Abstract
Background
The long-term safety results of the REALIZE (Ethicon Endo-Surgery, Inc., Cincinnati, OH) adjustable gastric band collected in this prospective, multicenter study in patients with morbid obesity are presented.
Objectives
To determine the reoperation rate, including band revisions, replacements, and explants, resulting from a serious adverse device-related event through years 4 and 5. Various efficacy measures were also assessed as secondary objectives.
Setting
Nine academic and/or private institutions.
Methods
The participating institutions enrolled 303 patients, who were then assessed on an annual basis, with 231 patients completing 5 years of follow-up. The study parameters included reoperation rates, changes in percentage of excess weight loss (%EWL), and changes in body mass index (BMI), as well as parameters of diabetes and dyslipidemia. Quality of life was assessed using the Short Form (SF)-36 and the Impact of Weight on Quality of Life-Lite questionnaires.
Results
The reoperation rate due to a serious adverse event in this population at 5 years after implantation with the REALIZE gastric band was 8.9%. The most common serious adverse event was band slippage, which affected 6.9% of the study population. The mean %EWL was 35.6% ± 26.84%, and the decrease in mean BMI was −7.01 ± 5.45 kg/m2 at 5 years. Patients experienced improvements in mean glycated hemoglobin and serum lipid levels, in addition to improvements in the quality of life measures.
Conclusion
No new safety concerns were identified during the 5 years of follow-up. Although the results of this study did not meet the predefined safety criteria of 8% or less, the safety profile and long-term effectiveness observed in this study are consistent with those in the current literature.
Keywords: Morbid obesity, Bariatric surgery, Gastric band, Weight loss, REALIZE adjustable gastric band, Laparoscopic adjustable gastric band
According to the World Health Organization, the worldwide prevalence of obesity has tripled since 1975 [1]. It is estimated that there were 1.9 billion adults (age 18+ years) who were overweight and 650 million adults who were obese in 2016 [1]. Obesity is associated with a wide variety of undesirable health outcomes. Being overweight or obese substantially raises an individual’s risk of mortality and morbidity from hypertension, dyslipidemia, type 2 diabetes (T2D), coronary heart disease, stroke, gall bladder disease, osteoarthritis, sleep apnea, respiratory problems, and endometrial, breast, prostate, and colon cancers [2]. In a more recent development, obesity, especially for those patients with a body mass index (BMI) ≥ 35 kg/m2, is a contributing factor to the severity of severe acute respiratory syndrome coronavirus 2 and the need for invasive mechanical ventilation [3].
Evidence has demonstrated that various strategies to reduce weight in overweight and obese individuals can successfully reduce the risks of mortality and morbidity [2,[4], [5], [6]]. Weight loss surgical procedures, including gastric bypass, sleeve gastrectomy, and adjustable gastric banding, exhibit the most substantial and durable responses among the available weight loss interventions. However, weight loss procedures are major operations with certain limitations and with both short- and long-term complications.
The purpose of this study is to present 5-year safety and effectiveness data for the REALIZE gastric band (manufactured by Ethicon Endo-Surgery, Inc.), as directed by the United States Food and Drug Administration (FDA). The primary objective was to assess the 5-year REALIZE gastric band reoperation rate, including band revisions, replacements, and explantations, resulting from serious adverse device-related events. The secondary (effectiveness) objectives were to evaluate changes in excess weight, quality of life, glycated hemoglobin (HbA1C) levels, and serum lipid levels 5 years after implantation of the REALIZE gastric band.
Methods
Study centers
We selected 9 academic and/or private practice clinical sites to participate in the study, based on their laparoscopic surgery experience and multidisciplinary team approach. All study centers followed a common protocol approved by their respective institutional review boards.
Study population
This was a prospective, single-arm, multicenter study to fulfill the FDA requirement to perform a postapproval study to evaluate the long-term safety and effectiveness of the REALIZE adjustable gastric band (clinicaltrials.gov: NCT 00543140). The initial study included 140 patients who had the device implanted under the original premarket approval (PMA) protocol (clinicaltrials.gov: NCT 00166205) and who reconsented to participate in the extended study. An additional 163 patients enrolled in a parallel protocol, for a total of 303 patients in the intent-to-treat population (ITT). Data were pooled from both protocols and data collection was conducted under similar conditions using the same device to assess safety and efficacy during the same assessment periods. A total of 231 (76.2%) patients completed the 5-year follow-up visit. The final study visit was completed on January 12, 2015. Male and female patients between 18 and 60 years of age (inclusive) who were considered appropriate candidates for a surgical weight loss intervention (body mass index [BMI] of >40 kg/m2 and ≤55 kg/m2, or a BMI ≥35 kg/m2 and <40 kg/m2 with ≥1 co-morbid conditions) were considered for inclusion in both the initial PMA and extension cohorts, as well as the parallel postmarket study cohort.
