Abstract
Background
The protection against aerosol transmission provided by masks vs face shields or in combination when speaking indoors is not well understood.
Methods
To simulate a human source, an aerosol generating system was made using a bacterial suspension in a nebulizer attached to an oxygen cylinder. A fan connected to the nebulizer created aerosols. Transmitted aerosols were detected using blood agar plates at 0.1524 and 1.8288 meters from source, simulating exposed person. The study was performed under controlled conditions at room temperature in a biohazard hood with high-efficiency particulate air (HEPA) filter and UV light.
Results
When face shields were used alone, significant numbers of bacterial colonies grew on blood agar plates. When a mask used alone for both the subjects (source and exposed), the blood agar yielded minimal colony forming units at both distances. When face shields were used in combination with masks, no significant improvement was observed as compared to masks alone.
Discussion
Our results were similar to what have been observed in related studies.
Conclusions
Surgical masks alone provided good protection, surpassing the protection provided by face shields alone. Both used together provided the best protection, although the combined protection was similar to surgical masks use alone.
Key Words: Aerosols, Respiratory particles, Nebulizer
Background
Covid-19, caused by the SARS CoV-2 virus, has resulted in over 67 million cases and more than 1.5 million deaths worldwide as of December 7, 2020.1 Despite various control efforts, the disease continues to spread globally and ongoing local transmission continues in many countries.2 Different transmission routes of SARS CoV-2 need to be better understood to research effective interventions to curtail disease spread.2
Like many other respiratory infectious diseases such as measles and influenza, evidence is suggesting that SARS CoV-2 is transmitted by expelling virus-containing droplets through sneezing or coughing.3, 4, 5 Studies have also found that breathing and talking, while not producing large droplets, can be more infectious for some diseases.5, 6, 7, 8 During a sneeze or cough, airway secretion droplets become airborne by expulsion from the mouth at high velocity.9 These droplets vary in size, and dispersion in the environment is also variable.9 , 10 Small respiratory particles from a patient coughing can remain airborne and spread widely through a room, leaving healthcare workers and others vulnerable to becoming infected through inhalation, especially if in the room for an extended period of time.9 , 11 The rate of air flow in a room will also affect how far droplets can travel.3
The key role of masks is in reducing the amount of aerosols and almost eliminating larger droplets, as well as splash and spray from infected persons.12 There have been multiple studies demonstrating that surgical masks provide infection protection.13, 14, 15 However, some experts believe that face shields have some advantages over masks: users report they are more comfortable, not as hot, ease breathing, and provide eye protection.12 , 16 Moreover, face shields demonstrate that they can reduce exposure to large aerosol particles by 96% in the short-term, and 68% with smaller aerosols.9 Some experts believe that face shields should be routinely used in the community for protection.16 The Swiss government, however, disagrees, citing observations in an unplanned exposure setting.17
In this study, we use a simulated environment to compare the use of masks and face shields in many different experiments to better understand these interventions and their protective roles, including source control. Our objective is to compare and contrast protection of mask vs face shields.
Our study uses a microbiological simulation of a “sick” person, emitting droplets and aerosols during talking and exhalation to assess the impact of mask and face shield in preventing airborne transmission to a “healthy individual” at distances of 0.1524 and 1.8288 meters. We also studied the effect of wearing masks and/or face shields by the healthy individual to determine whether such an approach would augment the protection provided by the mask. The use of masks and face shields in different scenarios was evaluated to better understand these interventions and their protective roles, including source control. Our objective is to evaluate the protection provided by masks and/or face shields on the simulated sick person and the simulated listener.
Methods
The study used a microbiological approach to simulate different scenarios in which a sick person talks to a healthy one: these experiments include exposure with no protection and exposure with prevention measures including using surgical masks and/or face shields at different distances.
