Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19) has infected 86,4 M patients and resulted in 1,86 M deaths worldwide. Severe COVID-19 patients have elevated blood levels of interleukin-6 (IL-6), IL-1β, tumor necrosis factor (TNF)α, IL-8 and interferon (IFN)γ.
Objective
To investigate the effect of antiviral treatment serum cytokines in severe COVID-19 patients.
Methods
Blood was obtained from 29 patients (aged 32–79 yr) with laboratory-confirmed COVID-19 upon admission and 7 days after antiviral (Favipiravir or Lopinavir/Ritonavir) treatment. Patients also received standard supportive treatment in this retrospective observational study. Chest computed tomography (CT) scans were evaluated to investigate lung manifestations of COVID-19. Serum was also obtained and cytokines levels were evaluated. 19 age- and gender-matched healthy controls were studied.
Results
Anti-viral therapy significantly reduced CT scan scores and the elevated serum levels of C-reactive protein (CRP) and lactate dehydrogenase (LDH). In contrast, serum levels of IL-6, IL-8 and IFNγ were elevated at baseline in COVID-19 subjects compared to healthy subjects with IL-6 (p = 0.006) and IL-8 (p = 0.011) levels being further elevated after antiviral therapy. IL-1β (p = 0.01) and TNFα (p = 0.069) levels were also enhanced after treatment but baseline levels were similar to those of healthy controls. These changes occurred irrespective of whether patients were admitted to the intensive care unit.
Conclusion
Antiviral treatments did not suppress the inflammatory phase of COVID-19 after 7 days treatment although CT, CRP and LDH suggest a decline in lung inflammation. There was limited evidence for a viral-mediated cytokine storm in these COVID-19 subjects.
Keywords: COVID-19, SARS-CoV-2, Cytokines storm, Flow cytometry
1. Introduction
In December 2019, an outbreak of severe acute respiratory syndrome coronavirus 2 was first reported in Wuhan, Hubei Province, China [1]. This novel coronavirus was announced a pandemic by the World Health Organization (WHO) on March 11th, 2020 This disease, named coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO), manifests clinically with fever, cough, muscle pain, fatigue, loss of taste and smell, diarrhea and pneumonia and results in death in susceptible subjects [2]. Approximately 20–30% of affected people may develop the severe form of disease and require further intervention in intensive care [3]. A high viral load and an intensified host immune response including both innate and acquired immunity contribute to COVID-19 pathogenesis in severe cases leading to organ damage and failure [4], [5].
The hyperactive host immunity in SARS-CoV-2 infection is characterized by lymphopenia, cytokine release storm (CRS), intracellular levels of nitric oxide (NO) in red blood cells and dysfunctional immune responses to virus-specific antigens [6]. Several studies have indicated that increased levels of serum proinflammatory cytokines were associated with pulmonary inflammation and lung and organ failure in COVID-19 disease [7]. Various mechanisms appear to be effective in reducing the number of lymphocytes in the disease, the most important being increased levels of IL-6, TNF-α and other cytokines [8], [9].
An effective immune response against viral infections occurs when cytotoxic T cells are properly activated to clear the virus and virus-infected cells and increasing the numbers of T cells and their function is essential for the recovery of COVID-19 patients [8]. IL-6, TNF-α and IL-1β have been implicated as key factors in the COVID-19 cytokine storm [1], [9] and in severe COVID-19 patients may lead to multi-organ failure or acute respiratory distress syndrome (ARDS) [3]. As such, many therapeutic strategies involve lowering cytokine levels [10], [11]. We hypothesized that anti-viral agents would reduce the cytokine storm and improve the clinical indices of patients with severe COVID-19.
2. Materials and methods
2.1. Study design
Confirmed COVID-19 patients (n = 29; aged 32–79) were enrolled into the study following admission to the Masih Daneshvari Hospital of Shahid Beheshti Medical University, Tehran between 10th April-9th May 2020. Serum samples were collected from all COVID-19 patients upon admission to Hospital and from 19 age- and gender-matched healthy volunteers were served as controls. Subjects were not infected with other pathogenic microorganisms such as hepatitis B (HBV), hepatitis C (HCV) and (human immunodeficiency virus) HIV. Demographic, clinical, treatment history and laboratory data were extracted from electronic medical records.
Severe COVID-19 disease was confirmed by the presence of at least one of the following: respiratory rate ≥30/min; blood oxygen saturation ≤93%; ratio of partial pressure of oxygen in arterial blood to the inspired oxygen fraction (PaO2/FiO2) < 300; lung infiltrates present in >50% of lung fields [12]. All patients provided written informed consent. This study was approved by the institutional ethics board of Masih Daneshvari Hospital of Shahid Beheshti Medical University (R.SBMU.NRITLD.REC.1399.123).
