Abstract
Introduction
Severe coronavirus 2019 disease (CoViD-19) may lead to respiratory failure and mechanical ventilation. Therefore, ventilator associated pneumonia (VAP) may complicate the course of the disease. The aim of the current article was to investigate possible predictive factors for bacterial VAP on a retrospective manner, in a cohort of mechanically ventilated CoViD-19 patients. Additionally, determinant factors of lethality were analyzed.
Methods
Medical records of patients hospitalized in the intensive care units (ICU) at the university hospital UZ Brussel during the epidemic were reviewed. VAP was defined following the National Healthcare Safety Network 2017 criteria. Univariate and multivariate logistic regressions analyses were performed.
Results
Among the 39 patients included in the study, 54% were diagnosed with bacterial VAP. Case fatality rate was 44%, but 59% of the deceased patients had a do-not-resuscitate status. Multivariate logistic regression for prediction of VAP showed significant differences in duration of ICU hospitalization and in minimal lung compliance.
Additional analyses were performed on CoViD-19 patients who were affected by bacterial respiratory superinfection. The responsible pathogens correspond to the commonly found bacteria in VAP. However, 71% of the isolated germs were multi-drug resistant and bacteraemia was reported in 38%. Multivariate analyses for prediction of lethality found significant difference in SOFA score.
Conclusions
Mechanically ventilated CoViD-19 patients might frequently develop VAP. Longer ICU hospitalization was associated with pulmonary superinfection in the current cohort. Moreover, decreased minimal lung compliance was correlated to VAP and higher SOFA score at VAP diagnosis was associated with lethality.
Keywords: Coronavirus 2019 disease, Severe acute respiratory syndrome coronavirus 2, Ventilator-associated pneumonia, Lung compliance, Multi-drug resistant bacteria
Uncommon abbreviations
- BIA
Bioelectrical impedance analysis
- CDC
Center of Disease and Control
- CCI
Charlson comorbidity index
- DNR
Do-not-resuscitate
- ECDC
European Center of Disease and Control definition
- EUCAST
European committee on antimicrobial susceptibility testing
- ECMO
Extra corporeal membrane oxygenation
- XDR
Extreme-drug resistant
- iVAC
Infectious ventilator associated condition
- NHSN
National Healthcare Safety Network
- SOFA
Sequential organ failure assessment
- SAPS
Simplified Acute Physiology Score
- spp
Species
- VIM
Verona integron-encoded metallo-β-lactamase
1. Introduction
A novel zoonotic coronavirus 2019, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), originated in Wuhan in mid-December 2019 and rapidly spread to the rest of the world. The virus explicitly affects the respiratory system, generating Coronavirus disease 2019 (CoViD-19). Respiratory failure with mechanical ventilation need was reported in 2.3% up to 33% of the affected patients [1,2].
Bacterial ventilator-associated pneumonia (VAP) is a common hospital acquired infection which occurs in 10% up to 33% of the mechanically ventilated patients [3]. Bacterial superinfections were described in 13.5%–44% of the CoViD-19 patients admitted to intensive care unit (ICU), with a case fatality rate of 50–100% [2,4,5]. However, the role of bacterial VAP in CoViD-19 patients has not yet been established. Prolonged mechanical ventilation may expose CoViD-19 patients to higher risk of pulmonary superinfection [4,5]. The current monocentric observational study has been designed to describe and investigate predictors of VAP in a cohort of mechanically ventilated CoViD-19 patients. Furthermore, discriminants for lethality in CoviD-19 patients with respiratory bacterial superinfection will be analyzed.
2. Materials and methods
2.1. Ethical considerations
The study was conducted in accordance with the study protocol, the Declaration of Helsinki and applicable regulatory requirements. We applied for approval of the protocol by the local Institutional Review Board and the Ethics Committee of the University Hospital Brussels approved the protocol (EC approval number: B1432020000208). In view of the retrospective nature of the study, which did not demand any deviation from standard clinical ICU care, and the fact that all data was anonymized, a waiver of informed consent was obtained.
2.2. Patient population
Medical records of patients hospitalized in ICU wards in our tertiary care hospital with a confirmed SARS-CoV-2 infection between March 1, 2020 up to May 31, 2020 were reviewed. All confirmed cases of CoViD-19, who were admitted to intensive care unit (ICU) and were mechanically ventilated within the selected period, were enrolled in the current study. All included patients had a positive polymerase chain reaction (PCR) for SARS-CoV-2 in nasopharyngeal swab or bronchial alveolar lavage fluid (BAL).
2.3. Variables definition
National Healthcare Safety Network (NHSN) 2017 criteria were used to define VAP, as these criteria are embraced by the Center of Disease and Control (CDC) [6]. Consequently, infectious ventilator associated condition (iVAC) was diagnosed whenever an oxygenation problem (positive end-expiratory pressure increase more or equal to three cmH2O or a rise in fraction of inspired oxygen of greater than or equal to 20 points for two or more days) occurred with hypothermia (temperature < 36 °C) or fever (temperature > 38 °C) or leucocytosis (>12,000 white blood cells/mm3) or leukocytopenia (<4000 white blood cells/mm3). Possible VAP was defined as iVAC combined with a qualitative pulmonary infection (endotracheal aspiration or BAL showing on gram stain >25 neutrophils and <10 epithelial cells per low power field). Finally, probable VAP was delineated as the sum of iVAC and a quantitative pulmonary infection (endotracheal aspiration or BAL growing respectively > 105 CFU/mL and >104 CFU/mL) [6].
Lung compliance was indirectly calculated for all-included patients, who were mechanically ventilated in volume or pressure-controlled modalities [7,8] and who were deeply sedated, with a Richmond agitation-sedation scale ranging between −4 and −5 [9].
