Abstract
COVID-19 is an acute respiratory syndrome caused by SARS-COV-2 which has now become a huge pandemic worldwide. The immunopathogenesis of COVID-19 has been established that increased serum levels of C-reactive protein (CRP), interleukin-6 (IL-6), and reduction of the CD4+ and the CD8+ T lymphocyte populations, are the most reported immunological findings in these patients. High levels of other inflammatory cytokines and chemokines such as IL-2 and IL-8 with an increased number of neutrophils and eosinophils may induce immune abnormalities in patients with COVID-19. There is growing evidence to obtain a deeper understanding of the immunopathogenesis of COVID-19 which will lay the foundation for the development of new potential therapies. However, specific and non-specific immunotherapies such as convalescent plasma (CP) are widely performed to treat patients with severe COVID-19, there is no definitive evidence to suggest the effectiveness of these treatments. Hence, this review aimed to highlight the current and most recent studies to identify the new immunotherapeutics for COVID-19 disease.
Keywords: COVID-19, Coronavirus, Immunotherapy, Lymphopenia, SARS-COV-2
1. Introduction:
By the end of 2019, severe pneumonia with unknown etiology was reported in Wuhan, China [1]. The cause of this pneumonia was a new single-stranded, positive-sense RNA virus Which is now called SARS-COV-2 causing COVID-19 disease [2]. Several clinical symptoms such as fever, coagulation dysfunction, refractory metabolic acidosis, multiple organ dysfunction syndromes (MODS), acute respiratory distress syndrome (ARDS), and high levels of inflammatory and anti-inflammatory mediators, were common in adults with COVID-19 [3]. The elderly and patients with diabetes and high blood pressure, cancer, or low immune function have a higher susceptibility and mortality [4]. During this pandemic, many studies have been performed to identify the immunopathogenesis of COVID-19. Increased levels of interleukin-6 and lymphopenia are more associated with severe acute respiratory syndrome caused by SARS-COV-2 [5]. However, the different mutation of SARS-CoV-2 spike protein leading to the viral increased transmission and severity of pathogenicity of COVID-19 [6]. On the other hand, both immunotherapy strategies and several different types of vaccines are being developed. Hence, in order to implement the most effective immunotherapy strategies, more in-deep studies are needed to understand the mechanism of the immunopathogenesis of COVID-19.
2. Immunopathogenesis of COVID-19
2.1. Virus recognition by pattern recognition receptors
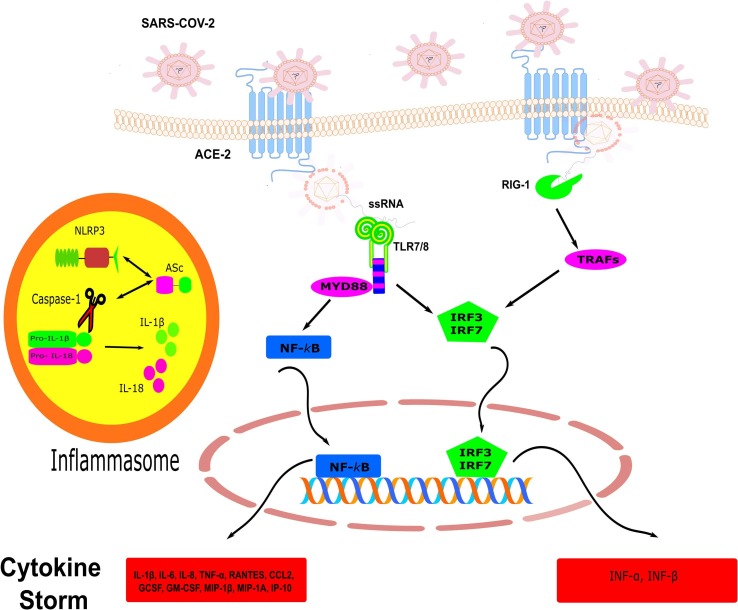
It has been established that the new coronavirus enters a variety of human cells using its spike protein which binds to the angiotensin-converting enzyme 2 (ACE2) [7]. SARS-CoV-2 like other RNA viruses are recognized by the intracellular receptors leading to disruption of the immune system in severe cases of COVID-19 [8], [9]. Generally, the immune responses to virus infections are mediated by both innate and adaptive immunity, particularly cellular and humoral immunity [10]. The viral single-strand (ssRNA) and double-strand (dsRNA) RNAs are recognized by different Toll-like receptor (TLR) especially TLR3, TLR7, TLR8 [11]. Binding of a viral RNA to TLR7 and TLR3 activates the adapter molecules, Toll/IL‐1 receptor adapter inducing IFN‐β (TRIF) and myeloid differentiation factor 88 (MyD88) [11], [12]. These signaling pathways induce the translocation of interferon‐regulatory factor (IRFs) and Nuclear-kappa B (NF‐κB) which leads to the production of inflammatory mediators including interferons (IFNα/β) and IL-1β [13]. It is hypothesized that SARS-COV-2 is also sensed by TLR7 or other receptors on lung resident cells and stimulates the production of pro-inflammatory and anti-inflammatory cytokines (Fig. 1 ).
Fig. 1.
The mechanism of recognition of SARS-COV-2. SARS-COV-2 binds to ACE −2 via S protein and enters into host cells. Ligation of TLR7/8 and RIG-1 by SARS-COV-2 ssRNA resulted in the recruitment of adaptor molecules such as MYD88 and TRAFs which in turn translocate transcriptions factors, NF-kB, and IRFs into the nucleus. This leads to the production of inflammatory mediators. The maturation of the Pro-inflammatory cytokine IL-1β and IL-18 are mediated by caspase 1 in NLRP3-inflammasome. ACE-2; angiotensin-converting enzyme-2, ssRNA; single-strand RNA, TLR; toll-like receptor, RIG-1; retinoic acid-inducible gene I, NLRP3; NOD‐like receptor family, pyrin domain containing 3, TRAF; Tumor necrosis factor (TNF) receptor-associated factor, IRF; Interferon (IFN) regulatory factor, IL; interleukin, GM-CSF; Granulocyte macrophage-colony stimulating factor, MIP1-β; macrophage inflammatory protein 1beta, IP-10; IFN-γ-inducible protein 10.