2.2. Laboratory examination of blood samples
15/29 COVID-19 patients were transferred to the intensive care unit (ICU) because they required high-flow nasal cannula or higher-level oxygen support measures to resolve their hypoxemia. Blood (3.5mls in tubes without anticoagulants) was obtained at baseline and 7 days after initiation of antiviral treatment. Serum samples were separated by centrifugation at 2000 rpm /20 min. Blood cell counts, coagulation profiles, serum biochemical tests (including kidney and liver function tests including creatine kinase (CK), lactate dehydrogenase (LDH), electrolytes, myocardial enzymes, ferritin and procalcitonin (PCT) levels) were obtained.
Serum cytokines were measured using ELISA kits for IL-1β (R&D Systems, Minneapolis, MN, USA), TNFα (R&D Systems), IL-8 (BD Biosciences, CA, USA), IFNγ (Thermo Fisher, Waltham, Massachusetts, United States), IL-6 (R&D Systems) according to the following Manufactures’ instructions. Specificity and cross reactivity for IL-8 was ≥10 ng/mL with no cross-reactivity seen (≥2pg/mL). IFN, IL-β, IL-6 (all at 50 ng/mL) exhibited no cross-reactivity.
2.3. Treatment protocol
All patients were under supportive care consisting of intravenous fluid and supplemental oxygen. As the recommendation of the Iranian national guideline of COVID-19 management at the time of the study [28], all patients received Favipiravir (600 mg three times a day) or lopinavir/ritonavir (Kaletra, 400/100 mg bid) for seven days. Dexamethasone was prescribed for one subject who developed moderate to severe ARDS.
All patients underwent a chest computed axial tomography (CT) scan on admission and 7 days after treatment. All chest CT scans were evaluated in a blinded fashion by one of the authors (PM). For comparing lung involvement in CT scans before and after treatment we used a semi-quantitative scoring system taking into account the most prevalent patterns of COVID-19 lung disease in CT scan (consolidation, ground glass opacity and crazy paving pattern) was used [13], [14], [15]. According to the anatomic structure in each patient right and left lungs were divided into the 5 lobes: Ieft upper lope (LUL), left lower lobe (LLL), right upper lobe (RVL), right middle lobe (RML) and right lower lobe (RLL) and each lobe was assigned a score: Score 0, 0% involvement, score 1, <5% involvement, score 2, 5% to <25% involvement, score 3, 25% to <50% involvement, score 4, 50% to <75% involvement and score 5, 75% or greater involvement. The total CT score was evaluated from all 5 lobes and sum plotted for each patient before and after treatment.
2.4. Statistical analysis
Analysis was performed using SPSS version 16.0 (SPSS, Inc. Chicago, USA) and GraphPad Prism software (version 6; 07 Graph Pad Software, Inc.). Non-parametric Mann-Whitney U test (Median, 95% confidence intervals (CI) was used for the variables without normal distribution. Parametric t-student test (mean ± SD) was used for the variables with normal distribution. Statistical Software p-values < 0.05 were considered as significant.
3. Results
3.1. Demographic and clinical characteristics of patients with COVID-19 and healthy control
Demographic and clinical characteristics of the all participants are shown in Table 1 . The mean age of the COVID-19 patients was similar (54.2 ± 2.53 years) to that of the healthy control population (53.6 ± 1.71 years). The groups were also matched for gender although COVID-19 patients had a greater propensity for co-morbid conditions including hypertension, diabetes and cardiovascular disease. The serum levels of LDH, ESR, CRP, CPK and troponin in COVID-19 patients before antiviral therapy on admission were 570.2 ± 54.6, 42.6 ± 5.2, 30.4 ± 5.1, 154 ± 29.6 and 0.02 ± 0.0 respectively (mean ± SD). The serum levels of LDH, ESR, CRP, CPK and troponin in COVID-19 patients after antiviral therapy were 382 ± 192, 33.8 ± 23.7, 19.8 ± 17.2, 107 ± 97 and 0.02 ± 0.0, respectively. The serum levels of LDH (p < 0.0001), ESR (p < 0.0001), CRP (p < 0.0023) and CPK (p < 0.0153), but not troponin (p = 0.9), were significantly reduced after antiviral therapy compared to before treatment (Table 1).
Table 1.
