Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide causing a pandemic with millions of infected people and deaths. Currently, the scientific community is working hard to develop a specific vaccine or treatment. However, since antibody production is an important part of the adaptive immune response, to develop vaccines and therapies, we must understand the antibody response to SARS-CoV-2 infection. In this work, we summarize the most important findings of antibody-mediated immunity against SARS-CoV-2 and highlight its role in the efficient use of plasma from convalescent patients and the direct application of antibodies as treatment.
Keywords: COVID-19, Humoral response, Seroconversion, Convalescent plasma
1. Introduction
The pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) continues to affect much of the world (Lagunas-Rangel and Chávez-Valencia, 2020). Despite all the research that has been done, there are still many unanswered questions about how the human body responds to SARS-CoV-2 infection. Among them, one that is important to answer in order to develop vaccines, new drugs, and use plasma from convalescent patients is how antibody-mediated immunity against this infectious agent develops.
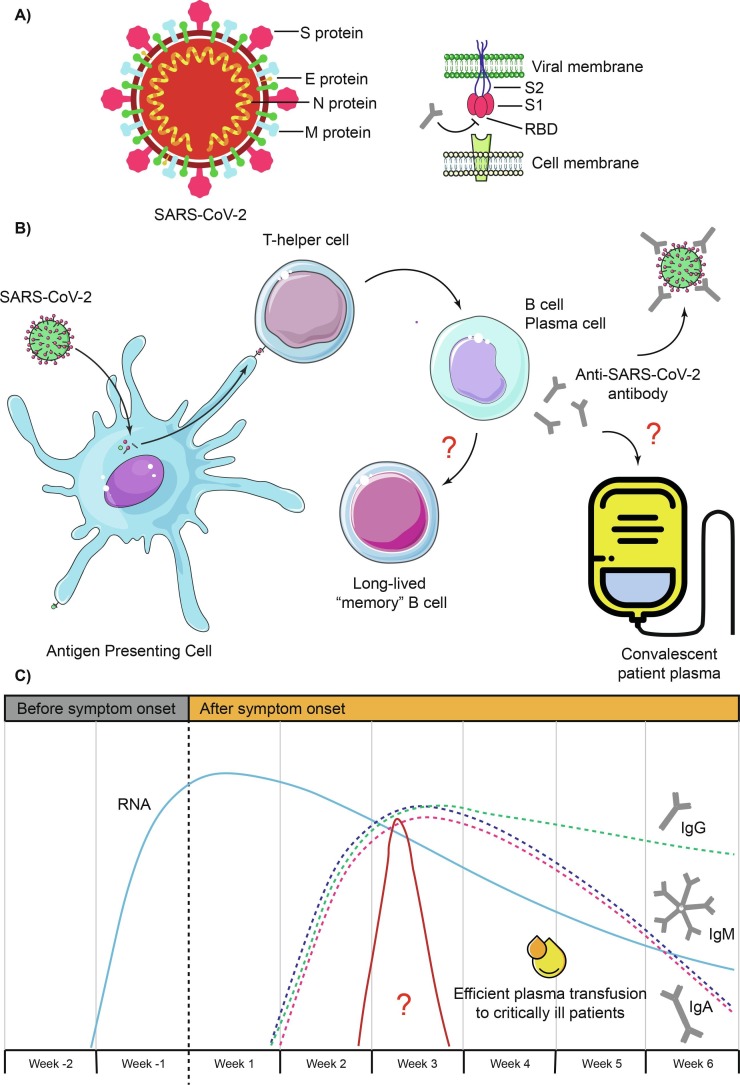
Overall, SARS-CoV-2 virus has four well-identified structural proteins which are: the spike (S) glycoprotein, the small envelope (E) glycoprotein, the membrane (M) glycoprotein, and the nucleocapsid (N) protein (Fig. 1 A). S protein is divided into 2 subunits called S1 and S2 and forms homotrimers that protrude from the viral surface. Remarkably, S protein mediates viral entry into the host cell by first binding to the angiotensin-converting enzyme 2 (ACE2) receptor through the receptor binding domain (RBD) that is part of the S1 subunit and then with the S2 subunit it fuses the viral and host membranes. Meanwhile, N protein binds to the virus RNA and participates in processes related to the viral replication cycle and the response of the infected cell. M protein stabilize the complex between protein N and viral RNA and promotes completion of the viral assembly. Finally, E protein plays a role in the production and maturation of the virus (Astuti and Ysrafil, 2020). Each of these proteins is capable of arousing an immune response that leads to the production of antibodies, to a greater or lesser degree. Although, those that stand out are protein S due to its role in viral entry, and protein N for its high concentration that facilitates its detection (Petherick, 2020).
Fig. 1.
A) Structure of SARS-CoV-2 and S protein. B) Proposed mechanism of antibody-mediated immunity during SARS-CoV-2 infection. C) Estimated time intervals and rates of viral RNA, IgG, IgM and IgA in patients infected with SARS-CoV-2. The appropriate interval for plasma transfusion from convalescent patients into severe patients is added. Because there are variations in the results of the analyzed studies, the time intervals should be considered only as approximations. Question marks indicate that it is suggested but not yet fully verified.
In this work, we summarize the most important findings of antibody-mediated immunity against SARS-CoV-2 and highlight its participation in the efficient use of plasma from convalescent patients and the direct application of antibodies as treatment.