It has been evidenced that the new coronavirus reduces both NK and CD8+ T cell count which is associated with severe pneumonia following SARS-COV-2 infection [14]. Also, suppression of the function of NK and CD8+ T cells is associated with increased expression of CD39, NKG2A, and programmed death-1 (PD-1). This functional exhaustion was directly related to the severity of COVID-19 disease [15]. Therefore, targeting NKG2A, PD- (L) 1, and CD39 can be considered to antiviral treatment in the early stages of new coronavirus infection [16].
Moreover, decreased number and function of CD4+ T lymphocyte in cases suffered from SARS-COV-2 resulted in a further reduction in NK cells and cytotoxic T lymphocytes (CTL) activity [17]. Because cytokines secreted by CD4+ T cells are required to maintain effective virus-specific cytotoxic T lymphocytes responses [18]. Also, T helper cells are a source of IFN-γ that activates NK cell subsets and enhances phagocytosis activity of macrophages which in turn induces resistance to viral infections [19].
2.2. Lymphopenia
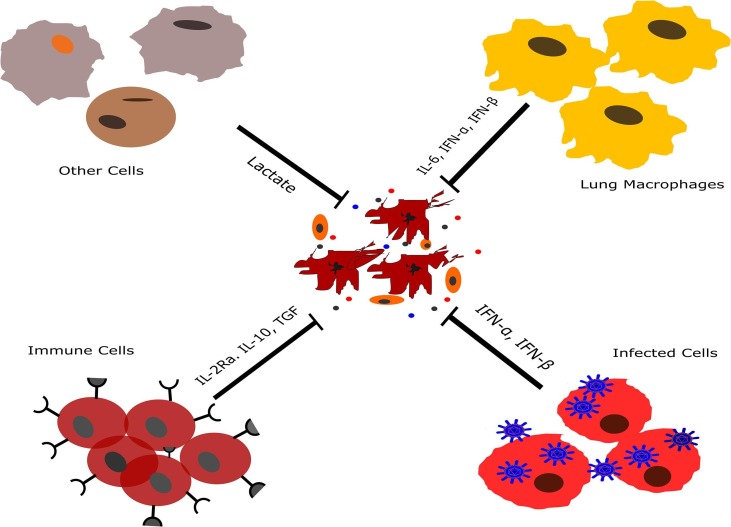
Lymphopenia is one of the most important features and common clinical symptoms in severe cases of COVID-19 disease. It has been proposed that the lymphocytopenia in patients with COVID-19 is caused by direct and indirect mechanisms, including the production of glucocorticoids, destruction of lymphocytes, a storm of cytokines, weakened vascular cell adhesion, and FAS-FASL interaction [20]. While the T cells function in COVID-19 disease is not completely explained; in some studies, decreased lymphocyte counts have been reported especially in ICU-patients [3], [21]. A recent study has reported ICU patients suffering from infection show lymphopenia (average lymphocyte count of 800 cells per milliliter) and hypercytokinemia. These Laboratory findings are common in COVID-19, SARS-CoV, and 2009 Pandemic Influenza virus diseases [22]. Lymphopenia is associated with decreased number of NK cells, T and B cells while restoration of lymphocytes in patients with COVID-19 is associated with viral clearance [14]. A case report study has demonstrated that a decreased number of peripheral CD38/HLA-DR double-positive T cells, CCR6 + Th17 cells, and CD8 T cells are associated with the hyperactivity of cytotoxic T lymphocytes and high concentrations of cytotoxic granules perforin and granulysin [23]. Decreased lymphocyte count in severe cases of COVID-19 could be influenced by two factors: first, direct cytotoxicity of the virus on immune cells such as T lymphocytes and second host factors including diabetes, hypertension, cardiovascular and cerebrovascular disease in the elderly or chronic diseases which causes endothelial dysfunction. Endothelial dysfunction leads to intercellular junctions impairment, which in turn decreases adhesion and migration of leukocytes from the blood [24]. Another hypothesis is that IL-6 may inhibit T-cell mediated immunity, which is associated with lung lesions in the acute phase in patients with COVID-19 (Fig. 2 ). Moreover, elevated levels of Lactate in COVID-19 patients may reduce the cytolytic activity of CTLs and also converts T helper cells into IL-17 secreting cells [25].
Fig. 2.
The probable mechanism of the lymphocyte decreasing in patients with COVID-19. Type I interferons can induce apoptosis in various types of cells such as lymphocytes. Also, IL-10 as an immunoregulatory cytokine suppresses T lymphocytes and other immune cells. Moreover, The activity of T-cells may reduce by high levels of lactate which is produced by different types of cells. IL; interleukin, IFN; interferon, IL-2R; interleukin-2 receptor.
Along with lymphopenia, abnormalities in granulocytes and monocytes have also been observed in COVID-19 patients [26]. It has been shown, the number of neutrophils and the ratio of neutrophils to lymphocytes are increased which may be due to a decrease in the lymphocytes count and infections following COVID-19 disease [26]. Also, many studies have reported a decreased number of monocytes, eosinophils, and basophils in patients with severe CoVID-19 [27], [28]. Zhang et al have detected that 53% of patients with COVID-19 have reduced absolute eosinophil counts and 81% had significant eosinophilia on the day of hospitalization [29].
2.3. Cytokine storm
The storm of cytokine is a state of an unexpected increase of pro-inflammatory cytokines which is the major cause of severe lung injury and the adverse outcomes of SARS-COV-2 infection [30]. From December of 2019 up to now, many studies have reported that both pro-inflammatory and anti-inflammatory plasma cytokines like IL-10, IL-2, IL-6, IL-4, IL1β, IL1RA, IL7, IL8, and tumor necrosis factor-alpha (TNF-α) are increased in patients with severe COVID-19 [3], [24]. Interestingly, it seems that when the number of T lymphocytes, especially the CD8+ are at the lowest level; the serum levels of IL-10, IL-2, IL-4, TNF-α, and IFN-γ are at the highest level [21], [24]. The levels of interleukin −2 receptor (IL-2R) and IL-6 were significantly different between patients with mild, moderate, and severe clinical symptoms [31]. In another study, lymphopenia was reported in 26 of 41 ICU and non-ICU patients with higher plasma levels of GSCF, IP10, MCP1, MIP1A, IL7, IL2, IL10, and TNFα in ICU patients with COVID-19 [5].