Demographic data of the study population and biochemical characteristics of serum components on admission to hospital.
| HC (n = 19) | Patients (BT) (n = 29) | ap value | Patients (AT) (n = 29) | p value (BT vs AT) | |
|---|---|---|---|---|---|
| Age, (yrs, mean ± SD) (range) | 53.6 ± 1.705 (40–64) | 54.45 ± 2.536 (32–79) | 0.8025 | – | – |
| Gender | |||||
| Female (n, mean ± SD) (age-range) | 11 (52.55 ± 2.44) (40–64) | 12 (53.73 ± 4.65) (32–79) | 0.9295 | – | – |
| Male (n, mean ± SD) (age-range) | 8 (54.89 ± 2.43) (40–63) | 17 (54.94 ± 3.21) (34–77) | 0.9196 | – | – |
| Comorbidities | |||||
| Hypertension | 4 | – | – | ||
| Chronic kidney disease | – | – | – | ||
| COPD | 3 | – | – | ||
| Cardiovascular disease | 6 | – | – | ||
| Diabetes | 8 | – | – | ||
| Malignancy | 1 | – | – | ||
| ARDS | – | – | – | ||
| ESR (mm/h, mean ± SD) | 5.6 ± 3.12 (1–11) | 42.55 ± 5.15 (5–100) | <0.0001**** | 33.76 ± 23.71 (4–89) | <0.0001**** |
| C-Reactive protein (mg/l, mean ± SD) | 10.6 ± 3.8 (6.8–19) | 30.41 ± 5.08 (0–107) | <0.0001**** | 19.79 ± 17.16 (0–60) | 0.0023** |
| Lactate Dehydrogenase (U/L, mean ± SD) | 307 ± 49.37 (222–378) | 570.2 ± 54.58 (231–1535) | <0.0001**** | 382 ± 192.6 (134–870) | <0.0001**** |
| Troponin (pg/ml, mean ± SD) | 0.02 ± 0.0 (0.02–0.02) | 0.02 ± 0.0 (0.02–0.02) | 0.9 | 0.02 ± 0.0 (0.02–0.02) | 0.9 |
| CPK (U/L, mean ± SD) | – | 154 ± 29.63 (5–671) | – | 107 ± 96.77 (5–450) | 0.0153* |
Abbreviations: ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; ESR, erythrocyte sedimentation rate, HC, Healthy control, CPK, Creatine phosphokinase, BT, before treatment, AT, after treatment.
p values indicate differences between healthy and COVID-19 patients. p < 0.05 was considered statistically significant.
CT scores improved with treatment (p < 0.05) irrespective of anti-viral therapy used or whether patients were in ICU or not. Further details are provided in the on-line supplement.
3.2. Serum cytokine levels at baseline and after anti-viral therapy
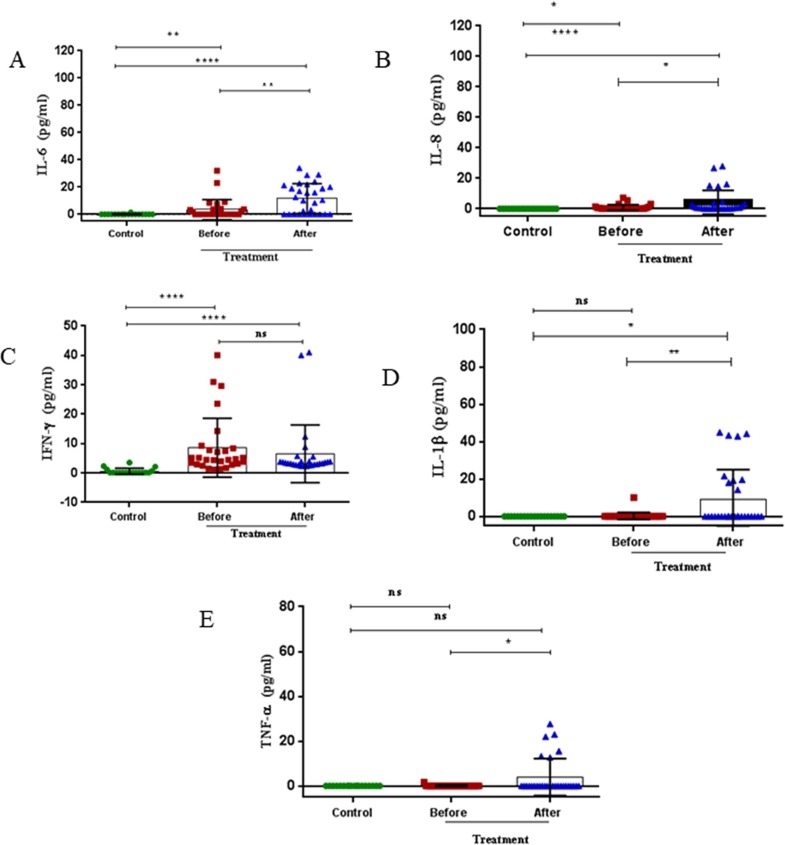
Serum levels of IL-6 were elevated at baseline in COVID-19 subjects [0.1 (0.1–27.5) pg/ml, p = 0.006] compared to healthy subjects [0.1 (0.1–1.46) pg/ml] with levels being further elevated after antiviral therapy [10.44 (0.1–31.55) pg/ml, p < 0.0001] (Fig. 1 A, Table 2 ). Serum IL-6 levels were significantly elevated after anti-viral therapy compared with levels before therapy (p < 0.01) (Fig. 1A, Table 2).
