Abstract
Background
There is a paucity of information on coronavirus disease 2019 (COVID-19) outcomes in asthmatics.
Objective
To identify risk factors associated with admission and subsequent mortality among COVID-19–infected asthmatics.
Methods
Adults at our institution with a positive polymerase chain reaction for COVID-19 between March 14 and April 27, 2020, were retrospectively identified. Comorbidities, laboratory results, and mortality rates during hospitalization were recorded.
Results
In total, 737 of 951 (77.5%) asthma patients with COVID-19 were seen in the emergency department (ED), and 78.8% of these ED patients (581 of 737) were admitted. Individuals with previously measured mean absolute eosinophil counts (AEC) ≥150 cells/μL were less likely to be admitted (odds ratio [OR] = 0.46, 95% confidence interval [CI]: 0.21-0.98, P = .04), whereas concomitant heart failure (CHF), chronic kidney disease (CKD), and chronic obstructive pulmonary disease (COPD) were risk factors for admission. Hospitalized patients with asthma with peak hospital-measured AEC ≥150 cells/μL (n = 104) were less likely to die compared with those whose AEC remained <150 cells/μL (n = 213) (mortality rate 9.6% vs 25.8%; OR = 0.006, 95% CI: 0.0001-0.64, P = .03). This group had also higher preadmission mean AEC (237 ± 181 vs 163 ± 147 cells/μL, P = .001, OR = 2012, 95% CI: 27.3-14,816). The mortality rate in patients with asthma alone (no associated CHF, CKD, COPD, diabetes, or hypertension) was similar to that of patients without asthma or any of these comorbidities.
Conclusions
In asthmatics, pre-existing eosinophilia (AEC ≥150 cells/μL) was protective from COVID-19–associated admission, and development of eosinophilia (AEC ≥150 cells/μL) during hospitalization was associated with decreased mortality. Preadmission AEC influenced the AEC trend during hospitalization. Having a Th2-asthma phenotype might be an important predictor for reduced COVID-19 morbidity and mortality that should be further explored in prospective and mechanistic studies.
Key words: COVID-19, Asthma, Eosinophilia, Mortality
Abbreviations used: ACE2, Angiotensin-converting enzyme 2; AEC, Absolute eosinophil count; CHF, Congestive heart failure; CI, Confidence interval; CKD, Chronic kidney disease; CLG, Clinical Looking Glass; COPD, Chronic obstructive pulmonary disease; COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; DM, Diabetes; ED, Emergency department; FEV1, Forced expiratory volume in 1 second; HTN, Hypertension; ICD, International Classification of Diseases; ICS, Inhaled corticosteroid; OR, Odds ratio; SARS-CoV-2, Severe acute respiratory syndrome coronavirus-2; TMPRSS2, Transmembrane protease serine 2
What is already known about this topic? Risk factors for COVID-19 severe outcomes in asthmatics are not known. Although asthma appears to be under-represented in the COVID-19 comorbidities, diabetes (DM), hypertension (HTN), congestive heart failure (CHF), and chronic kidney disease (CKD) have been associated with severe disease.
What does this article add to our knowledge? Eosinophilia was protective from admission and mortality in COVID-19 asthma patients. Overall, having an asthma diagnosis without associated CHF, CKD, DM, HTN, and chronic obstructive pulmonary disease did not increase the mortality risk from COVID-19.
How does this study impact current management guidelines? Having a Th2-asthma phenotype may be an important predictive factor for reduced COVID-19 morbidity and mortality, emphasizing the need of prospective and mechanistic studies to explore the exact role of eosinophils in COVID-19 mortality.
A viral pneumonia outbreak due to a novel coronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), now called coronavirus disease 2019 (COVID-19) infection, was recognized in Wuhan, China, at the end of 2019.1 Since then, more than half million deaths have been confirmed worldwide,2 with fatalities increasing daily. The presence of comorbidities such as diabetes (DM), cardiovascular disease, or hypertension (HTN) is associated with more severe complications and a higher case fatality rate in COVID-19.1 , 3 , 4 Although viral infections are known to trigger half of all asthma exacerbations and increase asthma morbidity and mortality,5 asthma appears to be under-represented in the comorbidities reported for patients with COVID-19.3 Similarly, asthma is absent among the top 10 COVID-19–associated comorbidities in New York state fatalities.6 The interaction between asthma and COVID-19 outcomes is not understood, and the prevalence of asthma among patients with COVID-19 infection varies largely based on the studied population, from 0.9% in Wuhan, China,7 to 1.92% in an Italian cohort,8 to 9% among admitted patients from New York with SARS-CoV-2 infection,9 and up to 17% of hospitalized patients from the COVID-19–Associated Hospitalization Surveillance Network.10 Among a case series of 24 critically ill patients with COVID-19 who were admitted to 9 hospital intensive care units during the first 3 weeks of the COVID-19 outbreak in the Seattle area, 3 patients (14%) had asthma.11 Similarly, a meta-analysis of COVID-19 hospitalizations in patients with asthma suggests that asthma prevalence among those hospitalized with COVID-19 appears to be similar to asthma prevalence in the general population.12
Asthma is 1 of the 5 conditions that is associated with a significantly lower life expectancy in the United States.13 Given the association between respiratory viral illnesses and asthma,5 it is important to carefully monitor patients with asthma in the COVID-19 epidemic. Recent data show that differences in angiotensin-converting enzyme 2 (ACE2), transmembrane protease serine 2 (TMPRSS2), and furin epithelial and airway gene expression (cofactors for SARS-CoV-2 infectivity) were unlikely to confer increased COVID-19 pneumonia risk in patients with asthma across all treatment intensities and severity.14 Moreover, expression of ACE2 and TMPRSS2 in sputum were decreased by the use of inhaled corticosteroids (ICS),15 suggesting a possible reason why patients with asthma do not seem to experience some of the more severe and life-threatening manifestations of the COVID-19 disease. Although asthma was not an independent risk factor for intubation among hospitalized patients with COVID-19 in a Colorado cohort,12 another study showed that self-reported asthma diagnosis in a Chicago, USA cohort was independently associated with prolonged duration of intubation for COVID-19,16 and recent use of oral corticosteroids was associated with higher risk of COVID-19–related death in a large England cohort.17 Therefore, there is a need to characterize COVID-19 outcomes in patients with asthma in different populations, to better understand the relationship between asthma and COVID-19.
Our tertiary health care facility is located in the Bronx county of New York City, USA, one of the COVID-19 epicenters in April 2020.18 The Bronx has the heaviest asthma burden of all New York State counties, and one of the highest in the United States.19 Although the COVID-19 literature provides frequent updates about this condition, a detailed study describing factors associated with admission and mortality in asthma patients with COVID-19 in an ethnically diverse population is lacking. Anecdotal observations during the surge of infections in our population suggested that allergic patients may have had milder COVID-19 disease and conversely allergic symptoms might have been milder during COVID-19 infection. We therefore hypothesized that patients with asthma with a Th2 phenotype characterized by elevated peripheral blood eosinophils would have better outcomes compared with patients who had a non-Th2 phenotype. In the present study, we sought to analyze the relationship between asthma and COVID-19 by identifying the factors predisposing to inpatient admission in our asthmatic population, and by comparing the mortality risk among admitted patients with only asthma and those with other coexistent chronic conditions such as DM, HTN, congestive heart failure (CHF), chronic kidney disease (CKD), which have been shown to be unique risk factors for severe complications of COVID-19.4 , 20, 21, 22
Methods
Data source
This is a retrospective study approved by the institutional review board of the Albert Einstein College of Medicine/Montefiore Medical Center (Bronx, NY). All adult patients (≥18 years old) who tested positive for SARS-CoV-2 infection by polymerase chain reaction at our institution between March 14 and April 27, 2020, were identified using Clinical Looking Glass (CLG; Looking Glass Clinical Analytics [Streamline Health, Atlanta, Ga]), a software application that stores electronic health record data from our medical system.23 All those patients who presented to the emergency department (ED) for COVID-19 symptoms and who had also been seen at least once in our health care system within the previous 10 years were included in the analysis. Of note, during that time period, all COVID-19 testing was limited to a few sites, mostly EDs. Therefore, the pool patients included symptomatic individuals of all severities. The primary outcome of this study was to identify the risk factors associated with admission from the ED in patients with asthma. The secondary outcome was to compare the mortality risk in admitted patients with asthma alone versus those with other associated comorbidities.