2. Antibody responses to SARS-CoV-2
Antibodies can help stop viral infection by different mechanisms that include neutralizing the virus by recognizing epitopes on its surface, blocking entry or fusion of the virus into the host cell, as well as improving the activity of other immune components such as complement, phagocytes and natural killer cells (Fig. 1B). Currently, it is known from some studies that serum viral antibodies increase only slightly in the early stage of the disease (first 4 days after the onset of symptoms) and, therefore, in few patients these are detectable (making diagnosis difficult by detecting antibodies). Subsequently, COVID-19 patients show a gradual increase in virus-specific IgG and IgM levels until the third week after the onset of symptoms, and then IgM levels begin to decrease, while IgG levels continue increasing, keeping anti-trimerized IgG S titers stable for approximately three months. IgM often peaks earlier than IgG. Virus-specific IgA has also been observed in COVID-19 patients with behavior similar to IgM, but peaking until day 20 after onset of symptoms (Iyer et al., 2020, Liu et al., 2020b, Long et al., 2020, Padoan et al., 2020, Wajnberg et al., 2020, Xiao et al., 2020). Apparently, antibody levels increase rapidly between days 7 to 10 after the symptoms onset and peak at days 17 to 19, with both antibodies being detectable beyond day 30 where IgG levels also start to drop (Long et al., 2020, To et al., 2020, Zeng et al., 2020c). Interestingly, SARS-CoV-2 infection also results in secretion of IgG, IgA and IgM in saliva against protein S and the RBD region, where only the IgG response persists up to three months after the onset of symptoms and it correlates well with serum titers (Isho et al., 2020). Although IgM levels contributed the most to the prediction of a recent infection in the early phase of the disease, IgG responses were the most predictive of infection 8 or more days after the onset of symptoms (Iyer et al., 2020). The concentration of antibodies in the serum of the patients has shown a negative correlation with the levels of viral RNA, which tells us that, in effect, they collaborate to neutralize and eliminate the virus (Fig. 1C) (Du et al., 2020, Zhang et al., 2020b). Additionally, low antibody levels at discharge increase the risk of redetectable viral RNA (Li et al., 2020).
While some articles report higher antibody responses in more severe cases, others do not. Some studies (Crawford et al., 2020, Qu et al., 2020, Seow et al., 2020, To et al., 2020) reported that in the first two weeks after symptom onset, severely ill patients had higher IgG levels than patients with moderate disease. Indeed, this can be explained since the high viral load in severe cases could activate extra follicular B cells and cause the production of antibodies earlier (Yongchen et al., 2020). Another study (Liu et al., 2020b) did not observe changes in the early phase, but until 15 days after the onset of symptoms, and another study (Wu et al., 2020a) found higher levels of IgG against protein S and N in patients without severe symptoms. Also, an earlier IgM response that also decreased more rapidly has been mentioned, and higher IgM and IgG levels in severe cases (Iyer et al., 2020, Long et al., 2020, Zeng et al., 2020c). Mucosal IgA has also been mentioned to have higher levels in severe patients (Cervia et al., 2020).
Approximately 97% of patients have showed seroconversion within 20 days after the onset of symptoms and the remaining 3% did not show seroconversion, where day 12 was the median day for IgG, IgM and IgA seroconversion (Iyer et al., 2020, Long et al., 2020, Zhao et al., 2020). In approximately half of the population, seroconversion against protein S, RBD, and protein N occurs simultaneously (Seow et al., 2020). Some asymptomatic patients also did not develop seroconversion presumably due to the low level of viral load (Lee et al., 2020, Yongchen et al., 2020). Interestingly, seroconversion has occurred in three different ways: synchronous seroconversion of both immunoglobulins (47.8%), IgM seroconversion later than that of IgG (40.5%), and IgM seroconversion earlier than that of IgG (11.6%) (Long et al., 2020, To et al., 2020). Most patients had earlier IgG and IgM seropositivity for anti-RBD than anti-N, and in both cases the IgG concentrations correlated better with the microneutralization assays than the IgM concentrations (To et al., 2020). Antibody responses to other virus proteins have been lower in patient serum (Ni et al., 2020).
SARS-CoV-2 N protein–directed antibodies have shown a cross reaction with the SARS-CoV N protein, as well as antibodies directed to the SARS-CoV 2 S and S1 proteins and the SARS-CoV counterparts (Long et al., 2020, Okba et al., 2020), but no cross reactivity has been detected between the SARS-CoV-2 RBD protein and the SARS-CoV RBD (Ju et al., 2020b, Ju et al., 2020a). This can be explained because the SARS-CoV-2 N protein shares 90.52% identity with SARS-CoV N protein, while the SARS-CoV-2 S protein shares a 76.42%, and where S1 subunit is less conserved (64%) than the S2 (90%). SARS-CoV-2 RBD region has 74% identity with the SARS-CoV counterpart, but the identity of the residues that have shown direct contact with the ACE2 receptor drops to 50% (Jaimes et al., 2020, Xu et al., 2020).
The competitive ability of ACE2 has correlated well with the neutralizing activities of the antibodies, although the antibodies analyzed so far have recognized epitopes in S1, both for the RBD region and for the N-terminal domain (NTD), and for S2, especially of the IgG class (Ju et al., 2020b, Liu et al., 2020a). Antibodies directed against the RBD region can prevent interaction with the ACE2 receptor, destabilize prefusion or restrict the conformational change of the S protein, in all cases preventing the entry of the virus, while for antibodies directed against NTD and S2 is unknown the mechanism of action (Gavor et al., 2020). Notably, antibodies identified in convalescent patients showed that only those targeting the RBD site with a dissociation constant (Kd) smaller or closer to that of ACE2/RBD showed potent neutralization effects against pseudoviruses and true SARS-CoV-2 virions (Cao et al., 2020). Despite the strong humoral response against the N protein, these antibodies have not been shown to be neutralizing for the virus, and also have a shorter half-life (Addetia et al., 2020, Grandjean et al., 2020, Wu et al., 2020a).
