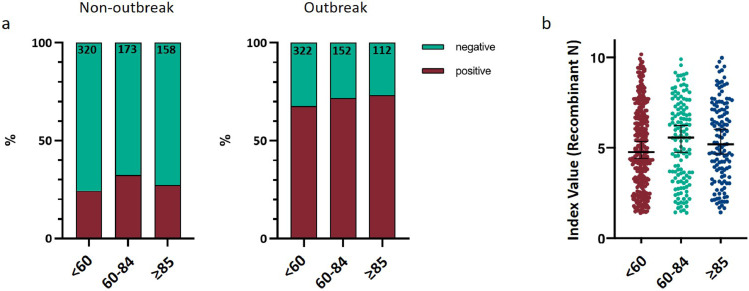
SARS-CoV-2 seropositivity rates did not differ by age in non-outbreak care homes (Kruskal-Wallis test p=0.84) or outbreak care homes (Kruskal Wallis test p=0.72) (Fig. 3a). Across all thirteen care homes there was no significant difference in reactivity to the N based assay by age for seropositive individuals (Kruskal Wallis test p=0.30) (Fig. 3b).
Fig. 3.
a) Summary data showing IgG serostatus to SARS-CoV-2 N protein by age for non-outbreak care homes (left panel, N=651) and outbreak care homes (right panel, N=586). Statistical analysis using Kruskal-Wallis test non outbreak homes p=0.84. Statistical analysis using Kruskal-Wallis test outbreak homes p=0.58. b) Summary data showing SARS-CoV-2 recombinant N IgG index value (Abbott®) for all seropositive individuals (n=586) for all care homes. Kruskal Wallis test p=0.30. Age bracket of <60 applied to encompass majority of staff. Categories of 60-84 and ≥85 applied to separate residents based on frequency distribution of age of residents from across the cohort and mean age of 85 years.
3.8. Genomics analysis of non-outbreak care homes
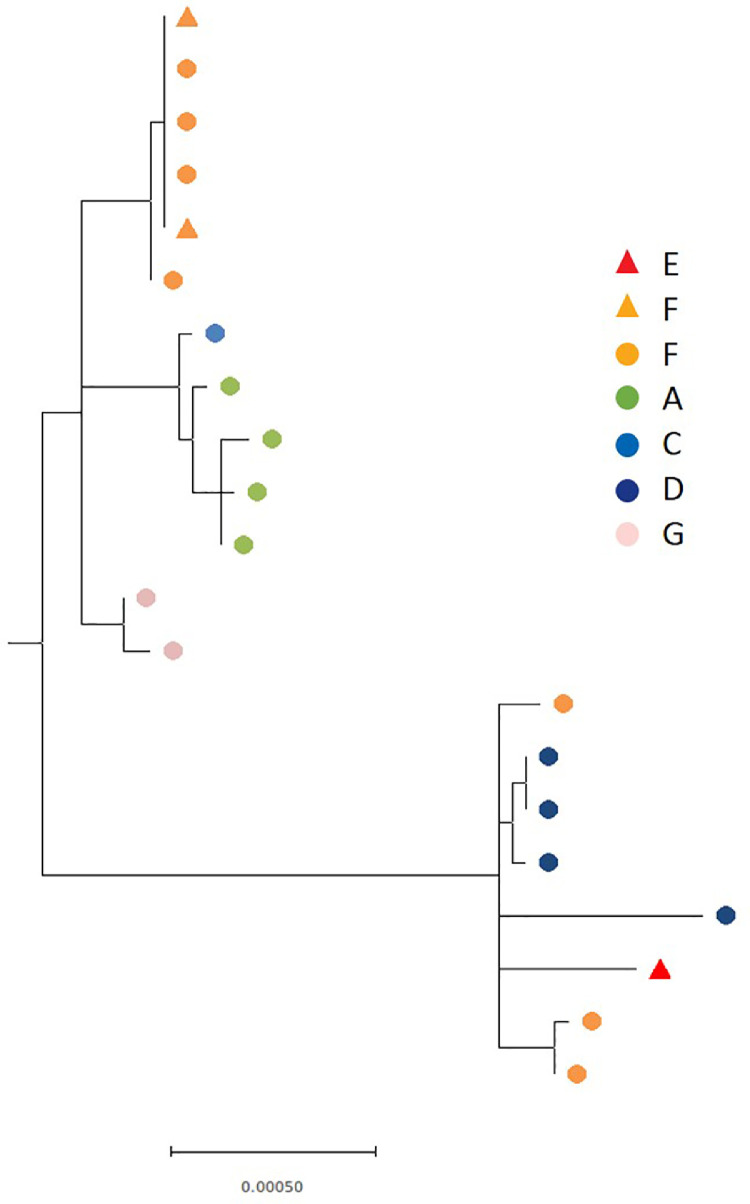
The 29 SARS-CoV-2 RT-PCR positive samples from the seven non-outbreak care homes (16 from care homes A-E, and 13 from care homes F-G) taken at time T0 underwent WGS, with 21 samples yielding whole genome sequences (Supplement Table 1). Different SARS-CoV-2 lineages were identified in different care homes (Fig. 4). Care home F had evidence of more than one introduction of SARS-CoV-2 into the home and findings consistent with onward local transmission between staff and residents as demonstrated by identical sequences in 3 residents and 2 staff (Fig. 4). Care homes A, C and G showed strains that were identical or differing by one or two single nucleotide polymorpshisms in at least two residents; local transmission cannot be inferred or excluded on the basis of these results.