Patient selection
Using CLG, we identified patients with prior International Classification of Diseases (ICD)-9/-10 diagnoses of asthma (codes are listed in this article's Online Repository at www.jaci-inpractice.org) who presented to the ED with symptoms suggestive of COVID-19 and tested positive for SARS-CoV-2. Patients who were admitted were identified. Length of stay was calculated from the time they arrived in the ED to the time of discharge or death. We collected data on demographic variables and prior diagnoses of CHF, CKD, chronic obstructive pulmonary disease (COPD), DM, HTN, allergic rhinitis, rhinosinusitis, food allergy, eczema, urticaria, and nasal polyposis (ICD-9/-10 codes used are listed in this article's Online Repository at www.jaci-inpractice.org). Asthma-related parameters included the last forced expiratory volume in 1 second (FEV1) before the COVID-19–positive test; recorded prescriptions for ICS, oral corticosteroids, montelukast, and antihistamines within the last year; receiving subcutaneous immunotherapy and receiving biological agents for asthma (omalizumab, dupilumab, mepolizumab, benralizumab, reslizumab) within the last 3 years. Laboratory results before the infection (mean of absolute eosinophil count [AEC], IgE, IgA, IgG, IgM, vitamin D levels) were also collected. Consistent with prior studies in asthmatics, AEC ≥ 150 cells/μL was used to define eosinophilic (Th2-high) asthma.24 , 25 Admitted patients with asthma without a prior AEC were included in the study to follow up the hospitalization course.
Statistical analysis
The data were analyzed using SPSS statistical software, version 26.0 (IBM Corp, Armonk, NY) and “R” Statistical programming language (Vienna, Austria). Descriptive statistics were applied to all variables. Any missing laboratory results or demographic data were interpreted as “missed data,” and this was reflected in the proportion, mean, and median calculations. A P value <.05 was used to determine statistical significance.
To identify the risk factors that were associated with a likelihood of admission from the ED (our first outcome), we performed binary logistic regression. The covariates included were age, race, gender, and smoking status.
For our second outcome, we performed an exploratory analysis using binary logistic regression to calculate the respective unadjusted odds ratios (ORs) and 95% confidence intervals (CIs) associated with the mortality risk in patients with asthma compared with admitted patients with other diagnoses (eg, CHF, CKD, COPD, DM, HTN). The variables included in this logistic regression model were age, race, gender, smoking status, IL-6, ferritin, D-dimer, and C-reactive protein (CRP) levels. For this specific analysis, Bonferroni correction for multiple comparisons was applied and adjusted and unadjusted P values are presented. We also used binary logistic regression to calculate the respective ORs and 95% CIs associated with the mortality risk in admitted patients with asthma based on the peak AEC during hospitalization. We chose to use the peak AEC rather than the average AEC during the hospitalization, because we observed that most patients had eosinopenia throughout the hospitalization, except in the days leading up to discharge. Therefore, the mean calculated across the duration of hospitalization would not have accurately reflected the AEC during the hospitalization. We partitioned patients based on whether their AEC increased above 150 cells/μL or stayed below this cutoff for the duration of the hospitalization. The administration of systemic corticosteroids is known to result in eosinopenia.26 Therefore, patients with asthma who received systemic corticosteroids during hospitalization (n = 242) were excluded for the purpose of this specific analysis. The variables included in this model were age, race, gender, smoking status, IL-6, ferritin, D-dimer, CRP levels, and associated comorbidities (COPD, HTN, DM, CHF, CKD).
A Kaplan-Meier curve was constructed comparing the survival probabilities in admitted patients with asthma with maximum hospital-measured AEC ≥150 cells/μL and those in whom AEC never increased above 150 cells/μL. Observations were right censored. A log-rank test was performed to compare the curves.
Results
Characteristics of patients included in the study
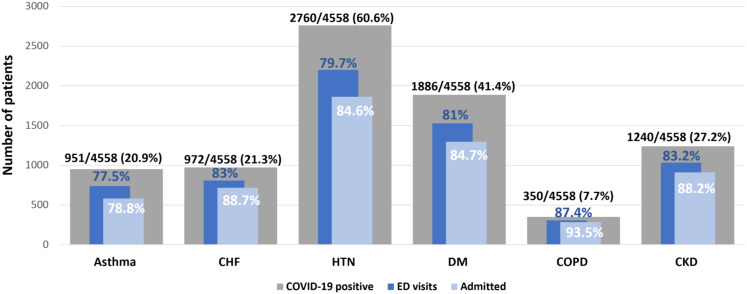
During the study period, 6445 patients tested positive for SARS-CoV-2. Of these, 4558 patients had been seen previously in our health care system at least once during the last 10 years before the COVID-19 infection (mean time between COVID-19 testing and first outpatient visit was 6.4 ± 3.1 years, with a median of 26 visits/person [interquartile range: 7-73]). In total, 951 of 4558 (20.9%) of COVID-19–infected patients had a diagnosis of asthma (Figure 1 ). Among patients with asthma, 77.5% (737 of 951) had been seen in the ED and 78.8% (581 of 737) of the ED-evaluated asthmatics were admitted. Among all COVID-19–positive patients (with and without asthma), HTN (60.6%) and DM (41.4%) were the most frequent associated comorbidities.
Figure 1.
Comorbidities, ED visits, and admission rates in COVID-19-positive patients included in the study (N = 4601). The gray bars represent associated comorbidities in patients with COVID-19, the dark blue bars represent patients with COVID-19 who presented to the ED, and the light blue bars represent patients with COVID-19 who were admitted. Different comorbidities are on the x-axis and number of patients on the y-axis. CHF, Congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; DM, diabetes; ED, emergency department; HTN, hypertension.
The characteristics of COVID-19–infected patients with asthma (n = 951) are presented in Table I . The majority (56%) were under the age of 65, female (68.2%), and of black (37.6%) or Hispanic (41.5%) origin. Patients who were admitted had a significantly lower oxygen saturation (SpO2%) (92.4 ± 7), compared with those who were discharged from the ED (96 ± 10, OR = 0.82, 95% CI: 0.76-0.89, P < .001). An elevated, pre-COVID-19 mean AEC ≥ 150 cells/μL was reported in 53.1% of patients with asthma. Common nonatopic comorbidities in the asthmatics included CHF (31%), CKD (36.5%), COPD (19%), DM (51.6%), and HTN (72.3%). A small proportion of asthma patients with COVID-19 were receiving subcutaneous aeroallergen immunotherapy (1.5%) or one of the new biological therapies for asthma (0.8%). Based on an analysis of the recorded ICD-9 and/or ICD-10 diagnosis codes, the majority (76.1%) of patients were categorized as “Unspecified” asthma.
Table I.