Notably, it has been mentioned that the percentage of B cells and plasmablasts is higher in COVID-19 patients and increases with the severity of the disease (Marcos‐Jiménez et al., 2020). RBD-specific B cells have been reported to vary from person to person among COVID-19 patients, but on average they represent approximately 0.005–0.065% of the total B cell population and 0.023–0.329% among memory subpopulations. Antibodies in COVID-19 patients are derived from broad and diverse families of antibody heavy and light chains and even in the same patient, the pattern of antibodies changed over time, only interdigitating between them, suggesting that patients develop different antibodies during SARS-CoV-2 infection, and these are related during the early stages of the disease. IgVH 3–30 was overrepresented and three clones were significantly enriched in these samples: antibodies with heavy-chain variable regions belonging to the VH1-2*06, VH3-48*02, and VH3-9*01 families, together with the corresponding light-chain kappa (Igκ) belonging to 2–40*01/2D-40*01, 3–20*01, and light-chain lambda (Igλ) to 2–14*02 with the respective joining segment kappa 4 (Jk4), Jk5 and joining segment lambda 1 (Jl1). This group of antibodies had a longer CDR3 length than other antibodies less present in the serum and were enriched with tyrosine residues in this region, indicating possible areas to establish hydrogen bonds and hydrophobic interactions with the surrounding residues. VH1-2*06 and VH3-9*01 showed a tendency to increase over time, while VH3-48*02, which has smallest CD3 region of these three, to decrease (Ju et al., 2020b, Ju et al., 2020a). The mean number of nucleotide mutations in the V genes for IGH and IGL was lower than in antibodies from individuals with chronic infections and similar to antibodies in individuals with primary circulating malaria or from non-antigen-enriched circulating IgG memory cells (Robbiani et al., 2020). Anti-N and anti-RBD IgG are mainly of the IgG1 isotype, and some patients presented the IgG3 isotype (Cao et al., 2020, Ni et al., 2020). Low reactivity was also detected for IgG2 and IgG4 (Amanat et al., 2020). In particular, for SARS-CoV-2 neutralizing antibodies, similar levels of somatic hypermutation were identified, regardless of the isolation time (Kreer et al., 2020).
It is not clear whether convalescent patients are at risk of “relapse” or “reinfection”, or how long protection lasts after infection. It has been seen that the presence of neutralizing antibodies from a previous infection was associated with protection against reinfection (Addetia et al., 2020), but at the same time, there have been cases where it is considered that there was reinfection (Vrieze, 2020). Experiments with Rhesus monkeys showed that infection with SARS-CoV-2 protects against reinfections in subsequent exposures, at least for short times, where the concentration of specific antibodies produced against the virus was much faster and higher in the re-exposure (Bao et al., 2020). Some studies reported that neutralizing antibody titers decrease 60 days after the onset of symptoms to reach a stable plateau and in some patients the decrease is quite drastic, but still appears to be sufficient to provide protection against SARS-CoV-2 (Crawford et al., 2020, Seow et al., 2020).
Although still in controversy, it is thought that IgG against SARS-CoV-2 can be transferred through the placenta from mother to fetus, but it is only identifiable in neonates for about 50 days and then disappears (Gao et al., 2020). IgM was also detected in infants, but it was not identified if it was due to any damage to the placenta, or if the virus crossed the placenta and the baby produced the antibodies, or if the antibodies were actually transferred from mother to child (Zeng et al., 2020a). This presence is also mentioned in other reports, disappearing 28 days after delivery (Cavaliere et al., 2020). Additionally, breast milk has also shown IgG and IgA antibodies against SARS-CoV-2 (Dong et al., 2020).
3. Antibodies as treatment
Because there are currently no vaccines or drugs that can be used specifically for the treatment of patients with COVID-19, treatment with the plasma of convalescent patients has emerged as a therapeutic option (Fig. 1B). Convalescent plasma treatment is a passive administration of polyclonal antibodies to provide immediate immunity and has previously been used as a treatment for some viral pathogens such as Ebolavirus, SARS-CoV and MERS CoV (Tiberghien et al., 2020). The US Food and Drug Administration (FDA) has approved the use of plasma from recovered patients, which contains antibodies against the SARS-CoV-2, to treat critically ill people (Tanne, 2020). Currently, the effectiveness of convalescent plasma is still unclear and there is insufficient data from well-controlled, adequately powered, randomized clinical trials to recommend either for or against its use for the treatment of COVID-19. Convalescent COVID-19 patients, who recovered from moderate illness, had detectable IgM and IgG levels (Duan et al., 2020, Shen et al., 2020, Zeng et al., 2020b, Zhang et al., 2020a, Zhang et al., 2020b). It has been reported that plasma-treated patients significantly dropped the virus load within the first 12 days after transfusion and improved its clinical characteristics. Gradually virus-specific IgG and IgM levels and their neutralizing activities increased in patients, which remained high for more than 7 days (Duan et al., 2020, Shen et al., 2020, Zhang et al., 2020a). IgG against protein S and the RBD region increased within 3 days after transfusion, with no apparent changes for IgG against protein N (Li et al., 2020). In some cases it is mentioned that the use of convalescent plasma was effective in very severe cases (Duan et al., 2020, Ye et al., 2020) and also that the effectiveness of plasma depends on the concentration of anti-RBD antibodies (Salazar et al., 2020). It is also necessary to note that plasma also contains other molecules such as anti-inflammatory cytokines, clotting factors, natural antibodies, defensins, pentraxins, and other undefined proteins obtained from donors that can help recipients (Rojas et al., 2020). Selected donors must have high antibody titers (at least 1:32) and until now recently recovered patients have been used (patients with 14 days after full recovery) because it is unknown how long these levels are maintained in patients (Epstein and Burnouf, 2020). Notably, one report mentions a considerable drop in antibody levels in patients who had previously seroconverted 60 days ago, including some patients reaching levels below the threshold for positivity. This limits the use of convalescent plasma and indicates that there is a short window to collect plasma from patients (Self et al., 2020). According to the passive immunotherapy used for the SARS-CoV, and also supported by the fact that transfusions after day 20 had no effect on recovery (Zeng et al., 2020b), the time used to apply the plasma transfusion to critically ill patients was before 14 days after the onset of symptoms (Duan et al., 2020). Regarding plasma doses, different protocols have been tested without clearly one being better than the other, for example, one study used two doses of 200–250 mL with an antibody titer of 1:40 (Shen et al., 2020), another trial used a dose of 200 mL with a neutralizing antibody titer of 1: 640 (Duan et al., 2020), and another four 200–250 mL units administered within 36 h with titers of at least 1:320 (Devos et al., 2020). To increase the level of protection, two or three doses of plasma are recommended. Furthermore, to obtain a cocktail of antibodies that could provide therapeutic benefit, plasma from two or more different donors could be used (Epstein and Burnouf, 2020). No serious adverse reactions have been recorded after plasma transfusion (Duan et al., 2020), and among those mentioned are non-hemolytic febrile reactions, circulatory overload associated with transfusions and allergic reactions and hypotensive (Nguyen et al., 2020).