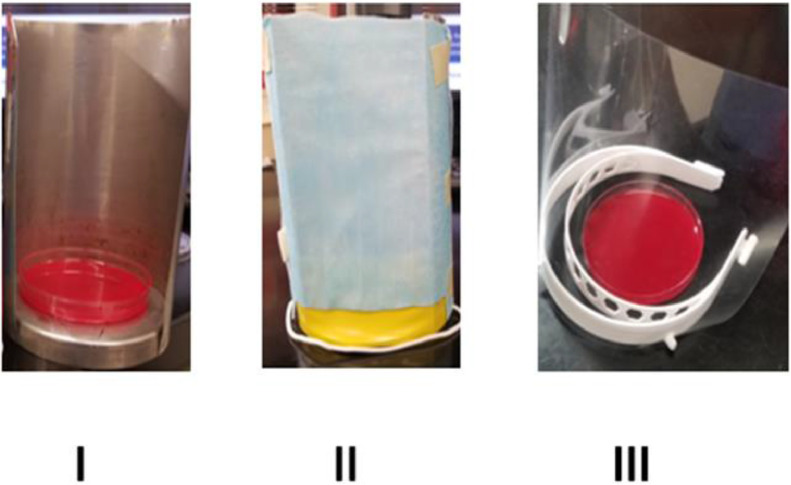
The study was performed under controlled conditions at room temperature and in a sealed Biosafety cabinet equipped with HEPA filter and UV light. The methodology for this study was inspired be a study by Lindsley et al9 as well as a demonstration performed by Dr Richard Davis at Providence Sacred Heart Medical Center.18 To study the impact of wearing mask or face shield in reducing aerosol transmission from a patient with respiratory tract infection to a healthy individual, we designed a set up to simulate emission of aerosols from the mouth of a sick person while talking and a semi-quantitative system to measure the amount of transmitted aerosols to a simulated healthy person (open blood agar Petri plate) in close proximity to the patient. A bacterial cell suspension was prepared from an over-night culture of Staphylococcus epidermidis in saline with concentration adjusted to 0.5 Mc Farland (1.5 × 108 cell/mL). Ten mL of cell suspension was added to a single use nebulizer with tight particle size distribution (Misty Max nebulizer 10, median aerodynamic diameter of 1.61 μm, geometric standard deviation of 2.18, Vyaire Medical) which was connected to an oxygen tank. The nebulizer was adjusted to an output of 8 liter/min for 30 seconds. The aerosolized bacterial cell suspension generated by the nebulizer was directed to a T junction tube where 1 end of T tube was connected to a fan and other end was connected to the narrow end of a funnel. The fan was adjusted to provide an airflow of 4 m/s at the wide end of the funnel where the aerosolized bacteria exit. This was left open or covered with mask, face shield, both, to test the efficacy of each modification in preventing aerosol transmission (Fig 1 ). The fan and nebulizer were turned on simultaneously during each experiment to generate an airflow similar to that of a talking person (4 m/s). Solid culture medium (blood agar Petri plates, Brain heart infusion agar with 5% sheep blood, 85 mm, Thermo Fisher Scientific/Remel)) simulating a healthy listener, were placed at 0.1524 and 1.8288 meters from the funnel were used to evaluate the number of aerosolized bacteria that reached the detection location. After 30 seconds the nebulizer and the fan were turned off, each experiment was concluded 2 minutes later by covering the culture petri with its lid. Before starting the next simulation, the air inside the biosafety cabinet was exhausted through the HEPA filter for 5 minutes. Culture plates were incubated at 35°C for 18 hours to allow the growth of bacteria and formation of visible colonies. The number of colonies on the culture media was used to evaluate the efficiency of mask and/or or face shield wear by the sick person in reducing the transmission of aerosols. In some simulations the effect of mask and/or face shield in protecting a simulated listener in close proximity to the emission source (sick patient) was evaluated by protecting the culture plates with mask and/or face shield (Fig 2 ). The resultant colony counts in such situations allowed us to compare effects of the protective equipment worn by the exposed listener.
Fig 1.
Oxygen cylinder and the nebulizer used to generate air current and to dispense bacteria simulating normal human speech.