Bacteria responsible for VAP were classified as sensitive, multi-drug resistant (MDR) or extreme-drug resistant (XDR), based on European Center of Disease and Control definition (ECDC) [10].
Lethality or case-fatality rate was defined as crude death percentage of CoViD-19 intubated patients during ICU hospitalization.
2.4. Patient data collection
Epidemiological, clinical, biological and microbiological data were collected from the medical records of enrolled patients during ICU admission, and at VAP diagnosis for cases diagnosed with probable VAP. Furthermore, comorbidities were classified using the Charlson comorbidity index (CCI), a validated tool to quantify the burden of comorbidities [11]. Bioelectrical impedance analysis (BIA) assessing the patient's fluid status, obtained the day of VAP diagnosis, was reported to estimate body fluid excess [12]. Average maximal and minimal lung compliance were calculated for all included patients during ICU hospitalization. Furthermore, lung compliance was assessed in patients affected by bacterial VAP at diagnosis. Previously validated prognostic scores (Apache II, Apache III, SAPS 1 and SAPS 2) were calculated for all the included patients at 48 h from ICU admission [13]. Finally, sequential organ failure assessment (SOFA) score was documented for probable bacterial VAP patients at time of diagnosis, in order to estimate the prognosis and the severity of the disease [14].
2.5. Statistical analysis
Positive outcome was defined as development of probable VAP in the first statistical analyses. Secondly, lethality was chosen as endpoint for CoViD-19 patients affected by bacterial probable VAP.
Data are expressed as median and interquartile range for continuous variable, numbers and proportions for categorical variables, as all the analyzed data were non-normal distributed. Mann-Whitney U tests and Chi-square or Fisher exact tests were performed on baseline characteristics respectively for the assessment of continuous and categorical data. Independent predictors of development of VAP were determined using univariate logistic regression analyses. Length of ICU hospitalization, lung compliance and the prognostic scores were chosen as these were previously correlated to onset of pneumonia and/or disease severity [8].
Further assessments were performed through multivariate logistic regression. Two statistical models were computed, the first included the variables age, sex, CCI and duration of ICU hospitalization. The variables age, sex, CCI and minimal lung compliance were chosen to compute the second model.
Additional analyses were performed only in the group of patients affected by bacterial probable VAP in order to predict lethality. Conform to our first analysis, univariate logistic regression was computed to estimate predictive factors. Prognostic scores, positive blood cultures, infection caused by MDR pathogen, need of renal replacement therapy or veno-venous extra corporeal membrane oxygenation (ECMO) were selected as these are related to disease severity or/and mortality [8,14,15]. Subsequently, a multivariate regression model was performed. The variables age, sex, CCI and SOFA score were used in this last statistical model.
All the analyses were performed with IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp, released in 2011.
3. Results
3.1. Baseline characteristics of the study population
Thirty-nine CoViD-19 mechanically ventilated patients were admitted in the ICU of the university hospital UZ Brussel between March 1, 2020 and May 31, 2020. The median age was 62 (IQR: 55–72) years and 72% (n = 28) were male, the median body mass index (BMI) was 29.0 (IQR: 26.5–36.5) kg/m2. The most frequently reported comorbidity was type 2 diabetes mellitus, 59% of the included patients. Three patients were considered to be immunosuppressed, as two patients were treated with tacrolimus and mycophenolate due to renal transplantation and one patient was on high dose hydrocortisone due to IgA nephropathy. These three patients were treated with high dose methylprednisolone during ICU hospitalization. According to local protocol, 30 patients received hydroxychloroquine, among them five patients were concomitantly treated with lopinavir/ritonavir. Moreover, one patient participated to a clinical study and received an interleukin-1 receptor antagonist. Forty-four percent (n = 17) of the included patients died of CoViD-19 or its complications, of which 11 patients affected by VAP and 6 without VAP (Table 1 ). Among the deceased patients, ten (59%) had do-not-resuscitate (DNR) status.
Table 1.
Baseline characteristics of mechanically ventilated CoViD-19 patients; Baseline characteristics of mechanically ventilated patients; BMI: body mass index; CCI: Charlson comorbidity index; ICU: intensive care units; SAPS: Simplified Acute Physiology Score; P/F ratio: oxygen partial pressure to fractional inspired oxygen ratio; ACEI: Angiotensin-converting-enzyme inhibitors; Data are expressed as median and interquartile range for continuous variable and numbers and proportions for categorical variables.