Recently, since plasma cannot be produced on a large-scale, the use of monoclonal neutralizing antibodies has also been suggested as a therapeutic and prophylactic strategy. These antibodies are mainly obtained by selecting neutralizing antibodies from patients infected with SARS-CoV-2 using different methodologies, although they can also be designed with bioinformatic models against specific antigens, to later be cloned and induce their proliferation (Cao et al., 2020). Particularly, the use of antibodies directed against the RBD region have shown good results in murine models to improve clinical characteristics and even prevent infection (Cao et al., 2020, Wu et al., 2020b). Furthermore, the use of a neutralizing antibody directed against a conserved S1 epitope, other than RBD, has also been proposed, which triggers NK-mediated antibody-dependent cellular cytotoxicity (ADCC), and also cross-reacts with SARS-CoV (Pinto et al., 2020). Likewise, an antibody cocktail with one antibody against the RBD region and the other against the NTD region has been shown to be effective in Rhesus monkeys and golden hamsters in reducing viral load and virus-induced pathological sequelae (Baum et al., 2020). Several candidate antibodies are currently in clinical trials, the vast majority of these correspond to monoclonal antibodies based on full-length IgG (although there are also therapies based on IgM/IgA, IgY), and the others can be bispecific or trispecific antibody designs, single domain antibody, polyclonal antibodies, with fusion protein or other variants (Yang et al., 2020). To optimize the results and the pharmacokinetics, modifications have been made in some antibodies which include the selective change of isotype and the modifications of the Fc region through the substitution of different glycans or amino acids (Hussen et al., 2020).
4. Conclusion
We want to highlight that many of the results presented in this work are preliminary, contain very small patient samples, or require more trials to be conclusive. In this way, we want to highlight the importance of studying antibody-mediated immunity against SARS-CoV-2 and leave some questions that we hope can be answered soon: What factors intervene so that a person does not carry out seroconversion? Is a lasting memory immunity generated after infection with SARS-CoV-2? Does the transfer of antibodies from the mother prevent infection of the fetus? What is the optimal time to apply the plasma of convalescent patients in critically ill patients? What is the optimal concentration of neutralizing antibodies that must be in the plasmas to be efficient? What precautionary strategies should be implemented for the safe administration of convalescent plasma therapy to mitigate the risk of SARS-CoV-2 transmission?
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
FALR is recipient of a doctoral scholarship (application number 2018-000012-01NACF-07226) from the National Council of Science and Technology, CONACyT.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.imbio.2021.152054.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Addetia, A., Crawford, K.H.D., Dingens, A., Zhu, H., Roychoudhury, P., Huang, M.-L., Jerome, K.R., Bloom, J.D., Greninger, A.L., 2020. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 58, 1–11. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed]
- Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., Jiang K., Arunkumar G.A., Jurczyszak D., Polanco J., Bermudez-Gonzalez M., Kleiner G., Aydillo T., Miorin L., Fierer D.S., Lugo L.A., Kojic E.M., Stoever J., Liu S.T.H., Cunningham-Rundles C., Felgner P.L., Moran T., García-Sastre A., Caplivski D., Cheng A.C., Kedzierska K., Vapalahti O., Hepojoki J.M., Simon V., Krammer F. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020;26(7):1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti I., Ysrafil Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metabolic Syndrome: Clin. Res. Rev. 2020;14(4):407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, L., Deng, W., Gao, H., Xiao, C., Liu, Jiayi, Xue, J., Lv, Q., Liu, Jiangning, Yu, P., Xu, Y., Qi, F., Qu, Y., Li, F., Xiang, Z., Yu, H., Gong, S., Liu, M., Wang, G., Wang, S., Song, Z., Liu, Y., Zhao, W., Han, Y., Zhao, L., Liu, X., Wei, Q., Qin, C., 2020. Lack of Reinfection in Rhesus Macaques Infected with SARS-CoV-2. bioRxiv. doi: 10.1101/2020.03.13.990226.
