Abstract
The spread of the corona virus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been intensifying in the past year, posing a huge threat to global health. There is an urgent need for effective drugs and vaccines to fight the COVID-19, but their advent may not be quite fast. Drug repurposing is a feasible strategy in the current situation, which could greatly shorten drug development time and help to response quickly to the novel virus outbreak. It has been reported that histamine H1 receptor antagonists have broad-spectrum antiviral effects. Therefore, in this study, we aim to screen potential drugs among histamine H1 receptor antagonists that may inhibit SARS-CoV-2 infection. Based on the model of angiotensin-converting enzyme 2 (ACE2) overexpressing HEK293T cell membrane chromatography (CMC), five FDA-approved histamine H1 receptor antagonists were found to have bioaffinity to ACE2. Then we determined the interaction between these drugs and ACE2 by frontal analysis and surface plasmon resonance (SPR), which consistently demonstrated that these hits bind to ACE2 at micromolar levels of affinity. Through the pseudovirus assay, we finally identified that doxepin could inhibit SARS-CoV-2 spike pseudovirus from entering the ACE2-expressing cell, reducing the infection rate to 25.82%. These preliminary results indicate that the histamine H1 receptor antagonist, doxepin, is a viable drug candidate for clinical trials. Therefore, we hope the work timely provides rational help for developing anti-SARS-CoV-2 drugs to control the rapid spread of SARS-CoV-2.
Keywords: Histamine H1 receptor antagonist, Doxepin, SARS-CoV-2, Angiotensin-converting enzyme 2, Drug repositioning
Graphical abstract
1. Introduction
In December 2019, there was an outbreak of pneumonia with unknown etiology in Wuhan, China. The virus was later confirmed to be a deadly novel beta-coronavirus, named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Zhu et al., 2020). Due to the high infectivity and the lack of effective antivirus drugs, the pandemic remains out of control, leading to the explosive spread of SARS-CoV-2 worldwide. According to data released by Johns Hopkins University, as of January 2021, corona virus disease 2019 (COVID-19) has swept across 191 countries or regions around the world. The number of confirmed cases worldwide has exceeded 88 million, of which more than 1.91 million people have died, and this number is still growing (JHU, 2020).
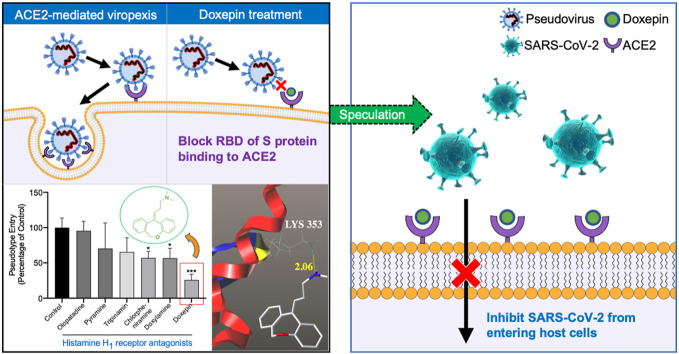
Angiotensin-converting enzyme 2 (ACE2), the key host cellular receptor of SARS-CoV-2 (Hoffmann et al., 2020), is primarily expressed in alveolar epithelial type II cells (Zhao et al., 2020). The binding of spike protein (S protein) to ACE2 is the first step of SARS-CoV-2 accessing into human cells (Shang et al., 2020). It is worth noting that the binding affinity of SARS-CoV-2 and human ACE2 is 10–20 times higher than that of S protein on SARS (Wrapp et al., 2020), which explains why the SARS-CoV-2 is highly contagious. Studies have shown that blocking the combination of SARS-Cov-2 spike and ACE2 can inhibit the infection of host cells (Wu et al., 2020), and therefore ACE2 is a critical target for the prevention and treatment of coronavirus (Gurwitz, 2020). Although vaccine is an effective means to block the binding of S protein to ACE2, the development period is relatively long (Corey et al., 2020). In addition, the S protein of SARS-CoV-2 may undergo a variety of mutations due to its high glycosylation, making vaccine development more difficult (Watanabe et al., 2020). Considering the severity of the pandemic, there is an urgent need to find other approaches to suppress the virus infection.
Drug repositioning is an effective alternative strategy in the current situation, which can accelerate the drug development and deployment of therapies for SARS-CoV-2 (Saul and Einav, 2020). For example, histamine H1 receptor antagonists directly down-regulate allergic inflammation by interfering with the effects of histamine H1 receptors on sensory neurons and small blood vessels, which are generally used for allergic rhinitis, allergic conjunctivitis, and allergic dermatitis (Simons and Simons, 2011).These drugs are widely available and relatively inexpensive, which make them ideal candidates for drug repurposing. Besides, it has been reported that histamine H1 receptor antagonists could inhibit the entry of Ebola viruses, Marburg viruses (Cheng et al., 2015), and influenza viruses (Xu et al., 2018) into host cells. Therefore, we speculate that there may be potential anti-SARS-CoV-2 drugs among histamine H1 receptor antagonists. Although it was previously proven that histamine H1 receptor antagonists have a wide range of antiviral properties, it remains unclear whether histamine H1 receptor antagonists could bind to ACE2 to inhibit SARS-CoV-2 infections.