| Parameters | Overall study populationc (n = 39) | Study characteristics considering probable VAP as primary endpoint |
||
|---|---|---|---|---|
| VAP patientsc (n = 21) | Non-VAP affected patients (n = 18) | P-value | ||
| Age, year | 62 (55–72) | 62 (58–71) | 64 (52–73) | 0.865 |
| CCI, index | 3 (2–5) | 4 (3–6) | 3 (1–4) | 0.107 |
| BMI, Kg/m2 | 29.0 (26.5–36.5) | 31.0 (37.0–27.0) | 28.0 (25.0–35.0) | 0.237 |
| ICU hospitalization length, days | 22 (10–34) | 29 (21–39) | 10 (7–24) | 0.004 |
| Intubation length, days | 21 (10–31) | 27 (18–34) | 10 (5–24) | 0.008 |
| Apache II, index | 17 (13–25) | 16 (14–28) | 18 (12–24) | 0.715 |
| Apache III, index | 47 (38–66) | 49 (38–70) | 47 (38–64) | 0.895 |
| SAPS 1, index | 9 (7–12) | 10 (7.7–14.0) | 8.5 (6.0–10.0) | 0.056 |
| SAPS 2, index | 28 (20–40) | 28 (21–40) | 27 (18–41) | 0.715 |
| Mean lung compliance, ml/cmH2O | 36.5 (25.1–55.6) | 31.5 (24.0–44.7) | 46.5 (36.9–66.5) | 0.032 |
| Maximal lung compliance, ml/cmH2O | 67.0 (48.0–123.5) | 71.0 (44.0–130.5) | 63.8 (48.3–97.2) | 0.785 |
| Minimal lung compliance, ml/cmH2O | 16.1 (12.0–24.7) | 14.0 (11.0–17.3) | 24.0 (17.5–36.5) | 0.001 |
| Mean P/F ratio | 172 (153–235) | 169 (145–238) | 209 (156–233) | 0.573 |
| Sex, male (%) | 28 (71.8%) | 15 (71.4%) | 13 (72.2%) | 0.725 |
| Active smoker, yes (%) | 3 (7.8%) | 2 (9.5%) | 1 (5.5%) | 1.000 |
| ACEI use, yes (%) | 18 (46.1%) | 13 (61.9%) | 5 (27.8%) | 0.106 |
| Immunosuppressant use, yes (%) | 3 (7.8%) | 3 (14.3%) | 0 (0.0%) | 0.243 |
| Diabetes, yes (%) | 23 (59.0%) | 15 (71.46%) | 8 (44.4%) | 0.209 |
| Chronic respiratory disease, yes (%) | 3 (7.8%) | 2 (9.5%) | 1 (5.5%) | 1.000 |
| Neoplasms, yes (%) | 3 (7.8%) | 3 (14.3%) | 0 (0.0%) | 0.243 |
| Chronic renal disease, yes (%) | 7 (17.9%) | 7 (33.3%) | 0 (0.0%) | 0.120 |
| Case fatality rate, yes (%) | 17 (43.6%) | 11 (52.4%) | 6 (33.3%) | 0.517 |
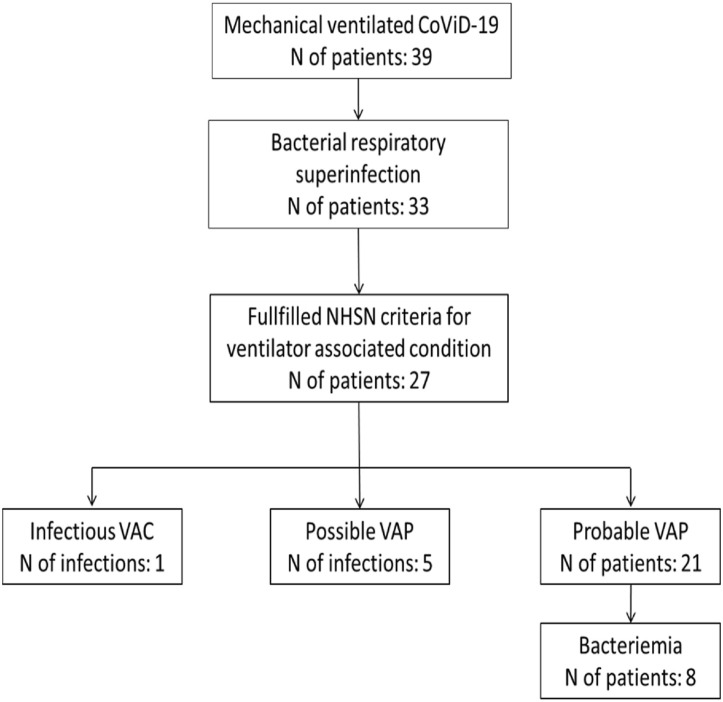
Thirty-three (85%) of the included cases were treated for bacterial respiratory superinfection. Among them, 27 patients were affected by VAP, fulfilling the criteria of NHSN 2017. One patient met the criteria of iVAC, but did not meet the definition of possible/probable VAP. Five patients fulfilled the criteria of possible VAP and 21 of probable VAP (Fig. 1 ). Only the latter group (n = 21) was further analyzed.
Fig. 1.
Study flowchart; CoViD-19: Coronavirus 2019 disease; NHSN: National Healthcare Safety Network; VAC: ventilator associated condition; VAP: ventilator associated pneumonia.