Key exclusion criteria for the PMA extension group included:
-
1.
Prior REALIZE explantation.
Key exclusion criteria for the parallel protocol included:
-
1.
Women who were currently pregnant or not practicing birth control;
-
2.
A previous malabsorptive or restrictive bariatric procedure;
-
3.
The presence of inflammatory disease or congenital or acquired anomalies of the gastrointestinal (GI) tract;
-
4.
Severe cardiopulmonary disease or other serious organic disease;
-
5.
Upper GI bleeding disorders;
-
6.
Gastroesophageal reflux disease;
-
7.
Use of long-term steroid treatment or steroids within 15 days of surgery;
-
8.
Being unable or unwilling to comply with the dietary restrictions required by this procedure;
-
9.
Having a known allergy to materials contained within the band or its injection port;
-
10.
The presence of a terminal illness with a life expectancy of ≤5 years;
-
11.
An inability to refrain from use of anticoagulants or aspirin within 15 days before surgery; or
-
12.
Acute or chronic infection (localized or systemic).
Surgical technique
The REALIZE adjustable gastric bands, also known as the Swedish adjustable gastric bands or the curved adjustable gastric bands (Ethicon Endo-surgery, Cincinnati, OH), were implanted in accordance with their respective Instructions for Use. The surgical technique has been previously described by Phillips et al. [7].
Statistical analyses
The sample size was selected to provide greater than 80% power, based on a 1-sided exact binomial test and a .05 significance level, to detect a significant difference from the performance goal of 8% after 4 and 5 years of implantation. The performance goal of 8% was based upon the safety results of the initial 3 years of this study, with input from the FDA. The reoperation rate in years 1–3 was ∼2% per year and was projected to be 4% for years 4 and 5. Doubling this rate established a 8% performance goal based upon an inferiority margin factor of 2. Secondary effectiveness parameters were summarized by postimplantation year. For continuous parameters, we used descriptive statistics, including means, standard deviations, medians, minimums, maximums, and 95% confidence intervals (CIs) of the means. Changes from baseline were summarized in a similar manner, with baseline defined as the last available measurement on or before the date of implantation. An analysis of results from the Short Form (SF)-36 Questionnaire and the Impact of Weight on Quality of Life (IWQOL-Lite) was consistent with the recommendations for these surveys, and the significance of changes from baseline was assessed using the matched-pairs t test. Categorical parameters were calculated as summaries with counts and percentages.
Ethical approval and informed consent
All procedures performed in this study were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Results
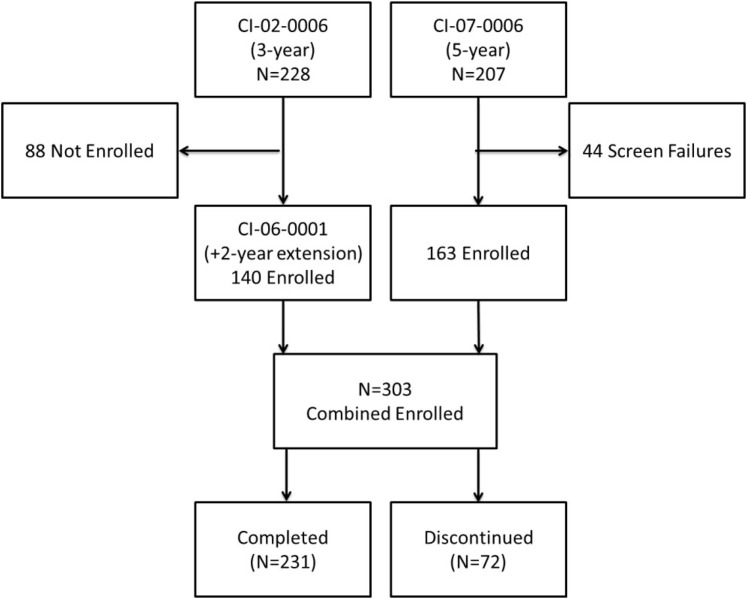
The disposition of patients showing the merging of the 2 cohorts is presented in Fig. 1 . Protocol CI-02-006 represents cohort from the PMA with the 2-year extension, and CI-07-006 represents the parallel cohort added as part of the postapproval study.