Fig. 4.
Maximum likelihood phylogeny of 21 SARS-CoV-2 genomes from individuals across the six non-outbreak care homes with PCR positive individuals. Coloured shapes are used to indicate the care home, with circles denoting residents and triangles staff. The phylogenetic tree was rooted using the midpoint of the phylogeny.
4. Discussion
In addition to widespread infection and transmission of SARS-CoV-2 in care homes reporting an outbreak of COVID-19 and in care homes reporting a single suspected or confirmed case of COVID-19 during the peak of the pandemic in London, England, we found infections in all five care homes that reported no cases or outbreaks of COVID-19 (A-E). Serological testing provided a more complete picture of the extent of virus spread in the non-outbreak care homes, with seropositivity rates ranging between 10.7% to 56.5% compared to SARS-CoV-2 RT-PCR positivity of 0 to 7.6%. These care homes reported no cases or outbreaks since the start of the pandemic despite heightened awareness of staff in these settings, and none of the residents were symptomatic when swabbed at T0. This, together with the high correlation in seropositivity rates between staff and residents within individual care homes and supported by WGS analysis, highlights the ability of SARS-CoV-2 to enter care homes and spread silently between staff and residents.
The variable seropositivity among staff and residents highlights the wide-ranging impact of SARS-CoV-2 on care homes. In particular, seropositivity rates among residents in homes A, C, D and E were as high as those reported in the care homes that experienced large outbreaks. Interestingly, though, there were very few deaths among residents in care homes A, C, D, E during the surveillance period. This may be a consequence of lower case-fatality rates associated with asymptomatic infection compared to symptomatic disease, as we have recently reported, although the reasons for predominance of asymptomatic or mild disease in this cohort remains unclear [4]
In contrast, residents in care homes B, F and G had seropositivity rates around or below the levels observed in the community. It is possible these care homes were in the early stages of an outbreak and that nasal swabbing as part of the care home investigation led to rapid identification and isolation of infected residents and staff members with reinforcement of IPC measures by the PHE investigation team, thus limiting the spread of the virus within the care homes [4,12]
Asymptomatic infection is now well-recognised as a manifestation of SARS-CoV-2 infection, although the proportion of asymptomatic infections reported is highly heterogenous across different populations and settings [[13], [14], [15], [16], [17]] The role of asymptomatic individuals in SARS-CoV-2 transmission remains unclear, but the finding of asymptomatic SARS-CoV-2 infection in care homes that did not report a single case of COVID-19 and genomic evidence of a small cluster of staff and residents infected with the same SARS-CoV-2 lineage in care home F provides strong evidence for silent transmission among asymptomatic residents and staff. This is also supported by the higher seropositivity rates compared to RT-PCR positivity in all the investigated care homes, confirming widespread asymptomatic infection and transmission throughout the course of the pandemic.