- Baum A., Ajithdoss D., Copin R., Zhou A., Lanza K., Negron N., Ni M., Wei Y.i., Mohammadi K., Musser B., Atwal G.S., Oyejide A., Goez-Gazi Y., Dutton J., Clemmons E., Staples H.M., Bartley C., Klaffke B., Alfson K., Gazi M., Gonzalez O., Dick E., Jr., Carrion R., Jr., Pessaint L., Porto M., Cook A., Brown R., Ali V., Greenhouse J., Taylor T., Andersen H., Lewis M.G., Stahl N., Murphy A.J., Yancopoulos G.D., Kyratsous C.A. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science. 2020;370(6520):1110–1115. doi: 10.1126/science:abe2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Su B., Guo X., Sun W., Deng Y., Bao L., Zhu Q., Zhang X.u., Zheng Y., Geng C., Chai X., He R., Li X., Lv Q.i., Zhu H., Deng W., Xu Y., Wang Y., Qiao L., Tan Y., Song L., Wang G., Du X., Gao N., Liu J., Xiao J., Su X.-D., Du Z., Feng Y., Qin C., Qin C., Jin R., Xie X.S. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020;182(1):73–84.e16. doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere A.F., Marchi L., Aquilini D., Brunelli T., Vasarri P.L. Passive immunity in newborn from SARS-CoV-2-infected mother. J. Med. Virol. 2020 doi: 10.1002/jmv.26609. jmv.26609. [DOI] [PubMed] [Google Scholar]
- Cervia C., Nilsson J., Zurbuchen Y., Valaperti A., Schreiner J., Wolfensberger A., Raeber M.E., Adamo S., Weigang S., Emmenegger M., Hasler S., Bosshard P.P., De Cecco E., Bächli E., Rudiger A., Stüssi-Helbling M., Huber L.C., Zinkernagel A.S., Schaer D.J., Aguzzi A., Kochs G., Held U., Probst-Müller E., Rampini S.K., Boyman O. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford, K.H.D., Dingens, A.S., Eguia, R., Wolf, C.R., Wilcox, N., Logue, J.K., Shuey, K., Casto, A.M., Fiala, B., Wrenn, S., Pettie, D., King, N.P., Chu, H.Y., Bloom, J.D., 2020. Dynamics of neutralizing antibody titers in the months after SARS-CoV-2 infection. medRxiv 1–13. doi: 10.1101/2020.08.06.20169367. [DOI] [PMC free article] [PubMed]
- Devos T., Geukens T., Schauvlieghe A., Ariën K.K., Barbezange C., Cleeren M., Compernolle V., Dauby N., Desmecht D., Grimaldi D., Lambrecht B.N., Luyten A., Maes P., Moutschen M., Romano M., Seyler L., Nevessignsky M.T., Vandenberghe K., van Griensven J., Verbeke G., Vlieghe E., Yombi J.C., Liesenborghs L., Verhamme P., Meyfroidt G. A randomized, multicentre, open-label phase II proof-of-concept trial investigating the clinical efficacy and safety of the addition of convalescent plasma to the standard of care in patients hospitalized with COVID-19: the Donated Antibodies Working agai. Trials. 2020;21:981. doi: 10.1186/s13063-020-04876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Chi X., Hai H., Sun L., Zhang M., Xie W.-F., Chen W. Antibodies in the breast milk of a maternal woman with COVID-19. Emerging Microbes Infect. 2020;9(1):1467–1469. doi: 10.1080/22221751.2020.1780952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z., Zhu F., Guo F., Yang B.o., Wang T. Detection of antibodies against SARS‐CoV‐2 in patients with COVID‐19. J. Med. Virol. 2020;92(10):1735–1738. doi: 10.1002/jmv.25820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L.i., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L.i., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. PNAS. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J., Burnouf T. Points to consider in the preparation and transfusion of COVID‐19 convalescent plasma. Vox Sang. 2020;115(6):485–487. doi: 10.1111/vox.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Hu X., Sun X., Luo X., Chen L. Possible intrauterine SARS-CoV-2 infection: positive nucleic acid testing results and consecutive positive SARS-CoV-2-specific antibody levels within 50 days after birth. Int. J. Infectious Diseases. 2020;99:272–275. doi: 10.1016/j.ijid.2020.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavor E., Choong Y.K., Er S.Y., Sivaraman H., Sivaraman J. Structural Basis of SARS-CoV-2 and SARS-CoV Antibody Interactions. Trends Immunol. 2020;41(11):1006–1022. doi: 10.1016/j.it.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean, L., Saso, A., Torres, A., Lam, T., Hatcher, J., Thistlethwayte, R., Harris, M., Best, T., Johnson, M., Wagstaffe, H., Ralph, E., Mai, A., Colijn, C., Breuer, J., Buckland, M., Gilmour, K., Goldblatt, D., Co-Stars Study Team, 2020. Humoral response dynamics following infection with SARS-CoV-2. medRxiv 1–17. doi: 10.1101/2020.07.16.20155663.
