Abstract
Background
Mechanical ventilation in intensive care for 48 h or longer is associated with the acute respiratory distress syndrome (ARDS), which might be present at the time ventilatory support is instituted or develop afterwards, predominantly during the first 5 days. Survivors of prolonged mechanical ventilation and ARDS are at risk of considerably impaired physical function that can persist for years. An early pathogenic mechanism of lung injury in mechanically ventilated, critically ill patients is inflammation-induced pulmonary fibrin deposition, leading to thrombosis of the microvasculature and hyaline membrane formation in the air sacs. The main aim of this study was to determine if nebulised heparin, which targets fibrin deposition, would limit lung injury and thereby accelerate recovery of physical function in patients with or at risk of ARDS.
Methods
The Can Heparin Administration Reduce Lung Injury (CHARLI) study was an investigator-initiated, multicentre, double-blind, randomised phase 3 trial across nine hospitals in Australia. Adult intensive care patients on invasive ventilation, with impaired oxygenation defined by a PaO2/FiO2 ratio of less than 300, and with the expectation of invasive ventilation beyond the next calendar day were recruited. Key exclusion criteria were heparin allergy, pulmonary bleeding, and platelet count less than 50 X 109/L. Patients were randomly assigned 1:1, with stratification by site and using blocks of variable size and random seed, via a web-based system, to either unfractionated heparin sodium 25 000 IU in 5 mL or identical placebo (sodium chloride 0·9% 5 mL), administered using a vibrating mesh membrane nebuliser every 6 h to day 10 while invasively ventilated. Patients, clinicians, and investigators were masked to treatment allocation. The primary outcome was the Short Form 36 Health Survey Physical Function Score (out of 100) of survivors at day 60. Prespecified secondary outcomes, which are exploratory, included development of ARDS to day 5 among at-risk patients, deterioration of the Murray Lung Injury Score (MLIS) to day 5, mortality at day 60, residence of survivors at day 60, and serious adverse events. Analyses followed the intention-to-treat principle. There was no imputation of missing data. The trial is registered with the Australian and New Zealand Clinical Trials Register, number ACTRN12612000418875 .
Findings
Between Sept 4, 2012, and Aug 23, 2018, 256 patients were randomised. Final follow-up was on Feb 25, 2019. We excluded three patients who revoked consent and one ineligible participant who received no intervention. Of 252 patients included in data analysis, the mean age was 58 years (SD 15), 157 (62%) were men, and 118 (47%) had ARDS. 128 (51%) patients were assigned to the heparin group and 124 (49%) to the placebo group, all of whom received their assigned intervention. Survivors in the heparin group (n=97) had similar SF-36 Physical Function Scores at day 60 compared to the placebo group (n=94; mean 53·6 [SD 31·6] vs 48·7 [35·7]; difference 4·9 [95% CI −4·8 to 14·5]; p=0·32). Compared with the placebo group, the heparin group had fewer cases of ARDS develop to day 5 among the at-risk patients (nine [15%] of 62 patients vs 21 [30%] of 71 patients; hazard ratio 0·46 [95% CI 0·22 to 0·98]; p=0·0431), less deterioration of the MLIS to day 5 (difference −0·14 [–0·26 to −0·02]; p=0·0215), similar day 60 mortality (23 [18%] of 127 patients vs 18 [15%] of 123 patients; odds ratio [OR] 1·29 [95% CI 0·66 to 2·53]; p=0·46), and more day 60 survivors at home (86 [87%] of 99 patients vs 73 [73%] of 100 patients; OR 2·45 [1·18 to 5·08]; p=0·0165). A similar number of serious adverse events occurred in each group (seven [5%] of 128 patients in the heparin group vs three [2%] of 124 patients in the placebo group; OR 2·33 [0·59 to 9·24]; p=0·23), which were a transient increase in airway pressure during nebulisation (n=3 in the heparin group), major non-pulmonary bleeding (n=2 in each group), haemoptysis (n=1 in the heparin group), tracheotomy site bleeding (n=1 in the heparin group), and hypoxaemia during nebulisation (n=1 in the placebo group).
Interpretation
In patients with or at risk of ARDS, nebulised heparin did not improve self-reported performance of daily physical activities, but was well tolerated and exploratory outcomes suggest less progression of lung injury and earlier return home. Further research is justified to establish if nebulised heparin accelerates recovery in those who have or are at risk of ARDS.
Funding
Rowe Family Foundation, TR and RB Ditchfield Medical Research Endowment Fund, Patricia Madigan Charitable Trust, and The J and R McGauran Trust Fund.
Introduction
Mechanical ventilation in intensive care for 48 h or longer is associated with the acute respiratory distress syndrome (ARDS).1 The diagnosis is usually made at the time of initiating ventilatory support or during the following 5 days.1 ARDS affects 23% of mechanically ventilated, critically ill patients and has a mortality of up to 46%.2, 3 Survivors of prolonged mechanical ventilation and ARDS have marked limitations in physical function, are more often readmitted to hospital, and have greater need of rehabilitation services and long-term care than those who required less than 48 h of mechanical ventilation.4, 5, 6, 7, 8, 9 Considerable physical limitations persist at 5 years after diagnosis and include difficulty undertaking basic activities, such as walking, bathing, and dressing.10 Dexamethasone has been found to improve survival in patients with established ARDS, but no pharmacological intervention as of yet, including corticosteroids, ketoconazole, dipyridamole, and aspirin, has been shown to both prevent ARDS and accelerate recovery from ARDS.11, 12, 13
The hallmark histological feature arising from the inflammatory response that causes ARDS is a fibrin mesh in the air sacs, known as a hyaline membrane, on which leucocytes attach and contribute to the development of diffuse alveolar damage.14, 15, 16 Another early manifestation of the inflammatory response is fibrin accumulation in pulmonary capillaries and venules, which leads to microvascular thrombosis,17, 18, 19 and the extent of this thrombosis correlates with the severity of acute lung injury.18, 20 Nebulised heparin targets pulmonary fibrin deposition. Phase 1 and 2 trials undertaken by our group in patients with acute lung injury and related conditions found that nebulised heparin significantly reduced pulmonary dead space, coagulation activation, microvascular thrombosis, and deterioration in the Murray Lung Injury Score (MLIS), and increased time free of ventilatory support.21, 22, 23, 24, 25, 26, 27 Heparin has other actions that might also be beneficial in this patient population. It has been shown to bind to bacterial and viral pathogens, demonstrating efficacy in animal models of pneumonia and limiting infectivity in-vitro of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).28, 29 Heparin also increases release of nitric oxide from the vascular endothelium, inhibits inflammatory pathways in the pulmonary and systemic circulation, reduces mucus tenacity, and prevents bronchospasm.30, 31, 32
Research in context.
Evidence before this study
An early and important mechanism in the pathogenesis of inflammation-induced lung injury is pulmonary fibrin deposition that leads to thrombosis of the microvasculature, hyaline membrane formation in the air sacs, and development of the acute respiratory distress syndrome (ARDS). Intensive care patients expected to require prolonged mechanical ventilation often present with ARDS or develop ARDS in the initial days after commencing ventilatory support, and face a prolonged period of recovery with considerable impairment of their physical function. We searched PubMed for articles published from database inception up to Sept 12, 2020, using the terms “acute respiratory distress syndrome” OR “adult respiratory distress syndrome” OR “acute lung injury” OR “ARDS” OR “mechanical ventilation”, AND “heparin”, AND “inhaled” OR “nebulised”, AND “randomised controlled trial” OR “randomised trial”. A phase 1 study in mechanically ventilated intensive care patients with acute lung injury or ARDS found nebulised heparin significantly reduced coagulation activation in the lungs. A subsequent randomised, double-blind, phase 2 study in 50 intensive care patients expected to require more than 48 h of mechanical ventilation found significant improvement in the Murray Lung Injury Score and the number of ventilator-free days with nebulised heparin. A multicentre trial of nebulised heparin 5000 IU every 6 h (a low dose) for prevention of ventilator-associated pneumonia did not find any improvement in clinical outcomes nor, however, did it raise safety concerns. No other completed randomised trials of nebulised heparin in patients with or at risk of developing ARDS were identified, reinforcing the need for a phase 3 trial.
Added value of this study
To our knowledge, this is the first multicentre trial to test the efficacy of nebulised heparin for reducing lung injury and improving the physical function recovery of mechanically ventilated intensive care patients with or at risk of developing ARDS. Blinded treatment with unfractionated heparin sodium 25000 IU or placebo was administered using a vibrating mesh membrane nebuliser every 6 h to day 10 while ventilated. The intervention was given in addition to usual care, including intravenous and subcutaneous heparin, and was well tolerated. The primary outcome, the Short Form 36 Health Survey Physical Function Score of survivors at day 60, was not improved. Secondary outcomes, which are exploratory, were consistent with reduced progression of lung injury, including fewer cases of new ARDS, less deterioration in the Murray Lung Injury Score, and faster recovery, with more survivors residing at home at day 60.
Implications of all the available evidence
Nebulised heparin can be safely administered to patients with or at risk of ARDS. Outcomes from early-phase clinical trials, and now secondary outcomes from a phase 3 clinical trial, suggest nebulised heparin might attenuate progression of lung injury and expedite recovery. Further research is required to establish whether nebulised heparin accelerates recovery in patients with or at risk of ARDS.