There are many factors that might explain differences in SARS-CoV-2 infection and antibody positivity rates between care homes,[18,19] but in our small but intensively investigated cohort we did not observe any significant differences in care home size, number of beds or staff numbers between the outbreak and non-outbreak homes. In addition, analysis of the CQC reports for these care homes did not highlight issues with IPC measures between the outbreak and non-outbreak homes. The six care homes that experienced large COVID-19 outbreaks, however, all provided nursing care compared to three of the seven non-outbreak care homes. Possible explanations include the level of care required for residents in nursing homes precluded effective IPC practices and residents had a higher risk of hospitalisation because of their underlying medical conditions. Early in the pandemic when there was limited testing for SARS-CoV-2, hospitalised residents returning to their care homes were considered a significant source of SARS-CoV-2 introduction. Contact patterns and behaviours inside and outside the care home are also likely to have contributed to the introduction and spread of SARS-CoV-2 in care homes, especially in the early pandemic phase when there were no restrictions of movement for staff, residents or visitors; this was not assessed as part of this investigation.
5. Strengths and limitations
The strength of these early, systematic and progressive care home investigations at the peak of the pandemic in London is the timeliness of the investigations and the comprehensive outcome follow-up. One limitation is that, because there was no testing for SARS-CoV-2 in care homes at the time, we had limited data information on potential cases or deaths in care homes reporting a case or an outbreak of COVID-19 before we began our investigations. Additionally, because of the large number of residents and staff investigated, we also did not collect extensive individual-level information such as co-morbidities, range and duration of individual symptoms or contact patterns between residents and staff. We used PCR testing to estimate the point prevalence of SARS-CoV-2 infection with the knowledge that its performance and utility is dependent on swabbing technique, timing of sampling in relation infection and assay performance [20] Another limitation was that, because serological tests were not available at the time, we did not collect blood samples at the initial investigation which would have allowed assessment of baseline seropositivity and assessment of seroconversion. Finally, only staff working in the care home at the time of testing were included at each time point; as such, those who were self-isolating at home in care homes with identified cases and outbreaks, or those not on shift at the time of testing were not included in the investigation.
6. Implications and conclusions
The high SARS-CoV-2 seropositivity rates among staff and residents in care homes with no recognised COVID-19 outbreaks demonstrates a significant role for asymptomatic infection leading to widespread silent transmission within care homes and highlights the futility of symptom-based screening. Following the London care home investigations, national guidelines have been implemented to recommend mass SARS-CoV-2 RT-PCR testing of care home staff and residents at regular intervals. This strategy will increase detection of asymptomatic infection, but may lead to false positive RT-PCR results, especially during periods of low community infection rates [[20], [21], [22]] Access to point of care devices to rapidly identify highly infectious individuals may alleviate some of the disruption care homes are currently experiencing. Ongoing investigations in the London care homes offer a unique opportunity to understand factors contributing to the varying outcomes between similar high-risk populations [1,18,23] Follow-up investigations are planned to inform the longevity of antibody responses and the role of cellular immunity, while regular RT-PCR screening will detect SARS-CoV-2 infections and help with our understanding of the risk and frequency of re-infections in care home residents and staff. These data will be critical for informing vaccine strategy in this highly vulnerable group.
Funding
This work was supported by Public Health England.
Data sharing statement
The investigation was conducted as Public Health England's duty to manage outbreaks in response the COVID-19 outbreak. There are no additional data for the Care Home Investigation in addition to what we have already reported. Viral genome data are available in the GISAID database.
Declaration of Competing Interests
The authors have nothing to disclose.
Acknowledgements
The authors are very grateful to the care home managers, their staff and the residents; without their support this investigation would not have been possible. In addition, the authors are indebted to Michael Lattimore for his continued assistance in coordinating sample collection and would like to thank the staff in the immunisation and countermeasures department, the virus reference department, PHE operations, the London Coronavirus Response Cell and Field services for their help and support with the investigation.
Footnotes
Key points: High rates of asymptomatic infection and transmission detected in care homes without identified COVID-19 cases highlights silent outbreaks and variable outcomes in these high-risk settings.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100038.
Contributor Information
Anna Jeffery-Smith, Email: anna.jefferysmith@phe.gov.uk.
Shamez Ladhani, Email: shamez.ladhani@phe.gov.uk.
Appendix. Supplementary materials
References
- 1.McMichael TM, Currie DW, Clark S. Epidemiology of covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2008–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belmin J, Um-Din N, Donadio C. Coronavirus disease 2019 outcomes in French nursing homes that implemented staff confinement with residents. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.17533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deaths involving COVID-19 in the care sector, England and Wales - Office for National Statistics.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/deathsinvolvingcovid19inthecaresectorenglandandwales/deathsoccurringupto12june2020andregisteredupto20june2020provisional#deaths-involving-covid-19-among-care-home-residents. Accessed October 11, 2020.
