Abstract
Background
Even though SARS-CoV-2 is a predominantly respiratory virus, several reports have described various neurological disorders, from the beginning of the pandemic. The first para-infectious myelitis case was described in Wuhan in February 2020. Nevertheless, data from registries and reviews are scarce.
Methods
A 40-year-old female with T5-T6 SARS-CoV-2 para-infectious myelitis is reported. A literature review of the published literature on the SARS-CoV-2 and para-infectious myelitis was done. Epidemiological, clinical, laboratory, image, treatment, and outcome data are described.
Results
Particular findings of our case are that Covid-19 was asymptomatic and anti-GD2/GD3 IgM was found. 18 para-infectious myelitis occurred over a wide age range (Beh et al., 2013-67), mean age 50.7±18.6 years, with 10/18 (55.6%) women. Covid-19 involvement was variable from asymptomatic cases to severe Covid-19 resulting in death. The mean time to establish myelitis from the onset of Covid-19 symptoms was 10.3 ±7.8 days (0-24). The most common clinical form was transverse myelitis (14/18 patients, 77.7%) and the most frequent radiological form was longitudinally extensive myelitis (11/17 patients, 64.7%). In CSF mild lymphocytosis (14/16, 87.5%) with low cellularity (40.9±49.7/μL) and elevated proteins (11/16, 77.8%, mean 145.0 mg±159.0/dL) were frequent. Oligoclonal bands were usually negative (7/9, 77.7%) and mirror pattern was found in 2/7 patients (33.3%). SARS-CoV-2 PCR in CSF was negative in 10/10 cases.
Conclusion
SARS-CoV-2 can cause myelitis by immune-mediated mechanisms. Clinical-radiological characteristics of Covid-19 para-infectious myelitis were variable and non-specific.
1. Introduction
From the coronavirus disease-2019 (Covid-19) outbreak in January 2020, numerous neurological problems have been reported given the frequent observations of neurological symptoms such as ageusia, anosmia, and encephalopathy. Moreover, stroke, encephalitis, myelitis, and syndromes with peripheral nerve involvement such as Guillain-Barré Syndrome (GBS) have been less commonly observed. Coronaviridae have been shown to have neurotropic and neuro-invasive capabilities. Nevertheless, the exact pathophysiology of the neurological involvement is still unknown. Only a few cases of myelitis related to Covid-19 have been reported: it remains unknown whether it is due to the direct invasion of the virus or it is a para-infectious phenomenon.
We report a case of a patient with anti-GD2/GD3 IgM and provide a comprehensive and updated review of all case reports of Covid-19-related myelitis to identify clinical, image, laboratory, and neurophysiological patterns.
2. Methods
We report a new case and conduct a review of the literature published up to October 2020 in PubMed and Embase®. The following indexing terms were used in the search strategy Medical Subject Headings (MeSH): (myelitis) AND (SARS-CoV-2 OR Coronavirus infections OR Covid-19 OR SARS virus) finding 73 articles. Embase® added 2 quotes that were not found in MEDLINE. The review was expanded by checking the relevant references of the selected articles.
LARA wrote the design and wrote the manuscript. IGS, IFB, and MRP reviewed the manuscript. The consensus of 3 of the 4 neurologists was reached in order to solve methodological and classification doubts.
Demographic variables such as age and sex, as well as descriptive variables of Covid-19 infection (upper respiratory infection or pneumonia), were evaluated based on the clinical and radiological findings presented in the cases. Serological status and pharyngeal PCR positivity were also examined. We have divided Covid-19 severity into stage I (early infection), stage IIA (pulmonary involvement without respiratory insufficiency), stage IIB (respiratory insufficiency), and stage III (systemic hyperinflammation) (Siddiqi et al., 2020). Transverse myelitis was defined according to the Transverse Myelitis Consortium group (Transverse Myelitis Consortium group, 2002). Nosological aspects were considered according to a Continuum review on Transverse Myelitis (Beh et al., 2013).
Variables concerning myelitis were: the time from the onset of Covid-19 symptoms to the first symptom of myelitis and the neurological symptoms manifested. Data from CSF was collected but only the first tap data were used. Quantitative data for CSF glucose and proteins were transformed to mg/dL for analysis and presentation. Outcome analysis was done considering closed categories such as: exitus, no improvement, slight improvement (when less than a half of the symptoms were recovered), moderate improvement (when more than a half of the symptoms were recovered), and complete improvement (when total recovery occurred) in the moment of discharge.