We hypothesised that in patients with or at risk of developing ARDS, nebulised heparin would attenuate the development of acute lung injury and thereby accelerate recovery of physical function, assessed by the Short Form 36 Health Survey (SF-36) Physical Function Score in survivors at day 60.8, 10
Methods
Study design and patients
This was an investigator-initiated, multicentre, double-blind, randomised, phase 3 trial conducted at nine hospitals in Australia (eight in Victoria and one in the Australian Capital Territory).
Eligible patients were aged 18 years or older, were receiving invasive mechanical ventilation via an endotracheal tube, had been intubated on the day of randomisation or the preceding day, had impaired oxygenation defined by an arterial partial pressure of oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) of less than 300 while invasively ventilated, were expected to require invasive ventilation beyond the end of the following day, and had active (heated) ventilator circuit humidification. These characteristics meet the oxygenation criteria for ARDS and are strongly associated with both the presence of ARDS and the risk of developing ARDS.1, 23, 33, 34
Exclusions for safety were pregnancy, heparin allergy, platelet count of less than 50 × 109/L, activated partial thromboplastin time (APTT) more than 80 s not due to anticoagulant therapy, uncontrolled bleeding, pulmonary bleeding, central nervous system bleeding, neurosurgery, epidural catheterisation, and hepatic encephalopathy or portal hypertension. Extra corporeal membrane oxygenation and high frequency oscillation ventilation were also exclusions as these could impede delivery of the intervention. Imminent death, anticipated death from an underlying cause within 90 days, treatment limitations, chronic dialysis, dementia, tracheostomy, home oxygen, home ventilation, and nerve injury or disease with likely prolonged ventilation were further exclusions as these could confound outcome assessment. A full list of eligibility criteria can be found in the appendix (p 5).
St Vincent's Hospital (Melbourne, VIC, Australia) Human Research Ethics Committee (HREC) granted ethics committee approval for eight of the nine participating centres, with ACT Health HREC (Canberra, ACT, Australia) granting approval for the remaining centre. Because all patients lacked capacity to consider participation at the time of eligibility, informed written consent was obtained from their substitute decision maker prior to enrolment, unless the substitute decision maker was unable to attend the hospital. In these circumstances, and in accordance with the approved consent procedures, verbal (telephone) consent was obtained before enrolment, and written consent was subsequently obtained. Additional information about the study design is available in the appendix (pp 4–9).
The protocol (version 1, version 2, and version 3) is available on Research Gate.26
Randomisation and masking
Patients were randomly assigned 1:1 to receive nebulised heparin (heparin group) or placebo (placebo group). Site research personnel performed randomisation using a central, password-protected, encrypted, web-based automated system hosted at the University of Sydney (Sydney, NSW, Australia). Randomisation was stratified by site. To ensure allocation concealment, blocks of variable size and a random seed were employed, and the random allocation sequence was developed by an independent researcher (GSD) and was not known to the investigators. Both study medications were manufactured by Pfizer (Sydney, NSW, Australia) and were presented by the manufacturer in ampoules of identical size, colour, shape, and material. Pharmacists at St Vincent's Hospital purchased the medications through the usual hospital procedures at market price and masked the ampoules according to the labelling requirements of Good Manufacturing Practice. The active treatment and the placebo were identical in appearance. The participants, clinical team, and investigators were masked to treatment allocation. The study medication was administered by clinicians under the supervision of site investigators and research coordinators, and all ampoules of study medication assigned to each participant were accounted for by the investigators and coordinators. There were no reports of unmasking including on the drug accountability logs.
Procedures
The study intervention was unfractionated heparin sodium 25 000 IU 5 mL or identical placebo (sodium chloride 0·9% 5 mL) every 6 h using a vibrating mesh membrane nebuliser (Aeroneb Solo, Aerogen, Galway, Ireland), but only while receiving invasive mechanical ventilation and only to day 10. Each dose was delivered over 10–15 mins. The heparin dose and nebulisation methodology were developed through earlier phase 1 and phase 2 studies by our group in mechanically ventilated, intensive care patients with ARDS and related conditions.21, 23, 27 An expiratory filter (Servo Duo Guard, Maquet Critical Care AB, Solna, Sweden) prevented the nebulised drug impairing ventilator function. Active (heated) ventilator circuit humidification was employed (appendix p 6). All other aspects of participant management, including investigations and use of intravenous and subcutaneous unfractionated heparin and low molecular weight heparin at prophylactic and therapeutic doses, were at the discretion of the treating physicians.
Outcomes
The primary outcome was the SF-36 Physical Function Score of survivors at day 60. This assesses patient (or proxy) reported limitations across ten physical activities; the minimum score is 0 and the maximum is 100. A lower score indicates worse function.35
Key prespecified secondary clinical efficacy endpoints were: development of new ARDS among at-risk patients to day 5, adjusted for the competing risk of death; deterioration in the MLIS to day 5; time to ventilator separation to day 28, and time to intensive care unit (ICU) separation to day 28, each adjusted for the competing risk of death and reported separately for all patients and survivors; tracheotomy, institution of new respiratory rescue therapies, and ICU readmission, each by day 28; vasopressor therapy-free days to day 28, and renal replacement therapy-free days to day 28, with non-survivors assigned 0 therapy-free days and reported separately for all patients and survivors; mortality at day 28; acute hospital length of stay and acute hospital discharge destination; mortality, place of residence, and quality of life, assessed using the EQ-5D-3L, at day 60 and at day 180; and proximate cause of death and SF-36 Physical Function Score of survivors at day 180.
Deterioration in the MLIS to day 5 was calculated by subtracting the baseline score from the highest score measured once daily on days 1 to 5. The score was calculated using all of the available score components for all patients while they were receiving invasive or non-invasive ventilatory support; respiratory compliance was calculated by tidal volume divided by the difference of peak inspiratory and expiratory pressures.36, 37
Development of ARDS was assessed once daily on days 1 to 5 in at-risk patients. ARDS was defined using the Berlin criteria; any level of invasive or non-invasive ventilatory support was taken as sufficient to satisfy positive end-expiratory pressure and continuous positive airway pressure criteria.38 Chest imaging was assessed using a structured tool (appendix p 7) by a blinded investigator (BD, TR, NS, FMPvH, ANG, SG, EJCB, TMEC, and CF) at each participating site trained using resources provided by the ARDS Task-Force.38
Ventilator separation was deemed to have occurred if the patient had not received either invasive or non-invasive ventilatory support at any time during the remainder of the day of stopping ventilatory support or the next day. If a participant achieved ventilator separation more than once, it was the final separation that was used to calculate the outcome. Similarly, if ICU separation occurred more than once, it was the final separation that was used to calculate the outcome. Deceased patients at day 28 were treated as though ventilated in the ICU at the end of day 28.
Prespecified serious adverse events were major bleeding,39 pulmonary bleeding with clinical deterioration, heparin-induced thrombocytopaenia confirmed by antibody testing, impaired function of the mechanical ventilator with clinical deterioration, and any serious adverse event considered by the site investigator to be at least possibly related to the study. Other prespecified safety outcomes were red cell transfusion, haemoglobin, and platelet count to day 10.
Analyses of secondary outcomes listed in the protocol26 that pertain to biomarkers of inflammation and to cost-effectiveness will be undertaken and reported in future publications.
A post-hoc analysis was performed to further explain the results in relation to the effect of the intervention on the primary outcome for patients with substantial limitations performing physical activities, based on a SF-36 Physical Function Score of less than 20 at day 60. In addition, deterioration in the MLIS to day 5 was assessed separately for patients with ARDS at baseline and for those at risk of developing ARDS.
Statistical analysis
To show a clinically important 10-point improvement in the primary outcome, a sample of 198 patients was required assuming an improvement in the score from 45 to 55 with SD 25, 80% power, and a two-sided significance level of 0·05 and, to allow for loss of physical function information due to death, 60-day mortality of 30% was assumed, necessitating enrolment of another 58 patients and giving a final target sample size of 256.40, 41 Analyses followed the intention-to-treat principle, considering all patients in the treatment group to which they were assigned, except for cases lost to follow-up or withdrawn. There was no imputation of missing data. The student t test assessed continuous outcomes, including the primary outcome. Development of ARDS to day 5, and ventilator and ICU separations to day 28, were assessed using the Fine-Gray methodology to account for the competing risk of death. Data for ARDS development are presented using cumulative incidence curves. Data for ventilator and ICU separations are presented using Kaplan-Meier curves with deceased patients treated as though ventilated in the ICU at the end of day 28.42, 43 Hazard ratio (95% CI), calculated by Cox regression, was used to assess day 180 survival and data are presented using Kaplan-Meier curves. Binary outcomes were compared using odds ratio (95% CI), assessed by logistic regression, except where the event rate in a group was 0 when exact logistic regression was used, and data are presented as the number out of the total-number-non-missing and percentage. Heterogeneity of treatment effect was assessed across ten baseline subgroups on the primary outcome and on ventilator separation to day 28, with 20 subgroup analyses in total (appendix p 7). All reported p values are two-sided, and a value less than 0·05 is taken to indicate statistical significance. Because of the potential for type 1 error due to multiple comparisons without adjustment, analyses of secondary endpoints should be interpreted as exploratory. We used Stata, version 15·1 (StataCorp, College Station, TX, USA).
