Abstract
Immunological memory is a mechanism to protect us against reinfection. Antibodies produced by B cells are integral to this defense strategy and underlie virtually all vaccine success. Here, we explain how B cell memory is generated by infection and vaccination, what influences its efficacy and its persistence, and how characterizing these parameters in the immune response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will help achieve protective immunity through vaccination.
Immunological memory is a mechanism to protect us against reinfection. Antibodies produced by B cells are integral to this defense strategy and underlie virtually all vaccine success. Here, we explain how B cell memory is generated by infection and vaccination, what influences its efficacy and its persistence, and how characterizing these parameters in the immune response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will help achieve protective immunity through vaccination.
Main Text
Basic concepts of immunological memory
Creating a memory requires an event to change the status quo in a way that persists beyond the event itself. For the immune system, events that induce memory include infections with viruses and bacteria as well as vaccinations. These challenges trigger immune responses that can leave a footprint of their occurrence that can then persist for decades. Spread over the many years of our lives, we will encounter many infectious threats—some once and others multiple times. But if the first encounter created an immunologically protective state that persists, these repeat exposures can pass essentially unnoticed, producing no or very mild symptoms of infection. During the first (or primary) response, our immune system “learns” how to neutralize the invading organism, and this knowledge is retained as immunological memory, providing ongoing protection from the pathogen; this is immunity. Immunity can exist as a shield, functioning to block infection at the outset, or it can be reactive, triggered into rapid action by re-exposure to a pathogen that has avoided or overwhelmed the shield. However, having immune memory to a pathogen does not necessarily mean having immunity to reinfection; sometimes, immune responses are directed at irrelevant targets on the pathogen, and sometimes, the protective components of the immune memory can diminish to a point of non-functionality. Knowing if and for how long memory confers immunity and the requirements for generating and sustaining such memory, be that from infection or vaccination, are challenging but critical questions to be answered in understanding how to prevent infection.
Memory is a cardinal feature of so-called adaptive immune responses, which are those involving lymphocytes, key cells of the immune system. When an infection triggers a lymphocyte response, it induces a small number of pre-existing B and T cells (two types of lymphocytes) to proliferate, creating a small army of cells specific for, and thus able to combat, the infectious agent. B and T cells are triggered to respond by recognizing a part of the pathogen through their antigen receptors with a certain strength. Each B cell and T cell expresses on their surface an antigen receptor that is different to all others such that all possible invaders have their own, pre-existing, matching B and T cells ready to respond if and when required. During the response, some pathogen-specific B cells are induced to differentiate and secrete their antigen receptors into the blood in form of antibodies (Abs), which circulate through the body, binding to the pathogen that triggered the response wherever it occurs. Once the pathogen has been subdued and the response is effectively over, most pathogen-specific B and T cells die, but a small number of these recent combatants—B cells, T cells, and Ab-secreting plasma cells (PCs)—persist as specialized, long-lived immune memory cells.
In responding to infection, our immune system uses information from the pathogen to determine the scale and composition of the response so as to efficiently combat the pathogen, minimizing the energetic cost of the response and the risk of collateral damage to self. This includes selecting for those B cells with the highest binding strength (affinity) for their specific targets (antigens) on the pathogen. It is from among these “best of the best” that immune memory cells are recruited. Ideally, re-infection by a pathogen would not require a repeat immune response as Abs secreted by persisting PCs could bind to the pathogen and block its life cycle at the outset. These pathogen-specific Abs would act as a shield against reinfection. If the amount of Abs in circulation drops, or if the pathogen varies from the initial infection, the shield may not be protective, and a re-run of the response would be required. This response, triggered by re-exposure to the same or a closely related pathogen, uses the memory B and T cells, incorporating the information acquired in the first response by starting with cells that have already been selected as being strongly reactive. This head start makes memory responses faster, larger, and of higher affinity than the initial response, allowing for rapid negation of the pathogen, often before symptoms develop.