Fig 2.
Simulation of different experiments for an individual. (A) No mask, no Shield, (B) Mask only, (C) Shield only.
Results
The results from various experiments in which the simulated sick and exposed individuals utilized various combinations of mask and/or shield, are shown in Table 1 . We repeated these experiments 3 times and used the average colony count from each scenario in our analysis. The results of different scenarios are presented as 1+ to 7 + to show the lowest and the highest possible colony count on blood agar plate. 1+ was used when number of colonies were 2-5 and 8+ would have been used to show surface of blood agar plate was almost completely covered by a confluent lawn of colonies (too many colonies to count). When neither simulated participants subjects wore mask or face shield (scenarios 1 and 2), the number of colonies was interpreted as 7+, because individual colonies, rather than a confluent lawn, predominated.
Table 1.
For evaluation of mask and face shield efficacy in preventing aerosol transmission, 17 different scenarios were tested
| Scenario | Subject A | Subject B | Distance | Growth Score | Colony forming units |
|---|---|---|---|---|---|
| 1 | No surgical mask, no face shield | No surgical mask, no face shield | 0.1524 Meters | 7+ |  |
| 2 | No surgical mask, no face shield | No surgical mask, no face shield | 1.8288 Meters | 7+ |  |
| 3 | Surgical mask | No surgical mask, no face shield | 0.1524 Meters | 5+ |  |
| 4 | Surgical mask | No surgical mask, no face shield | 1.8288 Meters | 4+ |  |
| 5 | No surgical mask, no face shield | Surgical mask | 0.1524 Meters | 5+ |  |
| 6 | No surgical mask, no face shield | Surgical mask | 1.8288 Meters | 1+ |  |
| 7 | Face shield | No surgical mask, no face shield | 01524 Meters | 6+ |  |
| 8 | Face shield | No surgical mask, no face shield | 1.8288 Meters | 5+ |  |
| 9 | Face shield | Face Shield | 0.1524 Meters | 5+ |  |
| 10 | Face shield | Face Shield | 1.8288 Meters | 4+ |  |
| 11 | Surgical mask & face shield | Surgical mask & face shield | 0.1524 Meters | 1+ |  |
| 12 | Surgical mask & face shield | Surgical mask & face shield | 1.8288 Meters | 1+ |  |
| 13 | Surgical mask & face shield | No surgical mask, no face shield | 1.8288 Meters | 2+ |  |
| 14 | Surgical mask | Face shield | 1.8288 Meters | 3+ |  |
| 15 | Surgical mask | Surgical mask & face shield | 1.8288 Meters | 1+ |  |
| 16 | Surgical mask | Surgical mask | 1.8288 Meters | 1+ |  |
| 17 | Surgical mask & face shield | Surgical mask | 1.8288 Meters | 1+ |  |
Growth score was assigned to each scenario based on colony count on the agar plate. Increasing the distance between the 2 subjects decreased the colony count. Highest growth score was obtained when none of the subjects used any protective measure and the lowest growth score was achieved when both subjects used mask or mask with face shield.
The highest colony counts were observed when neither the simulated sick person or the healthy listener were wearing mask or face shield and the 2 subjects were at 0.1524 meters from each other (7+; scenario 1). The colony count in this and all other tested scenarios reduced as the distance between 2 subjects increased from 0.1524 meters to 1.8288 meters. The colony counts reduced somewhat further when the healthy person used the face shield as the sole mean of protection (6+). An even greater reduction in colony count was observed when the sick person used the face shield (5+).
When only the healthy person wore a mask, the colony count was reduced to (4+). However, when the mask was worn only by the sick person, the colony count was reduced to (3+). The best result in prevention of aerosol transmission, shown by lowest colony count on the blood agar plate was obtained when both individuals worn masks (1+). Interestingly, the addition of face shields to masks produced results that were similar to those of wearing mask only by both individuals (1+).