Fig. 1.
Disposition of patients.
There were 9 participating institutions that contributed to this study. Table 1 lists the site numbers, the number of patients enrolled, and the number of patients who completed 60 months of follow-up.
Table 1.
Site listing and number of participating patients
| Site no. | Enrolled | Completed |
|---|---|---|
| 1 | 22 | 12 |
| 2 | 76 | 52 |
| 3 | 14 | 8 |
| 4 | 81 | 59 |
| 5 | 20 | 18 |
| 6 | 13 | 13 |
| 7 | 19 | 18 |
| 8 | 29 | 28 |
| 9 | 29 | 23 |
| Total | 303 | 231 |
Of the 303 patients, 72 did not complete a 5-year visit. The reasons for study discontinuation are listed in Table 2 .
Table 2.
Reasons for study discontinuation
| Reason for discontinuation | Intent-to-treat population, n = 303, n (%) |
|---|---|
| Lost to follow-up | 33 (45.8) |
| Withdrawal of consent | 13 (18.1) |
| Protocol violation | 10 (13.9) |
| Early band removal due to a complication | 9 (12.5) |
| SAE leading to removal of gastric band | 3 (4.2) |
| Lack of effectiveness leading to explant or additional obesity surgical procedure | 1 (1.4) |
| Death | 1 (1.4) |
| Other | 2 (2.8) |
| Total | 72 |
SAE = serious adverse event.
The complications or serious adverse events prompting device removal included device-related infections, band slippage, erosive gastritis due to band erosion, and impaired gastric emptying. There was 1 subject death in the study, during the second year of follow-up, due to myocardial infarction deemed unrelated to the study device or procedure. The demographic characteristics and baseline characteristics of the combined study population are presented in Table 3 . The majority of patients were female and Caucasian, not of Hispanic origin, with a mean age of 40.5 years. The combined study population had a mean weight of 272.2 pounds, the mean BMI was 44.1 kg/m2, and the mean waist circumference was 49.2 inches.
Table 3.
Demographic and baseline characteristics (n = 303)
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 61 (20.1) |
| Female | 242 (79.9) |
| Race | |
| Caucasian, not of Hispanic origin | 205 (67.7) |
| Asian/Pacific Islander | 8 (2.6) |
| Black, not of Hispanic origin | 27 (8.9) |
| Hispanic | 55 (18.2) |
| Other | 8 (2.6) |
| Age, yr | |
| Mean ± SD | 40.5 ± 9.90 |
| Range | 18–61 |
| Weight, pounds | |
| Mean ± SD | 272.2 ± 40.78 |
| Range | 187–412 |
| BMI, kg/m2 | |
| Mean ± SD | 44.1 ± 4.76 |
| Range | 35–55 |
| Waist circumference, in | |
| Mean ± SD | 49.2 ± 5.67 |
| Range | 37–72 |
SD = standard deviation; BMI = body mass index.
Of the 303 patients enrolled, 76 (25.1%) experienced at least 1 serious adverse event over the course of the study. Table 4 lists the most common serious adverse events reported over the 5 years of follow-up and the numbers of patients affected.
Table 4.
Most common serious adverse events
| Serious adverse event | ITT patients, n (%) |
|---|---|
| Band slippage | 21 (6.9) |
| Erosion into gastrointestinal tract | 4 (1.3) |
| Port displacement | 3 (1.0) |
| Catheter-related complication | 14 (4.6) |
| Gastric dilationa | 7 (2.3) |
| Vomitingb | 5 (1.6) |
| Hiatal herniac | 5 (1.6) |
ITT = intent-to-treat.
Gastric dilation was synonymous with pouch dilation, pouch enlargement, or prolapsed pouch.
Vomiting was defined as a forcible loss of stomach contents deemed related to the study device.
Hiatal hernia was defined as a protrusion of the upper stomach through the diaphragm into the chest cavity.