Given that an immune response is a life-or-death battle for a pathogen, microbes have evolved mechanisms to avoid or minimize recognition by the immune system, and many demonstrate co-evolution with immune responses. For example, a successful Ab response against a virus may focus on one specific target, called an epitope. If a virus mutates the epitope so it is no longer efficiently recognized by Abs, the variant may escape and be able to infect other individuals irrespective of their immunity to the initial strain. This could be prevented if there were sufficient breadth of recognition among the memory cells to react to the variant. Thus, while creating a memory of the initial pathogen is a crucial part of immunity, immune memory should have both specificity and the capacity to adapt to potential diversification of its targets.
Versatility through diversity: division of labor in “antibody memory”
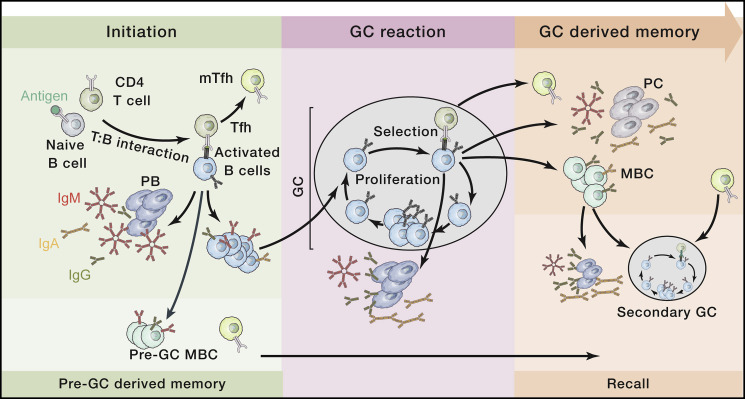
One of the most effective immune defense mechanisms of our body is the secretion of high-affinity Abs. Part of the effectiveness of Abs is that over the course of an infection, their affinity for antigen (binding strength) improves, and the class or isotype (immunoglobulin [Ig]M, IgG, IgA, IgE) changes, enabling Abs to better bind to and neutralize pathogens. Isotype refers to the section of the Ab that does not directly bind antigen but rather triggers other immune effector functions, such as activating the complement cascade or binding to receptors on immune cells to direct pathogen responses. Different isotypes activate different effector functions. Improvements in B cell affinity occur in specialized, transient, micro-anatomical structures called germinal centers (GCs), which develop in the secondary lymphoid organs such as lymph nodes and spleen following an immune challenge and are central to the production of immunological memory (Figure 1 ). Shortly after infection or vaccination, activated pathogen-specific CD4+ T cells and B cells interact. The T cells direct the B cells to proliferate, to switch the class of their antigen receptor (surface-bound Ab), to establish a GC, and for a fraction of these B cells, to differentiate into the initial form of Ab-secreting cells called plasmablasts (PBs). PBs appear within days of the infection and secrete Abs into the blood—and thus throughout the body—Abs that reflect the initial, best-available response to the pathogen (Figure 1, left). The B cells promote the differentiation of the T cells into specialized T follicular helper (Tfh) cells that thereafter direct the behavior of the B cells in the immune response.
Figure 1.
Generating protective antibody-mediated memory: the germinal center reaction
The initiation of a GC response takes place within a secondary lymphoid organ. Following T-B cell interaction, B cells rapidly proliferate, change their Ab class (isotype, e.g., IgM to IgG or IgA) and give rise to early PBs producing low-affinity Ab. At the same time, some cells will exit the immune response to become pre-GC MBCs. Remaining B cells go on to initiate and participate in the GC reaction where iterative cycles of proliferation, mutation, and selection increase the average antigen binding strength of the B cell receptors (affinity maturation). Throughout the response, some B cells will differentiate into short-lived PBs (secreting the now affinity-matured Abs), long-lived PCs, and MBCs. Upon recall, and if PC-derived Abs are insufficient for protection, both pre-GC and GC-derived MBCs can rapidly differentiate into PBs or initiate secondary GC responses together with mTfh.
Ab, antibody; PB, plasmablast; PC, plasma cell; MBC, memory B cell; GC, germinal center; Tfh, follicular helper T cell; mTfh, follicular helper memory T cell.