In this study, we used cell membrane chromatography (CMC), a method that can mimic the binding characteristics of drugs and receptors in vitro, to screen for histamine H1 receptor antagonists that can bind to ACE2. On this basis, further computer simulations and in vitro affinity experiments were performed to determine the binding effect, and finally investigated whether it can inhibit virus infection through a pseudovirus model. The results are expected to verify whether histamine H1 receptor antagonists could bind to ACE2, thereby inhibiting SARS-CoV-2 from entering cells.
2. Material and methods
2.1. Drugs and reagents
Doxepin, chlorpheniramine, doxylamine, tripinamin, pyramine, and olopatadine were purchased from TargetMol (Boston, USA). Dulbecco's Modification of Eagle's Medium (DMEM) with high glucose (Cat. No. SH30022.01), and fetal bovine serum (Cat. No. 16140071) were from HyClone (Logan, UT, USA). Penicillin–streptomycin solution was obtained from Xi'an Hat Biotechnology Co., Ltd (Xi'an, China). Puromycin was purchased from Meilunbio (Dalian, China). SARS-CoV-2 spike pseudovirus was purchased from Sino Biological (Beijing, China). Luciferase assay system was purchased from Proemga Biotech (Madison, USA).
2.2. Cell lines
ACE2-overexpressing HEK293T cell (ACE2-HEK293T) line was constructed by Genomeditech (Shanghai, China) and its over-expression of ACE2 has been verified in our previous research (Wang et al., 2020). ACE2-HEK293T was cultured at 37 °C and 5% CO2 in Dulbecco's modification of eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 4 μg/ml puromycin.
2.3. ACE2-HEK293T/cell membrane chromatography (CMC)
The CMC refers to making the cell membrane into a stationary phase and then using HPLC to study the interaction of drug and membrane receptor, which can mimic the binding of drug and receptor in vivo (Han et al., 2018). ACE2-HEK293T/CMC columns (n = 3) were prepared with ACE2-HEK293T cells following the published protocol (Hou et al., 2009). The CMC screening was performed via LC-30A (Shimadzu, Japan). Various histamine H1 receptor antagonists dissolved with methanol were injected into the ACE2-HEK293T/CMC column, respectively. ACE2-HEK293T/CMC column 10.0 mm × 2.0 mm, flow rate 0.2 ml/min, column temperature 37 °C, mobile phase 2 mM phosphate-buffer saline (pH 7.4), detection wavelength 254 nm. Components with retention characteristics on ACE2-HEK293T/CMC columns were considered capable of combining ACE2.
2.4. Frontal analysis
The frontal analysis was performed to determine the dissociation equilibrium constant (K D) that is an important affinity parameter for drug-receptor interaction (Ma et al., 2017; Sanghvi et al., 2011; Slon-Usakiewicz et al., 2005). 50 mM Phosphate buffer (pH 7.4) was used as the mobile phase A of CMC-HPLC system to equilibrate the column. The mobile phase B was obtained by dissolving doxepin into the mobile phase A. The doxepin solution have different concentrations ranging from 1 × 10-7 M to 2 × 10-6 M by adjusting the ratio of mobile phase A and B. Each doxepin solution was pumped to the column continuously until a breakthrough curve with a plateau was produced. Next, the system should be switch back to the mobile phase A to elute the retained doxepin from the column under the same condition. The series of breakthrough curve were analyzed and K D values were determined on the basis of following formula:
| (1) |
Next, using the K D of doxepin as a reference, the relative standard method was used to calculate the K D of other drugs. The K Dx was determined according to F. (2).
| (2) |
The derivation and interpretation of formulas have been described in previous literature (Ma et al., 2017).