An independent data safety monitoring board (DSMB) provided oversight (appendix p 7). The trial was registered prior to commencement on the Australian and New Zealand Clinical Trials Register, number 12612000418875.
Role of the funding source
The funders of the study, the drug supplier, and the equipment manufacturers and suppliers, had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Results
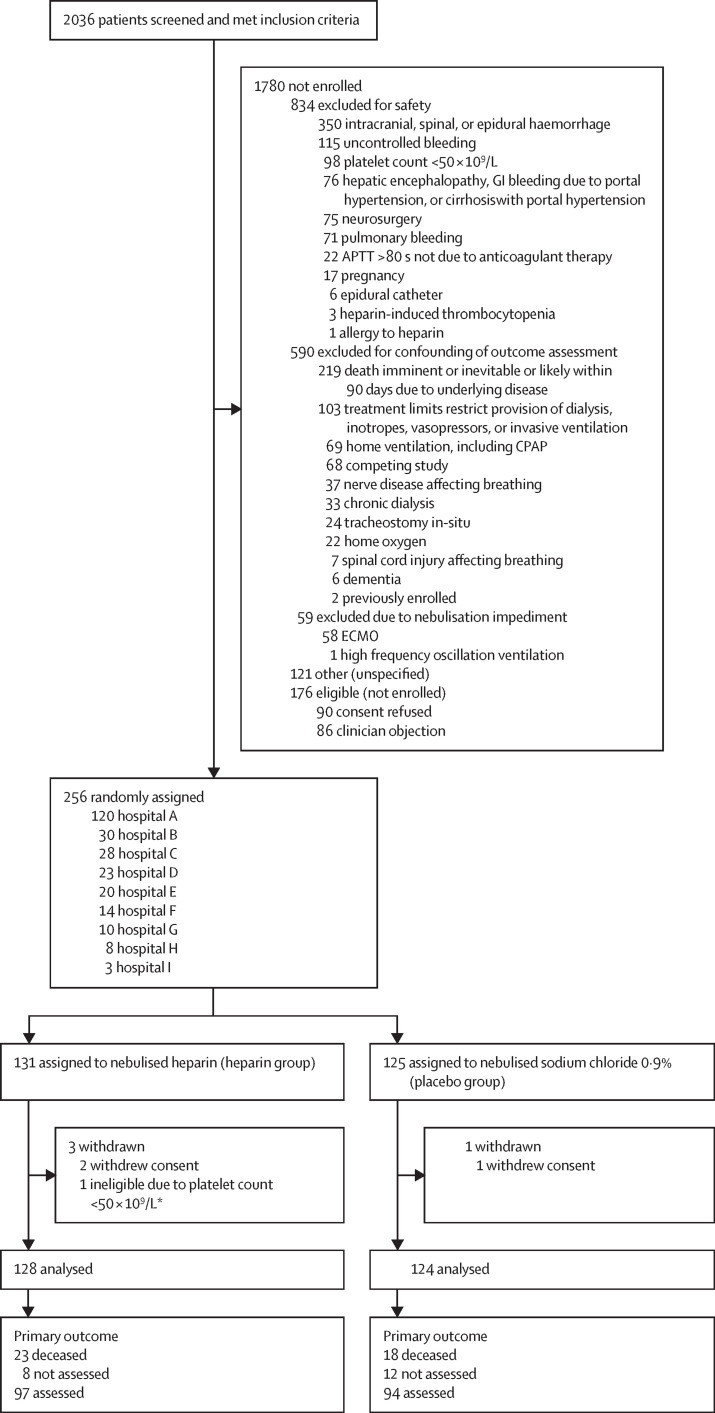
Screening was done from Sept 4, 2012, to Aug 23, 2018. Of 2036 patients who met the inclusion criteria, 256 (13%) were randomly assigned (n=131 to the heparin group; n=125 to the placebo group; figure 1 ; appendix p 9). We excluded two patients who revoked consent and one participant who was ineligible (due to thrombocytopaenia they did not receive study intervention). Data were therefore analysed for 252 patients (n=128 in the heparin group; n=124 in the placebo group). Four patients in the heparin group were transferred to a non-participating hospital ICU before day 28 (n=3 on day 1, preventing further study drug administration, and n=1 on day 18). Their health records at the receiving hospitals were examined to ascertain major outcomes. The DSMB met as scheduled after the 50th, 100th, and 200th enrolment, and reviewed the serious adverse events and reactions in each group while remaining masked to group allocation—they recommended the trial continue.
Figure 1.
Trial profile
The screening log was maintained at all study sites except Hospital H. Primary outcome was Short Form-36 Health Survey Physical Function Score of survivors at day 60. GI=gastrointestinal. APTT=activated partial thromboplastin time. ECMO=extra-corporeal membrane oxygenation. CPAP=continuous positive airway pressure. *Patient received no study intervention.
Baseline characteristics were similar in the two groups and the mean age of participants was 58 years (SD 15) (table 1 ; appendix p 10). Pneumonia was a risk for ARDS for at least 68% of each group and pulmonary aspiration for at least 13%. ARDS was present in 65 (51%) of 127 in the heparin group and 53 (43%) of 124 in the placebo group.
Table 1.
Baseline patient characteristics
| Heparin group (n=128) | Placebo group (n=124) | |||
|---|---|---|---|---|
| Age, years | 58 (15) | 59 (15) | ||
| Sex | ||||
| Female | 45 (35%) | 50 (40%) | ||
| Male | 83 (65%) | 74 (60%) | ||
| Body-mass index, kg/m2 | 30·2 (8·0);n=127 | 29·9 (8·1);n=123 | ||
| Comorbid conditions | ||||
| Ever smoked | 78 (61%) | 81 (65%) | ||
| COPD | 32 (25%) | 32 (26%) | ||
| Asthma | 21 (16%) | 20 (16%) | ||
| Other respiratory disease* | 14 (11%) | 17 (14%) | ||
| Leading risk factors for ARDS† | ||||
| Pneumonia | 87 (68%) | 91 (73%) | ||
| Sepsis, non-pulmonary | 20 (16%) | 18 (15%) | ||
| Inhalation of food or gastric contents | 16 (13%) | 20 (16%) | ||
| Cardiopulmonary bypass | 6 (5%) | 7 (6%) | ||
| Pancreatitis | 5 (4%) | 3 (2%) | ||
| APACHE II Score‡ | 23 (7) | 24 (7) | ||
| Therapies | ||||
| Inotrope or vasopressor infusion | 104 (81%) | 93 (75%) | ||
| Renal replacement therapy | 15 (12%) | 12 (10%) | ||
| Corticosteroid | 61 (48%) | 61 (49%) | ||
| Respiratory rescue therapies | ||||
| Neuromuscular blocker | 59 (46%) | 61 (49%) | ||
| Recruitment manoeuvre | 9 (7%) | 10 (8%) | ||
| Nitric oxide or prostacyclin | 8 (6%) | 10 (8%) | ||
| Prone positioning | 1 (1%) | 2 (2%) | ||
| Respiratory characteristics | ||||
| PaO2/FiO2 ratio§ | 185 (68); n=126 | 184 (84); n=123 | ||
| Respiratory rate, breaths/minute | 20 (6) | 19 (5); n=123 | ||
| Tidal volume, mL/kg pbw | 7·6 (1·8); n=127 | 7·8 (2·4); n=122 | ||
| PEEP, cmH2O | 10·2 (4·1) | 10·0 (3·1) | ||
| Peak inspiratory pressure, cmH2O | 25·1 (5·7);n=127 | 24·5 (5·2);n=122 | ||
| Acute lung injury assessments | ||||
| ARDS¶ | ||||
| At risk | 62 (49%); n=127 | 71 (57%) | ||
| Present | 65 (51%); n=127 | 53 (43%) | ||
| ARDS severity | ||||
| Mild | 19 (15%); n=127 | 16 (13%) | ||
| Moderate | 40 (31%); n=127 | 30 (24%) | ||
| Severe | 6 (5%); n=127 | 7 (6%) | ||
| Murray Lung Injury Score‖ | ||||
| Irrespective of ARDS status | 2·29 (0·64); n=127 | 2·19 (0·58) | ||
| If at risk of ARDS | 1·91 (0·57);n=62 | 1·95 (0·53);n=71 | ||
| If ARDS present | 2·63 (0·49);n=65 | 2·50 (0·50);n=53 | ||
Data are n (%) and mean (SD). The number of patients available for specific variables is stated in each cell if different from the total number of patients in the treatment group. APACHE=acute physiology age and chronic health evaluation. ARDS=acute respiratory distress syndrome. COPD=chronic obstructive pulmonary disease. PaO2/FiO2=arterial partial pressure of oxygen to fraction of inspired oxygen. PEEP=positive end-expiratory pressure. pbw=predicted body weight.
Documented history of bronchiectasis, pulmonary fibrosis, pulmonary sarcoidosis, other or unspecified obstructive or restrictive lung disease, recurrent or chronic lung infection, pneumonitis, alveolitis, lung cancer, or lung tissue resection.