Within GCs, B cells rapidly proliferate and, remarkably, deliberately mutate the DNA encoding the epitope-binding component of their antigen-binding receptor, potentially changing its affinity. This occurs as repeated cycles of proliferation, mutation, and selective survival of those B cells with improved binding affinity to antigen (Figure 1, center). This “selection of the fittest” continues for the duration of the response or until antigen receptor binding strength reaches a maximum, meaning that B cell affinity is improving as the response progresses. Importantly, B cell mutation and selection in the GC provides a capacity to counteract escape attempts that pathogens may make by mutating their targets of the Ab response. GCs progressively give rise to two forms of long-lived “Ab memory”: circulating memory B cells (MBCs) and PC-secreting high-affinity Ab (Figure 1, right). In addition, some Tfh cells differentiate into long-lived memory Tfh (mTfh), completing the three branches of GC-derived memory that provide layered, Ab-mediated protection against re-infection.
PCs produced by the GC ultimately reside in spleen and bone marrow (BM), where they can survive in specialized niches for periods of years to decades, continuously secreting Abs independently of pathogen presence. These circulating Abs will be protective as long as the amount is sufficient to neutralize a pathogen inoculum without activating an immune response and without symptoms developing (Figure 2 )—the amount depending on Ab affinity as well as the size of the inoculum. What controls the persistence of PCs, and thus the duration of Ab production, remains largely unknown, but there is a strong association between the type of immune response and the durability of the PCs produced; viral infections typically produce very long-lived PCs, while vaccines based on components of the virus (subunit vaccines) tend to be less long lived. In addition, and irrespective of whether or not the Abs are neutralizing, PC-derived Abs will decorate the target (opsonize), aiding recognition by other components of the immune system and if required, promoting a secondary immune response (Figure 2). These outcomes are dictated to an extent by the isotype of the Abs bound to the pathogen, as different Ab classes activate different aspects of the immune response. Ab class switching is directed by soluble mediators (cytokines) secreted by the CD4+ T cells that “help” the B cells in the early stages of the response (Figure 1). The cytokine profile of CD4+ T cells, established during their initial activation, is based on invariant characteristics of the pathogen and the infection. By programming CD4+ T cells using attributes of the pathogen, information about the pathogen is reflected in the class of Abs that the B cells, PBs, MBCs, and PCs produce. In this way, the most useful effector properties of the immune system are recruited for each infection independently of Ab specificity and affinity. Ab class also influences Ab half-life and tissue distribution.
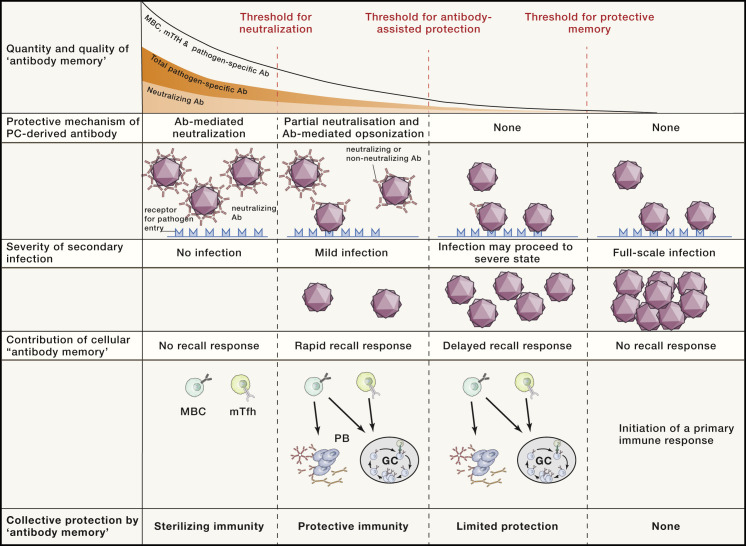
Figure 2.
Protective mechanisms of antibody-mediated memory during reinfection
In an infection primarily controlled by Abs, protective immunity depends on the quantity and quality of available Abs, MBCs, and mTfh cells. The presence of sufficiently high amounts of neutralizing Abs will result in sterilizing immunity, negating the requirement for a recall response. If the production of neutralizing Abs is insufficient or has waned and is thus exceeded by the pathogen load, an infection will occur. The remaining neutralizing Abs will still limit the extent of infection, and both neutralizing and non-neutralizing Abs will facilitate the initiation of secondary immune responses by increasing the visibility of the pathogen to the IS (opsonization). If circulating Abs have dropped below effective levels, the initial pathogen entry can occur largely unchecked, resulting in an infection of increased speed and magnitude before MBCs and mTfh cells can provide protection via a recall response. In some cases, Ab memory may completely dissipate, resulting in susceptibility to full-blown infection and the requirement for a primary immune response.