Reoperation rate
The primary safety endpoint was the reoperation rate, defined using the number of patients for whom reoperations were performed during years 4 and 5 as the numerator and the number of ITT patients as the denominator. For the purpose of this study, reoperations were defined as band explantations, band revisions, and band replacements resulting from a serious adverse device-related event. There were 27 qualified reoperations that fit the protocol definition, for a rate of 8.9% (27 of 303) with a 95% CI range of 6.0% to 12.7% (P = .6919 for comparison to the goal of <8%). As mentioned previously, 1 death occurred during this study, owing to myocardial infarction considered unrelated to the REALIZE gastric band device or procedure.
Secondary endpoints
While not the focus of this study, effectiveness parameters were collected and are summarized in Table 5 .
Table 5.
Weight loss and lab value results
| Variable | 1 yr, n = 295, mean (SD) | 2 yr, n = 276, mean (SD) | 3 yr, n = 271, mean (SD) | 4 yr, n = 160, mean (SD) | 5 yr, n = 240, mean (SD) |
|---|---|---|---|---|---|
| %EWL | 39.0 (19.29) | 42.6 (22.29) | 41.2 (24.87) | 37.4 (27.44) | 35.6 (26.84) |
| Reduction in BMI, kg/m2 | −7.80 (3.94) | −8.46 (4.52) | −8.21 (4.96) | −7.59 (5.57) | −7.01 (5.45) |
| Reduction in absolute weight, kg | −49.36 (26.16) | −52.30 (28.02) | −50.46 (30.52) | −46.74 (33.80) | −43.13 (32.49) |
| % With total weight reduction | 17.86 (8.70) | 19.24 (9.85) | 18.58 (10.93) | 17.02 (12.11) | 16.0 4 (11.92) |
| Reduction in HbA1C | −.40 (.69) | −.41 (.75) | −.27 (.67) | −.43 (.57) | −.22 (.82) |
| Increase in HDL, mg/dL | 7.6 (9.12) | 11.4 (9.46) | 10.2 (10.02) | 9.4 (11.44) | 9.3 (11.51) |
| Reduction in LDL, mg/dL | −3.8 (27.31) | −6.7 (27.68) | −8.2 (29.14) | −12.2 (31.17) | −7.3 (31.90) |
| Reduction in triglycerides, mg/dL | −42.0 (85.54) | −43.4 (89.63) | −41.6 (83.71) | −25.1 (122.66) | −28.2 (110.15) |
SD = standard deviation; EWL = excess weight loss; BMI = body mass index; HbA1C = glycated hemoglobin; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Of the 303 enrolled patients, at the beginning of the study 16.2% (49 of 303) met the definition of having diabetes, with an HbA1C level ≥6.5 percent or a medical history diagnosis of T2D, and over the duration of the study the proportion with diabetes decreased to 8.6%. The mean HbA1C level of this diabetic subgroup was 7.8 percent (range, 6.4–11.9 percent) at baseline, decreasing to 7.2 percent (range, 5.4–11.5 percent) at the last evaluable visit. Of these 49 patients, 46.9% (23 of 49) experienced a drop in HbA1C to <6.5 percent, from an average of 7.5 percent to 5.85 percent. Another 14 patients (28.6%) showed improvement but remained at or above the 6.5 percent threshold at the last evaluable study visit. The remaining 24.5% (12 of 49) of patients showed an increase in the HbA1C level over the study period, with a mean baseline value of 7.4 percent increasing to 9.1 percent. The follow-up compliance for this group of patients was good. The mean follow-up duration, measured from the implant date to the final study visit, among the 49 patients with diabetes was 4.8 years, and ranged from 1.98 to 5.23 years. In total, 45 (91.8%) patients with diabetes had at least 4 years of follow-up, 3 (6.1%) had 2 to 4 years of follow-up, and 1 (2.0%) had <2 years of follow-up.
Quality of life scores
Patients observed a general improvement in their quality of life, as measured by the SF-36 and IWQOL-Lite questionnaires, at every postsurgery visit. A higher number indicates a higher quality of life for both questionnaires. The SF-36 scores improved from baseline to 5 years in both dimensional components. The physical component summary increased significantly, from 41.8 ± 9.7 to 50.1 ± 9.8 (P < .0001). The mental component summary increased, but not enough to reach statistical significance (from 50.6 ± 10.0 to 52.0 ± 9.5; P < .1507). Results of the IWQOL-Lite questionnaires were also significant, with the mean transformed score increasing from 46.6 ± 20.0 at baseline to 79.1 ± 19.3 at 5 years (P < .0001).
The secondary effectiveness results seen in this study demonstrate that there is a reasonable assurance that the use of the device for its intended use and conditions of use will provide clinically significant effectiveness results in a significant portion of the target population.