- Hussen, J., Kandeel, M., Hemida, M.G., Al-Mubarak, A.I.A., 2020. Antibody-Based Immunotherapeutic Strategies for COVID-19. Pathogens 9, 917. doi: 10.3390/pathogens9110917. [DOI] [PMC free article] [PubMed]
- Isho B., Abe K.T., Zuo M., Jamal A.J., Rathod B., Wang J.H., Li Z., Chao G., Rojas O.L., Bang Y.M., Pu A., Christie-Holmes N., Gervais C., Ceccarelli D., Samavarchi-Tehrani P., Guvenc F., Budylowski P., Li A., Paterson A., Yue F.Y., Marin L.M., Caldwell L., Wrana J.L., Colwill K., Sicheri F., Mubareka S., Gray-Owen S.D., Drews S.J., Siqueira W.L., Barrios-Rodiles M., Ostrowski M., Rini J.M., Durocher Y., McGeer A.J., Gommerman J.L., Gingras A.-C. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci. Immunol. 2020;5:1–21. doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., Mills R., Teng E., Kamruzzaman M., Garcia-Beltran W.F., Astudillo M., Yang D., Miller T.E., Oliver E., Fischinger S., Atyeo C., Iafrate A.J., Calderwood S.B., Lauer S.A., Yu J., Li Z., Feldman J., Hauser B.M., Caradonna T.M., Branda J.A., Turbett S.E., LaRocque R.C., Mellon G., Barouch D.H., Schmidt A.G., Azman A.S., Alter G., Ryan E.T., Harris J.B., Charles R.C. Dynamics and significance of the antibody response to SARS-CoV-2 infection. medRxiv Prepr. Serv. Heal. Sci. 2020;1–31 doi: 10.1101/2020.07.18.20155374. [DOI] [Google Scholar]
- Jaimes J.A., André N.M., Chappie J.S., Millet J.K., Whittaker G.R. Phylogenetic analysis and structural modeling of SARS-CoV-2 spike protein reveals an evolutionary distinct and proteolytically sensitive activation loop. J. Mol. Biol. 2020;432(10):3309–3325. doi: 10.1016/j.jmb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju B., Zhang Q.i., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., Tang X., Yu J., Lan J., Yuan J., Wang H., Zhao J., Zhang S., Wang Y., Shi X., Liu L., Zhao J., Wang X., Zhang Z., Zhang L. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584(7819):115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- Ju, B., Zhang, Q., Ge, X., Wang, R., Yu, Jiazhen, Shan, S., Zhou, B., Song, S., Tang, X., Yu, Jinfang, Ge, J., Lan, J., Yuan, J., Wang, H., Zhao, J., Zhang, S., Wang, Y., Shi, X., Liu, L., Wang, X., Zhang, Z., Zhang, L., 2020b. Potent human neutralizing antibodies elicited by SARS-CoV-2 infection. bioRxiv 2020.03.21.990770. doi: 10.1101/2020.03.21.990770. [DOI] [PubMed]
- Kreer C., Zehner M., Weber T., Ercanoglu M.S., Gieselmann L., Rohde C., Halwe S., Korenkov M., Schommers P., Vanshylla K., Di Cristanziano V., Janicki H., Brinker R., Ashurov A., Krähling V., Kupke A., Cohen-Dvashi H., Koch M., Eckert J.M., Lederer S., Pfeifer N., Wolf T., Vehreschild M.J.G.T., Wendtner C., Diskin R., Gruell H., Becker S., Klein F. Longitudinal isolation of potent near-germline SARS-CoV-2-neutralizing antibodies from COVID-19 patients. Cell. 2020;182(4):843–854.e12. doi: 10.1016/j.cell.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas-Rangel F.A., Chávez-Valencia V. Laboratory findings that predict a poor prognosis in COVID-19 patients with diabetes: a meta-analysis. Endocrinología, Diabetes y Nutrición. 2020 doi: 10.1016/j.endinu.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-L., Liao C.-H., Liu P.-Y., Cheng C.-Y., Chung M.-Y., Liu C.-E., Chang S.-Y., Hsueh P.-R. Dynamics of anti-SARS-Cov-2 IgM and IgG antibodies among COVID-19 patients. J. Infect. 2020;81(2):e55–e58. doi: 10.1016/j.jinf.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Huang B., Wu M., Zhong A., Li L.u., Cai Y., Wang Z., Wu L., Zhu M., Li J., Wang Z., Wu W., Li W., Bosco B., Gan Z., Qiao Q., Wu J., Wang Q., Wang S., Xia X. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-19943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.-W., Sahi V., Figueroa A., Guo X.V., Cerutti G., Bimela J., Gorman J., Zhou T., Chen Z., Yuen K.-Y., Kwong P.D., Sodroski J.G., Yin M.T., Sheng Z., Huang Y., Shapiro L., Ho D.D. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584(7821):450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- Liu X., Wang J., Xu X., Liao G., Chen Y., Hu C.-H. Patterns of IgG and IgM antibody response in COVID-19 patients. Emerging Microbes Infect. 2020;9(1):1269–1274. doi: 10.1080/22221751.2020.1773324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.-X., Liu B.-Z., Deng H.-J., Wu G.-C., Deng K., Chen Y.-K., Liao P.u., Qiu J.-F., Lin Y., Cai X.-F., Wang D.-Q., Hu Y., Ren J.-H., Tang N.i., Xu Y.-Y., Yu L.-H., Mo Z., Gong F., Zhang X.-L., Tian W.-G., Hu L.i., Zhang X.-X., Xiang J.-L., Du H.-X., Liu H.-W., Lang C.-H., Luo X.-H., Wu S.-B., Cui X.-P., Zhou Z., Zhu M.-M., Wang J., Xue C.-J., Li X.-F., Wang L.i., Li Z.-J., Wang K., Niu C.-C., Yang Q.-J., Tang X.-J., Zhang Y., Liu X.-M., Li J.-J., Zhang D.-C., Zhang F., Liu P., Yuan J., Li Q., Hu J.-L., Chen J., Huang A.-L. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Marcos‐Jiménez, A., Sánchez‐Alonso, S., Alcaraz‐Serna, A., Esparcia, L., López‐Sanz, C., Sampedro‐Núñez, M., Mateu‐Albero, T., Sánchez‐Cerrillo, I., Martínez‐Fleta, P., Gabrie, L., Guerola, L. del C., Rodríguez‐Frade, J.M., Casasnovas, J.M., Reyburn, H.T., Valés‐Gómez, M., López‐Trascasa, M., Martín‐Gayo, E., Calzada, M.J., Castañeda, S., Fuente, H. de la, González‐Álvaro, I., Sánchez‐Madrid, F., Muñoz‐Calleja, C., Alfranca, A., 2020. Deregulated cellular circuits driving immunoglobulins and complement consumption associate with the severity of COVID‐19 patients. Eur. J. Immunol. null, eji.202048858. doi: 10.1002/eji.202048858. [DOI] [PMC free article] [PubMed]
- Nguyen, F.T., Akker, T., Lally, K., Lam, H., Lenskaya, V., Liu, S.T.H., Bouvier, N.M., Aberg, J.A., Rodriguez, D., Krammer, F., Strauss, D., Shaz, B.H., Rudon, L., Galdon, P., Jhang, J.S., Arinsburg, S.A., Baine, I., 2020. Transfusion reactions associated with <scp>COVID</scp> ‐19 convalescent plasma therapy for <scp>SARS‐CoV</scp> ‐2. Transfusion trf.16177. doi: 10.1111/trf.16177.