Yang Yang et al reported that when COVID-19 cases compared with healthy control, concentrations of both pro-and anti-inflammatory cytokines including IL-6, IFN-γ, IL-1ra, IL-18, IL-2ra, and IL-10 in the plasma samples were significantly increased. Increased plasma levels of MCP-3, IP-10, and IL-1ra are significantly associated with disease progression, severity, and fatal outcome of COVID-19 patients. The levels of these cytokines were highly correlated with the PaO2/FaO2 [32]. TH17 cells are involved in the cytokine storm phenomenon in patients with COVID-19 via induction of production of cytokines G-CSFIL-1b, IL-6, TNF-α MIP2A chemokines KC (Keratinocyte chemoattractant), IP10, MIP3A, IL-8 [33]. OX40 (CD134) is a potent costimulatory receptor that can activate T lymphocytes following engagement by its ligand on dendritic cells which promotes proliferation, effector function, and survival of T lymphocytes [34]. Previous studies have shown that OX40 has an important role in cytokine storm and recently some studies have reported increased expression of OX40 on CD4+ T in patients with COVID 19 [35], [36]. Although, monocytes and T cells are mainly the origins of cytokine storm in patients with COVID-19, the mechanism that explains the increased levels of different cytokines and chemokines needs to be addressed.
2.4. Anti-SARS-COV-2 antibodies
The first report of specific anti-SARS-COV-2 was performed by Zhikun Zeng et.al which SARS-CoV-2 specific IgM and IgG were detected at day 9 and 15 respectively after the onset of COVID-19 [37]. Another study has reported that anti-SARS-COV-2 IgG is detectable at the illness day 11 or the post-exposure 18–21 days [38]. Also, the levels of IgM and IgG antibodies that can neutralize the SARS-CoV-2 were detected in serum before symptomatic recovery [39]. The rates of seropositivity of Anti- surface spike protein receptor-binding domain (anti-RBD) IgG/IgM and Anti-nucleoprotein (anti-NP) IgG/IgM were detected 14 days or longer after symptom onset and anti-NP/RBD IgG levels correlated with virus neutralization titer [40]. Increased IgG response is related to the severity of COVID-19 So that the risk of death has more occurred in cases with neutrophil-to-lymphocyte ratio (NLR) High and IgGhigh phenotype [26]. The Anti-N, S1 IgG/ IgM responses to SARS-CoV-2 are detectable at the convalescent phase in 100% of COVID-19 patients. Also, the level of S1 IgG has a positive association with lactate dehydrogenase (LDH) and the age of the patients while negatively correlated with the Lymphocyte percentage [41]. However, the level of anti-SARS-CoV-2 IgG antibody in female patients with severe COVID-19 was higher than the anti-SARS-CoV-2 IgG antibody in male patients [42]. Nevertheless, antiviral IgM and IgG were shown to disappear on day 80 after disease onset [43]. Also, the rapid reduction of SARS-CoV-2 RBD-specific IgM, IgG, and IgA was reported in convalescent patients up to 14 weeks after discharge [44]. However, recent studies, have detected serum-specific SARS-VOV-2 IgG of healthcare workers and COVID-19 recovered patients up to 6 months [45], [46]. Because of differences in the receptor-binding domain (RBD) in the spike protein of SARS-CoV and SARS-COV-2, neutralizing antibodies such as CR3014 and m396 that target the ACE2 binding site of SARS-CoV specifically, failed to bind SARS-COV-2 spike protein. Fortunately, X. Tian et al. Suggested that CR3022, which can bind to the RBD of SARS-CoV-2, can be used alone or in combination with other neutralizing antibodies for the treatment or prevention of SARS-CoV-2 infections [47].
3. Current potential immunotherapy strategies for COVID-19
3.1. Convalescent plasma (CP) and intravenous immunoglobulin (IVIg)
Preventing and treating an infectious disease could be achieved with antibodies derived from naturally recovered individuals when the immune response and vaccine production takes time to develop. The convalescent plasma In 2014 and 2015 was recommended and established in the treatment of Ebola virus disease and Middle East respiratory syndrome coronavirus (MERS) [48], [49]. Recently, according to the National People's Health Commission of the People's Republic of China (NHCPRC), 91 of the 157 patients with COVID-19 had significant improvement in symptoms of fever, coughs, sputum, muscle pain, and weakness after plasma transfer collected from rehabilitated patients during 48 h of treatment. It has also reduced the level of virus antigens, increased blood oxygen saturation, and lymphocyte ratio [50]. A case study on four critically ill COVID-19 patients that received convalescent plasma and recovered from SARS-CoV-2 infection promises a potential therapy for the treatment of severe acute COVID-19 [51]. The increased number of lymphocytes and decreased C-reactive protein were seen post-transfusion of convalescent plasma compared to pre-transfusion [52]. The convalescent plasma has two important effects via its composition in the Improvement of covid 19 patients. An antiviral effect via neutralizing antibody (NABs) and an Immunomodulatory effect via anti-inflammatory cytokines and antibodies [53]. These antibodies control an overactive immune system (i.e., cytokine storm, th1/th17 ratio, complement activation, and regulation of a hypercoagulable state in COVID-19 patients [53]. Yun Xie et al in the first study on IVIG therapy have reported intravenous that administration of IVIG combined with a glucocorticoid as adjuvant treatment for 58 patients with severe COVID-19. This therapy within 48 h of admission to the ICU, reduced use of mechanical ventilation, hospital length of stay, with an improvement to achieve significant clinical effectiveness and improving 28-day mortality [54].