MRI lesions were measured in terms of length, localization, expansive aspect, meningeal involvement, and Gadolinium enhancement. Longitudinally extensive transverse myelitis (LETM) was considered when the lesion extends over 3 or more vertebral segments (Beh et al., 2013). Cases of myelitis that meet NMO (International Panel for NMO Diagnosis, 2015) criteria or with positive anti-myelin oligodendrocyte glycoprotein (anti-MOG) are presented in the same table (Appendix A: supplementary table) but they have not been taken into account when establishing a joint description of Covid-19 para-infectious myelitis. SPSS software, version 25 (SPSS, Chicago, IL), was used to collect and analyze data.
This report adheres to the Declaration of Helsinki. Patient data were obtained through inpatient and outpatient medical records at Hospital Universitario de Fuenlabrada (Madrid). Written informed consent was obtained.
3. Results
3.1. Case report
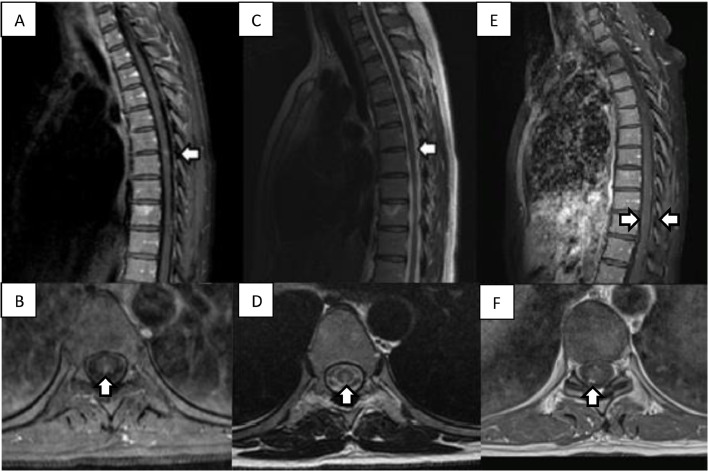
A 40-year-old woman with venous insufficiency, migraine, appendectomized, and splenectomized because of a traffic accident began with a feeling of numbness and hypoesthesia in both soles of the feet in June 2020. In the following days, these symptoms increased in intensity and ascended to knees, thighs, and perineum. No motor symptoms were present and mild urination urgency was noted. The neurological examination revealed hypoesthesia in perineum, distal third of both legs and feet as well as a moderate deficit of vibratory sensitivity in the ankles and knees. Her physical examination was otherwise normal. The patient had had neither symptoms of Covid-19 nor suspicious contacts. However, the admission screening test: polymerase chain reaction (PCR) and anti-SARS-CoV-2 IgG were positive, while anti-SARS-CoV-2 IgM was negative, which may indicate a final stage of infection. Brain and cervical MRI were normal, dorsal MRI showed a central 7 × 4 mm non-expansive T2-weighted hyperintense signal in T5-T6 level consistent with acute myelitis (Fig. 1 ). Administration of gadolinium showed enhancement (Fig. 1). Laboratory workup, including a complete blood count, complete metabolic panel, thyroid testing, and inflammatory markers, did not show pathological data, and serologies for Borrelia, syphilis, or HIV either. Cerebrospinal fluid (CSF) evaluation showed 20 cells, 100% mononuclear, with normal proteins (36 mg/dL). Immunoglobulins, ANAs, ACE, and ADA. CSF-specific oligoclonal bands, serum aquaporin-4 antibodies (anti-AQP4), and serum anti-myelin oligodendrocyte glycoprotein antibodies (anti-MOG) were also negative. IgM and IgG anti-gangliosides antibodies were tested and an IgM band corresponding to GD2 and GD3 was obtained. She was diagnosed with post-infectious myelitis secondary to SARS-CoV-2 infection. Treatment was initiated with intravenous (IV) methylprednisolone for 5 days and supportive care with complete recovery of the bladder dysfunction and mild recovery of the sensitive function. Hypoesthesia and a slight decrease in vibratory sensitivity persisted in a follow-up visit performed three months after the beginning of the symptoms. A control spinal MRI was done 5 months after admission. A meningeal diffuse gadolinium enhancement is observed (Fig. 1).
Fig. 1.