Characteristics of all asthmatics with a positive COVID-19 test
| Characteristics | Total patients with asthma with positive test for COVID-19 (N = 951) | ED presentation for COVID-19 infection (N = 737) |
P value¶ | OR¶ | 95% CI¶ | |
|---|---|---|---|---|---|---|
| Admitted (N = 581) | Not admitted (N = 156) | |||||
| Age at the time of COVID-19 positive (y), mean (±SD) | 60.5 (±17.07) | 64.91 (±15.4) | 54 (±16.7) | <.001 | 1.04 | 1.02-1.05 |
| Age groups (%) | ||||||
| 18-45 y | 17.5 | 9.8 | 26.3 | <.001 | 0.2 | 0.12-0.34 |
| 46-64 y | 38.5 | 36.8 | 42.9 | .001 | 0.5 | 0.33-0.75 |
| >65 y | 44 | 53.4 | 30.9 | |||
| Male (%) | 31.8 | 35.5 | 25.6 | .01 | 0.58 | 0.38-0.88 |
| Race (%) | ||||||
| Black | 37.6 | 37.5 | 37.8 | .62 | 0.8 | 0.33-1.93 |
| White | 6.5 | 6.9 | 4.5 | |||
| Hispanic | 41.5 | 41.3 | 44.2 | .58 | 0.78 | 0.32-1.88 |
| Asian | 2.1 | 2.4 | 3.2 | .49 | 0.62 | 0.15-2.43 |
| Other/unknown | 12.2 | 11.9 | 10.3 | .98 | 1.01 | 0.35-2.84 |
| Smoking status (%)∗ | ||||||
| Current smoker | 8.3 | 7.9 | 9.6 | .56 | 0.82 | 0.42-1.58 |
| Former smoker | 23.9 | 25 | 20 | .77 | 1.07 | 0.66-1.75 |
| Never smoker | 46.4 | 45.6 | 49.4 | |||
| Minimum SpO2 (%) in the first 24 h, mean (±SD) | 92.9 (±7) | 92.4 (±7) | 96 (±10) | <.001 | 0.82 | 0.76-0.89 |
| BMI (kg/m2), mean (±SD)∗ | 31.7 (±8.3) | 31.78 (±9) | 32.35 (±7.6) | .14 | 1.01 | 0.99-1.04 |
| Having BMI ≥30 kg/m2 (%) | 53.8 | 52.8 | 57.5 | .37 | 1.19 | 0.8-1.75 |
| Asthma severity (%)† | ||||||
| Mild intermittent | 14.6 | 12.9 | 17.9 | .76 | 0.92 | 0.55-1.53 |
| Mild persistent | 3.2 | 2.6 | 3.8 | .62 | 0.77 | 0.28-2.13 |
| Moderate persistent | 4.9 | 4.3 | 5.1 | .99 | 1.0 | 0.42-2.37 |
| Severe | 1.2 | 0.9 | 1.9 | .48 | 0.58 | 0.13-2.59 |
| Unspecified | 76.1 | 79.3 | 71.2 | |||
| FEV1 (%), mean (±SD)∗ | 79.1 (±60.3) | 79.6 (±68.5) | 77.4 (±22.7) | .89 | 0.9 | 0.9-1.009 |
| Prior AEC (cells/μL), mean (±SD)∗,‡ | 187 (±152) | 174 (±149) | 220 (±200) | .009 | 0.23 | 0.08-0.7 |
| Prior mean AEC ≥500 cells/μL (%) | 4.7 | 3.8 | 8.3 | .04 | 0.68 | 0.47-0.9 |
| Prior mean AEC ≥300 cells/μL (%) | 14.2 | 12.6 | 19.5 | .02 | 0.5 | 0.33-0.91 |
| Prior mean AEC ≥150 cells/μL (%) | 53.1 | 52.1 | 58.1 | .04 | 0.46 | 0.21-0.98 |
| Immunoglobulins level (kU/L), mean (±SD)∗,‡ | ||||||
| IgE | 520.3 (±1472) | 442 (±907) | 472.3 (±1023) | .81 | 1 | 0.99-1.001 |
| IgA | 297.4 (±220.7) | 309.3 (±210) | 290.3 (±299.2) | .84 | 1 | 0.99-1.002 |
| IgG | 1302 (±709) | 1322 (±741.2) | 1308.4 (±468.9) | .81 | 1 | 0.99-1.001 |
| IgM | 132.8 (±189.9) | 162.7 (±235) | 85.2 (±47.6) | .1 | 1.007 | 0.99-1.014 |
| Prior IgE >100 kU/L (%) | 47.8 | 42.9 | 56 | .68 | 1.26 | 0.41-3.91 |
| Vitamin D level (ng/mL), mean (±SD)∗,‡ | 26.9 (±14.5) | 27.9 (±15.6) | 24.6 (±12.1) | .55 | 1.006 | 0.98-1.02 |
| Vitamin D level <20 ng/mL (%) | 40 | 31.5 | 39.8 | .79 | 0.93 | 0.54-1.59 |
| Associated allergic comorbidities (%) | ||||||
| Allergic rhinitis | 23.7 | 21 | 29.5 | .10 | 0.71 | 0.47-1.07 |
| Eczema | 8 | 8.4 | 7.1 | .42 | 1.33 | 0.65-2.70 |
| Urticaria | 4.3 | 3.8 | 3.9 | .58 | 1.3 | 0.5-3.35 |
| Food allergy | 8.4 | 7.6 | 10.3 | .22 | 0.67 | 0.35-1.27 |
| Chronic sinusitis | 10.9 | 9.6 | 14.7 | .25 | 0.73 | 0.42-1.25 |
| Nasal polyps | 1.2 | 1 | 1.3 | .79 | 0.79 | 0.15-4.18 |
| Other comorbidities (%) | ||||||
| CHF | 31 | 36.8 | 19.9 | .04 | 1.61 | 1.01-2.56 |
| CKD | 36.5 | 43.5 | 24.4 | .03 | 1.61 | 1.04-2.51 |
| COPD | 19 | 23.9 | 10.3 | .017 | 2.06 | 1.14-3.74 |
| DM | 51.6 | 56.5 | 48.7 | .9 | 1 | 0.68-1.46 |
| HTN | 72.3 | 79.2 | 65.4 | .59 | 1.13 | 0.72-1.76 |
| Metabolic syndrome (BMI ≥30 kg/m2 and HTN and DM) | 21.2 | 27.7 | 26.3 | .84 | 1.04 | 0.68-1.57 |
| Prescription for ICS within the year prior, n (%) | 175 (18.4) | 114 (19.6) | 21 (13.5) | .11 | 1.51 | 0.9-2.56 |
| Strength of ICS, n (%)§ | ||||||
| Low dose | 15 (8.6) | 8 (7) | 1 (4.8) | .66 | 1.64 | 0.17-15.06 |
| Medium dose | 63 (36) | 43 (37.7) | 6 (28.6) | .44 | 1.53 | 0.51-4.53 |
| High dose | 97 (55.4) | 63 (55.3) | 14 (66.7) | .33 | 0.59 | 0.21-1.69 |
| Type of ICS, n (%) | ||||||
| Beclomethasone | 12 (6.9) | 7 (6.1) | 3 (14.3) | .33 | 0.47 | 0.1-2.16 |
| Budesonide | 52 (29.7) | 32 (28.1) | 6 (28.6) | .7 | 1.24 | 0.40-3.85 |
| Ciclesonide | 1 (0.5) | 0 | 0 | |||
| Fluticasone | 93 (53.1) | 64 (56.1) | 9 (42.9) | .53 | 1.36 | 0.5-3.71 |
| Mometasone | 17 (9.7) | 11 (9.6) | 3 (14.3) | .52 | 0.62 | 0.14-2.68 |
| Prescription for oral corticosteroids within the year prior, n (%) | 226 (23.8) | 139 (23.9) | 38 (24.4) | .84 | 1.04 | 0.68-1.6 |
| Prescription for montelukast within the year prior, n (%) | 104 (11) | 66 (11.4) | 14 (9) | .33 | 1.36 | 0.72-2.54 |
| Prescription for antihistamines within the year prior, n (%) | 133 (14) | 85 (14.7) | 29 (18.7) | .61 | 0.88 | 0.54-1.43 |
| On SCIT within the prior 3 y, n (%) | 17 (1.8) | 8 (1.4) | 3 (1.9) | .75 | 0.8 | 0.2-3.22 |
| On biologics within the prior 3 y, n (%)‖ | 8 (0.8) | 6 (1) | 1 (0.6) | .43 | 2.36 | 0.273-20.4 |
Bold values show statistical significance at P < 0.05.
AEC, Absolute eosinophil count; BMI, body mass index; CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; DM, diabetes mellitus; ED, emergency department; FEV1, forced expiratory volume in 1 second; HTN, hypertension; ICS, inhaled corticosteroids; OR, odds ratio; SCIT, subcutaneous immunotherapy; SD, standard deviation; SpO2, oxygen saturation.
Smoking status available in 456 admitted patients and in 123 nonadmitted patients; pulse oximetry was available in 580 admitted patients and 98 nonadmitted patients; BMI available in all patients; FEV1 available in 96 admitted patients and 33 nonadmitted patients; prior AEC available in 579 admitted patients and in 156 nonadmitted patients; prior IgE in 42 admitted patients and 25 nonadmitted patients; IgA available in 95 admitted patients and in 30 nonadmitted patients; IgG in 70 admitted patients and 21 nonadmitted patients; IgM in 78 admitted patients and 23 nonadmitted patients; and prior vitamin D level available in 333 admitted patients and 83 nonadmitted patients.
ICD-9/10 diagnoses, as gathered through CLG (Clinical Looking Glass).
Mean of prior laboratory results during the past 10 years before the ED visit for COVID-19.
The strength of the daily dose inhaler (50) is based on the last prescription found in the chart within the last year before COVID-19 infection.
Omalizumab, mepolizumab, benralizumab, reslizumab, dupilumab.
Model adjusted for age, race, gender, and smoking status.