3.2. Monoclonal antibody
3.2.1. TNF inhibitor
Thalidomide (α-N-[phthalimido] glutarimide) is an inhibitor of pro-inflammatory cytokines such as TNF-α and IL-8 through the inhibition of NF-κB [55]. This is known for its effects such as stimulating T cells, anti-inflammation, cell proliferation inhibition, and reducing lung injury and pulmonary fibrosis. Chengshui Chen et al. have reported that a patient who had developed severe COVID-19 pneumonia was treated with thalidomide in combination with low-dose methylprednisolone (glucocorticoid) within 1 to 8 days after treatment, the patient clinical condition improved, including increased oxygen index, relieving vomit, alleviating anxiety to reduce oxygen consumption, and lung exudation [56]. Furthermore, cytokine levels such as IL-6, IL-10, IFN-γ returned to the normal range 6 days after treatment. Interestingly, the absolute value of lymphocytes, T cells, D4+ T cells, CD8+ T cells, NK cells, and B cells also significantly increased 5 days after treatment [56].
3.2.2. IL-6 inhibitors
There are two classes of IL-6 inhibitors that were approved by the FDA-: monoclonal antibodies against IL-6 receptor (e.g., Sarilumab, Tocilizumab) and anti-IL-6 monoclonal antibodies (Siltuximab) [57].
Tocilizumab or Actemra® is an antagonist for interleukin (IL)-6 receptor that is approved in combination with methotrexate as a treatment for patients with severe active rheumatoid arthritis in elderly ages [58]. In COVID-19 patients a tremendous level of pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α, and MCP correlates to the pathogenesis of severe acute pneumonia-causing by SARS-COV-2 [3], [5]. More recently case report study has suggested two doses of Tocilizumab, at 8 mg/kg intravenously was able to reduce CRP and cytokine storm in a male with renal carcinoma who was suffering from COVID-19 [59]. Also, in patients with a severe case of COVID-19 who received Tocilizumab, clinical symptoms and clinical findings such as fever, lung lesion CRP, and the number of peripheral lymphocytes were returned to normal within a few days [60]. Moreover, the treatment of a 60-year-old man patient with multiple myeloma who was positive for COVID-19 with 8 mg/kg Tocilizumab, showed a decreased level of IL-6 and highly effective at preventing multiple myeloma [61]. This is promising that Tocilizumab could be an effective treatment of COVID-19 and more studies now in progress. Of course, studies have shown the adverse effects of Tocilizumab such as acute pancreatitis, hypertriglyceridemia, cytopenias, hypofibrinogenemia elevated ferritin, and lactate dehydrogenase in COVID-19 patients [62], [63].
A fully-human monoclonal antibody is Sarilumab (Kevzara) that inhibits the interleukin-6 (IL-6) signaling by blocking the IL-6 receptor. A phase 2/3 trial is currently ongoing to survey the efficacy and safety of Sarilumab in adult COVID-19 patients with serious complications [64].
Of course, lab abnormalities have been reported with Sarilumab and Tocilizumab treatment such as transient and/or reversible increase in liver enzymes, Aspartate AminoTransfrase and Alanin Amino Transferase that appear to be dose-dependent and rare occurrences of thrombocytopenia and neutropenia. Also, the risk for serious infections (bacterial or fungal infections) and bowel perforation have been reported with long-term use of these drugs [65].
Another recombinant human-mouse chimeric monoclonal antibody is Siltuximab that binds IL-6 and is approved by the FDA for use in Castleman’s disease patients. Siltuximab, inhibit IL-6 signaling by prevention the binding of IL-6 to both soluble and membrane-bound IL-6 receptors [66]. There was no data describing clinical experience with siltuximab in patients with SARS and MERS. Also, it is limited data describing the effect of siltuximab on patients with COVID-19 [65]. Up to now, 4 clinical trials of this drug are underway in Covid 19 patients [67].
3.2.3. IL-1 receptor antagonist
Anakinra is an interleukin IL-1 receptor antagonist. This recombinant antibody blocks IL-1α and IL-1β (proinflammatory cytokines) activity and is used to treat autoinflammatory disorders [68]. The advantage of Anakinera over other cytokine blockers is its short half-life and safety, which allows it to be discontinued for use in critically ill patients [69]. A retrospective cohort study in consecutive COVID-19 patients, ARDS, and hyper inflammation who received intravenous anakinra showed that treatment with high-dose anakinra was associated with serum C-reactive protein reduction and progressive improvements in respiratory function (72%). Also, the survival rate was 90% in the high-dose anakinra group but 56% in the standard group [70]. Currently, a phase 2/3 open-label, double-blind, multicenter clinical trial evaluating by Swedish Orphan Biovitrum is underway/has started in severe COVID-19 patients. Patients will receive intravenous anakinra 400 mg/day, divided into four daily doses (for 15 days) in combination with Emapalumab, Then safety and efficacy of anakinra at reducing hyper inflammation will be evaluated [71].
3.2.4. Complement inhibitor
Complement activation may be the cause of some pathophysiology aspects of COVID-19 infections, such as thrombotic microangiopathy (TMA) and acute kidney injury [72]. A study by Diao et al. Showed that C5b-9 complement deposition in the renal tubules of six patients with SARS-CoV-2 infection indicated the activation of the complement cascade in the kidney [73]. Eculizumab, as a monoclonal antibody, inhibiting C5b formation and prevents complement activation. Therefore, Ruchi Mahajan and colleagues presented the results of their study of a 14-year-old female as the first use of eculizumab to inhibit complement activation in children-mediated AKI associated with COVID-19 infection. The results of this study showed the Improvement of laboratory tests, clinical symptoms, and chest X-ray [72].
3.2.5. Anti-CD147 antibody
Meplazumab, is a humanized anti-CD147 antibody could block the infection of SARS-CoV via two mechanisms, inhibition of virus replication and suppression of inflammation storm. CD147 is a receptor for spike protein; so Meplazumab can block virus invasion and replication in the host cells [20].
On the other hand, CD147 is a receptor for the proinflammatory factor Cyclophilin A (CyPA). CyPA (e.g. activates and attracts leukocytes to the stimulus site in response to inflammatory stimuli [74]. Thus meplazumab can attenuate the chemotactic effect of CyPA via inhibition of the interaction of CyPA with CD147. Huijie Bian et al reported that adding meplazumab in patients with COVID-19 could decrease CRP, increased the virological clearance rate, promoted lymphocytopenia, and recovery chest radiography. They believe that there are two reasons for the short-term restoration of lymphocyte counts in patients treated with meplazumab. First, preventing virus invasion to keep lymphocytes survival and second, inhibiting the lymphocyte accumulation in pulmonary organ and lymphocyte elevation in peripheral blood [20].