2.5. Cytotoxicity assay
Cell Counting Kit (7Sea Biotech, Shanghai, China) was used to determine cell viability. 5 × 103 of ACE2-HEK293T cells seeded into 96-well plates were incubated for 24 h under standard conditions (37 °C and 5% CO2). Then the medium was replaced with serum-free DMEM or DMEM containing histamine H1 receptor antagonists at concentrations of 25, 50, 100, and 200 μM. The total volume in each well was 100 μl. ACE2-HEK293T cells were incubated in these solutions for 24 h followed by treatment with 10 μl of CCK8 in each well for another 1.5 h at 37 °C. The plates were shaken softly before the detection of the optical density at 450 nm (OD450) using a microplate reader (Bio-Rad, USA). At least three independent experiments were performed. The survival rate of ACE2-HEK293T cells was calculated using the following formula:
| (3) |
2.6. Pseudovirus neutralization assay
Pseudovirus is a chimeric virus particle that expresses a recombinant glycoprotein of another virus on the surface of a replication-defective virus. Pseudovirus neutralization assay can mimic the infectious process of the live virus, which has a good correlation with that measured by the wild type SARS-CoV-2 assay (Xiong et al., 2020). ACE2-HEK293T cells (5 × 104 per well) were seeded into white 96-well plates and cultured at 37 °C until cells were adherent. 5 μl of pseudovirus (104.4 TCID50/ml, 860 ng SARS-CoV-2 spike S1 protein/ml) was added to each well, and incubated in a 37 °C incubator for 8 h. After that, the culture medium containing the pseudovirus was aspirated, replaced with 200 μl of new medium, and incubated at 37 °C for 48 h. Finally, the medium in the 96-well plate were aspirated, 20 μl of cell lysate was added to each well, and shook gently. By adding 100 μl of luminescence solution, luciferase luminescence was detected by FlexStation 3 in a luminescence mode at 560 nm, with exposure time of 1 s.
2.7. Molecular docking
The crystal structure of the SARS-CoV-2 spike receptor-binding domain (S-RBD) bound with ACE2 was obtained from the PDB database (PDB code: 6M0J). Molecular docking were performed with SYBYL-X 2.0 (Tripos, St. Louis, USA) to investigate the interactions between drug and ACE2.The docking model of ACE2 used in the study was based on the X-ray Diffraction performed by Jun Lan et al. (2020).
2.8. Surface plasmon resonance (SPR) analysis
To detect the affinity of small molecules with ACE2, SPR analysis was performed through Open SPRTM (Nicoya, waterloo, Canada). Sensor chips coated in nitrilotriacetic acid (NTA) (Nicoya, waterloo Canada) were used for capture ACE2 protein containing a poly-histidine tag. 200 μl of 200 mM imidazole and 40 mM NiCl2 were sequentially injected onto the chip surface to prime the sensor surface and charge the NTA with Ni2+. ACE2 was immobilized on NTA chip by loading the ACE2 protein into sensor surface. The pump ran at a flow rate of 20 μl/min. After the baseline was stable, the sample to be analyzed was injected. Experimental data was processed through TraceDrawer and the OneToOne fitting model was used to calculate K D values.
2.9. Statistical analysis
Data were analyzed using GraphPad Prism Software 8.0 (GraphPad Software, Inc., San Diego, CA, USA) and presented as the mean ± standard error of the mean (S.D.). Significant differences were determined by one-way ANOVA and Dunnett's test. Two-tailed unpaired Student's t-test was used to conduct all analyses between two groups. Differences were deemed statistically significant at P < 0.05 (* P < 0.05, ** P < 0.01, *** P < 0.001).
3. Results
3.1. Screening of compounds with ACE2 binding activity by CMC
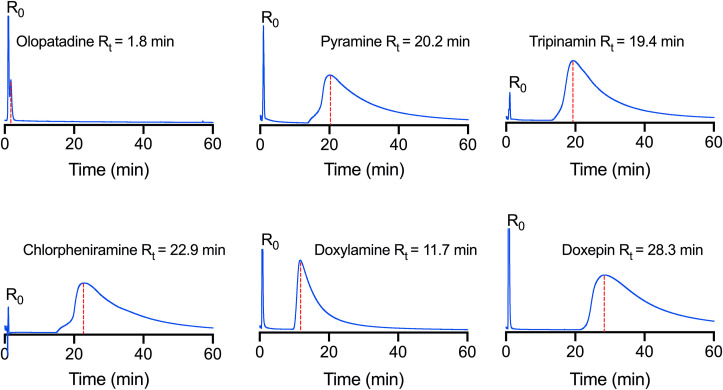
To discover histamine H1 receptor antagonists with ACE2 binding activity, cell membrane chromatography was used to randomly screen a series of histamine H1 receptor antagonists. We have screened out five active drugs form dozens of existing histamine H1 receptor antagonists and the results are shown in Fig. 1 . No significant retention was found in chromatogram of olopatadine, because its retention time is 1.8 min, which is very close to that of the solvent peak. Whereas pyramine, tripinamin, chlorpheniramine, doxylamine, and doxepin have strong retention on the ACE2-HEK293T/CMC column. Their retention time is 20.2, 19.4, 22.9, 11.7, and 28.3 min, respectively.
Fig. 1.
The chromatogram of histamine H1 receptor antagonists on the ACE2-HEK293T/CMC model. Experiments were repeated three times.