Discussion
The need for additional surgical intervention is of high concern when considering the long-term utility of an adjustable gastric band for weight loss. The primary goal of this study was to determine the reoperation rate (band revisions, band replacements, and explantations resulting from serious adverse events) of gastric banding at years 4 and 5 after implantation. The safety success criterion for this study was an observed device-related reoperation rate that was 8% or less for years 4 and 5 of the study. This postmarket safety study had a reoperation rate of 8.9%, with a 95% CI range of 6.0% to 12.7%. Even though this study failed to meet its safety success criterion, the safety performance of the REALIZE gastric band was acceptable and consistent with the reoperation rates found in literature. Gastric band complications, such as removals and revisions, have been researched and are well documented. The overall 14.2% (43 of 303) reoperation rate at 5 years in this investigation aligns with the reoperation rate of 13.5% at 5 years in a similar population studied by Biertho et al. [8]. Furbetta et al. [9] followed 1840 laparoscopic adjustable gastric band (LAGB) patients over a 5-year period, finding a reoperation rate of 5.9% through 3 years and a cumulative rate of 10% at 5 years. In a large study, Ibrahim et al. [10] reported that 18.5% of patients required a reoperation due to band complications at 4.5 years.
No new safety concerns were identified in this study; as such, all safety events were consistent with previously published data for at least 5 years of follow-up.
Secondary endpoints included an evaluation of the REALIZE band’s effectiveness. The REALIZE gastric band was effective in reducing excess weight loss in morbidly obese patients up to 5 years after implantation of the device, based on a percentage of excess weight loss (%EWL) of 35.6 ± 26.84% at 5 years post surgery. In this multicenter study, the mean %EWL across all sites ranged from 22.58 ± 14.54 to 52.44 ± 27.20. Table 6 shows the number of patients per site who completed the 5 year follow-up, and the corresponding mean %EWL.
Table 6.
Percentage of excess weight lost per site for completers at 60 months
| Site no. | No. ITT patients | No. patients completed 60 mo | % LTF | Mean %EWL at 60 mo | SD |
|---|---|---|---|---|---|
| 1 | 22 | 12 | 18.2 | 22.585 | 14.539 |
| 2 | 76 | 52 | 26.3 | 39.424 | 25.642 |
| 3 | 14 | 8 | 50 | 42.749 | 26.323 |
| 4 | 81 | 59 | 25.9 | 23.303 | 25.666 |
| 5 | 20 | 18 | 10 | 52.442 | 27.197 |
| 6 | 13 | 13 | 0 | 39.84 | 22.526 |
| 7 | 19 | 18 | 5.3 | 39.083 | 19.656 |
| 8 | 29 | 28 | 3.4 | 43.753 | 31.897 |
| 9 | 29 | 23 | 24.1 | 38.684 | 27.513 |
| Total | 303 | 231 |
ITT = intent-to-treat population; EWL = excess weight loss; SD = standard deviation; LTF = lost to follow up.
No discernible trends were apparent regarding the mean %EWL at 60 months and the percentage of patients completing the study. In the original PMA study, the mean %EWL at 3 years postsurgery was 41.2% [7], which is similar to the percentage seen in this postmarket extension study (i.e., 41.2%). This and other similarities are an indication of the validity of merging the populations reported herein. Table 4 lists the complete weight loss and lab value results. In the present study, the mean reduction in absolute weight at 5 years post surgery was −43.1 ± 32.5 pounds, while the mean percentage in weight reduction at 5 years post surgery was 16.0 ± 11.9%. This translated to a mean reduction in BMI of −7.0 ± 5.45 kg/m2 at 5 years post surgery.
In comparison to similar studies, the 5-year postsurgery mean %EWL of 35.6 with a BMI change of −7.0 kg/m2 suggests that the benefits of LAGB are durable as well as repeatable. Similarly, Vitiello et al. [11], noted a mean %EWL of 40.5% and a BMI change of −9.36 kg/m2 at 5 years. O’Brien et al. [12], in a large, long-term LAGB population study (n = 5235), showed a mean %EWL of 47.7% and a reduction in BMI of 8.5 points at 5 years.