Fig. 1.
Serum levels of cytokines at baseline in healthy and COVID-19 patients and following 7 days of anti-viral therapy in COVID-19 subjects. Serum levels of IL-6 (A), IL-8 (B), IFN-γ (C), IL-1β (D) and TNFα (E) were measured in healthy subjects (n = 19) and COVID-19 patients (n = 29) before and after antiviral therapy. Results are presented as dot blots of individual values for each subject with the median (5–95% percentiles). *p < 0.05, **p < 0.01, ***p < 0.001 and **** p < 0.0001.
Table 2.
Serum cytokine concentrations.
| Cytokine (pg/ml) | HC (n = 18) | BT (n = 29) | AT (n = 29) | p value HC vs. BT† | p value (HC vs. AT)† | p value (BT vs. AT)† |
|---|---|---|---|---|---|---|
| IL-6 | 0.1 (0.1–1.46) | 0.1 (0.1–27.5) | 10.44 (0.1–31.55) | 0.0067** | <0.0001**** | 0.0041** |
| IL-8 | 0.1 (0.1– 0.1) | 0.2 (0.015–6.4) | 0.6 (0.052–27.42) | 0.0114* | <0.0001**** | 0.0228* |
| IFN-γ | 0.1 (0.1–3.52) | 4.62 (1.2–35.98) | 3.41 (2.31–40.58) | <0.0001**** | <0.0001**** | 0.123 |
| IL-1β | 0.1 (0.1–0.1) | 0.1 (0.1–5.15) | 0.3 (0.1–44.6) | 0.99 | 0.0104* | 0.0019** |
| TNF-α | 0.1 (0.1–0.1) | 0.1 (0.1–0.888) | 0.1 (0.1–25.36) | 0.9 | 0.0691 | 0.0235* |
Values are presented as the median and 95% CI unless otherwise indicated.
Comparisons between the groups were performed using a Mann-Whitney U test.
The levels of serum IL-8 were also elevated at baseline in patients [0.2 (0.015–6.4) pg/ml] compared to healthy controls [0.1 (0.1–0.1) pg/ml, p = 0.011] and were further raised after 7 days of anti-viral therapy [0.6 (0.052–27.42) pg/ml, p < 0.0001] (Fig. 1B, Table 2). Serum IL-8 levels were significantly elevated after anti-viral therapy compared with levels before therapy (p < 0.05) (Fig. 1B, Table 2).
Furthermore, serum levels of IFN-γ were elevated at baseline in COVID-19 subjects [4.62 (1.2–35.98) pg/ml] compared to healthy subjects [0.1 (0.1–3.52) pg/ml, p < 0.001] with levels not being enhanced after antiviral therapy [3.41 (2.31–40.58) pg/ml] (Fig. 1C, Table 2).
In contrast, serum levels of IL-1β were not elevated at baseline in COVID-19 subjects [0.1)0.1–5.15) pg/ml] compared to healthy subjects [0.1 (0.1–0.19) pg/ml, p = 0.9]. However the levels of IL-1β were elevated after 7 days of antiviral therapy in COVID-19 patients [0.3 (0.1–44.64) pg/ml, p = 0.0019) (Fig. 1D, Table 2).
Serum levels of TNF-α was similar at baseline in COVID-19 subjects [0.1 (0.1–0.89) pg/ml] compared to healthy subjects [0.1 (0.1–0.1) pg/ml, p = 0.9) but elevated after antiviral therapy [0.1 (0.1–25.36) pg/ml, p = 0.0235) (Fig. 1E, Table 2).
3.3. Effect of ICU treatment on serum cytokine levels
Since some patients progressed to ICU whilst others did not, we assessed whether there were differences at baseline and after therapy according to ICU status. There was no significant difference in the baseline serum IL-6 level before and after therapy in non-ICU patients (Table 3 ). However, in ICU patients serum IL-6 levels were significantly elevated between baseline [0.1 (0.1–32) pg/ml] and after treatment [17 (1–34) pg/ml, p < 0.05) (Table 3).
Table 3.