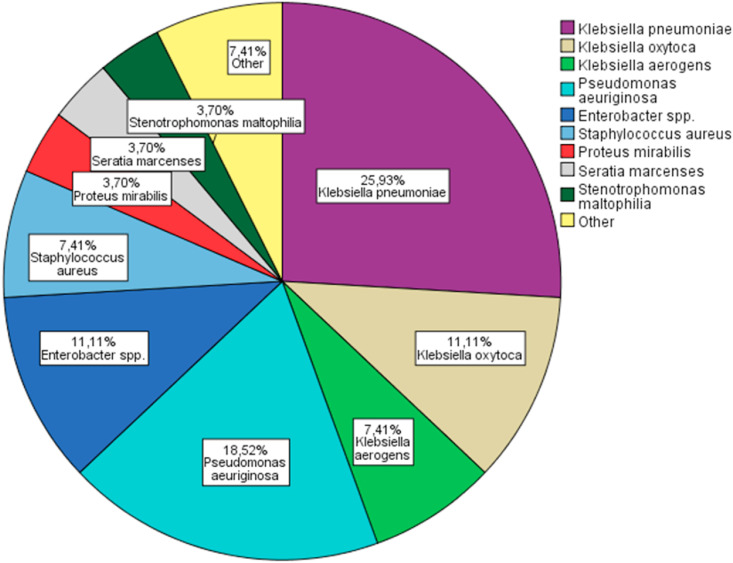
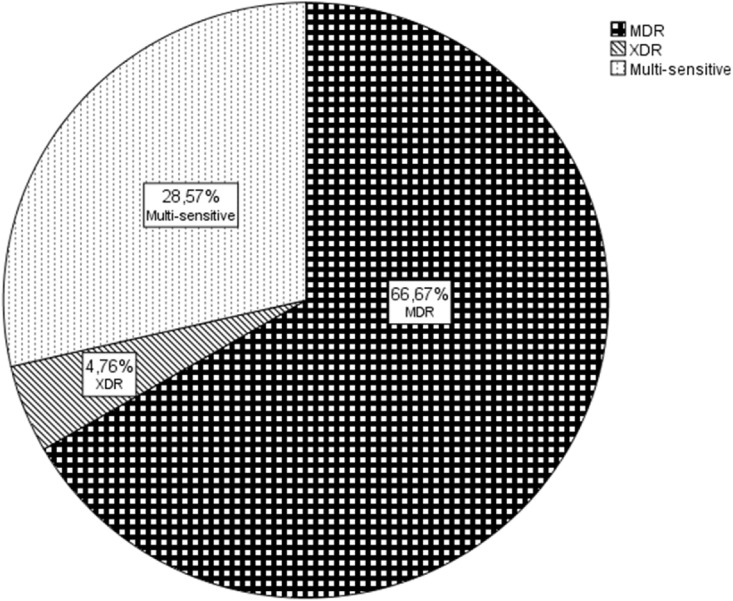
Baseline characteristics of CoViD 19 patients with probable bacterial VAP are reported in Table 2 . The median time at VAP onset was 16 (IQR: 7–22) days from ICU hospitalization and 13 (IQR: 7–21) days from intubation. Ninety percent of the VAP's were acquired five or more days from intubation and thus, were considered as late onset VAP. Twenty-seven bacterial pathogens were cultivated in 21 patients. The most reported pathogen was Klebsiella spp (44%). Particularly seven isolated germs were classified as Klebsiella pneumoniae, three as Klebsiella oxytoca and two as Klebsiella aerogenes. Pseudomonas aeruginosa was the second most found germ (18%), followed by Enterobacter spp. (11%) (Fig. 2 ). The majority of the superinfections were caused by MDR bacteria (67%) (Fig. 3 ), of which 29% were identified as Klebsiella spp with production of extended spectrum beta lactamase (ESBL). Specifically, five Klebsiella pneumoniae and one by Klebsiella oxytoca were classified as ESBL following the criteria European committee on antimicrobial susceptibility testing (EUCAST) [16]. Only one germ was defined as XDR: a Pseudomonas aeruginosa producer of Verona integron-encoded metallo-β-lactamase (VIM), which was resistant to all tested antibiotics except aztreonam. Thirty-eight percent (n = 8) of the affected patients had positive blood cultures. No other source of bacteraemia was found in these patients. During hospitalization or the week previous admission, 81% (n = 17) of the patients were exposed to antibiotics before the onset of VAP. In particular, ten patients received two antibiotic courses, seven were exposed to one antibiotic course and only 19% of the selected patients (n = 4) were antibiotic naïve. The preferred antibiotic for treatment of bacterial VAP was piperacillin-tazobactam, which was used in 42% (n = 10) of the documented infections, mainly in monotherapy. Other used antibiotic regimens were meropenem in 25% (n = 6), trimethoprim-sulfamethoxazole in 9% (n = 2) and cefepime in 9% (n = 2). Three patients had VAP caused by bacteria, which were not sensitive to the initial empiric treatment. A different, approriate antimicrobial was hence started after two to three days without any impact on lethality. One patient was affected by VAP caused by a Pseudomonas XDR, for which a combination of aztreonam and piperacillin-tazobactam was started. However, the pathogen was only sensitive to aztreonam, and the patient eventually died. Only four patients were de-escalated in function of microbiological results. The median antibiotic treatment length was seven (IQR: 4–8) days. Six patients died prematurely, before the termination of the antibiotic course. Nine patients (43%) needed renal replacement therapy as continuous veno-venous hemofiltration and three (14%) were treated with ECMO.
Table 2.
Baseline characteristics of mechanically ventilated CoViD-19 patients affected by probable VAP; Baseline characteristics of CoViD-19 patients affected by probable VAP at diagnosis of VAP; BMI: body mass index; CCI: Charlson comorbidity index; ICU: intensive care unit; VAP: ventilator-associated pneumonia; CRP: C reactive protein; WBC: white blood cells; NLR: neutrophil to lymphocyte ratio; IL-6: interleukin 6; SOFA: Sepsis-related Organ Failure Assessment; SAPS: Simplified Acute Physiology Score; P/F ratio: oxygen partial pressure to fractional inspired oxygen ratio; ECMO: Extracorporeal membrane oxygenation; Data are expressed as median and interquartile range for continuous variable and numbers and proportions for categorical variables.