A: T1 Post-Gadolinium MRI of the dorsal spine in sagittal view showing a patchy enhancement (white arrow). B: Post-Gadolinium axial T1 showing patchy enhancement. C: T2-weighted MRI of the T5-T6 lesion (white arrow). D: Axial T2-weighted MRI showing a dorsal position of the lesion. E: 5 months sagittal T1-post-gadolinium MRI showing a linear meningeal enhancement. F: 5 months MRI with post-Gadolinium axial T1 showing patchy meningeal enhancement.
3.2. Review
20 cases were identified, all epidemiological, clinical, diagnostic, treatment, and outcome characteristics of the patients with myelitis with a SARS-Cov-2 infection are shown in Appendix A: supplementary table. Two cases (Shaw et al., 2020; Zhou et al., 2019) were excluded from the analysis due to an alternative diagnosis of NMO or anti-MOG myelopathy. A summary of the epidemiological, clinical and radiological characteristics, treatment, and outcome of myelitis related to Covid-19 can be seen in Table 1 .
Table 1.
Frequency and summary of the epidemiological, clinical and diagnostic, treatment and outcome characteristics of myelitis related to Covid-19
| Female gender (n=18) | 55,6% | Mean age (n=18) | 50.7±18.6 |
| Covid-19 syndrome and complications | Covid-19 phases | ||
| Asymptomatic | 2 (11.1%) | Asymptomatic | 2 (11.1%) |
| Upper respiratory infection | 8 (44.4%) | Stage I | 8 (44.4%) |
| Pneumonia | 8 (44.4%) | Stage IIA | 6 (33.3%) |
| Pulmonary embolism | 1 (5.1%) | Stage IIB | 0 |
| Cardiac arrest and deaths | 2 (11.1%) | Stage III | 2 (11.1%) |
| SARS-CoV-2 diagnosis | CSF characteristics | ||
| PCR (n=18) | 13 (72.2%) | Mean pleocytosis (n=16) | 40.9±49.7/µL |
| IgG (n=5) | 5 (100%) | Mean proteinorrachia (n=16) | 145.0 mg±159.0/dL |
| IgM (n=4) | 2 (50%) | OCB (n=9) | 2 (22.2%). Mirror pattern. |
| Mean time to myelitis | 10.3 ±7.8 d | Median time to myelitis | 8 d |
| Neurological symptoms (n=18) | Myelitis syndrome | ||
| Motor involvement | 16 (88.9%) | Transverse myelitis | 14 (77.7%) |
| Sensitive involvement | 14 (77.7%) | Brown Sequard syndrome | 1 (5.5%) |
| Urinary dysfunction | 16 (88.8%) | Dorsal columns syndrome | 1 (5.5%) |
| MRI characteristics | Partial transverse myelitis | 2 (11.1%) | |
| Brain abnormalities (n=13) | 2 (35.4%) | Treatment | |
| Spinal cord abnormalities (n=17) | 16 (88.2%) | Corticosteroids (n=18) | 14 (77.8%) |
| Gd enhancement (n=12) | 6 (50%) | IVIgs (n=18) | 6 (33.3%) |
| Spinal lesion localization (n=18) | PLEX (n=18) | 8 (44.4%) | |
| - Cervical | 2 (12.5%) | Outcomes (n=18) | |
| - Thoracic | 6 (30.7%) | Complete recovery | 1 (5.6%) |
| - Cervico-thoracic | 8 (50.0%) | Mild recovery | 5 (27.7%) |
| Mean lesion length (segments) | 6.2±6.1(0-19) | Moderate recovery | 9 (50%) |
| Spinal cord swelling (n=14) | 8 (57.1%) | No improvement | 1 (5.6%) |
| LETM (n=17) | 11 (64.7 %) | Death | 2 (11.1%) |
Pharyngeal PCR was positive in 13/18 (72.2%). Positive IgG was found in 5/5 (100%) and IgM in 2/4 (50%) patients collected. All patients with negative PCR were serologically confirmed cases. In 10 patients SARS-Cov2 PCR in CSF were done with negative results.
Time to onset of the myelitis from the beginning of the first Covid-19 symptoms was mean 10.3 ±7.8 days, median 8 days.
Motor symptoms, including different degrees of paresis, were present in 16/18 (88.9%); and in 6/18 (33.3%) were complete plegias (quadriplegia or paraplegia); four of them were flaccid plegias (para and quadriplegias). Sensitive impairment was present in 14/18 (77.7%). Sphincter dysfunction (urinary retention, constipation, or bowel or urinary incontinence) was present in 16/18 (88.8%). Transverse myelitis was the most frequent syndrome (77.7% patients).