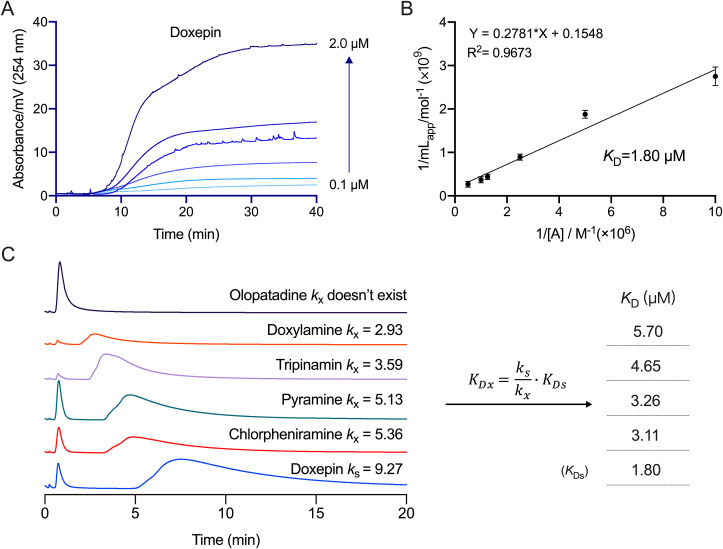
The frontier analysis method was used to determine the K D value. In this method, ACE2 on the cell membrane is closer to its actual spatial configuration, making it an effective technique to study the characteristics of drug-membrane receptor affinity. First, the K D value of doxepin-ACE2 was obtained through frontal analysis (Fig. 2 A and B) and found to be 1.80 μM. Then the K D values of other drugs were calculated by the relative standard method (Fig. 2C). The K D values of chlorpheniramine, pyramine, tripinamin, and doxylamine are 3.11, 3.26, 4.65, and 5.70 μM, respectively, indicating that these drugs have good affinity to ACE2. Since olopatadine doesn't reserve on the ACE2-HEK293T/CMC column, its K D cannot be calculated by this method.
Fig. 2.
The KD values of the histamine H1 receptor antagonists by ACE2-HEK293T/CMC model. (A) ACE2-HEK293T/CMC breakthrough curves of doxepin and the regression curves achieved by plotting mlapp versus 1/[A]. (B) The concentrations of drugs were 1 × 10-7, 2 × 10-7, 4 × 10-7, 8 × 10-7, 1 × 10-6, 2 × 10-6 M (from bottom to top), respectively. (C) The chromatograms of histamine H1 receptor antagonists on the ACE2-HEK293T/CMC column and the KD values of the drugs calculated by relative standard method. Experiments were repeated three times.
3.2. The effects of histamine H1 receptor antagonists on ACE2-HEK293T cells viability
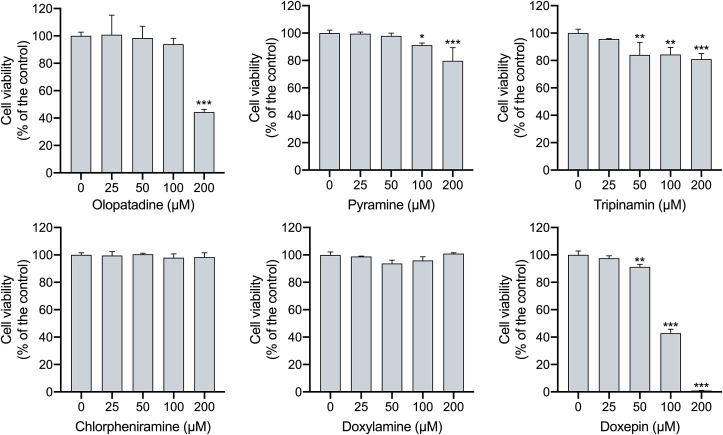
Cytotoxicity assay was performed to determine the drug concentration that is non-toxic to cells. The effects on ACE2-HEK293T cells viability of histamine H1 receptor antagonists at the concentration ranged 0–200 μM were detected by CCK-8 kit. It can be seen from Fig. 3 that chlorpheniramine and doxylamine shows sufficient safety at each test concentration. When the concentration is higher than 50 μM, tripinamin and doxepin begin to reduce cell the survival rate. Moreover, olopatadine and doxepin significantly deduces cell viability to 44.39% and 0.93% at 200 μM. Therefore, the concentration of 20 μM was used as the optimization of these drugs in subsequent experiments because it's non-toxic to ACE2-HEK293T cells.
Fig. 3.
Cytotoxic effects of histamine H1 receptor antagonists on ACE2-HEK293T cells. Experiments were repeated three times, and data are presented as the mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001, vs group of no histamine H1 receptor antagonists. n = 3.