- Ni L., Ye F., Cheng M.-L., Feng Y.u., Deng Y.-Q., Zhao H., Wei P., Ge J., Gou M., Li X., Sun L., Cao T., Wang P., Zhou C., Zhang R., Liang P., Guo H., Wang X., Qin C.-F., Chen F., Dong C. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52(6) doi: 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., Lamers M.M., Sikkema R.S., de Bruin E., Chandler F.D., Yazdanpanah Y., Le Hingrat Q., Descamps D., Houhou-Fidouh N., Reusken C.B.E.M., Bosch B.-J., Drosten C., Koopmans M.P.G., Haagmans B.L. Severe Acute Respiratory Syndrome Coronavirus 2−Specific Antibody Responses in Coronavirus Disease 2019 Patients. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoan A., Sciacovelli L., Basso D., Negrini D., Zuin S., Cosma C., Faggian D., Matricardi P., Plebani M. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: a longitudinal study. Clin. Chim. Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petherick A. Developing antibody tests for SARS-CoV-2. The Lancet. 2020;395(10230):1101–1102. doi: 10.1016/S0140-6736(20)30788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Park Y.-J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., Peter A., Guarino B., Spreafico R., Cameroni E., Case J.B., Chen R.E., Havenar-Daughton C., Snell G., Telenti A., Virgin H.W., Lanzavecchia A., Diamond M.S., Fink K., Veesler D., Corti D. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- Qu J., Wu C., Li X., Zhang G., Jiang Z., Li X., Zhu Q., Liu L. Profile of IgG and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;0954162:1–4. doi: 10.1093/cid/ciaa489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., Hägglöf T., Oliveira T.Y., Viant C., Hurley A., Hoffmann H.-H., Millard K.G., Kost R.G., Cipolla M., Gordon K., Bianchini F., Chen S.T., Ramos V., Patel R., Dizon J., Shimeliovich I., Mendoza P., Hartweger H., Nogueira L., Pack M., Horowitz J., Schmidt F., Weisblum Y., Michailidis E., Ashbrook A.W., Waltari E., Pak J.E., Huey-Tubman K.E., Koranda N., Hoffman P.R., West A.P., Jr, Rice C.M., Hatziioannou T., Bjorkman P.J., Bieniasz P.D., Caskey M., Nussenzweig M.C. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., Rojas-Villarraga A., Ramírez-Santana C., Díaz-Coronado J.C., Manrique R., Mantilla R.D., Shoenfeld Y., Anaya J.-M. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun. Rev. 2020;19(7):102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar E., Christensen P.A., Graviss E.A., Nguyen D.T., Castillo B., Chen J., Lopez B.V., Eagar T.N., Yi X., Zhao P., Rogers J., Shehabeldin A., Joseph D., Masud F., Leveque C., Olsen R.J., Bernard D.W., Gollihar J., Musser J.M. Significantly decreased mortality in a large cohort of Coronavirus Disease 2019 (COVID-19) patients transfused early with convalescent plasma containing high-titer anti-severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) spike protein IgG. Am. J. Pathol. 2020;1–18 doi: 10.1016/j.ajpath.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self W.H., Tenforde M.W., Stubblefield W.B., Feldstein L.R., Steingrub J.S., Shapiro N.I., Ginde A.A., Prekker M.E., Brown S.M., Peltan I.D., Gong M.N., Aboodi M.S., Khan A., Exline M.C., Files D.C., Gibbs K.W., Lindsell C.J., Rice T.W., Jones I.D., Halasa N., Talbot H.K., Grijalva C.G., Casey J.D., Hager D.N., Qadir N., Henning D.J., Coughlin M.M., Schiffer J., Semenova V., Li H., Thornburg N.J., Patel M.M., Rasheed M.A.U., Mills L., Lester S.N., Freeman B., Alston B., Ategbole M., Browning P., Bolcen S., Boulay D., Cronin L., David E., Desai R., Epperson M., Gorantla Y., Jia T., Maniatis P., Ortiz K., Park S.H., Patel P., Qin Y., Tatum H., Zellner B., Baughman A., Hart K.W., McClellan R., McHenry R., Johnson J., Fletcher A., Cordero K., Kozikowski L., De Souza L., Romain S., Ouellette S., Santana A., Thornton-Thompson S., Howell M., Peers J., Shelton S., Finck L., Soules K., Klausner M., Calderon-Morales X., Erickson H.L., Hendrickson A., Stang J., Maruggi E., Dunn A., Stenehjem E., Aston V., Bown M., Matheu M., Smith R., Krol O., Salar A., Kamel M., Nguyen K., Huynh P., Karow S., Bright M., Bookless H., Mullins S., Neidert K., McGowan D., Cassandra E., Brown E., Carlin C., Wemlinger T., Edwards B., Flores L., LaRose M., Ferbas K.J., Martin-Blais R., Aldrovandi G.M., Thompson O., Sehgal S. Decline in SARS-CoV-2 antibodies after mild infection among frontline health care personnel in a multistate hospital network – 12 States, April–August 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:1762–1766. doi: 10.15585/mmwr.mm6947a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J.A., Hemmings O., O’Byrne A., Kouphou N., Galao R.P., Betancor G., Wilson H.D., Signell A.W., Winstone H., Kerridge C., Huettner I., Jimenez-Guardeño J.M., Lista M.J., Temperton N., Snell L.B., Bisnauthsing K., Moore A., Green A., Martinez L., Stokes B., Honey J., Izquierdo-Barras A., Arbane G., Patel A., Tan M.K.I., O’Connell L., O’Hara G., MacMahon E., Douthwaite S., Nebbia G., Batra R., Martinez-Nunez R., Shankar-Hari M., Edgeworth J.D., Neil S.J.D., Malim M.H., Doores K.J. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020;5 doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Y., Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020;323:1582. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanne J.H. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020;368 doi: 10.1136/bmj.m1256. [DOI] [PubMed] [Google Scholar]