4. Cytokines and interferons therapy
Type 1 interferons have a broad antiviral activity and many studies have been performed on the treatment of Covid 19 patients with these cytokines, and most of them showed improvement in laboratory findings and clinical symptoms [59], [75]. According to Wang, Nan and colleagues' study, three patients with COVID-19 who received IFN-α-2b showed reduced viral load and increased anti-SARS -COV-2 Serum titration and chest X-ray improvement [76].
In China, treatment with 5 million unit of IFN-α by vapor inhalation twice a day accompanied by anti-viral drugs such as ribavirin is a guideline for the COVID-19 treatment [77].
Mansourabadi and colleagues reviewed 24 studies on the efficacy of type 1 interferon on COVID-19 patients and reported that treatment with this cytokine combined with other treatments (such as CP therapy, antiviral drugs, IVIg, MSC, and methylprednisolone) improved laboratory tests and clinical symptoms such as improved lymphocytopenia, improved pulmonary function, and decreased the SARS-CoV-2 RNA to an undetectable level [75].
Clinical trials have been recently registered to survey a combination of lopinavir/ritonavir with ribavirin and IFNβ1b administered subcutaneously (NCT04276688) or lopinavir/ritonavir and IFNα2b (ChiCTR2000029387) for COVID-19 treatment [78]. IFNβ1 may account for an easy and safe to upscale treatment for patients with COVID-19 in the early stages of SARS-CoV-2 infection. Similar treatments had a mixed efficiency against SARS-CoV viruses and MERS-CoV, but in vitro studies suggest that SARS-CoV-2 could be substantially more sensitive to IFN-I than other coronaviruses [78].
5. JAK signaling inhibitors
JAK signaling pathway inhibitors such as Baricitinib, Fedratinib, and ruxolitinib are powerful anti-inflammatory agents that are effective against the consequences of elevated cytokine levels in various diseases such as rheumatoid arthritis and myelofibrosis [79]. Generally, drugs that inhibit members of the numb-associated kinase (NAK) family (including AAK1 and GAK) such as Baricitinib reduce viral infection in vitro via inhibition of clathrin-mediated. Baricitinib have received more attention than others for the four following reasons: A) Anti-inflammatory properties B) The high affinity for NAKs C) Ability to ameliorate associated chronic inflammation in interferonopathies and D) High potential for combination therapy because of its low plasma protein binding and minimal interaction with cytochrome P enzymes and drug transporters. It is suggested that the use of Baricitinib in combination with antiviral drugs such as lopinavir or Ritonavir and Ramsudavir for patients with COVID-19 can reduce the inflammatory response of the host and reduce virus recurrence infection [80]. Th17 cells and secreted cytokines are one of the important participants in the cytokine storm and the pathology of Covid 19. Since this cell needs the JAK signaling pathway to differentiation and effector function; So Fedratinib (JAK2 inhibitor) could be used for reducing mortality of COVID-19 disease [81].
6. Cellular therapy
6.1. Mesenchymal stem cells (MSC)-based therapy
MSCs express anti-inflammatory and trophic factors like TGF-β, HGF, GAL, NOA1, LIF, BDNF, VEGF, EGF, FGF, and NGF indicating the immunomodulatory function of these cells [82]
Leng Z et al have reported that intravenous transplantation of ACE2- and TMPRSS2- Mesenchymal stem cells (MSCs) that are free from COVID-19 infection, improved the outcome of 7 enrolled patients with COVID-19 pneumonia 2–4 days after MSC transplantation. Also, the peripheral lymphocytes, regulatory DC cells, IL-10 were increased, and serum levels of TNF-α and CRP were decreased [83]. A 65 years old woman with severe pneumonia who received three doses of allogeneic stem cells along with conventional therapy showed improved symptoms and negative real-time PCR for Coronavirus [84].
6.2. NK cell-based therapy
NK cells can induce an antigen-independent immune response against malignant cells. An increasing number of clinical studies and scientific reports have shown promising antitumor effects when using NK cell-based immunotherapy [85], [86]. AS mentioned above, both the number and function of NK cells have been weakened in COVID-19 infection. Hence, NK cell-based therapy has been employed and approved in China to contribute to the antiviral defense and enhance the immune response, in COVID-19 disease. Kleo Pharmaceuticals Inc. entered into two research collaboration with south Korea-based GC Lab Cell and US-based Celularity Inc to investigate using COVID-19 Antibody Recruiting Molecule allogeneic NK Cell to combat COVID-19 [87]. Antibody Recruiting Molecule (ARM™) is a synthetic molecule and has three binding regions: spike protein binding region, a linker, and an antibody binding region. ARM can binds both to the virus and immune cells via FCγR, resulting in clearance of the virus by the NK cells and macrophage . ARM binds the spike protein on the coronavirus surface, preventing the virus from binding the ACE2 receptor on human cells. Also, this molecule can activate long-term immunity by delivering viral proteins to antigen-presenting cells [87].
7. Discussion
This study was conducted to review the recent evidence to investigate the immunopathogenesis and immunotherapeutic strategies in patients with COVID-19. The most likely mechanism of the immunopathogenesis of COVID-19 is that ligation of TLR3/7/8 by SARS-COV-2 recruitments compartments of downstream signaling pathway which induces the release of pro-inflammatory cytokines and chemokine’s resulting in fever, lung inflammation, and fibrosis [88], [89]. Studies have shown that increased levels of inflammatory cytokines are associated with the patient's condition. Yang Yang et al have reported that the plasma levels IL-6, IFN-γ, IP-10, IL-1ra, MCP-3, IL-18, IL-10, HGF, MIG, G, IL-2ra -CSF, and M−CSF, were significantly increased in COVID-19 patients compared with healthy control [30]. Moreover, ICU-patients had higher levels of GSCF, IP10, MCP1, MIP1A, IL7, IL2, IL10, and TNFα in comparison to non-ICU patients [5]. However, patients with mild, moderate, and severe clinical symptoms, had different levels of interleukin −2 receptor (IL-2R) and IL-6, the concentration of other inflammatory mediators such as hCRP, TNF-α, IL-1, IL-8, IL-10, and lymphocyte count [31]. This difference in cytokine levels and severity of COVID-19 may be due to physiological conditions, the immune system status, viral load, or management of the disease. Another hypothesis for this tremendous amount of cytokine production is an autoamplifying cytokine production called cytokine storm [90]. For instance, it has been reported that IL-8 and IL-6 in bronchoalveolar lavage fluids of patients with acute respiratory distress syndrome (ARDS) are significantly increased and the production and activation of these cytokines are intensified by TNF-α [91].