Myelitis was associated with encephalitis in 2 cases (Zoghi et al., 2020; Novi et al., 2020), with optic neuritis in 1 case (Novi et al., 2020), and with GBS in 3 cases (all of them with axonal-loss predominant polyneuropathy) (Maideniuc et al., 2020; Valiuddin et al., 2020; Masuccio et al., 2020).
Most patients presented with a mild pleocytosis 40.9±49.7/µL (Beh et al., 2013-150) usually at the expense of monocytes in 14/16 (87.5%) and neutrophils in 2/16 (12.5%). Moderate elevation of proteins in CSF, mean 145.0 mg±159.0/dL (Nakamura et al., 2017-573), were observed in 11/16 patients (68.8%). Oligoclonal bands were analyzed in 7 patients: mirror patterns of oligoclonal bands were found in 2/9 patients (22.2%).
Brain MRI was normal in 11/13 cases (64.6%). In 2/13 (15.4%) abnormalities were consistent with ADEM findings. Most of the patients presented abnormal spinal MRI except two patients (Zachariadis et al., 2020; Águila-Gordo et al., 2020) who did not show any pathological finding. Gadolinium use in cases without MRI lesion was not found. One patient showed a T1 hypointense necrotic lesion (Sotoca et al., 2020). Other radiological characteristics are shown in Table 1. Only our case showed meningeal contrast enhancement in a MRI performed 5 months later. Radicular uptake did not appear in any case. A control MRI is provided only in our case.
Corticosteroids were the most common treatment (14/18): the most frequent regimen was methylprednisolone 1g/day for 3 (1/15) or 5 days (8/15). However, prednisone adjusted by weight dose in a pediatric case (Kaur et al., 2020), or in a low dose (100mg/d) (Munz et al., 2020) are also registered. Dexamethasone 10 mg/10 days was used in 2 cases (Águila-Gordo et al., 2020; Zhao et al.,2020). 6/18 patients were treated with isolated corticosteroids. Other treatments registered were intravenous immunoglobulin (IVIg) in 6/18 patients at 0.4 g/kg/day for 5 days to 7 days (Baghbanian et al., 2020), and plasma exchange (PLEX) in 8/18 cases, frequently combined with corticosteroids (6/8). Duration of PLEX therapy varied from 2 days (1/7) (Sarma et al., 2020) to 7 sessions (1/7) (Kaur et al., 2020) being the most common number of sessions 5 (5/8).
Complete recovery was seen in 1/18(5.6%) (Chow et al., 2020), moderate improvement in 5/18 (27.7%) (Zhou et al., 2019; Novi et al., 2020; Munz et al., 2020; Sarma et al., 2020; AlKetbi et al., 2020), mild improvement in 9/18(50.0%) (Zoghi et al., 2020; Maideniuc et al., 2020; Valiuddin et al., 2020; Masuccio et al., 2020; Zachariadis et al., 2020; Águila-Gordo et al., 2020; Sotoca et al., 2020; Baghbanian et al., 2020), no improvement in 1/18(5.6%) (Kaur et al., 2020) and 2 deaths (11.1%) (Chakraborty et al., 2020; Abdelhady et al., 2020).
4. Discussion
We have presented a case with some unique characteristics: mielytis is developed in an asymptomatic Covid-19 patient, the patient presented a small lesion, and an anti-GD2/GD3 IgM was found. Previously, only a 3-year-old girl patient being asymptomatic for Covid-19 with a LETM was described (Kaur et al., 2020). Other cases had more or less severe Covid-19 symptoms. From the radiological point of view, although the majority of the patients registered to date had LETM, there are numerous cases described with small lesions, such as ours. In fact, we have found two cases without lesions detected in MRI (Zachariadis et al., 2020; Águila-Gordo et al., 2020). This may be because the myelitis lesions were very small. Viral myelitis without hypersignal in T2 due to polio or HTLV-1 or due to other etiologies such as trauma (also known as SCIWORA) or systemic lupus erythematosus have also been described (Kovacs et al., 2000). Nevertheless, other diagnosis explanations are possible.