Risk factors in the 7 days before randomisation. Patients could have multiple risk factors.
Scores on the APACHE II range from 0 to 71, with higher scores indicating more severe disease.
Eight patients in each group had PaO2/FiO2 ≥300.
Could neither confirm nor exclude ARDS in one participant with missing arterial blood gas data.
Murray Lung Injury Scores range from 0 to 4, with higher scores indicating more severe injury.
The heparin and placebo groups had similar time from intubation to randomisation (mean 17·2 h [SD 8·7] vs 17·5 h [8·9]). Participants in the heparin group received 95% of the total scheduled dose and participants in the placebo group received 98% (table 2 ). 38 (30%) of 128 participants in the heparin group and 26 (21%) of 124 in the placebo group had at least one dose of study medication withheld for any reason (appendix p 11). More patients in the heparin group than the placebo group had the study medication withheld because clinicians concluded the APTT was too prolonged (six [5%] of 128 patients vs 0 of 124; p=0·0324; table 2; appendix p 11).
Table 2.
Study medication, intravenous and subcutaneous heparin, and APTT to day 10
| Heparin group (n=128) | Placebo group (n=124) | Difference estimate (95% CI) | p value | ||
|---|---|---|---|---|---|
| Study medication* | |||||
| Time from intubation to randomisation, h | 17·2 (8·7) | 17·5 (8·9) | HR 1·04 (0·81 to 1·33) | 0·77 | |
| Time from randomisation to first dose, h | 1·5 (2·0) | 1·3 (2·5) | HR 0·84 (0·66 to 1·08) | 0·18 | |
| Total cumulative dose, mL† | 88 (56) | 96 (64) | MD −8 (−23 to 7) | 0·27 | |
| Treatment duration, days | 4·4 (2·8) | 4·8 (3·2) | MD −0·4 (−1·2 to 0·3) | 0·27 | |
| Percentage volume of total scheduled dose‡ | 94·7 (17·1) | 98·1 (18·1) | MD −3·4 (−7·7 to 1·0) | 0·13 | |
| Withheld; sputum too bloody§ | 8 (6%) | 2 (2%) | OR 4·07 (0·85 to 19·54) | 0·08 | |
| Withheld; APTT too prolonged§ | 6 (5%) | 0 | OR 8·20 (1·17 to undefined) | 0·0324 | |
| Intravenous and subcutaneous heparin | |||||
| Unfractionated heparin | |||||
| Any | 59 (46%) | 63 (51%) | OR 0·83 (0·50 to 1·36) | 0·45 | |
| Prophylactic¶ | 44 (35%); n=125 | 57 (46%) | OR 0·64 (0·38 to 1·06) | 0·08 | |
| Therapeutic¶ | 12 (10%); n=125 | 6 (5%) | OR 2·09 (0·76 to 5·75) | 0·15 | |
| Low molecular weight heparin | |||||
| Any | 91 (73%); n=125 | 87 (70%) | OR 1·14 (0·66 to 1·97) | 0·65 | |
| Prophylactic¶ | 68 (54%); n=125 | 68 (55%) | OR 0·98 (0·60 to 1·62) | 0·95 | |
| Therapeutic¶ | 23 (18%); n=125 | 19 (15%) | OR 1·25 (0·64 to 2·43) | 0·52 | |
| Unfractionated or low molecular weight heparin, or both | |||||
| Therapeutic¶ | 31 (25%); n=125 | 22 (18%) | OR 1·53 (0·83 to 2·83) | 0·18 | |
| APTT peak, s | |||||
| APTT peak | 52 (31); n=125 | 44 (20); n=122 | MD 7 (1 to 14) | 0·0260 | |
| If intravenous and subcutaneous unfractionated heparin—Any | 69 (38); n=56 | 51 (25); n=62 | MD 18 (6 to 30) | 0·0037 | |
| If intravenous and subcutaneous unfractionated heparin—Prophylactic¶ | 60 (34); n=44 | 50 (26); n=56 | MD 10 (−3 to 22) | 0·13 | |
| If intravenous and subcutaneous unfractionated heparin—Therapeutic¶ | 97 (77 to 148); n=12 | 59 (42 to 74); n=6 | MedD 38 (−17 to 93) | 0·16 | |
| If intravenous and subcutaneous unfractionated heparin—None‖ | 37 (9); n=69 | 37 (9); n=60 | MD 0 (−3 to 3) | 0·81 | |
Data are n (%), mean (SD), and median (IQR). The number of patients available for specific variables is stated in each cell if different from the total number of patients in the treatment group. APTT=activated partial thromboplastin time. HR=hazard ratio. MD=mean difference. MedD=median difference. OR=odds ratio.
Three patients, each in the heparin group, were transferred to a non-participating hospital intensive care unit on day 1, precluding further administration of the study drug; study medication usage is reported up to the time of transfer.
Calculated by the total cumulative volume administered divided by the expected volume every 24 h (20 mL).
Calculated by the volume of drug administered per hour of invasive ventilation divided by the expected volume. Dosing was intermittent (5 mL, every 6 h; expected mean 0·833 mL/hour) and commenced immediately making it is possible to receive more than 100% of the expected dose.
Withheld on at least one occasion.
Prophylactic unfractionated heparin if mean daily IV and SC dose ≤15 000 IU. Therapeutic unfractionated heparin if mean daily IV and SC dose >15 000 IU. Prophylactic LMWH if mean daily IV and SC dose ≤40 mg. Therapeutic low molecular weight heparin if mean daily intravenous and subcutaneous dose >40 mg.
All but three patients were administered low molecular weight heparin.
Intravenous or subcutaneous unfractionated heparin or low molecular weight heparin was administered to all but three patients, and at least 18% of patients in each group received these at therapeutic doses (table 2). Among patients exposed to intravenous or subcutaneous unfractionated heparin, the mean peak APTT was higher in the heparin group than the placebo group (69 [SD 38] vs 51 [25] s; mean difference 18 [95% CI 6 to 30]; p=0·0037). In other patients (ie, in those who did not receive intravenous or subcutaneous unfractionated heparin) it was similar (37 vs 37 s; mean difference 0 [–3 to 3]; p=0·81; table 2; appendix p 12). There were no significant differences in fluid balance and use of antimicrobials, sedatives, and corticosteroids (appendix p 13).
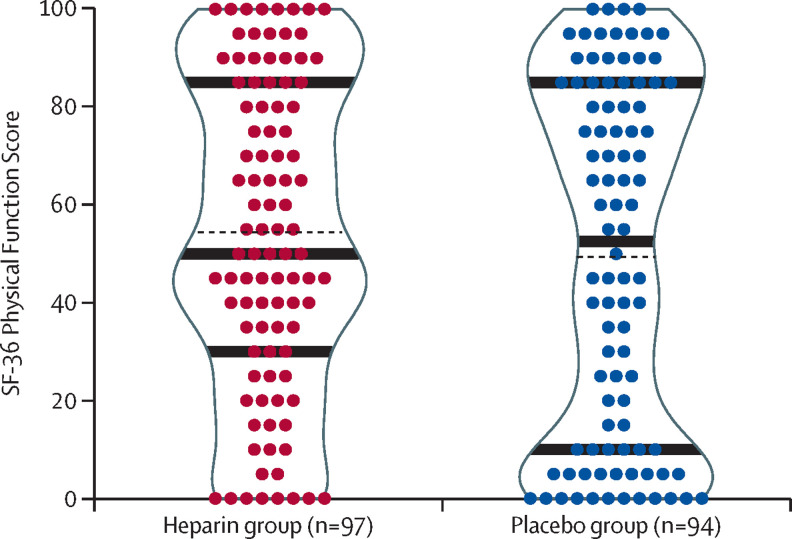
The primary outcome, SF-36 Physical Function Score at day 60, was not significantly different between the heparin group (n=97) compared with the placebo group (n=94; mean 53·6 [SD 31·6] vs 48·7 [35·7]; difference 4·9 [95% CI −4·8 to 14·5; p=0·32; figure 2 ; table 3 ). Excluding deceased patients, loss to follow-up meant the outcome could not be assessed in eight (8%) of 105 patients in the heparin group and 12 (11%) of 106 patients in the placebo group (figure 1; appendix p 19). Post-hoc analysis found a lower proportion of patients in the heparin group than in the placebo group had very poor physical function at day 60, based on a SF-36 Physical Function Score of less than 20 (16 [16%] of 97 patients vs 29 [31%] of 94 patients; odds ratio [OR] 0·44 [95% CI 0·22 to 0·88]; p=0·0210; figure 2).
Figure 2.
Scatter and violin density plots of the SF-36 of survivors at day 60
Mean (black dotted lines), and median (IQR) (black solid lines) are shown. SF-36=Short Form-36 Health Survey Physical Function Score.
Table 3.