In cases suffering from new coronavirus increased levels of pro-and anti-inflammatory cytokines are associated with a decrease in CD3 + TCR + CD4+ and CD8+ T lymphocytes. However, the number of exhausted PD-1 + CD8+ and Tim-3 + CD8 + subpopulation was increased in patients with severe COViD-19 [17]. Zhe Xu et al in a case report study have shown, despite decreased peripheral CD4 and CD8 T cells, these cells were hyperactivated (CD8 T cells with high concentrations of cytotoxic granules perforin and granulysin), and the severity of the disease is associated with excessive activity of lymphocytes [23]. The mechanisms of lymphopenia in patients with severe COVID-19 are not well understood. However, some studies have shown type I interferons modulate the S1P-S1P1 (Sphingosine-1-phosphate) system and induce internalization of S1P, a molecule involved in the distribution of lymphocytes, which in turn leads to lymphopenia [92], [93]. Another hypothesis is that the upregulation of angiotensin-converting enzyme 2 (ACE2) lead to the infection of lymphocytes and may lysis them after the viral excessive proliferation [94]. Also, elevated levels of lactate in patients with severe COVID-19 may reduce cytotoxicity activity of CD8 + lymphocytes and shift immune responses to Th17 immunity [25]. IL-6 and IL-23 induce the TH17 cell differentiation through the STAT3 transcription factor, JAK1, and JAK2. Therefore, it is promising that JAK2 inhibitor, Fedratinib hydrochloride (SAR302503) can suppress the production of several TH17 cytokines and possibly reduces the side effects of IL-6 which in turn attenuate TH17-related cytokine storm in COVID-19 [33].
The adaptive immunity plays a key role in response to the new coronavirus infection by the production of neutralizing anti-SARS-COV-2 IgG and IgM [26], [40]. Today it has been proven that the level of anti-SARS-COV-2 IgG/ IgM antibodies is associated with age, sex, and severity of infection [38], [42]. Moreover, Quan-Xin Long et al. have reported 15 out of 37 patients (40%) of asymptomatic individuals and 5 out of 37 patients (12.9%) of the symptomatic individuals became seronegative for IgG [95]. On the other hand, Huan Ma et al. have shown that convalescent COVID-19 patients contain a decline of IgM, IgG, and IgA-specific SARS-COV-2 antibodies 3 months after hospital discharge [44]. Therefore, due to the instability of anti-SARS-COV-2 antibodies in convalescent plasma and Intravenous immunoglobulin, the effectiveness of these treatments requires further clinical trial studies.
Xu, X., et al have reported, in patients with severe COVID-19 using Tocilizumab effectively improved clinical symptoms and laboratory findings such as whole blood cells (WBC) count, CRP, percentage of lymphocytes returned to normal levels [60]. Also, using Thalidomideas an anti-TNF inhibitor is known for its effects such as stimulating T cells, anti-inflammation, inhibiting cell proliferation, and maybe reduce lung injury and pulmonary fibrosis [56]. Hence, the combination of anti-inflammatory therapies, including glucocorticoids, Tocilizumab [59] and sarilumab [96] IL-6 inhibitors, JAK inhibitors such as Baricitinib [80], MSC-based therapy [83] may be more suitable for the treatment of patients with severe acute respiratory syndrome coronavirus 2 infections.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was supported by grant No 9929 from Shahroud University of Medical Sciences.Also, we would like to thank Dr. Moslem JafariSani for editing the final version of this study.
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su S., Wong G., Shi W., Liu J., Lai A.C., Zhou J., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng Y, Meng K, Guan H, Leng L, Zhu R, Wang B, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua xin xue guan bing za zhi. 2020;48:E004-E. [DOI] [PubMed]
- 4.Hu B., Zeng L.-P., Yang X.-L., Ge X.-Y., Zhang W., Li B., et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13(11) doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniloski Z., Guo X., Sanjana N.E. The D614G mutation in SARS-CoV-2 Spike increases transduction of multiple human cell types. BioRxiv. 2020 doi: 10.7554/eLife.65365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito T., Gale M., Jr Principles of intracellular viral recognition. Curr. Opin. Immunol. 2007;19(1):17–23. doi: 10.1016/j.coi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Bouayad A. Innate immune evasion by SARS-CoV-2: Comparison with SARS-CoV. Rev. Med. Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2135. [DOI] [PubMed] [Google Scholar]
- 10.Kanto T., Hayashi N. Immunopathogenesis of hepatitis C virus infection: multifaceted strategies subverting innate and adaptive immunity. Intern. Med. 2006;45(4):183–191. doi: 10.2169/internalmedicine.45.1530. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi O., Akira S. Innate immunity to virus infection. Immunol. Rev. 2009;227(1):75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung Y.-F., Chen C.-Y., Shih Y.-C., Liu H.-Y., Huang C.-M., Hsueh Y.-P. Endosomal TLR3, TLR7, and TLR8 control neuronal morphology through different transcriptional programs. J. Cell Biol. 2018;217(8):2727–2742. doi: 10.1083/jcb.201712113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S, editors. TLR signaling. Seminars in immunology; 2007: Elsevier. [DOI] [PubMed]
- 14.Patel A., Jernigan D.B. Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak—United States, December 31, 2019–February 4, 2020. Morb. Mortal. Wkly Rep. 2020;69(5):140. doi: 10.15585/mmwr.mm6905e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demaria O., Carvelli J., Batista L., Thibult M.-L., Morel A., André P., et al. Identification of druggable inhibitory immune checkpoints on Natural Killer cells in COVID-19. Cell. Mol. Immunol. 2020;17(9):995–997. doi: 10.1038/s41423-020-0493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiappelli F., Khakshooy A., Greenberg G. CoViD-19 Immunopathology and Immunotherapy. Bioinformation. 2020;16(3):219. doi: 10.6026/97320630016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalams S.A., Walker B.D. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 1998;188(12):2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam V.C., Lanier L.L. NK cells in host responses to viral infections. Curr. Opin. Immunol. 2017;44:43–51. doi: 10.1016/j.coi.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bian H., Zheng Z.-H., Wei D., Zhang Z., Kang W.-Z., Hao C.-Q., et al. Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. MedRxiv. 2020 [Google Scholar]
- 21.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiappini E., Galli L., Azzi A., Resti M., Bonsignori F., de Martino M. Lymphocytopenia as a marker for pandemic influenza A/H1N1 2009 virus infection in children. J. Med. Virol. 2011;83(1):1–4. doi: 10.1002/jmv.21930. [DOI] [PubMed] [Google Scholar]