Ab, antibody; PB, plasmablast; MBC, memory B cell; GC, germinal center; mTfh, follicular helper memory T cell.
In contrast to sessile PCs, MBCs recirculate through the body and can be rapidly activated following reinfection to produce Ab-secreting PBs or initiate secondary GCs (Figure 1). This can occur if the amount of Abs in circulation is insufficient to block the new infection or if infection is by a variant of the initial pathogen that has escaped recognition by the existing, highly specific Abs. MBCs can respond to variants because as a population, they possess a broader spectrum of affinity and reactivity than is present in the long-term secreted Abs, even incorporating the initial reactivity (Figure 1, left). MBCs can rapidly initiate a secondary GC reaction and produce PBs, which will secrete Abs that will promote antigen capture and feed forward to amplify the secondary responses. These new GCs can also drive improved affinity for the variant pathogen, producing fresh MBCs and PCs with improved specificity and affinity for the new challenge. Where long-lived PCs have diminished to the point that Abs are insufficient for neutralizing immunity (Figure 2), the response starts with MBCs that have a higher affinity and are more frequent than in the first response, so establishing protective amounts of Abs of the correct class is significantly faster than in the initial response. mTfhs function like MBCs in that their presence increases the likelihood and speed of secondary Ab responses. The mechanism of pathogen recognition by CD4+ T cells is very different to that of B cells, relying on linear protein determinants that are mostly distinct from the Abs' recognition sites. The linked but distinct recognition by B cells and CD4+ T cells combating the same pathogen fosters breadth and flexibility of the response, provides resilience against immune evasion, and offers a safety check on inappropriate immune responses, maximizing the likelihood of maintaining immunity (Figure 2).
Memory shaped by infection and vaccination
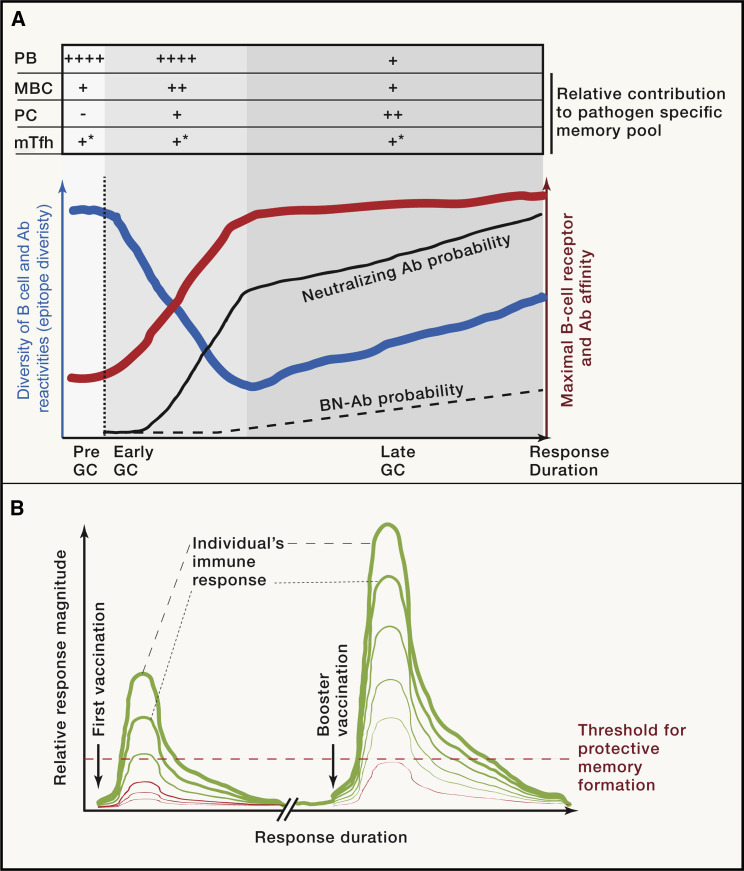
Ensuring the survival of the host is the principal goal of an immune response, and the formation of memory is subordinate to the course of infection. For example, if innate defense mechanisms are sufficient to hold an infection at bay, then an adaptive immune response will likely not be triggered, and as a result, lasting immunity may not be achieved. This comes at the cost of host susceptibility to re-infection and reflects a strategy for pathogens to keep circulating without the need for adaptation. When an adaptive immune response is initiated, the selection of Ab targets on the pathogen is guided by their physical properties and the host’s immune repertoire (the diversity of antigen-recognition receptors). Not every epitope is the target of an Ab response, and many viruses and bacteria have evolved to hide their most vulnerable regions, hindering effective neutralizing immune responses. So, having Abs specific to a pathogen does not imply immunity—the Abs have to be directed at appropriate targets that are retained unaltered by the pathogen. For example, influenza virus infection generates Ab responses that target several viral epitopes, some of which will block infection while others do little. For the influenza strain to successfully spread, it must alter those sites that are the targets of neutralizing Abs, but it can leave the remaining targets unaltered. Thus, although flu-specific Abs may be present, they may not block