- Tiberghien, P., Lamballerie, X., Morel, P., Gallian, P., Lacombe, K., Yazdanpanah, Y., 2020. Collecting and evaluating convalescent plasma for COVID‐19 treatment: why and how? Vox Sang. vox.12926. doi: 10.1111/vox.12926. [DOI] [PubMed]
- To K.-K.-W., Tsang O.-T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C., Yip C.-C.-Y., Cai J.-P., Chan J.-M.-C., Chik T.-S.-H., Lau D.-P.-L., Choi C.-Y.-C., Chen L.-L., Chan W.-M., Chan K.-H., Ip J.D., Ng A.-C.-K., Poon R.-W.-S., Luo C.-T., Cheng V.-C.-C., Chan J.-F.-W., Hung I.-F.-N., Chen Z., Chen H., Yuen K.-Y. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet. Infect. Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze J. More people are getting COVID-19 twice, suggesting immunity wanes quickly in some. Science (80-.) 2020 doi: 10.1126/science.abf7769. [DOI] [Google Scholar]
- Wajnberg, A., Amanat, F., Firpo, A., Altman, D.R., Bailey, M.J., Mansour, M., McMahon, M., Meade, P., Mendu, D.R., Muellers, K., Stadlbauer, D., Stone, K., Strohmeier, S., Aberg, J., Reich, D.L., Krammer, F., Cordon-Cardo, C., 2020. SARS-CoV-2 infection induces robust, neutralizing antibody responses that are stable for at least three months. medRxiv 1–13. doi: 10.1101/2020.07.14.20151126.
- Wu, J., Liang, B., Chen, C., Wang, H., Fang, Y., Shen, S., Yang, Xiaoli, Wang, B., Chen, L., Chen, Q., Wu, Y., Liu, J., Yang, Xuecheng, Li, W., Zhu, B., Zhou, W., Wang, H., Li, S., Lu, S., Liu, D., Li, H., Krawczyk, A., Lu, M., Yang, D., Deng, F., Dittmer, U., Trilling, M., Zheng, X., Xin Zheng, -Prof, Ulf Dittmer, -Prof, Fei Deng, -Prof, 2020. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19 Correspondence. medRxiv 2020.07.21.20159178. doi: 10.1101/2020.07.21.20159178.
- Wu, Y., Wang, F., Shen, C., Peng, W., Li, D., Zhao, C., Li, Z., Li, S., Bi, Y., Yang, Y., Gong, Y., Xiao, H., Fan, Z., Tan, S., Wu, G., Tan, W., Lu, X., Fan, C., Wang, Q., Liu, Y., Zhang, C., Qi, J., Gao, G.F., Gao, F., Liu, L., 2020. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science (80-.). eabc2241. doi:10.1126/science.abc2241. [DOI] [PMC free article] [PubMed]
- Xiao A.T., Gao C., Zhang S. Profile of specific antibodies to SARS-CoV-2: the first report. J. Infect. 2020;10–11 doi: 10.1016/j.jinf.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L., Wang Y., Guo X. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12:244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Liu W., Yu X., Wu M., Reichert J.M., Ho M. COVID-19 antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19. Antib. Ther. 2020;3:205–212. doi: 10.1093/abt/tbaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F., Xia X., Lv T. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J. Med. Virol. 2020;92:1890–1901. doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongchen, Z., Shen, H., Wang, X., Shi, X., Li, Y., Yan, J., Chen, Y., Gu, B., 2020. Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID-19 patients. Emerg. Microbes Infect. doi: 10.1080/22221751.2020.1756699. [DOI] [PMC free article] [PubMed]
- Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., Long X. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020 doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q.-L., Yu Z.-J., Gou J.-J., Li G.-M., Ma S.-H., Zhang G.-F., Xu J.-H., Lin W.-B., Cui G.-L., Zhang M.-M., Li C., Wang Z.-S., Zhang Z.-H., Liu Z.-S. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J. Infect. Dis. 2020;1–40 doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Chen L., Pan Y., Deng Q., Ye G., Li Y., Wang X. Re: Profile of specific antibodies to SARS-CoV-2: The first report. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Liu S., Tan T., Huang W., Dong Y., Chen L., Chen Q., Zhang L., Zhong Q., Zhang X., Zou Y., Zhang S. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest. 2020;1–5 doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Pang R., Xue X., Bao J., Ye S., Dai Y., Zheng Y., Fu Q., Hu Z., Yi Y. Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging (Albany. NY) 2020;12:1–7. doi: 10.18632/aging.103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., Qian S., Hong C., Wang F., Liu Y., Wang Z., He Q., Li Z., He B., Zhang T., Fu Y., Ge S., Liu L., Zhang J., Xia N., Zhang Z. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020;1–22 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.