| Parameters | CoViD-19 patients affected by VAP (n = 21) | Study characteristics considering all-cause mortality as primary endpoint |
||
|---|---|---|---|---|
| Deceased patients (n = 10) | Alive patients (n = 11) | P-value | ||
| Age, year | 62 (57–71) | 66 (58–76) | 60 (52–64) | 0.223 |
| CCI, index | 4 (2–6) | 4 (2–6) | 3 (3–6) | 0.705 |
| BMI, Kg/m2 | 30.0 (27.0–37.0) | 29.5 (28.7–34.7) | 33.0 (26.0–39.0) | 0.918 |
| ICU days at VAP diagnosis | 15 (7–20) | 16 (7–22) | 13 (7–20) | 1.000 |
| Mechanical ventilation days at diagnosis | 12 (7–19) | 13 (6–20) | 12 (7–20) | 0.863 |
| CRP, mg/L | 244 (137–373) | 302 (190–385) | 224 (80–248) | 0.132 |
| WBC, 103/μL | 13.0 (7.5–19.5) | 13.0 (6.0–22.7) | 13.0 (9.0–19.0) | 1.000 |
| NLR ratio | 10.0 (7.5–16.5) | 10.5 (7.2–16.2) | 9.0 (7.0–18.0) | 1.000 |
| D-dimers, ng/mL | 3379 (2101–5522) | 3344 (1277–5213) | 4206 (2245–5654) | 0.387 |
| Troponin, mcg/L | 0.060 (0.030–0.093) | 0.082 (0.039–0.163) | 0.060 (0.025–0.078) | 0.132 |
| IL-6, pg/mL | 92 (39–522) | 665 (76–911) | 50 (29–220) | 0.060 |
| Fluid excess, liter | 4.0 (0.0–6.0) | 4.5 (1.0–8.7) | 2.0 (0.0–6.0) | 0.788 |
| SOFA score, score | 7 (4–9) | 8 (8–10) | 6 (3–7) | 0.005 |
| Apache II, score | 16 (14–29) | 16 (16–28) | 16 (13–36) | 0.809 |
| Apache III, score | 48 (37–64) | 45 (34–70) | 48 (44–58) | 0.468 |
| SAPS 1, score | 10 (7–14) | 11 (7–15) | 9 (8–14) | 0.918 |
| SAPS 2, score | 28 (20–36) | 28 (18–40) | 29 (21–36) | 0.705 |
| P/F ratio | 137 (102–162) | 119 (97–142) | 160 (132–217) | 0.051 |
| Lung compliance, ml/cmH2O | 30.5 (23.0–38.5) | 28.0 (21.0–38.0) | 33.0 (27.0–63.0) | 0.383 |
| Sex, male, (%) | 14 (66.7%) | 7 (70.0%) | 7 (63.6%) | 1.000 |
| Diabetes, yes (%) | 14 (66.7%) | 6 (60.0%) | 8 (72.7%) | 0.659 |
| Renal replacement, yes (%) | 9 (42.8%) | 5 (50.0%) | 4 (36.4%) | 0.670 |
| ECMO, yes (%) | 3 (14.3%) | 1 (10.0%) | 2 (18.2%) | 1.000 |
| Multi-resistant drug VAP pathogen, yes (%) | 15 (71.4%) | 7 (70.0%) | 8 (72.7%) | 1.000 |
| Positive blood cultures, yes (%) | 8 (38.1%) | 2 (20.0%) | 6 (54.5%) | 0.138 |
Fig. 2.
Bacterial pathogens in probable VAP; VAP: ventilator associated pneumonia; spp: species.
Fig. 3.
Bacterial resistance in probable VAP; MDR: multi-drug resistant; XDR: extreme-drug resistant.
3.2. Predictive factors for probable VAP
The results of the univariate logistic regression for prediction of probable VAP, within the cohort of mechanical ventilated CoViD-19 patients, are reported in Table 3 . No significant differences were found in age, sex, BMI and medical history. The duration of ICU hospitalization was significantly longer in CoViD-19 patients, who developed VAP, with an OR (95%CI): 1.04 (1.01–1.09, p = 0.048). However, no major differences were found in duration of intubation. Furthermore, significant differences were found in minimal lung compliance between the two groups, with OR (95%CI): 0.86 (0.77–0.96, p = 0.007), but insignificant discrepancies were noted in average and maximal lung compliance. Finally, significant differences were appreciated in Simplified Acute Physiology Score 1 (SAPS) score for prediction of VAP development within the current cohort, but none in other severity scores.
Table 3.
Logistic regression analysis for the prediction of VAP; Logistic regression for prediction of probable VAP; OR: odds ratio; CI: confidence interval; BMI: body mass index; CCI: Charlson comorbidity index; ICU: intensive care units; SAPS: Simplified Acute Physiology Score; P/F ratio: oxygen partial pressure to fractional inspired oxygen ratio; ACEI: Angiotensin-converting-enzyme inhibitors; ‘-’ is used for ‘no observation’ or ‘not applicable’.
| Independent variables | Univariate regression analysis for prediction of probable VAP |
Multivariate regression analysis for prediction of probable VAP adjusted for age, sex and CCI |
||
|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Age | 1.01 (0.96–1.06) | 0.735 | - | - |
| CCI | 1.33 (0.95–1.85) | 0.970 | - | - |
| BMI | 1.07 (0.96–1.19) | 0.233 | - | - |
| ICU hospitalization length | 1.04 (1.01–1.09) | 0.048 | 1.06 (1.01–1.12) | 0.044 |
| Intubation length | 1.04 (0.99–1.09) | 0.072 | - | - |
| Apache II | 1.03 (0.96–1.10) | 0.424 | - | - |
| Apache III | 1.00 (0.98–1.03) | 0.752 | - | - |
| SAPS 1 | 1.24 (1.01–1.526) | 0.043 | - | - |
| SAPS 2 | 1.01 (0.96–1.05) | 0.701 | - | - |
| Mean lung compliance | 0.972 (0.94–1.01) | 0.106 | - | - |
| Maximal lung compliance | 1.01 (0.99–1.02) | 0.300 | - | - |
| Minimal lung compliance | 0.86 (0.77–0.96) | 0.007 | 0.82 (0.70–0.96) | 0.013 |
| Mean P/F ratio | 0.99 (0.98–1.00) | 0.305 | - | - |
| Sex | 0.66 (0.16–2.77) | 0.570 | - | - |
| Active smoker | 1.60 (0.13–19.28) | 0.711 | - | - |
| ACEI use | 3.47 (0.90–13.31) | 0.070 | - | - |
| Immunosuppressant use | - | - | - | - |
| Diabetes | 2.41 (0.65–8.92) | 0.187 | - | - |
| Chronic respiratory disease | 1.60 (0.13–19.28) | 0.711 | - | - |
| Neoplasms | - | - | - | - |
| Chronic renal disease | - | - | - | - |
Multivariate regression analysis found significant differences in length of ICU hospitalization OR (95%CI): 1.06 (1.01–1.12, p = 0.044) and minimal lung compliance OR (95%CI): 0.82 (0.70–0.96, p = 0.013) after adjustment for age, sex and CCI.