host infection. There is an important caveat to this: evading neutralization by changing a site targeted by the immune response is only possible to the extent that the alteration doesn’t interfere with the pathogen’s life cycle. Often, structures or proteins critical to the pathogen’s life cycle are invariant and conserved across related viral strains. Thus, Abs that recognize such targets can confer protection against multiple variants and are referred to as broadly neutralizing (BN)-Abs. As we’ve discussed, pathogens can disguise these sites, meaning that BN-Abs are often not generated or only generated after prolonged infection, as these hidden epitopes may require many rounds of B cell mutation and selection in the GC to achieve Abs with sufficient affinity to be neutralizing. Perhaps paradoxically, the opportunity for the immune system to generate BN-Abs may not arise if Abs that target other epitopes rapidly neutralize the infection (Figure 3 A).
Figure 3.
Immune response kinetics influence Ab diversity, affinity, and memory formation
(A) The initial diversity of B cell reactivities participating in an immune response is high, while their antigen binding affinity is generally low. During this pre-GC phase, pre-GC MBCs and short-lived PBs differentiate (producing low-affinity Abs). Once the GC reaction commences, affinity-based selection results in an increase in Ab affinity, while the diversity of targeted epitopes decreases. This is due to limited availability of resources (e.g., antigen, T cell help) that favors dominant epitopes to which high-affinity Abs can be rapidly generated. Once the GC reaches maximal Ab affinity, the response starts to re-diversify, now allowing more sub-dominant epitopes to be targeted. The probability of generating high-affinity neutralizing Abs increases rapidly during the first phase of the GC reaction, but BN-Abs often require prolonged immune responses due to their epitopes being sub-dominant. The response output is as staged: early on, rapid PB generation and MBC differentiation, while later, once affinity increases, long-lived PC generation.
(B) The immune response following vaccination is usually short lived, with the magnitude depending on the individual’s immune system. To achieve vaccine efficacy in a large fraction of the population, booster vaccination is often applied, recalling the memory cells already generated. The increased magnitude and duration of a secondary response improves memory generation to the point of sufficient quantity and quality for protection.
GC, germinal center; Ab, antibody; BN-Ab, broadly neutralizing Ab; MBC, memory B cell; mTfh, follicular memory T cell; PB, plasmablast; PC, plasma cell. (∗ when most mTfh differentiate is still largely unknown)
Another important parameter of the immune response, and therefore immunological memory, is the site of infection, such as respiratory tract, blood, or gut. For Abs, this can be reflected in choice of isotype (e.g., IgA is predominantly secreted into the mucosa, while IgG circulates in blood and lymphatics) and site of memory PC persistence (gut, spleen, BM). Pathogens do not necessarily complete their entire life cycle at one location, and both site and mechanism of initial entry may be independent of the remainder of the infection. Therefore, generating an immune response that prevents infection from happening may not necessarily be achieved by an immune response that targets a late-stage infection, as at that point, entry into the host is not a criterion for host survival.