Efficacy outcomes by treatment group
| Heparin group (n=128) | Placebo group (n=124) | Effect estimate (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| Primary outcome | ||||||
| SF-36 physical function score of survivors at day 60* | 53·6 (31·6); n=97 | 48·7 (35·7); n=94 | MD 4·9 (−4·8 to 14·5) | 0·32 | ||
| Secondary outcomes | ||||||
| Day 5 | ||||||
| Developed new ARDS† | 9 (15%); n=62 | 21 (30%); n=71 | HR 0·46 (0·22 to 0·98) | 0·0431 | ||
| Deterioration in Murray Lung Injury Score‡ | −0·05 (0·49); n=124 | 0·09 (0·48); n=123 | MD −0·14 (−0·26 to −0·02) | 0·0215 | ||
| Day 28 | ||||||
| New respiratory therapies | ||||||
| Neuromuscular blocker | 16 (24%); n=67 | 18 (29%); n=63 | OR 0·78 (0·36 to 1·72) | 0·54 | ||
| Recruitment manoeuvre | 14 (12%); n=115 | 10 (9%); n=114 | OR 1·44 (0·61 to 3·39) | 0·40 | ||
| Nitric oxide or nebulised prostacyclin | 7 (6%); n=117 | 10 (9%); n=114 | OR 0·66 (0·24 to 1·80) | 0·42 | ||
| Prone positioning | 3 (2%); n=127 | 3 (2%); n=122 | OR 0·96 (0·19 to 4·85) | 0·96 | ||
| ECMO | 0 | 1 (1%) | OR 0·97 (0 to 37·78) | 0·98 | ||
| Tracheotomy | 13 (10%) | 22 (18%) | OR 0·52 (0·25 to 1·09) | 0·09 | ||
| Time to ventilator separation, days§ | 9·9 (9·8) | 10·2 (10·1); n=123 | HR 1·01 (0·77 to 1·33) | 0·92 | ||
| Time to ventilator separation of survivors, days | 6·0 (5·5); n=106 | 7·5 (7·8); n=107 | HR 1·23 (0·93 to 1·62) | 0·14 | ||
| Time to ICU separation, days§ | 11·9 (9·3) | 12·6 (9·7); n=123 | HR 1·08 (0·82 to 1·42) | 0·59 | ||
| Time to ICU separation of survivors, days | 8·5 (6·0); n=106 | 10·2 (8·1); n=107 | HR 1·31 (0·99 to 1·74) | 0·06 | ||
| ICU readmission¶ | 1 (1%); n=103 | 9 (9%); n=101 | OR 0·10 (0·01 to 0·81) | 0·0306 | ||
| Deceased | 22 (17%) | 16 (13%); n=123 | OR 1·39 (0·69 to 2·79) | 0·36 | ||
| Day 60 | ||||||
| Survivors residing at home | 86 (87%); n=99 | 73 (73%); n=100 | OR 2·45 (1·18 to 5·08) | 0·0165 | ||
| Place of residence | ||||||
| Home | 86 (70%); n=122 | 73 (62%); n=118 | OR 1·47 (0·86 to 2·52) | 0·16 | ||
| Rehabilitation | 4 (3%); n=122 | 11 (9%); n=118 | OR 0·33 (0·10 to 1·07) | 0·06 | ||
| Hospital ward | 9 (7%); n=122 | 11 (9%); n=118 | OR 0·77 (0·31 to 1·94) | 0·59 | ||
| ICU or long-term ventilation | 0; n=122 | 5 (4%); n=118 | OR 0·14 (0 to 1·04) | 0·06 | ||
| Deceased | 23 (18%); n=127 | 18 (15%); n=123 | OR 1·29 (0·66 to 2·53) | 0·46 | ||
| Day 180 | ||||||
| Survivors residing at home | 89 (94%); n=95 | 87 (93%); n=94 | OR 1·19 (0·39 to 3·69) | 0·76 | ||
| Deceased | 28 (22%); n=126 | 24 (20%); n=120 | HR 1·15 (0·67 to 1·99) | 0·61 | ||
Data are n (%) and mean (SD). The number of patients available for specific variables is stated in each cell if different from the total number of patients in the treatment group. ARDS=acute respiratory distress syndrome. ECMO=extra-corporeal membrane oxygenation. HR=hazard ratio. ICU =intensive care unit. MD=mean difference. OR=odds ratio. SF-36=Short Form-36 Health Survey.
Scores on SF-36 Health Survey Physical Function range from 0 to 100, with higher scores indicating better function.
Assessed in those ARDS-free at randomisation. ARDS development was unknown in one patient in the heparin group, who died on day 1 before ARDS assessment. There were two deaths in the heparin group and one in the placebo group by day 5. Analysed using a competing-risk approach.
Calculated by subtracting the baseline score from the highest of the scores measured daily on days 1 to 5 while mechanically ventilated. Missing data is for one in each group where ventilator separation occurred by day 1, and for three in the heparin group transferred to another ICU on day 1. Calculated without adjustment for death for the six patients in each group who were deceased by day 5.
Non-survivors to day 28 were deemed to have never had separation from either the ventilator or the ICU. There were 22 deaths in the heparin group and 16 in the placebo group. Analysed using a competing-risk approach.
Assessed in those discharged alive from ICU prior to day 28.
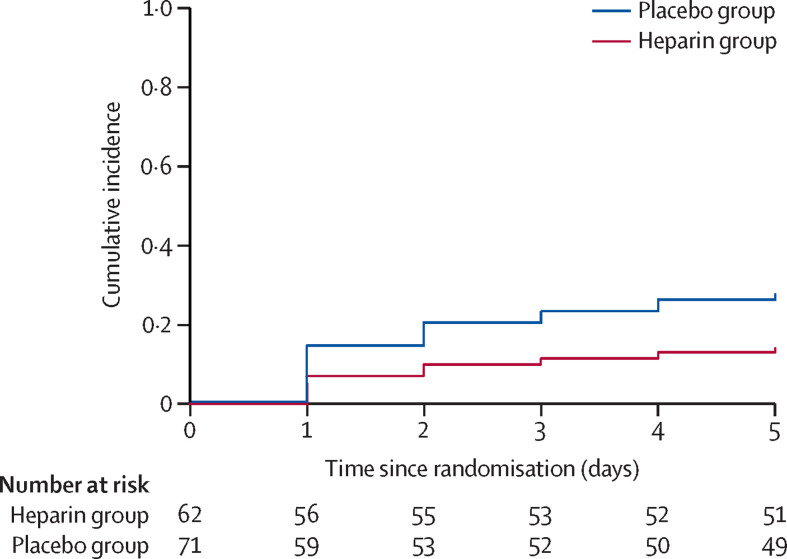
New ARDS developed by day 5 in nine (15%) of 62 patients in the heparin and 21 (30%) of 71 patients in the placebo group (hazard ratio [HR] 0·46 [95% CI 0·22 to 0·98]; p=0·0431; figure 3 ). Of the 30 at-risk patients who developed ARDS, 25 developed bilateral changes on chest imaging after enrolment. Two patients in each group had bilateral changes on chest imaging at baseline, but their PaO2/FiO2 did not satisfy ARDS criteria until after enrolment, and one patient in the heparin group did not meet either chest imaging or PaO2/FiO2 criteria until after enrolment.
Figure 3.
Development of ARDS by treatment group
Cumulative incidence curves for the development of ARDS by day 5. Of the 62 patients at risk of ARDS in the heparin group, two died before developing ARDS and nine developed ARDS compared with 71 patients at risk of ARDS in the placebo group, of whom one died before developing ARDS and 21 developed ARDS. ARDS=acute respiratory distress syndrome.
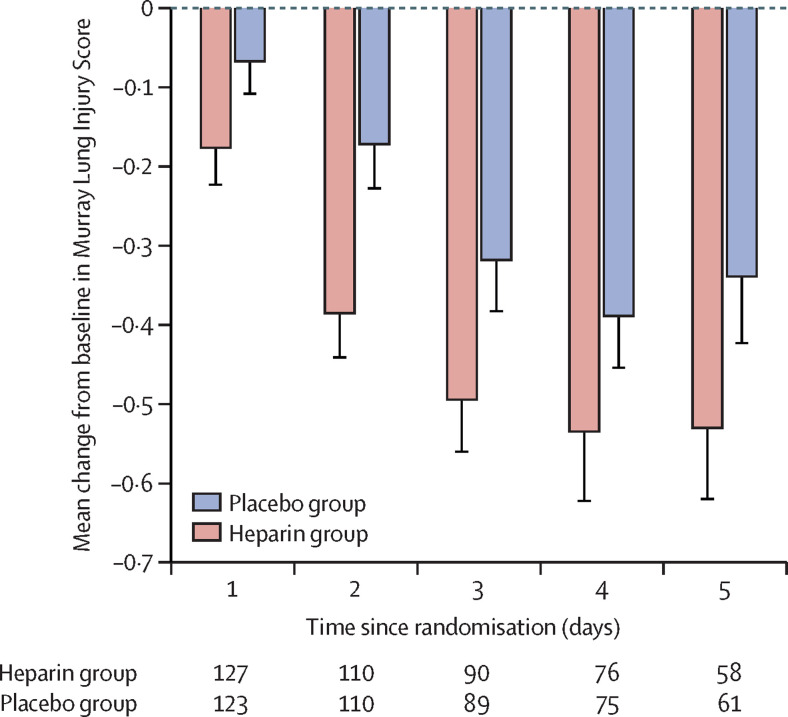
Deterioration in mean MLIS to day 5 was also reduced in the heparin group compared with the placebo group (−0·05 [SD 0·49] vs 0·09 [0·48]; mean difference −0·14 [95% CI −0·26 to −0·02]; p=0·0215; table 3). In post-hoc analysis, this lessening of deterioration in the MLIS was evident, although not statistically significant, in the point estimates of patients with ARDS at baseline (mean difference −0·16 [–0·33 to 0·01]; p=0·07) as well as in those at risk of ARDS (mean difference −0·10 [–0·28 to 0·07]; p=0·23; appendix p 14). Point estimates of the mean change from baseline MLIS on each day to day 5 favoured the heparin group (figure 4 ), and point estimates of the mean change from baseline for each component of the MLIS on each day to day 5 also favoured the heparin group (appendix p 15).