Pre-existing eosinophilia protects from admission in asthma patients with COVID-19
Table I shows the characteristics of patients with asthma who presented with COVID-19 infection symptoms and who were admitted from the ED (n = 581), compared with those individuals with asthma who were discharged from the ED (n = 156). Patients who were admitted were older (64.9 ±15.4 years vs 54 ±16.7 years, P < .05), and a higher proportion were men (35.6% vs 25.6%, P = .01). Patients with prior eosinophilia (mean AEC ≥ 150 cells/μL) were significantly less likely to be admitted from the ED (OR = 0.46; 95% CI: 0.21-0.98, P = .04), whereas asthma patients with comorbid CHF, CKD, and COPD were more likely to be hospitalized. No other significant asthma-related factors such as prior FEV1% (OR = 0.9, 95% CI: 0.9-1.009); prescription of oral corticosteroids within the year before COVID-19 (OR = 1.04, 95% CI: 0.68-1.6); asthma severity as gathered from the ICD-9/ICD-10 diagnoses (mild intermittent OR = 0.92, 95% CI: 0.55-1.53; mild persistent OR = 0.77, 95% CI: 0.28-2.13; moderate persistent OR = 1, 95% CI: 0.42-2.37; or severe OR = 0.58, 95% CI: 0.13-2.59); and proportion of patients receiving prescriptions for ICS (OR = 1.51; 95% CI: 0.9-2.56), or being prescribed monoclonal biologics for asthma (OR = 2.36, 95% CI: 0.273-20.4) or allergic immunotherapy (OR = 0.8, 95% CI: 0.2-3.22) were found to influence the odds of being admitted from the ED.
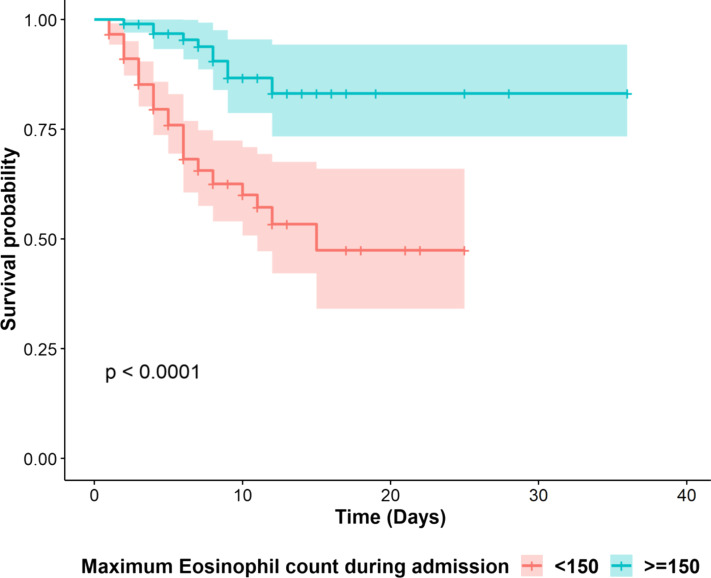
Increasing AEC ≥150 cells/μL in hospitalized patients with asthma protects from COVID-19 mortality
Overall, 85% of admitted patients with asthma had eosinopenia (AEC of 0 cells/μL) at the time of admission (data not shown). Those patients in whom AEC increased to a peak above 150 cells/μL (n = 104) were significantly less likely to die compared with admitted asthma individuals whose AEC remained below 150 cells/μL during the admission (n = 213) (mortality rate of 9.6% vs 25.8%, respectively; OR = 0.006, 95% CI: 0.0001-0.64, P = .03). The survival probability of these 2 groups was further compared by a Kaplan-Meier survival analysis (Figure 2 ), demonstrating a significant difference in survival between individuals whose AEC increased above 150 cells/μL compared with those whose AEC never peaked above 150 cells/μL (P < .0001) during hospitalization. In contrast, there was no relationship between lymphocyte count (OR = 0.9, 95% CI: 0.99-1.003, P = .66) or platelet count (OR = 0.74, 95% CI: 0.99-1.004, P = .74) and mortality risk in admitted patients. Similarly, there was no association between ICS dose and mortality in admitted patients with asthma in whom AEC increased above 150 cells/μL compared with those whose AEC never peaked above 150 cells/μL (medium dose: OR = 7.5, 95% CI: 0.21-263, P = .26; high dose: OR = 0.12, 95% CI: 0.004-4.75, P = .26) (models adjusted for age, race, gender, smoking status, IL-6, ferritin, CRP, D-dimer levels; because of the small number of patients on low-dose ICS, logistic regression could not be performed for this group).
Figure 2.
Kaplan-Meier curve of survival in patients with asthma with highest AEC <150 cells/μL (red) compared with patients with highest AEC ≥150 cells/μL (blue). The x-axis denotes days after admission and the y-axis is the probability of survival. The P value from the log-rank tests and 95% confidence intervals (shaded areas) are depicted. AEC, Absolute eosinophil count.
Table II shows the demographic and hospitalization characteristics of patients with asthma in whom AEC increased above 150 cells/μL during the admission and those in whom AEC never increased above 150 cells/μL. Patients with asthma in whom AEC increased above 150 cells/μL during admission had significantly higher mean pre-COVID-19 AEC (237 ± 181 vs 163 ± 147 cells/μL, OR = 2012, 95% CI: 27.3-14,816, P = .001) with higher proportion of women (63.4% vs 60.6%, OR = 5.44, 95% CI: 1.96-15.1, P = .001). Both groups had elevated inflammatory markers.
Table II.
Characteristics of admitted asthmatics in whom maximum AEC never increased above 150 cells/μL versus those in whom AEC increased above 150 cells/μL during the admission
| Characteristics | Patients in whom AEC never increased above 150 cells/μL (N = 213) | Patients in whom AEC increased ≥150 cells/μL (N = 104) | P value∗ | OR∗ | 95% CI∗ |
|---|---|---|---|---|---|
| Age (y), mean (±SD) | 65.7 (±17.4) | 64.4 (±13.6) | .83 | 0.99 | 0.96-1.02 |
| Female (%) | 63.4 | 60.6 | .001 | 5.44 | 1.96-15.1 |
| Race (%) | |||||
| African American | 38.5 | 32.7 | .1 | 0.47 | 0.19-1.15 |
| Caucasian | 8 | 5.8 | |||
| Hispanic | 40.8 | 43.3 | .74 | 1.15 | 0.48-2.78 |
| Other/unknown | 11.3 | 13.5 | .45 | 1.7 | 0.41-6.9 |
| Smoking status (%) | 2.2 | 0.26-18.5 | |||
| Current smoker | 7 | 10.6 | .46 | ||
| Former smoker | 22.5 | 27.9 | .3 | ||
| Never smoker | 46.5 | 41.3 | |||
| AEC before admission (cells/μL), mean (±SD) | 163 (±147) | 237 (±181) | .001 | 2012 | 27.3-14816 |
| IL-6 (pg/mL), median (IQR) | 39.1 (14.4-66.6) | 30.1 (20.5.6-62.6) | .15 | 0.99 | 0.99-1.001 |
| Ferritin (ng/mL), median (IQR) | 660 (293-1442 | 569 (320-1537) | .3 | 0.99 | 0.99-1.001 |
| CRP (mg/dL), median (IQR) | 7.4 (3.8-14) | 8.9 (4.8-14.4) | .44 | 1.02 | 0.96-1.08 |
| D-dimer (μg/mL), median (IQR) | 1.45 (0.8-3.1) | 2 (0.8-4) | .48 | 1.03 | 0.94-1.13 |
| Comorbidities (%) | |||||
| CHF | 35.7 | 37.5 | .9 | 1.06 | 0.38-2.96 |
| CKD | 39.9 | 45 | .95 | 0.97 | 0.35-2.62 |
| COPD | 18.3 | 19.2 | .7 | 1.23 | 0.41-3.69 |
| DM | 61 | 56.7 | .95 | 0.97 | 0.38-2.42 |
| HTN | 78.9 | 83.7 | .16 | 0.41 | 0.12-1.45 |
Bold values show statistical significance at P < 0.05.
AEC, Absolute eosinophil count; CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; DM, diabetes mellitus; HTN, hypertension; IQR, interquartile range; OR, odds ratio; SD, standard deviation.
Model adjusted for age, race, gender, smoking status, IL-6, ferritin, CRP, D-dimer levels, comorbidities (CHF, CKD, COPD, DM, HTN). IL-6 was checked in 87 patients in whom AEC never increased above 150 cells/μL and in 58 patients in whom AEC increased above 150 cells/μL. Ferritin was checked in 133 patients in whom AEC never increased above 150 cells/μL and in 83 patients in whom AEC increased above 150 cells/μL. CRP was checked in 172 patients in whom AEC never increased above 150 cells/μL and in 97 patients in whom AEC increased above 150 cells/μL. D-dimer was checked in 148 patients in whom AEC never increased above 150 cells/μL and in 92 patients in whom AEC increased above 150 cells/μL. Prior AEC was checked in 213 patients in whom AEC never increased above 150 cells/μL and in 104 patients in whom AEC increased above 150 cells/μL. Reference ranges: IL-6: <5 pg/mL; ferritin: 25-270 ng/mL; CRP <0.8 mg/dL; D-dimer: 0-0.5 μg/mL.