Many characteristics of an immune response are determined by the type of infection; however, diversity in crucial immune system components and age-related immune dysfunction can lead to variability in the immune response in each individual to the one pathogen. In an extreme form, a virus may be controlled by one person’s immune system but be fatal to another. More generally, it means there will be variation within a population in the quality and quantity of the immune response to a pathogen, a variability that will also occur in response to vaccination. Finding the right vaccine formulation and vaccination schedule to ensure a strong and long-lasting response that induces protective immune memory in as many individuals as possible is crucial for vaccine success (Figure 3B). Vaccines given in the absence of infection can utilize delivery schedules that promote lengthy immune responses to achieve long-lived protective immunity, as host survival at that time does not require rapid eradication of the pathogen. While current vaccination strategies can generate durable memory in the vast majority of individuals, there are groups such as the elderly and immunosuppressed who show poor immune responses to both vaccination and infection, which often correlates with being most at risk of severe disease, as currently exemplified by coronavirus disease 2019 (COVID-19). By analyzing responses to infection, we know that immunity operating through Abs has to comprise Ab reactivity that (1) limits the pathogen life cycle, (2) activates other components of the immune system evolved to negate the pathogen, (3) is distributed to appropriate parts of the body, (4) has sufficient affinity so that relatively low amounts of Abs will be effective in neutralizing a pathogen inoculum, and, ideally, (5) should be produced for many years.
Memorizing SARS-CoV-2: what we know so far
Immunity to infection requires an appropriate form of immunological memory at the time of re-exposure. For a pathogen that is primarily controlled by Abs, this can be either sufficient Abs in bodily fluids to suppress infection at the outset or it can be memory B and T cells combining to rapidly produce the PBs that will then secrete protective Abs. The central questions with regard to immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are as follows. Does infection or vaccination lead to the production of immune memory that will neutralize future infection? And how long does protection last, and is it capable of recognizing variants of SARS-CoV-2 that arise in the population?
We are still early in characterizing immune responses to SARS-CoV-2 and its vaccine candidates, meaning we lack the systematic population studies required to determine the full extent of protection provided by previous infection or vaccination. Encouragingly, confirmed reports of re-infection are very rare, and Abs induced by either infection or vaccine candidates include known neutralizing specificities. There is every reason to be optimistic of immunity to SARS-CoV-2 developing from infection and from vaccination. The vast majority of people recovering from SARS-CoV-2 infection developed T cell and B cell memory. The MBCs found in recovered patients up to 6 months post infection produced Abs that neutralized SARS-CoV-2, indicating that they could produce PCs secreting protective Abs when re-stimulated. Remarkably, one recent study noted that MBCs increased in number and affinity in the 6 months post infection, implying an ongoing immune response despite the apparent clearance of the virus. Despite these findings, the novelty of SARS-CoV-2 means that uncertainty remains as to the durability of Ab production, reflected in contrasting studies noting decline or sustained production of Abs. But even if Ab amounts diminish over time, it appears very likely that persistent MBCs and T cells will rapidly produce additional, even improved, Abs on re-exposure. Definitive assessment of these aspects of immunity will require examination of COVID-19 patients several years post recovery, measuring specific Abs and memory B and T cells and correlating these with re-infection.
Understanding the requirements for Abs to neutralize SARS-CoV-2 is of major importance in predicting immunity following either infection or vaccination. If SARS-CoV-2 infection takes a severe course, GC formation is impaired, which will impact affinity maturation and memory quality and quantity, but it is unclear if this occurs during mild or asymptomatic infection. Having said that, the Ab structure required for neutralizing SARS-CoV-2 may not be particularly complex, as several of the reported neutralizing Abs contained few mutations, suggesting that they can be generated in relatively short-term GCs. Another encouraging aspect is that coronaviruses “proofread” their relatively large genome to maintain integrity, thereby limiting diversification, which is in contrast to most other RNA viruses that continuously evolve by mutating their genome. Nevertheless, some naturally occurring and experimental SARS-CoV-2 variants did circumvent neutralization by specific Abs while retaining infectivity. Importantly, these cases did not represent escape from the totality of anti-SARS-CoV-2 Abs present in serum of recovered patients but rather from a single species of Ab. While this emphasizes the value of the diversity of the immune response to SARS-CoV-2, it also shows why monitoring closely the viral strains circulating in communities is critical so as to adjust vaccines if required.