- 23.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respiratory Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J.-P.-O., Lam D.S.C., Chen Y., Ting D.S.W. Novel Coronavirus disease 2019 (COVID-19): The importance of recognising possible early ocular manifestation and using protective eyewear. BMJ Publishing Group Ltd. 2020 doi: 10.1136/bjophthalmol-2020-315994. [DOI] [PubMed] [Google Scholar]
- 25.Chang C.-H., Pearce E.L. Emerging concepts in immunotherapy–T cell metabolism as a therapeutic target. Nat. Immunol. 2016;17(4):364. doi: 10.1038/ni.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B., Zhou X., Zhu C., Song Y., Feng F., Qiu Y., et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front. Mol. Biosci. 2020;7:157. doi: 10.3389/fmolb.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H., et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J.-j., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 30.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;102763 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L., Liu H., Liu W., Liu J., Liu K., Shang J., et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua jie he he hu xi za zhi= Zhonghua jiehe he huxi zazhi= Chin. J. Tuberculosis Respiratory Dis. 2020;(43) doi: 10.3760/cma.j.issn.1001-0939.2020.0005. E005-E. [DOI] [PubMed] [Google Scholar]
- 32.Zheng C. Time course of lung changes at chest CT during recovery from Coronavirus Disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.K. Shen, Y. Yang, T. Wang, D. Zhao, Y. Jiang, R. Jin, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World J. Pediatr. (2020) 1–9. [DOI] [PMC free article] [PubMed]
- 34.Curti B.D., Kovacsovics-Bankowski M., Morris N., Walker E., Chisholm L., Floyd K., et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73(24):7189–7198. doi: 10.1158/0008-5472.CAN-12-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., et al. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+ CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. BioRxiv. 2020 [Google Scholar]
- 36.Croft M., So T., Duan W., Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol. Rev. 2009;229(1):173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao A.T., Gao C., Zhang S. Profile of specific antibodies to SARS-CoV-2: the first report. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee N.-Y., Li C.-W., Tsai H.-P., Chen P.-L., Syue L.-S., Li M.-C., et al. A case of COVID-19 and pneumonia returning from Macau in Taiwan: Clinical course and anti-SARS-CoV-2 IgG dynamic. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thevarajan I., Nguyen T.H., Koutsakos M., Druce J., Caly L., van de Sandt C.E., et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26(4):453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.To K.K.-W., Tsang O.T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang H-w, Li Y., Zhang H-n, Wang W., Men D., Yang X., et al. Global profiling of SARS-CoV-2 specific IgG/IgM responses of convalescents using a proteome microarray. medRxiv. 2020 doi: 10.1038/s41467-020-17488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng F., Dai C., Cai P., Wang J., Xu L., Li J., et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J. Med. Virol. 2020 doi: 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu A., Wang W., Zhao X., Zhou X., Yang D., Lu M., et al. Disappearance of antibodies to SARS-CoV-2 in a-COVID-19 patient after recovery. Clin. Microbiol. Infect. 2020;26(12):1703–1705. doi: 10.1016/j.cmi.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma H., Zhao D., Zeng W., Yang Y., Hu X., Zhou P., et al. Decline of SARS-CoV-2-specific IgG, IgM and IgA in convalescent COVID-19 patients within 100 days after hospital discharge. Sci. China Life Sci. 2020;1–4 doi: 10.1007/s11427-020-1805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Figueiredo-Campos P., Blankenhaus B., Mota C., Gomes A., Serrano M., Ariotti S., et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur. J. Immunol. 2020;50(12):2025–2040. doi: 10.1002/eji.202048970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J., et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020;5(12):1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Organization W.H. World Health Organization; 2014. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks: interim guidance for national health authorities and blood transfusion services. [Google Scholar]
- 49.Arabi Y., Balkhy H., Hajeer A.H., Bouchama A., Hayden F.G., Al-Omari A., et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4(1):1–8. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Law P.K. Emergent serum therapy and antibody medicine to counteract sudden attacks of COVID-19 and other pathogenic epidemics. Sci. Res. Publ. 2020 [Google Scholar]
- 51.Zhang B., Liu S., Tan T., Huang W., Dong Y., Chen L., et al. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest. 2020 doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. medRxiv. 2020 [Google Scholar]
- 53.Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun. Rev. 2020;102554 doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Y., Cao S., Li Q., Chen E., Dong H., Zhang W., et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amirshahrokhi K., Khalili A.-R. The effect of thalidomide on ethanol-induced gastric mucosal damage in mice: involvement of inflammatory cytokines and nitric oxide. Chem. Biol. Interact. 2015;225:63–69. doi: 10.1016/j.cbi.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 56.C. Chen, F. Qi, K. Shi, Y. Li, J. Li, Y. Chen, et al. Thalidomide combined with low-dose glucocorticoid in the treatment of COVID-19 pneumonia, (2020). [DOI] [PMC free article] [PubMed]
- 57.Khan F., Fabbri L., Stewart I., Robinson K., Smyth A.R., Jenkins G. Sarilumab and Siltuximab for coronavirus-related infections. medRxiv; Tocilizumab: 2020. A systematic review of Anakinra. [Google Scholar]
- 58.Oldfield V., Dhillon S., Plosker G.L. Tocilizumab. Drugs. 2009;69(5):609–632. doi: 10.2165/00003495-200969050-00007. [DOI] [PubMed] [Google Scholar]
- 59.Michot J.-M., Albiges L., Chaput N., Saada V., Pommeret F., Griscelli F., et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann. Oncol. 2020 doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X., Song K., Tong F., Fei M., Guo H., Lu Z., et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4(7):1307. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morrison A.R., Johnson J.M., Ramesh M., Bradley P., Jennings J., Smith Z.R. Letter to the Editor: Acute hypertriglyceridemia in patients with COVID-19 receiving tocilizumab. J. Med. Virol. 2020 doi: 10.1002/jmv.25907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Radbel J., Narayanan N., Bhatt P.J. Use of tocilizumab for COVID-19 infection-induced cytokine release syndrome: A cautionary case report. Chest. 2020 doi: 10.1016/j.chest.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.TARRYTOWN NY. First Patient Outside U.S. Treated in Global Kevzara® (sarilumab) Clinical Trial Program for Patients with Severe COVID-19. https://wwwdrugscom/clinical_trials/first-patient-outside-u-s-treated-global-kevzara-sarilumab-clinical-trial-program-patients-severe-18499html. 2020.