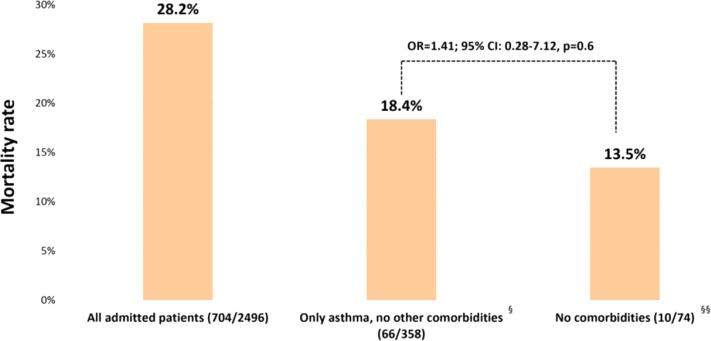
Mortality rate in asthmatics was similar to nonasthmatics with no associated comorbidities
We also assessed the influence on mortality of various suspected comorbidities of COVID-19, previously identified as risk factors for admission in patients with asthma. During the study period, there were 2496 admitted patients with COVID-19 who were previously seen in our health care system (data not shown). The overall mortality rate in this cohort was 28.2% (704 of 2496) (Figure 3 ). The mortality rate in patients with asthma alone (no associated CHF, CKD, COPD, DM, or HTN) (18.4%, 66 of 358) was similar to nonasthmatics who did not have any of these associated comorbidities (13.5%, 10 of 74, OR = 1.41; 95% CI: 0.28-7.12, P = 0.6) (Figure 3).
Figure 3.
Mortality risk in admitted patients with asthma with COVID-19 was not different from those without any comorbidities. Mortality rates and risk in asthmatics with no other comorbidities (no CHF, CKD, COPD, DM, HTN) versus those individuals without any underlying conditions (no asthma, CHF, CKD, COPD, DM, HTN) were compared. CHF, Congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; DM, diabetes; HTN, hypertension; OR, odds ratio.
Patients with asthma had lower mortality rates compared with patients with COPD
Patients with COPD (but no associated asthma) had a higher COVID-19–associated mortality rate (48.3%), when compared with asthma patients with no associated COPD (OR = 1.87; 95% CI: 1.15-3.04, unadjusted P = .01, adjusted P = .06). Patients with codiagnoses of asthma and COPD had a higher mortality rate (41%) compared with asthmatics with no COPD (24.2%, OR = 3.2; 95% CI: 1.32-7.79, unadjusted P = .01, adjusted P = .06). Patients with COPD had a higher mortality rate (48.3%) than those with no asthma or COPD (26.5%, OR = 2.08; 95% CI: 1.01-4.28, unadjusted P = .04, adjusted P = .2).There was no difference in the mortality rates between patients with asthma (24.2%) and those with no asthma or COPD (26.5%, P > .05).
Patients with asthma had lower mortality rates compared with patients with CHF
Patients with coexistent asthma and CHF had higher mortality rate (41.1%) compared with asthma patients without CHF (20.7%) (OR = 2.29; 95% CI: 1.009-5.22, unadjusted P = .04, adjusted P = .2). Subjects with a prior diagnosis of CHF (but no associated asthma diagnosis) had a higher mortality rate (39%) than asthma patients without CHF (20.7%, OR = 1.953; 95% CI: 1.02-3.74, unadjusted P = .04, adjusted P = .2) or nonasthma patients without CHF (24.3%, OR = 2.22; 95% CI: 1.41-3.48, unadjusted P <.001, adjusted P = .005). The mortality rate was not significantly different between asthmatics and nonasthmatics without CHF.
Similar analyses comparing mortality rates in asthma patients with and without comorbid HTN, DM, and CKD were performed. None of these comorbid conditions was found to affect mortality in admitted patients with asthma (data not shown).
Discussion
During the Spring of 2020, the Bronx was one of the epicenters of COVID-19 infection in the United States.18 It is an area that is ethnically diverse, with a majority African American and Hispanic population.27 Historically, this region has a high incidence of asthma.19 In our cohort, a diagnosis of asthma was found in one-fifth of all positive COVID-19 cases, which represents the highest percentage of COVID-19 patients with asthma described to date.
Findings from the present study indicate that in symptomatic asthmatics with COVID-19 infection, the Th2-asthma phenotype characterized by peripheral blood eosinophilia (≥150 cells/μL) was associated with decreased hospital admissions. Development of eosinophilia (AEC ≥ 150 cells/μL) during hospitalization was associated with decreased in-patient mortality from COVID-19 infection in patients with asthma. Furthermore, patients with asthma with higher pre-COVID-19 peripheral blood eosinophilia had higher odds of developing a peak eosinophil count ≥150 cells/μL while hospitalized for COVID-19 infection. To our knowledge, this is the first study demonstrating a potential protective role of eosinophilia in asthma patients with COVID-19. The exact role of eosinophils in SARS-CoV-2 infection is not understood. Current literature, although limited, shows that many admitted patients have eosinopenia,28, 29, 30 similar to what we observed in our cohort, which may serve as a prognostic factor for developing more severe COVID-19 infection.31 In this regard, improvement in eosinopenia before discharge was described in 10 patients receiving lopinavir for COVID-19, and this was considered a possible indicator of COVID-19 improvement.29 Eosinophilic asthma (characterized by Th2 inflammation) has been well recognized, and pathophysiologic and interventional studies indicate that it is a distinct clinical entity.32 , 33 Our findings, along with recent results from in vitro and in vivo studies showing interactions between Th2 inflammation and COVID-19 host ACE2 gene expression, suggest that this asthma phenotype might be an important predictive factor for COVID-19 morbidity and mortality that should be further explored. For instance, bronchial epithelium-ACE2 expression is decreased in patients with asthma with high levels of allergic sensitization,34 whereas increased ACE2 expression was found in patients with asthma with low blood eosinophils (serum cutoff of either 150 or 300 eosinophils/μL).35 Moreover, IL-13 significantly reduced ACE2 expression in airway epithelial cells from both asthmatic and nonasthmatic atopic groups.36 Together, these findings suggest that T2-low patients with asthma may have higher risk for COVID-19 severe outcomes,35 possibly because of increased capacity for viral binding. In addition, we show that admitted patients with asthma who developed peak AEC ≥ 150 cells/μL during their hospitalizations also had higher mean AECs before COVID-19 compared with those in whom peak of AEC never increased above 150 cells/μL, suggesting that hospitalized patients with the T2-asthma phenotype might have less severe COVID-19 outcomes. These findings also suggest that it would be worthwhile to assess the outcomes of COVID-19 in patients with other eosinophilic disorders, as well as the role of eosinophils in COVID-19 outcomes in the general population.
In our study, the overall risk of mortality in patients with asthma alone (no associated CHF, CKD, COPD, DM, or HTN) was similar to nonasthmatics who did not have any of these associated comorbidities. This finding is somewhat surprising given the fact that viruses, including human coronaviruses,37 are common triggers of viral-induced asthma exacerbations, resulting in high morbidity and mortality.5 However, in 2003, SARS did not appear to increase asthma exacerbation in children.37 Although our findings that the mortality rates in asthma and nonasthma patients are similar, as shown also in a large asthma Chicago cohort,38 the risk of dying from COVID-19 in patients with asthma may be influenced by other comorbidities, such as associated COPD and CHF. Because of the retrospective nature of our study, a precise diagnosis of COPD could not be made (as opposed to having a more severe asthma endotype, for example). Nevertheless, our results confirm previous findings that patients with COPD might have a poor outcome after COVID-19 infection. It is of note that the cytokine milieu in COPD and CHF is dominated by the overabundance of Th1 and Th17 cytokines as opposed to Th2 cytokines.39, 40, 41, 42 A recent study showed that a comorbid COPD diagnosis (as gathered through ICD-9/-10 diagnoses) in a cohort of patients with asthma from New England was a strong risk factor for hospitalization,43 highlighting the importance of distinguishing asthma from other chronic pulmonary diseases.44 It is known that patients with overlapping diagnoses of asthma and COPD have a high rate of exacerbations and hospitalizations.45 Although only 1.5% of patients had a diagnosis of COPD in a large Chinese cohort of 1590 cases,46 COPD was found among the risk factors of reaching the composite morbidity end points of the study (admission to an intensive care unit or invasive ventilation or death). None of the patients had a diagnosis of asthma in this Chinese study. Similarly, patients with chronic respiratory disorders (other than asthma) had a higher risk for COVID-19–related death.17 It is not known if smoking status influenced these outcomes47 , 48 or if pre-existing lung pathology leads to high mortality rates. It is also not fully known if prior use of inhaled or oral corticosteroids has any role on COVID-19 outcomes in patients with COPD or asthma. Similar to the findings from other cohorts,38 in our cohort of patients with asthma, we did not find any association between inhaler use or the type of the inhalers and the odds of being admitted for COVID-19. Although it has been reported that medium dose of ICS decreases blood AEC,49 we did not find that the use of medium-dose or high-dose ICS influenced the mortality in admitted patients with asthma in whom AEC increased above 150 cells/μL compared with those whose AEC never peaked above 150 cells/μL. This finding is similar to the results from a large cohort, where regular ICS use was not found to protect against COVID-19–related death among patients with asthma or COPD.50 Likewise, we did not find an association between oral corticosteroid use in the year before COVID-19 infection and the risk of being admitted for COVID-19. On the other hand, prior use of oral corticosteroids in asthmatics was found to pose a higher risk for COVID-19–related death in one large English study.17 We did not assess if there were any differences in inhaler and oral corticosteroid prescriptions in patients with asthma versus those with COPD.