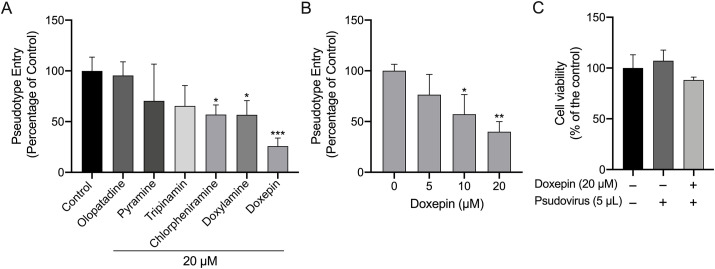
3.3. The effect of histamine H1 receptor antagonists on the entering of SARS-CoV-2 spike pseudovirus into ACE2-HEK293T cells
Pseudovirus infection tests were performed to detect potential anti-SARS-CoV-2 entry inhibitors. The results shows that pseudovirus infection rates on ACE2-HEK293T cells incubated with tripinamin, pyramine, and olopatadine didn't have statistically significant difference compared with the control group. Whereas chlorpheniramine, doxylamine, and doxepin significantly reduced the virus infection rate (56.91%, 56.75%, and 25.82%). As is shown in Fig. 4 A, doxepin displays the strongest inhibition of viral entry, while olopatadine had almost no inhibitory effect. Therefore, we conducted further pseudovirus test on different concentrations of doxepin, and the results showed that doxepin inhibited the entry of pseudovirus into ACE2-HEK293T cells in a dose-dependent manner (Fig. 4B). Simultaneously, cell viability was assayed using pseudovirus-treated samples with the same processing steps as the pseudovirus neutralization assay experiment. As shown in Fig. 4C, the pseudovirus has no obvious effect on the viability of ACE2-HEK293T cells, either in the doxepin treatment group or in the vehicle group. This result rules out the possibility of pseudovirus ultimately affecting luminescence by reducing cell viability.
Fig. 4.
(A) Inhibition on the entrance of SARS-CoV-2 spike pseudovirus after incubation with histamine H1 receptor antagonists at a non-cytotoxic concentration (20 μM) in ACE2-HEK293T cells. (B) Inhibition on the entrance of SARS-CoV-2 spike pseudovirus after incubation with doxepin at the concentrations of 0, 5, 10, and 20 μM in ACE2-HEK293T cells. Data are presented as mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001, vs group of no histamine H1 receptor antagonists. n = 3. (C) Cell viability of ACE2-HEK293T cells treated with pseudovirus.
3.4. Molecular docking conformation and interaction of histamine H1 receptor antagonists with ACE2
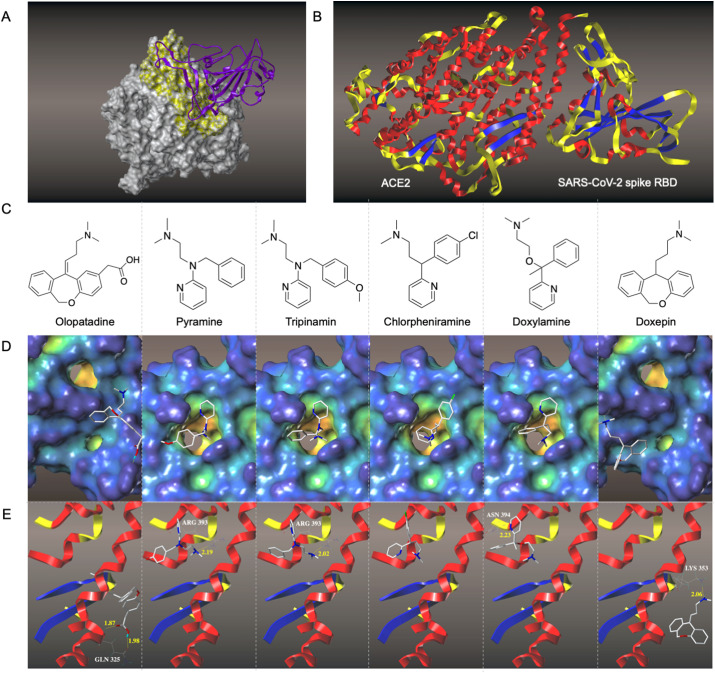
To investigate the interactions of histamine H1 receptor antagonists with ACE2, molecular docking test was performed. The docking posture SARS-CoV-2 spike RBD-ACE2 calculated by the Surflex-Dock mode of the SYBYL-X 2.0 is shown in Fig. 5 A. The binding site of the SARS-CoV-2 spike RBD is located at the bottom of the small lobe of ACE2 (Fig. 5B). The structures of the histamine H1 receptor antagonists are shown in Fig. 5C. It can be seen from Fig. 5D that pyramine, tripinamin, chlorpheniramine, and doxylamine have similar binding cavities on ACE2 with the typical dimethylamino group in the structure of the histamine H1 receptor antagonist extending into the binding pocket, while the binding cavities of doxepin and olopatadine are different from them. Doxepin's and olopatadine's binding sites that are closer to the section where SARS-CoV-2 spike RBD contacts ACE2. Since these drugs could reach into the pocket or form hydrogen bonds (Fig. 5E) with the amino acid residues of ACE2, which indicates the good interaction between these drugs and ACE2.
Fig. 5.