Comparison of pre- and post-treatment cytokine levels according to ICU status.
| Cytokine (pg/ml) |
ICU |
p value |
non-ICU |
p value | p value BT (ICU-non-ICU) | p value AT (ICU-non-ICU) | ||
|---|---|---|---|---|---|---|---|---|
| BT | AT | BT | AT | |||||
| IL-6 | 0.1 (0.1–32) | 17 (1–34) | 0.0165* | 0.1 (0.1–7.7) | 1.68 (0.1–29.1) | 0.1459 | 0.4092 | 0.14 |
| IL-8 | 0.3 (0.01–7.2) | 1.56 (0.07–28) | 0.19 | 0.1 (0.02–1.5) | 0.45 (0.03–3.6) | 0.021* | 0.0617 | 0.13 |
| IFN-γ | 5.2 (1.5–30.98) | 3.89 (2.7–41) | 0.4671 | 4.52 (1.2–40.7) | 2.9 (2.3–12.36) | 0.1353 | 0.46 | 0.003*** |
| IL-1β | 0.1 (0.1–10.2) | 0.1 (0.1–45) | 0.0063** | 0.1 (0.1–0.1) | 0.1 (0.1–18.13) | 0.4815 | >0.99 | 0.02* |
| TNF-α | 0.1 (0.1–1.67) | 0.1 (0.1–27.71) | 0.0421* | 0.1 (0.1–0.1) | 0.1 (0.1–12.75) | >0.99 | >0.99 | 0.0738 |
Values are presented as the median (5–95% percentile).
†p value < 0.05 was determined by Mann-Whitney U test.
AT: After treatment.
BT: Before treatment.
Similarly, there were no differences between serum IL-8 levels in ICU patients and non-ICU patients at baseline and after anti-viral therapy (Table 3). In addition, anti-viral therapy did not significantly affect serum IL-8 levels in patients in ICU but there was a small but significant increase in serum IL-8 levels in non-ICU subjects [0.1 (0.02–1.5) vs 0.45 (0.03–3.6) pg/ml, p < 0.05] (Table 3).
Serum IFNγ concentrations did not differ at baseline between patients in ICU or not in ICU and anti-viral therapy had no effect on serum levels of IFNγ (Table 3). However, comparison of serum IFNγ levels after 7 days of anti-viral therapy showed a significantly reduced level in non-ICU subjects [2.9 (2.3–12.36) pg/ml] compared to those in ICU subjects [3.89 (2.7–41) pg/ml, p = 0.003) ] (Table 3).
There were no significant differences at baseline between ICU and non-ICU patients in the serum levels of IL-1β (Table 3). Anti-viral therapy significantly enhanced serum IL-1β in patients in ICU [0.1 (0.1–45) vs 0.1 (0.1–10.2) pg/ml, p = 0.0063] but not in non-ICU subjects [0.1 (0.1–18.13 vs 0.1 (0.1–0.1) pg/ml, p = 0.482] which resulted in levels being greater in ICU patients compared to non-ICU patients after treatment (p < 0.05) (Table 3).
Serum TNFα levels before therapy were similar between subjects in ICU and those not in ICU (Table 3). Anti-viral therapy resulted in a small, but significant increase in serum TNFα levels in ICU patients [0.1 (0.1–27.71) vs. 0.1 (0.1–1.67) pg/ml, p < 0.05] but not in non-ICU subjects [0.1 (0.1–12.75) vs. 0.1 (0.1–0.1) pg/ml, p > 0.99] (Table 3).
3.4. Antiviral treatment on serum cytokine levels
We evaluated the effect of the two different anti-viral therapies on serum cytokine levels. Cytokine levels varied considerably between individual patients but there were no significant differences in baseline levels of any cytokine measured between ICU or non-ICU subjects on Kaletra or Favipirivir treatment (Table 4A and Table 4B ). In non-ICU patients, Kaletra treatment resulted in a significant decrease in serum IFN-γ levels (2.59 (2.3–3.83) versus 7.46 (2.48–40.07) pg/ml, p = 0.007) with no effect on the levels of the other cytokines measured (Table 4A and Table 4B). In contrast, Faviprivir had no significant effect on any cytokine studied. Favipriavir also had no significant effect on cytokine levels in patients treated within the ICU whereas Kaletra significantly enhanced the expression of IL-1β (19.6 (0.1–44.27) versus 0.1 (0.1–0.1) pg/ml, p = 0.021) in COVID-19 patients in the ICU (Table 4A and Table 4B).
Table 4A.
Comparison of cytokine levels according to antiviral received.