One limitation of the present study is that although these findings include the COVID-19 outcomes in a large cohort of patients with asthma, the data are retrospective and are limited only to those patients who presented to the ED with symptomatic COVID-19. In addition, we used only a physician diagnosis of asthma, as reflected through ICD-9/-10 codes, as opposed to spirometry findings, bronchodilator response, or positive methacholine challenge test to make a diagnosis of asthma. Nevertheless, our real-life setting results could be a useful starting point for additional prospective and/or cohort studies to further assess if there is any difference in COVID-19 mortality based on asthma phenotype and to further investigate if there is any association between specific asthma-related factors such as asthma control or the use of different therapies (such as ICS, monoclonal biologics, or immunotherapy) before the SARS-CoV-2 infection, and subsequent COVID-19 infection outcomes.
Our finding that prior eosinophilia (AEC ≥150 cells/μL) decreases the odds of admission in asthma patients with COVID-19 and mortality in admitted patients also raises an important clinical question about the outcome of asthmatics being treated with monoclonal biological agents that target eosinophils and/or Th2 pathways.51 Our cohort had too few patients on these medications to allow for an analysis of this question. Another limitation is that we do not know the exact effect of corticosteroid administration during hospitalization on the AEC. Because protocols for the use of systemic corticosteroids had not been clearly established at the stage of the pandemic when these data were obtained, we chose to exclude the patients who had been treated with systemic corticosteroids at any point during the course of the admission to reduce the confounding effect of steroids on inpatient AEC levels. Finally, it is unclear if the season of the year played any role in COVID-19 outcomes in patients with asthma. It is known that asthma-related ED visits and hospitalizations peak during the spring in the New York area because of tree pollination.52 This year, in New York City, the tree pollination season overlapped the peak COVID-19 infection rates.
In conclusion, in our cohort of asthma patients with COVID-19, eosinophilia was a protective factor for hospital admission and for mortality. Overall, having an asthma diagnosis alone without some associated common comorbidity (CHF, CKD, COPD, DM, HTN) did not increase the rate and risk of mortality from SARS-CoV-2 infection, whereas having associated COPD and CHF appeared to increase mortality in asthma patients with COVID-19. Further prospective and mechanistic studies are needed to explore the exact role of eosinophils in COVID-19 mortality, as well as the influence of different asthma characteristics on outcomes of patients with asthma and COVID-19 infection.
Footnotes
No funding was received for this work.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository
ICD-9/-10 diagnoses of different medical conditions used for the study (alphabetical order):
| Allergic rhinitis: |
|---|
| 472.0-CHRONIC RHINITIS |
| 477.0-RHINITIS DUE TO POLLEN |
| 477.8-ALLERGIC RHINITIS NEC |
| 477.9-ALLERGIC RHINITIS NOS |
| 477-ALLERGIC RHINITIS |
| J30.1-Allergic rhinitis due to pollen |
| J30.2-Other seasonal allergic rhinitis |
| J30.81-Allergic rhinitis due to animal (cat) (dog) hair and dander |
| J30.89-Other allergic rhinitis |
| J30.9-Allergic rhinitis, unspecified |
| Asthma: |
|---|
| 493.00-EXTRINSIC ASTHMA, UNSPECIFIED |
| 493.90-ASTHMA, UNSPECIFIED |
| 493.92-ASTHMA, UNSPECIFIED, W/ACUTE EXACERBATION |
| J45.20-Mild intermittent asthma, uncomplicated |
| J45.21-Mild intermittent asthma with (acute) exacerbation |
| J45.22-Mild intermittent asthma with status asthmaticus |
| J45.30-Mild persistent asthma, uncomplicated |
| J45.31-Mild persistent asthma with (acute) exacerbation |
| J45.40-Moderate persistent asthma, uncomplicated |
| J45.41-Moderate persistent asthma with (acute) exacerbation |
| J45.42-Moderate persistent asthma with status asthmaticus |
| J45.50-Severe persistent asthma, uncomplicated |
| J45.51-Severe persistent asthma with (acute) exacerbation |
| J45.901-Unspecified asthma with (acute) exacerbation |
| J45.909-Unspecified asthma, uncomplicated |
| Congestive heart failure: |
|---|
| 398.91-RHEUMATIC HEART FAILURE |
| 428.0-CONGESTIVE HEART FAILURE, UNSPECIFIED |
| 428.1-LEFT HEART FAILURE |
| 428.20-UNSPECIFIED SYSTOLIC HEART FAILURE |
| 428.21-ACUTE SYSTOLIC HEART FAILURE |
| 428.22-CHRONIC SYSTOLIC HEART FAILURE |
| 428.23-ACUTE ON CHRONIC SYSTOLIC HEART FAILURE |
| 428.30-UNSPECIFIED DIASTOLIC HEART FAILURE |
| 428.31-ACUTE DIASTOLIC HEART FAILURE |
| 428.32-CHRONIC DIASTOLIC HEART FAILURE |
| 428.33-ACUTE ON CHRONIC DIASTOLIC HEART FAILURE |
| 428.9-HEART FAILURE NOS |
| 428-HEART FAILURE |
| I11.0-Hypertensive heart disease with heart failure |
| I13.0-Hypertensive heart and chronic kidney disease with heart failure and stage 1 through stage 4 chronic |
| I13.2-Hypertensive heart and chronic kidney disease with heart failure and with stage 5 chronic kidney disease |
| I50.20-Unspecified systolic (congestive) heart failure |
| I50.21-Acute systolic (congestive) heart failure |
| I50.22-Chronic systolic (congestive) heart failure |
| I50.23-Acute on chronic systolic (congestive) heart failure |
| I50.30-Unspecified diastolic (congestive) heart failure |
| I50.31-Acute diastolic (congestive) heart failure |
| I50.32-Chronic diastolic (congestive) heart failure |
| I50.33-Acute on chronic diastolic (congestive) heart failure |
| I50.42-Chronic combined systolic (congestive) and diastolic (congestive) heart failure |
| I50.43-Acute on chronic combined systolic (congestive) and diastolic (congestive) heart failure |
| I50.810-Right heart failure, unspecified |
| I50.811-Acute right heart failure |
| I50.812-Chronic right heart failure |
| I50.9-Heart failure, unspecified |
| Chronic kidney disease: |
|---|
| 585.1-CHRONIC KIDNEY DISEASE, STAGE I |
| 585.2-CHRONIC KIDNEY DISEASE, STAGE II (MILD) |
| 585.3-CHRONIC KIDNEY DISEASE, STAGE III (MODERATE) |
| 585.4-CHRONIC KIDNEY DISEASE, STAGE IV (SEVERE) |
| 585.9-CHRONIC KIDNEY DISEASE, UNSPEC |
| I13.0-Hypertensive heart and chronic kidney disease with heart failure and stage 1 through stage 4 chronic |
| N18.1-Chronic kidney disease, stage 1 |
| N18.2-Chronic kidney disease, stage 2 (mild) |
| N18.3-Chronic kidney disease, stage 3 (moderate) |
| N18.4-Chronic kidney disease, stage 4 (severe) |
| N18.9-Chronic kidney disease, unspecified |
| Chronic obstructive pulmonary disease: |
| J44-Other chronic obstructive pulmonary disease |
| J44.0-Chronic obstructive pulmonary disease with (acute) lower respiratory infection |
| J44.1-Chronic obstructive pulmonary disease with (acute) exacerbation |
| Diabetes: |
|---|
| 250.00-DIABETES MELLITUS W/O MENTION COMPL TYPE II |