Molecular docking identifies possible binding pockets for histamine H1 receptor antagonists in ACE2. (A). 3D structure of SARS-CoV-2 spike RBD-ACE2 complexes. Complex structure of SARS-CoV-2 spike RBD (purple ribbon) and ACE2 (gray surface) are shown. The active binding site residues are shown in yellow surfaces. (B) Docked pose of SARS-CoV-2 spike RBD-ACE2. (C) Structures of histamine H1 receptor antagonists. (D) Surface representation of the best ranked docking pose of histamine H1 receptor antagonists in ACE2 binding pocket. (E) Residues involved in binding are displayed as sticks, with hydrogen bonds shown as dashed lines.
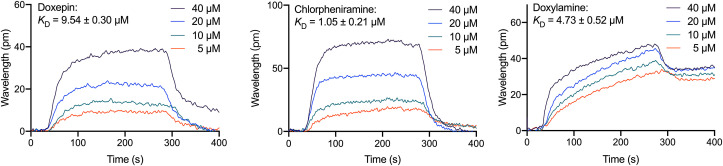
3.5. Analysis of bimolecular interactions between ACE2 and histamine H1 receptor antagonists
SPR was used to monitor the interactions between molecules and determine the binding specificity between ACE2 and the identified ligand molecules. In this study, the ACE2 protein was immobilized on a carboxyl sensor chip and the identified molecules were passed over the sensor's surface. Then the SPR sensorgrams of the compounds were recorded at the specific concentrations. Chlorpheniramine, doxepin, and doxylamine increased the SPR sensorgrams significantly and in a dose-dependent manner (Fig. 6 ). The dissociation constant K D was calculated by fitting the kinetic data at various concentrations of these drugs. The K D values of doxepin, chlorpheniramine, and doxylamine were recorded as 9.54 ± 0.30, 10.5 ± 2.1, and 47.3 ± 5.2 μM, showing a good interaction between the immobilized ACE2 and these drugs. The K D value of doxepin is the smallest among the tested drugs, in agreement with its strong activity of inhibiting pseudovirus entry.
Fig. 6.
Binding response curves and KD of doxepin, chlorpheniramine, and doxylamine to ACE2 proteins by SPR. Drugs were injected at the indicated concentration. Experiments were repeated three times.
4. Discussion
Due to the urgency of the increasingly serious global pandemic, screening drugs with antiviral potential from existing drugs could shorten the drug development cycle. Studies have shown that histamine H1 receptor antagonists have the antivirus potential, however whether they play a role in inhibiting SARS-CoV-2 infections is still unclear. Considering that the retention time of the compound on cell membrane chromatography reflects its affinity to membrane receptors (Yuan et al., 2005), we speculated that the drugs with potent retention in ACE2-HEK293T/CMC column may inhibit SARS-CoV-2 from infecting host cells by binding to ACE2. In this study, we first screened out five antiviral drug candidates from histamine H1 receptor antagonists used in clinical applications. Olopatadine, without obvious retention behavior, was used as a control. Then a drug concentration that does not affect cell viability for subsequent experiments was determined through cytotoxicity tests. Subsequently, we conducted pseudovirus infection tests with the selected drugs. After incubating ACE2-HEK293T cells with doxepin, the proportion of virus-infected cells were significantly reduced in a dose-dependent manner. Finally, the interaction and the K D of these drugs to ACE2 were investigated through molecular docking and SPR. These results suggested that doxepin has good binding ability to ACE2, indicating that it may inhibit the SARS-CoV-2 from entering host cells by binding to ACE2.
Drug repositioning is currently one of the main research and development strategies for drugs of SARS-CoV-2. Based on this rule, scientists all over the world are looking for specific drugs that can prevent the new coronavirus effectively. A large number of clinical trials have been conducted on chloroquine, hydroxychloroquine, remdesivir, favipiravir, bromhexine and other marketed drugs. However, some drugs are not as effective as expected (Geleris et al., 2020; Spinner et al., 2020; White et al., 2020). The world is still waiting for the emergence of specific drugs, which means that we need to further expand the scope of reposition of old drugs to meet the challenges of new virus. There have been reports on the screening of drugs that have an affinity with the main protease from the marketed drugs (Odhar et al., 2020; Udrea et al., 2020), and the screening of natural compounds which can inhibit ACE2 (Muchtaridi et al., 2020). Herein, we put forward the hypothesis of screening antiviral drugs from histamine H1 receptor antagonists that have been used in clinical practice with sufficient safety. In our study, we show for the first time, that histamine H1 receptor antagonists can bind to ACE2 and inhibit viral infection of host cells, which is a valuable supplement the research of antiviral drugs targeting ACE2. On the other hand, SARS-CoV-2 can activate mast cells in the submucosa of the respiratory tract to degranulate and release histamine, which may lead to aggravation of respiratory system inflammation (Kritas et al., 2020). Considering that antihistamines can prevent airway inflammation and bronchoconstriction caused by histamine, they may relieve the respiratory symptoms of COVID-19, although there is currently no clinical evidence that antihistamines are beneficial to COVID-19 patients. We hope our novel discovery will lead to more research to reveal its details.