| Cytokine (Pg/ml) | non-ICU | p value | non-ICU | p value | ICU | p value | ICU | p value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Kaletra (Kal) |
Favipiravir (Fav) |
Kaletra (Kal) |
Favipiravir (Fav) |
|||||||||
| BT (n = 7) | AT (n = 7) | BT (n = 7) | AT (n = 7) | BT (n = 7) | AT (n = 7) | BT(n = 8) | AT(n = 8) | |||||
| IL-6 | 1.94 (0.1–3.67) | 15.7 (0.1–29.1) | 0.0915 | 0.1 (0.1–7.7) | 0.1 (0.1–16.0) | 0.7308 | 0.1 (0.1–32) | 19 (0.1–34.0) | 0.0862 | 3.87 (0.1–23) | 10.28 (0.1–24) | 0.1497 |
| IL-8 | 0.1 (0.02–0.3) | 0.3 (0.1–0.5) | 0.0892 | 0.1 (0.02–1.5) | 0.7 (0.03–3.6) | 0.0769 | 0.3 (0.01–7.2) | 0.7 (0.07–28.0) | 0.8048 | 0.3 (0.1–3.19) | 3.5 (0.2–26.8) | 0.06 |
| IFN-γ | 7.46 (2.48–40.1) | 2.59 (2.3–3.83) | 0.007*** | 3.01 (1.2–5.2) | 3.11 (2.7–12.36) | 0.6991 | 5.2 (1.7–30.98) | 3.9 (2.99–41.0) | 0.8741 | 4.4 (1.5–29.6) | 3.7 (2.7–8.9) | 0.2786 |
| IL-1β | 0.1 (0.1–0.1) | 0.1 (0.1–18.13) | 0.4615 | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | >0.99 | 0.1 (0.1–0.1) | 19.6 (0.1–44.27) | 0.021* | 0.1 (0.1–10.2) | 0.1 (0.1–45) | 0.4667 |
| TNF-α | 0.1 (0.1–0.1) | 0.1 (0.1–12.75) | >0.99 | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | >0.99 | 0.1 (0.1–0.1) | 0.1 (0.1–27.7) | 0.1923 | 0.1 (0.1–1.67) | 0.1 (0.1–23) | 0.467 |
Table 4B.
Associated p values for group comparisons.
| Cytokine |
p value (ICU vs non-ICU) |
p value (ICU vs non-ICU) |
p value (Kaletra vs Faviprivir, AT) | ||
|---|---|---|---|---|---|
| Kaletra (BT) | Favipiravir (BT) | Kaletra (AT) | Favipiravir (AT) | All patients | |
| IL-6 | 0.1026 | 0.0797 | 0.6818 | 0.0044** | 0.069 |
| IL-8 | 0.1515 | 0.3164 | 0.6865 | 0.1796 | 0.0444* |
| IFN-γ | 0.8759 | 0.0593 | 0.007*** | 0.2151 | 0.7773 |
| IL-1β | >0.99 | >0.99 | 0.4667 | 0.46 | 0.0722 |
| TNF-α | >0.99 | >0.99 | 0.1923 | 0.4667 | 0.4649 |
p < 0.05 was considered significant as determined by a Mann- Whitney U test.
BT: before treatment; AT: After treatment.
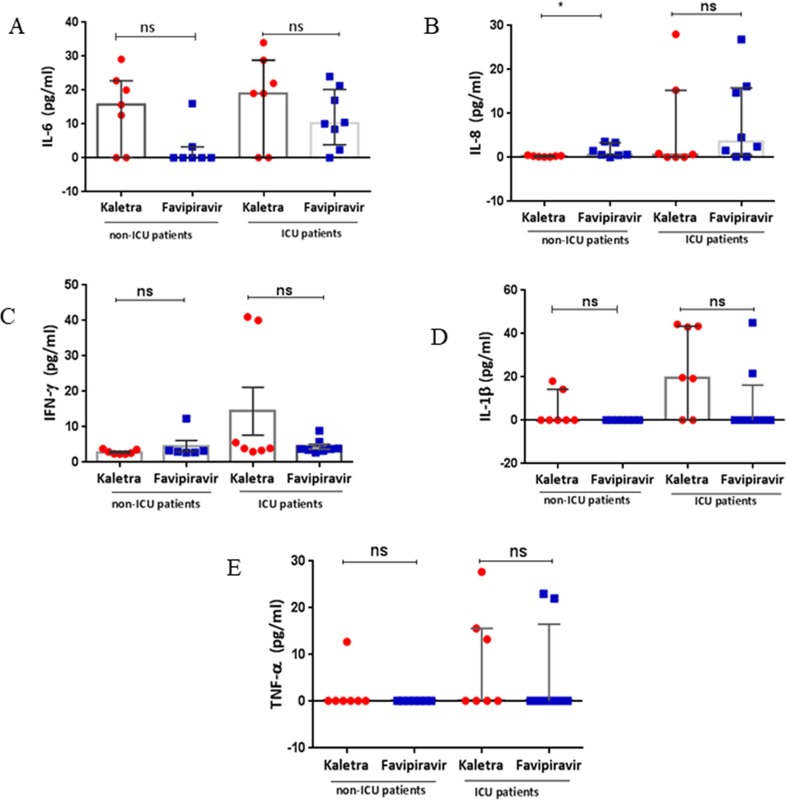
Serum IL-8 levels were significantly lower in non-ICU patients treated with Kaletra (0.3 (0.1–0.5) pg/ml) compared with those treated with Favipiravir (0.7(0.03–3.6) pg/ml) (p = 0.0444) (Table 4B. Fig. 2 B). This difference was not observed in ICU patients. There were no significant differences in post-treatment levels of IL-6 (Fig. 2A), IFN-γ (Fig. 2C), IL-1β (Fig. 2D) or TNFα (Fig. 2E) between patients treated with Kaletra or Favipirivir.