Discussion
This experiment was simple in its concept and execution, using materials and equipment that were readily available in a clinical lab. However, because it is a simulation, its obvious limitations need to be highlighted to help interpretation and guide future studies.
The first limitation was that there were no actual humans in this experiment. The simulated listener (petri plate) could not inhale. The lack of air being aspirated around and through the mask and/or face shield means that their ability to trap or redirect the nebulized bacteria is probably overestimated. Thus, the results presented here probably represent best case experiments. Although simulated speech is able to give valuable information regarding aerosol spread and mask/shield protection, variations in speech and breathing patterns, volume, head size, etc. cannot be captured this way.19 Second, our model tested spread of a bacteria in a nebulized solution, and not viruses. Recent information has indicated that SARS CoV-2 is transmitted by aerosols and droplets,20 , 21 and we could not simulate various droplet sizes. Virus quantification in this type of experiment would be very cumbersome and use of SARS CoV-2 was not possible due to safety requirements. Thus, bacteria better served the purpose of this model. Third, surgical masks were the only mask type used in the simulation, and this is a limitation only because cloth masks are so widely used in the general population.19 However, cloth masks vary widely and could not be represented in a single experiment.
Face shields have certain advantages that make their use attractive, such as eye protection, preventing face touching, and prolonging the life of a mask if both are worn together.12 They are also reported to be cooler and less claustrophobic if worn alone.12 , 16 Our observations fell into line with what others have already observed, demonstrating that face shields alone, including use in source control, were not effective in preventing the transmission of aerosols generated by the nebulize.9 , 17 Of note, Swiss hospitality workers became ill while wearing face shields only, resulting in the government discouraging the use of face shields alone.17 The Swiss real-life observation aligns well with our simulated face shield results (Table 1, experiments 7, 8, 9, and 10).
Experiment 16 (Table 1) of our results demonstrated that surgical masks provided good protection from nebulized bacteria, similar to what others have observed.13, 14, 15 , 22 A real-life example that aligns with our results is a hair salon in Missouri that practiced universal masking, and had 2 SARS CoV-2 infected, masked hairdressers in contact with over 100 masked clients. Half of the clients were tested, all were negative, and none ever reported symptoms.15
Surprisingly, when assessing the combined protection of surgical mask and face shield, experiment 12 (Table 1), the level of protection was not enhanced in comparison to experiment 16 (Table 1) using surgical masks alone. This result reinforces the fact that a surgical mask serves as an effective intervention, providing protection from aerosols. Our results do not support the hypothesis that face shields would provide significant passive eye protection.
Professionals working in high-risk settings with exposure to aerosols and droplets, such as dentists, would benefit from the use of face shields, or perhaps goggles, which this experiment could not simulate, as added eye protection.23 The shields would also protect the mask surface from contamination, prolonging the life of the mask. Ethical considerations preclude planning a study demonstrating SARS CoV-2 transmission through eyes, and the likelihood of finding a “natural experiment” is low. However, this simulation indicates that surgical masks worn by all are more likely to provide significant protection to uninfected participants in indoor conversations. Outdoor situations with varying degrees of sun and wind were beyond the scope of this simulation. The role of the face shields in preventing transmission is not supported, and if transmission via the eyes is felt to be a significant factor, goggles might provide better protection than a face shield.
Conclusion
In summary, and in agreement with other reports, face shields worn alone do not appear to provide a significant level of protection. The mask alone provides such a significant improvement that it is difficult to tell, based on this simple simulation, whether the protection afforded by a surgical mask combined with a face shield is improved. Face shields might provide eye protection if when worn as a supplement to the mask, but the role of the eyes in transmission of SARS CoV-2 is not well established.24 , 25 However, some evidence indicates that SARS CoV-2 transmission may occur through the ocular conjunctival epithelium.23 , 26
Footnotes
Financial support: None reported.
Conflicts of interest: None to report.