Furthermore, as far as we know, the case we have presented is the first case with this particular immune-phenotype (anti-GD2 IgM and anti-GD3 IgM) related to myelitis. Anti-GD2 and anti-GD3 are antibodies against gangliosides (D refers to disialic gangliosides) which are expressed in peripheral nerves and central nervous system, particularly in the cerebellum. This type of anti-gangliosides is usually seen in GBS and Chronic Inflammatory Demyelinating Polyneuropathy (CIDP). Nevertheless, anti-GM1 (another anti-ganglioside) has been found in cases of para-infectious myelitis and ADEM after Campylobacter jejuni enteritis (Llamas et al., 2018; Gaig et al., 2005). It may be also possible to find an anti-GD2/GD3 IgM in an acute myelitis context. In any case, this result could be due to a cross-reaction against other glycoproteins or sphyngolipids present in the central nervous system. On the contrary, we have found a meningeal enhancement in a follow-up MRI. Therefore, a radicular subclinical involvement is also possible. Another case report of para-infectious Covid-19 myelitis and Guillain-Barré syndrome has found an anti-GD1b IgM (Masuccio et al., 2020).
In our opinion, the presence of anti-MOG (Zhou et al., 2019), anti-GD1/GD2/GD3 antibodies (Masuccio et al., 2020), or anti-GM1 (Moriguchi et al., 2020; Kim et al., 2017) proves that an erroneous humoral response can damage structural peptides of the central or peripheral nervous system by auto-antibodies in some patients.
This is the first specific review of para-infectious myelitis related to Covid-19 infection. Several reviews discussing the neurological complications of Covid-19 have been recently published; however, myelitis seems to be overlooked (Gklinos et al., 2020). Maybe due to the low incidence, no myelitis was reported in the first neuro-covid registry with 841 Covid-19 cases (Romero-Sánchez et al., 2020). In another study (Frontera et al., 2020) with 12,990 Covid-19 patients, no coexistence of any meningitis, encephalitis, myelitis, or myelopathy was found. Nevertheless, the latency between the beginning of the myelitis and the Covid-19 infection or the respiratory asymptomatic cases could lead to a misdiagnosis of the entity.
The symptoms onset latency, the development of anti-SARS-CoV-2 IgG, and the lymphocytosis in CSF suggest a para-infectious or dysimmune mechanism. In 1 case (Zhou et al., 2019) a positive anti-MOG antibody was observed, a fact that is known to occur in response to infections (Vieira et al., 2017; Nakamura et al., 2017; Nakamura Y Nakajima et al., 2017). Secondly, direct viral invasion of the spinal cord has not been proven since PCR, despite being a non-validated technique in CSF, has been repeatedly negative in all documented cases of myelitis. Moreover, positive CSF-PCR has only been observed in 2 encephalitis cases (Virhammar et al., 2020; Moriguchi et al., 2020).
Some authors have speculated that severe Covid-19 and neurological complications as myelitis (Sotoca et al., 2020; Baghbanian et al., 2020; Chakraborty et al., 2020) are due to a “cytokine storm” and they share pathophysiological mechanisms with severe Covid-19. Additionally, the cytokine storm could also trigger an indirect immune response that affects the central nervous system (CNS) (Kim et al., 2017). This mechanism may explain the cases of myelitis that died (Chakraborty et al., 2020; Abdelhady et al., 2020). Precisely in these cases, latency in the development of myelitis and the onset of respiratory symptoms was shorter and multisystemic involvement suggests severe Covid-19 and, therefore, common mechanisms. However, recent evidence (Leisman et al., 2020) found that the degree of cytokinaemia is markedly less than in other disorders associated with elevated cytokines (sepsis, cytokine release syndrome, acute respiratory distress syndrome unrelated to Covid-19) in which neurological involvement is scarce. Moreover, the cytokine storm theory could not explain the presence of myelitis or other immune-mediated neurological syndromes in asymptomatic or mild Covid-19 cases, as it can be observed in our review.
Associations have been found with other neurological problems such as encephalitis, optic neuritis, and GBS syndrome (Zhou et al., 2019; Zoghi et al., 2020; Novi et al., 2020; Maideniuc et al., 2020). Demyelination of the CNS or peripheral nervous system can occur simultaneously or sequentially (Thomas et al., 1987). All these syndromes have a similar para-infectious and immune-mediated genesis and auto-antibodies are common. In fact, the myelitis itself can be considered within the spectrum of manifestations of ADEM. Another classic example of this immune-mediated central and peripheral involvement would be the Bickerstaff brainstem encephalitis-Miller-Fisher syndrome-GBS spectrum.