| 250.01-DIABETES MELLITUS W/O MENTN COMPL,TYPE I(JUVE) |
| 250.02-DIABETES MELLITUS W/O MENTN CMPL,TYPE II |
| 250.03-DIABETES MELLITUS W/O MENTN COMPL, TYPE I (JUVE) |
| 250.12-DIABETES W KETOACIDOSIS,TYPE II/UNSPEC TY, UNCNTRL |
| 250.13-DIABETES W KETOACIDOSIS,TYPE I(JUVE), UNCONTROLLED |
| 250.1-DIABETES W KETOACIDOSIS |
| 250.20-DIABETES W HYPEROSMOLARITY,TYPE II/UNSPEC, NOT STT |
| 250.22-DIABETES W HYPEROSMOLARITY,TYPE II/UNSPC, UNCONTRL |
| 250.40-DIABETES W RENAL MANIF,TYPE II/UNSPEC,NOT STTD UNC |
| 250.41-DIABETES W RENAL MANIF,TYPE I(JUV), NOT STAT UNCON |
| 250.42-DIABETES W RENAL MANIF,TYPE II/UNSPC,UNCONTROLLED |
| 250.50-DIABETES W OPHTHALMIC MANIF,TYPE II/UNSPC,NOT S UN |
| 250.51-DIABETES W OPHTALMIC MANIF,TYPE I(JUV),NOT ST UNCO |
| 250.52-DIABETES W OPHTHALMIC MANIF,TYPE II/UNSPC,UNCONTRL |
| 250.53-DIABETES W OPHTHALMIC MANIF,TYPE I(JUV), UNCONTROL |
| 250.60-DIABETES W NEUROLOGICAL MANIF,TYPE II,NOT ST UNCON |
| 250.61-DIABETES W NEUROLOGL MANIF,TYPE I(JUV),NOT ST UNCO |
| 250.62-DIABETES W NEUROLGL MANIF,TYPE II, UNCONTROLLED |
| 250.70-DIABETES W PERIPHRL CIRC DISOR,TYPE II,N S UNCONTL |
| 250.72-DIABETES W PERIPHRL CIRC DISOR,TYPE II, UNCONTROL |
| 250.7-DIABETES W CIRCULAT DIS |
| 250.80-DIABETES W OTH SPEC MANIF,TYPE II, NOT ST UNCONTRL |
| 250.81-DIABETES W OTH SPEC MANIF,TYPE I, NOT STAT UNCONTL |
| 250.82-DIABETES W OTH SPEC UNAIF,TYPE II, UNCONTROLLED |
| 250.83-DIABETES W OTH SPEC MANIF,TYPE I,UNCONTROLLED |
| 250.8-DIABETES W MANIFEST NEC |
| 250.90-DIABETES W UNSPEC COMPLC,TYPE II, NOT ST UNCONTROL |
| 250.92-DIABETES W UNSPEC COMPL,TYPE II,UNCONTROLLED |
| 250.93-DIABETES W UNSPEC COMPL,TYPE I, UNCONTROLLED |
| 250.9-DIABETES W COMPLIC NOS |
| 250-DIABETES MELLITUS |
| 357.2-NEUROPATHY IN DIABETES |
| 648.03-DIABETES-ANTEPARTUM |
| E08.00-Diabetes mellitus due to underlying condition with hyperosmolarity without nonketotic hyperglycemic- |
| E08.21-Diabetes mellitus due to underlying condition with diabetic nephropathy |
| E08.22-Diabetes mellitus due to underlying condition with diabetic chronic kidney disease |
| E08.3553-Diabetes mellitus due to underlying condition with stable proliferative diabetic retinopathy, bilate |
| E08.42-Diabetes mellitus due to underlying condition with diabetic polyneuropathy |
| E08.8-Diabetes mellitus due to underlying condition with unspecified complications |
| E08.9-Diabetes mellitus due to underlying condition without complications |
| E09.65-Drug or chemical induced diabetes mellitus with hyperglycemia |
| E09.9-Drug or chemical induced diabetes mellitus without complications |
| E10.21-Type 1 diabetes mellitus with diabetic nephropathy |
| E10.22-Type 1 diabetes mellitus with diabetic chronic kidney disease |
| E10.40-Type 1 diabetes mellitus with diabetic neuropathy, unspecified |
| E10.9-Type 1 diabetes mellitus without complications |
| E11.00-Type 2 diabetes mellitus with hyperosmolarity without nonketotic hyperglycemic-hyperosmolar coma (NK |
| E11.10-Type 2 diabetes mellitus with ketoacidosis without coma |
| E11.21-Type 2 diabetes mellitus with diabetic nephropathy |
| E11.22-Type 2 diabetes mellitus with diabetic chronic kidney disease |
| E11.29-Type 2 diabetes mellitus with other diabetic kidney complication |
| E11.311-Type 2 diabetes mellitus with unspecified diabetic retinopathy with macular edema |
| E11.319-Type 2 diabetes mellitus with unspecified diabetic retinopathy without macular edema |
| E11.3291-Type 2 diabetes mellitus with mild nonproliferative diabetic retinopathy without macular edema, righ |
| E11.3293-Type 2 diabetes mellitus with mild nonproliferative diabetic retinopathy without macular edema, bila |
| E11.3393-Type 2 diabetes mellitus with moderate nonproliferative diabetic retinopathy without macular edema, |
| E11.3532-Type 2 diabetes mellitus with proliferative diabetic retinopathy with traction retinal detachment no |
| E11.3591-Type 2 diabetes mellitus with proliferative diabetic retinopathy without macular edema, right eye |
| E11.36-Type 2 diabetes mellitus with diabetic cataract |
| E11.39-Type 2 diabetes mellitus with other diabetic ophthalmic complication |
| E11.40-Type 2 diabetes mellitus with diabetic neuropathy, unspecified |
| E11.42-Type 2 diabetes mellitus with diabetic polyneuropathy |
| E11.43-Type 2 diabetes mellitus with diabetic autonomic (poly)neuropathy |
| E11.51-Type 2 diabetes mellitus with diabetic peripheral angiopathy without gangrene |
| E11.52-Type 2 diabetes mellitus with diabetic peripheral angiopathy with gangrene |
| E11.59-Type 2 diabetes mellitus with other circulatory complications |
| E11.620-Type 2 diabetes mellitus with diabetic dermatitis |
| E11.621-Type 2 diabetes mellitus with foot ulcer |
| E11.649-Type 2 diabetes mellitus with hypoglycemia without coma |
| E11.65-Type 2 diabetes mellitus with hyperglycemia |
| E11.69-Type 2 diabetes mellitus with other specified complication |
| E11.8-Type 2 diabetes mellitus with unspecified complications |
| E11.9-Type 2 diabetes mellitus without complications |
| E13.10-Other specified diabetes mellitus with ketoacidosis without coma |
| E13.22-Other specified diabetes mellitus with diabetic chronic kidney disease |
| E13.39-Other specified diabetes mellitus with other diabetic ophthalmic complication |
| E13.621-Other specified diabetes mellitus with foot ulcer |
| E13.9-Other specified diabetes mellitus without complications |
| Eczema: |
|---|
| 373.31-ECZEM DERMATITIS EYELID |
| 691.8-OTHER ATOPIC DERMATITIS |
| 691-ATOPIC DERMATITIS |
| H01.119-Allergic dermatitis of unspecified eye, unspecified eyelid |
| H01.136-Eczematous dermatitis of left eye, unspecified eyelid |
| H01.139-Eczematous dermatitis of unspecified eye, unspecified eyelid |
| L20.82-Flexural eczema |
| L20.83-Infantile (acute) (chronic) eczema |
| L20.84-Intrinsic (allergic) eczema |
| L20.89-Other atopic dermatitis |
| L20.9-Atopic dermatitis, unspecified |
| L30.9-Dermatitis, unspecified |
| Food allergy: |
|---|
| T78.07XA-Anaphylactic reaction due to milk and dairy products, initial encounter |
| V15.04-ALLERGY TO SEAFOOD |
| V15.05-ALLERGY TO OTHER FOODS |
| Z91.010-Allergy to peanuts |
| Z91.011-Allergy to milk products |
| Z91.012-Allergy to eggs |
| Z91.013-Allergy to seafood |
| Z91.018-Allergy to other foods |
| Nasal polyposis: |
|---|
| J33.0-Polyp of nasal cavity |
| J33.8-Other polyp of sinus |
| J33.9-Nasal polyp, unspecified |
Urticaria:
| Urticaria: |
|---|
| 708.0-ALLERGIC URTICARIA |
| 708.1-IDIOPATHIC URTICARIA |
| 708.2-URTICARIA FROM COLD/HEAT |
| 708.3-DERMATOGRAPHIC URTICARIA |
| 708.9-URTICARIA NOS |
| 708-URTICARIA |
| L50.0-Allergic urticarial |
| L50.1-Idiopathic urticarial |
| L50.8-Other urticarial |
| L50.9-Urticaria, unspecified |
16.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Johns Hopkins Coronavirus Resource Center COVID-19 case tracker. https://coronavirus.jhu.edu/ Available from: Accessed May 30, 2020.