References
- 1.The New York Times . 2020. Coronavirus Map: Tracking the Global Outbreak. [Google Scholar]
- 2.Chia PY, Coleman KK, Tan YK, et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nature Commun. 2020;11:2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei J, Li Y. Airborne spread of infectious agents in the indoor environment. Am J Infect Control. 2016;44(suppl 9):S102–S108. doi: 10.1016/j.ajic.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asadi S, Wexler AS, Cappa CD, Barreda S, Bouvier NM, Ristenpart WD. Aerosol emission and superemission during human speech increase with voice loudness. Sci Rep. 2019;9:2348. doi: 10.1038/s41598-019-38808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie YL X., Chwang A.T.Y., Ho P.L., Seto W.H. How far droplets can move in indoor environments – revisiting the Wells evaporation–falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 7.Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10:105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 8.Duguid JP. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. Epidemiol Infect. 2009;44:471–479. doi: 10.1017/s0022172400019288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindsley WG, Noti JD, Blachere FM, Szalajda JV, Beezhold DH. Efficacy of face shields against cough aerosol droplets from a cough simulator. J Occup Environ Hyg. 2014;11:509–518. doi: 10.1080/15459624.2013.877591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gralton J, Tovey E, McLaws M-L, Rawlinson WD. The role of particle size in aerosolised pathogen transmission: a review. J Infect. 2011;62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsley WG, King WP, Thewlis RE, et al. Dispersion and exposure to a cough-generated aerosol in a simulated medical examination room. J Occup Environ Hyg. 2012;9:681–690. doi: 10.1080/15459624.2012.725986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.University of California SF; 2020. UCSF Covid Medicine Grand Rounds. How the Virus Gets in and How to Block It: Aerosols, Droplets, Masks, Face Shields, and More. [Google Scholar]
- 13.Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi M, Havlir D. The time for universal masking of the public for coronavirus disease 2019 is now. Open Forum Infect Dis. 2020, ofaa131;7 doi: 10.1093/ofid/ofaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendrix MJ WC, Findley K, Trotman R. Center for Disease Control and Prevention; Springfield, Missouri: 2020. Absence of Apparent Transmission of SARS-CoV-2 from Two Stylists After Exposure at a Hair Salon with a Universal Face Covering Policy. [DOI] [PubMed] [Google Scholar]
- 16.Perencevich EN, Diekema DJ, Edmond MB. Moving personal protective equipment into the community: face shields and containment of COVID-19. JAMA. 2020;323:2252–2253. doi: 10.1001/jama.2020.7477. [DOI] [PubMed] [Google Scholar]
- 17.The Local Switzerland. 'Only those with plastic visors were infected': Swiss government warns against face shields, 2020. Available at:https://www.thelocal.ch/20200715/only-those-with-plastic-visors-were-infected-swiss-government-warns-against-face-shields. Accessed February 8, 2021.
- 18.Davis R. Spokane, WA: Providence Sacred Heart Laboratory. 2020. Are you still on the fence about wearing a mask?https://www.providence.org/news/uf/619927061 Available at: Accessed March 12, 2020. [Google Scholar]
- 19.Fischer EP, Fischer MC, Grass D, Henrion I, Warren WS, Westman E. Low-cost measurement of facemask efficacy for filtering expelled droplets during speech. Sci Adv. 2020;6:eabd3083. doi: 10.1126/sciadv.abd3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 22.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Z-y Ge, L-m Yang, J-j Xia, X-h Fu, Y-z Zhang. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J Zhejiang Univ Sci B. 2020;21:361–368. doi: 10.1631/jzus.B2010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho D, Low R, Tong L, Gupta V, Veeraraghavan A, Agrawal R. COVID-19 and the ocular surface: a review of transmission and manifestations. Ocul Immunol Inflamm. 2020;28:726–734. doi: 10.1080/09273948.2020.1772313. [DOI] [PubMed] [Google Scholar]
- 25.Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu C-W, Liu X-F, Jia Z-F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)30313-5. e39-e39. [DOI] [PMC free article] [PubMed] [Google Scholar]