- 65.Interleukin-6 Inhibitors. https://wwwcovid19treatmentguidelinesnihgov/immune-based-therapy/immunomodulators/interleukin-6-inhibitors/. 2020.
- 66.Ataie-Kachoie P., Pourgholami M.H., Morris D.L. Inhibition of the IL-6 signaling pathway: a strategy to combat chronic inflammatory diseases and cancer. Cytokine Growth Factor Rev. 2013;24(2):163–173. doi: 10.1016/j.cytogfr.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 67.Siltuximab. https://clinicaltrialsgov/ct2/results?cond=COVID-19&term=Siltuximab&cntry=&state=&city=&dist. 2020.
- 68.Furst D.E. Anakinra: review of recombinant human interleukin-I receptor antagonist in the treatment of rheumatoid arthritis. Clin. Ther. 2004;26(12):1960–1975. doi: 10.1016/j.clinthera.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 69.Cavalli G., Pappalardo F., Mangieri A., Dinarello C.A., Dagna L., Tresoldi M. Treating life-threatening myocarditis by blocking interleukin-1. Crit. Care Med. 2016;44(8):e751–e754. doi: 10.1097/CCM.0000000000001654. [DOI] [PubMed] [Google Scholar]
- 70.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. The Lancet. Rheumatology. 2020 doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sobi to initiate a clinical study to evaluate whether anakinra and emapalumab may relieve complications associated with severe COVID-19 disease. https://wwwsobicom/en/press-releases/sobi-initiate-clinical-study-evaluate-whether-anakinra-and-emapalumab-may-relieve. 2020.
- 72.Mahajan R., Lipton M., Broglie L., Jain N.G., Uy N.S. Eculizumab treatment for renal failure in a pediatric patient with COVID-19. J. Nephrol. 2020;33(6):1373–1376. doi: 10.1007/s40620-020-00858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diao B., Feng Z., Wang C., Wang H., Liu L., Wang C., et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. MedRxiv. 2020 doi: 10.1038/s41467-021-22781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dawar F.U., Wu J., Zhao L., Khattak M.N.K., Mei J., Lin L. Updates in understanding the role of cyclophilin A in leukocyte chemotaxis. J. Leukoc. Biol. 2017;101(4):823–826. doi: 10.1189/jlb.3RU1116-477R. [DOI] [PubMed] [Google Scholar]
- 75.Mansourabadi A.H., Sadeghalvad M., Mohammadi-Motlagh H.-R., Rezaei N. The immune system as a target for therapy of SARS-CoV-2: a systematic review of the current immunotherapies for COVID-19. Life Sci. 2020;118185 doi: 10.1016/j.lfs.2020.118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang N., Zhan Y., Zhu L., Hou Z., Liu F., Song P., et al. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe. 2020;28(3) doi: 10.1016/j.chom.2020.07.005. pp. 455–64. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discoveries Therapeut. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 78.Sallard E., Lescure F.-X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N., Florence A., et al. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;104791 doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduction Targeted Therapy. 2020;5(1):1–8. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet. Infect. Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5(6):485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 83.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Disease. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liang B., Chen J., Li T., Wu H., Yang W., Li Y., et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine. 2020;99(31) doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng M., Chen Y., Xiao W., Sun R., Tian Z. NK cell-based immunotherapy for malignant diseases. Cell. Mol. Immunol. 2013;10(3):230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.F. Fang, W. Xiao, Z. Tian (Eds.). NK cell-based immunotherapy for cancer. Seminars in immunology; 2017: Elsevier. [DOI] [PubMed]
- 87.A Simple Approach to Convert IVIG into Neutralizing Anti-Coronavirus Antibodies. https://wwwkleopharmaceuticalscom/our-pipeline/covid-19/. 2020.
- 88.Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 89.Onofrio L., Caraglia M., Facchini G., Margherita V., Placido S.D., Buonerba C. Toll-like receptors and COVID-19: a two-faced story with an exciting ending. Future. Science. 2020 doi: 10.2144/fsoa-2020-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.B.G. Chousterman, F.K. Swirski, G.F. Weber, (Eds.), Cytokine storm and sepsis disease pathogenesis. Seminars in immunopathology; 2017: Springer. [DOI] [PubMed]
- 91.Blackwell T., Christman J. Sepsis and cytokines: current status. Br. J. Anaesth. 1996;77(1):110–117. doi: 10.1093/bja/77.1.110. [DOI] [PubMed] [Google Scholar]
- 92.Liu C.H., Thangada S., Lee M.-J., Van Brocklyn J.R., Spiegel S., Hla T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell. 1999;10(4):1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kamphuis E., Junt T., Waibler Z., Forster R., Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108(10):3253–3261. doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- 94.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12(1):1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 96.Benucci M., Giannasi G., Cecchini P., Gobbi F.L., Damiani A., Grossi V., et al. COVID-19 pneumonia treated with Sarilumab: A clinical series of eight patients. J. Med. Virol. 2020 doi: 10.1002/jmv.26062. [DOI] [PMC free article] [PubMed] [Google Scholar]