Recap
A hallmark of immune responses to pathogens is to create a memory of the response, which is the persistence of small numbers of pathogen-specific B and T cells and the PCs that secrete pathogen-specific Abs. Immune memory may block future infections without symptoms developing either by continued production of neutralizing Abs from long-lived PCs or, if that Ab amount diminishes, by recalling memory B and T cells to rapidly produce new PBs and thus restore high-affinity, neutralizing Abs in circulation. Protection from infection is also generated through vaccination, relying also on the continued or restored production of pathogen-specific, neutralizing Abs. The key challenges in establishing protective immune memory to a pathogen are producing high-affinity neutralizing Abs, maintaining production of such Abs, and counteracting any ongoing variability of the pathogen. The immune system has evolved to address these issues in the generation of memory, including affinity maturation in GCs, potentially life-long survival of PCs in specialized niches, and breadth of reactivity in MBCs. While these attributes frequently occur in response to infection, establishing PC—and thus Ab—longevity through vaccination remains inconsistent. Overall, however, the prospects of widespread immunity to SARS-CoV-2, either by infection or by vaccination, appear to be very strong, with durability being the remaining unknown that can only be answered with time.
Acknowledgments
D.T. was funded by National Health and Medical Research Council (NHMRC) Australia Investigator Award (APP1175411) and I.Q. by an Early Postdoc Mobility fellowship (P2ZHP3_164964) and an Advanced Postdoc Mobility fellowship (P300PA_177893) provided by the Swiss National Science Foundation and a Peter Doherty Early Career fellowship (APP1145136) provided by NHMRC Australia.
Declaration of Interests
The authors declare no competing interests.
Recommended Reading
- Akkaya M., Kwak K., Pierce S.K. B cell memory: building two walls of protection against pathogens. Nat. Rev. Immunol. 2020;20:229–238. doi: 10.1038/s41577-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanna I.J., Slifka M.K. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol. Rev. 2010;236:125–138. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juno J.A., Tan H.X., Lee W.S., Reynaldi A., Kelly H.G., Wragg K., Esterbauer R., Kent H.E., Batten C.J., Mordant F.L., et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020;26:1428–1434. doi: 10.1038/s41591-020-0995-0. [DOI] [PubMed] [Google Scholar]
- Li Q., Wu J., Nie J., Zhang L., Hao H., Liu S., Zhao C., Zhang Q., Liu H., Nie L., et al. The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity. Cell. 2020;182:1284–1294. doi: 10.1016/j.cell.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.F., Sahi V., Figueroa A., et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- Mesin L., Ersching J., Victora G.D. Germinal Center B Cell Dynamics. Immunity. 2016;45:471–482. doi: 10.1016/j.immuni.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtha W.E., Tedder T.F., Johnson S., Bhattacharya D., Diamond M.S. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J. Exp. Med. 2011;208:2599–2606. doi: 10.1084/jem.20110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph H.E., Barreiro L.B. Herd Immunity: Understanding COVID-19. Immunity. 2020;52:737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.J., Webster R.H., Tarlinton D.M. How intrinsic and extrinsic regulators of plasma cell survival might intersect for durable humoral immunity. Immunol. Rev. 2020;296:87–103. doi: 10.1111/imr.12895. [DOI] [PubMed] [Google Scholar]
- Rodda L.B., Netland J., Shehata L., Pruner K.B., Morawski P.M., Thouvenel C., Takehara K.K., Eggenberger J., Hemann E.A., Waterman H.R., et al. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell. 2020;184:169–183. doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora G.D., Wilson P.C. Germinal center selection and the antibody response to influenza. Cell. 2015;163:545–548. doi: 10.1016/j.cell.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C., Muecksch F., Rutkowska M., Hoffmann H.H., Michailidis E., et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife. 2020;9 doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel F., Shlomchik M. Memory B Cells of Mice and Humans. Annu. Rev. Immunol. 2017;35:255–284. doi: 10.1146/annurev-immunol-041015-055531. [DOI] [PubMed] [Google Scholar]