- 3.Halpin D.M.G., Faner R., Sibila O., Badia J.R., Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med. 2020;8:436–438. doi: 10.1016/S2213-2600(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Sullivan S.M. Asthma death, CD8+ T cells, and viruses. Proc Am Thorac Soc. 2005;2:162–165. doi: 10.1513/pats.200502-016AW. [DOI] [PubMed] [Google Scholar]
- 6.New York State Department of Health COVID-19 tracker-fatalities. https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Fatalities?%3Aembed=yes&%3Atoolbar=no Available from: Accessed May 30, 2020.
- 7.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lombardi C., Roca E., Bigni B., Cottini M., Passalacqua G. Clinical course and outcomes of patients with asthma hospitalized for severe acute respiratory syndrome coronavirus 2pneumonia: a single-center, retrospective study. Ann Allergy Asthma Immunol. 2020;125:707–709. doi: 10.1016/j.anai.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg S., Kim L., Whitaker M., O'Halloran A., Cummings C., Holstein R. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus Disease 2019—COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the Seattle Region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broadhurst R., Peterson R., Wisnivesky J.P., Federman A., Zimmer S.M., Sharma S. Asthma in COVID-19 hospitalizations: an overestimated risk factor? Ann Am Thorac Soc. 2020;17:1645–1648. doi: 10.1513/AnnalsATS.202006-613RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia H., Zack M.M., Thompson W.W. The effects of diabetes, hypertension, asthma, heart disease, and stroke on quality-adjusted life expectancy. Value Health. 2013;16:140–147. doi: 10.1016/j.jval.2012.08.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradding P., Richardson M., Hinks T.S.C., Howarth P.H., Choy D.F., Arron J.R. ACE2, TMPRSS2, and furin gene expression in the airways of people with asthma—implications for COVID-19. J Allergy Clin Immunol. 2020;146:208–211. doi: 10.1016/j.jaci.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters M.C., Sajuthi S., Deford P., Christenson S., Rios C.L., Montgomery M.T. COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202:83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahdavinia M., Foster K.J., Jauregui E., Moore D., Adnan D., Andy-Nweye A.B. Asthma prolongs intubation in COVID-19. J Allergy Clin Immunol Pract. 2020;8:2388–2391. doi: 10.1016/j.jaip.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan R. The Bronx, long a symbol of American poverty, is now New York City's coronavirus capital. The Washington Post. April 21, 2020. https://www.washingtonpost.com/national/bronx-new-york-yankee-stadium-coronavirus/2020/04/21/3b38b460-8182-11ea-a3ee-13e1ae0a3571_storyhtml Available from: Accessed May 30, 2020.
- 19.New York State Department of Health Information on asthma in New York state. https://www.health.ny.gov/statistics/ny_asthma/ Available from: Accessed May 30, 2020.
- 20.Henry B.M., Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52:1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14:247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellin E., Fletcher D.D., Geberer N., Islam S., Srivastava N. Democratizing information creation from health care data for quality improvement, research, and education-the Montefiore Medical Center Experience. Acad Med. 2010;85:1362–1368. doi: 10.1097/ACM.0b013e3181df0f3b. [DOI] [PubMed] [Google Scholar]
- 24.Katz L.E., Gleich G.J., Hartley B.F., Yancey S.W., Ortega H.G. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc. 2014;11:531–536. doi: 10.1513/AnnalsATS.201310-354OC. [DOI] [PubMed] [Google Scholar]
- 25.Bakakos A., Loukides S., Bakakos P. Severe eosinophilic asthma. J Clin Med. 2019;8:1375. doi: 10.3390/jcm8091375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altman L.C., Hill J.S., Hairfield W.M., Mullarkey M.F. Effects of corticosteroids on eosinophil chemotaxis and adherence. J Clin Invest. 1981;67:28–36. doi: 10.1172/JCI110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Census Bureau. QuickFactsBronx County (Bronx Borough), New York. https://www.census.gov/quickfacts/bronxcountybronxboroughnewyork Available from: Accessed May 30, 2020.
- 28.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K. Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 31.Lindsley A.W., Schwartz J.T., Rothenberg M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146:1–7. doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akar-Ghibril N., Casale T., Custovic A., Phipatanakul W. Allergic endotypes and phenotypes of asthma. J Allergy Clin Immunol Pract. 2020;8:429–440. doi: 10.1016/j.jaip.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yancey S.W., Keene O.N., Albers F.C., Ortega H., Bates S., Bleecker E.R. Biomarkers for severe eosinophilic asthma. J Allergy Clin Immunol. 2017;140:1509–1518. doi: 10.1016/j.jaci.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Jackson D.J., Busse W.W., Bacharier L.B., Kattan M., O'Connor G.T., Wood R.A. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146:203–206.e3. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camiolo M., Gauthier M., Kaminski N., Ray A., Wenzel S.E. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J Allergy Clin Immunol. 2020;146:315–324.e7. doi: 10.1016/j.jaci.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimura H., Francisco D., Conway M., Martinez F.D., Vercelli D., Polverino F. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146:80–88.e8. doi: 10.1016/j.jaci.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Bever H.P., Chng S.Y., Goh D.Y. Childhood severe acute respiratory syndrome, coronavirus infections and asthma. Pediatr Allergy Immunol. 2004;15:206–209. doi: 10.1111/j.1399-3038.2004.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chhiba K.D., Patel G.B., Vu T.H.T., Chen M.M., Guo A., Kudlaty E. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:307–314.e4. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai Y.H., Ma Z.J., Lu X.Y., He E.L., You M.Y. Study on the effect and mechanism of the dysfunction of CD4(+) T cells in the disease process of chronic cardiac failure. Asian Pac J Trop Med. 2016;9:682–687. doi: 10.1016/j.apjtm.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Barnes P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y., Kumar R.K., Thomas P.S., Herbert C. Th1/17-biased inflammatory environment associated with COPD alters the response of airway epithelial cells to viral and bacterial stimuli. Mediators Inflamm. 2019;2019:7281462. doi: 10.1155/2019/7281462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponce-Gallegos M.A., Ramírez-Venegas A., Falfán-Valencia R. Th17 profile in COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2017;12:1857–1865. doi: 10.2147/COPD.S136592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L., Foer D., Bates D.W., Boyce J.A., Zhou L. Risk factors for hospitalization, intensive care and mortality among patients with asthma and COVID-19. J Allergy Clin Immunol. 2020;146:808–812. doi: 10.1016/j.jaci.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore W.C., Meyers D.A., Wenzel S.E., Teague W.G., Li H., Li X. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menezes A.M.B., Montes de Oca M., Pérez-Padilla R., Nadeau G., Wehrmeister F.C., Lopez-Varela M.V. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145:297–304. doi: 10.1378/chest.13-0622. [DOI] [PubMed] [Google Scholar]
- 46.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu W., Tao Z.W., Wang L., Yuan M.L., Liu K., Zhou L. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Lian N. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol. 2020;92:1915–1921. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jabbal S., Lipworth B.J. Blood eosinophils: the forgotten man of inhaled steroid dose titration. Clin Exp Allergy. 2018;48:93–95. doi: 10.1111/cea.13057. [DOI] [PubMed] [Google Scholar]
- 50.Schultze A., Walker A.J., MacKenna B., Morton C.E., Bhaskaran K., Brown J.P. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106–1120. doi: 10.1016/S2213-2600(20)30415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Busse W., Chupp G., Nagase H., Albers F.C., Doyle S., Shen Q. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: Indirect treatment comparison. J Allergy Clin Immunol. 2019;143:190–200.e20. doi: 10.1016/j.jaci.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 52.Witonsky J., Abraham R., Toh J., Desai T., Shum M., Rosenstreich D. The association of environmental, meteorological, and pollen count variables with asthma-related emergency department visits and hospitalizations in the Bronx. J Asthma. 2019;56:927–937. doi: 10.1080/02770903.2018.1514627. [DOI] [PubMed] [Google Scholar]