Our review allows us to recognize that Covid19 para-infectious myelitis can appear from asymptomatic to severe Covid-19 patients. The degree of spinal involvement was highly variable ranging from ad-integrum improvements (Chow et al., 2020) and little radiological involvement (Novi et al., 2020; Zachariadis et al., 2020) to severe clinical forms and almost complete involvement of the spinal cord (Sotoca et al., 2020; Kaur et al., 2020; Sarma et al., 2020; AlKetbi et al., 2020). However, the most common phenotype was LETM. The myelitis symptoms were not specific since they ranged from flaccid, para and tetraplegia to partial affectations of the spinal cord with hemisensitive symptoms or posterior cord symptoms as can be seen in our case. The myelitis was radiologically characterized by a T2 weighted hyperintense lesion with variable degrees of patchy Gadolinium enhancement. Meningeal or radicular enhancement was not documented in any case in acute phase, unlike infectious myelitis caused by herpes simplex viru, Epstein-Barr virus, varicella zoster virus, Mycobacterium tuberculosis, Lyme disease, and parasitic diseases. Meningeal contrast uptake was seen in a follow-up MRI performed in our case without a negative clinical meaning.
The non-specific characteristic of the Covid-19 related myelitis makes the diagnosis challenging, being mandatory to include a wide range of differential diagnosis including other causes of infectious, inflammatory and paraneoplastic myelitis. In the case of Covid-19, epidemiological suspicion in the context of an outbreak of the disease, the presence of a dry cough, fever, headache, anosmia, ageusia, and bilateral pneumonia with interstitial involvement must raise the suspicion. However, other etiologic agents can give similar symptoms: para-infectious mycoplasma pneumoniae myelitis can be preceded by different forms of respiratory infection and pneumonia. Moreover, prodromes such as fever and previous general malaise are not uncommon in NMO spectrum disorder (Chitnis et al., 2016).
The treatments used can be divided in two categories: treatments addressed to the cause (antibiotics and antivirals) and immunological treatments aimed at reducing inflammation and the pathological immune response that causes myelitis. Some of these treatments were used until the para-infectious etiology was clarified and others were used in the context of the first wave of Covid-19. However, later efficacy was not proven for treatments with lopinavir/ritonavir (Cao et al., 2020) and hydroxychloroquine (Beigel et al., 2020) or the efficacy found was slight, as in the case of remdensivir (Scott et al., 2011). The immunological treatments used were corticosteroids, immunoglobulins and PLEX, as well as rituximab in very severe cases. There are no clinical trials that confirm the usefulness of these treatments, however, once the infectious origin has been excluded, treatment with corticosteroids should be started as soon as possible (methylprednisolone 1 g for 3 to 7 days) and PLEX is indicated if corticosteroid treatment fails (Scott et al., 2011). The response to these treatments is variable and sequelae are expected. Given the recent case reports, long-term follow-up data are not available. Many patients, due to the preference for LETM, variable degree of motor, sphincter, or sensitive sequelae, will have to undergo rehabilitation and receive adequate symptomatic treatments.
This review has some limitations: it is based on recently published clinical cases, some data may be missing, and the extrapolations that we have done may be incomplete. Although the patients have been exhaustively studied in most cases, there is no evidence that anti-NMO or anti-MOG determination has been determined in all cases and anti-GFAP was performed only in some patients. Secondly, it may exist a publication bias, in which the publication of more striking or serious cases is favored. In this sense, we think that prospective records are necessary to confirm our conclusions. Finally, most cases show only hospitalization data and subsequent follow-ups have been scarce, given the time elapsed from the start of the pandemic. We consider that the follow-up of these cases is important because some of them may later progress to multiple sclerosis or NMO (Chan et al., 2006; Smith et al., 2020).
5. Conclusion
We have described a case of para-infectious myelitis with anti-GD2/GD3 IgM related to Covid-19 asymptomatic infection. Myelitis in the context of Covid-19 infection is a rare but a serious complication. Myelitis can affect from asymptomatic to severe cases of Covid-19, making the diagnosis challenging. Although the pathophysiology is unknown, we hypothesized an immuno-mediated mechanism, possibly by auto-antibodies.
CRediT authorship contribution statement
Luis Alberto Rodríguez de Antonio: Conceptualization, Methodology, Formal analysis, Writing - review & editing. Inés González-Suárez: Data curation, Writing - review & editing. Inés Fernández-Barriuso: Data curation, Writing - review & editing. María Rabasa Pérez: Data curation, Writing - review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest in this article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2021.102783.