Improvements in various metabolic factors were maintained at 5 years post surgery. Mean reductions in HbA1C; reductions in low-density lipoprotein, total cholesterol, and triglycerides; and a mean increase in high-density lipoprotein were all maintained at 5 years post surgery, with similar results as those described in the 3-year study. In the 49 patients meeting the definition of being diabetic due to an HbA1C level of ≥6.5 percent or a medical history diagnosis of T2D, almost half (46.9%) reduced their HbA1C to nondiabetic levels during the study.
In addition to an acceptable reoperation rate, an acceptable %EWL, and improvements in metabolic factors, these changes were accompanied by lasting improvements in the quality of life at 5 years postsurgery, based on SF-36 Physical Component and domain scores and the IWQOL-Lite total transformed score and domain scores.
There were several limitations in this investigation. There was no comparison group, but the subject functioned as his/her own control, with results pre- and posttreatment. The results of this study may not be generalizable to clinical practice, as the study was done in specialized centers and may not represent all sites of care. For various reasons, with the most common being loss to follow-up, 24% (72 of 303) of our patient group did not complete the entire study.
Conclusions
This postmarket safety study had a reoperation rate of 8.9%, which did not meet the arbitrary, predetermined safety success criterion of 8%. Even so, the general safety profile, reoperation rate, and effectiveness endpoints that were observed in this study are consistent with those reported in current literature. No unexpected findings were observed.
Acknowledgments
The authors wish to acknowledge professional medical writing services from Natalie Edwards. Funding for this study was provided by Ethicon Endo-Surgery, Inc., the manufacturer of the REALIZE Adjustable Gastric Band.
Disclosures
The study sponsor, Ethicon Endo-Surgery, Inc., was involved in the study design and the collection, analysis, and interpretation of data. Edward Phillips owns Johnson and Johnson stock. Jaime Ponce and Scott Cunneen are paid consultants for Ethicon. Michael Schwiers, Jason Waggoner, and Janet DeMarchi are Ethicon employees. Sunil Bhoyrul, Moises Jacobs, Eddie Gomez, Mark Kipnes, and Robert Marema have no conflicts of interest.
Footnotes
Funding for this study was provided by Ethicon Endo-Surgery, Inc., the manufacturer of the REALIZE Adjustable Gastric Band. The study sponsor was involved in the study design and the collection, analysis, and interpretation of data.
References
- 1.World Health Organization. Fact sheet on Obesity and Overweight. 2020. Here is the link: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 2.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 3.Simonnet A., Chetboun M., Poissy J., LICORN and the Lille Coronavirus Disease 2019 (COVID-19) and Obesity study group High prevalence of obesity in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuneen S.A. Review of meta-analytic comparisons of bariatric surgery with a focus on laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2008;4(3 Suppl):S47–S55. doi: 10.1016/j.soard.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Korenkov M., Shah S., Sauerland S., Duenschede F., Junginger T. Impact of laparoscopic adjustable gastric banding on obesity co-morbidities in the medium- and long-term. Obes Surg. 2007;17:679–683. doi: 10.1007/s11695-007-9118-y. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien P.E., McPhail T., Chaston T.B., Dixon J.B. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16:1032–1040. doi: 10.1381/096089206778026316. [DOI] [PubMed] [Google Scholar]
- 7.Phillips E., Ponce J., Cunneen S.A. Safety and effectiveness of REALIZE adjustable gastric band: 3 year prospective study in the United States. Surg Obes Relat Dis. 2009;5(5):588–597. doi: 10.1016/j.soard.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Biertho L., Steffen R., Branson R. Management of failed adjustable gastric banding. Surgery. 2005;137(1):33–41. doi: 10.1016/j.surg.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Furbetta N., Gragnani F., Flauti G., Guidi F., Furbetta F. Laparoscopic adjustable gastric banding on 3566 patients up to 20-year follow-up: long-term results of a standardized technique. Surg Obes Relat Dis. 2019;15(3):409–416. doi: 10.1016/j.soard.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim A.M., Thumma J.R., Dimick J.B. Reoperation and Medicare expenditures after laparoscopic gastric band surgery. JAMA Surg. 2017;152(9):835–842. doi: 10.1001/jamasurg.2017.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitiello A., Pilone V., Ferraro L., Forestieri P. Is the sleeve gastrectomy always a better procedure? Five-year results from a retrospective matched case-control study. Obes Surg. 2018;28(8):2333–2338. doi: 10.1007/s11695-018-3161-8. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien P.E., Hindle A., Brennan L. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 2019;29(1):3–14. doi: 10.1007/s11695-018-3525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]