Figure 4.
Change from baseline Murray Lung Injury Score of mechanically ventilated patients
Error bars indicate lower boundary of SE. At baseline, there were 128 patients in the heparin group and 124 patients in the placebo group; loss of data is due to death (n=6 in each group by day 5), transfer to another intensive care unit (n=3 in the heparin group after day 1), and separation from mechanical ventilation (n=61 in the heparin group; n=57 in the placebo group by day 5)
The groups had similar requirements for new respiratory rescue therapies. Fewer patients in the heparin group than the placebo group received a tracheotomy, although this difference was not significant (table 3). There were six more deaths in the heparin group than the placebo group by day 28 (table 3). When those deceased by day 28 were deemed not to have achieved ventilator separation or ICU separation, the groups had similar time to ventilator separation to day 28 and similar time to ICU separation to day 28 (table 3; appendix pp 16–17).
For the 106 survivors in the heparin group and the 107 in the placebo group to day 28, mean days to ventilator separation were 6·0 (SD 5·5) and 7·5 (7·8), respectively, and mean days to ICU separation were 8·5 (6·0) and 10·2 (8·1), respectively (table 3; appendix pp 16–17). Among those discharged alive from the ICU before day 28, significantly fewer patients in the heparin group than in the placebo group were readmitted to the ICU by day 28 (table 3). Other outcomes at day 28 and at hospital discharge were similar (appendix p 18).
The heparin and placebo groups had similar day 60 mortality, but more survivors at day 60 were at home in the heparin group than the placebo group (table 3). The groups did not differ significantly in their EQ-5D-3L domains at day 60 (appendix p 20). With respect to the day 180 EQ-5D-3L anxiety or depression domain, the groups were different and there was a higher frequency of extreme problems in the heparin group, but there were no significant differences between the groups in the other EQ-5D-3L domains at day 180 (appendix p 21). The groups had similar survival and other outcomes at day 180 (table 3; appendix pp 21–22).
The total number of patients for whom a serious adverse event was recorded was similar in each group (seven [5%] of 128 patients in the heparin group vs three [2%] of 124 patients in the placebo group; OR 2·33 [95% CI 0·59–9·24]; p=0·23; table 4 ). The serious adverse events were: three patients in the heparin group, with severe pre-existing bronchospasm, had a transient increase in airway pressure during nebulisation, and after the second case a safety alert was disseminated to investigators; there were two instances of major non-pulmonary bleeding in each group; in the heparin group, one patient had haemoptysis and one had tracheotomy site bleeding; and in the placebo group, one patient had hypoxaemia during nebulisation. Two patients in each group had heparin antibody testing and clinical evaluation of possible heparin-induced thrombocytopaenia; there were no confirmed cases. There were no cases of impaired function of the mechanical ventilator with clinical deterioration. Red cell transfusion and transfusion volume were similar in the groups (table 4), and changes in haemoglobin and platelet count were also similar in the groups (appendix p 13).
Table 4.
Safety outcomes by treatment group
| Heparin group (n=128) | Placebo group (n=124) | Effect estimate (95% CI) | p | ||
|---|---|---|---|---|---|
| Red cell transfusion* | 32 (26%); n=125 | 31 (25%) | OR 1·03 (0·58 to 1·83) | 0·91 | |
| Red cell transfusion volume, mL* | 898 (783); n=32 | 733 (576); n=31 | MD 165 (−181 to 511) | 0·34 | |
| Serious adverse event | |||||
| Any type | 7 (5%) | 3 (2%) | OR 2·33 (0·59 to 9·24) | 0·23 | |
| Major non-pulmonary bleeding | 2 (2%) | 2 (2%) | OR 0·97 (0·13 to 6·98) | 0·97 | |
| Transient increased airway pressure during nebulisation | 3 (2%) | 0 | OR 3·77 (0·40 to undefined) | 0·26 | |
| Haemoptysis | 1 (1%) | 0 | OR 0·97 (0·02 to und.) | 1·00 | |
| Tracheotomy site bleeding | 1 (1%) | 0 | OR 0·97 (0·02 to undefined) | 1·00 | |
| Hypoxaemia during nebulisation | 0 | 1 (1%) | OR 0·97 (0 to 37·78) | 0·98 | |
| Heparin-induced thrombocytopaenia† | 0 | 0 | .. | .. | |
| Ventilator or circuit dysfunction with clinical deterioration | 0 | 0 | .. | .. | |
Data are n (%) and mean (SD). The number of patients available for specific variables is stated in each cell if different from the total number of patients in the treatment group. MD=mean difference. OR=odds ratio.
Packed red cells and whole blood. Assessed to day 10.
Two patients in each group underwent heparin antibody testing and clinical evaluation of possible heparin-induced thrombocytopaenia. There were no confirmed cases.
We did not find obvious heterogeneity of treatment effect across any subgroup for the primary outcome (appendix p 23), but a potential treatment interaction was observed for the secondary outcome of ventilator separation to day 28 in a subgroup defined by the median baseline MLIS, with a relative advantage for the effect of heparin among patients with a MLIS of 2·25 or higher (p=0·0439 for interaction; appendix p 24).
Discussion
To our knowledge, this is the first multicentre trial to test the efficacy of nebulised heparin for reducing lung injury and thereby improving the physical function recovery of mechanically ventilated, critically ill patients with or at risk of ARDS. The intervention was given in addition to usual care, which included administration of intravenous and subcutaneous heparin, and was well tolerated. The SF-36 Physical Function Score of survivors at day 60, the primary endpoint, did not improve more in the heparin than the placebo group. Conversely, analyses of other outcomes showed that the heparin group had fewer cases of ARDS developing among at-risk patients, less deterioration in the MLIS, and faster recovery, with more survivors residing at home at day 60, than the placebo group, all of which were consistent with mitigation of lung injury. However, these results were not corrected for multiple comparisons and should be regarded as exploratory.
Although the study groups had similar time to ventilator separation and similar time to ICU separation, when the components of these composite outcomes were examined separately, a small numerical increase in mortality was identified in the heparin group while, among the survivors, the point estimates for time to ventilator separation and time to ICU separation favoured the heparin group by 1·5 days for time to ventilator separation and by 1·7 days for time to ICU separation, when compared with the placebo group. The tracheotomy rate was also numerically lower in the heparin group than the placebo group (10% vs 18%), and although the number of patients readmitted to the ICU was small, significantly fewer patients in the heparin group were readmitted to the ICU compared with the placebo group.
We had hypothesised that nebulised heparin would attenuate progression of lung injury and thereby improve physical function at day 60. We observed a non-significant 4·9-point increase in the mean SF-36 Physical Function Score of survivors—less than the hypothesised 10-point increase—indicating that average function across a broad range of physical activities was not improved. However, post-hoc analysis found that significantly fewer patients in the heparin group than the placebo group had a very low SF-36 Physical Function Score (<20). Patients with very poor physical function are more likely to require prolonged hospitalisation and transfer to a post-care facility.35, 44, 45 That more survivors in the heparin group were found to be residing at home at day 60 than the placebo group, accords with fewer in the heparin group recording a very low SF-36 Physical Function Score.46
We found evidence that the benefit of treatment with nebulised heparin might be greater for those with more severe lung injury at baseline. Compared with the placebo group, in the heparin group mitigation of deterioration in the MLIS was evident in the point estimates of patients with baseline ARDS and among those who were at risk of ARDS, but the mitigation of deterioration was greater in those with baseline ARDS. Also, there was heterogeneity of treatment effect on the rate of separation from the ventilator, with a relative advantage in the heparin group for patients with a baseline MLIS of 2·25 or more. The study enrolled, according to the inclusion criteria, a relatively heterogenous group of patients. The MLIS at baseline ranged from 0·25 to 3·50. A future study should consider a more homogenous group based on the severity of lung injury when establishing eligibility criteria and when estimating the potential treatment effect.
Our results are consistent with those of a phase 2 study that used a similar methodology and showed reduced progression of the MLIS (unpublished), numerically fewer cases of new ARDS, and more ventilator-free days.23, 26 Our results are also consistent with a small, double-blind study47 of intubated patients with acute exacerbation of chronic obstructive pulmonary disease that reported significantly more ventilator-free days with nebulised heparin, and a case-control study48 of patients with inhalation injury that too reported an increase in ventilator-free days with nebulised heparin. A study of nebulised heparin49 for ventilator-associated pneumonia did not find an improvement in clinical outcomes, but the heparin dose was 20% of the dose used in this study, and the nebulisation methodology was not standardised.
