The COVID-19 pandemic is a global emergency. Identifying populations who are at risk of severe complications is crucial in developing special measures to prevent or reduce severe illness and mortality in vulnerable patients.1 Emerging evidence indicates that alpha1-proteinase inhibitor might inhibit infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). α1-Antitrypsin also has anticoagulation effects and can protect against inflammation, cell death, and the formation of neutrophil extracellular traps, so this multifunctional protein has been considered as a candidate for COVID-19 treatment.2 Several clinical trials of alpha1-proteinase inhibitor have been initiated. An urgent need exists to address the possibility that patients with α1-antitrypsin deficiency (AATD) might be at increased risk of SARS-CoV-2 infection and development of severe COVID-19.
Human α1-antitrypsin is the most abundant serine proteinase inhibitor in human plasma and is encoded by the SERPINA1 gene, which is located on human chromosome 14q32.1. In individuals with one of several inherited mutations in SERPINA1, low circulating α1-antitrypsin concentrations increase the risk of destructive diseases, particularly emphysema and bronchiectasis. Infusion of alpha1-proteinase inhibitor has been shown to have therapeutic benefits in patients with AATD.3
TMPRSS2 is a cell membrane-bound protease that facilitates entry of viruses (including SARS-CoV-2) into host cells by proteolytically cleaving and activating viral envelope glycoproteins. Preliminary evidence indicates that alpha1-proteinase inhibitor might impede SARS-CoV-2 infection by inhibiting TMPRSS2 activity.4 Alpha1-proteinase inhibitor is being tested as a treatment for patients with COVID-19 in four clinical trials, in Saudi Arabia (NCT04385836), Spain (NCT04495101), the USA (NCT04547140), and Ireland (EudraCT 2020-001391-15). In patients with COVID-19 who were admitted to the intensive care unit (ICU), higher ratios of interleukin (IL)-6 to α1-antitrypsin predicted a prolonged ICU stay and higher mortality, whereas lower ratios of IL-6 to α1-antitrypsin were associated with clinical resolution.5
According to the Johns Hopkins University and Medicine Coronavirus Resource Center, Spain, Italy, France, and the UK have reported Europe's highest numbers of confirmed COVID-19 cases and deaths. The numbers of individuals with PI*ZZ, PI*SZ, and PI*MZ genotypes of AATD in these countries are also the highest in Europe.6 Vianello and Braccioni used data from the Italian registry for AATD to show that the geographical distribution of people with AATD is similar to that of the confirmed distribution of people with COVID-19.7 A significant positive correlation was reported between the combined frequencies of the PI*SZ genotype in 67 countries and their reported COVID‐19 mortality.8 These observations are intriguing, but interpretations are challenged by many confounding factors (eg, socioeconomic status and ethnicity) and further investigations are required.
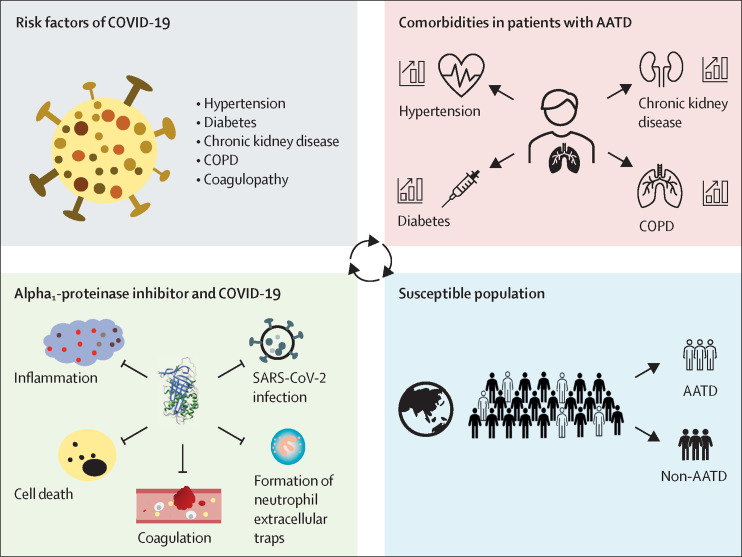
The comorbidities of AATD9 mirror key risk factors predisposing patients to severe COVID-19.10 Patients with AATD have a higher prevalence of hypertension, chronic kidney disease, chronic obstructive pulmonary disease (COPD), and diabetes than do members of the general population.9 These diseases are not only independently associated with increased prevalence of COVID-19 but also associated with poor prognosis in patients with the disease.
We propose that patients with AATD are a susceptible population for COVID-19. First, for patients with AATD who do not have enough functional α1-antitrypsin, TMPRSS2 would be activated more easily, allowing SARS-CoV-2 entry into cells. Second, α1-antitrypsin has inhibitory effects on thrombin and plasmin, so AATD could be associated with an increased risk of coagulation disorder.11 Third, insufficient anti-inflammation, anti-cell death, anti-protease, and anticoagulation effects of α1-antitrypsin could increase the likelihood of severe acute lung injury. Patients with AATD who are infected with SARS-CoV-2 might, therefore, have worse outcomes than do members of the general population (figure ). Since patients with AATD might have an increased risk of adverse outcomes from COVID-19, we propose the following call to action for the management of patients with AATD, with or without COVID-19.
Figure.