Histamine H1 receptor antagonists have been found to inhibit the entry of a variety of viruses, but their mechanisms seems to be inconsistent. Studies have shown that the inhibition of Ebola virus and Marburg virus entry into cells by antihistamine drugs does not depend on the classical antagonism of histamine receptors, but inhibits the entry of viruses into host cells by interacting with glycoproteins on the virus surface (Cheng et al., 2015). It has been reported that carbinoxamine and chlorpheniramine do not interfere with HA-mediated virus attachment and NA-mediated virus release, but other steps of influenza virus entry (Xu et al., 2018). In our study, to further determine the effect of doxepin in inhibiting virus entry into cells, we analyzed its docking conformation with ACE2. Doxepin displays a bonding to the S protein at the molecular level, via hydrophobic interactions and hydrogen bonding. The hydrogen bond can be seen with Lys353, which is a residue that interact with the SARS-CoV-2 S protein RBD directly (Muchtaridi et al., 2020). Thereby doxepin prevented the S protein from contacting ACE2, which may be one of the reasons why it inhibited virus from entering into the cells. Interestingly, although olopatadine also formed hydrogen bonds with an important residue Gln325, its K D to ACE2 was much lower than doxepin. Therefore, no obvious inhibition of virus entry into cells was observed in pseudovirus assay. Due to the limited number of biosafety level 3 laboratories that can handle true viruses, our research is still at the computer or in vitro stage, and no further detailed research is available. It is recommended to compare our results with true virus tests conducted in vitro and in vivo, and provide evidence for the final attempt at clinical trials.
In conclusion, we found that doxepin could inhibit the entry of pseudoviruses into cells by binding ACE2. Thus, doxepin can be used as a potential anti-SARS-CoV-2 drug candidate. On the other word, our work provides a new perspective for the world to make good use of the existing drugs to respond to the challenge of the surge in number of the novel coronavirus infections.
Availability of data
All data generated or analyzed during this study are included in this published article.
CRediT authorship contribution statement
Shuai Ge: Conceptualization, Methodology, Investigation, Writing - original draft. Xiangjun Wang: Data curation, Investigation. Yajing Hou: Investigation, Software. Yuexin Lv: Formal analysis, Visualization. Cheng Wang: Investigation, Validation. Huaizhen He: Writing - review & editing, Supervision, Resources, All authors read and approved the final manuscript.
Declaration of competing interest
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (Grant Number: 81930096, 81903573), the Postdoctoral Research Foundation of China (Grant Number: 2018M643682), and the Natural Science Basic Research Plan in Shaanxi Province of China (Grant Number: 2020JQ-089).
References
- Cheng H., Lear-Rooney C.M., Johansen L., Varhegyi E., Chen Z.W., Olinger G.G., Rong L. Inhibition of Ebola and Marburg virus entry by G protein-coupled receptor antagonists. J. Virol. 2015;89:9932–9938. doi: 10.1128/JVI.01337-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L., Mascola J.R., Fauci A.S., Collins F.S. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368:948–950. doi: 10.1126/science.abc5312. [DOI] [PubMed] [Google Scholar]
- Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D.K., Kubin C., Barr R.G., Sobieszczyk M.E., Schluger N.W. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N. Engl. J. Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020;81:537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Lv Y., Wei F., Fu J., Hu Q., Wang S. Screening of bioactive components from traditional Chinese medicines using cell membrane chromatography coupled with mass spectrometry. Phytochem. Anal. 2018;29:341–350. doi: 10.1002/pca.2756. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Zhou M., Jiang Q., Wang S., He L. A vascular smooth muscle/cell membrane chromatography-offline-gas chromatography/mass spectrometry method for recognition, separation and identification of active components from traditional Chinese medicines. J. Chromatogr. A. 2009;1216:7081–7087. doi: 10.1016/j.chroma.2009.08.062. [DOI] [PubMed] [Google Scholar]
- JHU COVID-19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins university (JHU) 2020. https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- Kritas S.K., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Conti P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J. Biol. Regul. Homeost. Agents. 2020;34:9–14. doi: 10.23812/20-Editorial-Kritas. [DOI] [PubMed] [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Ma W., Yang L., Lv Y., Fu J., Zhang Y., He L. Determine equilibrium dissociation constant of drug-membrane receptor affinity using the cell membrane chromatography relative standard method. J. Chromatogr. A. 2017;1503:12–20. doi: 10.1016/j.chroma.2017.04.053. [DOI] [PubMed] [Google Scholar]