The most likely mechanism by which heparin limits inflammation-induced lung injury is through moderation of pulmonary fibrin deposition, leading to a reduction of hyaline membrane formation in the alveoli, and a reduction of thrombosis in the pulmonary microvasculature. The hyaline membrane acts as a physical barrier that restricts the diffusion of gases and contributes to alveolar collapse by impairing surfactant. Histological studies in ARDS showed that the extent of pulmonary microvascular thrombosis correlated with the severity of acute lung injury.18, 20 Microvascular thrombosis increases lung dead space, and the increase in the dead space has been shown to be an independent marker of mortality in ARDS.50 Microvascular thrombosis has also been found to cause increased pulmonary vascular resistance, which might result in right heart failure.51 A trial of dexamethasone13 in patients with ARDS showed both a survival benefit and reduction in ventilator duration. Dexamethasone has been shown to reduce sepsis-induced coagulation activation in the lungs, among other anti-inflammatory actions.52 The effect of heparin in limiting progression to ARDS observed in this study was predominantly due to fewer patients developing bilateral pulmonary infiltrates, suggesting heparin might also limit progression of pulmonary oedema.53 Of particular current interest, the spike protein of SARS-CoV-2 and other associated coronaviruses have been shown to bind to heparan sulphate receptors expressed by respiratory epithelial cells, leading to cellular invasion. Heparin also binds to the spike protein of SARS-CoV-2, preventing cellular adhesion and invasion.54 In addition, post-mortem studies and lung biopsies of patients with SARS-CoV-2 infection and ARDS have shown hyaline membranes in the alveolar spaces and extensive pulmonary microvascular thrombi, suggesting a potential role for nebulised heparin in the treatment of COVID-19 pneumonia, which is currently under clinical investigation.28, 29, 55, 56, 57, 58
Nebulised heparin moderately increased the peak APTT of patients concomitantly administered intravenous or subcutaneous unfractionated heparin but had no effect on the peak APTT of other patients, almost all of whom received intravenous or subcutaneous low molecular weight heparin. Three patients, each with severe pre-existing bronchospasm and assigned heparin, experienced an increase in airway pressure during nebulisation. In all cases the study drug was reintroduced without event and without unmasking. We postulate that heparin might have transiently increased airflow resistance by accumulating on the luminal surface of the terminal bronchioles. The principal safety concern at the outset of the study was bleeding. Reassuringly, there was only a single case of haemoptysis. This case, which occurred in the heparin group, was perceived by the site investigator to be medically important but was not associated with clinical deterioration. The groups had similar blood transfusion requirements and there was no difference in the number of reports of major non-pulmonary bleeding.
Strengths of the CHARLI study include the double-blind design and the preliminary trials undertaken to establish the dose and nebulisation method.21, 23, 27 There are also limitations. Most importantly, several outcomes, including the primary outcome, were assessed only in survivors. Because survival is a post-randomisation event, and because there is some evidence that the timing and rate of death was differential (not different) between study groups, outcomes assessed only in survivors should not be regarded as randomised comparisons and might be subject to bias. Another limitation related to the primary outcome is loss to follow-up, which was just under 10%. We cannot exclude the possibility that the non-respondents of each group are systematically different to the respondents, with outcomes that are better or worse, but any differences would have to be large to have an effect on the result. Another potential limitation is that randomisation was not stratified by ARDS status and this might have given rise to imbalance in the baseline characteristics of the patients at risk of ARDS. But patients at risk of ARDS in each group had a similar baseline MLIS, which is a predictor of ARDS, and the proportion of patients that developed ARDS was 30% in the placebo group, consistent with expectations for a high-risk population.1, 59, 60, 61 A further potential limitation is that the lead site recruited 45% of patients and this might affect the generalisability of findings. Randomisation was stratified by site and this should reduce the risk of differences due to between-centre variation in ventilation and extubation practices. There was a 17-h period from intubation to randomisation, consistent with the inclusion criteria, and the delays inherent to a clinical trial where patients must be screened and consent obtained. It seems plausible that earlier administration, which would be feasible in clinical practice, might arrest the development of inflammation-induced injury sooner and lead to better patient outcomes, but subgroup analyses did not show evidence of an interaction between the study treatment and the duration of pre-randomisation invasive ventilation for either the primary outcome or the rate of ventilator separation (appendix pp 23–24). The results of secondary analyses should be interpreted cautiously as there were many secondary analyses, and these were performed without correction for multiple comparisons, increasing the possibility that observed differences are due to chance. Finally, although the statistical analysis plan was agreed upon before the database lock, its publication in advance would have provided greater transparency.
In conclusion, the CHARLI study found that nebulised heparin therapy, compared with placebo, in invasively ventilated intensive care patients with impaired oxygenation and the expectation of invasive ventilation beyond the next calendar day, did not improve the SF-36 Physical Function of survivors at day 60. Nebulised heparin was well tolerated and secondary outcomes were consistent with attenuation of lung injury, including fewer cases of ARDS among the at-risk patients, less deterioration in the MLIS, and faster recovery, with more survivors residing at home at day 60. Further research is justified to establish whether nebulised heparin accelerates recovery in patients with or at risk of ARDS.
Data sharing
All data needed to evaluate the conclusions in this Article are presented in the main text or the appendix. The trial protocol and supporting documents can be obtained by contacting the corresponding author. Requests from bone-fide researchers for de-identified individual raw data that underlie the results reported here can be made to the corresponding author for consideration by the study management committee. Such requests should be accompanied by a proposal that includes a detailed rationale and analysis plan, and which complies with the participant consent. Institutional ethics review board approval and execution of a data sharing agreement will be required prior to sharing of data. Sharing of data will occur via a secure data access system, and the data dictionary defining each field in the set will be provided. Data will not be available until 12 months after publication of this Article.
Acknowledgments
Acknowledgements
We wish to acknowledge financial support provided by the Rowe Family Foundation, TR and RB Ditchfield Medical Research Endowment Fund, Patricia Madigan Charitable Trust, and The J and R McGauran Trust Fund (Sydney, NSW, Australia). We are grateful to Aerogen Ltd (Galway, Ireland) who supplied nebuliser equipment at no cost. We thank the members of the Data Safety Monitoring Board led by Matthew Anstey. For their assistance with the purchase and masking of the study drug we are grateful to Daniel Lim and Yves Lorenzo. For his assistance developing and managing the study database we thank David Reid. For their assistance with preparation of funding applications we thank Robert Duncan Hite and Marcus Schultz. This work is dedicated to the study patients and their families, and to our own families and colleagues.
Contributors
BD, RJS, DJC, and JDS conceived and designed the study, developed the study protocol, secured funding for the study, and managed the trial. GSD designed the randomisation website and prepared the random allocation sequence. BD, TR, CMM, NS, FMPvH, ANG, SG, EJCB, TMEC, and CF recruited patients and collected data. BD, RJS, DJC, JDS, and JLM prepared the statistical analysis plan, can confirm the integrity of the data and the accuracy of the data analysis, and wrote the first draft of the manuscript. All authors had full access to all the data reported in the study, were involved in critically revising the manuscript for important intellectual content, and gave final approval of the version to be published. BD took final responsibility to submit for publication.
Declaration of interests
DJC reports that St Vincent's Institute of Medical Research received infrastructure support during the conduct of the study from the Victorian Government's Operational Infrastructure Support Program. All other authors declare no competing interests.