Fig. 2.
Serum levels of cytokines in patients treated with Favipiravir or Kaletra. Serum levels of IL-6 (A), IL-8 (B), IFN-γ (C), IL-1β (D) and TNFα (E) were measured in non-ICU and ICU patients after 7 days of antiviral treatment. Results are presented as dot blots of individual values for each subject with the median (5–95% percentiles) also indicated. *p < 0.05; ns, not significant.
Serum IL-6 levels were higher in Favipiravir-treated patients in ICU compared to non-ICU patients (p = 0.0044) whilst IFN-γ levels were higher in Kaltera-treated patients in the ICU compared to non-ICU patients (p = 0.007) (Table 4A and Table 4B , Fig. 2). No other effects of anti-viral therapy was seen on serum cytokine expression.
4. Discussion
Antiviral therapy resulted in improvements in lung CT scores after 7 days irrespective of whether subjects were in ICU or not. In addition, the raised blood levels of ESR, CRP, LDH and CPK seen in COVID-19 patients compared to those in healthy control subjects were also reduced by anti-viral therapy. In contrast, serum levels of IL-6, IL-8 and IFN-γ were elevated at baseline in COVID-19 subjects compared to healthy subjects with IL-6 and IL-8 levels being further elevated after antiviral therapy. IL-1β and TNFα levels were also enhanced after anti-viral treatment but baseline levels were similar to those of healthy controls. Antiviral therapy significantly raised IL-6, IL-1β and TNFα levels in ICU patients and IL-8 levels in non-ICU patients.
Two major families of IFNs exist: type 1 IFNs include IFN-α, IFN-β, and IFN-ω, which are encoded by a family of more than 20 genes and share the type 1 cellular receptor and type II IFN (IFN-γ) which is encoded by a single gene, has a separate cellular receptor, and is produced by T cells and NK cells. IFNγ triggers its antiviral actions in vivo by exerting cellular effects at multiple levels [16], [17]. Severe COVID‐19 patients have a higher IL‐6/IFNγ ratio than in patients with moderate disease, which could be related to an enhanced cytokine storm favoring lung damage. In addition, a suppressed T-cell immunity exists in patients with severe COVID-19 based on decreased T-cell numbers and abnormal IFNγ expression by T-lymphocytes [6], [18], [19]. Continuous high levels of cytokines observed in COVID-19 patients may imply a key inflammatory drive in response to infection in these subjects [20]. However, anti-IL-6 therapies have been tested in COVID-19 patients with limited success [21].
Several cytokines in addition to IL-6 and IFNγ are elevated during the proposed cytokine storm in COVID-19 patients and these contribute to tissue damage in the respiratory tract and in other organs. TNFα is important in nearly all acute inflammatory reactions, acting as an amplifier of inflammation. IL-1β is also a highly active pro-inflammatory cytokine, and monotherapy blocking IL-1β activity is used to treat inflammatory diseases including rheumatoid arthritis and inherited auto-inflammatory syndromes such as cryopyrin-associated syndromes [22]. IL-8 is a potent pro-inflammatory cytokine playing a key role in the recruitment and activation of neutrophils during inflammation, and, given the frequent neutrophilia observed in patients infected with SARS-CoV-2, it is possible that IL-8 contributes to COVID-19 pathophysiology [23].
To our knowledge, this is the first report regarding the ineffectiveness of antiviral medication on modulating cytokine release in COVID-19 patients. Thwaites and colleagues analyzed 619 hospitalized and 39 milder, non-hospitalized COVID-19 patients and reported higher levels of angiopoietin-2, CXCL10, and GM-CSF in plasma [24]. In addition, they detected mediators of endothelial cell injury in the early stages of disease and reported that inflammatory cytokines and markers of lung injury were higher in patients with a poor clinical outcome [24]. Zhou and co-workers showed that treatment with IFNα2b, in presence or absence of umifenovir, a broad-spectrum antiviral, decreased elevated blood levels of inflammatory mediators such as IL-6 and CRP [25]. We show a reduction in serum CRP, LDH, ESR and CPK, but not IL-6, in our study which may reflect a dose-dependent difference in local versus systemic effects or reflect the differences in antivirals used in our study and these others.
The lack of IFN-γ induction seen in the current study with antiviral treatment may due to the lymphopenia induction induced by COVID-19 virus but additional information regarding the impact upon blood total blood lymphocyte counts are necessary to test this hypothesis. However, the significant rise in serum IL-1β, IL-6, TNF-α and IL-8 levels observed after 7 days of antiviral therapy treatment suggests an inability of the anti-viral therapy to suppress systemic inflammation despite the improvement in CT scores and other inflammatory markers associated with COVID-19 infection. The use of corticosteroids such as dexamethasone is used as supportive treatment for COVID-19 patients and appears to be highly effective [26], [27]. The results from our study are not confounded by co-treatment with dexamethasone since only one subject was given this therapy.