3.3. Predictive factors for lethality
The results of univariate logistic regression for prediction of lethality within the CoViD-19 patients diagnosed with probable VAP are reported in Table 4 . No major discrepancies were noticed for the variables age, sex, BMI and medical history. Furthermore, patients affected by VAP caused by MDR pathogens or with bacteraemia had no significant higher fatality rate. Deceased patients had a significantly higher SOFA score at VAP diagnosis, with OR (95%CI): 1.86 (1.03–3.36, p = 0.039). In the current cohort, mechanically ventilated patients affected by VAP with a SOFA score higher than seven had a sensitivity of 80% and a specificity of 82% for lethality. No meaningful discrepancies were noted in either other severity score, or lung mechanics laboratory variables. No significant differences were found in renal replacement therapy or ECMO or body fluid excess at VAP diagnosis.
Table 4.
Logistic regression analysis for the prediction of lethality; Logistic regression for prediction of lethality within CoViD-19 patients affected by VAP; OR: odds ratio; CI: confidence interval; CCI: Charlson comorbidity index; BMI: body mass index; ICU: intensive care unit; VAP: ventilator-associated pneumonia; CRP: C reactive protein; WBC: white blood cells; ACEI: Angiotensin-converting-enzyme inhibitors; IL-6: interleukin 6; SOFA: Sepsis-related Organ Failure Assessment; SAPS: Simplified Acute Physiology Score; P/F ratio: oxygen partial pressure to fractional inspired oxygen ratio; ECMO: Extracorporeal membrane oxygenation; ‘-’ is used for ‘no observation’ or ‘not applicable’.
| Independent variables | Univariate regression analysis for prediction of lethality |
Multivariate regression analysis for prediction of lethality adjusted for age, sex and CCI |
||
|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Age | 1.07 (0.97–1.18) | 0.158 | - | - |
| CCI | 1.06 (0.72–1.56) | 0.759 | - | - |
| BMI | 0.96 (0.85–1.08) | 0.502 | - | - |
| ICU days at VAP diagnosis | 0.99 (0.91–1.08) | 0.821 | - | - |
| Mechanical ventilation days at VAP diagnosis | 0.98 (0.90–1.07) | 0.606 | - | - |
| CRP | 1.01 (0.99–1.01) | 0.142 | - | - |
| WBC | 1.00 (0.93–1.08) | 0.988 | - | - |
| D-dimers | 1.00 (1.00–1.00) | 0.998 | - | - |
| Troponin | 1.02 (0.99–1.04) | 0.102 | - | - |
| IL-6 | 1.00 (1.00–1.01) | 0.074 | - | - |
| SOFA score | 1.86 (1.03–3.36) | 0.039 | 2.70 (1.08–6.73) | 0.034 |
| Apache II | 0.98 (0.91–1.05) | 0.573 | - | - |
| Apache III | 0.99 (0.95–1.03) | 0.506 | - | - |
| SAPS 1 | 0.99 (0.84–1.18) | 0.940 | - | - |
| SAPS 2 | 0.99 (0.92–1.06) | 0.754 | - | - |
| P/F ratio | 0.97 (0.94–1.00) | 0.052 | - | - |
| Lung compliance | 0.95 (0.87–1.04) | 0.282 | - | - |
| Fluid excess | 1.14 (0.78–1.67) | 0.489 | - | - |
| Sex | 1.33 (0.21–8.29) | 0.758 | - | - |
| Diabetes | 0.56 (0.09–3.52) | 0.538 | - | - |
| Renal replacement | 1.75 (0.31–10.02) | 0.530 | - | - |
| ECMO | 0.50 (0.04–6.55) | 0.597 | - | - |
| Multi-resistant drug VAP pathogen | 0.87 (0.13–5.82) | 0.890 | - | - |
| Positive blood cultures | 0.21 (0.03–1.47) | 0.115 | - | - |
Finally, multivariate logistic regression for prediction of lethality showed a significantly higher SOFA score after adjustment for age, sex and CCI, with OR (95%CI): 2.70 (1.08–6.73, p = 0.034).
4. Discussion
During a period of three months, 39 CoViD-19 patients required mechanically ventilation at the ICU of the university hospital UZ Brussel. As previously reported, the majority of the inclusions were male patients and the most frequent comorbidity was diabetes mellitus [2,5]. According to different reports, the incidence of VAP roughly ranges between 10% up to 33% of the mechanically ventilated patients [3,6,17]. In our study, more than the half of the examined subjects (54%) developed VAP, following the strict criteria of NHNS for probable VAP. It could be speculated that a protracted ICU hospitalization and a longer intubation period may be responsible for the greater rate of VAP [18]. In this cohort, the duration of ICU hospitalization in mechanically ventilated CoViD-19 patients was associated with VAP development after adjusting for age, sex and comorbidities.