Supplementary Material
References
- 1.Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817–1824. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 2.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 4.Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:538–544. doi: 10.1164/rccm.200505-693OC. [DOI] [PubMed] [Google Scholar]
- 5.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 6.Iwashyna TJ. Trajectories of recovery and dysfunction after acute illness, with implications for clinical trial design. Am J Respir Crit Care Med. 2012;186:302–304. doi: 10.1164/rccm.201206-1138ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chelluri L, Im KA, Belle SH, et al. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med. 2004;32:61–69. doi: 10.1097/01.CCM.0000098029.65347.F9. [DOI] [PubMed] [Google Scholar]
- 8.Oeyen SG, Vandijck DM, Benoit DD, Annemans L, Decruyenaere JM. Quality of life after intensive care: a systematic review of the literature. Crit Care Med. 2010;38:2386–2400. doi: 10.1097/CCM.0b013e3181f3dec5. [DOI] [PubMed] [Google Scholar]
- 9.Quality of Life After Mechanized Ventilation in the Elderly Study Investigators 2-month mortality and functional status of critically ill adult patients receiving prolonged mechanical ventilation. Chest. 2002;121:549–558. doi: 10.1378/chest.121.2.549. [DOI] [PubMed] [Google Scholar]
- 10.Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 11.Beitler JR, Schoenfeld DA, Thompson BT. Preventing ARDS: progress, promise, and pitfalls. Chest. 2014;146:1102–1113. doi: 10.1378/chest.14-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kor DJ, Carter RE, Park PK, et al. Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department: the LIPS-A randomized clinical trial. JAMA. 2016;315:2406–2414. doi: 10.1001/jama.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villar J, Ferrando C, Martínez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 14.Castro CY. ARDS and diffuse alveolar damage: a pathologist's perspective. Semin Thorac Cardiovasc Surg. 2006;18:13–19. doi: 10.1053/j.semtcvs.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003;31(suppl):S213–S220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 16.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 17.Blaisdell FW. Pathophysiology of the respiratory distress syndrome. Arch Surg. 1974;108:44–49. doi: 10.1001/archsurg.1974.01350250036009. [DOI] [PubMed] [Google Scholar]
- 18.Tomashefski JF, Jr, Davies P, Boggis C, Greene R, Zapol WM, Reid LM. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983;112:112–126. [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon B. The role of microvascular thrombosis in sepsis. Anaesth Intensive Care. 2004;32:619–629. doi: 10.1177/0310057X0403200502. [DOI] [PubMed] [Google Scholar]
- 20.Greene R, Zapol WM, Snider MT, et al. Early bedside detection of pulmonary vascular occlusion during acute respiratory failure. Am Rev Respir Dis. 1981;124:593–601. doi: 10.1164/arrd.1981.124.5.593. [DOI] [PubMed] [Google Scholar]
- 21.Dixon B, Schultz MJ, Hofstra JJ, Campbell DJ, Santamaria JD. Nebulized heparin reduces levels of pulmonary coagulation activation in acute lung injury. Crit Care. 2010;14:445. doi: 10.1186/cc9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon B, Campbell DJ, Santamaria JD. Elevated pulmonary dead space and coagulation abnormalities suggest lung microvascular thrombosis in patients undergoing cardiac surgery. Intensive Care Med. 2008;34:1216–1223. doi: 10.1007/s00134-008-1042-7. [DOI] [PubMed] [Google Scholar]
- 23.Dixon B, Schultz MJ, Smith R, Fink JB, Santamaria JD, Campbell DJ. Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: a randomized controlled trial. Crit Care. 2010;14:R180. doi: 10.1186/cc9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon B, Opeskin K, Stamaratis G, et al. Pre-operative heparin reduces pulmonary microvascular fibrin deposition following cardiac surgery. Thromb Res. 2011;127:e27–e30. doi: 10.1016/j.thromres.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 25.Dixon B, Smith R, Santamaria JD, et al. A trial of nebulised heparin to limit lung injury following cardiac surgery. Anaesth Intensive Care. 2016;44:28–33. doi: 10.1177/0310057X1604400106. [DOI] [PubMed] [Google Scholar]
- 26.Dixon B, Smith R. Nebulised heparin for lung injury–clinical protocol V1, V2, and V3. Jan 8, 2020. https://www.researchgate.net/publication/348310801_Neb_Hep_Protocol-V1_V2_V3
- 27.Dixon B, Santamaria JD, Campbell DJ. A phase 1 trial of nebulised heparin in acute lung injury. Crit Care. 2008;12:R64. doi: 10.1186/cc6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mycroft-West C, Su D, Elli S, et al. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 receptor binding domain undergoes conformational change upon heparin binding. Thromb Haemost. 2020;120:1700–1715. doi: 10.1055/s-0040-1721319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mycroft-West CJ, Su D, Pagani I, et al. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the surface protein (spike) S1 receptor binding domain with heparin. bioRxiv. 2020 doi: 10.1101/2020.04.28.066761. published online May 8. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Brown JR, Varki A, Esko JD. Heparin's anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L- and P-selectins. J Clin Invest. 2002;110:127–136. doi: 10.1172/JCI14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed T, Garrigo J, Danta I. Preventing bronchoconstriction in exercise-induced asthma with inhaled heparin. N Engl J Med. 1993;329:90–95. doi: 10.1056/NEJM199307083290204. [DOI] [PubMed] [Google Scholar]
- 32.Shute JK, Calzetta L, Cardaci V, di Toro S, Page CP, Cazzola M. Inhaled nebulised unfractionated heparin improves lung function in moderate to very severe COPD: a pilot study. Pulm Pharmacol Ther. 2018;48:88–96. doi: 10.1016/j.pupt.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Jia X, Malhotra A, Saeed M, Mark RG, Talmor D. Risk factors for ARDS in patients receiving mechanical ventilation for >48 h. Chest. 2008;133:853–861. doi: 10.1378/chest.07-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soto GJ, Kor DJ, Park PK, et al. Lung injury prediction score in hospitalized patients at risk of acute respiratory distress syndrome. Crit Care Med. 2016;44:2182–2191. doi: 10.1097/CCM.0000000000002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Needham DM, Wozniak AW, Hough CL, et al. Risk factors for physical impairment after acute lung injury in a national, multicenter study. Am J Respir Crit Care Med. 2014;189:1214–1224. doi: 10.1164/rccm.201401-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 37.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 38.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 39.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 40.Elliott D, McKinley S, Alison J, et al. Health-related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care. 2011;15:R142. doi: 10.1186/cc10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox CE, Carson SS, Lindquist JH, Olsen MK, Govert JA, Chelluri L. Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation: a prospective cohort study. Crit Care. 2007;11:R9. doi: 10.1186/cc5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodet-Contentin L, Frasca D, Tavernier E, Feuillet F, Foucher Y, Giraudeau B. Ventilator-free day outcomes can be misleading. Crit Care Med. 2018;46:425–429. doi: 10.1097/CCM.0000000000002890. [DOI] [PubMed] [Google Scholar]
- 43.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200:828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardner AK, Ghita GL, Wang Z, et al. The development of chronic critical illness determines physical function, quality of life, and long-term survival among early survivors of sepsis in surgical ICUs. Crit Care Med. 2019;47:566–573. doi: 10.1097/CCM.0000000000003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson C, Laubscher S, Burns R. Validation of the short form 36 (SF-36) health survey questionnaire among stroke patients. Stroke. 1996;27:1812–1816. doi: 10.1161/01.str.27.10.1812. [DOI] [PubMed] [Google Scholar]
- 46.Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time–measuring what matters to patients and payers. N Engl J Med. 2017;377:4–6. doi: 10.1056/NEJMp1703423. [DOI] [PubMed] [Google Scholar]
- 47.Ashoor TM, Hasseb AM, Esmat IM. Nebulized heparin and salbutamol versus Salbutamol alone in acute exacerbation of chronic obstructive pulmonary disease requiring mechanical ventilation: a double blind randomised controlled trial. Korean J Anesthesiol. 2020 doi: 10.4097/kja.19418. published online Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McIntire AM, Harris SA, Whitten JA, et al. Outcomes following the use of nebulized heparin for inhalation injury (HIHI Study) J Burn Care Res. 2017;38:45–52. doi: 10.1097/BCR.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 49.Bandeshe H, Boots R, Dulhunty J, et al. Is inhaled prophylactic heparin useful for prevention and management of pneumonia in ventilated ICU patients? The IPHIVAP investigators of the Australian and New Zealand Intensive Care Society Clinical Trials Group. J Crit Care. 2016;34:95–102. doi: 10.1016/j.jcrc.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 51.Cooper JR, Jr, Abrams J, Frazier OH, et al. Fatal pulmonary microthrombi during surgical therapy for end-stage heart failure: possible association with antifibrinolytic therapy. J Thorac Cardiovasc Surg. 2006;131:963–968. doi: 10.1016/j.jtcvs.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Bartko J, Schoergenhofer C, Schwameis M, et al. Dexamethasone inhibits endotoxin-induced coagulopathy in human lungs. J Thromb Haemost. 2016;14:2471–2477. doi: 10.1111/jth.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chimenti L, Camprubí-Rimblas M, Guillamat-Prats R, et al. Nebulized heparin attenuates pulmonary coagulopathy and inflammation through alveolar macrophages in a rat model of acute lung injury. Thromb Haemost. 2017;117:2125–2134. doi: 10.1160/TH17-05-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clausen TM, Sandoval DR, Spliid CB, et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183:1043–1057. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18:1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciceri F, Beretta L, Scandroglio AM, et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22:95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dixon B, Smith R, Artigas A, et al. Can nebulised heparin reduce time to extubation in SARS CoV 2 The CHARTER study protocol. medRxiv. 2020 doi: 10.1101/2020.04.28.20082552. published online May 12. (preprint). [DOI] [Google Scholar]
- 59.Kangelaris KN, Calfee CS, May AK, Zhuo H, Matthay MA, Ware LB. Is there still a role for the lung injury score in the era of the Berlin definition ARDS? Ann Intensive Care. 2014;4:4. doi: 10.1186/2110-5820-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ying J, Zhou D, Gu T, Huang J. Endocan, a risk factor for developing acute respiratory distress syndrome among severe pneumonia patients. Can Respir J. 2019;2019 doi: 10.1155/2019/2476845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Festic E, Carr GE, Cartin-Ceba R, et al. Randomized clinical trial of a combination of an inhaled corticosteroid and beta agonist in patients at risk of developing the acute respiratory distress syndrome. Crit Care Med. 2017;45:798–805. doi: 10.1097/CCM.0000000000002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in this Article are presented in the main text or the appendix. The trial protocol and supporting documents can be obtained by contacting the corresponding author. Requests from bone-fide researchers for de-identified individual raw data that underlie the results reported here can be made to the corresponding author for consideration by the study management committee. Such requests should be accompanied by a proposal that includes a detailed rationale and analysis plan, and which complies with the participant consent. Institutional ethics review board approval and execution of a data sharing agreement will be required prior to sharing of data. Sharing of data will occur via a secure data access system, and the data dictionary defining each field in the set will be provided. Data will not be available until 12 months after publication of this Article.