Appendix. Supplementary materials
References
- Águila-Gordo D, Flores-Barragán JM, Ferragut-Lloret F, Portela-Gutierrez J, et al. Acute myelitis and SARS-CoV-2 infection. A new etiology of myelitis? J Clin Neurosci. 2020;80:280–281. doi: 10.1016/j.jocn.2020.07.074. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhady M, Elsotouhy A, Vattoth S. Acute Flaccid Myelitis in COVID-19. BJR Case Rep. 2020;6 doi: 10.1259/bjrcr.20200098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlKetbi R, AlNuaimi D, AlMulla M, AlTalai N, et al. Acute myelitis as a neurological complication of Covid-19: A case report and MRI findings. Radiol Case Rep. 2020;15(9):1591–1595. doi: 10.1016/j.radcr.2020.06.001. Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghbanian SM, Namazi F. Post COVID-19 longitudinally extensive transverse myelitis (LETM)-a case report. Acta Neurol Belg. 2020:1–2. doi: 10.1007/s13760-020-01497-x. Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beh SC, Greenberg BM, Frohman T, Frohman EM. Transverse myelitis. Neurol Clin. 2013;31(1):79–138. doi: 10.1016/j.ncl.2012.09.008. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY Collaborative Group Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022926. Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Wang Y, Wen D, Liu W, et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty U, Chandra A, Ray AK, Biswas P. COVID-19-associated acute transverse myelitis: a rare entity. BMJ Case Rep. 2020;13(8) doi: 10.1136/bcr-2020-238668. Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Tsang KL, Fong GC, Ho SL, et al. Idiopathic inflammatory demyelinating disorders after acute transverse myelitis. Eur J Neurol. 2006;13(8):862–868. doi: 10.1111/j.1468-1331.2006.01376.x. Aug. [DOI] [PubMed] [Google Scholar]
- Chitnis T, Ness J, Krupp L, Waubant E, et al. Clinical features of neuromyelitis optica in children: US Network of Pediatric MS Centers report. Neurology. 2016;86(3):245–252. doi: 10.1212/WNL.0000000000002283. Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CCN, Magnussen J, Ip J, Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. 2020;13(8) doi: 10.1136/bcr-2020-236720. Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera JA, Sabadia S, Lalchan R, Fang T, et al. A Prospective Study of Neurologic Disorders in Hospitalized COVID-19 Patients. Neurology. 2020 Oct 5. [Google Scholar]
- Gaig C, Valldeoriola F, Saiz A. Acute disseminated encephalomyelitis associated with Campylobacter jejuni infection and antiganglioside GM1 IgG antibodies. J Neurol. 2005;252(5):613–614. doi: 10.1007/s00415-005-0701-7. May. [DOI] [PubMed] [Google Scholar]
- Gklinos P. Neurological manifestations of COVID-19: a review of what we know so far. J Neurol. 2020;267(9):2485–2489. doi: 10.1007/s00415-020-09939-5. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729. Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Mason JA, Bajracharya M, McGee J, et al. Transverse Myelitis in a Child With COVID-19. Pediatr Neurol. 2020;112:5–6. doi: 10.1016/j.pediatrneurol.2020.07.017. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Heo JH, Kim HO, Song SH, et al. Neurological Complications during Treatment of Middle East Respiratory Syndrome. J Clin Neurol. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs B, LaVerty T, Brent L, et al. Transverse myelopathy in systemic lupus erythematosus: an analysis of 14 cases and review of the literature. Ann Rheum Dis. 2000;59:120–124. doi: 10.1136/ard.59.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisman DE, Ronner L, Pinotti R, Taylor M, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;S2213-2600(20):30404–30405. doi: 10.1016/S2213-2600(20)30404-5. Oct 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamas Y, Hazel K, Nicholson P, Share Costelloe L. Longitudinally extensive transverse myelitis after Campylobacter jejuni enteritis. Pract Neurol. 2018;18(2):143–145. doi: 10.1136/practneurol-2017-001777. Apr. [DOI] [PubMed] [Google Scholar]