Gattinoni et al. previously reported two different phenotypes of physio-pathological respiratory tract involvement in CoViD-19 patients [19]. SARS-CoV-2 pneumonia type L, which provokes hypoxia, but minimally affects lung compliance, and SARS-CoV-2 pneumonia type H, similar to classic acute respiratory distress syndrome (ARDS), which induces hypoxia and decreases lung compliance. Further hypotheses based on Italian and Spanish experience speculate that vasoconstriction due to hypoxia and pulmonary micro-embolization might be responsible for pneumonia with “normal” compliance (>40 ml/cmH2O), whereas acute lung injury occurring in later stage of disease and/or bacterial superinfection might lead to pneumonia with reduced compliance [20]. In the current cohort, lower average lung compliance was detected in CoViD-19 patients affected by VAP (31.5 ml/cmH2O) in comparison with those who did not develop pulmonary superinfection (46.5 ml/cmH2O). However, this finding was not significant in univariate analyses. Moreover, decreased minimal lung compliance was significantly correlated with VAP occurrence, independently from age, sex and comorbidities. Further investigations are needed to confirm these results.
In the current cohort, univariate analysis showed a greater SAPS 1 score at 48 h from ICU admission in CoViD-19 patients affected by VAP, however the same result could not be found with SAPS 2 or Apache II–III scores.
Case fatality rate was 44%. Fifty-two percent of the VAP patients and 33% of the non-VAP inclusions died. No significant discrepancies in lethality between the two groups were found, possibly due to the small sample size.
As expected, gram negative rods were the most detected VAP associated pathogens [3,17]. Klebsiella spp. Was the most involved bacteria, followed by Pseudomonas spp., with the large majority of isolated germs being MDR (71%). As previously reported, the higher rate of resistances might be explained by the late onset of VAP [3,17]. Ninety percent of the patients, affected by probable VAP, developed bacterial pulmonary superinfection five or more days after intubation. Moreover, 81% of the patients affected by VAP had previously been exposed to one or more antibiotic regimens before VAP diagnosis. Inappropriate or unnecessary broad antibiotic treatment at hospital admission in hospitalized CoViD-19 patients could result in higher resistance levels and might increase mortality rates [20], [21], [22]. Therefore, prompt antibiotic de-escalation or cessation should be considered following microbiologic results and antimicrobial stewardship principles should be respected particularly during the CoViD-19 epidemic [21].
An unexpected high rate of positive blood cultures was found in the current cohort. Thirty-eight percent of the VAP patients developed a significant bacteremia, without any extra pulmonary source of infection identified. Nonetheless, positive blood cultures were not associated with higher lethality as previously reported, again possibly due to the small sample size [18].
Greater SOFA score at diagnosis may be correlated with higher fatality rate. As observed in the present study, a higher SOFA score was significantly correlated to lethality in VAP affected CoViD-19 patients independently from age, sex and comorbidities. Furthermore, a SOFA score, greater than seven, had a considerable predictive power for lethality.
The strengths of this study are the rigorous study design and structure. Variables were strictly defined. Particularly, the definition of VAP was in accordance with NHSN classification and the bacterial resistances classification followed the ECDC criteria [6,10]. To the best of our knowledge, this is the first cohort study focused on bacterial VAP's in CoViD-19 patients [4,23]. Finally, the current study enforces the evidence-based knowledge on critical ill CoViD-19 patients, affected by bacterial VAP, and provides possible risk factor for lethality in these patients.
This study has several limitations. First, due to the retrospective design of this study, some confounding factors could not have been excluded. Furthermore, the difference between upper respiratory ways colonisation and bacterial VAP is extremely challenging in severe CoViD-19 patients [24]. In the current article, NHNS classification was used to differentiate the two entities. However, this could have under or overestimated the real number of bacterial VAP. Second, a relative low number of patients were included, which may undermine the power of this study. Multicentre large prospective observational trials should be performed to shed more light on this topic.
5. Conclusion
In summary, we found a high number of bacterial VAP's in mechanically ventilated CoViD-19 patients which may be due to the longer ICU hospitalization. Furthermore, decreased minimal lung compliance might be associated with bacterial respiratory superinfection in CoViD-19 mechanically ventilated patients. We believe that the implementation of an antimicrobial stewardship team could enhance judicious antibiotic use, prevent antibiotic overuse, and perhaps reduce ICU hospitalization duration. Restricted antimicrobial use and early de-escalation might lower resistances and airway colonisation with MDR pathogens. Furthermore, preventive measures should be considered to limit decreases in lung compliance. Strict protective ventilation might delay lung damages and lowering of the lung compliance. We observed the expected distribution of bacteria responsible for VAP in these CoViD-19 patients, however a greater number of MDR pathogen and bacteraemia was reported in the current cohort. SOFA score at VAP diagnosis may predict lethality with considerable specificity and sensitivity.
Ethics approval and consent to participate
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was conducted in accordance with the study protocol, the Declaration of Helsinki and applicable regulatory requirements. We applied for approval of the protocol by the local Institutional Review Board and Ethics Committee of the University Hospital Brussels approved the protocol (EC approval number: B1432020000208). In view of the retrospective nature of the study, which did not demand a deviation from standard clinical ICU care, and the fact that all data was anonymized, informed consent from the patient or the next of kin was not essential.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
MM: concept, study design, data collection, data analysis and interpretation, writing and revision. JVL: study design, writing and revision. AM: data collection and data analysis. DP and MLNGM: writing and revision. All authors have given final approval of the version to be submitted.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
MLNGM is a member of the medical advisory Board of Pulsion Medical Systems (now fully integrated in Getinge, Solna, Sweden) and Serenno Medical (Tel Aviv, Israel), consults for Baxter, Maltron, ConvaTec, Acelity, Spiegelberg and Holtech Medical.
All other authors declare that they hve no competing interests in relation to the content published in this manuscript.