- 4.Ladhani SN, Chow JY, Janarthanan R. Investigation of SARS-CoV-2 outbreaks in six care homes in London, April 2020. EClinicalMedicine. 2020;0(0) doi: 10.1016/j.eclinm.2020.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronavirus (COVID-19) in the UK: UK Summary. https://coronavirus.data.gov.uk/?_ga=2.22164795.1499401167.1602438389-1329507269.1584618230. Accessed October 12, 2020.
- 6.Tang S et al. Mass testing after a single suspected or confirmed case of COVID-19 in London care homes: implications for policy and practice. Submitted. [DOI] [PMC free article] [PubMed]
- 7.Ladhani SN, Jeffery-Smith A, Patel M. High prevalence of SARS-CoV-2 antibodies in care homes affected by COVID-19: Prospective cohort study, England. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100597. November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niu P, Lu R, Zhao L. Three novel real-time RT-PCR assays for detection of COVID-19 virus. China CDC Wkly. 2020;2(25):453–457. doi: 10.46234/ccdcw2020.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quick J. 2020. Spanish journal of legal medicine ARTIC coronavirus method development community 1 more workspace ARTIC amplicon sequencing protocol for MinION for nCoV-2019. January. [DOI] [Google Scholar]
- 10.Shu Y, McCauley J. GISAID: Global initiative on sharing all influenza data – from vision to reality. Eurosurveillance. 2017;22(13):30494. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phe . 2020. Serological surveillance - summary report 3, 6 May 2020. [Google Scholar]
- 12.Lee MH, Lee GA, Lee SH, Park YH. Effectiveness and core components of infection prevention and control programmes in long-term care facilities: a systematic review. J Hosp Infect. 2019;102(4):377–393. doi: 10.1016/j.jhin.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Patel MC, Chaisson LH, Borgetti S. Asymptomatic SARS-CoV-2 infection and COVID-19 mortality during an outbreak investigation in a skilled nursing facility. Clin Infect Dis. June 2020:1–7. doi: 10.1093/cid/ciaa763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Guo M, Wu F. Factors associated with asymptomatic infection in health-care workers with severe acute respiratory syndrome coronavirus 2 infection in Wuhan, China: a multicentre retrospective cohort study. Clin Microbiol Infect. 2020;1 doi: 10.1016/j.cmi.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivett L, Sridhar S, Sparkes D. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020;9 doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arons MM, Hatfield KM, Reddy SC. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020 doi: 10.1056/NEJMoa2008457. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennelly SP, Dyer AH, Noonan C. Asymptomatic carriage rates and case fatality of SARS-CoV-2 infection in residents and staff in Irish nursing homes. Age Ageing. 2020 doi: 10.1093/ageing/afaa220. September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton JK, Bayne G, Evans C. Evolution and effects of COVID-19 outbreaks in care homes: a population analysis in 189 care homes in one geographical region of the UK. Lancet Heal Longev. 2020;1(1):e21–e31. doi: 10.1016/s2666-7568(20)30012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emmerson C, Adamson J, Turner D. Risk factors for outbreaks of COVID-19 in care homes following hospital discharge: a national cohort analysis. SSRN Electron J. 2020 doi: 10.2139/ssrn.3677861. October. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson J, Whiting PF, Brush JE. Interpreting a covid-19 test result. BMJ. 2020;369:m1808. doi: 10.1136/bmj.m1808. [DOI] [PubMed] [Google Scholar]
- 21.Ladhani SN, Chow JY, Atkin S. Regular mass screening for SARS-CoV-2 infection in care homes already affected by COVID-19 outbreaks: implications of false positive test results. J Infect. 2020;0(0):4840. doi: 10.1016/j.jinf.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regular retesting rolled out for care home staff and residents - GOV.UK. https://www.gov.uk/government/news/regular-retesting-rolled-out-for-care-home-staff-and-residents. Accessed October 12, 2020.
- 23.Ladhani SN, Jeffery-Smith AJ, Patel M. High prevalence of SARS-CoV-2 antibodies in care homes affected by COVID-19; a prospective cohort study in England. medRxiv. 2020 doi: 10.1101/2020.08.10.20171413. August2020.08.10.20171413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.