- Maideniuc C, Memon AB. Acute necrotizing myelitis and acute motor axonal neuropathy in a COVID-19 patient. J Neurol. 2020 doi: 10.1007/s00415-020-10145-6. Aug 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuccio FG, Barra M, Claudio G, Claudio S. A rare case of acute motor axonal neuropathy and myelitis related to SARS-CoV-2 infection. J Neurol. 2020:1–4. doi: 10.1007/s00415-020-10219-5. Sep 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Harii N, Goto J, Harada D, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz M, Wessendorf S, Koretsis G, Tewald F, et al. Acute transverse myelitis after COVID-19 pneumonia. J Neurol. 2020;267(8):2196–2197. doi: 10.1007/s00415-020-09934-w. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Iwasaki Y, Takahashi T, Kaneko K, et al. A case of MOG antibodypositive bilateral optic neuritis and meningoganglionitis following a genital herpes simplex virus infection. Mult Scler Relat Disord. 2017;17:148–150. doi: 10.1016/j.msard.2017.07.023. [DOI] [PubMed] [Google Scholar]
- Nakamura Y Nakajima H, Tani H, Hosokawa T, et al. Anti-MOG antibody-positive ADEM following infectious mononucleosis due to a primary EBV infection: a case report. BMC Neurol. 2017;17:76. doi: 10.1186/s12883-017-0858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novi G, Rossi T, Pedemonte E, Saitta L, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(5) doi: 10.1212/NXI.0000000000000797. Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020;95(8) doi: 10.1212/WNL.0000000000009937. Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma D, Bilello LA. A Case Report of Acute Transverse Myelitis Following Novel Coronavirus Infection. Clin Pract Cases Emerg Med. 2020;4(3):321–323. doi: 10.5811/cpcem.2020.5.47937. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TF, Frohman EM, De Seze J, Gronseth GS, et al. Evidence-based guideline: clinical evaluation and treatment of transverse myelitis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;77(24):2128–2134. doi: 10.1212/WNL.0b013e31823dc535. Dec 13. [DOI] [PubMed] [Google Scholar]
- Shaw VC, Chander G, Puttanna A. Neuromyelitis optica spectrum disorder secondary to COVID-19. Br J Hosp Med (Lond) 2020;81(9):1–3. doi: 10.12968/hmed.2020.0401. Sep 2. [DOI] [PubMed] [Google Scholar]
- Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transpl. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Jaakonmäki N, Nylund M, Kupila L, et al. Frequency and etiology of acute transverse myelitis in Southern Finland. Mult Scler Relat Disord. 2020;46 doi: 10.1016/j.msard.2020.102562. Oct 7. [DOI] [PubMed] [Google Scholar]
- Sotoca J, Rodríguez-Álvarez Y. COVID-19-associated acute necrotizing myelitis. Neurol Neuroimmunol Neuroinflamm. 2020;7(5) doi: 10.1212/NXI.0000000000000803. Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PK, Walker RW, Rudge P, Morgan-Hughes JA, et al. Chronic demyelinating peripheral neuropathy associated with multifocal central nervous system demyelination. Brain. 1987;110(Pt 1):53–76. doi: 10.1093/brain/110.1.53. [DOI] [PubMed] [Google Scholar]
- Transverse Myelitis Consortium group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499–505. doi: 10.1212/wnl.59.4.499. [DOI] [PubMed] [Google Scholar]
- Valiuddin H, Skwirsk B, Paz-Arabo P. Acute transverse myelitis associated with SARS-CoV-2: A Case-Report. Brain Behav Immun Health. 2020;5 doi: 10.1016/j.bbih.2020.100091. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J, Sequeira J, Brito M. Postinfectious anti-myelin oligodendrocyte glycoprotein antibody positive optic neuritis and myelitis. J Child Neurol. 2017;32:996–999. doi: 10.1177/0883073817724927. [DOI] [PubMed] [Google Scholar]
- Virhammar J, Kumlien E, Fällmar D, Frithiof R, et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020;95(10):445–449. doi: 10.1212/WNL.0000000000010250. Sep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariadis A, Tulbu A, Strambo D, Dumoulin A, et al. Transverse myelitis related to COVID-19 infection. J Neurol. 2020 doi: 10.1007/s00415-020-09997-9. Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Huang J, Dai D, Feng Y, et al. Acute myelitis after SARS-cov-2 infection: a case report. Medrxiv. https://www.medrxiv.org/content/10.1101/2020.03.16.20035105v1.full.pdf.
- Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ et al. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Optic Neuritis and Myelitis in COVID-19. J Neuroophthalmol 40:3 (398-402). [DOI] [PMC free article] [PubMed]
- Zoghi A, Ramezani M, Roozbeh M, Darazam IA, et al. A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult Scler Relat Disord. 2020;24(44) doi: 10.1016/j.msard.2020.102324. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.