Acknowledgements
We would like to thank the medical students working at the ICU, for their precious support collecting the BIA data. Furthermore, we would like to express gratitude for the logistic support offered by the ICU coordinating study team and the study nurses Claire and Lieve.
MLNGM is member of the Executive Committee of the Abdominal Compartment Society, formerly known as the World Society of Abdominal Compartment Syndrome (https://www.wsacs.org/). He is co-founder, past-president and current treasurer of WSACS. He is co-founder and president of the International Fluid Academy (IFA). The mission statement of the IFA is to foster education, promote research on fluid management and hemodynamic monitoring, and thereby improve survival of critically ill by bringing together physicians, nurses, and others from throughout the world and from a variety of clinical disciplines. The IFA is integrated within the not-for-profit charitable organization iMERiT, International Medical Education and Research Initiative, under Belgian law. The content of the IFA website (http://www.fluidacademy.org) is based on the philosophy of FOAM (Free Open Access Medical education – #FOAMed). The site recently received the HONcode quality label for medical education (https://www.healthonnet.org/HONcode/Conduct.html?HONConduct519739).
References
- 1.Guan W., Ni Z., Hu Yu, Liang W., Ou C., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., et al. Clinical characteristics of covid-19 in New York city. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhodes N.J., Cruce C.E., O'Donnell J.N., Wunderink R.G., Hauser A.R., et al. Resistance trends and treatment options in Gram-negative ventilator-associated pneumonia. Curr Infect Dis Rep. 2019;20(2):3. doi: 10.1007/s11908-018-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clancy Cornelius J., Hong Nguyen M. COVID-19, superinfections and antimicrobial development: what can we expect? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa524. ciaa524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Yu Y., Xu J., Shu H., Xia J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spalding M., Cripps M., Minshall C. Ventilator-associated pneumonia: new definitions. Crit Care Clin. 2017;33(2):277–292. doi: 10.1016/j.ccc.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hess D. Respiratory mechanics in mechanically ventilated patients. Respir Care. 2014;59(11):1773–1794. doi: 10.4187/respcare.03410. [DOI] [PubMed] [Google Scholar]
- 8.Kock K., Maurici R. Respiratory mechanics, ventilator-associated pneumonia and outcomes in intensive care unit. World J Crit Care Med. 2018;7(1):24–30. doi: 10.5492/wjccm.v7.i1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sessler C.N., Grap M.J., Ramsay M. Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care. 2008;12 doi: 10.1186/cc6148. Suppl 3(Suppl 3):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 11.Quan H., Li B., Couris C.M., Fushimi K., Graham P., et al. Practice of epidemiology updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;15(173):676–682. doi: 10.1093/aje/kwq433. (6) [DOI] [PubMed] [Google Scholar]
- 12.Malbrain M.L.N.G., Huygh J., Dabrowski W., De Waele J., Staelens A., et al. The use of bio-electrical impedance analysis (BIA) to guide fluid management, resuscitation and deresuscitation in critically ill patients: a bench-to-bedside review. Anaesthesiol Intensive Ther. 2014;46(5):381–391. doi: 10.5603/AIT.2014.0061. [DOI] [PubMed] [Google Scholar]
- 13.Breslow M.J., Badawi O. Severity scoring in the critically ill: Part 1--interpretation and accuracy of outcome prediction scoring systems. Chest. 2012;141(1):245–252. doi: 10.1378/chest.11-0330. [DOI] [PubMed] [Google Scholar]
- 14.Raith E.P., Udy A.A., Bailey M. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. J Am Med Assoc. 2019;317(3):290–300. doi: 10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]
- 15.Bercault N., Boulain T. Mortality rate attributable to ventilator-associated nosocomial pneumonia in an adult intensive care unit: a prospective case-control study. Crit Care Med. 2001;29(12):2303–2309. doi: 10.1097/00003246-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Polsfuss S., Bloemberg G., Giger J., Meyer V., Hombach M. Comparison of European Committee on Antimicrobial Susceptibility Testing (EUCAST) and CLSI screening parameters for the detection of extended-spectrum -lactamase production in clinical Enterobacteriaceae isolates. J Antimicrob Chemother. 2012;67(1):159–166. doi: 10.1093/jac/dkr400. [DOI] [PubMed] [Google Scholar]
- 17.Patil H.V., Patil V.C. Incidence, bacteriology, and clinical outcome of ventilator-associated pneumonia at tertiary care hospital. J Nat Sci Biol Med. 2017;8(1):46–55. doi: 10.4103/0976-9668.198360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunac A., Sifri Z.C., Mohr A.M., Horng H., Lavery R.F., et al. Bacteremia and ventilator-associated pneumonia: a marker for contemporaneous extra-pulmonic infection. Surg Infect. 2014;15(2):77–83. doi: 10.1089/sur.2012.030. [DOI] [PubMed] [Google Scholar]
- 19.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rello J., Storti E., Belliato M., Serrano R. Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers. Eur Respir J. 2020;55(5):2001028. doi: 10.1183/13993003.01028-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee C., Kadri S.S., Dekker J.P., Danner R.L., Chen H.C., et al. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bengoechea J.A., Bamford C.G. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol Med. 2020;26 doi: 10.15252/emmm.202012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Póvoa H.C., Chianca G.C., Iorio N.L. COVID-19: an alert to ventilator-associated bacterial pneumonia. Infect Dis Ther. 2020;30:1–4. doi: 10.1007/s40121-020-00306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.François B., Laterre P.F., Luyt C.E. J. ChastreThe challenge of ventilator-associated pneumonia diagnosis in COVID-19 patients. Crit Care. 2020;24(1):289. doi: 10.1186/s13054-020-03013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.