Our current data suggests that antiviral therapy is able to improve lung-associated clinical features of disease as determined by CT scans as well as blood markers such as ESR, CRP, LDH and CPK irrespective of the severity of the disease. This effect was independent of an action on systemic blood cytokines that are up-regulated by viral infection. These data suggest that the systemic inflammatory drive seen in these patients is either no longer driven by an ongoing infection or that other driver mechanisms exist for the enhanced cytokine expression seen in these patients. These may reflect the host response to the initial infection that remains despite the potential absence of ongoing viral replication. A limitation of our study was that we did not obtain PCR analysis for SAR-CoV-2 presence in all patients after anti-viral therapy. Moreover, the small sample size in this study is another limitation of the study and larger studies should be conducted to confirm the results.
The local effect of therapy on the CT imaging and on serum CRP and LDH measures of COVID-19 infection without reducing serum cytokine levels supports the concept of a local virus-induced lung inflammation. Furthermore, it suggests a systemic inflammatory response that is prolonged even though local lung inflammation is decreased. In this retrospective single-centre observational study, antiviral therapy has a significant local effect in the lung but appears unable to suppress the systemic cytokine storm and control the down-stream inflammatory cascade. Further studies are needed to confirm this data.
5. Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Dr. Masih Daneshvari Hospital, and all patients gave signed informed consent.
6. Consent for publication
All authors have read the manuscript and consent to publication in the Journal.
Funding
This study was supported by internal funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2021.107407.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Wang C., et al. Cytokine levels in the body fluids of a patient with COVID-19 and acute respiratory distress syndrome: a case report. Ann. Intern. Med. 2020;173(6):499–501. doi: 10.7326/L20-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng M., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortaz E., et al. The immune response and immunopathology of COVID-19. Front. Immunol. 2020;11:2037. doi: 10.3389/fimmu.2020.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 2005;5(12):917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catanzaro M., et al. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target Ther. 2020;5(1):84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin C., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm' in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diao B., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmoudi S., et al. Immunologic features in coronavirus disease 2019: functional exhaustion of T cells and cytokine storm. J. Clin. Immunol. 2020;40(7):974–976. doi: 10.1007/s10875-020-00824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta P., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee D.W., et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan F., et al. Time course of lung changes on chest CT during recovery from novel coronavirus (COVID-19) pneumonia. Radiology. 2019;259(2020):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang Y.C., et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology. 2005;236(3):1067–1075. doi: 10.1148/radiol.2363040958. [DOI] [PubMed] [Google Scholar]
- 15.Ding X., et al. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur. J. Radiol. 2020;127 doi: 10.1016/j.ejrad.2020.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.J. Vilcek, G.C. Sen, Interferons and other cytokines, in: B.N. Fields, D.M. Knipe, P.M. Howley, (Eds.) Fields' Virology, Lippincott-Raven Publishers, Philadelphia, pp. 375–399.
- 17.Kundig T. Hengartner, H, Zinkernagel, RM, T cell-dependent IFN-gamma exerts an antiviral effect in the central nervous system but not in peripheral solid organs. J. Immunol. Ref. 1993;150:2316–2321. [PubMed] [Google Scholar]
- 18.Chen G., et al. Clinical and immunological features of severe and moderate coronavirus disease. J. Clin. Invest. 2020 Jul;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu B., et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J. Infect. 2020;81(1):e51–e60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephens D.S., McElrath M.J. COVID-19 and the path to immunity. JAMA. 2020;324(13):1279–1281. doi: 10.1001/jama.2020.16656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullard A. Anti-IL-6Rs falter in COVID-19. Nat. Rev. Drug Discovery. 2020;19(9):577. doi: 10.1038/d41573-020-00141-w. [DOI] [PubMed] [Google Scholar]
- 22.Dinarello C.A., Simon A., van der Meer J.W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baggiolini M., Walz A., Kunkel S.L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Invest. 1989;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thwaites R.S., et al. Elevated antiviral, myeloid and endothelial inflammatory markers in severe COVID-19. MedRxiv. 2020 [Google Scholar]
- 25.Zhou Q., et al. Interferon-alpha2b treatment for COVID-19. Front. Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with covid-19 - preliminary report. N. Engl. J. Med. 2020;1–11 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Backer D., Azoulay E., Vincent J.L. Corticosteroids in severe COVID-19: a critical view of the evidence. Crit. Care. 2020;24(1):627. doi: 10.1186/s13054-020-03360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marjani Majid, et al. Nritld protocol for the management of patients with covid-19 admitted to hospitals. Tanaffos. 2020;19(2):91–99. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.