Patients with AATD as a population that is susceptible to COVID-19
AATD=α1-antitrypsin deficiency. COPD=chronic obstructive pulmonary disease. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Clinicians should screen patients who are recovering from COVID-19 for AATD to examine the effects of various AATD genotypes on the incidence and severity of COVID-19. Measurements of α1-antitrypsin concentrations in plasma, frequently used for clinical screening purposes, can detect severe deficiencies but might miss carriers whose plasma α1-antitrypsin concentrations are near the normal range (ie, 1·0–2·7 g/L). Studies of patients who have recovered from COVID-19 should include assessment of SERPINA1 status via genotyping. A prevalence of deficiency genotypes that is higher than expected in patients who have recovered from COVID-19, particularly if associated with increased disease severity, would establish the clinical importance of AATD.
Public health agencies should recommend that patients with a high risk of AATD be screened for the SERPINA1 genotypes at the same time as screening and testing for COVID-19 to establish the association between different SERPINA1 genotypes and incidence of SARS-CoV-2 infection. Screening would include foremost the family members of individuals with known AATD and people with disorders that are commonly associated with AATD, particularly the many unscreened patients with COPD. Multiple registries, both locally and internationally, collect data for COVID-19 or AATD; collaborations among these agencies should be advocated.
Patients with AATD and their immediate family members should be aware of the higher risk of infection by SARS-CoV-2 and the possibility of worse clinical outcomes, if they are infected, than in the general population. Clinicians should pay special attention to patients with AATD and their immediate family members, if diagnosed with COVID-19. Plasma α1-antitrypsin concentrations should be measured for these patients. For individuals receiving augmentation therapy at standard doses (ie, 60 mg/kg per week), clinicians should plan to continue infusions should patients become acutely ill. Such patients might require admission to hospital to continue receiving infusions from health-care providers with appropriate personal protective equipment. Higher than standard maintenance doses should be considered.
Although clinical trials have been done to examine the potential benefits of alpha1-proteinase inhibitor in patients with COVID-19, basic and translational research is needed to study the mechanisms of alpha1-proteinase inhibitor's therapeutic benefits and its safety, timing, dosing, limitations, and use in combination with other concurrent therapeutics.
As AATD represents a large population in countries that have a high incidence of COVID-19 and high mortality associated with the disease, these actions for patients with AATD could help to reduce COVID-19-related morbidity and mortality. As the COVID-19 pandemic continues, strategies are needed to address the risks for patients with AATD as a matter of urgency.
For more on the Johns Hopkins University and Medicine Coronavirus Resource Center see https://coronavirus.jhu.edu
Acknowledgments
CY, AW, and ML declare no competing interests. KRC has received consultation fees from CSL Behring, Grifols, Kamada, and Takeda; research grants from CSL Behring, Grifols, and Kamada; and lecture fees from the Alpha1 Foundation, CSL Behring, and Grifols. ML is funded by the Canadian Institutes of Health Research (PJT-148847) and Government of Ontario (RE-08–029). The funders had no role in preparing this Comment.
References
- 1.Maier BF, Brockmann D. Effective containment explains subexponential growth in recent confirmed COVID-19 cases in China. Science. 2020;368:742–746. doi: 10.1126/science.abb4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang C, Keshavjee S, Liu M. Alpha-1 antitrypsin for COVID-19 treatment: dual role in antiviral infection and anti-inflammation. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.615398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McElvaney NG, Burdon J, Holmes M, et al. Long-term efficacy and safety of α1 proteinase inhibitor treatment for emphysema caused by severe α1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE) Lancet Respir Med. 2017;5:51–60. doi: 10.1016/S2213-2600(16)30430-1. [DOI] [PubMed] [Google Scholar]
- 4.Azouz NP, Klingler AM, Rothenberg ME. Alpha 1 antitrypsin is an inhibitor of the SARS-CoV-2–priming protease TMPRSS2. bioRxiv. 2020 doi: 10.1101/2020.05.04.077826. published online Oct 7. (preprint, version 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McElvaney OJ, McEvoy NL, McElvaney OF, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco I, de Serres FJ, Fernandez-Bustillo E, Lara B, Miravitlles M. Estimated numbers and prevalence of PI*S and PI*Z alleles of alpha1-antitrypsin deficiency in European countries. Eur Respir J. 2006;27:77–84. doi: 10.1183/09031936.06.00062305. [DOI] [PubMed] [Google Scholar]
- 7.Vianello A, Braccioni F. Geographical overlap between alpha-1 antitrypsin deficiency and COVID-19 infection in Italy: casual or causal? Arch Bronconeumol. 2020;56:609–610. doi: 10.1016/j.arbr.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Shapira G, Shomron N, Gurwitz D. Ethnic differences in alpha-1 antitrypsin deficiency allele frequencies may partially explain national differences in COVID-19 fatality rates. FASEB J. 2020;34:14160–14165. doi: 10.1096/fj.202002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greulich T, Nell C, Hohmann D, et al. The prevalence of diagnosed α1-antitrypsin deficiency and its comorbidities: results from a large population-based database. Eur Respir J. 2017;49 doi: 10.1183/13993003.00154-2016. [DOI] [PubMed] [Google Scholar]
- 10.Ji HL, Zhao R, Matalon S, Matthay MA. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev. 2020;100:1065–1075. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanash HA, Ekström M, Wagner P, Piitulainen E. Cause-specific mortality in individuals with severe alpha 1-antitrypsin deficiency in comparison with the general population in Sweden. Int J Chron Obstruct Pulmon Dis. 2016;11:1663–1669. doi: 10.2147/COPD.S109173. [DOI] [PMC free article] [PubMed] [Google Scholar]