- Muchtaridi M., Fauzi M., Khairul Ikram N.K., Mohd Gazzali A., Wahab H.A. Natural flavonoids as potential angiotensin-converting enzyme 2 inhibitors for anti-SARS-CoV-2. Molecules. 2020;25 doi: 10.3390/molecules25173980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odhar H.A., Ahjel S.W., Albeer A., Hashim A.F., Rayshan A.M., Humadi S.S. Molecular docking and dynamics simulation of FDA approved drugs with the main protease from 2019 novel coronavirus. Bioinformation. 2020;16:236–244. doi: 10.6026/97320630016236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghvi M., Moaddel R., Wainer I.W. The development and characterization of protein-based stationary phases for studying drug-protein and protein-protein interactions. J. Chromatogr. A. 2011;1218:8791–8798. doi: 10.1016/j.chroma.2011.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul S., Einav S. Old drugs for a new virus: repurposed approaches for combating COVID-19. ACS Infect. Dis. 2020;6:2304–2318. doi: 10.1021/acsinfecdis.0c00343. [DOI] [PubMed] [Google Scholar]
- Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons F.E., Simons K.J. Histamine and H1-antihistamines: celebrating a century of progress. J. Allergy Clin. Immunol. 2011;128:1139–1150. doi: 10.1016/j.jaci.2011.09.005. e1134. [DOI] [PubMed] [Google Scholar]
- Slon-Usakiewicz J.J., Dai J.R., Ng W., Foster J.E., Deretey E., Toledo-Sherman L., Redden P.R., Pasternak A., Reid N. Global kinase screening. Applications of frontal affinity chromatography coupled to mass spectrometry in drug discovery. Anal. Chem. 2005;77:1268–1274. doi: 10.1021/ac048716q. [DOI] [PubMed] [Google Scholar]
- Spinner C.D., Gottlieb R.L., Criner G.J., Arribas Lopez J.R., Cattelan A.M., Soriano Viladomiu A., Ogbuagu O., Malhotra P., Mullane K.M., Castagna A., Chai L.Y.A., Roestenberg M., Tsang O.T.Y., Bernasconi E., Le Turnier P., Chang S.C., SenGupta D., Hyland R.H., Osinusi A.O., Cao H., Blair C., Wang H., Gaggar A., Brainard D.M., McPhail M.J., Bhagani S., Ahn M.Y., Sanyal A.J., Huhn G., Marty F.M., Investigators G.-U.-. Effect of remdesivir vs standard care on clinical status at 11 Days in patients with moderate COVID-19: a randomized clinical trial. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udrea A.M., Avram S., Nistorescu S., Pascu M.L., Romanitan M.O. Laser irradiated phenothiazines: new potential treatment for COVID-19 explored by molecular docking. J. Photochem. Photobiol., B. 2020;211:111997. doi: 10.1016/j.jphotobiol.2020.111997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Han S., Liu R., Meng L., He H., Zhang Y., Wang C., Lv Y., Wang J., Li X., Ding Y., Fu J., Hou Y., Lu W., Ma W., Zhan Y., Dai B., Zhang J., Pan X., Hu S., Gao J., Jia Q., Zhang L., Ge S., Wang S., Liang P., Hu T., Lu J., Wang X., Zhou H., Ta W., Wang Y., Lu S., He L. Chloroquine and hydroxychloroquine as ACE2 blockers to inhibit viropexis of 2019-nCoV Spike pseudotyped virus. Phytomedicine. 2020;79:153333. doi: 10.1016/j.phymed.2020.153333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Allen J.D., Wrapp D., McLellan J.S., Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369:330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.J., Watson J.A., Hoglund R.M., Chan X.H.S., Cheah P.Y., Tarning J. COVID-19 prevention and treatment: a critical analysis of chloroquine and hydroxychloroquine clinical pharmacology. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wang F., Shen C., Peng W., Li D., Zhao C., Li Z., Li S., Bi Y., Yang Y., Gong Y., Xiao H., Fan Z., Tan S., Wu G., Tan W., Lu X., Fan C., Wang Q., Liu Y., Zhang C., Qi J., Gao G.F., Gao F., Liu L. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368:1274–1278. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H.L., Wu Y.T., Cao J.L., Yang R., Liu Y.X., Ma J., Qiao X.Y., Yao X.Y., Zhang B.H., Zhang Y.L., Hou W.H., Shi Y., Xu J.J., Zhang L., Wang S.J., Fu B.R., Yang T., Ge S.X., Zhang J., Yuan Q., Huang B.Y., Li Z.Y., Zhang T.Y., Xia N.S. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg. Microb. Infect. 2020;9:2105–2113. doi: 10.1080/22221751.2020.1815589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Xia S., Pu J., Wang Q., Li P., Lu L., Jiang S. The antihistamine drugs carbinoxamine maleate and chlorpheniramine maleate exhibit potent antiviral activity against a broad spectrum of influenza viruses. Front. Microbiol. 2018;9:2643. doi: 10.3389/fmicb.2018.02643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B.X., Hou J., He L.C., Yang G.D. Evaluation of drug-muscarinic receptor affinities using cell membrane chromatography and radioligand binding assay in Guinea pig jejunum membrane. Acta Pharmacol. Sin. 2005;26:113–116. doi: 10.1111/j.1745-7254.2005.00015.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020;202:756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus I., Research T. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.